Abstract
Prolonged Exposure (PE) therapy is one of the most efficacious, evidence-based treatments for posttraumatic stress disorder (PTSD). A key component of PE involves in vivo exposures (IVEs) during which patients approach situations or activities in “real life” that are safe but avoided because they elicit a fear response. Despite their critical role in treatment, little research has focused on IVEs. This gap in knowledge is primarily due to the fact that IVEs are typically conducted by patients in between therapy sessions, leaving clinicians reliant upon patient self-report. This approach has numerous shortcomings, which the current study addresses by leveraging technology to develop an innovative device that allows for physiological, biomarker-driven, therapist-guided IVEs. The new system enables clinicians to virtually accompany patients during IVEs and provides real-time physiological (heart rate, skin conductance) and self-report (subjective units of distress) data that clinicians can use to modify the exposure and optimize therapeutic value. This Small Business Innovation Research (SBIR) Phase I project aims to: (1) integrate physiological sensors and live audio/visual streaming into a system for clinicians to guide patients during IVEs; (2) determine feasibility and acceptability of the system; and (3) conduct a pilot randomized clinical trial among veterans with PTSD (N = 40) to evaluate the preliminary efficacy of the system in reducing PTSD symptoms during PE. This paper describes the rationale, design, and methodology of the Phase I project. The findings from this study have the potential to innovate clinical practice, advance the science of exposure therapy, and improve clinical outcomes.
Keywords: Posttraumatic stress disorder, PTSD, Technology, Physiology, Military, Veterans
Abbreviations: CAPS-5, Clinician-Administered PTSD Scale for DSM-5; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; FDA, U.S. Food and Drug Administration; IRB, Institutional Review Board; MINI, Mini International Neuropsychiatric Interview; MUSC, Medical University of South Carolina; PCL-5, PTSD Checklist for DSM-5; PTSD, posttraumatic stress disorder; RCT, randomized controlled trial; VA, U.S. Department of Veterans Affairs; U.S., United States
1. Introduction
Military veterans are at increased risk of developing posttraumatic stress disorder (PTSD), a debilitating and chronic mental health disorder [[1], [2], [3], [4]]. If left untreated, PTSD increases risk of other psychiatric disorders (e.g., substance use disorders), medical problems, social/family impairment, employment problems, and suicidal ideation and attempts [[5], [6], [7], [8]].
Prolonged Exposure (PE) therapy is one of the most effective treatments for PTSD, with response rates ranging from 65 to 80% [[9], [10], [11]]. A key component of PE involves in vivo exposures (IVEs) during which patients approach safe, but avoided, stimuli in “real life” (e.g., crowded stores, driving) because they remind the patient of the trauma or increase PTSD symptoms. IVEs are crucial to exposure therapy for PTSD (and all anxiety disorders) to ensure that new knowledge and behaviors learned in the therapy session are successfully transferred to patients' “real world” environment. Although IVEs are a key treatment component, their content is “invisible” to the clinician, as they typically occur outside the office and without professional assistance. This poses a major logistical challenge because there is no way for the clinician to monitor or guide the patient in this critical aspect of treatment. In rare cases, clinicians leave the office and go to IVE sites with patients. More commonly, especially during the COVID-19 pandemic, patients are given in vivo assignments to complete on their own before the next session and the clinician is reliant upon patient self-report of the IVE. Unfortunately, some patients fail to attempt IVEs given avoidance symptoms, or when they do attempt the exercise, they are under-engaged or do not remain in the situation for a sufficient length of time. Physiological indicators of distress, such as skin conductance and heart rate, are objective indices of therapeutic engagement and extinction learning, and several studies show that greater activation and reactivity before and during PE therapy sessions (i.e., imaginal exposures) is associated with improved treatment outcomes [[12], [13], [14], [15], [16]]. However, physiological activation during IVEs has not yet been investigated.
The current study leverages technology to mitigate current limitations and address gaps in knowledge by enabling therapists to virtually accompany and modify IVEs, in real-time, based on patient-specific physiological and subjective data to optimize engagement and maximize the therapeutic value of IVEs. This paper describes the design and methodology of an ongoing randomized controlled trial (RCT) to evaluate adjunctive technology in reducing PTSD severity among military veterans.
2. Research objectives and hypotheses
The primary research objective of the current study is to address key gaps in knowledge regarding IVEs during PE therapy by developing and evaluating a technology-based system that will (1) enable clinicians to virtually accompany patients during IVEs to enhance engagement and successful completion of the exercises, and (2) provide clinicians with real-time streaming of physiological biomarkers of affective engagement (i.e., galvanic skin response [GSR] and heart rate [HR]) and subjective units of distress (SUDS). Therapists will use this information to guide and modify the exercises in real-time to address under- or over-engagement, thereby maximizing therapeutic value of IVEs. To accomplish this, Zeriscope, a small business with experience developing cutting-edge mobile technology platforms for health care, is partnering with clinical researchers at a Southeastern academic medical institution to develop and preliminarily evaluate the technology device (“Bio Ware”) among veterans with PTSD.
Aim 1 of the project is to integrate physiological biomarker sensors (GSR, HR) with SUDS and live audio/visual streaming into a device for office-based clinicians to use and communicate with patients during IVE. Two-way audio allows the patient and clinician to communicate with one another. The video stream is one-way and only allows the clinician to see the patient's environment. This requires development in three domains: (1) patient interface, (2) clinician “dashboard,” and (3) cloud-based storage and analytics. Aim 2 of the project is to determine feasibility and acceptability of the new system in a small sample of veterans (n = 5). Finally, Aim 3 is to conduct a pilot randomized controlled trial (RCT) of treatment-seeking veterans with current PTSD (N = 40) to evaluate the acceptability and preliminary efficacy of the device and investigate predictors of positive treatment outcomes.
3. Materials and methods
3.1. Research design
This study is a Small Business Innovation Research (SBIR) Phase I grant to develop and evaluate a novel technology system to enhance Prolonged Exposure (PE) therapy for the treatment of PTSD. End-user input will be used to guide the process and tailor the system to ensure maximum relevance and patient acceptability. A pilot RCT will be conducted where PE will be delivered for up to 12 sessions. Outcome measures will evaluate feasibility and acceptability, as well as self-reported and clinician-rated PTSD symptoms.
3.2. General procedures of the technology system build and beta testing
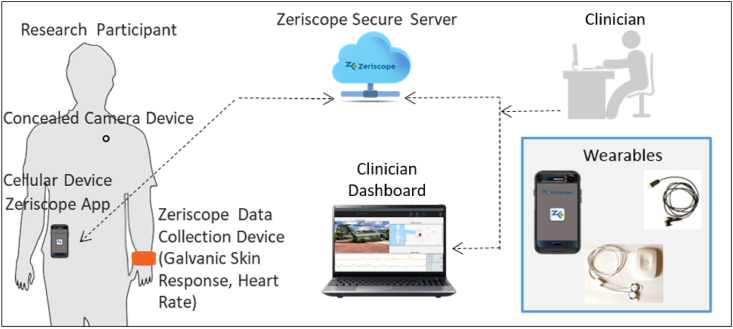
Zeriscope will design, build, and test a multidimensional data acquisition and storage system to capture real-time biomarkers of engagement and self-report during IVEs. This innovation will be realized using wearables linked to a cloud-based system for live streaming data, online storage, and analysis. The system will include a customized (1) patient interface, (2) clinician interface, and (3) cloud-based system (see Fig. 1). The patient interface will consist of (a) wearables that enable continuous collection of quantitative (e.g., heart rate, galvanic skin conductance) and qualitative data (e.g., video) with as little burden as possible to the patient, and (b) software application on a mobile phone. The system will include: (a) local (phone) session storage when no cellular or Wi-Fi service is available, (b) keypad for self-report of SUDS, (c) geo-location, (d) application login and authentication, and (e) intuitive user control and notification features. The wearables will be comfortable and designed to be inconspicuous (e.g., camera looks like a shirt button). The clinician interface will include a “dashboard” or display (see Fig. 1) to enable live video of the patient's environment; two-way audio to facilitate communication between patient and clinician; real-time streaming of HR, GSR, and subjective ratings of distress (SUDS); and a secure system to store and review completed IVEs offline. Clinicians will be able to demarcate moments of interest during IVEs (e.g., with a time stamp or event marker) on the clinician dashboard, which may be peaks in the patient's physiological or subjective levels. Event markers will be stored and can be reviewed in the future to help prepare for subsequent IVEs to optimize engagement and efficiency. The dashboard will include summary statistics for each IVE, such as the total number of minutes spent in the exercise, as well as peak HR, GSR, and SUDS ratings. The cloud-based system will be HIPAA-compliant and designed to rapidly store quantitative and qualitative data from IVEs. The database will be secure and backed up daily.
Fig. 1.
Overview of the Bio Ware technology-based system for therapist-guided in vivo exposures (IVEs).
Beta testing will be used to preliminarily evaluate the feasibility and acceptability of the new system with 5 veterans who have previously completed PE. Potential participants will be given a full description of the study and asked to read and sign an IRB-approved informed consent form before any study procedures take place. Participants will receive instructions for turning on/off the system. Feasibility will be assessed by evaluating if participants can turn on/off the system in ≤5 min. Each participant will then complete one “mock” IVE and provide feedback on perceptions of comfort, usability, helpfulness, acceptability, and patient satisfaction. Subjects will be recruited from clinician referral and flyers and receive $25 for volunteering in the beta testing. Based on the beta testing, final system modifications or improvements will be made in preparation for the RCT. The remaining sections below describe the methods and design of the RCT for this project.
3.3. General procedures of the RCT
Interested individuals will be screened by telephone or in person. Individuals who meet inclusion/exclusion criteria will be invited for a comprehensive baseline assessment (see Table 1) to determine eligibility for enrollment. Potential participants will be given a full description of the study and asked to read and sign an IRB-approved informed consent form before any study procedures occur. All eligible and interested participants will then receive 10 to 12, 90-min sessions of PE therapy (described below). Following the treatment phase, participants will be asked to complete a one-month follow-up visit. All participants receive a kit with a phone to use during the study. Ineligible individuals are referred clinically for treatment.
Table 1.
Assessment measures and timeline.
| Instrument | Purpose/Domain | BSL | Weekly | End of TX | 1-Month Follow-Up |
|---|---|---|---|---|---|
| Informed Consent | Obtain informed consent | X | |||
| Demographics Form | Characterize sample demographics | X | |||
| MINI International Neuropsychiatric Interview | Assess DSM-5 psychiatric disorders | X | |||
| Adverse Events: AEs | Monitor AEs and safety | X | X | X | |
| Life Events Checklist | Assess lifetime trauma exposure | X | |||
| Clinician Administered PTSD Scale: CAPS-5 | PTSD symptom severity (clinician-rated) | X | X | X | |
| PTSD Checklist: PCL-5 | PTSD symptom severity (self-report) | X | X | X | X |
| Beck Depression Inventory-II: BDI-II | Measure depressive symptoms | X | X | X | X |
| Client Satisfaction Questionnaire-8: CSQ | Assess satisfaction with treatment | X | |||
| Helping Alliance Questionnaire: HAQ-II | Measure therapeutic alliance | X | |||
| System Usability Survey: SUS | Assess usability and comfort | X | X | ||
| In Vivo Exposures: IVEs | Assess biological and self-report indices of engagement (HR, GSR, SUDS), feasibility, and acceptability | X |
Note. BSL = baseline, TX = treatment, HR = heart rate, GSR = galvanic skin response, SUDS = Subjective Units of Distress Scale.
Participants. Participants (N = 40) will be U.S. military veterans between the ages of 18 and 65 with current PTSD. Participants will be recruited from the Ralph H. Johnson VA Medical Center in Charleston, SC, local veterans' groups, and online social media advertisements (e.g., Facebook). Inclusion criteria include being able to comprehend English and meet DSM-5 diagnostic criteria for current (i.e., past 6 months) PTSD as assessed by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5; [17]. Participants may also meet criteria for a mood disorder (except unstable bipolar disorder) or anxiety disorder. The inclusion of participants with affective and other anxiety disorders is essential because of the marked frequency of the co-existence of mood and other anxiety disorders among patients with PTSD [18]. Individuals taking psychotropic medications will be required to be maintained on a stable dose for at least four weeks before study initiation. Individuals with clinically significant medical or psychiatric conditions that in the opinion of the investigators may adversely affect safety will be excluded and referred for treatment to ensure they receive the appropriate level of clinical care. Participants enrolled in ongoing evidence-based behavioral therapy for PTSD will be excluded.
Prolonged Exposure (PE) Therapy and Procedures for using Bio Ware During IVEs. PE will be delivered based on the manual developed by Foa and colleagues [19]. During session 1, the Study Therapist will provide the rationale and an overview of PE, review trauma history, instruct the patient on breathing retraining, and provide a brief overview of the device to be used in the study. Prior to session 2, a brief (15–30 min) in-service appointment will be scheduled with a Bio Ware technician to familiarize the patient with the device, provide instructions on how to properly use the equipment, and answer any questions. In-service training with the Bio Ware technician was chosen for this first project, however it is feasible that therapists can provide the device training. A step-by-step instruction manual will also be provided to participants. Session 2 includes a review of common reactions to trauma, introduction of SUDS, in vivo hierarchy development, and a brief review (∼5 min) of how to use the device. Starting in session 3 through the final session of treatment, in vivo “homework” is reviewed, the next in vivo assignments are planned, and imaginal exposure is conducted followed by emotional processing. During the final session, the therapist and patient review treatment progress and discuss potential next steps depending on each patient's needs (e.g., couples therapy, vocational counseling). Participants receive 10 or 12 sessions of PE, determined by reductions in the PCL-5 assessment [20]. Participants with less than a 10-point reduction on the PCL-5 by session 10 will be offered 2 additional sessions. Study Therapists will have a master's or doctoral degree and attend weekly supervision during the trial. Sessions will be recorded, and a subset (10%) randomly selected and rated for fidelity to the manual using existing checklists.
Participants will be randomly assigned, using a randomization scheme developed by a statistician not associated with the project, to either (1) therapist-guided IVEs or (2) record-only IVEs. In the therapist-guided group, study therapists will schedule therapist-guided IVE sessions between PE treatment sessions, starting after session 2. During therapist-guided IVE sessions, therapists will log into the online clinician dashboard to connect with the patient using the Bio Ware wearable device and start the IVE. Therapists will use the clinician dashboard to view the patient's real-time physiological data (e.g., HR, GSR) and their SUDS (recorded every ∼5 min for therapist-guided IVEs) and use this information to modify the patient's behavior during the exposure. For example, if a patient is not exhibiting increased HR, GSR, or SUDS during the exercise, the therapist can make recommendations to help increase the patient's engagement (e.g., instruct the patient go down a more crowed shopping aisle, instruct the patient to sit with their back to the door instead of facing it). Therapists will virtually accompany patients to at least three IVEs during the first few weeks of treatment and may attend one IVE each week as needed. Therapists and patients will collaboratively discuss which IVEs are the most appropriate to select as therapist-guided IVEs. After selecting these IVEs, therapists and patients will find an appropriate time to schedule the IVE. IVEs can be completed outside of normal office hours (e.g., weekends, evenings) if needed. In the record-only group, participants will be instructed to wear the device during their IVEs, but the therapist will not virtually accompany them, nor will the therapist review the recorded IVEs. The record-only condition is similar to standard PE with the exception that the participants wear the device, which records physiological activity and SUDS from IVE activities. Consistent with standard PE, patients report on the IVE to the therapist at the next session. As part of this project, therapists do not watch recorded IVEs in the record-only condition. In the case that participants in either condition do not have Wi-Fi or cellular service, hot spots will be provided for the duration of the study.
Primary Outcome Measures. Primary clinical outcomes include the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5 [17]; for clinician-rated, and the PTSD Checklist for DSM-5 (PCL-5 [20]; for self-reported PTSD symptoms. The CAPS-5 is a semi-structured diagnostic interview and gold standard for assessing PTSD. The CAPS-5 is rated on a 5-point scale (0 = absent to 4 = extreme/incapacitating) with a total score ranging from 0 to 80. The CAPS-5 will be administered at the baseline, end-of-treatment, and at 1-month follow-up. The PCL for DSM-5 is a 20-item self-report measure that will be used assesses PTSD severity weekly and at one month follow-up. The PCL-5 has excellent psychometric characteristics.
Secondary Outcome Measures. Secondary outcome measures include IVE related assessments of psychophysiological reactivity (HR and GSR), which will be measured continuously during IVEs using the patient worn data acquisition system (Zeriscope Inc.). Subjective units of distress (SUDS; 0 to 100 range) will also be collected via numeric keypad every 5 min during IVEs for the therapist-guided IVEs. Pre, post, and peak SUDS will be collected via numeric keypad for record-only IVEs. Finally, self-report questionnaires will assess acceptability by measuring comfort, willingness to use, utility, and client satisfaction [16,[21], [22], [23]]. Information regarding additional measures can be found in Table 1.
Compensation. Participants will receive $25 for volunteering in the beta testing. In the RCT, participants will receive $50 for baseline and follow-up visits, and up to $400 for completing the weekly visits.
3.4. Hypotheses and data analytic plan
We hypothesize that Bio Ware will be feasible and acceptable, as evidenced by at least 80% of participants (1) being able to turn on/off the equipment in ≤5 min and (2) reporting positive perceptions toward the device via self-report assessments. We hypothesize that participants in the therapist-guided group will evidence greater reductions in PTSD severity at the end of treatment as compared to the record-only group, as measured by the CAPS-5 and PCL-5. However, given the small sample size and use of an evidence-based therapy (PE) in both treatment conditions, differences between groups may not reach statistical significance. Bootstrapping will be employed, and effect sizes will be calculated to estimate differences in PTSD reductions within and between treatment groups to inform future research. In addition, we hypothesize that higher levels of physiological (GSR, HR) and subjective (SUDS) engagement during IVEs will be associated with greater reductions in PTSD symptoms at end of treatment.
All analyses will be performed on the intent-to-treat (ITT) sample consisting of all randomized subjects. Given the Phase I developmental nature of the project, participants will be randomized in a 3:1 fashion to allow for more experience and data collection from the therapist-guided condition. Baseline clinical and demographic characteristics are collected, and contrasts will be performed between treatment groups. Baseline characteristics that are associated with the primary outcome measures will be included as covariates in subsequent analyses.
To test the hypothesis that the device will be feasible and acceptable, we will examine the percentage of participants who are able to turn on/off the equipment in ≤5 min and participant perceptions of ease, comfort, utility, and acceptability using self-report measures (see Table 1). To test the hypothesis that participants in the therapist-guided group will evidence significantly greater pre-to post-treatment reductions in PTSD severity as compared to the record-only group, we will use independent generalized linear mixed effects models to examine CAPS-5 and PCL-5 scores at end of treatment by group. Models will be adjusted, respectively, for baseline PCL-5 and CAPS-5. Group will be entered as the predictor variable. Bootstrapping will be employed to enhance statistical power. In addition, effect sizes will be calculated for within group and between group changes in PTSD symptoms. To test the hypothesis that higher levels of physiological and subjective engagement will predict greater treatment response, we will calculate mean HR, GSR, and SUDS responses during IVEs and use multilevel modeling to analyze these variables as predictors of PCL-5 and CAPS-5 at end of treatment within and between treatment groups.
Exploratory analyses will examine group differences in the rate of reduction in PTSD symptoms, attrition, and durability of effects at 1-month follow up. Data from both groups will be harnessed to preliminarily explore the development of patient-specific algorithms predicting optimal engagement during IVEs, allowing each patient to be fitted with a precise algorithm for IVEs personalized to their biological and subjective responding.
4. Discussion
This paper describes the research design and methodology for an ongoing SBIR Phase I project that aims to improve PE therapy for PTSD using biomarker-driven technology. While PE is a highly effective, evidence-based treatment for PTSD, an estimated one-third of patients who complete PE remain symptomatic and drop-out rates are a concern in this population [24]. Advances in mobile technology can help address these shortcomings and improve treatment outcomes.
Over the last decade, there has been a notable increase in the use of digital technologies to improve PTSD treatment. Acierno and colleagues employed telehealth delivery of PE to overcome access barriers to care (e.g., transportation problems, travel costs) with comparable efficacy and patient satisfaction [[25], [26], [27], [28], [29]]. This is now widely used given the ongoing COVID-19 pandemic [[30], [31], [32]]. The advent of mHealth applications (e.g., PE Coach [33]; provides psychoeducation, self-management tools, and homework tracking. Importantly, these applications are rated by clinicians and patients as feasible, acceptable, and highly promising [[34], [35], [36], [37]]. Virtual Reality (VR) for PTSD, which was pioneered by Rothbaum and colleagues, uses computer-generated, simulated environments presented to patients via head-mounted displays to facilitate exposures [[38], [39], [40], [41]]. Importantly, research consistently shows that digital interventions are as effective as face-to-face interventions when coupled with human support [[42], [43], [44]]. Thus, while some people may be able to fully benefit from standalone digital interventions, the majority require human support or coaching to achieve more consistent engagement and positive outcomes. Taken together, this growing body of literature suggests patients with PTSD are open to and can benefit substantially from technology-enhanced services.
The technology-based system being investigated in the current study adds to the growing body of research in this area by being among the first to target IVEs in the patient's real-world environment, bringing biometric and behavioral data “from the field” into the clinician's office and treatment planning in ways that have not previously been possible. Clinicians will be able to use objective biomarkers of engagement during the IVEs to evaluate and modify patients' behaviors during IVEs in real-time to optimize outcomes. This new technology system may increase patients' willingness, confidence, and ability to attempt and complete exposures effectively (e.g., remain in the situation for the full 45 min, reduce or eliminate safety behaviors during the exposure). The new system also serves as a means of accountability, which can help enhance motivation (e.g., the patient knows the clinician will be virtually meeting them at the scheduled time for the IVE so they are more likely participate in the exercise). Clinicians will no longer have to wait a week or more for the patient to return to the next therapy session and provide a retrospective account of the IVEs over the past week, which is then subject to memory biases, social desirability, or other issues affecting accuracy. The advantages provided through this new technology system may ultimately improve the efficiency and efficacy of PE and help reduce attrition.
The study is limited by small sample size. Because this initial study is designed to determine feasibility and provide information on preliminary efficacy, this study will estimate effect sizes for subsequent, larger trials. Given the disproportionately high rates of PTSD among military veterans, this study is focused on veterans and we anticipate the majority of participants (90%) will be male, which limits our ability to conduct in-depth examination of sex differences. However, we will explore engagement and outcomes by sex to inform future research with larger samples of females. In addition, conducting therapist-guided IVEs in real-time will increase therapists' time commitment. Strategies for addressing this potential limitation will be explored by gaining feedback from therapists participating in the trial. Such feedback will inform future iterations on how to best use the Bio Ware wearable technology. For example, it may be feasible for other mental health professionals or support staff to conduct the guided IVEs under the supervision and direction of the therapist, who can prescribe the specific IVE activity, but not conduct it. A summary report of the IVE (e.g., amount of time spent in IVE, physiological engagement, SUDS levels) could then be shared with the therapist for treatment planning purposes. Finally, the investigative team considered including a third standard PE group (no patient worn Bio Ware device) but given the scope of this project and the Phase I emphasis on product refinement and testing in a clinical population, the team decided to include the proposed two groups, which will provide a wealth of novel data to inform a larger Phase II project.
In summary, the current study will develop and evaluate a novel technology system to be used during PE, in particular in vivo exercises, for the treatment of PTSD to enhance clinical outcomes. The findings from this study will provide seminal data on IVEs which will generate new knowledge and novel insights to inform clinical practice and accelerate the science in the field of exposure therapy for PTSD. The ability to capture multidimensional data in real-time and in real-world settings will provide an ecologically-valid and nuanced understanding of patient-specific physiological, behavioral and affective responses during IVEs. Results from this study will inform the development of an innovative, biomarker-driven technology system to address critical gaps and current limitations in exposure therapy for PTSD. The findings will also provide new knowledge to enhance the efficiency and effectiveness of exposure therapy for PTSD as well as anxiety disorders, conferring strong potential for therapeutic expansion. The findings from this study have the potential to make a significant and transformative impact on clinical practice.
Funding
This research was supported by grants from the National Institute of Mental Health (R43 MH122045), National Institute on Drug Abuse (T32 DA007288, R25 DA020537, U54 DA016511), National Institute on Alcohol Abuse and Alcoholism (K23 AA027307) and the Office of Research on Women’s Health and the National Institute on Child Health and Human Development (K12 HD055885).
Disclaimer
The views expressed herein are solely those of the authors and do not reflect an endorsement by or the official policy or position of the Ralph H. Johnson VA, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, the Department of Defense, the Department of Veterans Affairs, or the U.S. Government.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: B.H. and R.J.A. are Co-Founders of Zeriscope, Inc.
Contributor Information
Sudie E. Back, Email: backs@musc.edu.
Ron Acierno, Email: ronald.acierno@uth.tmc.edu.
Tanya C. Saraiya, Email: saraiya@musc.edu.
Bill Harley, Email: bill.harley@zeriscope.com.
Bethany Wangelin, Email: wangelin@musc.edu.
Amber M. Jarnecke, Email: jarnecka@musc.edu.
Lisa M. McTeague, Email: mcteague@musc.edu.
Delisa G. Brown, Email: browdg@musc.edu.
Elizabeth Santa Ana, Email: santaana@musc.edu.
Alex O. Rothbaum, Email: rothbaum@musc.edu.
Robert J. Adams, Email: adamsrj@musc.edu.
Data availability
No data was used for the research described in the article.
References
- 1.Kessler R.C., Demler O., Frank R.G., Olfson M., Pincus H.A., Walters E.E.…Zaslavsky A.M. Prevalence and treatment of mental disorders, 1990 to 2003. N. Engl. J. Med. 2005;352(24):2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the national epidemiologic survey on Alcohol and related conditions. J. Anxiety Disord. 2011;25(3):456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schein J., Houle C., Urganus A., Cloutier M., Patterson-Lomba O., Wang Y.…Davis L.L. Prevalence of post-traumatic stress disorder in the United States: a systematic literature review. Curr. Med. Res. Opin. 2021:1–11. doi: 10.1080/03007995.2021.1978417. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi R.B., Post E.P., Sun H., Pomerantz A., Saxon A.J., Piette J.D.…Nelson K. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am. J. Publ. Health. 2015;105(12):2564–2569. doi: 10.2105/AJPH.2015.302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black S.A., Gallaway M.S., Bell M.R., Ritchie E.C. Prevalence and risk factors associated with suicides of Army soldiers 2001–2009. Mil. Psychol. 2011;23(4):433–451. doi: 10.1080/08995605.2011.590409. [DOI] [Google Scholar]
- 6.Chan D., Cheadle A.D., Reiber G., Unützer J., Chaney E.F. Health care utilization and its costs for depressed veterans with and without comorbid PTSD symptoms. Psychiatr. Serv. 2009;60(12):1612–1617. doi: 10.1176/ps.2009.60.12.1612. [DOI] [PubMed] [Google Scholar]
- 7.Fang S.C., Schnurr P.P., Kulish A.L., Holowka D.W., Marx B.P., Keane T.M., Rosen R. Psychosocial functioning and health-related quality of life associated with posttraumatic stress disorder in male and female Iraq and Afghanistan war veterans: the VALOR registry. J. Wom. Health. 2015;24(12):1038–1046. doi: 10.1089/jwh.2014.5096. [DOI] [PubMed] [Google Scholar]
- 8.Kachadourian L.K., Nichter B., Herzog S., Norman S.B., Sullivan T., Pietrzak R.H. Non-suicidal self-injury in US military veterans: results from the national health and resilience in veterans study. Clin. Psychol. Psychother. 2021 doi: 10.1002/cpp.2673. [DOI] [PubMed] [Google Scholar]
- 9.McLean C.P., Foa E.B. Prolonged exposure therapy for post-traumatic stress disorder: a review of evidence and dissemination. Expert Rev. Neurother. 2011;11(8):1151–1163. doi: 10.1586/ern.11.94. [DOI] [PubMed] [Google Scholar]
- 10.Mouilso E.R., Tuerk P.W., Schnurr P.P., Rauch S.A.M. Addressing the gender gap: prolonged exposure for PTSD in veterans. Psychol. Serv. 2016;13(3):308–316. doi: 10.1037/ser0000040. [DOI] [PubMed] [Google Scholar]
- 11.Powers M.B., Halpern J.M., Ferenschak M.P., Gillihan S.J., Foa E.B. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin. Psychol. Rev. 2010;30(6):635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Jaycox L.H., Foa E.B., Morral A.R. Influence of emotional engagement and habituation on exposure therapy for PTSD. J. Consult. Clin. Psychol. 1998;66(1):185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- 13.Norrholm S.D., Jovanovic T., Gerardi M., Breazeale K.G., Price M., Davis M.…Rothbaum B.O. Baseline psychophysiological and cortisol reactivity as a predictor of PTSD treatment outcome in virtual reality exposure therapy. Behav. Res. Ther. 2016;82:28–37. doi: 10.1016/j.brat.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauch S.A., King A.P., Abelson J., Tuerk P.W., Smith E., Rothbaum B.O.…Liberzon I. Biological and symptom changes in posttraumatic stress disorder treatment: a randomized clinical trial. Depress. Anxiety. 2015;32(3):204–212. doi: 10.1002/da.22331. [DOI] [PubMed] [Google Scholar]
- 15.Rothbaum B.O., Price M., Jovanovic T., Norrholm S.D., Gerardi M., Dunlop B.…Ressler K.J. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am. J. Psychiatr. 2014;171(6):640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wangelin B.C., Tuerk P.W. Taking the pulse of Prolonged Exposure therapy: physiological reactivity to trauma imagery as an objective measure of treatment response. Depress. Anxiety. 2015;32(12):927–934. doi: 10.1002/da.22449. [DOI] [PubMed] [Google Scholar]
- 17.Weathers F.W., Blake D.D., Schnurr P.P. Clinician-administered PTSD scale for DSM-5 (CAPS-5) 2013. www.ptsd.va.gov Retrieved from Interview available from: the National Center for PTSD at. [DOI] [PMC free article] [PubMed]
- 18.Spinhoven P., Penninx B.W., van Hemert A.M., de Rooij M., Elzinga B.M. Comorbidity of PTSD in anxiety and depressive disorders: prevalence and shared risk factors. Child Abuse Negl. 2014;38(8):1320–1330. doi: 10.1016/j.chiabu.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Foa E.B., Hembree E.A., Rothbaum B.O., Rauch S.A.M. second ed. Oxford University Press; 2019. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. [DOI] [Google Scholar]
- 20.Weathers F.W. The PTSD checklist for DSM-5 (PCL-5) 2013. www.ptsd.va.gov Retrieved from Scale available from: the National Center for PTSD at.
- 21.Attkisson C.C., Greenfield T.K. third ed. vol. 3. Lawrence Erlbaum Associates Publishers; Mahwah, NJ, US: 2004. pp. 799–811. (The UCSF Client Satisfaction Scales: I. The Client Satisfaction Questionnaire-8. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults). [Google Scholar]
- 22.Brooke J. In: Usability Evaluation in Industry. Jordan P.W., Thomas B., Weerdmeester B.A., McClelland, editors. Taylor & Francis; London: 1996. SUS: a “quick and dirty” usability scale; pp. 189–194. [Google Scholar]
- 23.Luborsky L., Barber J.P., Siqueland L., Johnson S., Najavits L.M., Frank A., Daley D. The revised helping alliance questionnaire (HAq-II) : psychometric properties. J. Psychother. Pract. Res. 1996;5(3):260–271. [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards-Stewart A., Smolenski D.J., Bush N.E., Cyr B.A., Beech E.H., Skopp N.A., Belsher B.E. Posttraumatic stress disorder treatment dropout among military and veteran populations: a systematic review and meta-analysis. J. Trauma Stress. 2021;34(4):808–818. doi: 10.1002/jts.22653. [DOI] [PubMed] [Google Scholar]
- 25.Acierno R., Knapp R., Tuerk P., Gilmore A.K., Lejuez C., Ruggiero K.…Foa E.B. A non-inferiority trial of Prolonged Exposure for posttraumatic stress disorder: in person versus home-based telehealth. Behav. Res. Ther. 2017;89:57–65. doi: 10.1016/j.brat.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frueh B.C., Monnier J., Yim E., Grubaugh A.L., Hamner M.B., Knapp R.G. A randomized trial of telepsychiatry for post-traumatic stress disorder. J. Telemed. Telecare. 2007;13(3):142–147. doi: 10.1258/135763307780677604. [DOI] [PubMed] [Google Scholar]
- 27.Gros D.F., Lancaster C.L., López C.M., Acierno R. Treatment satisfaction of home-based telehealth versus in-person delivery of prolonged exposure for combat-related PTSD in veterans. J. Telemed. Telecare. 2016;24(1):51–55. doi: 10.1177/1357633X16671096. [DOI] [PubMed] [Google Scholar]
- 28.Tuerk P.W., Yoder M., Ruggiero K.J., Gros D.F., Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J. Trauma Stress. 2010;23(1):116–123. doi: 10.1002/jts.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuen E.K., Gros D.F., Price M., Zeigler S., Tuerk P.W., Foa E.B., Acierno R. Randomized controlled trial of home-based telehealth versus in-person Prolonged Exposure for combat-related PTSD in veterans: preliminary results. J. Clin. Psychol. 2015;71(6):500–512. doi: 10.1002/jclp.22168. [DOI] [PubMed] [Google Scholar]
- 30.Fina B.A., Wright E.C., Rauch S.A.M., Norman S.B., Acierno R., Cuccurullo L.J.…Foa E.B. Conducting prolonged exposure for PTSD during the COVID-19 pandemic: considerations for treatment. Cognit. Behav. Pract. 2021;28(4):532–542. doi: 10.1016/j.cbpra.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morland L.A., Wells S.Y., Glassman L.H., Greene C.J., Hoffman J.E., Rosen C.S. Advances in PTSD treatment delivery: review of findings and clinical considerations for the use of telehealth interventions for PTSD. Curr. Treat. Options Psychiatr. 2020:1–21. doi: 10.1007/s40501-020-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells S.Y., Morland L.A., Wilhite E.R., Grubbs K.M., Rauch S.A.M., Acierno R., McLean C.P. Delivering Prolonged Exposure therapy via videoconferencing during the COVID-19 pandemic: an overview of the research and special considerations for providers. J. Trauma Stress. 2020;33(4):380–390. doi: 10.1002/jts.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reger G.M., Hoffman J., Riggs D., Rothbaum B.O., Ruzek J., Holloway K.M., Kuhn E. The "PE coach" smartphone application: an innovative approach to improving implementation, fidelity, and homework adherence during prolonged exposure. Psychol. Serv. 2013;10(3):342–349. doi: 10.1037/a0032774. [DOI] [PubMed] [Google Scholar]
- 34.Botella C., Serrano B., Banos R.M., Garcia-Palacios A. Virtual reality exposure-based therapy for the treatment of post-traumatic stress disorder: a review of its efficacy, the adequacy of the treatment protocol, and its acceptability. Neuropsychiatric Dis. Treat. 2015;11:2533–2545. doi: 10.2147/ndt.S89542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erbes C.R., Stinson R., Kuhn E., Polusny M., Urban J., Hoffman J.…Thorp S.R. Access, utilization, and interest in mHealth applications among veterans receiving outpatient care for PTSD. Mil. Med. 2014;179(11):1218–1222. doi: 10.7205/MILMED-D-14-00014. [DOI] [PubMed] [Google Scholar]
- 36.Gilmore A.K., Wilson S.M., Skopp N.A., Osenbach J.E., Reger G. A systematic review of technology-based interventions for co-occurring substance use and trauma symptoms. J. Telemed. Telecare. 2017;23(8):701–709. doi: 10.1177/1357633X16664205. [DOI] [PubMed] [Google Scholar]
- 37.Gould C.E., Kok B.C., Ma V.K., Zapata A.M.L., Owen J.E., Kuhn E. Psychological Services; 2018. Veterans Affairs and the Department of Defense Mental Health Apps: A Systematic Literature Review. [DOI] [PubMed] [Google Scholar]
- 38.Beidel D.C., Frueh B.C., Neer S.M., Bowers C.A., Trachik B., Uhde T.W., Grubaugh A. Trauma management therapy with virtual-reality augmented exposure therapy for combat-related PTSD: a randomized controlled trial. J. Anxiety Disord. 2019;61:64–74. doi: 10.1016/j.janxdis.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Gerardi M., Cukor J., Difede J., Rizzo A., Rothbaum B.O. Virtual reality Exposure therapy for post-traumatic stress disorder and other anxiety disorders. Curr. Psychiatr. Rep. 2010;12(4):298–305. doi: 10.1007/s11920-010-0128-4. [DOI] [PubMed] [Google Scholar]
- 40.Maples-Keller J.L., Bunnell B.E., Kim S.J., Rothbaum B.O. The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harv. Rev. Psychiatr. 2017;25(3):103–113. doi: 10.1097/hrp.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLay R.N., Wood D.P., Webb-Murphy J.A., Spira J.L., Wiederhold M.D., Pyne J.M., Wiederhold B.K. A randomized, controlled trial of virtual reality-graded exposure therapy for post-traumatic stress disorder in active duty service members with combat-related post-traumatic stress disorder. Cyberpsychol., Behav. Soc. Netw. 2011;14(4):223–229. doi: 10.1089/cyber.2011.0003. [DOI] [PubMed] [Google Scholar]
- 42.Baumeister H., Reichler L., Munzinger M., Lin J. The impact of guidance on Internet-based mental health interventions—a systematic review. Internet Interv. 2014;1(4):205–215. [Google Scholar]
- 43.Cuijpers P., Donker T., van Straten A., Li J., Andersson G. Is guided self-help as effective as face-to-face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychol. Med. 2010;40(12):1943–1957. doi: 10.1017/s0033291710000772. [DOI] [PubMed] [Google Scholar]
- 44.Fairburn C.G., Patel V. The impact of digital technology on psychological treatments and their dissemination. Behav. Res. Ther. 2017;88:19–25. doi: 10.1016/j.brat.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.