Abstract
Stainless steel coupons were treated with skim milk and subsequently challenged with individual bacterial suspensions of Staphylococcus aureus, Pseudomonas fragi, Escherichia coli, Listeria monocytogenes, and Serratia marcescens. The numbers of attached bacteria were determined by direct epifluorescence microscopy and compared with the attachment levels on clean stainless steel with two different surface finishes. Skim milk was found to reduce adhesion of S. aureus, L. monocytogenes, and S. marcescens. P. fragi and E. coli attached in very small numbers to the clear surfaces, making the effect of any adsorbed protein layer difficult to assess. Individual milk proteins α-casein, β-casein, κ-casein, and α-lactalbumin were also found to reduce the adhesion of S. aureus and L. monocytogenes. The adhesion of bacteria to samples treated with milk dilutions up to 0.001% was investigated. X-ray photoelectron spectroscopy was used to determine the proportion of nitrogen in the adsorbed films. Attached bacterial numbers were inversely related to the relative atomic percentage of nitrogen on the surface. A comparison of two types of stainless steel surface, a 2B and a no. 8 mirror finish, indicated that the difference in these levels of surface roughness did not greatly affect bacterial attachment, and reduction in adhesion to a milk-treated surface was still observed. Cross-linking of adsorbed proteins partially reversed the inhibition of bacterial attachment, indicating that protein chain mobility and steric exclusion may be important in this phenomenon.
Adhesion of microorganisms to food processing equipment surfaces and the problems it causes are a matter of concern to the food industry. Biofilms have the potential to act as a chronic source of microbial contamination which may compromise food quality and represent a significant health hazard. To control these problems, it has been recognized that a greater understanding of the interaction between microorganisms and food-processing surfaces is required (7, 8, 18, 24, 25, 33, 34). Several groups have reported the ability of bacteria to attach to surfaces commonly found in the food processing environment, such as rubber and stainless steel (10, 17, 21, 22, 28). The increased resistance of these sessile organisms towards disinfectants and sanitizing agents (13) often exacerbates the problems caused by microbial fouling and can contribute to the inefficacy of cleaning in place systems (4).
Development of adsorbed layers, often termed “conditioning” of a surface, is considered to be the first stage in biofilm formation and has been widely demonstrated (9). Because this conditioning film is likely to change the physicochemical properties of the substratum (20, 32) and thus to influence bacterial attachment, an understanding of these initial interactions is crucial in identifying control measures. A variety of proteins, including milk proteins (2, 3), have been shown to affect bacterial adhesion to surfaces such as polystyrene (12); hydroxyapatite (26); glass (1), rubber, and stainless steel (16, 19, 27); silica (2); and medical implants (30). The nature of the effect appears to vary with the organism, substratum, and protein under investigation.
This study investigates the effect of preadsorbed, skim milk and its individual major protein constituents on the adhesion of a number of bacterial species to stainless steel surfaces.
MATERIALS AND METHODS
Bacterial maintenance and growth.
Staphylococcus aureus (University of Surrey Culture Collection [USCC] 1500, ASCDP 2), Listeria monocytogenes (F6861), Serratia marcescens (USCC 2156), Escherichia coli (USCC 2879), and Pseudomonas fragi (NCIMB 11082) were all maintained on plastic beads (Protect; Technical Service Consultant Limited, Lancashire, United Kingdom) stored at −70°C. To produce cultures, a single bead was placed into 50 ml of nutrient broth (Oxoid, Basingstoke, Hampshire, United Kingdom) in a 250-ml conical flask and incubated overnight at 25°C and 170 rpm. This culture was then used to inoculate a second flask of nutrient broth to give an initial concentration of 103 CFU/ml, which was incubated under the same conditions until the required growth phase was reached. Growth curve determinations were made by enumerating each of the organisms on nutrient agar (Oxoid) by using the Miles and Misra technique (23).
Cleaning of stainless steel samples.
Coupons of stainless steel AISI 304 with a 2B finish (Rigidised Metals, Ltd., Middlesex, United Kingdom) and no. 8 mirror finish (Parker Steel, Andover, United Kingdom) were cut to dimensions of 2.5 by 1.0 cm (1.25 by 1.00 cm for X-ray photoelectron spectroscopy [XPS] samples) and boiled in a 2% solution of RBS 25 (Medline Scientific, Ltd.) for 30 min. Coupons were then sonicated (38 kHz, Kerry ultrasonication unit; Kerry, Hertfordshire, United Kingdom) in the same solution for 30 min and then rinsed (five times) by vortexing in 10 ml of sterile reverse osmosis (RO) water. Degreasing in acetone for 30 min was followed by rinsing, as previously described, and drying in a laminar air flow cabinet. The stainless steel coupons were flame sterilized with ethanol and rinsed briefly in sterile RO water immediately prior to use. This procedure was found by XPS to reduce contaminating carbon to extremely low levels.
Treatment of stainless steel coupons with skim milk.
Three cleaned stainless steel samples were placed horizontally into a glass beaker, avoiding overlap, and 5 ml of ultrahigh-temperature-treated skim milk was added. The samples were gently swirled at approximately 40 rpm, and adhesion was allowed to take place for 2 h at 20°C. At the end of this period, the samples were each rinsed (twice) in 10 ml of sterile RO water by gently rocking three times. When investigating the effect of precoating samples with a range of milk dilutions, samples were treated as described above by using three samples for each milk dilution.
Treatment of stainless steel samples with individual milk proteins.
Each of the following proteins, α-casein, β-casein, κ-casein, and α-lactalbumin, was used to treat three stainless steel coupons. The proteins were freeze-dried extracts of bovine milk (Sigma, Poole, Dorset, United Kingdom) and solutions were prepared at a concentration of 0.5 mM (allowing for purity status, resulting in solutions which are the equivalent of 0.5 mM 100% protein) by adding 5 ml of sterile RO water. The solution was poured over the samples, and adhesion was allowed to take place over 2 h at 20°C, with swirling at 40 rpm. At the end of the adhesion period, the samples were removed and rinsed twice in sterile RO water as described previously.
Exposure of milk-treated samples to glutaraldehyde.
Triplicate milk-treated stainless steel samples were exposed to either 5 or 10% solutions of glutaraldehyde (prepared by dilution of a stock solution [Agar Scientific, Ltd., Essex, United Kingdom] with sterile RO water). Samples were left in the glutaraldehyde solution for a period of 2 h (20°C), after which they were rinsed three times in sterile RO water. The results were compared against those from a number of controls. These comprised samples which had received no milk treatment, a sample treated with milk as described previously, and a milk-treated sample exposed to sterile RO water in lieu of a glutaraldehyde solution.
Bacterial adhesion.
Bacterial suspensions were prepared from early-stationary-phase cultures. These were centrifuged at approximately 900 × g for 15 min (room temperature) and resuspended in sterile 1/4-strength Ringer’s solution. This washing process was carried out twice more. The cells were finally resuspended in sterile 1/4-strength Ringer’s solution (or a variety of salt solutions, as specified in the Results section) and adjusted to a concentration of 108 CFU/ml (optical density at 620 nm). Protein-treated and untreated samples were placed vertically in individual sterile bottles (25 ml). Ten milliliters of bacterial suspension was added to each bottle and incubated for 2 h at 20°C under static conditions. At the end of this period, the samples were removed and gently rinsed twice by rocking three times in sterile 1/4-strength Ringer’s solution. Adhesion to both protein-treated and untreated samples was determined on three replicate coupons. For certain of the bacterial species, tests were also carried out in a modified suspension solution. This involved substituting the Ringer’s suspension medium and rinse solution with Ringer’s solution plus 0.1% glucose or maximum recovery diluent (MRD [1 g of bacteriological peptone per liter and 8.5 g of NaCl per liter]) plus 0.1% glucose. Postadhesion rinse solutions were also modified accordingly. Glucose solutions were made up separately, filter sterilized, and added to media after autoclaving.
Enumeration of adhered population.
Stainless steel samples were placed in 10 ml of 0.0025% acridine orange solution and left to stain for 30 min. They were then rinsed twice with 10 ml of RO water and allowed to air dry. A Leitz Dialux 20 fluorescence microscope [Leica Microsystems (UK) Limited, Milton Keynes, United Kingdom] fitted with an I 2 filter block was used to view the samples under oil immersion, using a nonfluorescing immersion oil. Twenty fields were counted for each of the three replicate coupons, unless otherwise stated.
XPS analysis of milk-coated stainless steel.
Stainless steel coupons (1.25 by 1.0 cm) were coated with various milk dilutions and rinsed, as previously described. The samples were allowed to air dry in a laminar air flow cabinet and then placed in a desiccator under vacuum, until analyzed. Samples were mounted onto specimen stubs with double-sided tape. XPS data were collected with a VG ESCALAB Mk.II spectrometer interfaced to a VGS 5000S data system based on a DEC PDP 11/73 computer (VG Scientific, Crawley, United Kingdom). The operating conditions were as follows: the X-ray source (A1 Kα [1486.6 eV] radiation) was operated at a power level of 450 W (i.e., 13-kV potential and 34-mA emission current). The spectrometer was operated in the fixed analyzer transmission mode at a pass energy of 50 eV (survey spectra) or 20 eV (high-resolution spectra). The base pressure in the sample chamber during analysis was approximately 3 × 10−3 mPa. Survey spectra were obtained with one scan; high-resolution spectra were integrated over 1 to 25 scans, depending on the intensity of the spectral region of interest. Spectral analysis was carried out using the standard VGS 500s software for quantification and peak fitting; quantification was based on peak areas calculated from the high-resolution spectra.
Surface roughness measurements.
Cleaned samples of 2B and no. 8 mirror finish stainless steel were analyzed with a Zeiss confocal laser-scanning microscope (CLSM) (Zeiss, Hertfordshire, United Kingdom) to measure the Ra value (defined below). A 633-nm wavelength beam with a ×50 objective and a ×100 zoom was used to analyze five random areas for each sample, and an average was calculated. The Ra value provides the arithmetical average value of all departures from the mean line throughout the sampling length. The equation used to calculate a value for Ra is given as:
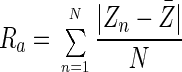 |
where Zn is the sample height for each data point and Z̄ is the mean height for a roughness curve containing N data points.
RESULTS
Effect of milk and milk proteins on bacterial adhesion.
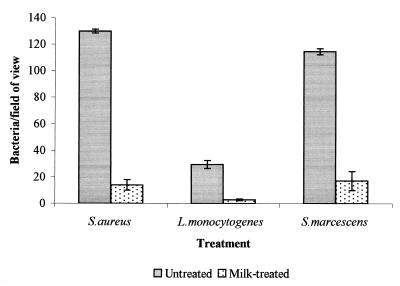
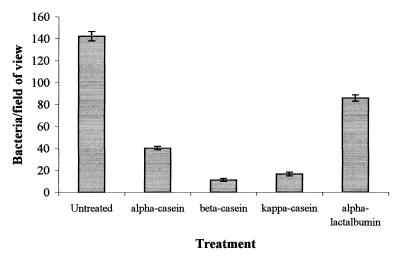
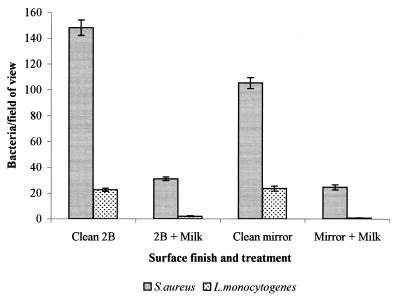
Pretreatment of stainless steel coupons (2B finish) with skim milk was shown to substantially reduce attachment of S. aureus, S. marcescens, and L. monocytogenes (Fig. 1). E. coli and P. fragi attached in very small numbers to both clean and pretreated surfaces alike (less than 1 organism/field of view). Pretreatments conducted with the individual milk proteins α-, β-, and κ-casein and α-lactalbumin, at equal concentrations also reduced attachment of S. aureus compared with attachment to the untreated surface (Fig. 2). However, this effect was least marked with α-lactalbumin. A similar effect was observed with L. monocytogenes (data not shown).
FIG. 1.
Adhesion of a number of bacterial species to both untreated stainless steel 2B samples and samples which have been treated with skim milk (bars represent the standard error of the mean).
FIG. 2.
Effect upon the adhesion of S. aureus of pretreating stainless steel samples with individual milk proteins (0.5 mM) (bars represent the standard error of the mean).
Effect of suspension medium.
Attachment of S. aureus to the cleaned steel surface was critically dependent on the ionic strength of the suspending medium. With RO-purified water, attachment was less than 1% of that observed with 1/4-strength Ringer’s solution. Individual components of Ringer’s solution gave increasing attachment in proportion to their contribution to the overall ionic strength (Table 1). Sodium chloride, at 40 mM the major component in Ringer’s solution, gave levels of attachment to clean stainless steel that were 81% of those seen when Ringer’s solution was used. When solutions of salts of other divalent and monovalent cations were used at similar molar concentrations (20 to 40 mM), they all showed a similar enhancement of attachment to the clean stainless steel; however, salts of divalent cations showed an increase of attachment to milk-treated surfaces, particularly marked with calcium, an effect that was not apparent with monovalent cations (Table 2).
TABLE 1.
Effect of 1/4-strength Ringer’s solution and its components on attachment levels of S. aureus to clean and milk-treated stainless steel
| Suspending solution | Ionic strength (103) | Effect of solution on steela:
|
|||||
|---|---|---|---|---|---|---|---|
| Clean
|
Milk treated
|
||||||
| No. of bacteria/field | % of control | ±SE | No. of bacteria/field | % of control | ±SE | ||
| 1/4-strength Ringer’s solution | 43.8 | 1,178 | 100 | 34 | 3 | 0.3 | <1 |
| RO purified water | 8 | 0.7 | <1 | 3 | 0.3 | <1 | |
| NaCl (40 mM) | 40 | 954 | 81.0 | 24 | 6 | 0.5 | <1 |
| KCl (1.4 mM) | 1.4 | 14 | 1.2 | <1 | 2 | 0.2 | <1 |
| CaCl2 (0.6 mM) | 1.8 | 27 | 2.3 | 1 | 3 | 0.3 | <1 |
| NaHCO3 (0.6 mM) | 0.6 | 4 | 0.3 | <1 | 1 | 0.1 | 2 |
Results represent the average of 36 fields from three different samples ± standard error (SE) of the mean. The experiments were performed under rotating conditions.
TABLE 2.
Effect of different salt solutions on the attachment levels of S. aureus to clean and milk-treated stainless steel
| Suspending solution | Effect of solution on steela:
|
|||||
|---|---|---|---|---|---|---|
| Clean
|
Milk treated
|
|||||
| No. of bacteria/field | % of control | ±SE | No. of bacteria/field | % of control | ±SE | |
| 1/4-strength Ringer’s solution | 1,294 | 100 | 31 | 5 | 0.4 | <1 |
| NaCl (40 mM) | 1,054 | 81.5 | 24 | 6 | 0.5 | <1 |
| CaCl2 (40 mM) | 1,109 | 85.7 | 2 | 630 | 48.7 | 9 |
| CaCl2 (20 mM) | 1,213 | 93.7 | 12 | 646 | 49.9 | 14 |
| FeCl2 (20 mM) | 939 | 72.6 | 32 | 247 | 19.1 | 10 |
| KCl (40 mM) | 1,042 | 80.5 | 9 | 3 | 0.2 | <1 |
| MgCl2 (20 mM) | 1,089 | 84.2 | 31 | 68 | 5.3 | 6 |
| MnCl2 (20 mM) | 1,366 | 105.6 | 36 | 42 | 3.2 | 4 |
Results represent the average of 36 fields from three different samples ± standard error (SE) of the mean. The experiments were performed under rotating conditions.
P. fragi exhibited low adhesion to clean, untreated stainless steel. Little difference in attachment was observed when the suspension medium of 1/4-strength Ringer’s solution was supplemented with 0.1% glucose or replaced with MRD plus 0.1% glucose (data not shown).
Effect of precoating the surface with milk dilutions.
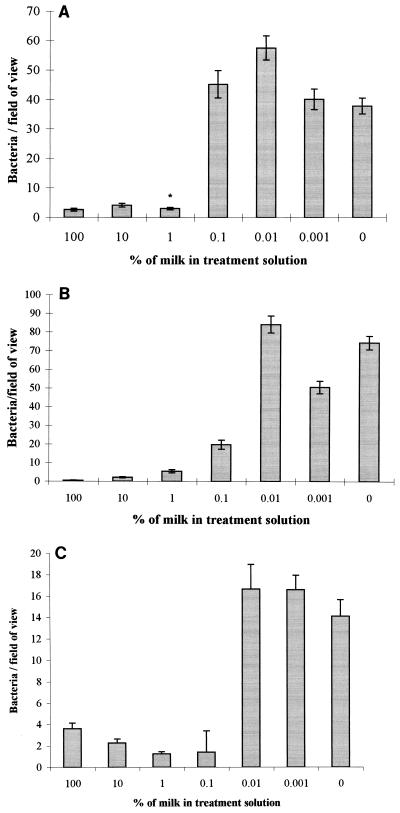
Treating stainless steel (2B finish) with a range of milk dilutions prior to bacterial contact was investigated. The level of S. aureus attachment remained relatively constant for steel treated with milk and 10 and 1% milk solutions. Between treatments with 1 and 0.01% milk solutions, the numbers adhering increased sharply. A similar effect was also observed for S. marcescens and L. monocytogenes, although with these organisms, the step-like increase in attached numbers was observed between treatments with 0.1 and 0.01% milk solutions and the numbers of S. marcescens cells adhering at the higher dilutions were lower (around 16 per field) (Fig. 3).
FIG. 3.
Adhesion of S. aureus (A), L. monocytogenes (F6861) (B), and S. marcescens (C) to stainless steel samples with a 2B surface finish which have been pretreated with a range of milk dilutions (bars represent the standard error of the mean). An asterisk indicates that the results represent the average of 40 readings from two replicate samples.
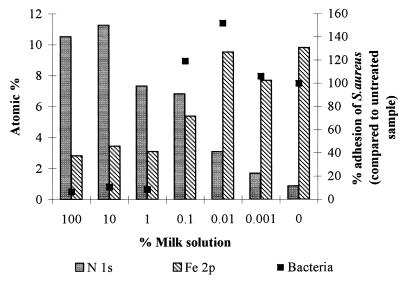
XPS analysis of the relative atomic composition of the top 3 to 5 nm of the different surfaces generally showed an increasing nitrogen content with increasing concentrations of milk applied. When treated with undiluted milk, the iron signal was reduced to background levels. The data obtained from these analyses, combined with the bacterial adhesion data for S. aureus, suggested that as the amount of nitrogen (protein) at the surface decreased, the bacterial attachment increased (Fig. 4). Since there was an inverse relationship between the relative levels of nitrogen and iron, bacterial attachment and the amount of iron detected followed a similar trend.
FIG. 4.
XPS analysis of stainless steel 2B surfaces indicating levels of nitrogen (N 1s) and iron (Fe 2p) following treatment with a range of milk dilutions, combined with adhesion data for S. aureus.
Surface roughness and wettability.
Average Ra values for the 2B finish and the no. 8 mirror finish, as determined by using the CLSM, were 0.412 ± 0.011 and 0.035 ± 0.004 μm (mean ± standard error), respectively. Contact angle measurements underwater with octane droplets (15) on a 2B surface finish, before and after milk treatment, showed a small decrease (approximately 100) in contact angle, indicating a slight decrease in surface hydrophilicity.
Numbers of S. aureus and L. monocytogenes cells attaching to both surfaces, with and without milk treatment, are presented in Fig. 5. Numbers of S. aureus cells adhering to the polished surface were 29% less than for the rougher 2B finish. L. monocytogenes cells adhered in lower numbers, with no difference between the two surface finishes. Bacterial adhesion to both surface finishes was reduced substantially following milk treatment (by 117 [79%] and 81 [77%] bacteria/field of view for S. aureus and by 20 [96%] and 22 [97%] bacteria/field of view for L. monocytogenes on the 2B and no. 8 finishes, respectively).
FIG. 5.
Adhesion of S. aureus and L. monocytogenes to stainless steel surfaces with either a 2B or a no. 8 mirror surface finish, with and without surface pretreatment with undiluted milk.
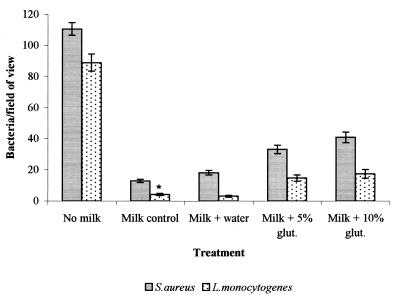
Effect of glutaraldehyde on adhesion to milk-treated stainless steel.
Cross-linking of proteins on milk-treated steel with glutaraldehyde increased the subsequent attachment of S. aureus and L. monocytogenes. After cross-linking, numbers of S. aureus and L. monocytogenes cells attaching were greater than those attaching to the native milk-treated surface, an effect more marked with L. monocytogenes. These levels represented 37.1 and 19.7%, respectively, of the levels obtained with a clean stainless steel surface (Fig. 6).
FIG. 6.
Effect upon the adhesion of S. aureus and L. monocytogenes of treating a preadsorbed milk layer with glutaraldehyde (glut.) for 2 h compared with that of an untreated control (bars represent the standard error of the mean). An asterisk indicates that the results represent the average of 40 readings from two replicate samples.
DISCUSSION
Milk is a highly perishable commodity which can be frequently in contact with stainless steel surfaces during its processing and storage. Skim milk was used in this work to minimize the effects of fat and to focus on the role of proteins in bacterial attachment, since these are likely to interact most with the hydrophilic surface of stainless steel. A number of organisms were chosen for use in the study due to their association with foods and potential as food-borne pathogens or as food-spoilage organisms.
Adhesion to the milk-treated stainless steel varied with the organism used. With the gram-positive S. aureus and L. monocytogenes cells and the gram-negative S. marcescens cells, attachment was reduced to levels ≤20% of clean surface values. In contrast, the gram-negative E. coli and P. fragi cells adhered in small numbers to the clean stainless steel surface, with less than 1 organism per field of view, making any effect of the protein film difficult to assess. While it has previously been found that gram-negative organisms attached to stainless steel in larger numbers than gram-positive organisms (27), this was with different strains and under experimental conditions very different from those used here. The results presented here demonstrate very clearly the effects of a conditioning layer and of the ionic composition of the suspending medium on attachment.
None of the proteins studied was shown to augment attachment of the bacteria used. In 1976, Fletcher (12) found that while preadsorbed serum albumin, gelatin, fibrinogen, and pepsin all inhibited bacterial attachment to petri dishes, the basic proteins, protamine, and histone had no effect. Austin and Bergeron (4) suggested that contact with milk solids is one reason for lower attachment on the inside diameter of gaskets in milk processing equipment.
In this work, adsorbed protein was clearly inhibiting the initial attachment of bacteria. As the concentration of milk used to treat the stainless steel surface was reduced, the iron signal initially remained stable, indicating surface coverage with a protein layer thicker than the iron photoelectron escape depth. However, below 1% milk, there was a sharp increase in the iron signal, as well as a simultaneous increase in bacterial attachment. This suggested a possible dual effect of protein directly inhibiting bacterial attachment while increased availability of surface iron promotes it. The presence of high concentrations of ferrous ions in the suspending medium produced a 27.4% reduction in adhesion to clean stainless steel, possibly as a result of its competition with surface iron for bacterial surface binding sites (Table 2). With milk-treated steel, however, ferrous ions in solution increased attachment, presumably as a result of their interaction with surface-adsorbed protein, either by acting as a bridging cation between protein and bacterium or by cross-linking the protein molecules and thus reducing their potential for interaction with the bacteria. Increased attachment as a result of protein cross-linking was demonstrated by glutaraldehyde treatment, which for L. monocytogenes resulted in at least a fourfold increase in bacterial numbers adhering to milk-treated steel.
The ionic composition of the suspending medium had the most effect when clean stainless steel was exposed to bacteria, presumably as a result of the dissolved cations shielding the surface-negative charge on bacteria and steel. With adsorption of a conditioning layer, bacterial adhesion was decreased and was less affected by the ionic composition of the suspension medium, with the exception of iron (discussed above) and calcium. Since calcium is a component of native milk proteins, its reintroduction may cause conformational changes in the adsorbed proteins facilitating attachment.
It has been suggested that surface roughness may play an important role in the adhesion of microorganisms by protecting from shear forces and increasing available surface area (5, 6, 14, 31). In the present study, greater numbers of S. aureus adhered to the untreated steel with the rougher surface. For L. monocytogenes, little difference between the two surfaces was observed. Scanning electron microscopy micrographs showed that organisms did not orient themselves exclusively along polishing lines (not shown). This may reflect the fact that our system was static while the above observations were made in flowing systems in which higher shear forces may be experienced. Duddridge and Pritchard (11) suggested that in a static system, a rougher substratum offers the prospect of a larger surface area available for adsorption. When adhesion to both rough and smooth milk-treated surfaces was examined, it was noted that the substratum topography still has an important effect, because adhesion to a milk-treated rougher surface was greater than adhesion to a milk-treated smooth surface. This effect has also been noted by Taylor et al. (29).
The above results demonstrate the important role that preconditioning layers of adsorbed organics can play in the attachment of microorganisms to food processing surfaces. In particular, the observation that layers can inhibit attachment suggests that a pretreatment with macromolecules possessing the required properties could offer at least a short-term solution to the problem of biofouling in the food industry and its attendant problems.
ACKNOWLEDGMENTS
This work was supported by studentships from the Ministry for Agriculture Fisheries and Food (M.F.L. and L.-M.B.).
We also acknowledge the assistance of J. Watts and S. Greaves (School of Mechanical and Materials Engineering, University of Surrey) with the XPS work and the staff of the Micro-Structural Studies Unit (University of Surrey) for their cooperation with CLSM and scanning electron microscopy.
REFERENCES
- 1.Abbott A, Rutter P R, Berkeley R C W. The influence of ionic strength, pH and a protein layer on the interaction between Streptococcus mutans and glass surfaces. J Gen Microbiol. 1983;129:439–445. doi: 10.1099/00221287-129-2-439. [DOI] [PubMed] [Google Scholar]
- 2.Al-Makhlafi H, McGuire J, Daeschel M. Influence of preadsorbed milk proteins on adhesion of Listeria monocytogenes to hydrophobic and hydrophilic silica surfaces. Appl Environ Microbiol. 1994;60:3560–3565. doi: 10.1128/aem.60.10.3560-3565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Makhlafi H, Nasir A, McGuire J, Daeschel M. Adhesion of Listeria monocytogenes to silica surfaces after sequential and competitive adsorption of bovine serum albumin and β-lactoglobulin. Appl Environ Microbiol. 1995;61:2013–2015. doi: 10.1128/aem.61.5.2013-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin J W, Bergeron G. Development of bacterial biofilms in dairy processing lines. J Dairy Res. 1995;62:509–519. doi: 10.1017/s0022029900031204. [DOI] [PubMed] [Google Scholar]
- 5.Baker J H. Factors affecting the colonisation of various surfaces in a river. Can J Microbiol. 1984;30:511–515. [Google Scholar]
- 6.Beeftink H H, Staugaard P. Structure and dynamics of anaerobic bacterial aggregates in a gas-lift reactor. Appl Environ Microbiol. 1986;52:1139–1146. doi: 10.1128/aem.52.5.1139-1146.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryers J D. Biofilms and the technological implications of microbial cell adhesion. Colloids Surf. 1994;2:9–23. [Google Scholar]
- 8.Carpentier B, Cerf O. Biofilms and their consequences with particular reference to hygiene in the food industry. J Appl Bacteriol. 1993;75:499–511. doi: 10.1111/j.1365-2672.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain A H L. The role of adsorbed layers in bacterial adhesion. In: Melo L F, Bott T R, Fletcher M, Capdeville B, editors. Biofilms—science and technology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 59–67. [Google Scholar]
- 10.Czechowski M H. Bacterial attachment to Buna-N gaskets in milk processing equipment. Aust J Dairy Technol. 1990;45:113–114. [Google Scholar]
- 11.Duddridge J E, Pritchard A M. Microbial corrosion. London, United Kingdom: The Metals Society; 1983. pp. 28–35. [Google Scholar]
- 12.Fletcher M. The effects of proteins on bacterial attachment to polystyrene. J Gen Microbiol. 1976;94:400–404. doi: 10.1099/00221287-94-2-400. [DOI] [PubMed] [Google Scholar]
- 13.Frank J F, Koffi R A. Surface adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitisers and heat. J Food Prot. 1990;53:550–554. doi: 10.4315/0362-028X-53.7.550. [DOI] [PubMed] [Google Scholar]
- 14.Geesey G G, Costerton J W. Microbiology of a northern river: bacterial distribution and relationship to suspended sediment and organic carbon. Can J Microbiol. 1979;25:1058–1062. doi: 10.1139/m79-162. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton W C. A technique for the characterisation of hydrophilic solid surfaces. J Coll Interface Sci. 1972;40:219–222. [Google Scholar]
- 16.Helke D M, Somers E B, Wong A C L. Attachment of Listeria monocytogenes and Salmonella typhimurium to stainless steel and Buna-N in the presence of milk and individual milk components. J Food Prot. 1993;56:479–484. doi: 10.4315/0362-028X-56.6.479. [DOI] [PubMed] [Google Scholar]
- 17.Holah J T. Industrial monitoring: hygiene in food processing. In: Melo L F, Bott T R, Fletcher M, Capdeville B, editors. Biofilms—science and technology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 645–659. [Google Scholar]
- 18.Hood S K, Zottola E A. Biofilms in food processing. Food Cont. 1995;6:9–18. [Google Scholar]
- 19.Hood S K, Zottola E A. Growth media and surface conditioning influence the adherence of Pseudomonas fragi, Salmonella typhimurium, and Listeria monocytogenes cells to stainless steel. J Food Prot. 1997;60:1034–1037. doi: 10.4315/0362-028X-60.9.1034. [DOI] [PubMed] [Google Scholar]
- 20.Johal S. Ph.D. thesis. Bacterial adhesion to food processing surfaces in the meat industry. Guildford, United Kingdom: University of Surrey; 1988. [Google Scholar]
- 21.Krysinski E P, Brown L J, Marchisello T J. Effect of cleaners and sanitisers on Listeria monocytogenes attached to product contact surfaces. J Food Prot. 1992;55:246–251. doi: 10.4315/0362-028X-55.4.246. [DOI] [PubMed] [Google Scholar]
- 22.Mafu A A, Roy D, Goulet J, Magny P. Attachment of Listeria monocytogenes to stainless steel, glass, polypropylene and rubber surfaces after short contact times. J Food Prot. 1990;53:742–746. doi: 10.4315/0362-028X-53.9.742. [DOI] [PubMed] [Google Scholar]
- 23.Miles A A, Misra S S. The estimation of the bacteriocidal power of the blood. J Hyg. 1938;38:732–748. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notermans S, Dormans J A M A, Mead G C. Contribution of surface attachment to the establishment of micro-organisms in food processing plants: a review. Biofouling. 1991;5:21–36. [Google Scholar]
- 25.Pontefract R D. Bacterial adherence: its consequence in food processing. Can Inst Sci Technol J. 1991;24:113–117. [Google Scholar]
- 26.Reynolds E C, Wong A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect Immun. 1983;39:1285–1290. doi: 10.1128/iai.39.3.1285-1290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speers J G S, Gilmour A. The influence of milk and milk components on the attachment of bacteria to farm dairy equipment surfaces. J Appl Bacteriol. 1985;59:325–332. doi: 10.1111/j.1365-2672.1985.tb03326.x. [DOI] [PubMed] [Google Scholar]
- 28.Suárez B, Ferreirós C M, Criado M-T. Adherence of psychrotrophic bacteria to dairy equipment surfaces. J Dairy Res. 1992;59:381–388. doi: 10.1017/s002202990003065x. [DOI] [PubMed] [Google Scholar]
- 29.Taylor R L, Verran J, Lees G C, Ward A J P. The influence of substratum topography on bacterial adhesion to polymethyl methacrylate. J Mater Sci. 1998;9:17–22. doi: 10.1023/a:1008874326324. [DOI] [PubMed] [Google Scholar]
- 30.Vaudaux P E, Francois P, Proctor R A, McDevitt D, Foster T J, Albrecht R M, Lew D P, Wabers H, Cooper S L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verran J, Maryan C J. Retention of Candida albicans on acrylic and silicone of different surface topographies. J Prosthet Dent. 1997;77:535–539. doi: 10.1016/s0022-3913(97)70148-3. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, McGuire J, Kolbe E. Use of the equilibrium contact angle as an index of contact surface cleanliness. J Food Prot. 1991;54:879–884. doi: 10.4315/0362-028X-54.11.879. [DOI] [PubMed] [Google Scholar]
- 33.Zottola E A. Microbial attachment and biofilm formation: a new problem for the food industry? Food Technol. 1994;48:107–114. [Google Scholar]
- 34.Zottola E A, Sasahara K C. Microbial biofilms in the food industry—should they be a concern? Int J Food Microbiol. 1994;23:125–148. doi: 10.1016/0168-1605(94)90047-7. [DOI] [PubMed] [Google Scholar]