Abstract
Organic sulfur compounds are present in all aquatic systems, but their use as sources of sulfur for bacteria is generally not considered important because of the high sulfate concentrations in natural waters. This study investigated whether dimethylsulfoniopropionate (DMSP), an algal osmolyte that is abundant and rapidly cycled in seawater, is used as a source of sulfur by bacterioplankton. Natural populations of bacterioplankton from subtropical and temperate marine waters rapidly incorporated 15 to 40% of the sulfur from tracer-level additions of [35S]DMSP into a macromolecule fraction. Tests with proteinase K and chloramphenicol showed that the sulfur from DMSP was incorporated into proteins, and analysis of protein hydrolysis products by high-pressure liquid chromatography showed that methionine was the major labeled amino acid produced from [35S]DMSP. Bacterial strains isolated from coastal seawater and belonging to the α-subdivision of the division Proteobacteria incorporated DMSP sulfur into protein only if they were capable of degrading DMSP to methanethiol (MeSH), whereas MeSH was rapidly incorporated into macromolecules by all tested strains and by natural bacterioplankton. These findings indicate that the demethylation/demethiolation pathway of DMSP degradation is important for sulfur assimilation and that MeSH is a key intermediate in the pathway leading to protein sulfur. Incorporation of sulfur from DMSP and MeSH by natural populations was inhibited by nanomolar levels of other reduced sulfur compounds including sulfide, methionine, homocysteine, cysteine, and cystathionine. In addition, propargylglycine and vinylglycine were potent inhibitors of incorporation of sulfur from DMSP and MeSH, suggesting involvement of the enzyme cystathionine γ-synthetase in sulfur assimilation by natural populations. Experiments with [methyl-3H]MeSH and [35S]MeSH showed that the entire methiol group of MeSH was efficiently incorporated into methionine, a reaction consistent with activity of cystathionine γ-synthetase. Field data from the Gulf of Mexico indicated that natural turnover of DMSP supplied a major fraction of the sulfur required for bacterial growth in surface waters. Our study highlights a remarkable adaptation by marine bacteria: they exploit nanomolar levels of reduced sulfur in apparent preference to sulfate, which is present at 106- to 107-fold higher concentrations.
Sulfur is essential to all organisms because of its ubiquity in proteins and other important biomolecules. The most familiar sulfur compounds in organisms are the protein amino acids methionine and cysteine, both of which contain sulfur in the reduced (−II) oxidation state. Methionine in particular has many important biological functions, including that as the principal methyl donor in biosynthesis via its conjugate S-adenosylmethionine (6, 46). Bacteria, fungi, and plants can synthesize methionine from inorganic sulfur (i.e., SO42− and H2S), but most animals cannot synthesize methionine de novo and must therefore obtain it from the diet. To date, studies that have examined sulfur assimilation by aquatic microorganisms have focused on sulfate, which is present at high concentrations in most natural waters, especially seawater (∼28 mM SO42−). Because sulfate contains sulfur in its most oxidized state (+VI), whereas most of the sulfur in biomolecules is reduced, the use of sulfate represents an energetic cost to microorganisms, not only for transport into the cells but also for the required 8-electron reduction to the level of sulfide.
From a bioenergetics point of view, use of relatively more reduced substrates by microorganisms could provide the greatest growth yield (56). Prereduced sulfur might therefore be favored over oxidized forms. Evidence that marine bacteria cultures prefer reduced sulfur comes from several studies that have found repression of sulfate incorporation into proteins by the addition of lower levels of reduced organic sulfur compounds. For example, 10 μM methionine inhibited the uptake and incorporation of 1 mM sulfate in Halomonas halodurans (formerly Pseudomonas halodurans) (9), and 10 μM cystine inhibited sulfate uptake in the gliding bacterium Cytophaga johnsonae (17). Enzymatic pathways that could utilize extracellular reduced sulfur compounds such as hydrogen sulfide and methanethiol (MeSH) for sulfur amino acid biosynthesis have been identified in cultures of bacteria (27, 52) and plants (49), but the operation and importance of these pathways in natural systems have not been investigated.
Dimethylsulfoniopropionate [(CH3)2S+CH2CH2COO−; DMSP] is one of the most abundant reduced sulfur compounds present in oxygenated surface waters of the marine environment (39, 45). A variety of unicellular algae and macroalgae produce DMSP mainly as an intracellular osmolyte (38), although other functions are also recognized (47, 60). The degradative metabolism of DMSP has come under close scrutiny because it is the major biogenic precursor of marine dimethylsulfide (DMS), a volatile sulfur compound that contributes significantly to the global atmospheric sulfur cycle and possibly to climate regulation (7). Lyase enzymes found in marine bacteria and some algae catalyze the production of DMS from DMSP (11, 53, 54, 61). Recent work, however, suggests that DMS is a minor product of overall DMSP degradation in seawater (5, 35, 39), indicating that alternative fates for the sulfur of DMSP are important. Kiene (30) reported that MeSH (CH3SH) was a major degradation product of DMSP and that this compound was lost rapidly from seawater, possibly through biological activity. MeSH arises from a demethylation/demethiolation pathway of DMSP degradation that is independent of the DMS-producing lyase pathway (55). Because the turnover of DMSP in marine surface waters is rapid (up to 120 nM day−1) (31, 33), and much of this may be metabolized without net sulfur gas production, the fate of sulfur from DMSP is of considerable interest from ecological and biogeochemical perspectives.
Studies of the fate of DMSP and its degradation products have been hampered by the lack of commercially available radiolabeled compounds. We therefore synthesized [35S]DMSP and [35S]MeSH and undertook a study to trace the fate of sulfur during the uptake and degradation of these compounds in natural marine microbial communities. Here we tested whether the sulfur in DMSP or its degradation product MeSH was utilized by marine bacterioplankton for biomass production. We characterized the main sulfur products formed, and by use of bacterial cultures, inhibitors, and differential radiolabeling, we investigated the pathway by which DMSP and MeSH sulfur was incorporated. The results suggest that the sulfur from DMSP is efficiently incorporated via MeSH into methionine and bacterial proteins, and that DMSP is a major and hitherto unrecognized source of reduced sulfur for marine bacterioplankton.
MATERIALS AND METHODS
Radiochemicals.
[35S]DMSP (specific activity, 0.81 to 3.4 Ci mmol−1; 1,800 to 7,500 dpm pmol−1) was synthesized by the alga Platymonas subcordiformis (UTEX-171) after administration of l- [35S]methionine (specific activity, 1,100 Ci mmol−1) in F/2 medium and was purified to >98% radiochemical purity according to procedures outlined by Kiene et al. (32). [35S]MeSH and [methyl-3H]MeSH were generated in 4-ml serum bottles from [35S]methionine and l-[methyl-3H]methionine, respectively, by using methionine gamma lyase (Sigma; EC 4.4.1.11). Specific activities of the 3H- and 35S-labeled MeSH were those of the parent methionine (100 and 1,100 Ci mmol−1, respectively), but in some cases this was adjusted to 1 Ci mmol−1 by addition of unlabeled methionine to the reaction vial. The gaseous labeled MeSH was removed by gas-tight syringe from the reaction vial and injected through a layer of Parafilm which was used to cover the opening of Teflon incubation bottles containing seawater. Labeled MeSH quickly dissolved in the water after mild shaking. Total added radioactivity was determined by counting 1- to 2-ml subsamples of the water sample within 5 min of isotope addition. Incubation bottles were closed with screw caps, and loss of volatile radioactivity during incubations with labeled MeSH or DMSP was <5% of the total added activity.
Seawater collection and processing.
Coastal seawater samples used for microbial uptake experiments were collected in the vicinity of Dauphin Island, Alabama, located in the northern Gulf of Mexico (30°15′N, 88°05′W). Whole, unfiltered water samples were used within 1 to 2 h of collection. In some experiments, seawater filtrate cultures were used so that the microbial community would consist mainly of heterotrophic bacteria and also so that endogenous organic substrates would be at low concentrations. The cultures were generated by filtering seawater through Whatman GF/F glass fiber filters (nominal retention, >0.7 μm) by using gravity only. Prior to the initiation of uptake experiments, the filtrate cultures were incubated in the dark for 24 to 48 h, during which time endogenous substrates (including DMSP and MeSH) became depleted (<1 nM) and bacterial abundances typically doubled from 1 × 106 to 2 × 106 cells · ml−1 or 2 × 106 to 4 × 106 cells · ml−1. Microscopic examination showed that filtrate cultures contained mainly free-living bacteria, with few microzooplankton or photoautotrophs observed at the time experiments were carried out.
Incubations were carried out in Teflon bottles held in the dark at 25 to 27°C. When used, heat-killed samples were held at 80°C for 1 h, then returned to 27°C prior to isotope additions. After the addition of substrate to the water samples, 5-ml subsamples were withdrawn by pipette at selected times and transferred to a 10-place Hoefer filtration manifold set up with 0.2-μm-pore-size nylon filters (MSI, Inc.). The samples were filtered by using a gentle vacuum (5 to 10 cm of Hg). Total uptake of the substrate was determined by counting filters in an Ecolume scintillation cocktail (ICN Biomedicals) after rinsing three times with 1 ml of filtered seawater (FSW) (pore size, 0.2 μm) of the same salinity as the sample. For measurement of the amount of 35S incorporated into macromolecules, parallel filters at each time point were first treated with ice-cold 5% trichloroacetic acid (TCA) (37). Tests showed that other, more elaborate treatment protocols, including rinses of hot TCA followed by 80% ethanol, which would be more specific for the protein fraction (as opposed to all macromolecules), produced results that were within 10% of those obtained with the cold 5% TCA procedure. For simplicity we routinely used the cold 5% TCA procedure. Radioactivity on filters was determined by liquid scintillation counting (Packard Tri-Carb model 2500 TR). Most data are presented as the percentage of the added 35S recovered in filterable fractions.
Proteinase tests.
A broad-spectrum proteinase (proteinase K; Fisher Scientific) was used to test whether the TCA-insoluble 35S-labeled material produced from DMSP and MeSH was protein. Seawater samples were treated with either [35S]DMSP or [35S]MeSH and were incubated for 24 h to allow uptake and incorporation of the substrates. For each substrate, 5-ml subsamples of the water were filtered through four separate 0.2-μm-pore-size nylon filters as described above. Each filter was rinsed with cold 5% TCA followed by five rinses with 1 ml of FSW (pore size, 0.2 μm). Duplicate filters were then covered with 5 ml of either Tris buffer alone (pH 7.8) or Tris buffer containing proteinase K (20 μg per ml). The solutions were left to stand on the filters for 30 min at room temperature before the solution was drawn through by vacuum. Each filter was rinsed again five times with 1 ml of FSW before being counted in Ecolume. The proteinase K solution used was tested for effectiveness at solubilizing 35S-labeled proteins by incubating a seawater sample with [35S]methionine (48). The labeled cells were filtered onto nylon membranes and subjected to the TCA rinse followed by proteinase K treatment (as above). This test showed that the proteinase K treatment solubilized (removed) 85% ± 1% of the 35S activity captured on TCA-rinsed filters.
HPLC of radiolabeled hydrolysis products.
After a 24-h incubation with either [35S]DMSP or [35S]MeSH, water samples were filtered onto 0.2-μm-pore-size Nuclepore polycarbonate or Magna Nylon (MSI) membranes and rinsed with ice-cold 5% TCA so that only the macromolecular fraction was retained. Filters were subjected to acid (HCl) hydrolysis under N2 at 105°C for 24 h. After evaporation of HCl, the products were taken up in pure water and a 50-μl aliquot was injected into a high-pressure liquid chromatograph (HPLC) for separation on a Whatman Partisil SCX column by using 50 mM KH2PO4 (pH 3.0) as the eluent. Fractions were collected by hand at various times during the elution of sample from the HPLC. Fractions were counted for radioactivity in Ecolume. For confirmation of peak identity, a second aliquot of the same sample was treated with a small volume of authentic [35S]methionine or l-[3H]methionine (NEN), and this too was subjected to HPLC analysis. Authentic methionine (labeled or unlabeled) eluted at a retention time of 5.15 min. Some methionine degradation products (i.e., methionine sulfoxide) might have been present in the radioactive stock, as well as the hydrolyzed samples, since l-methionine is reported to undergo oxidative degradation during aqueous storage (NEN product information).
Effects of sulfur cycle intermediates and inhibitors.
Seawater filtrate cultures (size fraction, <0.7 μm) were used to test the effects of certain sulfur compounds and metabolic inhibitors on incorporation of sulfur from [35S]DMSP and [35S]MeSH. These potential inhibitor compounds were added to the final concentrations indicated in Table 2, 10 to 15 min prior to [35S]DMSP or [35S]MeSH addition. The rate of 35S incorporation (determined from least-squares regression of three to four time points) from either [35S]DMSP or [35S]MeSH into TCA-insoluble material was measured in inhibited and noninhibited (control) samples. The percent inhibition was calculated as [1 − (inhibited rate/control rate)] × 100. Data for all inhibitors listed in Table 2 were collected on several different dates, with different water samples from the coastal Gulf of Mexico. All [35S]MeSH data were from different water samples than data for [35S]DMSP. All inhibitor stocks were prepared fresh on the day of use.
TABLE 2.
Effects of potential inhibitors on the rate of [35S]DMSP or [35S]MeSH incorporation into TCA-insoluble macromolecules by seawater bacterioplanktona
| Inhibitor compound and concnb (nM) | % Inhibition of rate of 35S incorporationc from:
|
|
|---|---|---|
| [35S]DMSP | [35S]MeSH | |
| Chloramphenicol (93,000) | 96 | ND |
| l-Methionined (20) | 91 | 89 |
| 3-Methiolpropionic acidd (20) | 47 | 54 |
| dl-Homocysteine (20) | 54 | 62 |
| 3-Mercaptopropionic acidd (20) | 51 | ND |
| MeSH (15) | 83 | ND |
| l-Cysteine (20) | 36 | 18 |
| 2-Ketomethiolbutyrated (20) | 77 | ND |
| Cystathionine (20) | 48 | 47 |
| Sodium sulfide (20) | 36 | ND |
| Sodium sulfide (85) | 46 | ND |
| DMSPd (20) | 82 | |
| Vinylglycine (20) | 62 | 25 |
| Propargylglycine (20) | 57 | 31 |
| Propargylglycine (50) | 68 | ND |
| Propargylglycine (100) | 73 | ND |
| Glucose (20) | −8 | 7 |
| Glycine (20) | −5 | 0 |
Seawater filtrates (<0.7 μm) consisting primarily of free-living bacterioplankton were used. Data were collected on several different dates, with different water samples from coastal waters of the Gulf of Mexico. All [35S]MeSH data were from water samples different from those used for [35S]DMSP tests.
Inhibitor compounds were added at the concentrations indicated, 10 to 15 min prior to [35S]DMSP or [35S]MeSH addition.
Relative to untreated controls. For each compound, measurements were obtained at multiple time points during incubations lasting 1 to 1.5 h (in the dark at 26°C), and the rate of incorporation was determined by least-squares regression. ND, not determined.
Low levels of MeSH may have been produced from these compounds during the incubation.
Tests of direct incorporation of MeSH into methionine.
A seawater filtrate culture (salinity, 30 ppt) was used for these tests. Water samples (in duplicate) were treated with ∼1 nM [35S]MeSH or [methyl-3H]MeSH. Total uptake and uptake into TCA-insoluble macromolecules were determined over several hours. After 16 h of incubation, TCA-rinsed filters with material from the [methyl-3H]MeSH-treated sample were subjected to acid hydrolysis. The hydrolysis products were separated by HPLC, and the radioactive peak fractions were collected as described above.
Assimilation of sulfur from DMSP and MeSH by bacterial isolates.
The 13 isolates tested were members of the “Roseobacter group,” a genetic lineage within the α subdivision of the division Proteobacteria that is abundant in coastal marine environments and amenable to isolation and culturing (21). More than half of these isolates were obtained nonselectively on yeast extract media, but most, if not all, members of this group can metabolize organic sulfur compounds, including DMSP (20). Isolate designations are those given previously (20). Isolate ISM was described by Fuhrman et al. (15). Cultures were grown aerobically on a modified basal salt medium (20 ppt) with Tris buffer (pH 7.5) and with vitamins and Fe-EDTA (21). Glucose (5 mM) was the carbon source. Cultures were in exponential growth (A580, 0.2 to 0.4) at the time incorporation was measured. Subsamples (2 ml) of the growing cultures were incubated in sealed 14-ml serum bottles, treated with either 2 nM [35S]DMSP or <0.01 nM (i.e., tracer level) [35S]MeSH, and incubated for 6 h. Incorporation was determined by treatment of filtered cells for 5 min with 5% TCA and assaying for the radioactivity remaining on the filter after rinsing with sterile medium.
DMSP turnover in situ.
Dissolved DMSP turnover rates in seawater were estimated with tracer additions of [35S]DMSP to freshly collected seawater from the northern and central Gulf of Mexico as described by Kiene and Linn (33). Data were collected on the R/V Pelican in September 1997. In parallel with DMSP turnover measurements, bacterial carbon production was estimated from [3H]thymidine incorporation on the assumptions that 2 × 1018 cells were produced per mol of thymidine incorporated and each cell contained 20 fg of C (42). Sulfur demand by the bacterioplankton was estimated by using a molar C/S ratio in marine bacteria of 248 (10). Only surface water (depth, 1 m) data are reported here. Further details on DMSP turnover in the Gulf of Mexico will be presented elsewhere (33).
RESULTS
Uptake and incorporation of DMSP sulfur.
When tracer levels (<0.12 nM) of dissolved [35S]DMSP were added to coastal seawater samples from the Gulf of Mexico, the radiolabel rapidly appeared in a particulate (>0.2-μm) fraction, presumably bacteria, and reached stable levels within 3 h (Fig. 1). The decrease in apparent uptake after 1 to 2 h was due to depletion of the added [35S]DMSP. The balance of 35S, other than that recovered in particulates, can be accounted for in volatile and dissolved nonvolatile products, as will be described elsewhere (34). Results from size fractionation of whole seawater incubated with [35S]DMSP and experiments with seawater filtrate cultures (<0.7 μm) supported the dominant role of bacteria in DMSP uptake (data not shown). In coastal-seawater samples, bacterioplankton incorporated approximately 30% of the total added [35S]DMSP into a macromolecular fraction (insoluble in cold 5% TCA) (Fig. 1). Heat-killed samples showed insignificant DMSP uptake or incorporation compared with live controls (Fig. 1). The higher total uptake of 35S, compared with that incorporated into macromolecules, is indicative of the presence of other 35S-labeled compounds in the cells. In other tests we have found that a large fraction of the non-TCA-insoluble 35S in cells is untransformed [35S]DMSP, which accumulated to varying degrees in bacterial cells from different samples (data not shown). The uptake and incorporation data presented in Fig. 1 are representative of a larger number of samples we have tested (>20 different samples over 1 year, including samples from temperate ocean waters), in which the fraction of added [35S]DMSP taken up ranged from 15 to 85% and the fraction incorporated into TCA-insoluble macromolecules ranged from 15 to 40%.
FIG. 1.
Time course showing total uptake and incorporation into TCA-insoluble macromolecules of 35S from [35S]DMSP by bacterioplankton in a coastal seawater sample from the Gulf of Mexico (salinity, 18 ppt; 27°C). The seawater sample was amended with <0.12 nM [35S]DMSP, which was less than 10% of the natural concentration (1.5 nM). Error bars represent the range of duplicate determinations. Water samples were incubated in the dark. Heat-killed samples were held at 80°C for 1 h, then returned to 26°C prior to isotope additions.
Incorporation of sulfur from DMSP and MeSH by bacterial cultures.
A survey of 13 isolates from the α-subdivision of the Proteobacteria, selected for their ability to utilize organic sulfur compounds (20) and as representatives of a numerically dominant group of bacteria in coastal seawater (21), showed that each of these isolates could metabolize DMSP to DMS but only 5 isolates could also produce MeSH from DMSP (Table 1) (20). Only those isolates (5 of 13) which could form MeSH from DMSP also incorporated significant amounts of [35S]DMSP into macromolecules (Table 1). In contrast, all of the isolates incorporated a large fraction (49 to 100%) of added [35S]MeSH. Separate tests showed that [35S]DMS was incorporated into biomass only slowly, or not at all, by these isolates or by seawater assemblages (data not shown), despite the fact that all of the isolates could produce DMS from DMSP and some could consume the DMS (20). This evidence suggested that DMSP must first be converted to MeSH via the demethylation/demethiolation pathway before significant incorporation of the sulfur into macromolecules is possible.
TABLE 1.
Incorporation of 35S from either [35S]DMSP or [35S]MeSH into the protein (TCA-insoluble) fraction by marine bacterial isolatesa
| Isolate | Production from DMSP of:
|
% of added 35S assimilated into TCA-insoluble fraction from:
|
||
|---|---|---|---|---|
| DMS | MeSH | [35S]DMSP | [35S]MeSH | |
| Group 1 | ||||
| DSS-3 | + | + | 80 | 86 |
| ISM | + | + | 46 | 49 |
| GAI-5 | + | + | 53 | 88 |
| DSS-8 | + | + | 55 | 84 |
| GAI-109 | + | + | 49 | 113b |
| Group 2 | ||||
| EE-36 | + | − | 3 | 74 |
| DSS-1 | + | − | 4 | 87 |
| DSS-2 | + | − | 1 | 84 |
| Sulfitobacter pontiacus ChLG 10 | + | − | 2 | 78 |
| Sagittula stellata E-37 | + | − | 4 | 74 |
| GAI-16 | + | − | 7 | 78 |
| GAI-21 | + | − | 5 | 86 |
| GAI-37 | + | − | 4 | 83 |
All isolates are in the Roseobacter group, a phylogenetic cluster of marine bacteria in the α-subdivision of the division Proteobacteria. Cultures were in exponential growth at the time assimilation was measured.
This culture produced extracellular mucilage that made pipetting difficult, possibly leading to overestimation of the percent incorporation.
Uptake and incorporation of [35S]MeSH by seawater microorganisms.
Because the bacterial cultures showed the ability to incorporate sulfur from MeSH, and because DMSP was recently shown to be degraded to MeSH in aerobic seawater (30), experiments were undertaken to test whether natural seawater assemblages could utilize trace levels (<1 nM) of [35S]MeSH. In coastal seawater with a natural pool of 0.5 nM MeSH, added tracer-level [35S]MeSH (<0.01 nM) was rapidly taken up and incorporated into macromolecules by bacterioplankton (Fig. 2). The total uptake and incorporation were substantially inhibited by heat treatment (Fig. 2), indicating that biological activity was responsible for the uptake. Filtration of water samples through 0.2-μm-pore-size membranes prior to substrate addition produced results similar to heat treatment (data not shown). The incorporation of 35S from MeSH into macromolecules represented most (>85%) of the total uptake, indicating little pooling of nonreacted [35S]MeSH in the cells. Similar results were obtained with temperate coastal waters from Nova Scotia (data not shown).
FIG. 2.
Time course showing total uptake and incorporation into TCA-insoluble macromolecules of 35S from [35S]MeSH by bacterioplankton in a coastal seawater sample from the Gulf of Mexico (salinity, 18 ppt; 27°C). The seawater sample was amended with <0.01 nM [35S]MeSH, which was less than 10% of the natural concentrations (∼0.5 nM). Error bars represent the range of duplicate determinations. Water samples were incubated in the dark. Heat-killed samples were held at 80°C for 1 h, then returned to 26°C prior to isotope additions.
TCA-insoluble 35S is in proteins and mostly in methionine.
Recovery of 35S in cold 5% TCA-insoluble material strongly suggested that the sulfur had been incorporated into a macromolecular, possibly protein fraction. When filters used to capture TCA-insoluble 35S cell material derived from either [35S]DMSP or [35S]MeSH were treated with proteinase K for 30 min, more than 75% of the 35S activity was solubilized, suggesting that the sulfur had been incorporated into proteins (Fig. 3). Further evidence that sulfur from these compounds was incorporated into bacterial proteins came from experiments in which chloramphenicol, a well-known inhibitor of protein synthesis in prokaryotes, inhibited the rate of [35S]DMSP incorporation by 96% in short-term (<2-h) assays (Table 2).
FIG. 3.
Effects of proteinase K treatment on retention of 35S activity on filters used to capture TCA-insoluble materials labeled after metabolism of [35S]DMSP and [35S]MeSH by whole-seawater microbial communities. Control filters (solid bars) were treated with the same Tris buffer (pH 7.8) that was used to prepare the proteinase K solution.
To test whether specific labeling of sulfur amino acids was taking place, we incubated samples from the Gulf of Mexico with [35S]DMSP or [35S]MeSH and collected the resulting labeled, TCA-insoluble cell material on filters. The TCA-rinsed filters were subjected to acid hydrolysis, and the resulting amino acids were separated by cation-exchange HPLC. The major fraction (>60%) of [35S]DMSP-derived radioactivity eluted from the column was associated with methionine (retention time, 5.15 min [Fig. 4A]). Lesser amounts of radioactivity were found in a peak corresponding to the retention time of cysteine (4.4 min) and in unidentified compounds. Overall, we could account for approximately 50% of the 35S activity initially present on TCA-rinsed filters as [35S]methionine. Results for [35S]MeSH-derived proteins were similar; >60% of the TCA-insoluble 35S on filters was associated with [35S]methionine (Fig. 4B).
FIG. 4.
Identification by HPLC of [35S]methionine (retention time = 5.15 min) produced from either [35S]DMSP (A) or [35S]MeSH (B) by natural bacterioplankton assemblages from coastal waters of the Gulf of Mexico. Heavy line with open circles, radiochromatogram (0.25- to 1.0-ml fractions) of the hydrolyzed samples of TCA-insoluble material generated from either [35S]DMSP or [35S]MeSH. Lighter line, radiochromatogram of the same sample, but with added [35S]methionine. The peak of radioactivity at 5.15 min coeluted with unlabeled l-methionine as determined by UV absorbance detection. Some methioninie degradation products (i.e., methionine sulfoxide) might have been present in the radioactive stock, as well as the hydrolyzed samples. The small peak at 4.4 min corresponds to the retention time of cysteine.
Effects of methionine on DMSP uptake and incorporation.
If methionine is a product of DMSP metabolism, exogenous l-methionine could compete with DMSP as a source of sulfur for bacterial biomass production. Addition of 10 nM methionine to a seawater filtrate culture 15 min before the addition of [35S]DMSP did not affect the initial uptake of DMSP (Fig. 5). However, after the 1st h of incubation, less particulate 35S activity was recovered in the samples treated with methionine. The divergence in the two treatments over time probably resulted from the lower rate of incorporation of [35S]DMSP into macromolecules in the presence of methionine, which was revealed by the 66%-lower level of 35S in TCA-insoluble material produced in the presence of methionine (Fig. 5). Overall the results of this experiment suggest that methionine did not inhibit the uptake of DMSP but did inhibit the incorporation of sulfur from DMSP into proteins.
FIG. 5.
Comparison of the total uptake of [35S]DMSP with time by a seawater filtrate culture in the presence (▴) or absence (●) of 10 nM l-methionine. Cell materials in 5-ml subsamples at each time were collected on 0.2-μm-pore-size nylon filters. At 6 h of incubation, an additional set of subsamples were filtered, and the filters were rinsed with 5% cold TCA, in order to measure the amount of cellular 35S in macromolecules. These data are presented as open circles (without methionine) and open triangles (with methionine). Data points represent the means of duplicate determinations. Error bars indicate the ranges but are smaller than the symbols in most cases. l-Methionine was added 15 min prior to addition of [35S]DMSP. The seawater used for this experiment had a salinity of 28, and the incubation was carried out in the dark at 25°C.
Effects of sulfur cycling intermediates and inhibitors.
The incorporation of [35S]DMSP and [35S]MeSH into macromolecules of natural bacterioplankton was strongly inhibited by nanomolar concentrations of compounds known to be associated with sulfur amino acid metabolism, including hydrogen sulfide, methionine, cysteine, cystathionine, homocysteine, 3-mercaptopropionate, and 3-methiolpropionate (Table 2). In contrast, compounds like glucose and glycine, which are not directly involved in sulfur metabolism, had no significant inhibitory effects. These findings suggest that incorporation of DMSP and MeSH involves biochemical pathways central to sulfur amino acid biosynthesis in marine bacteria. When we added propargylglycine, a known inhibitor of cystathionine γ-synthetase (25), and vinylglycine, a substrate for this enzyme (26), at 20 to 100 nM to seawater filtrate cultures, strong inhibition of DMSP and MeSH incorporation into protein was observed (Table 2).
Evidence for direct incorporation of MeSH into methionine.
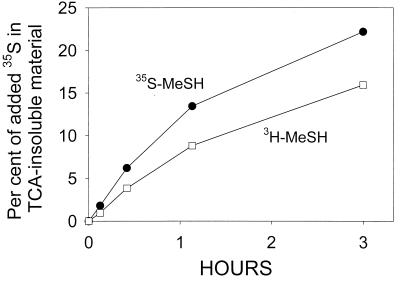
To test whether seawater microorganisms incorporated the methyl and sulfur moieties from MeSH into methionine, we conducted uptake experiments with both [35S]MeSH and [methyl-3H]MeSH. When [35S]MeSH was added to seawater samples, it was taken up and incorporated into TCA-insoluble cell material with a time pattern identical to that of [35S]MeSH in parallel samples, but at a somewhat slower initial rate (62 to 64%) (Fig. 6). The total amount of 3H taken up after 6 h (data not shown) also reflected this lower percentage. Despite the somewhat lower rate of incorporation, approximately 70% of the total TCA-insoluble 3H activity captured on filters was shown by HPLC analysis of acid hydrolysis products to be in the form of [3H]methionine (Fig. 7). No 3H labeling was associated with the retention time of cysteine, as had been observed in the radiochromatograms of [35S]MeSH-labeled cell materials (see Fig. 4). The latter observation might explain the lower incorporation rate of [methyl-3H]MeSH compared with that of the sulfur label.
FIG. 6.
Comparison of uptake into TCA-insoluble macromolecules of [35S]MeSH and [methyl-3H]MeSH in a seawater filtrate culture. Both substrates were added at ∼1 nM. Results are means of duplicate determinations at each time, and the ranges are smaller than the symbols in all cases. The initial rate of uptake in the [methyl-3H]MeSH was 62% that in the [35S]MeSH sample. Samples were incubated in the dark at 25°C.
FIG. 7.
Identification by HPLC of [3H]methionine (retention time = 5.15 min) produced from [methyl-3H]MeSH by a seawater filtrate culture from coastal waters of the Gulf of Mexico. Heavy line with open circles, radiochromatogram (0.25- to 1.0-ml fractions) of the hydrolyzed samples of TCA-insoluble material generated from [methyl-3H]MeSH. Lighter line, radiochromatogram of the same sample, but with added [methyl-3H]methionine.
DMSP turnover in situ and its contribution to sulfur demand.
Natural DMSP turnover in surface waters of the Gulf of Mexico, measured with tracer additions of [35S]DMSP, ranged from 2.8 to 33 nM day−1 (Table 3). Simultaneous estimates of bacterial carbon production (based on [3H]thymidine incorporation into DNA) in the same samples ranged from 140 to 8,220 nM of C day−1. By using a literature value of 248 for the molar ratio of C to S in marine bacteria (10), bacterial sulfur demand was calculated to be 0.6 to 32.9 nM S day−1. Each mole of DMSP contains 1 mol of reduced sulfur; therefore, at most of the stations sampled, the DMSP turnover provided more reduced sulfur (mean, 459%) than the amount required for bacterial growth in these waters (Table 3). Of the total DMSP turnover, not all will be incorporated, and unfortunately we did not measure the fraction of DMSP turnover that was incorporated into protein during this cruise. If, however, we assume that 25% of the S from DMSP was incorporated (the mean incorporation efficiency measured throughout our study), the contribution of DMSP to sulfur demand ranged from 23 to 209%, with a mean of 115% (Table 3).
TABLE 3.
Rates of bacterial carbon production and DMSP turnover and the potential contribution of DMSP turnover and incorporation to bacterial sulfur demand in surface waters of the Gulf of Mexicoa
| Station no. | Date (mo/day/yr) | LATb | LONc | Total depth (m) | Bacterial carbon production (nM day−1) | Calculated bacterial sulfur demand (nM day−1) | Dissolved DMSP turnover rate (nM day−1) | Calculated DMSP incorporation rated rate (nM day−1) | Potential contribution to bacterial sulfur demand (%) by:
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| DMSP turnover | DMSP incorporation | |||||||||
| 2 | 9/24/97 | 28 22.82 | 88 17.97 | 2,280 | 140 | 0.6 | 2.8 | 0.7 | 496 | 123 |
| 5 | 9/24/97 | 28 23.02 | 88 19.94 | 143 | 156 | 0.6 | 3.2 | 0.8 | 518 | 129 |
| 8 | 9/25/97 | 27 54.14 | 83 32.63 | 35 | 420 | 1.7 | 12.8 | 3.2 | 759 | 188 |
| 10 | 9/26/97 | 27 04.72 | 85 19.56 | 3,184 | 413 | 1.7 | 4.9 | 1.2 | 297 | 74 |
| 12 | 9/27/97 | 26 21.57 | 87 25.28 | 2,964 | 148 | 0.6 | 2.8 | 0.7 | 475 | 118 |
| 14 | 9/28/97 | 27 35.76 | 88 50.99 | 1,833 | 293 | 1.2 | 9.9 | 2.5 | 842 | 209 |
| 18 | 9/29/97 | 28 36.54 | 90 29.19 | 27 | 1,225 | 4.9 | 10.9 | 2.7 | 223 | 55 |
| 23 | 9/30/97 | 28 53.12 | 89 26.20 | 15 | 8,219 | 32.9 | 31.0 | 7.8 | 94 | 23 |
| Mean | 463 | 115 | ||||||||
All samples were collected from a 1-m depth by using a Niskin bottle attached to a CTD. DMSP turnover was estimated with nonperturbing tracer additions of [35S]DMSP to 50-ml water samples. Incubation times were less than 1 h. Bacterial carbon production levels were estimated from [3H]thymidine incorporation by assuming that 2 × 1018 cells were produced per mol of thymidine incorporated and that each cell contained 20 fg of C. Sulfur demand was estimated by using a value of 248 for the molar C/S ratio in marine bacteria (10).
LAT, latitude (N, degree and minute).
LON, longitude (W, degree and minute).
At 25% efficiency.
DISCUSSION
Marine bacteria are known to be important consumers of dissolved DMSP (31, 41), and it is generally believed that bacteria convert DMSP into volatile sulfur compounds such as DMS and MeSH (30). The fates of these gases and other products of DMSP metabolism are poorly known. The results of the present study show that incorporation into particulates was a major short-term fate of the sulfur from DMSP in the subtropical waters of the Gulf of Mexico. We were able to trace the sulfur into methionine found in the protein fraction of bacterioplankton, suggesting that the sulfur from DMSP and MeSH was used for assimilatory purposes. We have obtained similar results with temperate waters from Nova Scotia (data not shown), suggesting that the use of DMSP as a sulfur source by marine bacterioplankton is a general phenomenon in marine surface waters. As far as we are aware, this is the first report demonstrating the use of DMSP as a source of sulfur in marine bacteria. Evidence from field measurements in the Gulf of Mexico further suggests that DMSP (via MeSH) is a quantitatively important source of reduced sulfur for bacterioplankton (Table 3). These findings significantly advance our understanding of sulfur cycling in surface seawater and add new dimensions to the cycling of DMSP, which is already recognized as a biogeochemically important sulfur compound.
Sulfur sources in bacteria.
It is generally assumed that most bacteria in nature acquire sulfur in the form of sulfate, which is plentiful in most aquatic systems. Few studies, however, have actually examined sulfur uptake and assimilation in nature. Cuhel and coworkers (8–10) studied sulfate uptake by both bacterial cultures and natural marine populations. They found that 35SO42− was taken up into a variety of intracellular pools, of which the major one was protein. Sulfate appeared to be used by all isolates tested and by natural assemblages, but no comparisons were made in the natural samples between the uptake of sulfate and other potential sources of sulfur. With a culture of H. halodurans (formerly P. halodurans), Cuhel et al. (9) found that 10 μM methionine significantly inhibited the uptake of 1 mM 35SO42−. Likewise, in the gliding bacterium C. johnsonae, 10 μM cystine was found to inhibit sulfate incorporation into protein but had less of an effect on sulfate incorporation into sulfonolipids (17). These findings suggest that reduced organic sulfur compounds might be used in preference to sulfate for protein synthesis in nature. A major difficulty in using 35SO42− to measure sulfur incorporation by natural seawater bacteria is the huge isotope dilution resulting from the seawater sulfate pool (28 mM). To overcome this dilution, Cuhel et al. used additions of 25 μCi of 35SO42− ml−1 in samples of hundreds of milliliters (9). The requirement for such high levels of isotope prohibited direct comparisons of sulfate uptake with DMSP or MeSH uptake in the present study. Such comparisons will be needed, however, to test whether DMSP or MeSH is used in preference to sulfate, as our data suggest.
Bacteria capable of incorporating sulfur from DMSP or MeSH have been isolated from coastal seawater (Table 1) (20). From the screening of the isolates, we can deduce that a key step in the incorporation of DMSP sulfur into protein amino acids is the production of MeSH, which is produced in the demethylation/demethiolation pathway of DMSP degradation (55): (CH3)2S+CH2CH2COOO− → CH3SCH2CH2COO− → CH3SH + other products DMSP 3-methiolpropionate MeSH
Bacterial strains isolated from coastal seawater incorporated DMSP sulfur into protein only if they were capable of degrading DMSP to MeSH, whereas MeSH was rapidly incorporated into macromolecules by all tested strains (Table 1) and by natural bacterioplankton (Fig. 2). Thus, the expression of the demethylation/demethiolation pathway appears to be essential for organisms to directly utilize DMSP as a source of sulfur for protein amino acids. In mixed assemblages DMSP might supply sulfur even to those organisms unable to utilize it directly. Extracellular release of MeSH has been observed during DMSP degradation in seawater (30, 34), and this might provide a pool of available MeSH to organisms able to use it as a source of sulfur. Data from our limited survey of bacterial isolates (Table 1) suggest that the use of MeSH for protein synthesis is widespread in marine bacteria from the α subdivision of the Proteobacteria.
Evidence for direct incorporation of MeSH into methionine and the role of cystathionine γ-synthetase.
Several lines of evidence suggest that marine bacterioplankton incorporate MeSH directly into methionine by the action of cystathionine γ-synthetase. The potential pathways of sulfur incorporation from MeSH into sulfur amino acids are shown in Fig. 8. We found that both the methyl and sulfur moieties of MeSH are incorporated with high efficiency into methionine by natural assemblages of bacterioplankton (Fig. 4 and 7). These results strongly suggest that the methiol group (CH3S-H) of MeSH was incorporated as a unit directly into methionine. If MeSH were first converted to H2S and the H2S were incorporated into sulfur amino acids, then the methyl group would be most unlikely to follow the sulfur into methionine (Fig. 8). The fact that we observed primarily methionine, rather than cysteine, being produced from DMSP and MeSH further supports a major role for direct incorporation of MeSH into methionine. Seawater bacterioplankton, and isolates from the numerically important Roseobacter group (21), apparently utilize cystathionine γ-synthetase for incorporation of MeSH into methionine and eventually protein (Fig. 2; Table 1). Tests carried out with seawater showed that low concentrations (20 to 100 nM) of propargylglycine or vinylglycine inhibited incorporation of [35S]DMSP and [35S]MeSH into bacterial macromolecules (Table 2). Both these compounds are reported to inhibit the activity of cystathionine γ-synthetase, with propargylglycine functioning as a suicide inhibitor whereas vinylglycine is a substrate for the enzyme. The enzyme cystathionine γ-synthetase is present in diverse bacteria (26–28, 51) and plants (18, 19, 49) and is best known for catalyzing the reaction of cysteine with O-substituted (acetyl or succinyl) homoserine to form cystathionine. Several enzymological studies have observed that cystathionine γ-synthetase is a versatile enzyme capable of utilizing a wide variety of thiols in place of cysteine. When MeSH is the thiol substrate, methionine is formed directly, bypassing other intermediates such as homocysteine and cystathionine (27). This reaction has been known for some time (14), but its use for sulfur assimilation by organisms in nature has not been documented previously.
FIG. 8.
Potential pathways of sulfur incorporation from MeSH into methionine. Reaction 1 is the scheme proposed as the major one used to incorporate sulfur from DMSP (via MeSH) into bacterial methionine and ultimately protein. Pathway 2 may also operate to a lesser extent and may explain the labeling of cysteine with the sulfur from DMSP and MeSH.
In the field, MeSH may be produced from any number of sources, but phytoplankton DMSP is likely to be the main precursor in the euphotic zone of the ocean (30). The conversion of DMSP to methionine by bacteria completes a cycle of sorts, in that methionine is the precursor of the sulfur, methyl groups, and carbon chain of DMSP in all known biosynthetic pathways for this compound (16). Such interconversions of organic sulfur compounds by microorganisms are common in nature (29, 55). Interestingly, ours is not the first study to link DMSP to methionine synthesis. Durell et al. (12) and Ishida and Kadota (24) presented evidence that DMSP could donate a methyl group to homocysteine in the synthesis of methionine in mammals and heterotrophic algae. Such a transmethylation reaction, however, would not transfer the sulfur from DMSP to methionine and so would not explain the results we obtained with [35S]DMSP in seawater.
Ecological and biogeochemical implications.
The present study focused exclusively on marine microorganisms, but some of our findings might shed light on sulfur acquisition by freshwater microorganisms as well. While DMSP appears to be restricted primarily to marine habitats, MeSH is present in all aquatic environments (22, 29), being produced from protein (i.e., methionine) degradation and also sulfide methylation (44). Use of MeSH for methionine synthesis might therefore be important to aquatic microbes from diverse habitats. In this regard, we have obtained preliminary evidence that freshwater bacterioplankton readily incorporate tracer levels of [35S]MeSH into TCA-insoluble macromolecules (34a).
Our finding that a substantial fraction of the natural DMSP turnover results in incorporation of sulfur into bacterioplankton rather than production of sulfur gases has important biogeochemical implications. DMS, a climatically active sulfur gas (7) and a major source of reduced sulfur in the atmosphere (3), is produced from DMSP in seawater but appears to be a minor product under most circumstances (30). Assimilation of DMSP sulfur into organisms limits the potential production of DMS; therefore, factors such as the relative predominance of DMSP-assimilating bacteria (e.g., group 1 in Table 1) and overall bacterial sulfur demand (e.g., growth rate) are likely to influence the amounts of DMS formed from DMSP in seawater. The presence of alternative reduced-sulfur compounds (methionine, cysteine, homocysteine, etc. [see Table 2 and Fig. 5]) will probably affect the metabolic fate of DMSP as well. Likewise, dissolved DMSP concentrations are likely to affect the fraction of DMSP converted to DMS. If DMSP is available in excess of bacterial sulfur demand, a larger fraction of the DMSP could be degraded to DMS and possibly escape from the sea to the atmosphere to influence the climate (7).
Our data strongly suggest that DMSP supplies a major fraction of the sulfur utilized for biomass production by bacterioplankton in marine surface waters (Table 3). Comparison of bacterial sulfur demand with measurements of natural DMSP turnover, as determined with tracer additions of [35S]DMSP, suggested that DMSP turnover provided more reduced sulfur than what was required for bacterial growth in Gulf of Mexico surface waters (Table 3). Cycling of DMSP in excess of bacterial sulfur demand would support production of DMS and dissolved nonvolatile compounds (34) and is consistent with the fact that we observed only 15 to 40% of the sulfur from DMSP being incorporated into macromolecules (see Fig. 1). If the mean incorporation factor of 25% is applied to the DMSP turnover rates, the resulting sulfur incorporation rates compare closely with the sulfur demand calculated from thymidine-based growth rates (mean contribution, 115% [Table 3]). While these calculations lead to the conclusion that DMSP is an important, if not major, source of sulfur for bacterioplankton, there is some uncertainty in our estimates. One source of uncertainty lies in the C/S ratio that we used. Fagerbakke et al. (13) used X-ray microanalysis of individual bacterial cells and determined that natural populations and cultured bacteria had a mean molar C/S ratio of 86, about threefold lower than the ratio of 248 obtained by Cuhel et al. (10). The lower ratio would make the bacteria especially rich in sulfur (even more than in phosphorus; C/P ratio, ∼106). If this lower ratio of C to S is used in our calculations, the contribution of DMSP to bacterial sulfur demand diminishes by a factor of about 3, but the conclusion that DMSP is an important source of sulfur (8 to 65% of demand; mean, 40%) could still be supported. Additionally, there could be uncertainties in the conversion of thymidine-based growth rates to bacterial carbon production (used for estimating sulfur demand). However, in recent tests we have used bacterial carbon production rates based on [3H]leucine incorporation into protein and reached similar conclusions with regard to DMSP being a major sulfur source for bacterioplankton (62).
Importance of DMSP sulfur to the food web.
Our work suggests a fascinating adaptation by the marine microbial community: they utilize trace amounts of reduced sulfur available in DMSP (∼1 to 10 nM) instead of the oxidized sulfate, which at ∼28 mM is nearly 107-fold more abundant. Marine bacterioplankton exploit the nanomolar levels of reduced sulfur available in DMSP, presumably for the purpose of saving energy over that required for reduction of sulfate. Direct incorporation of MeSH into methionine would save the reducing power needed to convert sulfate to sulfide and would also save the energy (and carbon) that would be required to methylate the sulfur during methionine synthesis. Although the energetics of sulfur cycling in aerobic bacteria are expected to contribute only a small fraction of the total energy budget of the cells, even small efficiencies are probably beneficial to bacteria in the competitive, low-nutrient environment of the ocean. Our observation of efficient use of prereduced sulfur by bacterioplankton would be consistent with observations that marine bacterioplankton (36) and phytoplankton (58) prefer reduced nitrogen (NH4+) over oxidized nitrogen (NO3−) and also with bioenergetics models (56) that suggest that aquatic bacteria might optimize growth rates by use of the most-reduced substrates. Inorganic reduced-sulfur compounds, including hydrogen sulfide, which is present at picomolar to nanomolar levels in seawater (57), might also be used by bacterioplankton, since sulfide can be incorporated by the same pathway as MeSH (Fig. 8) (27). Likewise, sulfur amino acids and other organic sulfur compounds are probably scavenged by the bacterioplankton such that reduced sulfur is efficiently recycled within the food web. Some sulfate assimilation by bacteria undoubtedly occurs (9), perhaps by organisms that cannot assimilate DMSP (or MeSH) or even by those that can, when DMSP is present at low levels or absent (i.e., below the euphotic zone), or to meet non-reduced-sulfur requirements (e.g., sulfonate, sulfate esters, etc.) (17).
Much of the work on DMSP to date has focused on its role in trace gas production (i.e., DMS and MeSH). The results reported here point to an important but previously unrecognized role for DMSP in the ecology of marine microorganisms: that is, as a major source of reduced sulfur for sulfur amino acid synthesis. In particular, DMSP and its degradation product MeSH seem to support production of methionine by a very efficient mechanism that highlights an elegant adaptation of the marine microbial community. Because methionine is an important nutritional amino acid for bacteria and higher organisms, this finding could have broader implications for marine food web dynamics. That methionine in particular is a major product of DMSP metabolism is of interest because methionine is required in the diets of many marine animals and DMSP is abundant in the euphotic zone and has a turnover time of minutes to hours (31, 40). DMSP-derived methionine produced by bacteria is likely to be passed up the food chain because bacterioplankton are an important food source for heterotrophic nanoplankton, which in turn are important links to higher trophic levels (4, 50). We also speculate that incorporation of DMSP sulfur may not be restricted to bacterioplankton. Previous studies (5, 59) have shown that when protozoans graze on DMSP-containing phytoplankton, accumulations of volatile products are low, suggesting possible incorporation of the DMSP sulfur by the grazers. Our findings may also help to explain the long-known trophic transfer of DMSP from phytoplankton to higher levels of the food chain. Filter feeding shellfish (1, 23), fin fish (2, 43), and other marine animals are known to accumulate phytoplankton-derived DMSP from the diet and to retain it in nondigestive tissues. DMSP (and MeSH) might serve as a source(s) of reduced sulfur (with or without the help of bacteria) which can be utilized by animals.
ACKNOWLEDGMENTS
Thanks are extended to John Foster for providing leads to information on cystathionine synthetase. David Kirchman provided very helpful advice on setting up the proteinase K tests and on the protein hydrolysis/amino acid HPLC analysis. Jed Fuhrman graciously provided the bacterial isolate ISM. Robert Moore and Phil Morneau were kind enough to arrange for collection and transport of seawater from Nova Scotia to Alabama. Brian Jones and Ted Stets provided helpful comments. The captain and crew of the R/V Pelican facilitated work in the Gulf of Mexico.
This study was funded by the Chemical and Biological Oceanography programs of the NSF through grants OCE-95-30378 (to R.P.K.) and OCE-97-30745 (to M.A.M.).
REFERENCES
- 1.Ackman R G, Hingley H J. The occurrence and retention of dimethyl-β-propiothetin in some filter-feeding organisms. J Fish Res Board Can. 1968;25:267–284. [Google Scholar]
- 2.Ackman R G, Hingley J, May A W. Dimethyl-β-propiothetin dimethyl sulfide in Labrador cod. J Fish Res Board Can. 1967;24:457–461. [Google Scholar]
- 3.Andreae M O. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar Chem. 1990;30:1–29. [Google Scholar]
- 4.Azam F, Fenchel T, Field J, Gray J, Meyer-Reil L, Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser. 1983;10:257–263. [Google Scholar]
- 5.Belviso S, Kim S K, Rassoulzadegan F, Krajka B, Nguyen B C, Mihalopoulos N, Buat-Menard P. Production of dimethylsulfonium propionate (DMSP) and dimethylsulfide (DMS) by a microbial food web. Limnol Oceanogr. 1990;35:1810–1821. [Google Scholar]
- 6.Cantoni G L. Onium compounds and their biological significance. In: Florkin M, Mason H S, editors. Comparative biochemistry. New York, N.Y: Academic Press; 1960. pp. 181–241. [Google Scholar]
- 7.Charlson R J, Lovelock J E, Andreae M O, Warren S G. Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate. Nature. 1987;326:655–661. [Google Scholar]
- 8.Cuhel R L, Jannasch H W, Taylor C D. Microbial growth and macromolecular synthesis in the northwestern Atlantic Ocean. Limnol Oceanogr. 1983;28:1–18. [Google Scholar]
- 9.Cuhel R L, Taylor C D, Jannasch H W. Assimilatory sulfur metabolism in marine microorganisms: considerations for the application of sulfate incorporation into protein as a measurement of natural population protein synthesis. Appl Environ Microbiol. 1982;43:160–168. doi: 10.1128/aem.43.1.160-168.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuhel R L, Taylor C D, Jannasch H W. Assimilatory sulfur metabolism in marine microorganisms: sulfur metabolism, protein synthesis, and growth of Alteromonas luteo-violaceus and Pseudomonas halodurans during perturbed batch growth. Appl Environ Microbiol. 1982;43:151–159. doi: 10.1128/aem.43.1.151-159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza M P, Yoch D C. Purification and characterization of DMSP lyase from an Alcaligenes-like dimethylsulfide-producing marine isolate. Appl Environ Microbiol. 1995;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durell J, Anderson D G, Cantoni G L. The synthesis of methionine by enzymic transmethylation. I. Purification and properties of thetin homocysteine methylpherase. Biochim Biophys Acta. 1957;26:270–282. doi: 10.1016/0006-3002(57)90005-7. [DOI] [PubMed] [Google Scholar]
- 13.Fagerbakke K M, Heldal M, Norland S. Content of carbon, nitrogen, oxygen, sulfur, and phosphorus in native aquatic and cultured bacteria. Aquat Microb Ecol. 1996;10:15–27. [Google Scholar]
- 14.Flavin M, Slaughter C. Enzymatic synthesis of homocysteine or methionine directly from O-succinylhomoserine. Biochim Biophys Acta. 1967;132:400–405. doi: 10.1016/0005-2744(67)90158-1. [DOI] [PubMed] [Google Scholar]
- 15.Fuhrman J A, Lee S H, Masuchi Y, Davis A A, Wilcox R M. Characterization of marine prokaryotic communities via DNA and RNA. Microb Ecol. 1994;28:133–145. doi: 10.1007/BF00166801. [DOI] [PubMed] [Google Scholar]
- 16.Gage D, Rhodes D, Nolte K, Hicks W, Leustek T, Cooper A, Hanson A. A new route for the synthesis of dimethylsulfoniopropionate in marine algae. Nature. 1997;387:891–894. doi: 10.1038/43160. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore D F, Godchaux W I I I, Leadbetter E R. Regulation of sulfate assimilation in Cytophaga johnsonae. Arch Microbiol. 1989;152:387–392. [Google Scholar]
- 18.Giovanelli J. Sulfur amino acids of plants: an overview. Methods Enzymol. 1987;143:419–426. [Google Scholar]
- 19.Giovanelli J, Mudd S H, Datko A H. Homocysteine biosynthesis in plants. In: Cavallini D, Gaull G E, Zappia V, editors. Natural sulfur compounds: novel biochemical and structural aspects. New York, N.Y: Plenum Press; 1980. pp. 81–92. [Google Scholar]
- 20.González J M, Kiene R P, Moran M A. Transformation of sulfur compounds by an abundant lineage of marine bacteria in the α-subclass of the class Proteobacteria. Appl Environ Microbiol. 1999;65:3810–3819. doi: 10.1128/aem.65.9.3810-3819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González J M, Moran M A. Numerical dominance of a group of marine bacteria in the α-subclass of the class Proteobacteria in coastal seawater. Appl Environ Microbiol. 1997;63:4237–4242. doi: 10.1128/aem.63.11.4237-4242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henatsch J J, Juttner F. Occurrence and distribution of methane thiol and other volatile organic sulphur compounds in a stratified lake with anoxic hypolimnion. Arch Hydrobiol. 1990;119:315–323. [Google Scholar]
- 23.Iida H, Tokunaga T. Dimethyl sulfide and dimethyl-β-propiothetin in shellfish. Bull Jpn Soc Sci Fish. 1986;52:557–563. [Google Scholar]
- 24.Ishida Y, Kadota H. Participation of dimethyl-β-propiothetin in transmethylation reaction in Gyrodinium cohnii. Bull Jpn Soc Sci Fish. 1968;34:699–705. [Google Scholar]
- 25.Johnston M, Jankowski D, Marcotte P, Tanaka H, Esaki N, Soda K, Walsh C. Suicide inactivation of bacterial cystathionine γ-synthase and methionine γ-lyase during processing of l-propargylglycine. Biochemistry. 1979;18:4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- 26.Johnston M, Marcotte P, Donovan J, Walsh C. Mechanistic studies with vinylglycine and β-haloaminobutyrates as substrates for cystathionine γ-synthetase from Salmonella typhimurium. Biochemistry. 1979;18:1729–1738. doi: 10.1021/bi00576a015. [DOI] [PubMed] [Google Scholar]
- 27.Kanzaki H, Kobayashi M, Nagasawa T, Yamada H. Purification and characterization of cystathionine γ-synthase type II from Bacillus sphaericus. Eur J Biochem. 1987;163:105–112. doi: 10.1111/j.1432-1033.1987.tb10742.x. [DOI] [PubMed] [Google Scholar]
- 28.Kanzaki H, Nagasawa T, Yamada H. Insight into the active site of Streptomyces cystathionine γ-lyase based on the results of studies on its substrate specificity. Biochim Biophys Acta. 1987;913:45–50. doi: 10.1016/0167-4838(87)90230-5. [DOI] [PubMed] [Google Scholar]
- 29.Kiene R P. Microbial cycling of organosulfur gases in marine and freshwater environments. In: Adams D, Seitzinger S, Crill P, editors. Cycling of reduced gases in the hydrosphere. Vol. 23. Stuttgart, Germany: E. Schweitzerbart’sche Verlagsbuchhandlung (Naglele u. Obermiller); 1995. pp. 137–151. [Google Scholar]
- 30.Kiene R P. Production of methanethiol from dimethylsulfoniopropionate in marine surface waters. Mar Chem. 1996;54:69–83. [Google Scholar]
- 31.Kiene R P. Turnover of dissolved DMSP in estuarine and shelf waters from the Northern Gulf of Mexico. In: Kiene R, Visscher P, Keller M, Kirst G, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum; 1996. pp. 337–349. [Google Scholar]
- 32.Kiene R P, Hoffmann Williams L P, Walker J E. Seawater microorganisms have a high affinity glycine betaine uptake system which also recognizes dimethylsulfoniopropionate. Aquat Microb Ecol. 1998;15:39–51. [Google Scholar]
- 33.Kiene, R. P., and L. Linn. Turnover of dissolved DMSP and its relationship with bacterial production in the Gulf of Mexico. Submitted for publication.
- 34.Kiene, R. P., and L. J. Linn. On the fate of DMSP-sulfur in seawater: tracer studies with dissolved 35S-DMSP. Submitted for publication.
- 34a.Kiene, R. P., and L. J. Linn. Unpublished data.
- 35.Kiene R P, Service S K. Decomposition of dissolved DMSP and DMS in estuarine waters: dependence on temperature and substrate concentration. Mar Ecol Prog Ser. 1991;76:1–11. [Google Scholar]
- 36.Kirchman D L. The uptake of inorganic nutrients by heterotrophic bacteria. Microb Ecol. 1994;28:255–271. doi: 10.1007/BF00166816. [DOI] [PubMed] [Google Scholar]
- 37.Kirchman D L, K’Nees E, Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol. 1985;49:599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirst G O. Osmotic adjustment in phytoplankton and macroalgae: the use of dimethylsulfoniopropionate (DMSP) In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum; 1996. pp. 121–129. [Google Scholar]
- 39.Kwint R L J, Kramer K J M. Annual cycle of the production and fate of DMS and DMSP in a marine coastal system. Mar Ecol Prog Ser. 1996;134:217–224. [Google Scholar]
- 40.Kwint R L J, Quist P, Hansen T A, Dijkhuizen L, Kramer K J M. Turnover of dimethylsulfoniopropionate and dimethylsulfide in the marine environment: a mesocosm experiment. Mar Ecol Prog Ser. 1996;145:223–232. [Google Scholar]
- 41.Ledyard K M, Dacey J W H. Microbial cycling of DMSP and DMS in coastal and oligotrophic seawater. Limnol Oceanogr. 1996;41:33–40. [Google Scholar]
- 42.Lee S, Fuhrman J A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microbiol. 1987;53:1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levasseur M, Keller M D, Bonneau E, D’Amours D, Bellows W K. Oceanographic basis of a DMS-related Atlantic cod (Gadus morhua) fishery problem: blackberry feed. Can J Fish Aquat Sci. 1994;51:881–889. [Google Scholar]
- 44.Lomans B P, Smolders A J P, Intven L C, Pol A, Op den Camp H J M, van der Drift C. Formation of dimethyl sulfide and methanethiol in anoxic freshwater sediments. Appl Environ Microbiol. 1997;63:4741–4747. doi: 10.1128/aem.63.12.4741-4747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malin G, Turner S, Liss P S, Holligan P, Harbour D. Dimethyl sulfide and dimethylsulphoniopropionate in the Northeast Atlantic during the summer coccolithophore bloom. Deep-Sea Res. 1993;40:1487–1508. [Google Scholar]
- 46.Maw G A. The biochemistry of sulphonium salts. In: Stirling C J M, Patai S, editors. The chemistry of the sulphonium group. New York, N.Y: John Wiley & Sons, Ltd.; 1981. pp. 703–770. [Google Scholar]
- 47.Noordkamp D J B, Schotten M, Gieskes W W C, Forney L J, Gottschal J C, van Rijssel M. High acrylate concentrations in the mucus of Phaeocystis globosa colonies. Aquat Microb Ecol. 1998;16:45–52. [Google Scholar]
- 48.Nystrom T, Marden P, Kjelleberg S. Relative incorporation rates of leucine and methionine during starvation survival of two bacteria isolated from marine waters. FEMS Microbiol Ecol. 1986;38:285–292. [Google Scholar]
- 49.Ravenel S, Droux M, Douce R. Methionine biosynthesis in higher plants. I. Purification and characterization of cystathionine γ-synthase from spinach chloroplasts. Arch Biochem Biophys. 1995;316:572–584. doi: 10.1006/abbi.1995.1077. [DOI] [PubMed] [Google Scholar]
- 50.Sherr E, Sherr B. Role of microbes in pelagic food webs: a revised concept. Limnol Oceanogr. 1988;33:1225–1227. [Google Scholar]
- 51.Simon M, Hong J-S. Direct homocysteine biosynthesis from O-succinylhomoserine in Escherichia coli: an alternate pathway that bypasses cystathionine. J Bacteriol. 1983;153:558–561. doi: 10.1128/jb.153.1.558-561.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soda K. Microbial sulfur amino acids: an overview. Methods Enzymol. 1987;143:453–459. doi: 10.1016/0076-6879(87)43080-2. [DOI] [PubMed] [Google Scholar]
- 53.Stefels J, Dijkhuizen L. Characteristics of DMSP-lyase in Phaeocystis sp. (Prymnesiophyceae) Mar Ecol Prog Ser. 1996;131:307–313. [Google Scholar]
- 54.Steinke M, Daniel C, Kirst G O. DMSP lyase in marine micro- and macroalgae: intraspecific differences in cleavage activity. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum; 1996. pp. 317–324. [Google Scholar]
- 55.Taylor B F, Visscher P T. Metabolic pathways involved in DMSP degradation. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum; 1996. pp. 265–276. [Google Scholar]
- 56.Vallino J J, Hopkinson C S, Hobbie J E. Modeling bacterial utilization of dissolved organic matter: optimization replaces Monod growth kinetics. Limnol Oceanogr. 1996;41:1591–1609. [Google Scholar]
- 57.Walsh R S, Cutter G A, Dunstan W M, Radford-Knoery J, Elder J T. The biogeochemistry of hydrogen sulfide: phytoplankton production in the surface ocean. Limnol Oceanogr. 1994;39:941–948. [Google Scholar]
- 58.Wheeler P A, Kokkinakis S A. Ammonium recycling limits nitrate use in the oceanic subartic Pacific. Limnol Oceanogr. 1990;35:1267–1278. [Google Scholar]
- 59.Wolfe G V, Sherr E B, Sherr B F. Release and consumption of DMSP from Emiliania huxleyi during grazing by Oxyrrhis marina. Mar Ecol Prog Ser. 1994;111:111–119. [Google Scholar]
- 60.Wolfe G V, Steinke M, Kirst G O. Grazing-activated chemical defence in a unicellular marine alga. Nature. 1997;387:894–897. [Google Scholar]
- 61.Yoch D C, Ansede J H, Rabinowitz K S. Evidence for intracellular and extracellular dimethylsulfoniopropionate (DMSP) lyases and DMSP uptake sites in two species of marine bacteria. Appl Environ Microbiol. 1997;63:3182–3188. doi: 10.1128/aem.63.8.3182-3188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zubkov, M. V., L. J. Linn, and R. P. Kiene. Unpublished data.