Abstract
Introduction
Invasive candidiasis is a serious infection seen in hospitalized or immunocompromised patients. Mortality rates for candidemia can be as high as 30–60%. Candida auris is an emerging species of Candida and is increasingly becoming a global public health problem.
Methods
This was a retrospective observational study, in which we analyzed 79 episodes of candidemia. Blood cultures were done using the Bactec™ FX blood culturing instrument (Becton, Dickinson and Company Sparks, Maryland, USA). Species identification was done using VITEK® 2 YST panels (bioMérieux Inc., Durham, North Carolina, USA). Antifungal susceptibility testing was performed using VITEK® 2 AST-YSO8 panels (bioMérieux Inc., Durham, North Carolina, USA).
Results
Among the 79 episodes, the most common species was found to be C. auris (43.03% of all the episodes). Candida tropicalis was found to be the second most common species in patients admitted to our hospital with candidemia. All the isolates of C. auris were resistant to fluconazole, while 32.35 % of the isolates were also resistant to amphotericin B. Crude mortality in patients with C. auris candidemia was higher than the crude mortality for the other species.
Conclusion
This is the first study from India where C. auris was seen as the most predominant species among patients admitted with candidemia. This is a serious issue given the high rates of fluconazole resistance, mortality, and cost of therapy associated with C. auris bloodstream infections. Urgent attention needs to be diverted to infection control practices and antimicrobial stewardship programs.
How to cite this article
Prayag PS, Patwardhan S, Panchakshari S, Rajhans PA, Prayag A. The Dominance of Candida auris: A Single-center Experience of 79 Episodes of Candidemia from Western India. Indian J Crit Care Med 2022;26(5):560–563.
Keywords: Candida, Candida auris, Candidemia, Invasive candidiasis
Introduction
Invasive candidiasis is a serious infection seen in hospitalized or immunocompromised patients and can be associated with significant mortality. Mortality rates for candidemia can be as high as 30–60%.1 The distribution of Candida species can vary by geographic locations, and so can the rates of resistance to antifungal drugs. Different species of Candida have varying levels of susceptibility and tolerance to different classes of antifungal agents, and hence, correct characterization of species is important for selecting the right antifungal agent for empiric therapy.
In a large multicenter epidemiological study of candidemia in the intensive care unit (ICU) published in 2015, Candida tropicalis (C. tropicalis) was found to be the most common species.2 Also in the same study, public-sector hospitals reported a higher presence of Candida auris (C. auris) than private-sector hospitals (8.2 vs 3.9%).2 The rate of azole resistance in this study was 11.8%.2
In a study of 114 isolates of Candida species from a tertiary center in India published in 2018, C. tropicalis was the most prevalent species (39.4%), followed by C. auris (17.5%), Candida albicans (C. albicans) (14%), and Candida parapsilosis (C. parapsilosis) (11.4%).3 This study demonstrated the rising incidence of C. auris, which was the second most common species in this study.
C. auris is an emerging species of Candida, which is first isolated from Japan in 2009.4 It is increasingly becoming a global public health problem. It has been isolated from six continents so far, with five genetic clades described: the South Asia Clade (I), the East Asia Clade (II), the South Africa Clade (III), the South America Clade (IV), and a potential fifth clade recently reported from Iran.5,6 The South Asian Clade of C. auris (prevalent in India) is very often resistant to fluconazole and may be resistant to polyene antifungals, while echinocandin resistance is rare.7 It presents a considerable diagnostic and therapeutic challenge in India.
In this retrospective observational study, we analyzed 79 episodes of candidemia (in 79 patients) admitted to a tertiary care center in western India, with respect to the relative prevalence of species, antifungal susceptibility rates, risk factors, and the therapy used. We also analyzed the adherence to the practices of line removal, fundoscopy, echocardiography, and repeating the blood cultures to document clearance, as well as involving an infectious diseases (ID) specialist in the management of these patients.
Materials and Methods
This was a retrospective observational study conducted in a large 900-bed tertiary care multispecialty center in Western India. Ethics committee approval was obtained prior to the study. Due to the retrospective nature of the study, the requirement for obtaining patient consent was waived.
Isolation and Identification of Candida
Blood cultures were done using the Bactec™ FX blood culturing instrument (Becton, Dickinson and Company Sparks, Maryland, USA). Ten milliliters of blood was inoculated into BD Bactec™ Aerobic/F or BD Bactec™ Mycosis IC/F blood culture vials, which were loaded into the machine with onboard incubation at 37°C and continuously monitored for growth. Candida was identified in bottles flagging positive by Gram stain showing yeast forms, subculture onto blood agar or Sabouraud's dextrose agar to obtain colonies, and germ tube test to differentiate between albicans and non-albicans species. Species identification was done using VITEK® 2 YST panels (bioMérieux Inc., Durham, North Carolina, USA). Serial blood cultures were advised to document clearance of candidemia. In patients with central lines in situ, paired blood cultures were drawn to determine the differential time to positivity.
Antifungal Susceptibility Testing
This was performed using VITEK® 2 AST-YSO8 panels (bioMérieux Inc., Durham, North Carolina, USA). Drugs tested were fluconazole, voriconazole, amphotericin B, caspofungin, micafungin, and 5-flucytosine. Interpretation of the obtained minimum inhibitory concentrations (MICs) as susceptible, resistant, or susceptible dose-dependent was done with reference to the clinical breakpoints and interpretive categories via the Clinical and Laboratory Standards Institute (CLSI) and EUCAST standards of broth microdilution. Susceptibility interpretation of C. auris was done with tentative CDC breakpoints and published tentative ECVS in multicentric Indian studies.8
Data regarding the risk factors, treatment, and outcomes were obtained from the hospital's electronic medical records.
Results
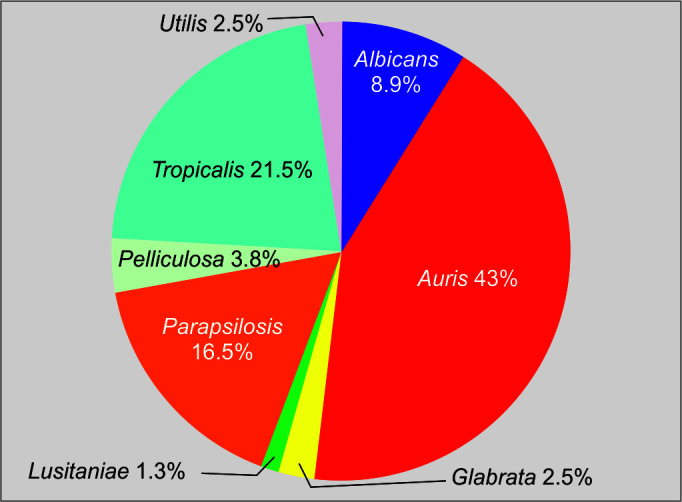
A total of 79 episodes of candidemia were studied from December 2019 to July 2021. The most common species was found to be C. auris. C. tropicalis was found to be the second most common species in our hospital (Fig. 1 and Table 1).
Fig. 1.
Species distribution
Table 1.
The relative prevalence of the species of candida
| Species of candida | Number (%) |
|---|---|
| C. auris | 34/79 (43.03) |
| C. tropicalis | 17/79 (21.51) |
| C. parapsilosis | 13/79 (16.45) |
| C. albicans | 7/79 (8.86) |
| C. glabrata | 2/79 (2.53) |
| C. lusitaniae | 1/79 (1.26) |
| C. utilis | 2/79 (2.53) |
| C. pelliculosa | 3/79 (3.79) |
All but one of the patients were on broad-spectrum antibiotics at the time of developing candidemia. Of note, 62.02% of patients were colonized with Candida (either in the sputum or in the urine) at the time of developing candidemia (Table 2).
Table 2.
Prevalence of risk factors in patients with candidemia
| Risk factor | Number (%) |
|---|---|
| Broad-spectrum antibiotics | 78/79 (98.73) |
| Diabetes mellitus | 43/79 (54.43) |
| Kidney failure on dialysis | 20/79 (25.31) |
| Neutropenia | 7/79 (8.86) |
| Central line | 72/79 (91.13) |
| Steroids | 35/79 (44.30) |
| Immunosuppressive therapy | 22/79 (27.84) |
| Total parenteral nutrition | 9/79 (11.39) |
| Abdominal surgery | 22/79 (27.84) |
| Colonization with candida | 49/79 (62.02) |
All the isolates of C. auris were resistant to fluconazole. Nearly one third of the isolates of C. auris were resistant to amphotericin B (Table 3). Crude mortality for C. auris was higher than the crude mortality for the other species (Tables 4 and 5).
Table 3.
Antifungal susceptibility rates for the different species isolated in our center
| Species | Fluconazole resistance (%) | Amphotericin resistance (%) | Caspofungin resistance (%) | Micafungin resistance (%) | Voriconazole resistance (%) | 5-Flucytosine resistance (%) |
|---|---|---|---|---|---|---|
| C. auris | 34/34 (100) | 11/34 (32.35) | 2/34 (5.88) | 1/34 (2.94) | 2/34 (5.88) | 5/34 (14.70) |
| C. tropicalis | 2/17 (11.76) | 2/17 (11.76) | 0/17 (0) | 0/17 (0) | 0/17 (0) | 0/17 (0) |
| C. parapsilosis | 7/13 (53.84) | 0/13 (0) | 0/13 (0) | 1/13 (7.69) | 0/13 (0) | 0/13 (0) |
| C. albicans | 0/7 (0) | 0/7 (0) | 0/7 (0) | 0/7 (0) | 0/7 (0) | 0/7 (0) |
| C. glabrata | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 1/2 (50) | 1/2 (50) |
| C. lusitaniae | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| C. utilis | 1/2 (50) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| C. pelliculosa | 1/3 (33.33) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| All species | 45/79 (56.96) | 13/79 (16.45) | 2/79 (2.53) | 2/79 (2.53) | 3/79 (3.79) | 6/79 (7.59) |
Table 4.
Crude mortality for various species associated with candidemia
| Species of candida | Day 28 mortality (%) |
|---|---|
| C. auris | 11/34 (32.35) |
| C. tropicalis | 5/17 (29.41) |
| C. parapsilosis | 4/ 13 (30.76) |
| C. albicans | 3/17 (17.64) |
| C. glabrata | 1/2 (50) |
| C. lusitaniae | 0/1 (0) |
| C. utilis | 0/2 (0) |
| C. pelliculosa | 0/3 (0) |
| All species | 24/79 (30.38) |
| Non-C. auris species | 13/45 (28.88) |
Table 5.
Adherence to certain practices, such as line removal, performing a fundoscopic eye examination, echocardiography, involving infectious diseases (ID) specialists, and sending repeat blood cultures in patients with candidemia
| Action | Number of patients in which performed (%) | Remarks/findings |
|---|---|---|
| Removal of the CVC | 65/72 (90.27) | |
| Fundoscopy | 41/79 (51.89) |
|
| Transthoracic echocardiography | 65/79 (82.27) | |
| Repeat blood cultures | Culture repeated once: 65/79 (82.27) Culture repeated twice: 37/79 (46.83) |
|
| Involving an ID specialist in the management | 67/79 (84.81) |
Discussion
Our study reflects the changing epidemiology of candidemia in India. Unlike previous large studies of Candida infections from India, our study showed that C. auris was the most common Candida species in patients admitted to our center with candidemia. C. tropicalis, which has previously been shown to be the most common species in India, was the second most prevalent species in patients admitted with candidemia to our center. To the best of our knowledge, this is the first study in India that shows C. auris as the most predominant species. Of note, 19 of 34 patients were referred to our center from outside. The rise of C. auris is alarming, and stringent infection control measures need to be implemented across the country. The VITEK 2 automated identification system (bioMérieux) has only recently included C. auris in its database (version 8.01). Laboratories across the country use a variety of methods and commercial systems, and it is very likely that C. auris is currently being underreported. Adaptation of methods, like VITEK 2 (version 8.01) or identification using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS), is essential to determine the true burden of this problem.
Table 2 shows the risk factors present in patients who developed candidemia in this study. Of note, except one patient, all were on broad-spectrum antibiotics at the time of being found to have candidemia. This calls for meticulous implementation of antimicrobial stewardship programs and reducing the use of unwarranted antibiotic therapy.
The fluconazole resistance rate for C. auris in our study was 100%, which is consistent with the rates described previously for the South Asian Clade.7 The predominance of C. auris and the very high rates of fluconazole resistance make fluconazole a poor choice for empiric therapy for candidemia in our center. This impacts antimicrobial stewardship programs adversely. Amphotericin resistance was seen in 32.35% of the isolates of C. auris in this study, which is higher than the previously reported rates from India.7 Resistance was seen only in two isolates for caspofungin and in one isolate for micafungin. This is in congruence with previously published data, which makes echinocandins the preferred therapeutic option for C. auris. However, this increases the cost of treating patients with candidemia, as echinocandins are significantly more expensive than fluconazole. Echinocandins have poor penetration into the central nervous system, genitourinary system, as well as the aqueous and vitreous humors, and hence, infections at these sites with C. auris are particularly challenging.
The Infectious Diseases Society of America (IDSA) guidelines for the management of candidemia strongly recommend removal of the central venous catheter (CVC).9 Among patients who had a CVC, the compliance for removal in our center was 90.27%. The IDSA guidelines for candidemia also strongly recommend a dilated retinal examination for all patients with candidemia, preferably performed by an ophthalmologist, within the first week of therapy to establish whether endophthalmitis is present.9 Among the 79 patients with candidemia in our center, 41 patients underwent an eye examination. Of these, three patients showed evidence of Candida endophthalmitis. None of these three patients had candidemia with C. auris (one with C. parapsilosis and two patients with C. albicans). This has clinical significance as the management of endophthalmitis requires using drugs that achieve adequate concentrations at the site of infection and considering intravitreal therapy.
There is evidence to suggest that consultation with an ID expert improves mortality in patients with candidemia.10 Among patients with candidemia in our center, 84.81% were referred to ID specialists. Involving ID specialists in the management of candidemia should be a part of the protocol. Also 82.87% of patients in this study underwent repeat blood cultures. Clearance of candidemia is important to document since persistent candidemia can be a sign of endocarditis or failure of therapy (which may be related to resistance to antifungal agents or their inability to achieve optimal levels at the site of infection).
Limitations of this study include its retrospective nature and the relatively smaller number of isolates, which makes it difficult to assess the factors affecting mortality. Also, this is a single-center experience, and collaborative studies from multiple centers are needed to confirm whether C. auris is now the predominant species in India. Also, the antifungal susceptibility testing was done using VITEK® 2 AST-YSO8 panels (bioMérieux Inc., Durham, North Carolina, USA). This has been reported to be less reliable for detecting resistance to caspofungin.11
However, the rise of C. auris shown in this study is startling. With the high rates of azole resistance and the predominance of C. auris, there is an urgent need to establish robust infection control practices as well as antimicrobial stewardship programs. Further larger studies need to be planned in India to study the rise of C. auris, its epidemiology, risk factors, and attributable mortality.
Conclusion
This is the first study from India where C. auris was seen as the most predominant species among patients admitted with candidemia. This is a serious issue given the high rates of fluconazole resistance, mortality, and cost of therapy associated with C. auris bloodstream infections. Urgent attention needs to be diverted to infection control practices and antimicrobial stewardship programs.
Orcid
Parikshit S Prayag https://orcid.org/0000-0003-2102-7627
Sampada Patwardhan https://orcid.org/0000-0003-0998-5742
Shweta Panchakshari https://orcid.org/0000-0002-5708-3287
Prasad A Rajhans https://orcid.org/0000-0002-0111-6123
Amrita Prayag https://orcid.org/0000-0002-2498-9576
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Flevari A, Theodorakopoulou M, Velegraki A, Armaganidis A, Dimopoulos G. Treatment of invasive candidiasis in the elderly: a review. Clin Interv Aging. 2013;8:1199–1208. doi: 10.2147/CIA.S39120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41(2):285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 3.Mathur P, Hasan F, Singh PK, Malhotra R, Walia K, Chowdhary A. Five-year profile of candidemia at an Indian trauma centre: High rates of Candida auris blood stream infections. Mycoses. 2018;61(9):674–680. doi: 10.1111/myc.12790. [DOI] [PubMed] [Google Scholar]
- 4.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64(2):134–140. doi: 10.1093/cid/ciw691. 27988485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis. 2019;25(9):1780–1781. doi: 10.3201/eid2509.190686. 31310230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73(4):891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 8.Arendry MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. Comparison of EUCAST and CLSI reference microdilution MICS of eight antifungal compounds for C. auris and associated tentative epidemiological cut off values. Antimicrob Agents Chemother. 2017;61:e00485–e00517. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi T, Marra AR, Schweizer ML, Eyck PT, Wu C, Alzunitan M, et al. Impact of infectious disease consultation in patients with candidemia: a retrospective study. Systematic literature review, and meta-analysis. Open Forum Infect Dis. 2020;7(9):ofaa270. doi: 10.1093/ofid/ofaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astvad KM, Perlin DS, Johansen HK, Jensen RH, Arendrup MC. Evaluation of caspofungin susceptibility testing by the new Vitek 2 AST-YS06 yeast card using a unique collection of FKS wild-type and hot spot mutant isolates, including the five most common candida species. Antimicrob Agents Chemother. 2013;57(1):177–182. doi: 10.1128/AAC.01382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


