Abstract
Background: The neutrophil–lymphocyte count ratio (NLR) has emerged as a potential prognostic tool for different diseases. In the current coronavirus disease (COVID-19) pandemic, the NLR may be a useful tool for risk scarification and the optimal utilization of limited healthcare resources. However, there is no consensus regarding the optimal value of NLR, and the association with disease severity and mortality. Thus, this study aims to systematically analyze the current evidence of the utility of baseline NLR as a predictive tool for mortality, disease severity in COVID-19 patients. Methods: A compendious screening of electronic databases up to June 15, 2021, was done after enlisting the protocol in PROSPERO (CRD42020202659). Studies evaluating the utility of baseline NLR in COVID-19 are included for this review as per the PRISMA statement. Results: We retrieved a total of 13112 and 12986 COVID-19 patients for survivability and severity over 90 studies. The expired and critically sick patients had elevated baseline NLR on admission, in comparison to survivors and noncritical patients. (SMD = 3.82; 95% CI: 2.79-4.85; I2 = 100% and SMD = 1.42; 95% CI: 1.22-1.63; I2 = 95%, respectively). The summary receiver operating curve analysis for mortality (AUC = 0.87; 95% CI: 0.86-0.87; I2 = 94.7%), and severity (AUC = 0.82; 95% CI: 0.80-0.84; I2 = 79.7%) were also suggestive of its significant predictive value. Conclusions: The elevated NLR on admission in COVID-19 patients is associated with poor outcomes.
Keywords: COVID-19, SARS-CoV-2, Neutrophil to Lymphocyte ratio
Introduction
The global healthcare system is going through an extraordinary crisis due to the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Identification of rapid and reliable clinical biomarkers for risk stratification and optimal utilization of the limited resources is the burning need of the moment.
Of late the neutrophil–lymphocyte count ratio (NLR), a systemic inflammatory indicator has generated a lot of interest regarding the potential prognostic role in several clinical conditions including acute respiratory distress syndrome, liver diseases, cardiovascular disease, and malignancies.1–6
Usually, the neutrophil count increases, and the lymphocyte count decreases with the advancement of any inflammatory condition. The NLR, which seems to be more sensitive than the isolated value of absolute neutrophil, or lymphocyte count in bacterial as well as viral pneumonia, is a marker of the systemic inflammatory response.7,8
Multiple recent studies have found the increase in NLR is consistent with critical illness and mortality, particularly in inflammatory diseases.9 A recent meta-analysis also found NLR as a potential prognostic biomarker in sepsis patients and an elevated NLR in deceased than in survivors (SMD = 1.18, 95% CI: 0.42-1.94)9
Thus, the NLR on admission may be beneficial for early risk stratification and the necessary prioritization of resources. However, there is no consensus regarding the association between NLR and clinical prognosis.
Thus, we aim to comprehensively analyze the current evidence of the utility of baseline NLR in COVID-19 management as per the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA-P) guidelines.10,11
Methods
Protocol and Registration
We prospectively enlisted the protocol for this review in PROSPERO (ID: CRD42020202659) and did not deviate from the published protocol.
Search Strategy
SS and PK carried out the comprehensive search individually in “PubMed,” “Medline,” and “Embase” databases, Google Scholar (https://scholar.google.com), and preprint platforms MedRxiv (https://www.medrxiv.org) from January first, 2020 to June 15, 2021, with the following terminologies: (“COVID-19”) OR (“SARS-CoV-2”) AND (“NLR” OR “neutrophil-lymphocyte count ratio” OR “neutrophil to lymphocyte ratio”).
Inclusion and Exclusion Criteria
Prospective as well as retrospective articles presenting clinical data for the utility of baseline NLR in COVID-19 patients were included for full-text review. Full articles in other than English languages were also retrieved using Google Translate (https://translate.google.com).
Cohort studies, cross-sectional studies, case series, and randomized controlled trials were incorporated. The reference section of selected articles for inclusion was also searched to identify any additional studies for potential inclusion. The primary objective under evaluation was mortality and severity.
The editorials, letters, articles without retrievable full text, and necessary data, were excluded (PRISMA flow diagram).10,11
Study Selection
PK and SS scrutinized every title and abstracts separately to determine whether they met the incorporating criteria, followed by evaluating the full-text of studies, fulfilled the said criteria. The difference in point of view was sorted out by consulting with the other researcher (AKS).
Data Extraction
SS and PK extracted the following data: study design (retrospective vs prospective), country/region of study, sample size, baseline NLR, disease extremity, and fatality in COVID-19 patients from the incorporated studies using a spreadsheet and substantiate the accuracy independently. The number of events & the overall number of patients per group, and the mean ± SD were extracted for dichotomous and continuous data, respectively. In the absence of a consensus definition and grading of COVID-19 severity across the studies, we considered any patient either with mechanical ventilation or a ratio of the partial pressure of arterial blood oxygen (PaO2)/oxygen concentration (FiO2) ≤300 mmHg as severe/critically ill and the rest all as nonsevere patients.
Risk of Bias Assessment
PK and SS assessed each included study for potential bias independently. The opinion of the third researcher (AKS) was sorted to resolve any different point of view. We applied the “Risk of Bias in Non-randomized Studies—of Interventions” (ROBINS-I)12 tool to assess the risk of bias in nonrandomized studies. It comprises 7 domains: “bias due to confounding,” “selection of participants, classification of interventions,” “deviations from intended interventions,” “missing data,” “measurement of outcomes,” and “selection of the reported result.” Each domain is graded as “Low,” “Moderate,” “Serious,” and “Critical.”
Quality of the Evidence
PK and AKS examined the quality of evidence independently and classified each outcome as “High,” “Moderate,” “Low,” or “Very low” depending upon the 5 downgrading factors (“study limitations, consistency of effect, imprecision, indirectness, and publication bias”) and 3 upgrading factors (“large magnitude of the effect, dose-response relation, and plausible confounders or biases”) as per the “Grading of Recommendations Assessment, Development, and Evaluation” (GRADE) tool.13–20
Data Synthesis
SS and PK used Review Manager version 5.4 and Medcalc software 16.2 for conducting this frequentist meta-analysis. The standardized mean difference, and area under the receiver operating curve along with respective 95% confidence intervals (CIs) were calculated as per the “Cochrane Handbook for Systematic Reviews of Interventions.”21 Statistical heterogeneity was evaluated with the I2 statistic, > 50% indicating substantial heterogeneity. Begg's test, Egger's test along the funnel plot were used to evaluate the potential publication bias.
Results
Basic Characteristics
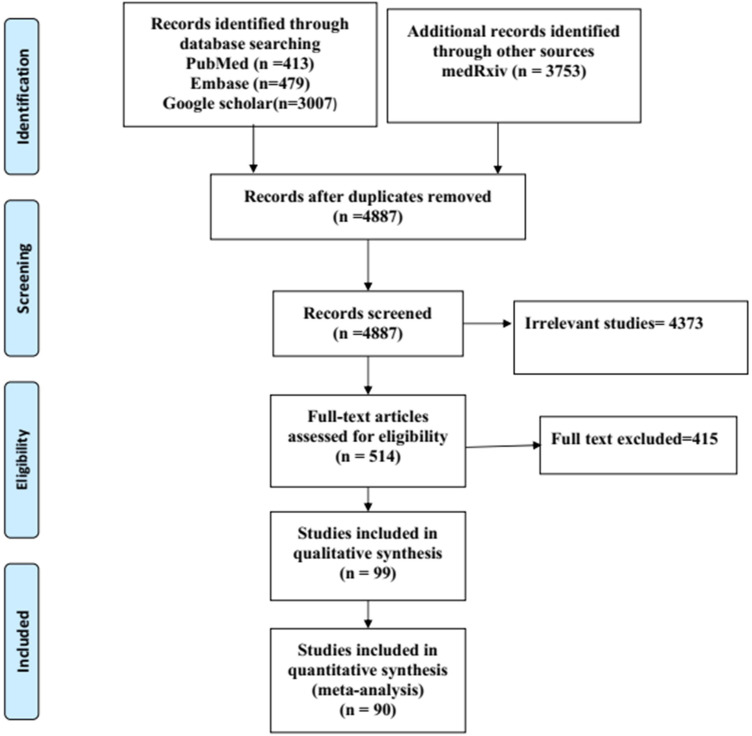
A total of 90 studies22–111 (82 retrospectives, 5 prospective, 3 cross-sectional) out of 7352 identified publications were included after satisfying the inclusion criteria (Figure 1). While 44 articles41,42,44,46,47,49,50–71,73–76,78–80,101,103–106,108–111 assessed baseline NLR as a predictor for determining only severity, 32 articles24,25,29–34,38–40,43,45,48,77,82–93,96–98,100,102 assessed only mortality, 14 studies22,23,26–28,35–37,72,81,94,95,99,107 addressed both survivability and severity. Out of the 46 articles, assessed survivability 36 were with only dichotomous data, 10 with only receiver operating curve, and 11 with both types of data. Among the 58 studies assessing severity 13 studies also assessed receiver operating curve.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-2009 flow diagram.
A total of 68.8% (n = 62) of the included studies were from China, 6.6% (n = 6) were from European countries, 5.5% (n = 5) from the United States and 18.8% (n = 17) were from other Asian countries (Turkey, Pakistan, Iran, India, and Bangladesh) (Table 1).
Table 1.
Characteristics of Included Studies for Quantitative Synthesis.
| SN | Author, Year | Type of study, center | Country | Total no. of patients | Outcome |
|---|---|---|---|---|---|
| 1. | Asghar et al,22 2020 | Retrospective, SC | Pakistan | 100 | NLR increasing with disease severity, NLR (AUC: 0.806, PPV: 95.8%) for mortality |
| 2. | Chen et al,23 2020 | Retrospective, SC | China | 132 | The mortality rate of COVID-19 patients is associated with the lower lymphocytes and higher NLR |
| 3. | Chen et al,24 2020 | Retrospective, SC | China | 363 | High NLR value was associated with disease severity, progression and an overall poor prognosis |
| 4. | Chen et al,25 2020 | Retrospective, MC | China | 1859 | High NLR associated with risk of in-hospital death in persons with COVID-19 |
| 5. | Chen et al,26 2020 | Retrospective, MC | China | 548 | Nonsurvivors kept a high level or showed an upward trend for neutrophils |
| 6. | Cheng et al,27 2020 | Retrospective, SC | China | 456 | Higher levels of NLR at admission were associated with a poor prognosis of individuals with moderate COVID-19 |
| 7. | Huang et al,28 2020 | Retrospective, SC | China | 299 + 45 | Serum albumin level was inversely correlated to NLR, hypoalbuminemia is associated with the outcome of COVID-19 |
| 8. | Li et al,29 2020 | Retrospective, SC | China | 93 | The mortality rate of COVID-19 monotonously increased with chest CT scores, which positively correlated with the neutrophil-to-lymphocyte ratio, neutrophil percentage, |
| 9. | Luo et al,30 2020 | Retrospective, SC | China | 298 | Patients with severe or critical illness tended to exhibit elevated NLR |
| 10. | Pakos et al,31 2020 | Retrospective, SC | USA | 242 | NLR was positively associated with death (OR = 1.038; 95% CI: 1.003-1.074, P = .031 |
| 11. | Ye et al,32 2020 | Retrospective, SC | China | 349 | The rising trend in D-dimer and NLR, or the test results higher than the critical values may indicate a risk of death for participants with COVID-19 |
| 12. | Yan et al,33 2020 | Retrospective, SC | China | 1004 | NLR appears to be a significant prognostic biomarker of outcomes in critically ill patients with COVID-19. |
| 13. | Yang et al,34 2020 | Retrospective, SC | China | 226 | Higher NLR was also found to increase COVID-19 patients’ mortality risk. |
| 14. | Zhang et al,35 2020 | Retrospective, SC | China | 315 | NLR >8.0 (HR 4.56, 95% CI: 2·25-9·23; P < .0001)was associated with 28-day mortality |
| 15. | Zhang et al,36 2020 | Retrospective, SC | China | 60 | Higher CRP and NLRs with diffuse lung involvement were more likely to die of COVID-19 |
| 16. | Zhang et al,37 2020 | Retrospective, MC | China | 516 | Older age, high lactate dehydrogenase, NLR, and direct bilirubin level were independent predictors of 28-day mortality in adult hospitalized patients with confirmed COVID-19. |
| 17. | Tatum et al,38 2020 | Prospective, SC | USA | 125 | NLR is a prognostic factor for endotracheal intubation upon hospital admission and an independent predictor for risk of mortality in SARS-CoV-2 patients |
| 18. | Chen et al,39 2020 | Retrospective, SC | China | 681 | Patients with a high NLR (>6.66) combined with myocardial injury are highly predictive of mortality. |
| 19. | Ok et al,40 2020 | Retrospective, SC | Turkey | 139 | NLR may be associated with disease severity, and routine use of these parameters may be beneficial in the evaluation of the disease. |
| 20. | Song et al,41 2020 | Retrospective, SC | China | 84 | NLR >6.1 has a sensitivity of 76.2% and specificity of 88.1% for predicting mortality in COVID-19 patients |
| 21. | Huang et al,42 2020 | Retrospective, SC | China | 415 | The NLR of patients in the severe group had 1.729-fold higher than that of the no-severe group (OR 1.729; 95% CI: 1.050-2.847, P = .031) |
| 22. | Sun et al,43 2020 | Retrospective, SC | China | 116 | Patients with COVID-19 have lower counts of lymphocytes, eosinophils, platelets, and higher neutrophil-lymphocyte ratio (NLR) in comparison to controls (P < .001). |
| 23. | Fu et al,44 2020 | Retrospective, SC | China | 75 | The dynamic change of NLR and D-dimer levels can distinguish severe COVID-19 cases from mild/moderate. |
| 24. | Yang et al,45 2020 | Retrospective, SC | China | 93 | Elevated age and NLR can be considered independent biomarkers for indicating poor clinical outcomes. |
| 25. | Wang et al,46 2020 | Retrospective, SC | China | 45 | The combined NLR and RDW-SD may help clinicians to predict the severity of COVID-19 patients |
| 26. | Peng et al,47 2020 | Retrospective, SC | China | 220 | Compared with nonsevere patients, the severe ones had significantly higher levels of neutrophil percentage (74.9% vs 62.1%; P < .001), NLR (4.1 vs 2.1; P < .001) |
| 27. | Zhang et al,48 2020 | Retrospective, SC | China | 652 | NLR + SaO2 is an appropriate and promising method for predicting severe illness |
| 28. | Zhang et al,49 2020 | Retrospective, SC | China | 80 | Compared with nonsevere patients, the severe ones had significantly higher levels of neutrophil percentage |
| 29. | Chen et al, 202050 | Retrospective, SC | China | 139 | ↑NLR in severely ill COVID-19 patients |
| 30. | Chen et al,51 2020 | Retrospective, SC | China | 296 | The NLR was higher in the severe group |
| 31. | Chen et al,52 2020 | Retrospective, MC | China | 291 | ↑NLR in severely ill COVID-19 patients |
| 32. | Ding et al,53 2020 | Retrospective, SC | China | 72 | NLR from day 5 after admission was found to be positively correlated with the duration of hospitalization |
| 33. | Gong et al,54 2020 | Retrospective, MC | China | 189 | Early identification of patients who will progress to severe COVID-19, |
| 34. | Hou et al,55 2020 | Retrospective, SC | China | 49 | The NLR was higher in the severe group |
| 35. | Kong et al,56 2020 | Retrospective, SC | China | 40 | Compared with mild/moderate COVID-19 cases, severe cases had a higher NLR |
| 36. | Kong et al,57 2020 | Retrospective, SC | China | 210 | NLR was identified as an early risk factor for severe COVID-19 illness. |
| 37. | Liao et al,58 2020 | Retrospective, MC | China | 380 | The NLR, platelet count, D-dimer, and prothrombin time might provide a reliable and convenient method for classifying and predicting the severity and outcomes of patients with COVID-19. |
| 38. | Liu et al,59 2020 | Retrospective, SC | China | 134 | The NLR was higher in the severe group |
| 39. | Liu et al,60 2020 | Prospective, SC | China | 122 | Age ≥ 50 and NLR ≥ 3.13 are predicted to develop a critical illness. |
| 40. | Liu et al,61 2020 | Retrospective, SC | China | 61 | The NLR was significantly associated with mortality in patients with COVID-19 |
| 41. | Ma et al,62 2020 | Retrospective, SC | China | 37 | The NLR was higher in the severe group |
| 42. | Ma et al,63 2020 | Retrospective, SC | China | 149 | NLR ≥ 2.22 could be utilized as a predicting indicator for the early recognition COVID-19 and facilitate detection timely. |
| 43. | Peng et al,64 2020 | cross-sectional study, MC | China | 190 | NLR may be a reliable marker to evaluate the disease severity of COVID-19. |
| 44. | Peng et al,65 2020 | Retrospective, SC | China | 112 | Critical patients are characterized by lower lymphocyte counts. |
| 45. | Qin et al,66 2020 | Retrospective, SC | China | 452 | Surveillance of NLR is helpful in the early screening of critical illness, diagnosis, and treatment of COVID-19 |
| 46. | Shang et al,67 2020 | Retrospective, SC | China | 443 | NLR, CRP, and platelets can effectively assess the severity of COVID-19, among which NLR is the best predictor of severe COVID-19, |
| 47. | Song et al,68 2020 | Retrospective, SC | China | 73 | The NLR was significantly higher in the COVID-19 patients. |
| 48. | Wang et al, 202069 | Retrospective, SC | China | 138 | The NLR was higher in the severe group. |
| 49. | Wang et al,70 2020 | Retrospective, SC | China | 323 | The potential risk factors of males, older age, with comorbidities, low T lymphocyte level and high level of NLR, CRP, IL-6. |
| 50. | Wang et al,71 2020 | Retrospective, SC | China | 30 | The NLR was higher in the severe group. |
| 51. | Wang et al,72 2020 | Retrospective, SC | China | 131 | The NLR was significantly associated with mortality in patients with COVID-19 |
| 52. | Wei et al,73 2020 | Retrospective, SC | China | 167 | Decline in T lymphocytes and significant increases in the levels of inflammatory factors, including CRP and IL-6, can be associated with severe infection |
| 53. | Wu et al,74 2020 | Retrospective, SC | China | 270 | ↑NLR in severely ill COVID-19 patients |
| 54. | Xie et al,75 2020 | Retrospective, SC | China | 97 | Eosinophil counts had a good value for COVID-19 prediction, even higher when combined with NLR. |
| 55. | Xie et al,76 2020 | Retrospective, MC | China | 373 | The NLR was higher in the severe group. |
| 56. | Xu et al, 202077 | Retrospective, MC | China | 338 | NLR qualifies as an independent predictor of disease progression in COVID-19 patients. |
| 57. | Zhang et al,78 2020 | Retrospective, SC | China | 148 | NLR may act as a predictive tool to discriminate between severe and nonsevere COVID-19 patients |
| 58. | Zhang et al,79 2020 | Retrospective, SC | China | 115 | ↑NLR in severely ill COVID-19 patients |
| 59. | Zhou et al,80 2020 | Retrospective, SC | China | 304 | NLR, PLR, troponin-I, creatinine, and BUN are important indicators for severity grading in COVID-19. |
| 60. | Zhu et al,81 2020 | Retrospective, SC | China | 127 | NLR, fibrinogen, C-reaction protein (CRP), IL-6, interleukin-10 (IL-10), and interferon-γ (IFN-γ) in the severe group were significantly higher. |
| 61. | Archana et al, 202182 | Cross-sectional, SC | India | 302 | NLR had a sensitivity of 85% and specificity of 51% in predicting mortality of COVID-19 patients. |
| 62. | Asgar et al,83 2020 | Retrospective, SC | Pakistan | 191 | Elevated NLR is positively correlated with morbidity and mortality of COVID-19 patients (AUC: 0.860, PPV: 91.1%) |
| 63. | Baqi et al,84 2021 | Retrospective, SC | Pakistan | 299 | NLR, C-reactive protein (CRP), and lactate dehydrogenase (LDH) were higher among the deceased COVID-19 patients |
| 64. | Bisso et al,85 2020 | Retrospective, SC | Argentina | 168 | NLR was higher among nonsurvivors. |
| 65. | Cervantes et al,86 2021 | Cross sectional,SC | Israel | 337 | NLR ≥ 8.5 increased the probability of death in severe COVID-19 (odds ratio 11.68). |
| 66. | Lopez-Escobar et al,87 2021 | Retrospective, MC | Spain | 1955 | NLR is useful in predicting in-hospital mortality risk due to COVID-19 (0.873 [95% CI: 0.849-0.898]) |
| 67. | Güneysu et al,88 2020 | Retrospective, SC | Turkey | 169 | NLR ≥ 3.9 can be used as an early predictor of mortality in COVID-19 patients |
| 68. | Prasetya et al,89 2021 | Retrospective, MC | Indonesia | 391 | NLR ≥ 6 at hospital admission can be a good predictor for poor outcomes in COVID-19 patients. |
| 69. | Kalabin et al,90 2021 | Retrospective, SC | USA | 184 | NLR and PLR have no statistically significant predictive role in suspecting COVID-19 mortality. |
| 70. | Kaufmann et al,91 2021 | Retrospective, SC | Austria | 423 | COVID-19 patients with elevated NLR values had a higher frequency of in-hospital mortality |
| 71. | Nasir et al,92 2021 | Retrospective, SC | Bangladesh | 99 | Nonsurvivors had a high level of NLR (9.76) in comparison to survivors (5.9) at admission. |
| 72. | Nicholson et al,93 2021 | Retrospective, MC | USA | 1042 | NLR was significantly high among the deceased COVID-19 patients. |
| 73. | Pujani et al,94 2021 | Prospective, SC | India | 506 | NLR has an excellent prognostic role in predicting severity and mortality. |
| 74. | Rasyid et al,95 2021 | Retrospective, SC | Indonesia | 295 | NLR can be considered as an early predictive factor of COVID-19 disease progression. |
| 75. | Rokni et al,96 2020 | Retrospective, SC | Iran | 233 | Nonsurvivors had a high level of NLR (11.08) in comparison to survivors (4.69) at admission. |
| 76. | Ruiz et al,97 2020 | Retrospective, SC | Spain | 119 | COVID-19 patients with initial elevated NLR at admission had a poor outcome. |
| 77. | Allahverdiyev et al,98 2020 | Retrospective, SC | Turkey | 455 | The mortality rate of COVID-19 positively correlated with higher NLR (OR = 1.261, 95% CI: 1.054-1.509, P = .011) |
| 78. | Yufei et al,99 2020 | Retrospective, SC | China | 191 | Elevated NLR was found to be an independent risk factor for COVID-19. |
| 79. | Ghazanfari et al,100 2021 | Retrospective, MC | Iran | 79 | NLR showed a significant association with the mortality of COVID-19 patients |
| 80. | Jian-bo Xu et al,101 2020 | Retrospective, MC | China | 76 | NLR has not been proven as an independent predictor of survival in patients with COVID-19. |
| 81. | Zhi-Yong Zeng et al,102 2021 | Prospective, SC | China | 352 | NLR at admission can be used as a predictor for disease severity and mortality in COVID-19 patients. |
| 82. | Wang P et al., 2020103 | Retrospective, MC | China | 441 | NLR and D dimer (≥1 μg/mL) helps to predict the severity of COVID-19 patients. |
| 83. | Xia et al,104 2020 | Retrospective, SC | China | 63 | NLR can be used as an early warning signal for severe COVID-19 |
| 84. | Mousavi-Nasab et al,105 2020 | Retrospective, SC | Iran | 70 | NLR and CRP are potential early markers for assessing the prognosis and severity of COVID-19 patients |
| 85. | Sepulchre et al,106 2020 | Retrospective, SC | Belgium | 198 | Elevated NLR in COVID-19 Patients had a higher rate of in-hospital mortality |
| 86. | Tahtasakal et al,107 2021 | Retrospective, SC | Turkey | 534 | An elevated baseline NLR, CRP, troponin I, LDH are associated with increased severity. |
| 87. | Asan et al,108 2021 | Retrospective, SC | Turkey | 695 | Initial NLR was associated with the severity of COVID-19 disease |
| 88. | Imran et al,109 2021 | Prospective, SC | Pakistan | 63 | NLR can be used as an early warning signal for deteriorating severe COVID-19 |
| 89. | Bastung et al,110 2020 | Retrospective, SC | Turkey | 191 | Elevated D-dimer, NLR, and CRP were significant laboratory predictors of severe prognosis in COVID-19 patients. |
| 90. | Mingming Fe et al,111 2020 | Retrospective, SC | China | 72 | NLR can be used to stratify the severity of COVID-19 patient |
Abbreviations: SC, single center; Mc, multicenter; NLR, neutrophil-to-lymphocyte ratio.
Out of the 90 studies, 77 were peer-reviewed, and 13 were preprints and 25 studies had a moderate degree of bias (Figure 2).
Figure 2.
Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I) assessment for the included non-randomized cohort studies.
Meta-Analyses
Mortality
Mortality was evaluated in 36 articles with a total of 13 112 patients. A significantly exacerbated risk of mortality is found in patients with increased NLR on admission in comparison to the control group. (SMD = 3.82; 95% CI: 2.79-4.85; I2 = 100%) (Figure 3a).
Figure 3.
(a) The impact of the NLR on mortality in COVID-19 patients and (b) summary receiver operating curve analysis of the NLR on mortality in COVID-19 patients.
Abbreviations: NLR, neutrophil–lymphocyte count ratio; COVID-19, coronavirus disease 2019.
Summary Receiver Operating Curve Analysis
Twenty-one studies with a total of 8431 patients assessed ROC with optimum NLR cut-off on admission (ranging 3.19-11.75) for mortality. Raised NLR on admission suggestive of significant predictive value (AUC = 0.87; 95% CI: 0.84-0.91; I2 = 83.2%) (Figure 3b).
Severity
Fifty-eight studies with a total of 12 986 patients were included for assessing the severity of COVID-19. Severely ill patients are associated with elevated baseline NLR. (SMD = 1.42; 95% CI: 1.22-1.63; I2 = 95%), (Figure 4a).
Figure 4.
(a) The impact of NLR on disease severity in COVID-19 patients and (b) summary receiver operating curve analysis of the NLR on disease severity in COVID-19 patients.
Abbreviations: NLR, neutrophil–lymphocyte count ratio; COVID-19, coronavirus disease 2019.
Summary Receiver Operating Curve Analysis
Thirteen studies with a total of 2160 patients assessed ROC with optimum NLR cut-off on admission (ranging 2.3-10.1) for severity. Raised NLR on admission suggestive of significant predictive value (AUC = 0.82; 95% CI: 0.80-0.84; I2 = 79.7%) (Figure 4b).
The heterogeneity across studies assessing the severity and mortality was remarkable.
Quality of Evidence
The quality of evidence on the utility of raised NLR on COVID-19 outcome was low. Significant indirectness in terms of the difference in population, and outcome measures were noted (Table 2).
Table 2.
GRADE Evidence Profile of COVID-19 Studies.
| Out come | No. of participants | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Quality of evidence (Grade) | Relative effect | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | Raised NLR | Control | ||||||||
| Mortality | 13 112 | 2223 | 10 889 | No | No | Yes | No | None | Low ⊕⊕⊝⊝ | SMD = 3.82 (95% CI: 2.79-4.85) |
| Severity | 12 433 | 3538 | 8895 | No | No | Yes | No | None | Low ⊕⊕⊝⊝ | SMD = 1.40 (95% CI: 1.19-1.60) |
Publication Bias
While qualitatively a publication bias is likely as per the Funnel plot for the studies on COVID-19 mortality due to smaller studies with large effect (Supplemental Figure 1), the Begg's test (P = .01) and Egger's test (0.23) indicate a mild risk of publication bias quantitatively.
Discussion
We discovered low-quality evidence with variability for the baseline elevated NLR on admission as a potential predictor of poor outcomes in COVID-19 patients.
Similarly, the severe COVID-19 patients have been reported to have increased, neutrophilia, lymphopenia, and thrombocytopenia than those with milder disease.112 Most of these patients were reported to develop ARDS and thereby required intensive care unit (ICU) admission.113,114 Thus, the raised NLR could be a potential cost-effective biomarker for predicting the disease severity as it indicates a combination of relative neutrophilia and lymphopenia in near real-time without any specific assay requirement unlike other biomarkers: D-dimer, IL6, C-reactive protein, and so on.
A recent meta-analysis also reported severe COVID-19 patients had a higher NLR value (SMD: 2.80, 95% CI: 2.12-3.48) in comparison to patients with nonsevere disease. They have also found raised NLR values in the expired in comparison to the survivors (SMD: 3.72, 95% CI: 0.53-6.90).115
Similarly, Feng et al116 have found that that elevated NLR is associated with disease severity in COVID-19 patients. (OR = 2.50, 95% CI: 2.04-3.06, P < .001).
The current study not only found that baseline elevated NLR was associated with mortality and disease severity but also quantified the predictive value through Summary Receiver operating curve analysis.
While Zhang et al117 have reported NLR ≥ 8 is associated with increased 28-day mortality (HR 9.74, 95% CI: 5.96-15.94) in the Univariable Cox regression model of 516 COVID-19 patients, Li et al118 have reported the cut-off NLR ≥ 4.5 and 6.5 for severity (AUC 0.86, 95% CI: 0.83-0.89) and mortality (AUC 0.92, 95% CI: 0.89-0.94).
There is no NLR consensus regarding the optimal cut-off value for determining the elevated level, particularly for COVID-19 patients. The wide variation implies that optimal cut-off values may vary in different populations as previously NLR has been found to vary with ethnicity, age, and sex.119–121
Strengths and Limitations
This study is one of the substantial and compendious reviews of the effectiveness of baseline NLR at admission in COVID-19 patients for predicting the mortality and severity and can be contemplated for decision making at present.
However, the majority of the included studies are retrospective (n = 82) in nature, originated from China (n = 62), and associated with significant heterogeneity probably due to the use of different cut-off values of NLR. The outcome of the disease could be impacted by other confounding factors: comorbid conditions, frailty, gender, etc also, which we could not assess due to the unavailability of appropriate data. We also acknowledged that few included studies are preprint and not peer-reviewed (n = 13), and the optimum value of NLR is yet to be standardized and information in this context is still evolving.
Conclusion
NLR is a promising tool for risk stratification and prompt decision making about intensifying the management, further studies for assessing the suitable cut-off points of NLR to utilize the already constrained healthcare resources during the ongoing pandemic are the need of the hour.
Supplemental Material
Supplemental material, sj-jpg-1-jic-10.1177_08850666211045626 for The Impact of Neutrophil-Lymphocyte Count Ratio in COVID-19: A Systematic Review and Meta-Analysis by Soumya Sarkar, Puneet Khanna and Akhil Kant Singh in Journal of Intensive Care Medicine
Footnotes
Author Contributions: SS: Conceptualization, Search strategy, Study selection, Data extraction, Data synthesis, Risk of bias assessment, and Drafted the manuscript. PK: Conceptualization, Search strategy, Study selection, Risk of bias assessment, Quality of the evidence assessment, and Editing. AKS: Study selection, Data extraction, Risk of bias assessment, Quality of the evidence assessment, and Editing.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Puneet Khanna https://orcid.org/0000-0002-9243-9963
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Wang Y, Ju M, Chen C, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J Thorac Dis. 2018;10(1):273–282. doi:10.21037/jtd.2017.12.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riche F, Gayat E, Barthelemy R, Le Dorze M, Mateo J, Payen D. Reversal of neutrophil-to-lymphocyte count ratio in early versus late death from septic shock. Crit care . 2015;19:439.Published 2015 Dec 16. doi:10.1186/s13054-015-1144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98–102. doi:10.1016/j.jns.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 4.Peng Y, Li Y, He Y, et al. The role of neutrophil to lymphocyte ratio for the assessment of liver fibrosis and cirrhosis: a systematic review. Expert Rev Gastroenterol Hepatol. 2018;12(5):503–513. doi:10.1080/17474124.2018.1463158 [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Li L, Wang X, Wang C, Wang D. Neutrophil-to-lymphocyte ratio and platelet count predict long-term outcome of stage IIIC epithelial ovarian cancer. Cellular Physiol Biochem: Int J Experim Cellular Physiol, Biochem, and Pharmacol. 2018;46(1):178–186. doi:10.1159/000488420 [DOI] [PubMed] [Google Scholar]
- 6.Vidal AC, Howard LE, de Hoedt A, et al. Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control. 2018;29(6):581–588. doi:10.1007/s10552-018-1031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 8.Han Q, Wen X, Wang L, et al. Role of hematological parameters in the diagnosis of influenza virus infection in patients with respiratory tract infection symptoms. J Clin Lab Anal. 2020;34(5):e23191. doi:10.1002/jcla.23191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–647. doi: 10.1016/j.ajem.2019.10.023. Epub Nov 18, 2019. PMID: 31785981. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. Br Med J. 2016;355:i4919.Published 2016 Oct 12. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt G, Oxman AD, Akl EA, et al. GRADE Guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 14.Norris SL, Meerpohl JJ, Akl EA, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016;79:150–158.e1.Epub 2016 Jul 14. doi:10.1016/j.jclinepi.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–415. doi:10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Montori V, et al. GRADE Guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64(12):1277–1282. doi:10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi:10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi:10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. doi:10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Sultan S, et al. GRADE Guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. doi:10.1016/j.jclinepi.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 21.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019 Oct 3;10:ED000142. doi:10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asghar MS, Haider Kazmi SJ, Ahmed Khan N, et al. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi [published correction appears in Cureus. 2020 Aug 6;12(8):c34]. Cureus. 2020;12(6):e8712. Published Jun 20, 2020. doi: 10.7759/cureus.8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Yi ZJ, Chang L, et al. The characteristics and death risk factors of 132 COVID-19 pneumonia patients with comorbidities: a retrospective single center analysis in Wuhan. China. medRxiv. 2020:2020.2005.2007.20092882 [Google Scholar]
- 24.Cheng F, Zheng F, Xie Ret al. Dynamics of neutrophil-to-lymphocyte ratio is associated with disease severity, progression and prognosis of COVID-19 in Wuhan, China (5/17/2020). Available at https://ssrn.com/abstract=3605274 or doi:10.2139/ssrn.3605274.
- 25.Chen L, Yu J, He W, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34(8):2173–2183. doi: 10.1038/s41375-020-0911-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146(1):89–100. doi: 10.1016/j.jaci.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng B, Hu J, Zuo X, et al. Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect. 2020;26(10):1400–1405. doi: 10.1016/j.cmi.2020.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity . J Med Virol. 2020; 92(10): 2152–2158. doi: 10.1002/jmv.26003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Yang L, Gui S, et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics. 2020;10(14):6113–6121. Published May 15, 2020. doi: 10.7150/thno.46569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo X, Zhou W, Yan Xet al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020;71(16):2174–2179. doi: 10.1093/cid/ciaa641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pakos IS, Lo KB, Salacup G, et al. Characteristics of peripheral blood differential counts in hospitalized patients with COVID-19. Eur J Haematol. 2020;105(6):773–778. . doi: 10.1111/ejh.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye W, Chen G, Li X, et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21(1):169. doi: 10.1186/s12931-020-01428-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan X, Li F, Wang X, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J Med Virol.2020;92(11):2573–2581. doi: 10.1002/jmv.26061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Q, Zhou Y, Wang X, et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: a propensity score-matching analysis. Respir Res. 2020;21(1):172. doi: 10.1186/s12931-020-01435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Guo M, Duan L, et al. Short term outcomes and risk factors for mortality in patients with COVID-19 in Wuhan. China: a Retrospective Study. 2020Available at SSRN: https://ssrn.com/abstract=3551390 or doi:10.2139/ssrn.3551390 . [Google Scholar]
- 36.Zhang N, Xu X, Zhou LY, et al. Clinical characteristics and chest CT imaging features of critically ill COVID-19 patients . Eur Radiol. 2020. doi: 10.1007/s00330-020-06955-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Guo M, Duan L, et al. Development and validation of a risk factor-based system to predict short-term survival in adult hospitalized patients with COVID-19: a multicenter, retrospective, cohort study. Crit Care . 2020;24(1):438.doi:10.1186/s13054-020-03123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-lymphocyte ratio and outcomes in Louisiana COVID-19 patients . Shock. 2020;54(5):652–658. doi: 10.1097/SHK.0000000000001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen FF, Zhong M, Liu Yet al. et al. Zhang Y: the characteristics and outcomes of 681 severe cases with COVID-19 in China. J Crit Care. 2020 Dec;60:32–37. doi:10.1016/j.jcrc.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ok F, Erdogan O, Durmus E, Carkci S, Canik A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID-19 patients. J Med Virol. 2021. Feb;93(2):786–793. doi: 10.1002/jmv.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H, Bai T, Shi J, Yang J. Predictive value of multiple in ammatory indexes on the prognosis of patients with corona virus disease 2019. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2020;28(06):13–16. [Google Scholar]
- 42.Huang S, Huang M, Li X, Zhang T, Lu H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio for predicting clinical outcomes in COVID-19. medRxiv. 2020.05.04.20090431. [Google Scholar]
- 43.Sun S, Cai X, Wang Het al. et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020 Aug;507:174–180. doi: 10.1016/j.cca.2020.04.024.Epub Apr 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu J, Kong J, Wang W, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb Res. 2020 Aug;192:3–8. doi: 10.1016/j.thromres.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020. Jul;84:106504. doi: 10.1016/j.intimp.2020.106504. Epub Apr 13, 2020. PMID: 32304994; PMCID: PMC7152924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Deng R, Gou L, et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. 2020;8(9):593. doi: 10.21037/atm-20-3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng F, Lei S, Wu C, et al. Neutrophil Percentage and Neutrophil-to-Monocyte Ratio as Independent Risk Factors in the Severity of COVID-19, 06 August 2020, PREPRINT (Version 1) available at Research Square [+ 10.21203/rs.3.rs-52622/v1+] [DOI]
- 48.Zhang Z, Zeng X, Wang J, et al. NLR combined with SaO2 predict severe illness among COVID-19 patients: a currently updated model, 26 August 2020, PREPRINT (Version 1) available at Research Square [+ 10.21203/rs.3.rs-64080/v1+] [DOI]
- 49.Zhang C, Qin L, Li K, et al. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. 2020;10:318. Published Jun 5, 2020. doi: 10.3389/fcimb.2020.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Tong J, Xiang J, Hu J. Retrospective study on the epidemiological characteristics of 139 patients with novel coronavirus pneumonia on the effects of severity. Chongqing Med. 2020. [Epub ahead of print]. [Google Scholar]
- 51.Chen X, Ou J, Huang Y, et al. Diagnostic roles of several parameters in corona virus disease 2019. Lab Med. 2020. [Epub ahead of print]. [Google Scholar]
- 52.Chen X, Zheng F, Qing Y, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv. 2020. 2020.03.03.20030353 [Google Scholar]
- 53.Ding X, Yu Y, Lu B, et al. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clin Chem Lab Med. 2020;58(8):1365–1371. [DOI] [PubMed] [Google Scholar]
- 54.Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou K, Zhang N, Li T, Zhou M, Shi X, Zhao G. CT Features of coronavirus disease 2019 (COVID-19) in different stages and its correlation with neutrophil-lymphocyte (NLR) and T lymphocyte subsets. Radiol Pract. 2020;35:272–276. doi: 10.13609/j.cnki.1000-0313.2020.03.00 [DOI] [Google Scholar]
- 56.Kong J, Wang T, Di Z, et al. Analysis of hematological indexes of COVID-19 patients from fever clinics in Suzhou, China. Int J Lab Hematol. 2020;42(5):e204–e206. doi: 10.1111/ijlh.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong M, Zhang H, Cao X, Mao X, Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect. 2020;148:e139. Published Jul 9, 2020. doi: 10.1017/S0950268820001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and 0prognosis evaluation of COVID-19: a retrospective cohort study. The Lancet Haematology. 2020;7(9):e671–e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10(12):5613–5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's Retrospective study. J Infect. 2020;81(2):318-356. doi: 10.1016/j.jinf.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, S Nannan Fan Y Wang J, et al. Predictive Value of the Neutrophil-to-Lymphocyte Ratio(NLR) for Diagnosis and Worse Clinical Course of the COVID-19: Findings from Ten Provinces in China . 2020. SSRN: https://ssrn.com/abstract=3569838 or doi:10.2139/ssrn.3569838
- 64.Peng J, Qi D, Yuan G, et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): a multicenter, cross-sectional study. J Clin Lab Anal. 2020;34(10):e23475. doi: 10.1002/jcla.23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(6):450–455. doi: 10.3760/cma.j.cn112148-20200220-00105 [DOI] [PubMed] [Google Scholar]
- 66.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shang W, Dong J, Ren Y, et al. The value of clinical parameters in predicting the severity of COVID-19 . J Med Virol. 2020;92(10):2188-2192. doi: 10.1002/jmv.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song C-Y, Xu J, He J-Q, Lu Y-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv. 2020. 2020.2003.2005.20031906. [Google Scholar]
- 69.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.[published correction appears in JAMA. 2021 Mar 16;325(11):1113]. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F, Qu M, Zhou X, et al. The timeline and risk factors of clinical progression of COVID-19 in Shenzhen, China. J Transl Med. 2020;18(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Li Q, Yin Y, et al. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Front Immunol. 2020;11:2063. doi:10.3389/fimmu.2020.02063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Li X, Shang Y, et al. Ratios of neutrophil-to-lymphocyte and platelet-to-lymphocyte predict all-cause mortality in inpatients with coronavirus disease 2019 (COVID-19): a retrospective cohort study in a single medical centre. Epidemiol Infect. 2020;148:e211. Published Sep 9, 2020. doi: 10.1017/S0950268820002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei YY, Wang RR, Zhang DW, et al. Risk factors for severe COVID-19: evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020;81(1):e89-e92. doi: 10.1016/j.jinf.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu S, Du Z, Shen S, et al. Identification and validation of a novel clinical signature to predict the prognosis in confirmed COVID-19 patients. Clin Infect Dis. 2020.;71(12):3154-3162. doi:10.1093/cid/ciaa793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie G, Ding F, Han L, Yin D, Lu H, Zhang M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2021;76(2):471-482. doi:10.1111/all.14465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie L, Wu Q, Lin Q, et al. Dysfunction of adaptive immunity is related to severity of COVID-19: a retrospective study. Ther Adv Respir Dis. 2020;14:1753466620942129. doi:10.1177/1753466620942129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, Hu S, Li S, et al. A Composite Index Predicts Disease Progression in Early Stages of COVID-19: a Propensity score-matched Cohort Study, 28 May 2020, PREPRINT (Version 1) available at Research Square [+ 10.21203/rs.3.rs-30635/v1+] [DOI] [PMC free article] [PubMed]
- 78.Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients With COVID-19. Front Mol Biosci. 2020;7(157), Published Jul 3, 2020. doi: 10.3389/fmolb.2020.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city. China. Liver Int: Official J Int Assoc Study Liver. 2020;40(9):2095-2103. doi:10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Guo S, He Y, et al. COVID-19 is distinct from SARS-CoV-2-negative community-acquired pneumonia. Front Cell Infect Microbiol. 2020;10(322). doi:10.3389/fcimb.2020.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. Epub 2020 Apr 22. doi:10.1016/j.ijid.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Archana B, Shyamsunder S, Das R. Validity of markers and indexes of systemic inflammation in predicting mortality in COVID 19 infection: a hospital based cross sectional study. medRxiv. Published online January 1, 2021:2021.03.30.21254635. doi: 10.1101/2021.03.30.21254635 [DOI] [Google Scholar]
- 83.Asghar MS, Khan NA, Haider Kazmi SJ, et al. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: a retrospective comparative analysis. J Community Hosp Intern Med Perspect. 2020;10(6):514–520. Published Oct 29, 2020. doi: 10.1080/20009666.2020.1816276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baqi S, Naz A, Sayeed MA, et al. Clinical characteristics and outcome of patients with severe COVID-19 pneumonia at a public sector hospital in Karachi, Pakistan. Cureus. 2021;13(2):e13107. Published Feb 3, 2021. doi: 10.7759/cureus.13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bisso IC, Huespe I, Lockhart C, et al. Clinical characteristics of critically ill patients with COVID-19. medRxiv. Published online January 1, 2020:2020.12.09.20246413. doi: 10.1101/2020.12.09.20246413 [DOI] [PubMed] [Google Scholar]
- 86.Montiel-Cervantes LA, Medina G, Pilar Cruz-Domínguez M, et al. Poor survival in COVID-19 associated with lymphopenia and higher neutrophil-lymphocyte ratio. Isr Med Assoc J. 2021;23(3):153–159. [PubMed] [Google Scholar]
- 87.López-Escobar A, Madurga R, Castellano JM, et al. Risk score for predicting in-hospital mortality in COVID-19 (RIM score). Diagnostics (Basel). 2021;11(4):596. Published Mar 26, 2021. doi: 10.3390/diagnostics11040596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Güneysu F, Guner NG, Erdem AF, Durmus E, Durgun Y, Yurumez Y. Can COVID-19 mortality be predicted in the emergency room? J Coll Physicians Surg Pak. 2020;30(9):928–932. doi: 10.29271/jcpsp.2020.09.928 [DOI] [PubMed] [Google Scholar]
- 89.Prasetya IB C, Lorens JO, Sungono V, El-Khobar KE, Wijaya RS. Prognostic value of inflammatory markers in patients with COVID-19 in Indonesia. Clin Epidemiol Glob Health. 2021;11:100803.Epub 2021 Jun 8. doi: 10.1016/j.cegh.2021.100803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalabin A, Mani VRK, Valdivieso SC, Donaldson B. Role of neutrophil-to-lymphocyte, lymphocyte-to-monocyte and platelet-to-lymphocyte ratios as predictors of disease severity in COVID-19 patients. Infez Med. 2021Mar 1;29(1):46–53. [PubMed] [Google Scholar]
- 91.Kaufmann CC, Ahmed A, Brunner U, et al. Red cell distribution width Upon hospital admission predicts short-term mortality in hospitalized patients With COVID-19: a single-center experience. Front Med (Lausanne). 2021 Mar 18;8:652707. doi: 10.3389/fmed.2021.652707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasir M, Perveen RA, Nazneen R, Zahan T, Ahmad SN, Chowdhury AS. Paradox of predictors in critically ill COVID-19 patients: outcome of a COVID-dedicated intensive care unit. medRxiv. Published online January 1, 2021:2021.04.23.21256009. doi: 10.1101/2021.04.23.21256009 [DOI] [Google Scholar]
- 93.Nicholson CJ, Wooster L, Sigurslid HH, et al. Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to mass general Brigham: the VICE and DICE scores. E Clin Med. 2021. Mar;33:10765. doi: 10.1016/j.eclinm.2021.100765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pujani M, Raychaudhuri S, Verma N, et al. Association of hematologic biomarkers and their combinations with disease severity and mortality in COVID-19—an Indian perspective. Am J Blood Res. 2021;11(2):180–190. Published Apr 15, 2021. [PMC free article] [PubMed] [Google Scholar]
- 95.Rasyid H, Sangkereng A, Harjianti T, Soetjipto AS. Impact of age to ferritin and neutrophil-lymphocyte ratio as biomarkers for intensive care requirement and mortality risk in COVID-19 patients in Makassar, Indonesia. Physiol Rep. 2021;9(10):e14876. doi: 10.14814/phy2.14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rokni M, Ahmadikia K, Asghari S, Mashaei S, Hassanali F. Comparison of clinical, para-clinical and laboratory findings in survived and deceased patients with COVID-19: diagnostic role of inflammatory indications in determining the severity of illness. BMC Infect Dis. 2020;20(1):869. Published Nov 23, 2020. doi: 10.1186/s12879-020-05540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruiz SJ, Ventura PS, Vázquez JMC, et al. Prognostic implications of neutrophillymphocyte ratio in COVID-19. Eur J Clin Invest. 2021 Jan;51 (1):e13404. 10.1111/eci.13404 [DOI] [PubMed] [Google Scholar]
- 98.Allahverdiyev S, Quisi A, Harbalioglu H, et al. The neutrophil to lymphocyte ratio and in-hospital all-cause mortality in patients with COVID-19. Eur J Therapeut. 2020;26(3):251–256. doi:10.5152/eurjther.2020.20067 [Google Scholar]
- 99.Yufei Y, Mingli L, Xuejiao L, et al. Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19). Scand J Clin Lab Invest. 2020;80(7):536–540. doi: 10.1080/00365513.2020.1803587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghazanfari T, Salehi MR, Namaki S, et al. Interpretation of hematological, biochemical, and immunological findings of COVID-19 disease: biomarkers associated with severity and mortality. Iran J Allergy Asthma Immunol. 2021;20(1):46–66. Published Feb 11, 2021. doi: 10.18502/ijaai.v20i1.5412 [DOI] [PubMed] [Google Scholar]
- 101.Xu J, Xu C, Zhang R, et al. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci Rep. 2020;10(1):15058. doi: 10.1038/s41598-020-72164-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng Z-Y, Feng S-D, Chen G-P, Wu J-N. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19: a prospective cohort study. BMC Infect Dis. 2021;21(1):80. doi: 10.1186/s12879-021-05796-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang P, Sha J, Meng M, et al. Risk factors for severe COVID-19 in middle-aged patients without comorbidities: a multicentre retrospective study. J Transl Med. 2020;18(1):461. doi: 10.1186/s12967-020-02655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xia X, Wen M, Zhan S, He J, Chen W. [An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19]. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(3):333–336.Chinese. doi: 10.12122/j.issn.1673-4254.2020.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mousavi-Nasab SD, Mardani R, Nasr Azadani H, et al. Neutrophil to lymphocyte ratio and C-reactive protein level as prognostic markers in mild versus severe COVID-19 patients. Gastroenterol Hepatol Bed Bench. 2020;13(4):361–366. [PMC free article] [PubMed] [Google Scholar]
- 106.Sepulchre E, Pittie G, Stojkovic V, et al. COVID-19: contribution of clinical characteristics and laboratory features for early detection of patients with high risk of severe evolution [published online ahead of print, Sep 16, 2020]. Acta Clin Belg. 2020:1–7. doi: 10.1080/17843286.2020.1822078 [DOI] [PubMed] [Google Scholar]
- 107.Tahtasakal CA, Oncul A, Sevgi DY, et al. Could we predict the prognosis of the COVID-19 disease? J Med Virol. 2021;93(4):2420–2430. doi: 10.1002/jmv.26751 [DOI] [PubMed] [Google Scholar]
- 108.Asan A, Üstündağ Y, Koca N, et al. Do initial hematologic indices predict the severity of COVID-19 patients? Turk J Med Sci. 2021;51(1):39–44. Published Feb 26, 2021. doi: 10.3906/sag-2007-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity. Int J Clin Pract. 2021;75(4):e13698. doi: 10.1111/ijcp.13698 [DOI] [PubMed] [Google Scholar]
- 110.Bastug A, Bodur H, Erdogan S, et al. Clinical and laboratory features of COVID-19: predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950. doi: 10.1016/j.intimp.2020.106950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fei M, Tong F, Tao X, Wang J. [Value of neutrophil-to-lymphocyte ratio in the classification diagnosis of coronavirus disease 2019]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(5):554–558.Chinese. doi: 10.3760/cma.j.cn121430-20200413-00506. [DOI] [PubMed] [Google Scholar]
- 112.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi:10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 113.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943.[published correction appears in JAMA Intern Med. 2020 Jul 1;180(7):1031].doi:10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chan AS, Rout A. Use of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19. J Clin Med Res. 2020;12(7):448–453. doi: 10.14740/jocmr4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng X, Li S, Sun Q, et al. Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med (Lausanne). 2020;7:301. Published Jun 9, 2020. doi: 10.3389/fmed.2020.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang S, Guo M, Duan L, et al. Development and validation of a risk factor-based system to predict short-term survival in adult hospitalized patients with COVID-19: a multicenter, retrospective, cohort study. Crit Care. 2020;24:438. doi: 10.1186/s13054-020-03123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Res Square. 2020. doi: 10.21203/rs.3.rs-71611/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361.doi:10.1371/journal.pone.0112361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore). 2018;97[26]:e11138. doi:10.1097/MD.0000000000011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu L, Zou S, Wang C, Tan X, Yu M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in south China. BMC Cardiovasc Disord. 2019;19(1):125–125. doi:10.1186/s12872-019-1110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-jic-10.1177_08850666211045626 for The Impact of Neutrophil-Lymphocyte Count Ratio in COVID-19: A Systematic Review and Meta-Analysis by Soumya Sarkar, Puneet Khanna and Akhil Kant Singh in Journal of Intensive Care Medicine