Abstract
BACKGROUND
Para-aortic lymph nodes (PALN) are found in the aortocaval groove and they are staged as metastatic disease if involved by pancreatic ductal adenocarcinoma (PDAC). The data in the literature is conflicting with some studies having associated PALN involvement with poor prognosis, while others not sharing the same results. PALN resection is not included in the standard lymphadenectomy during pancreatic resections as per the International Study Group for Pancreatic Surgery and there is no consensus on the management of these cases.
AIM
To investigate the prognostic significance of PALN metastases on the oncological outcomes after resection for PDAC.
METHODS
This is a retrospective cohort study of data retrieved from a prospectively maintained database on consecutive patients undergoing pancreatectomies for PDAC where PALN was sampled between 2011 and 2020. Statistical comparison of the data between PALN+ and PALN- subgroups, survival analysis with the Kaplan-Meier method and risk analysis with univariable and multivariable time to event Cox regression analysis were performed, specifically assessing oncological outcomes such as median overall survival (OS) and disease-free survival (DFS).
RESULTS
81 cases had PALN sampling and 17 (21%) were positive. Pathological N stage was significantly different between PALN+ and PALN- patients (P = 0.005), while no difference was observed in any of the other characteristics. Preoperative imaging diagnosed PALN positivity in one case. OS and DFS were comparable between PALN+ and PALN- patients with lymph node positive disease (OS: 13.2 mo vs 18.8 mo, P = 0.161; DFS: 13 mo vs 16.4 mo, P = 0.179). No difference in OS or DFS was identified between PALN positive and negative patients when they received chemotherapy either in the neoadjuvant or in the adjuvant setting (OS: 23.4 mo vs 20.6 mo, P = 0.192; DFS: 23.9 mo vs 20.5 mo, P = 0.718). On the contrary, when patients did not receive chemotherapy, PALN disease had substantially shorter OS (5.5 mo vs 14.2 mo; P = 0.015) and DFS (4.4 mo vs 9.8 mo; P < 0.001). PALN involvement was not identified as an independent predictor for OS after multivariable analysis, while it was for DFS doubling the risk of recurrence.
CONCLUSION
PALN involvement does not affect OS when patients complete the indicated treatment pathway for PDAC, surgery and chemotherapy, and should not be considered as a contraindication to resection.
Keywords: Para-aortic lymph node, Pancreatectomy, Survival, Pancreatic adenocarcinoma, Chemotherapy, Lymph node sampling
Core Tip: Currently there is no consensus on the prognostic significance of para-aortic lymph node (PALN) involvement in pancreatic ductal adenocarcinoma (PDAC), which is staged as metastatic disease (M1). Our study has demonstrated that patients with PALN involvement have comparable oncological outcomes, overall survival (OS) and disease free survival, to ones without PALN disease, when the appropriate treatment pathway is competed (surgery and chemotherapy). Multivariable risk analysis did not identify PALN involvement as an independent predictor for OS, while it doubled the risk of disease recurrence. Our data support that PALN involvement should not be considered a contraindication to resection for PDAC.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) presents as localised disease for only a small subset of patients for whom only 20% are eligible for resection[1] with 5-year survival of 6.8%[2]. Nodal status is amongst the most important prognostic indicators. Early lymph node involvement can be as common as 90% and may lead to tumour recurrence even after complete resection[3]. Survival difference has been demonstrated between N0 and lymph node positive disease within variances of lymph node ratio[4] and nodal stations[5] However, para-aortic lymph nodes found in the aortocaval groove (PALN, station Ln16b1) are distinct from regional lymph node stations and are staged as distant metastatic (M1) disease[6]. PALN metastases are found in 14%-18% of pancreatic head/uncinate PDAC at resection[7]. The exact significance and management of PALN is yet to be fully determined. Within the literature, various studies have alluded to PALN metastases being associated with poor prognosis, whereas others have failed to replicate this effect[8,9] and a meta-analysis[10] has only concluded the need for intra-operative assessment of PALN. A consensus statement from the International Study Group for Pancreatic Surgery (ISGPS) supported standard lymphadenectomy for pancreatic resections, as evidence do not support any benefit with an extended approach[11]. There was no recommendation to include PALN in standard lymphadenectomy, however it was acknowledged that PALN may be included in the resection plane based on individual practice. Currently, whether intra-operative assessment should be undertaken or whether there is sufficient evidence that resection should be abandoned depends on surgeon or unit policy.
The aim of this study was to determine the prognostic significance of PALN metastases on the oncological outcomes after pancreatic resections for PDAC.
MATERIALS AND METHODS
The study was conducted in line with STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines[12]. It was conducted at the University Hospitals of Birmingham, a tertiary specialist centre for the treatment of pancreatic cancer, after departmental approval. Staging of the tumours was based on the NCCN staging criteria[13]. The unit adopts a policy of fast-track[14] upfront surgery approach for resectable and borderline resectable PDAC with venous only involvement as supported by the United Kingdom National Institute for Care and Health Excellence[15], patients with borderline tumours with arterial involvement and locally advanced PDAC undergo neoadjuvant chemotherapy before resection is contemplated. All patients are referred for adjuvant chemotherapy after resection. In the early part of the study gemcitabine-based regimens were used both in the neoadjuvant and adjuvant setting. In the more recent years, modified FOLFIRINOX has been the preferred regimen, with gemcitabine-based regimens as back-up option depending on patients’ status and tolerance. PALN were sampled from the infra-renal, aortacaval lymph nodes and more specifically from the level of the third part of the duodenum to the angle of the left renal vein (station 16). PALN sampling was performed at the discretion of the operating surgeon. Over the last 3 years of the study 3 surgeons sampled PALN routinely, accounting for 36% of the cases in the study. Pre-operative staging included a computer tomography (CT) with IV contrast of the thorax, abdomen and pelvis and endoscopic ultrasound (EUS) with fine needle aspiration when preoperative cytological diagnosis was required. Magnetic resonance imaging (MRI) liver and positron emission tomography/CT (PET/CT) were used selectively if there were concerns for metastatic disease based on the CT scan. The management of all cases was discussed and agreed in the hepatopancreaticobiliary multidisciplinary meeting. Follow-up of patients was determined from time of diagnosis until disease recurrence or death. The study cohort included all patients that had PALN sampling during pancreatic resection for PDAC between 2011 and 2020. Clinical, radiological and pathological data were obtained from the hospital’s electronic records and the departmental prospectively maintained database. The American Joint Committee on Cancer 8th edition was used for tumor-node-metastasis (TNM) staging statistical analysis. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up and disease free survival as the time from resection to diagnosis of disease recurrence.
The cohort characteristics are presented with standard descriptive statistical analysis. One way Anova, Chi-Square and Mann-Whitney U tests were used as appropriate to compare variables and outcomes between PALN positive and negative subgroups, with statistical significance set at P < 0.05. Exact statistics were used for all tests to account for small sample size. Survival analysis was performed with the Kaplan-Meier method and log rank test was used to compare survival curves. Univariable and multivariable time to event analyses were performed using the Cox proportional hazard model to determine risk factors for median OS and disease-free survival (DFS). Variables were subjected to a univariable analysis first and those with P < 0.2 were introduced into a multivariable model. Hazard ratios and associated 95% confidence intervals (CI) were calculated. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using the software package SPSS Statistics for Windows (version 25.0; SPSS Inc., Chicago, IL, United States).
RESULTS
During the study period there were 81 patients who underwent pancreatectomies for PDAC where PALN were sampled. PALN metastasis was identified in 17 (21%) cases. The median sampled LNs were 2 (range 1-7) and median positivity ratio 0.5 (range 0.14-1). Patient, tumour and post-operative parameters for the whole cohort, as well as for the PALN positive and negative subgroups, are displayed in Table 1. Pathology N stage (pN) was significantly different between patients with PALN positive and negative disease (P = 0.005). All patients with PALN metastases also had regional lymph node disease, with 82% having pN2 disease (in contrast to 45% of PALN negative patients). There was no difference observed in any of the other characteristics. PALN sampling did not cause any significant morbidity in terms of chyle leak or post-pancreatectomy haemorrhage.
Table 1.
Patient demographics, operative and pathological characteristics and outcomes
|
Factors
|
Total (n = 81)
|
PALN+ (n = 17)
|
PALN- (n = 64)
|
P
value
|
| Demographics | ||||
| Age (median and range in years) | 69 (43-84) | 68.8 (61-72.3) | 69 (61-75) | 0.404 |
| Gender, male (%) | 38 (47) | 12 (71) | 33 (52) | 0.171 |
| BMI (kg/m2) | 25.1 (22.0-27.8) | 26.2 (22.2-27.8) | 24.9 (21.9-27.7) | 0.413 |
| Non-smoker (%) | 13 (73) | 14 (82) | 46 (72) | 0.462 |
| Preoperative CA19-9 levels (KU/L) | 286 (2-36000) | 410 (14-2784) | 252 (2-36000) | 0.594 |
| Charlson comorbidity index | 4 (3-5) | 4 (3-5) | 4.5 (3-5) | 0.079 |
| Preoperative radiological stage n (%) | ||||
| Resectable | 41 (51) | 8 (47) | 33 (52) | 0.601 |
| Borderline resectable | 31 (38) | 8(47) | 23 (36) | |
| Locally advanced | 9 (11) | 1 (6) | 8 (12) | |
| Operation, n (%) | ||||
| Distal pancreatectomy | 1 (1) | 0 | 1 (1) | 0.681 |
| Total pancreatectomy | 14 (17) | 2 (12) | 12 (19) | |
| Pancreaticoduodenectomy | 66 (82) | 15 (88) | 51 (80) | |
| Vein resection | 33 (41) | 7 (41) | 26 (41) | 0.310 |
| Arterial resection | 3 (4) | 1 (6) | 2 (3) | 0.842 |
| Pathological staging, n (%) | 1.000 | |||
| pT1 | 13 (16) | 3 (18) | 10 (16) | 0.951 |
| pT2 | 46 (57) | 9 (53) | 37 (58) | |
| pT3 | 21 (26) | 5 (29) | 16 (25) | |
| pT4 | 1 (1) | 0 | 1 (1) | |
| pN0 | 14 (79) | 0 | 14 (22) | 0.005 |
| pN1 | 24 (30) | 3 (18) | 21 (33) | |
| pN2 | 43 (53) | 14 (82) | 29 (45) | |
| Resection margin, n (%) | ||||
| Negative | 39 (48) | 6 (35) | 33 (52) | 0.282 |
| Positive | 42 (52) | 11 (65) | 31 (48) | |
| Perineural invasion | 66 (83) | 15 (88) | 51 (80) | 0.722 |
| Perivascular invasion | 59 (73) | 13 (77) | 46 (72) | 1.000 |
| Chemotherapy, n (%) | 55 (74) | 12 (71) | 43 (67) | 0.746 |
| Neoadjuvant therapy | 13 (16) | 2 (12) | 11 (17) | 0.726 |
| Adjuvant chemotherapy | 49 (66) | 12 (71) | 37 (58) | 0.553 |
| Post-operative complications, n (%) | ||||
| Clavien Dindo category ≥ 3 | 10 (12) | 2 (11.8) | 8 (12.5) | 0.549 |
| Chyle leak | 1 (1) | 0 | 1 (1.56) | 0.835 |
| Perioperative haemorrhage | 2 (2) | 0 | 2 (3.13) | 0.712 |
| Comprehensive complication index | 0 (0-20.9) | 0 (0-20.9) | 0 (0-20.9) | 0.083 |
| Hospital length of stay (median and range in days) | 9 (1-76) | 8 (5-30) | 10 (1-76) | 0.138 |
BMI: Body mass index; PALN: Para-aortic lymph nodes.
Radiological detection of PALN
Amongst patients with metastatic PALN on pathology, there was no modality of investigation which detected this during preoperative staging (CT 1/81, EUS 0/5 or PET 0/3).
OS
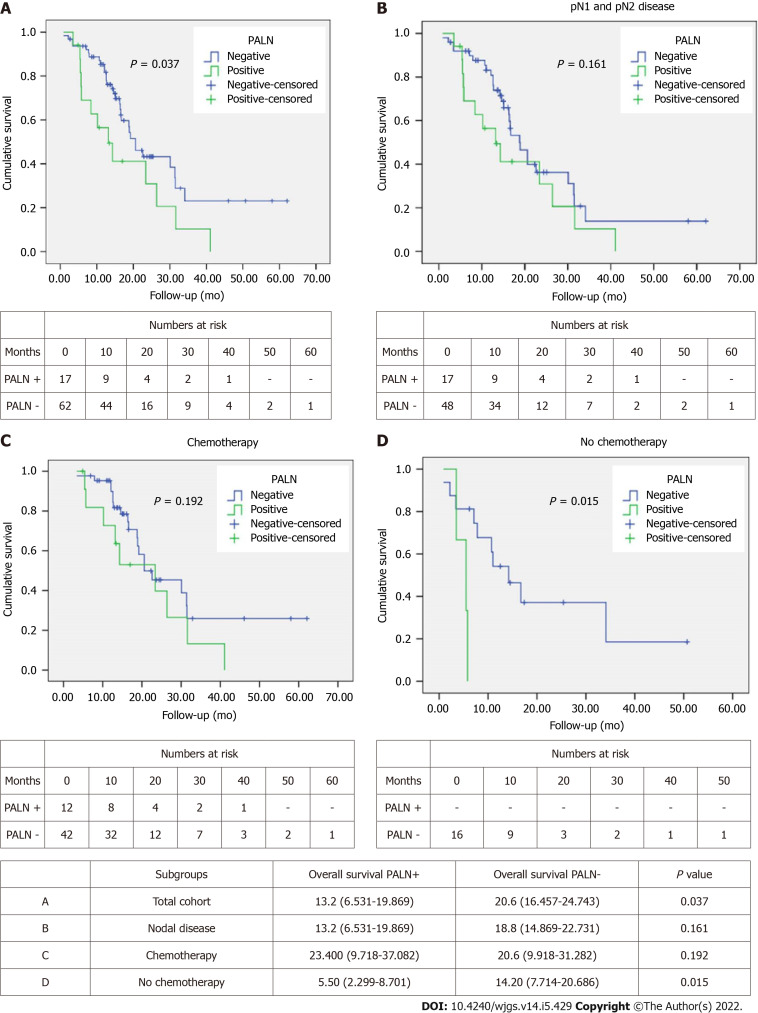
OS was better in PALN negative patients with a median of 20.6 mo compared to 13.2 mo in PALN positive patients (P = 0.037) (Figure 1A). However, OS among patients with lymph node disease (pN1 and pN2) was comparable between PALN positive and negative cases (13.2 mo vs 18.8 mo, P = 0.161) (Figure 1B).
Figure 1.
Kaplan Meier curves comparing overall survival in patients with para-aortic lymph nodes(+) vs para-aortic lymph nodes(-). A: Total cohort; B: Patients with positive nodal disease; C: Patients who received chemotherapy; D: Patients who did not receive chemotherapy. PALN: Para-aortic lymph nodes.
Similarly, when patients were stratified based on receipt of chemotherapy, either in the neoadjuvant or the adjuvant setting, no difference in OS was observed between PALN positive and negative patients who had chemotherapy (23.4 mo vs 20.6 mo, P = 0.192). Interestingly OS of PALN positive patients was slightly longer by about 3 mo (Figure 1C). On the contrary, when patients did not receive chemotherapy, PALN metastatic disease had substantially shorter OS (5.5 mo vs 14.2 mo; P = 0.015) (Figure 1D).
Univariable Cox regression analysis showed that pT, pN, presence of PALN metastases, resection margin status and receipt of chemotherapy were associated with OS (Table 2). Multivariable analysis identified pT, pN, margin status and receipt of chemotherapy as independent predictors of survival (Table 2). Of note PALN positivity was not identified as an independent prognostic factor for OS.
Table 2.
Risk analysis for overall survival
|
Univariable
|
Multivariable
|
|||
|
P
value
|
HR (CI)
|
P
value
|
HR (CI)
|
|
| Age | 0.668 | 0.994 (0.966-1.022) | ||
| Sex | 0.359 | 0.756 (0.416-1.374) | ||
| Preoperative CA19-9 | 0.626 | 1.000 (1.000-1.000) | ||
| Pre-operative stage | 0.949 | 0.986 (0.641-1.517) | ||
| Resection type | 0.517 | 1.141 (0.765-1.702) | ||
| Venous resection | 0.659 | 1.146 (0.625-2.104) | ||
| Arterial resection | 0.327 | 2.045 (0.489-8.559) | ||
| pT | 0.001 | 2.148 (1.368-3.371) | 0.008 | |
| pT1 | 0.842 | 1.114 (0.385-3.226) | ||
| pT2 | 0.115 | 2.459 (0.803-7.536) | ||
| pT3 | 0.008 | 31.275 (2.491-392.605) | ||
| pN | 0.002 | 2.195 (1.337-3.604) | 0.004 | |
| pN1 | 0.329 | 2.332 (0.427-12.740) | ||
| pN2 | 0.016 | 7.564 (1.459-39.224) | ||
| PALN positivity | 0.041 | 1.970 (1.028-3.776) | ||
| Margin status | 0.007 | 2.331 (1.261-4.308) | 0.049 | 1.986 (1.003-3.932) |
| Perineural invasion | 0.212 | 1.691 (0.741-3.861) | ||
| Perivascular invasion | 0.464 | 1.278 (0.663-2.461) | ||
| Chemotherapy | 0.033 | 0.487 (0.251-0.944) | 0.002 | 0.283 (0.129-0.622) |
All parameters with P < 0.200 on univariable entered into multivariable model. HR: Hazard ratios; CI: Confidence intervals; PALN: Para-aortic lymph nodes.
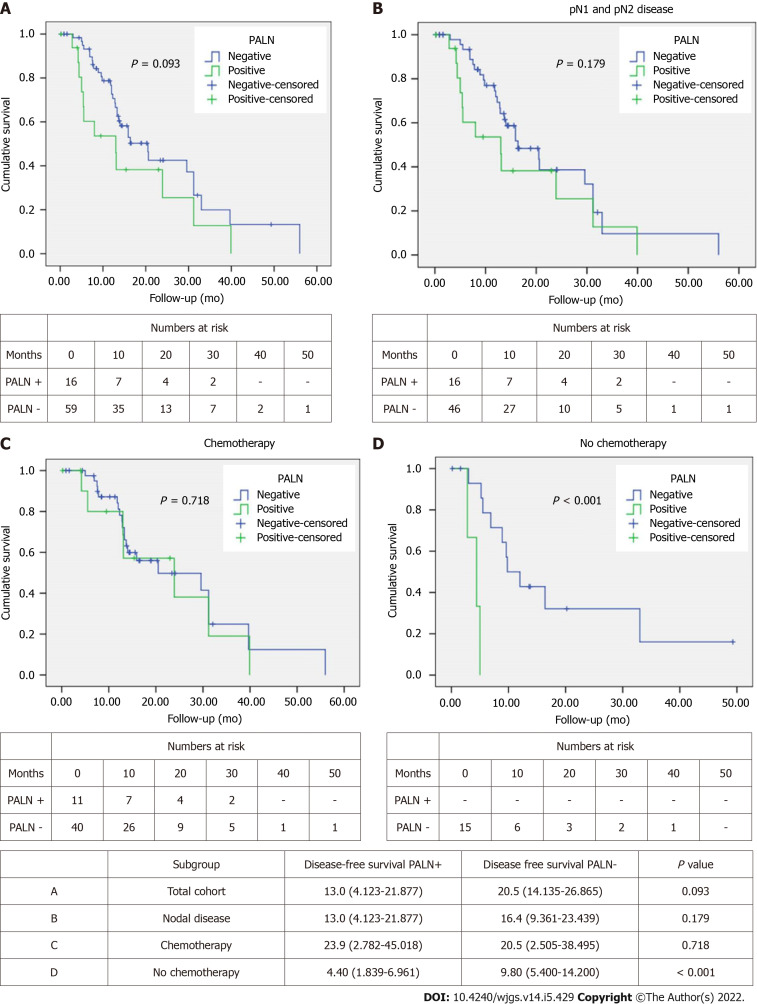
Disease-free survival
Median DFS in the PALN positive group was 13 mo compared to 20.5 mo in the PALN negative one (Figure 2A). This approached but did not achieve statistical significance (P = 0.093). However, among patients with lymph node disease (pN1 and pN2), DFS was comparable between PALN positive and negative cases (13 mo vs 16.4 mo, P = 0.179) (Figure 2B).
Figure 2.
Kaplan Meier curves comparing disease-free survival in patients with para-aortic lymph nodes(+) vs para-aortic lymph nodes(-). A: Total cohort b; B: Patients with positive nodal disease; C: Patients who received chemotherapy; D: Patients who did not receive chemotherapy. PALN: Para-aortic lymph nodes.
When the patients were stratified based on receipt of chemotherapy, either in the neoadjuvant or the adjuvant setting, no difference in DFS was observed between PALN positive and negative patients that had chemotherapy (23.9 mo vs 20.5 mo, P = 0.718). Interestingly DFS of PALN positive patients was slightly longer by about 3 mo (Figure 2C). When patients did not receive chemotherapy, PALN metastatic disease had substantially shorter DFS (4.4 mo vs 9.8 mo; P < 0.001) (Figure 2D).
Univariable Cox regression analysis showed that pT, resection margin status and receipt of chemotherapy were associated with DFS. Age, pN, PALN metastases, perineural and perivascular invasion approached but did not achieve significance (Table 3). On multivariable analysis PALN positivity was identified as an independent predictor of DFS, doubling the risk of recurrence. Other predictors were age, pT, margin status, PNI and chemotherapy (Table 3).
Table 3.
Risk analysis for disease-free survival
|
Univariable
|
Multivariable
|
|||
|
P
value
|
HR (CI)
|
P
value
|
HR (CI)
|
|
| Age | 0.129 | 0.979 (0.952-1.006) | 0.023 | 0.964 (0.933-0.995) |
| Sex | 0.881 | 0.955 (0.526-1.736) | ||
| Preoperative CA19-9 | 0.773 | 1.000 (1.000-1.000) | ||
| Preoperative stage | 0.943 | 1.016 (0.656-1.573) | ||
| Resection type | 0.215 | 1.272 (0.869-1.862) | ||
| Venous resection | 0.739 | 0.899 (0.481-1.681) | ||
| Arterial resection | 0.567 | 0.048 (0.000-1578.950) | ||
| pT | 0.002 | 2.102 (1.308-3.378) | 0.004 | |
| pT1 | 0.265 | 1.726 (0.661-4.509) | ||
| pT2 | 0.015 | 3.689 (1.287-10.576) | ||
| pT3 | 0.002 | 49.543 (4.018-610.815) | ||
| pN | 0.121 | 1.387 (0.917-2.097) | ||
| PALN positivity | 0.101 | 1.748 (0.896-3.410) | 0.045 | 2.287 (1.018-5.136) |
| Margin status | 0.032 | 1.927 (1.057-3.514) | 0.007 | 2.48 (1.275-4.822) |
| Perineural invasion | 0.103 | 2.084 (0.862-5.036) | 0.041 | 2.938 (1.045-8.255) |
| Perivascular invasion | 0.152 | 1.657 (0.830-3.308) | ||
| Chemotherapy | 0.047 | 0.509 (0.261-0.992) | 0.001 | 0.242 (0.105-0.559) |
All parameters with P < 0.200 on univariable entered into multivariable model. HR: Hazard ratios; CI: Confidence intervals; PALN: Para-aortic lymph nodes.
DISCUSSION
The prognostic significance of PALN positivity has long been an area of debate. The anatomic location of PALN in the aortocaval groove and away from the peri-pancreatic area has resulted in staging these as extra-regional lymph nodes and therefore metastatic disease on TNM if involved[16]. On the other hand, PALN (LN16b1) drain lymph nodes around groups 13 and 14[7,17,18] which are commonly involved in PDAC and therefore PALN could be considered the next lymph node station involved in cases of node positive disease. Furthermore, one theory that has been proposed to explain PALN acting similarly to nodal disease rather than metastatic is that LN16 involvement is due to local invasion through the fascia of Treitz[19] and this is why it is also associated with a high incidence of positive resection margins[9,19]. In this case, PALN excision may allow extensive mesopancreas dissection[20]. The published evidence on the significance of PALN positive disease and its impact in oncological outcomes is conflicting. A consensus statement from the ISGPS suggested that extended lymphadenectomy is not indicated in pancreatic resections[11]. The same group defined standard lymphadenectomy for pancreaticoduodenectomy to include lymph nodes in the hepatoduodenal ligament (stations 5, 6, 8a, 12b, 12c), pancreaticoduodenal groove (stations 13 and 17), right side of the superior mesenteric artery (stations 14a and 14b) and for distal pancreatectomy those along the splenic artery (station 11), along the inferior border of the pancreas (station 18) and in the splenic hilum (station 10), with station 9 to be included only in pancreatic body tumours. Resection of PALN (station 16) was not recommended based on the reported poor outcomes of patients with PALN positive disease. Nonetheless, it was acknowledged that PALN may be included in the resection plane based on individual practice. Some studies have stated no impact of PALN involvement on survival[7,19,21] with others suggested the opposite and even abandoning resection if this is identified intra-operatively upon sampling[8,22,23]. A confounding flaw in many studies is the comparison of survival between PALN+ and PALN-, where the latter group includes a subgroup of N0 patients with invariably better survival rates. A meta-analysis by Agalianos et al[9] made a pertinent comparison of PALN+ with pN1 PALN- patients, showing that survival rates at 1 and 2 years were significantly worse in PALN+ group. This was contested by Hempel et al[6] who showed that the OS of PALN+ and pN1 PALN- patients were not significantly different. In our study all PALN positive patients also had regional lymph node disease, whereas 22% of PALN negative patients were staged as pN0. No significant difference in OS and DFS was identified in regional lymph node positive (pN1 and pN2) PALN positive patients compared to PALN negative ones. Given that resection in the presence of nodal disease has been shown to prolong survival[24-28] there is no indication on this basis to abandon resection.
The appropriateness of PALN+ being termed M1 disease has also been challenged where long term survival after PALN+ resection has been achieved by various studies[6,29,30] including a multicentre study of 102 (12.4%) PALN+ which has shown survival of 2 years of PALN+ patients[20]. Our study covers a 10 year period during which the chemotherapy practice has changed from single agent gemcitabine to gemcitabine combined with capecitabine and more recently FOLFIRINOX. This along with the fact that approximately 30% of patients did not receive any systemic treatment can explain the OS of 20.6 mo in PALN negative and 13.2 mo in PALN positive patients. However, in patients who received chemotherapy, whether NAT or adjuvant chemotherapy, this disparity disappeared. Furthermore, on multivariable analysis PALN positivity was not an independent predictor for OS. Interestingly, OS was slightly longer in the PLAN positive patients after chemotherapy (23.4 mo vs 20.6 mo). This may reflect a treatment selection bias by the oncology teams as patients with more aggressive disease received more commonly chemotherapy in the adjuvant period (71% for PALN positive disease compared to 58% for PALN negative), even though this difference did not reach statistical significance. During the same time period, patients diagnosed with metastatic disease intra-operatively had a medial OS of 14.1 mo after palliative treatment (6.1 mo if they did not receive any palliative treatment), which is substantially less than the 23.4 mo OS recorded for PALN+ patients with chemotherapy.
Similarly, DFS was only worse in PALN positive patients if they did not receive any systemic treatment. However, in patients that had systemic treatment DFS was slightly longer in PALN positive patients (23.9 mo vs 20.5 mo). Similar to OS, this is most likely a reflection of oncological treatment selection bias. Furthermore, the fact that PALN positivity was identified on multivariable analysis as an independent predictor of DFS, doubling the risk for recurrence, is not an unexpected finding, as nodal disease is a well established prognostic factor for recurrence of PDAC.
The survival benefit of completion of the treatment pathway (surgery and chemotherapy) in patients with PDAC is well established and the sequence of chemotherapy is based on preoperative staging (neoadjuvant or adjuvant setting)[31,32]. With regards to PALN involvement, this is further supported by the results of this study as well as others on PALN disease[20,33,34]. Therefore, the comparable OS and DFS after completion of the whole treatment, surgery and chemotherapy, suggest that PALN should not be considered as a contraindication for resection if identified intra-operatively. The substantially worse OS in patients who did not receive any chemotherapy, stresses the importance of considering PALN positive disease in preoperative staging as an indication for NAT. Pre-operative CA19-9 Levels have been associated with PALN+[20,23,35]. Nonetheless, preoperative staging investigations have a very low sensitivity for this in the current as well as other studies to provide the required confirmation. The sensitivity of CT and MRI has been suggested to be close to zero for PALN+[36] while 18F-flurodeoxyglucose positron emission tomography (FDG-PET) was shown to have sensitivity 37%-50%[37-39]. EUS is used for staging of nodal involvement with accuracy reaching around 65%[40,41] though one small study of 21 patients with PALN+ was shown to have 95% sensitivity[42]. In our study only one case of PALN metastasis was identified on preoperative staging scans, while operative excisional sampling upstaged the diagnosis in 21% of the cases without increasing the risk of peri-operative complications.
The limitations of this study include its retrospective and single centre nature, as well as the selection bias associated with intra-operative PALN sampling. Additionally, as the study covers a 10 year period with changes in the preferred systemic treatment regimens for PDAC, systemic treatment selection time bias is inevitable. The small number of PALN positive patients precluded a subgroup analysis of types and duration of NAT or adjuvant chemotherapy. Despite these limitations, the study accurately reflects the practice around PALN over the previous decade and the results clearly add to the body of evidence advocating against considering PALN involvement in the absence of evidence of distant metastases as unresectable disease and against treating these patients with palliative intent.
CONCLUSION
This study suggests that PALN sampling is safe and should be routinely performed during resection of PDAC for accurate staging, even in the absence of involvement in the pre-operative imaging. PALN involvement does not affect OS when patients complete the indicated treatment pathway (surgery and chemotherapy) and occult involvement identified intra-operatively should not be considered as a contraindication to resection. Future studies should focus on improving pre-operative diagnosis and on the value of NAT for these cases.
ARTICLE HIGHLIGHTS
Research background
Pancreatic ductal adenocarcinoma (PDAC) presents as localised disease for only a small subset of patients for whom only 20% are eligible for resection with 5-year survival of 6.8%. Nodal status is amongst the most important prognostic indicators. Para-aortic lymph nodes found in the aortocaval groove (PALN) are staged as distant metastatic (M1) disease and are found in 14%-18% of pancreatic head/uncinate PDAC at resection. Various studies have alluded to PALN metastases being associated with poor prognosis, whereas others have failed to replicate this effect and a meta-analysis has only concluded the need for intra-operative assessment of PALN. A consensus statement from the International Study Group for Pancreatic Surgery supported standard lymphadenectomy for pancreatic resections, which does not include PALN.
Research motivation
Currently, whether intra-operative assessment of PALN should be undertaken or whether there is sufficient evidence that resection should be abandoned depends on surgeon or unit policy.
Research objectives
The aim of this study was to determine the prognostic significance of PALN metastases on the oncological outcomes after pancreatic resections for PDAC.
Research methods
This is a retrospective cohort study of data from a prospectively maintained database on consecutive patients undergoing pancreatectomies for PDAC where PALN was sampled between 2011 and 2020 in a tertiary specialist centre. The study was conducted in line with STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines. Staging of the tumours was based on the NCCN staging criteria. PALN were sampled from the infra-renal, aortacaval lymph nodes and more specifically from the level of the third part of the duodenum to the angle of the left renal vein (station 16). PALN sampling was performed at the discretion of the operating surgeon. Over the last 3 years of the study 3 surgeons sampled PALN routinely, accounting for 36% of the cases in the study. Follow-up of patients was determined from time of diagnosis until disease recurrence or death. OS was defined as the time from diagnosis to death or last follow-up and disease free survival as the time from resection to diagnosis of disease recurrence.
The cohort characteristics are presented with standard descriptive statistical analysis. One way Anova, Chi-Square and Mann-Whitney U tests were used as appropriate for statistical comparisons with statistical significance set at P < 0.05. Exact statistics were used for all tests to account for small sample size. Survival analysis was performed with the Kaplan-Meier method and log rank test was used to compare survival curves. Univariable and multivariable time to event analyses were performed using the Cox proportional hazard model to determine risk factors for median OS and disease-free survival (DFS). Variables were subjected to a univariable analysis first and those with P < 0.2 were introduced into a multivariable model. Hazard ratios and associated 95% confidence intervals were calculated. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using the software package SPSS Statistics for Windows (version 25.0; SPSS Inc., Chicago, IL, United States).
Research results
81 cases had PALN sampling and 17 (21%) were positive. Pathological N stage was significantly different between PALN+ and PALN- patients (P = 0.005), while no difference was observed in any of the other characteristics. Preoperative imaging diagnosed PALN positivity in one case. OS and DFS were comparable between PALN+ and PALN- patients with lymph node positive disease (OS: 13.2 mo vs 18.8 mo, P = 0.161; DFS: 13 mo vs 16.4 mo, P = 0.179). No difference in OS or DFS was identified between PALN positive and negative patients when they received chemotherapy either in the neoadjuvant or in the adjuvant setting (OS: 23.4 mo vs 20.6 mo, P = 0.192; DFS: 23.9 mo vs 20.5 mo, P = 0.718). On the contrary, when patients did not receive chemotherapy, PALN disease had substantially shorter OS (5.5 mo vs 14.2 mo; P = 0.015) and DFS (4.4 mo vs 9.8 mo; P < 0.001). PALN involvement was not identified as an independent predictor for OS after multivariable analysis, while it was for DFS doubling the risk of recurrence.
Research conclusions
This study suggests that PALN sampling is safe and should be routinely performed during resection of PDAC for accurate staging, even in the absence of involvement in the pre-operative imaging. PALN involvement does not affect OS when patients complete the indicated treatment pathway (surgery and chemotherapy) and occult involvement identified intra-operatively should not be considered as a contraindication to resection.
Research perspectives
Future studies should focus on improving pre-operative diagnosis and on the value of NAT for these cases.
Footnotes
Institutional review board statement: The study was reviewed and approved for publication by the Departmental Institutional Review Board.
Informed consent statement: As this was an anonymised retrospective cohort study over a period of 10 years, individual consent forms were not required based on the policy of Queen Elizabeth Hospital and the UK on ethics and research.
Conflict-of-interest statement: All the Authors have no conflict of interest related to the manuscript.
STROBE statement: the authors have reviewed the STROBE statement-checklist of items. The written material was prepared concordant with the STROBE statement-check list of items.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 21, 2021
First decision: March 13, 2022
Article in press: April 21, 2022
Specialty type: Surgery
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang L, China; Kuo SH, Taiwan; Nguyen LT, Viet Nam S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
Contributor Information
Rupaly Pande, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Shafiq Chughtai, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Manish Ahuja, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Rachel Brown, Department of Pathology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
David C Bartlett, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Bobby V Dasari, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Ravi Marudanayagam, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Darius Mirza, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Keith Roberts, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
John Isaac, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Robert P Sutcliffe, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Nikolaos A Chatzizacharias, Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom. chatzizacharias@gmail.com.
Data sharing statement
The original anonymous dataset is available upon request from the corresponding author.
References
- 1.Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476–e485. doi: 10.1016/S1470-2045(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 4.Malleo G, Maggino L, Capelli P, Gulino F, Segattini S, Scarpa A, Bassi C, Butturini G, Salvia R. Reappraisal of Nodal Staging and Study of Lymph Node Station Involvement in Pancreaticoduodenectomy with the Standard International Study Group of Pancreatic Surgery Definition of Lymphadenectomy for Cancer. J Am Coll Surg. 2015;221:367–79.e4. doi: 10.1016/j.jamcollsurg.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845–847. doi: 10.1245/s10434-017-6025-x. [DOI] [PubMed] [Google Scholar]
- 6.Hempel S, Plodeck V, Mierke F, Distler M, Aust DE, Saeger HD, Weitz J, Welsch T. Para-aortic lymph node metastases in pancreatic cancer should not be considered a watershed for curative resection. Sci Rep. 2017;7:7688. doi: 10.1038/s41598-017-08165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Ueno K, Tajima H, Elnemr A, Miwa K. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer. 1999;85:583–590. doi: 10.1002/(sici)1097-0142(19990201)85:3<583::aid-cncr8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Doi R, Kami K, Ito D, Fujimoto K, Kawaguchi Y, Wada M, Kogire M, Hosotani R, Imamura M, Uemoto S. Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer. World J Surg. 2007;31:147–154. doi: 10.1007/s00268-005-0730-5. [DOI] [PubMed] [Google Scholar]
- 9.Agalianos C, Gouvas N, Papaparaskeva K, Dervenis C. Positive para-aortic lymph nodes following pancreatectomy for pancreatic cancer. Systematic review and meta-analysis of impact on short term survival and association with clinicopathologic features. HPB (Oxford) 2016;18:633–641. doi: 10.1016/j.hpb.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paiella S, Sandini M, Gianotti L, Butturini G, Salvia R, Bassi C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2016;42:616–624. doi: 10.1016/j.ejso.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andrén-Sandberg A, Asbun HJ, Bockhorn M, Büchler MW, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 13.NCCN Cancer Network Clinical Practice Guidelines in Oncology: Pancreatic adenocarcinoma. Version 2.2021. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455 .
- 14.Roberts KJ, Prasad P, Steele Y, Marcon F, Faulkner T, Cilliers H, Dasari B, Abradelo M, Marudanayagam R, Sutcliffe RP, Muiesan P, Mirza DF, Isaac J. A reduced time to surgery within a 'fast track' pathway for periampullary malignancy is associated with an increased rate of pancreatoduodenectomy. HPB (Oxford) 2017;19:713–720. doi: 10.1016/j.hpb.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 15.NICE Pancreatic cancer in adults: diagnosis and ancreatic cancer in adults: diagnosis and management management NICE guideline. 2018. Available from: https://www.nice.org.uk/guidance/ng85/resources/pancreatic-cancer-in-adults-diagnosis-and-management-pdf-1837696373701 .
- 16.Isaji S, Murata Y, Kishiwada M. New Japanese Classification of Pancreatic Cancer. In: Neoptolemos J, Urrutia R, Abbruzzese J, Büchler M. (eds) Pancreatic Cancer. Springer, New York, 2018: 1021-1037. [Google Scholar]
- 17.Kayahara M, Nagakawa T, Kobayashi H, Mori K, Nakano T, Kadoya N, Ohta T, Ueno K, Miyazaki I. Lymphatic flow in carcinoma of the head of the pancreas. Cancer. 1992;70:2061–2066. doi: 10.1002/1097-0142(19921015)70:8<2061::aid-cncr2820700808>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Ohta T, Kitagawa H, Kayahara M, Kinami S, Ninomiya I, Fushida S, Fujimura T, Nishimura G, Shimizu K, Yi S, Miwa K. Sentinel lymph node navigation surgery for pancreatic head cancers. Oncol Rep. 2003;10:315–319. [PubMed] [Google Scholar]
- 19.Connor S, Bosonnet L, Ghaneh P, Alexakis N, Hartley M, Campbell F, Sutton R, Neoptolemos JP. Survival of patients with periampullary carcinoma is predicted by lymph node 8a but not by lymph node 16b1 status. Br J Surg. 2004;91:1592–1599. doi: 10.1002/bjs.4761. [DOI] [PubMed] [Google Scholar]
- 20.Sho M, Murakami Y, Motoi F, Satoi S, Matsumoto I, Kawai M, Honda G, Uemura K, Yanagimoto H, Kurata M, Fukumoto T, Akahori T, Kinoshita S, Nagai M, Nishiwada S, Unno M, Yamaue H, Nakajima Y. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: a multicenter study on 822 patients. J Gastroenterol. 2015;50:694–702. doi: 10.1007/s00535-014-1005-4. [DOI] [PubMed] [Google Scholar]
- 21.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–66; discussion 366. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz L, Lupinacci RM, Svrcek M, Lesurtel M, Bubenheim M, Vuarnesson H, Balladur P, Paye F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg. 2014;101:530–538. doi: 10.1002/bjs.9444. [DOI] [PubMed] [Google Scholar]
- 23.Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- 24.Delcore R, Rodriguez FJ, Forster J, Hermreck AS, Thomas JH. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. Am J Surg. 1996;172:463–8; discussion 468. doi: 10.1016/S0002-9610(96)00237-1. [DOI] [PubMed] [Google Scholar]
- 25.van Geenen RC, van Gulik TM, Offerhaus GJ, de Wit LT, Busch OR, Obertop H, Gouma DJ. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. 2001;27:549–557. doi: 10.1053/ejso.2001.1162. [DOI] [PubMed] [Google Scholar]
- 26.Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, Bassi C, Dervenis C, Fernandez-Cruz L, Lacaine F, Buckels J, Deakin M, Adab FA, Sutton R, Imrie C, Ihse I, Tihanyi T, Olah A, Pedrazzoli S, Spooner D, Kerr DJ, Friess H, Büchler MW European Study Group for Pancreatic Cancer. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 28.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, Mazzeo F. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Masui T, Kubota T, Aoki K, Nakanishi Y, Miyamoto T, Nagata J, Morino K, Fukugaki A, Takamura M, Sugimoto S, Onuma H, Tokuka A. Long-term survival after resection of pancreatic ductal adenocarcinoma with para-aortic lymph node metastasis: case report. World J Surg Oncol. 2013;11:195. doi: 10.1186/1477-7819-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrikhande SV, Kleeff J, Reiser C, Weitz J, Hinz U, Esposito I, Schmidt J, Friess H, Büchler MW. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2007;14:118–127. doi: 10.1245/s10434-006-9131-8. [DOI] [PubMed] [Google Scholar]
- 31.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 32.Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, van Tienhoven G Dutch Pancreatic Cancer Group. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946–958. doi: 10.1002/bjs.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperti C, Gruppo M, Blandamura S, Valmasoni M, Pozza G, Passuello N, Beltrame V, Moletta L. Para-aortic node involvement is not an independent predictor of survival after resection for pancreatic cancer. World J Gastroenterol. 2017;23:4399–4406. doi: 10.3748/wjg.v23.i24.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada S, Nakao A, Fujii T, Sugimoto H, Kanazumi N, Nomoto S, Kodera Y, Takeda S. Pancreatic cancer with paraaortic lymph node metastasis: a contraindication for radical surgery? Pancreas. 2009;38:e13–e17. doi: 10.1097/MPA.0b013e3181889e2d. [DOI] [PubMed] [Google Scholar]
- 35.Gaedcke J, Gunawan B, Grade M, Szöke R, Liersch T, Becker H, Ghadimi BM. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg. 2010;395:451–458. doi: 10.1007/s00423-009-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai H, Doi R, Kanazawa H, Kamo N, Koizumi M, Masui T, Iwanaga Y, Kawaguchi Y, Takada Y, Isoda H, Uemoto S. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. Int J Clin Oncol. 2010;15:294–300. doi: 10.1007/s10147-010-0066-5. [DOI] [PubMed] [Google Scholar]
- 37.Asagi A, Ohta K, Nasu J, Tanada M, Nadano S, Nishimura R, Teramoto N, Yamamoto K, Inoue T, Iguchi H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas. 2013;42:11–19. doi: 10.1097/MPA.0b013e3182550d77. [DOI] [PubMed] [Google Scholar]
- 38.Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, Rinta-Kiikka I, Alanen K, Borra RJ, Puolakkainen PA, Nuutila P, Ovaska JT. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 39.Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, Jinnouchi S, Aikou T. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 40.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A, Feu F, Montanyà X, Fernández-Cruz L, Navarro S. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 41.Li JH, He R, Li YM, Cao G, Ma QY, Yang WB. Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: a meta-analysis. Dig Surg. 2014;31:297–305. doi: 10.1159/000368089. [DOI] [PubMed] [Google Scholar]
- 42.Kurita A, Kodama Y, Nakamoto Y, Isoda H, Minamiguchi S, Yoshimura K, Kuriyama K, Sawai Y, Uza N, Hatano E, Uemoto S, Togashi K, Haga H, Chiba T. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest Endosc. 2016;84:467–475.e1. doi: 10.1016/j.gie.2016.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original anonymous dataset is available upon request from the corresponding author.