Abstract
Objective:
We retrospectively compared the outcomes of patients with severe aplastic anemia (SAA) who received haploidentical hematopoietic stem cell transplantation (haplo-HSCT) combined or not combined with umbilical cord-derived mesenchymal stem cells (UC-MSCs).
Materials and Methods:
A total of 101 patients with SAA were enrolled in this study and treated with haplo-HSCT plus UC-MSC infusion (MSC group, n=47) or haplo-HSCT alone (non-MSC group, n=54).
Results:
The median time to neutrophil engraftment in the MSC and non-MSC group was 11 (range: 8-19) and 12 (range: 8-23) days, respectively (p=0.049), with a respective cumulative incidence (CI) of 97.82% and 97.96% (p=0.101). Compared to the non-MSC group, the MSC group had a lower CI of chronic graft-versus-host disease (GVHD) (8.60±0.25% vs. 24.57±0.48%, p=0.048), but similar rates of grades II-IV acute GVHD (23.40±0.39% vs. 24.49±0.39%, p=0.849), grades III-IV acute GVHD (8.51±0.17% vs. 10.20±0.19%, p=0.765), and moderate-severe chronic GVHD (2.38±0.06% vs. 7.45±0.18%, p=0.352) were observed. The estimated 5-year overall survival (OS) rates were 78.3±6.1% and 70.1±6.3% (p=0.292) while the estimated 5-year GVHD-free, failure-free survival (GFFS) rates were 76.6±6.2% and 56.7±6.9% (p=0.045) in the MSC and non-MSC groups, respectively.
Conclusion:
In multivariate analysis, graft failure was the only adverse predictor for OS. Meanwhile, graft failure, grades III-IV acute GVHD, and moderate-severe chronic GVHD could predict worse GFFS. Our results indicated that haplo-HSCT combined with UC-MSCs infusion was an effective and safe option for SAA patients.
Keywords: Severe aplastic anemia, Haploidentical, Hematopoietic stem cell transplantation, Mesenchymal stem cells
Abstract
Amaç:
Göbek kordonundan elde edilen mezenkimal kök hücrelerin (UK-MKH’ler) infüzyonu ile kombine edilmiş ve edilmemiş, haploidentik hematopoietik kök hücre nakli (haplo-HKHN) yapılan şiddetli aplastik anemili (ŞAA) hastalarının sonuçları geriye dönük olarak karşılaştırıldı.
Gereç ve Yöntemler:
Bu çalışmaya ŞAA’lı toplam 101 hasta alındı ve hastalar haplo-HSCT ile birlikte UK-MKH infüzyonu (MKH grubu, N=47) ve tek başına haplo-HKHN (MKH olmayan grup, N=54) şeklinde iki gruba ayrıldı.
Bulgular:
MKH alan ve almayan gruplarda nötrofil engraftmanı için medyan süreler sırasıyla, 11 (aralık, 8-19) ve 12 (aralık, 8-23) gün (p=0,049) ve kümülatif insidans (CI) sırası ile %97,82 ve %97,96 (p=0,101) bulundu. MKH almayan grupla karşılaştırıldığında, MKH alan grupta daha düşük kronik graft-versus-host hastalığı (cGVHD) CI saptandı (%8,60±0,25’e karşı %24,57±0,48, p=0,048), ancak II-IV akut GVHD (aGVHD) (%23,40±0,39-%24,49±0,39, p=0,849), III-IV aGVHD (%8,51±0,17 ve %10,20±0,19, p=0,765) ve orta-şiddetli cGVHD (%2,38±0,06 vs. %7,45±0,18, p=0,352) gruplarında fark görülmedi. MKH alan ve MKH almayan gruplarda tahmini beş yıllık genel sağkalım oranları %78,3±6,1 ve %70,1±6,3 (p=0,292) iken, GVHD’siz, hastalıksız sağkalım (GFFS) oranları sırasıyla, %76,6 ±%6,2 ve %56,7±6,9 (p=0,045) idi.
Sonuç:
Çok değişkenli analizde greft yetmezliği, OS için tek olumsuz gösterge idi. Bu arada, greft yetmezliği, III-IV aGVHD ve orta-şiddetli cGVHD arasında daha kötü GFFS’yi öngörebilir. Sonuçlarımız, UC-MKH infüzyonu ile birlikte haplo-HKHN’nin ŞAA hastaları için etkili ve güvenli bir seçenek olduğunu gösterdi.
Introduction
Severe aplastic anemia (SAA) is a rare, life-threatening bone marrow (BM) failure syndrome characterized by pancytopenia and hypoplastic bone marrow. The incidence is 2-2.4 per million per year in Europe but higher in China, affecting 7.4 patients per million [1].
Although hematopoietic stem cell transplantation (HSCT) from a human leukocyte antigen (HLA)-matched related donor (MRD) is the first choice for SAA patients who are <40 years old, it is difficult to search for HLA-matched siblings in China [2]. Immunosuppressive therapy (IST) with antithymocyte globulin (ATG) and cyclosporin A (CsA) is recommended as the first-line treatment for SAA patients who lack an appropriate MRD and those aged >40 years [3]. However, most patients cannot be cured by IST. Previous studies showed that response rates were in the range of 40%-60% and 25% of responders subsequently relapsed [4,5,6]. Therefore, the treatment of SAA patients who lack sibling donors or fail to respond to IST is extremely challenging.
Stem cells from haploidentical family donors have become the most common source of HSCT in China due to the advantage of immediate availability for almost any patient [7]. Haploidentical HSCT (haplo-HSCT) has greatly developed in recent years. With improvements in donor selection, conditioning regimens, and graft-versus-host disease (GVHD) prophylaxis, studies comparing haplo-HSCT with MRD-HSCT in young patients have revealed limited differences in overall survival (OS) [6,8,9]. However, the higher incidence of graft failure (GF) and GVHD has limited the clinical application of haplo-HSCT for SAA patients [6,10,11,12].
Mesenchymal stem cells (MSCs) have been shown to support hematopoiesis and display potent immunomodulatory properties for treating GVHD after HSCT [13,14]. It was reported that human umbilical cord-derived MSCs (UC-MSCs) have higher activity levels of proliferation and differentiation in comparison with bone marrow-derived MSCs (BM-MSCs) [11]. Thus, UC-MSCs are highly promising for use in haplo-HSCT. In recent years, some studies have reported the co-transplantation of haplo-HSCT and UC-MSCs with favorable outcomes in cases of SAA [11,12,15,16]. To the best of our knowledge, there are still no head-to-head studies exploring the efficiency and safety of haplo-HSCT with and without UC-MSCs. In the present study, we report the outcomes of haplo-HSCT with or without third-party UC-MSCs for the treatment of SAA patients in our centers from June 2014 to June 2021.
Materials and Methods
Patients
Between June 2014 and June 2021, 101 consecutive SAA patients who underwent haplo-HSCT in our transplant unit were enrolled in this study. Informed consent was obtained from all patients or their parents and the study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University. All patients met the following inclusion criteria: (1) diagnosed with SAA or very severe aplastic anemiaSAA according to the guidelines of the International Aplastic Anemia Study Group; (2) no HLA-identical sibling donor; (3) no serious infectious disease or acute hemorrhaging; (4) absence of severe liver, renal, lung, and heart diseases; (5) Eastern Cooperative Oncology Group score of 0-2 points. HLA compatibility was determined by high-resolution DNA techniques for the HLA-A, B, C, DRB1, and DQB1 loci. Donors were ranked on the basis of HLA match, age (younger preferred), gender (same preferred), and health status (better preferred).
Conditioning Regimen
Patients who underwent haplo-HSCT were placed on regimens including fludarabine (Flu)/cyclophosphamide (Cy)/ATG or Bu/Cy/ATG. Patients with acute SAA received the following regimen: intravenous fFlu at 30 mg/m2/day on days -5 and -2; intravenous c (Cy) at 50 mg/kg/day on days -5 to -2; and intravenous ATG (rabbit, Genzyme Polyclonals SAS, Lyon, France) at 2.5 mg/kg/day on days -5 to -2. For patients with chronic SAA (SAA-II), or, in other words, patients who had progressed to SAA from non-SAA [17], the same protocol for Cy and ATG was applied as above with the addition of busulfan (Bu) at 3.2 mg/kg/day from day -7 to -6.
All patients underwent testing for donor-specific anti-HLA antibodies (DSA) before transplantation. If the DSA results were positive (MFI >5000), the patient received rituximab (once a week, 4 times) and plasmapheresis (2-3 times). The DSA results were then reviewed again and transplantation occurred if the value dropped below 5000. Among the 101 patients enrolled in this study, 5 patients had positive DSA results. After treatment, all of these patients had negative DSA results.
Stem Cell Collection and Infusion
All donors were injected with granulocyte colony-stimulating factor (G-CSF) subcutaneously at 10 µg/kg/day for 4-5 continuous days. BM grafts were collected by BM aspiration in an operating room on day +1. The target volume was 10 to 20 mL/kg of the recipient’s weight. In cases of ABO incompatibility, red blood cells were removed by sedimentation with Hespan. Peripheral blood stem cells (PBSCs) were collected with a COBE Spectra device (Gambro BCT, Lakewood, CO, USA) on day +2. The target mononuclear cell) count from BM and peripheral blood was ≥5x108/kg and the target CD34+ cell count was ≥2x106/kg of the recipient’s weight. An additional collection of PBSCs was performed on day +3 if the initial cell numbers were insufficient. UC-MSCs were purchased from the Shanghai Cord Blood Bank. A total of 1x106/kg UC-MSCs was infused 4 h before the infusion of the graft on day +1.
GVHD Prophylaxis
In the protocol applied for these patients, prophylaxis for acute GVHD (aGVHD) included CsA, mycophenolate mofetil (MMF), and short-term methotrexate (MTX). CsA was administrated intravenously twice daily at a dose of 1.5 mg/kg/day from day -7 until bowel function returned to normal, at which time patients received oral CsA. A target trough blood concentration of 200-300 ng/mL was maintained for ≥1 year after haplo-HSCT. CsA was gradually tapered thereafter and was withdrawn completely in the following 2-3 months. MMF was given orally at 500 mg every 12 h (250 mg for children) from day -7 to +30 and subsequently at 250 mg from day +30 to +90. MTX was administrated intravenously at a dose of 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11. If GVHD occurred in any organ, CsA and MMF were continued and adjusted to therapeutic concentrations.
Definitions and Post-transplantation Evaluations
Neutrophil engraftment was defined as occurring on the first of 3 consecutive days with an absolute neutrophil count of >0.5x109/L. Platelet engraftment was defined as occurring on the first day of a platelet count of >20x109/L without transfusion for 7 consecutive days. Short-tandem repeat polymerase chain reaction of peripheral whole blood was performed monthly to assess hematopoietic chimerism from the time of neutrophil recovery.
Primary GF (PGF) was defined as the failure to achieve neutrophil engraftment before day +28 after transplantation. Secondary GF was defined as graft loss after initial engraftment, with the recipient experiencing pancytopenia and hypocellular BM without moderate to severe aGVHD [15]. Delayed platelet recovery was defined as platelet engraftment achieved after day +28. aGVHD was defined according to the criteria proposed by the 1994 Consensus Conference on aGVHD Grading [18], while chronic GVHD (cGVHD) was defined according to the National Institutes of Health Consensus Conference on cGVHD [19].
Diagnoses of cytomegalovirus (CMV) infection, CMV pneumonia, Epstein-Barr virus (EBV) infection, and EBV-associated post-transplant lymphoproliferative disorders (PTLDs) were based on standard clinical criteria.
OS was defined as the time from the date of haplo-HSCT to the date of death or last follow-up. GVHD-free, failure-free survival (GFFS) was defined as survival without grades III-IV aGVHD, moderate-severe cGVHD, or HSCT failure. Death, primary or secondary GF, and relapse were considered as HSCT failures.
Supportive Care
All patients were admitted to class 100 laminar flow clean wards after a skin-care bath. Patients received oral antibiotics (levofloxacin for adults, gentamycin for children) for gastrointestinal decontamination before transplantation. Fluconazole, acyclovir, and trimethoprim/sulfamethoxazole were administered to prevent fungal, viral, and Pneumocystis pneumonia infection, respectively. Heparin and prostaglandin E1 were administrated to prevent veno-occlusive disease. Human immunoglobulin was administered intravenously at a dose of 10 g once a week from day 0 to day +100 after the transplantation.
Recombinant human G-CSF was given at a dose of 5 µg/kg/day from day +3 until neutrophil recovery. Irradiated erythrocytes and platelets were given to maintain a hemoglobin level of >60 g/L and a platelet count of >20x109/L.
Statistical Analysis
The date of last follow-up for all surviving patients was December 31, 2021. Patient baseline characteristics were compared by chi-square test for categorical variables and by Mann-Whitney U and Kruskal-Wallis tests for continuous data. OS and GFFS were calculated using the Kaplan-Meier method and the groups were compared using the log-rank test. The cumulative incidence (CI) method was used to calculate the incidence of aGVHD and cGVHD with death and GF as competing risks according to the competing risk model. We used univariate and multivariate analyses to determine whether any of the selected factors were predictive of OS and GFFS. In multivariate analysis, all factors with values of p<0.1 in univariate analysis were included in the Cox regression model. Values of p<0.05 were considered to be significant. SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
Results
Characteristics of Patients and Donors
SAA patients undergoing haplo-HSCT with UC-MSC infusion (MSC group, n=47) or without UC-MSC infusion (non-MSC group, n=54) were enrolled in this study. Characteristics of the patients and their donors were compared between groups as shown in Table 1. The two cohorts were similar with regard to age distribution, sex ratio, disease severity, interval between diagnosis and transplantation, and previous treatments. No differences were found in the baseline characteristics of donors and grafts between the two groups.
Table 1. Characteristics of patients with severe anaplastic anemia, their donors, and grafts.

Engraftment
Forty-seven patients (100%) in the MSC group and 49 patients (90.74%) in the non-MSC group survived for 28 days. The five patients who experienced early mortality before engraftment were not considered in the subsequent analysis. One patient in each group experienced PGF. Two patients died suddenly before a second HSCT could be performed. The CIs of 28-day neutrophil engraftment were 97.82±0.06% and 97.96±0.06% in the MSC and non-MSC groups, respectively (p=0.101). The median times to neutrophil engraftment were 11 (range: 8-19) and 12 (range: 8-23) days, respectively (p=0.049). Forty-three and 48 patients achieved platelet engraftment in the MSC and non-MSC groups, respectively, with respective CIs of platelet engraftment of 91.49±0.19% and 91.84±0.16% (p=0.345). The median times to platelet engraftment were 12 (range: 7-43) and 14 (range: 6-70) days, respectively (p=0.047). In the MSC group, 2 patients (4.26%) experienced secondary GF and 3 patients (6.38%) had delayed platelet recovery. In the non-MSC group, 3 (6.12%) and 6 (12.24%) patients experienced secondary GF and delayed platelet recovery, respectively. The patients who experienced secondary GF subsequently died due to infection or cerebral hemorrhage. No significant differences were observed between groups in terms of PGF, secondary GF, or delayed platelet recovery (p>0.05, Table 2).
Table 2. Comparison of clinical outcomes between the groups after haploidentical hematopoietic stem cell transplantation.

GVHD
Table 2 presents the incidence and severity of GVHD for both groups. Patients with primary engraftment were included in the aGVHD analysis. In the MSC group, 17 patients experienced aGVHD after HSCT. These included 6 patients with grade I, 7 with grade II, 3 with grade III, and 1 with grade IV aGVHD. Of the 49 patients in the non-MSC groups, 21 experienced aGVHD after HSCT, including 9 with grade I, 7 with grade II, 4 with grade III, and 1 with grade IV. At 100 days after transplantation, the CIs of grade II-IV aGVHD for the MSC and non-MSC groups were 23.40±0.39% and 24.49±0.39%, respectively (p=0.849, Figure 1a). The CIs of grade III-IV aGVHD in the MSC and non-MSC groups were 8.51±0.17% and 10.20±0.19%, respectively (p=0.765, Figure 1b).
Figure 1.
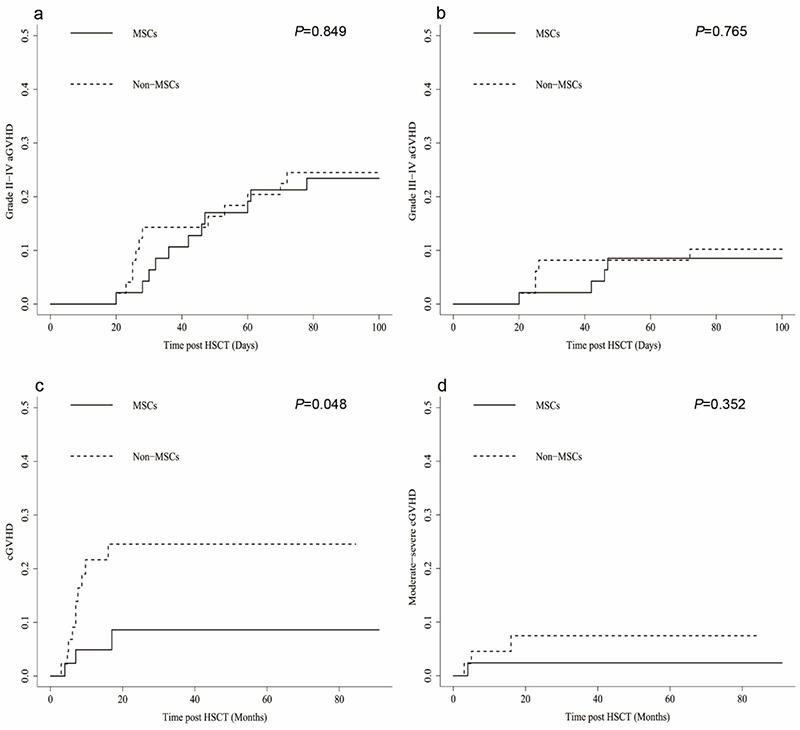
Cumulative incidences of graft-versus-host disease (GVHD) for the two groups. a) Cumulative incidences of grade II-IV acute GVHD (aGVHD): mesenchymal stem cell (MSC) group 23.40±0.39% vs. non-MSC group 24.49±0.39% (p=0.849). b) Cumulative incidences of grade III-IV aGVHD: MSC group 8.51±0.17% vs. non-MSC group 10.20±0.19% (p=0.765). c) Cumulative incidences of chronic GVHD (cGVHD): MSC group 8.60±0.25% vs. non-MSC group 24.57±0.48% (p=0.048). d) Cumulative incidences of moderate-severe cGVHD: MSC group 2.38±0.06% vs. non-MSC group 7.45±0.18% (p=0.352).
HSCT: Hematopoietic stem cell transplantation.
Forty-two patients in the MSC group and 44 in the non-MSC group with survival longer than 100 days after transplantation were assessed for cGVHD. In the MSC group, 2 patients had mild cGVHD, 2 had moderate cGVHD, and none had severe cGVHD. In the non-MSC group, 7 patients had mild cGVHD, 2 had moderate cGVHD, and 1 had severe cGVHD. Patients in the MSC group had a lower CI of cGVHD than the non-MSC group (8.60±0.25% vs. 24.57±0.48%, p=0.048, Figure 1c). However, there was no significant difference in the CIs of moderate-severe cGVHD between the two groups (2.38±0.06% vs. 7.45±0.18%, p=0.352, Figure 1d).
Infectious Complications
The most common infections after transplantation were the reactivation of CMV and EBV, pulmonary infections, septicemia, and herpes zoster (Table 2).
Fourteen of the 47 patients (29.8%) in the MSC group experienced CMV reactivation following transplantation as detected by DNA testing. In the non-MSC group, 15 of 54 patients (27.8%) experienced CMV reactivation. There was no significant difference in CMV reactivation between the two groups (p=0.824, Table 2). Viral infections were treated with foscarnet or ganciclovir and gamma globulin. In the non-MSC group, one patient developed CMV retinitis and recovered with an infusion of CMV-specific cytotoxic T lymphocytes. Other patients who did not develop CMV disease were mostly cured after antiviral treatment.
As shown in Table 2, 31 patients in the MSC group (66.0%) and 30 patients in the non-MSC group (55.6%) experienced EBV reactivation (p=0.286). Nineteen patients (10 in the MSC group and 9 in the non-MSC group) who were not successfully treated with antiviral drugs were treated with rituximab. EBV copy numbers decreased to the normal range in most cases. However, one patient in the non-MSC group only recovered with EBV-specific cytotoxic T lymphocytes. Three patients (6.4%) and 4 patients (7.4%) progressed to EBV-associated PTLD, respectively, in the MSC and non-MSC groups (p=1.000). All patients with PTLD received rituximab treatment, and some patients received it in combination with chemotherapy. Three patients with PTLD in the non-MSC group died of complications resulting from immunochemotherapy on day +274, day +205, and day +134, respectively. Others recovered from PTLD with the regression of enlarged lymph nodes and EBV copy numbers that declined to normal values.
In the MSC group, 14 patients (29.8%) experienced pulmonary infections, 7 patients (14.9%) experienced septicemia, and 2 patients (4.3%) experienced herpes zoster. In the non-MSC group, 16 patients (29.6%) experienced pulmonary infections, 11 patients (20.4%) experienced septicemia, and 10 patients (18.5%) experienced herpes zoster. The incidence of herpes zoster was higher in the non-MSC group than the MSC group (p=0.027), but there was no significant difference in the incidence of pneumonia or sepsis between the groups (p>0.05, Table 2).
Transplantation-Related Mortality
During a median follow-up period of 29.5 (range: 6.5-91) months, 10 and 14 patients died of transplantation-related mortality (TRM) in the MSC and non-MSC groups, respectively, with a median time to death of 91 (range: 31-270) and 76 (range: 1-274) days (p=0.334). The CIs of TRM in the MSC and non-MSC groups were 21.7±6.1% and 27.2±6.2% (p=0.424, Figure 2). Severe infection was the primary cause of death in both groups (Table 2). In the MSC group, 4 patients died of infection (3 pulmonary infections and 1 case of septicemia), 3 died of GVHD (1 severe aGVHD and 2 severe cGVHD), 2 of GF (1 PGF and 1 secondary GF), and 1 of thrombotic microangiopathy. In the non-MSC group, the causes of TRM included infection in 5 cases (4 pulmonary infections and 1 case of septicemia), GF in 3 cases (1 PGF and 2 secondary GF), regimen-related toxicities in 3 cases, GVHD in 2 cases (1 each of severe aGVHD and cGVHD), and leukoencephalopathy syndrome in 1 case.
Figure 2.
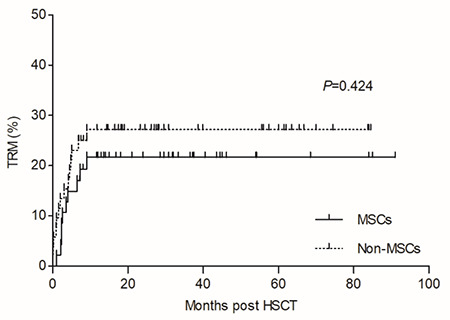
Cumulative incidences of transplantation-related mortality (TRM) during follow-up in the mesenchymal stem cell (MSC) group and non-MSC group were 21.7±6.1% and 27.2±6.2%, respectively (p=0.424).
HSCT: Hematopoietic stem cell transplantation.
Survival
The estimated 5-year OS rates in the MSC and non-MSC groups were 78.3±6.1% and 70.1±6.3%, respectively, and the difference was not significant (p=0.292, Figure 3a). However, the estimated 5-year GFFS rate in the MSC group was significantly higher than that of the non-MSC group (76.6±6.2% vs. 56.7±6.9%, respectively, p=0.045) (Figure 3b). In univariate analysis, age, disease severity, donor-recipient relationship, graft type, and GF significantly predicted OS and GFFS (Tables 3 and 4). Meanwhile, grade II-IV aGVHD, grade III-IV aGVHD, and moderate-severe cGVHD also predicted worse GFFS (Table 4). In multivariate Cox regression analysis, only GF was found to be predictive of worse OS (p=0.000, Table 3), while GF, grade III-IV aGVHD, and moderate-severe cGVHD were significantly associated with lower GFFS (p<0.05, Table 4).
Figure 3.
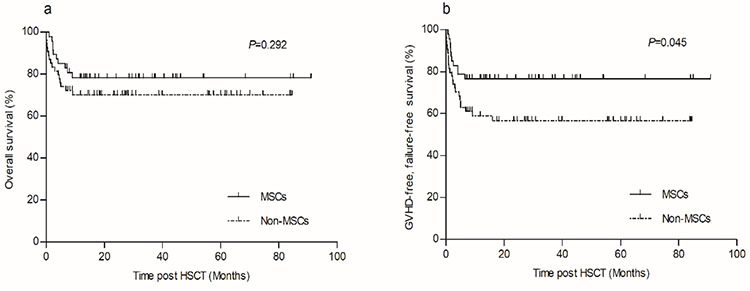
Overall survival and graft-versus-host disease (GVHD)-free, failure-free survival of the two groups. a) Comparison of estimated 5-year overall survival between mesenchymal stem cell (MSC) group and non-MSC group (78.3±6.1% vs. 70.1±6.3%, p=0.292). b) Comparison of estimated 5-year GVHD-free, failure-free survival between MSC group and non-MSC group (76.6±6.2% vs. 56.7±6.9%, p=0.045).
HSCT: Hematopoietic stem cell transplantation.
Table 3. Univariate and multivariate analysis of factors associated with overall survival.

Table 4. Univariate and multivariate analysis of factors associated with graft-versus-host disease-free, failure-free survival.

Discussion
Haplo-HSCT is usually considered as a salvage treatment option for SAA patients after failed IST. In recent years, some studies have evaluated haplo-HSCT as an upfront therapy for newly diagnosed SAA patients [6,20,21]. The currently available data reflect a favorable survival outcome of haplo-HSCT in China. However, higher incidences of GF and GVHD have limited its clinical applications [7]. Numerous trials have been undertaken to overcome these challenges, including the co-transplantation of MSCs [13,22,23,24]. MSCs possess the capacity to differentiate into various types of cells, modulate immune response, and support hematopoiesis. They are currently widely used in cases of hematological disease, and especially in treating aplastic anemia and GF, promoting HSC engraftment, and preventing and suppressing GVHD [25]. Given the encouraging results, some researchers have tried co-transplanting MSCs with HSCs in haplo-HSCT for SAA patients [9,12,13,14,26,27]. Due to the rarity of SAA, however, there are still no trials directly evaluating the role of MSCs in haplo-HSCT for SAA patients. To the best of our knowledge, we present the first prospective, head-to-head study comparing the efficacy and safety of haplo-HSCT combined with or not combined with MSC infusion. Our results have demonstrated that co-transplantation of MSC could shorten the engraftment time, reduce the incidence of cGVHD, and extended the GFFS.
GF is always a major risk of HSCT in cases of SAA, occurring more frequently in these cases than in other malignant diseases. In earlier years, the GF rate was as high as 70% [28]. In recent years, the GF rate has significantly fallen to less than 10% due to improved conditioning regimens, the use of G-CSF-mobilized grafts, and cell therapy strategies such as cord-blood units and MSC infusions [27,29,30,31,32]. MSCs have been shown to support hematopoiesis in vitro and in vivo [25]. Some researchers combined haplo-HSCT with MSC infusions in a series of single-arm trials, and the promising results included engraftment rates ranging from 93.2% to 98.9% [9,11,12,13,14,15]. Although the current incidence of engraftment appears to be higher than the historically reported values, there are no head-to-head studies to date to confirm this. Our results showed no statistical differences in the CIs of neutrophil engraftment (97.82±0.06% vs. 97.96±0.06%, p=0.101) or platelet engraftment (91.49±0.19% vs. 91.84±0.16%, p=0.345) in the MSC and non-MSC groups. However, patients in the MSC group experienced faster neutrophil engraftment (11 (range: 8-19) vs. 12 (range: 8-23) days, p=0.049) and platelet engraftment (12 (range: 7-43) vs. 14 (range: 6-70) days, p=0.047) compared to those in the non-MSC group. Our results thus indicate that co-transplantation of MSCs could enhance engraftment in HSCT.
GVHD is another major challenge in haplo-HSCT for SAA. MSC applications in the field of GVHD treatment have achieved great success due to the immunoregulatory properties of these cells [33,34]. However, the efficacy of MSCs for GVHD prophylaxis varies in different reports [25,35]. In a phase II study, 37 patients with hematological malignant diseases were randomly divided into a standard GVHD prophylaxis group (Group 1) and a group receiving standard GVHD prophylaxis combined with MSCs (Group 2). Results showed that the incidence of grade II-IV aGVHD was lower in Group 2 compared to Group 1 (5.3% vs. 38.9%, p=0.002) [36]. There have been no controlled studies to date to confirm the efficacy of MSCs for GVHD prophylaxis in SAA patients, although some studies reported that co-transplantation of haplo-HSCs and MSCs in SAA patients was safe and effective [12,13,14,15]. The reported CIs of grade II-IV aGVHD, grade III-IV aGVHD, and cGVHD were previously found to be approximately 23.5%-29.3%, 4.9%-11.4%, and 18.2%-26.8%, respectively [12,13,14,15]. These results did not appear to differ significantly from the historical findings. On the other hand, a meta-analysis concluded that MSCs may make little or no difference for the risk of GVHD compared to treatment without MSCs in haplo-HSCT for SAA patients [16]. Our results showed that the CI of cGVHD in the MSC group was significantly lower compared to that of the non-MSC group (8.60±0.25% vs. 24.57±0.48%, p=0.048). However, there were no statistical differences in the CIs of grade II-IV aGVHD, grade III-IV aGVHD, or moderate-severe cGVHD between the two groups. Another phase II multicenter, randomized, double-blind controlled study similarly demonstrated that prophylactic infusion of MSCs after haplo-HSCT may reduce the incidence of cGVHD in hematological malignant diseases (27.4% vs. 49%, p=0.021) [37]. Our data and previous studies thus suggest that MSCs co-transplanted during HSCT decrease the incidence of cGVHD to some extent.
Infection is another major obstacle to survival, and particularly lethal viral infections. Accordingly, another issue of concern is whether MSCs increase the incidence of infection. In the past, some studies suggested that MSCs did increase the risk of infection by suppressing T-cell response and increasing the secretion of some proinflammatory cytokines [38,39]. However, our results showed that the incidences of all types of infections did not increase after MSC administration. The incidence of CMV and EBV reactivation in the MSC group was 29.8% and 66.6%, respectively, and these values were consistent with some recent studies [14,40]. However, other studies reported a higher risk of CMV reactivation (51.7% to 65.9%) and a lower risk of EBV reactivation (22.7% to 31.8%) [2,12,13], which seems to be contrary to our results. This may be related to different conditioning regimens and protocols for GVHD prophylaxis. No significant differences were observed between the MSC and non-MSC groups in the present study in terms of EBV-associated PTLD, pulmonary infections, septicemia, and other non-infection complications. Thus, our data indicate that MSC applications are safe.
The rate of TRM in the MSC group was 21.7%, comparable to the rate observed in the non-MSC group (27.2%, p=0.424). Infection, GF, and GVHD were the most common causes of death. We also observed comparable rates of OS between the two groups (78.3±6.1% vs. 70.1±6.3%, p=0.292), with GF being the main adverse factor. These findings were consistent with previous reports [12,31]. Surprisingly, we found that the estimated 5-year GFFS was significantly improved in the MSC group compared to the non-MSC group (76.6±6.2% vs. 56.7±6.9%, p=0.045). Possible explanations for this finding include the MSCs promoting faster engraftment to reduce the chance of infection or MSCs reducing the risk of cGVHD to improve the quality of life. GF, grade III-IV aGVHD, and moderate-severe cGVHD were the factors that predicted worse GFFS. These findings suggest the need to reduce GF and GVHD, which may improve survival outcomes. Co-transplantation of MSCs during HSCT is a good attempt in this direction, although some results were not satisfactory.
Our study has several limitations. First, certain shortcomings are inherent in retrospective, single-center studies. A prospective, multicenter, head-to-head clinical trial should be performed to confirm our results. Second, other factors that may affect engraftment and GVHD, such as variables of the patient populations and conditioning regimens, should be controlled in future studies. Third, many factors may influence the effects of MSC treatment, such as the source of the MSCs (BM-MSCs versus UC-MSCs), the frequency of MSC infusions (once, twice, or more), therapeutic schedules, and the timing of MSC administrations. All of these factors require more studies to explore their impacts on efficacy. Despite these limitations, however, our study offers remarkable comparative evidence of the value of the prophylactic use of MSCs in haplo-HSCT treatment for SAA patients.
Conclusion
The work presented here is the first head-to-head, retrospective study to date to explore the efficacy and safety of the co-transplantation of UC-MSCs during haplo-HSCT for the treatment of patients with SAA. The encouraging results suggest that infusion of MSCs after haplo-HSCT can promote engraftment, reduce the incidence of cGVHD, and improve GFFS. The prophylactic use of MSCs combined with haplo-HSCT was found to be an effective and safe method for the treatment of patients with SAA.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LY19H290003), the Zhejiang Provincial Medical and Health Science and Technology Project (Nos. 2020KY196, 2018277310), the Foundation of Zhejiang Province Chinese Medicine Science and Technology Planes (Nos. 2017ZB030, 2020ZA044), and the Key Project of the 2017 School Research Fund of Zhejiang Chinese Medical University (No. 2017ZZ02).
Footnotes
Ethics
Ethics Committee Approval: This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University.
Informed Consent: All patients signed informed consent forms in accordance with the Declaration of Helsinki.
Authorship Contributions
Surgical and Medical Practices: H-F.Z., X-F.S., H.L., L-L.H.; Concept: H-F.Z.; Design: H-F.Z.; Data Collection or Processing: H-F.Z., X-F.S., H.L., L-L.H.; Analysis or Interpretation: X-F.S., H.L., L-L.H.; Writing: X-F.S., H.L., L-L.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Zhu XF, He HL, Wang SQ, Tang JY, Han B, Zhang DH, Wu LQ, Wu DP, Li W, Xia LH, Zhu HL, Liu F, Shi HX, Zhang X, Zhou F, Hu JD, Fang JP, Chen XQ, Ye TZ, Liang YM, Jin J, Zhang FK. Current treatment patterns of aplastic anemia in China: a prospective cohort registry study. Acta Haematol. 2019;142:162–170. doi: 10.1159/000499065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ElGohary G, El Fakih R, de Latour R, Risitano A, Marsh J, Schrezenmeier H, Gluckman E, Hochsmann B, Pierri F, Halkes C, Alzahrani H, De la Fuente J, Cesaro S, Alahmari A, Ahmed SO, Passweg J, Dufour C, Bacigalupo A, Aljurf M. Haploidentical hematopoietic stem cell transplantation in aplastic anemia: a systematic review and meta-analysis of clinical outcome on behalf of the severe aplastic anemia working party of the European group for blood and marrow transplantation (SAAWP of EBMT) Bone Marrow Transplant. 2020;55:1906–1917. doi: 10.1038/s41409-020-0897-2. [DOI] [PubMed] [Google Scholar]
- 3.Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC; British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. doi: 10.1111/bjh.13853. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Giammarco S, Sica S. Bone marrow transplantation versus immunosuppressive therapy in patients with acquired severe aplastic anemia. Int J Hematol. 2016;104:168–174. doi: 10.1007/s12185-016-2037-8. [DOI] [PubMed] [Google Scholar]
- 5.Xu LP, Zhang XH, Wang FR, Mo XD, Han TT, Han W, Chen YH, Zhang YY, Wang JZ, Yan CH, Sun YQ, Zuo SN, Huang XJ. Haploidentical transplantation for pediatric patients with acquired severe aplastic anemia. Bone Marrow Transplant. 2017;52:381–387. doi: 10.1038/bmt.2016.281. [DOI] [PubMed] [Google Scholar]
- 6.Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, Liu QF, Wang JM, Wang X, Jiang M, Zhang X, Wu DP, Huang XJ. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10:25. doi: 10.1186/s13045-017-0398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye L, Zhang F, Kojima S. Current insights into the treatments of severe aplastic anemia in China. Int J Hematol. 2020;112:292–299. doi: 10.1007/s12185-020-02955-1. [DOI] [PubMed] [Google Scholar]
- 8.Dufour C, Veys P, Carraro E, Bhatnagar N, Pillon M, Wynn R, Gibson B, Vora AJ, Steward CG, Ewins AM, Hough RE, de la Fuente J, Velangi M, Amrolia PJ, Skinner R, Bacigalupo A, Risitano AM, Socie G, Peffault de Latour R, Passweg J, Rovo A, Tichelli A, Schrezenmeier H, Hochsmann B, Bader P, van Biezen A, Aljurf MD, Kulasekararaj A, Marsh JC, Samarasinghe S. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br J Haematol. 2015;171:585–594. doi: 10.1111/bjh.13614. [DOI] [PubMed] [Google Scholar]
- 9.Yue C, Ding Y, Gao Y, Li L, Pang Y, Liu Z, Zhang H, Xiao Y, Jiang Z, Xiao H. Cotransplantation of haploidentical hematopoietic stem cells and allogeneic bone marrow-derived mesenchymal stromal cells as a first-line treatment in very severe aplastic anemia patients with refractory infections. Eur J Haematol. 2018;100:624–629. doi: 10.1111/ejh.13060. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Wang S, Wang J, Liu L, Su Y, Lu Z, Zhang X, Zhang Y, Zhong JF, Peng L, Liu Q, Lu Y, Gao L, Zhang X. Optimal donor for severe aplastic anemia patient requiring allogeneic hematopoietic stem cell transplantation: a large-sample study from China. Sci Rep. 2018;8:2479. doi: 10.1038/s41598-018-20853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Liu Z, Wu Y, Yang X, Cao Y, Li X, Yan B, Li S, Da W, Wu X. Clinical evaluation of haploidentical hematopoietic combined with human umbilical cord-derived mesenchymal stem cells in severe aplastic anemia. Eur J Med Res. 2018;23:12. doi: 10.1186/s40001-018-0311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Wu X, Wang S, Xia L, Xiao H, Li Y, Li H, Zhang Y, Xu D, Nie D, Lai Y, Wu B, Lin D, Du X, Jiang Z, Gao Y, Gu X, Xiao Y. Co-transplantation of mesenchymal stem cells makes haploidentical HSCT a potential comparable therapy with matched sibling donor HSCT for patients with severe aplastic anemia. Ther Adv Hematol. 2020;11:2040620720965411. doi: 10.1177/2040620720965411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Zhang Y, Xiao H, Yao Z, Zhang H, Liu Q, Wu B, Nie D, Li Y, Pang Y, Fan Z, Li L, Jiang Z, Duan F, Li H, Zhang P, Gao Y, Ouyang L, Yue C, Xie M, Shi C, Xiao Y, Wang S. Cotransplantation of bone marrow-derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone Marrow Transplant. 2017;52:704–710. doi: 10.1038/bmt.2016.347. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Yu H, Cao F, Liu Z, Liu Z, Feng W, Liu X, Yu Y, Xiao Y, Li L, Zhou J. Donor-derived marrow mesenchymal stromal cell co-transplantation following a haploidentical hematopoietic stem cell transplantation trail to treat severe aplastic anemia in children. Ann Hematol. 2019;98:473–479. doi: 10.1007/s00277-018-3523-2. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Han DM, Zheng XL, Yan HM, Xue M, Liu J, Zhu L, Li S, Mao N, Guo ZK, Ning HM, Wang HX, Zhu H. A study of human leukocyte antigen-haploidentical hematopoietic stem cells transplantation combined with allogenic mesenchymal stem cell infusion for treatment of severe aplastic anemia in pediatric and adolescent patients. Stem Cells Transl Med. 2021;10:291–302. doi: 10.1002/sctm.20-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Tu J, Zhao J, Pan H, Fang L, Shi J. Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia?-a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12:106. doi: 10.1186/s13287-021-02170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Q, Sha P, Chen H, Shen H, Qin L, Li Z, Wu T, Wang Z. Modified immunosuppressive therapy with porcine antilymphocyte globulin plus delayed cyclosporine A in children with severe aplastic anemia. Int J Hematol. 2018;107:64–68. doi: 10.1007/s12185-017-2321-2. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus- Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu ZL, Zhou M, Jia JS, Mo WJ, Zhang XH, Zhang YP, Wang Y, Li YM, Huang XJ, Wang SQ, Xu LP. Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia. Bone Marrow Transplant. 2019;54:1319–1326. doi: 10.1038/s41409-018-0410-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Guo Z, Liu XD, He XP, Yang K, Chen P, Chen HR. Comparison of haploidentical hematopoietic stem cell transplantation and immunosuppressive therapy for the treatment of acquired severe aplastic anemia in pediatric patients. Am J Ther. 2017;24:e196–e201. doi: 10.1097/MJT.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 22.Huang XJ. HLA-mismatched/haploidentical hematopoietic stem cell transplantation: a field in which Chinese doctors are making great contributions. Chin Med J (Engl) 2010;123:1235–1240. [PubMed] [Google Scholar]
- 23.Ciceri F, Lupo-Stanghellini MT, Korthof ET. Haploidentical transplantation in patients with acquired aplastic anemia. Bone Marrow Transplant. 2013;48:183–185. doi: 10.1038/bmt.2012.231. [DOI] [PubMed] [Google Scholar]
- 24.Liu LM, Zhang YM, Zhou HF, Wang QY, Qiu HY, Tang XW, Han Y, Fu CC, Jin ZM, Sun AN, Miao M, Wu DP. Outcome of combination of HLA-haploidentical hematopoietic SCT with an unrelated cord blood unit for 127 patients with acquired severe aplastic anemia. Zhonghua Xue Ye Xue Za Zhi. 2018;39:624–628. doi: 10.3760/cma.j.issn.0253-2727.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao K, Liu Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol. 2016;9:46. doi: 10.1186/s13045-016-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Wang Z, Xue M, Liu J, Yan H, Guo Z. Co-transfusion of haplo-identical hematopoietic and mesenchymal stromal cells to treat a patient with severe aplastic. Cytotherapy. 2010;12:563–565. doi: 10.3109/14653241003695059. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P, Yan P, Liu Z, Wang J, Jiang S, Wu X, Gao C, Da W, Han Z. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res. 2014;12:132–138. doi: 10.1016/j.scr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Wagner JL, Deeg HJ, Seidel K, Anasetti C, Doney K, Sanders J, Sullivan KM, Storb R. Bone marrow transplantation for severe aplastic anemia from genotypically HLA-nonidentical relatives. An update of the Seattle experience. Transplantation. 1996;61:54–61. doi: 10.1097/00007890-199601150-00012. [DOI] [PubMed] [Google Scholar]
- 29.Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, Zhang XH, Wang Y, Wang FR, Wang JZ, Huang XJ. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant. 2012;47:1507–1512. doi: 10.1038/bmt.2012.79. [DOI] [PubMed] [Google Scholar]
- 30.Xu LP, Wang SQ, Wu DP, Wang JM, Gao SJ, Jiang M, Wang CB, Zhang X, Liu QF, Xia LH, Wang X, Huang XJ. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematol. 2016;175:265–274. doi: 10.1111/bjh.14225. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Duan F, Xiao H, Wu X, Wang S, Xu D, Liu Q, Fan Z, Nie D, Lai Y, Wu B, Lin D, Du X, Weng J, Jiang Z, Pang Y, Ouyang L, Liu Z, Zhang L, Han N, Chen L, Xiao Y. Therapeutic outcomes of haploidentical allogeneic hematopoietic stem cell transplantation in patients with severe aplastic anemia: a multicenter study. Transplantation. 2018;102:1724–1731. doi: 10.1097/TP.0000000000002200. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Qiu H, Tang X, Han Y, Fu C, Jin Z, Chen S, Sun A, Miao M, Wu D. Combination of haploidentical haematopoietic stem cell transplantation with an unrelated cord-blood unit in patients with severe aplastic anemia: a report of 146 cases. Bone Marrow Transplant. 2020;55:2017–2025. doi: 10.1038/s41409-020-0874-9. [DOI] [PubMed] [Google Scholar]
- 33.Kuzmina LA, Petinati NA, Vasilieva VA, Dovydenko MV, Drokov MY, Davydova YO, Kapranov NM, Sats NV, Chabaeva YA, Kulikov SM, Gaponova TV, Drize NI, Parovichnikova EN, Savchenko VG. Multipotent mesenchymal stromal cells application for acute graft versus host disease treatment. Ter Arkh. 2020;92:23–30. doi: 10.26442/00403660.2020.07.000757. [DOI] [PubMed] [Google Scholar]
- 34.Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, Nemecek E, Neudorf S, Prasad V, Prockop S, Quigg T, Satwani P, Cheng A, Burke E, Hayes J, Skerrett D; MSB-GVHD001/002 Study Group. A phase 3, single-arm, prospective study of remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26:845–854. doi: 10.1016/j.bbmt.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L, Chen S, Yang P, Cao H, Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10:182. doi: 10.1186/s13287-019-1287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, Svinareva DA, Drize NJ, Savchenko VG. Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease-a phase II study. Stem Cells Int. 2012;2012:968213. doi: 10.1155/2012/968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, Su Y, Yang T, Li H, Liu Y, Zhang C, Gao L, Zhu L, Wen Q, Wang P, Chen X, Zhong J, Zhang X. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol. 2016;34:2843–2850. doi: 10.1200/JCO.2015.65.3642. [DOI] [PubMed] [Google Scholar]
- 38.Kogler G, Radke TF, Lefort A, Sensken S, Fischer J, Sorg RV, Wernet P. Cytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cells. Exp Hematol. 2005;33:573–583. doi: 10.1016/j.exphem.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Forslow U, Blennow O, LeBlanc K, Ringden O, Gustafsson B, Mattsson J, Remberger M. Treatment with mesenchymal stromal cells is a risk factor for pneumonia-related death after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2012;89:220–227. doi: 10.1111/j.1600-0609.2012.01824.x. [DOI] [PubMed] [Google Scholar]
- 40.Zu Y, Zhou J, Fu Y, Fang B, Liu X, Zhang Y, Yu F, Zuo W, Zhou H, Gui R, Li Z, Liu Y, Zhao H, Zhang C, Song Y. Feasibility of reduced-dose posttransplant cyclophosphamide and cotransplantation of peripheral blood stem cells and umbilical cord-derived mesenchymal stem cells for SAA. Sci Rep. 2021;11:253. doi: 10.1038/s41598-020-80531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


