Abstract
Adventitious shoot (AS) regeneration accelerates plant reproduction and genetic transformation. WOX11 is involved in many biological processes, but its regulation of AS regeneration has not been reported. Here, we showed that the genotype and CK/IAA ratio of apple leaves were the key factors that affected their capacity for AS formation. Moreover, the expression level of MdWOX11 was negatively correlated with the capacity for AS formation. Phenotypic analysis of MdWOX11 transgenic plants showed that overexpression of MdWOX11 inhibited AS formation. Endogenous hormone analysis demonstrated that the contents of auxin (IAA), cytokinin (CK), and abscisic acid (ABA) were higher in MdWOX11-RNAi plants than in MdWOX11-OE transgenic plants. We used RNA sequencing to examine the transcriptional responses of genes in MdWOX11-RNAi and MdWOX11-OE transgenic apple plants at different AS stages. We identified 8066 differentially expressed genes and focused our analysis on those involved in the IAA, CK, ABA, and gibberellin (GA) hormone signaling pathways. The expression of genes related to the CK signaling pathway and shoot development was higher in GL-3 than in MdWOX11-OE transgenic plants during the callus and AS emergence stages. However, the expression of MdCKX5 was higher in MdWOX11-OE transgenic plants than in GL3 and MdWOX11-RNAi transgenic plants. Yeast one-hybrid (Y1H) assays, dual-luciferase reporter assays, and ChIP-qPCR showed that MdWOX11 binds to the promoter of MdCKX5, and a dual-luciferase reporter assay showed that MdWOX11 enhanced the promoter activity of MdCKX5. We concluded that MdCKX5 acts downstream of MdWOX11 to control AS formation, and we built a regulatory model of the suppression of AS formation by MdWOX11 in apple.
Introduction
Plant regeneration refers to the process by which cells, tissues, or structures that have been damaged or partly subjected to environmental stress respond by self-repair or structural replacement [1]. The acquisition of regeneration ability depends on the totipotency or pluripotency of plant cells and helps to ensure the developmental plasticity of the plant. Adventitious shoot (AS) regeneration is not only an effective approach for rapid plant propagation but also provides an experimental system for studies of plant development and genetic transformation. The AS developmental process has four main stages: the acquisition of cell pluripotency, the formation of bud primary meristematic tissue, the establishment of restricted bud primordium cells, and the germination and elongation of the bud [2]. To date, the capacity to regenerate an entire plant from AS organs through in vitro culture has been reported in various plants, including Arabidopsis [3], tomato [4], poplar [5], and others. Dependable processes for AS formation have been developed for model plants, but methods of improving AS formation in apple remain to be studied.
Apple (Malus domestica) is a major commercial fruit tree cultivated worldwide. Apple genetic transformation provides a new approach for apple breeding; the breeding cycle can be shortened and breeding efficiency improved by combining genetic transformation technology with conventional breeding. The leaf disc method is used for apple genetic transformation, meaning that regenerated buds must be obtained from the leaf. Therefore, establishing a complete and efficient in vitro leaf regeneration system is a prerequisite for successful genetic transformation. AS regeneration in apples is affected by multiple factors, including genotype, explant maturity, medium, hormone concentration, culture conditions, and so on. Genotype is the decisive factor for the regeneration of explants, and genotypes differ significantly in regeneration efficiency under identical culture conditions [6]. Nonetheless, the AS regeneration ability of apple leaves from different genotypes requires further clarification.
It is well known that a high ratio of auxin (IAA) to cytokinin (CK) promotes root regeneration, whereas a high ratio of CK to IAA promotes bud regeneration [7]. In general, AS formation proceeds in two sequential steps: first, callus formation is promoted by culture on callus induction medium (CIM) with a high IAA level, and then calli are transferred to shoot induction medium (SIM) with a high CK level to produce AS. IAA and CK are the two most important hormones that influence AS formation [8]. Studies have found that the size of the shoot apical meristem (SAM) and the formation of primordial organ units are negatively affected in CK-deficient plants, providing direct evidence that CK enhances SAM activity [9]. Exogenous gibberellin (GA) has been found to reduce the formation of buds, whereas the GA biosynthesis inhibitor paclobutrazol can improve the bud regeneration rate [10]. In addition, abscisic acid (ABA) promotes shoot regeneration in Arabidopsis [11]. Nonetheless, the role of these hormones in the AS regeneration of apples has not been widely studied.
Some studies have shown that bud regeneration depends on the induction of WUS and CLV3 expression in the callus [12]. In addition, WUSCHEL-RELATED HOMEOBOX GENE 5 (WOX5) expression is inhibited during bud regeneration [13]. WUS, WOX5, and WOX11 all belong to the WOX family; whether WOX11 regulates AS regeneration has not been reported. Most research on WOX11 has focused on the regulation of adventitious root formation. For example, WOX11 promotes crown root development and de novo root organogenesis in rice and Arabidopsis [14, 15]; it also participates in crown shoot emergence and development [16]. The WOX11–LBD16 pathway promotes pluripotency acquisition in Arabidopsis callus cells [17]. However, the regulatory mechanism by which WOX11 influences AS development in woody plants is poorly understood.
Hormone-related genes are also involved in AS formation. Some studies have demonstrated that IAA-related genes such as auxin response factors (ARFs), YUCCA, and GH3 participate in AS regeneration. ARF5 may influence callus bud formation through the transcriptional activation of STM and CRF2 [18]. Transgenic plants overexpressing ARF10 showed a higher level of bud regeneration than wild-type explants, and this was accompanied by high expression levels of CLV3, CUCs, and WUS [19, 20]. High expression levels of the IAA synthesis-related gene YUCCA promote AS regeneration ability [21], and this ability was significantly inhibited in the pin1 mutant [12]. In apple, the overexpression of MsGH3.5 inhibits shoot development through the IAA and CK pathways [22]. Many components of CK biosynthesis and signal transduction pathways are considered to be key drivers of AS regeneration. AHK2, AHK3, and AHK4 encode CK-receptor histidine kinases that play an active role in AS generation. Deletion of AHK2, AHK3, and AHK4 genes in mutants caused a decrease in AS regeneration ability, even when the mutants were induced on a medium with high CK levels [23, 24]. The CK oxidase gene CKX5 is expressed in the meristem [25], and CKX1-overexpressing plants exhibit slow shoot growth and lower CK content [26]. A-ARR genes negatively regulate the CK signaling pathway, and AS regeneration ability is improved in an A-ARR mutant [27]. The B-ARR genes play a positive regulatory role in the CK pathway [28, 29], and B-ARR genes regulate WUS expression during shoot regeneration in Arabidopsis [30]. How CK-related genes regulate AS regeneration in apples remains to be studied.
At present, the mechanism by which MdWOX11 regulates AS formation in apples is not well characterized. In the present study, we show that there are differences in the capacity for AS formation among apple leaves of different genotypes and that an appropriate CK/IAA ratio promotes AS formation. We further demonstrate that overexpression of MdWOX11 inhibits the emergence and development of AS, and we use RNA sequencing (RNA-seq) to document changes in gene expression associated with the overexpression and suppression of MdWOX11. Finally, we show that MdCKX5 acts downstream of MdWOX11 to control AS formation. This study sheds light on the mechanism by which MdWOX11 regulates AS formation and provides insight into the overall regulation of AS formation in apple rootstocks.
Results
AS regeneration in different apple rootstocks
To analyze the effect of genotype on AS formation, fully-opened leaves were selected from terminal microshoots of different apple rootstocks. Results showed that AS regeneration ability differed significantly among apple leaves of different rootstocks when a hormone combination of 2 mg·L−1 thidiazuron (TDZ) and 0.5 mg·L−1 1-naphthaleneacetic acid (NAA) was used. On average, 3.5 AS were produced from each leaf of Malus prunifolia (MP) stem cuttings, whereas 4.1 were produced from M26 leaves and 1.6 from SZ23 leaves. Under the same culture conditions, the AS regeneration number of MP was much higher than that of SZ23, but the regeneration ability of SZ23 was significantly higher than that of SH6. Overall, the AS regeneration abilities of the rootstocks were ranked as follows: M26 > MP > Malus zumi > T337 > SZ23 > SH6 (Figure 1a–g). We next analyzed the expression of MdWOX11 in these apple rootstocks when the leaves were cultured on regenerative medium and found that it was highest in SH6 and lowest in MP, which had high AS regeneration ability. The expression of MdWOX11 was therefore negatively correlated with AS regeneration ability (Figure 1h).
Figure 1.
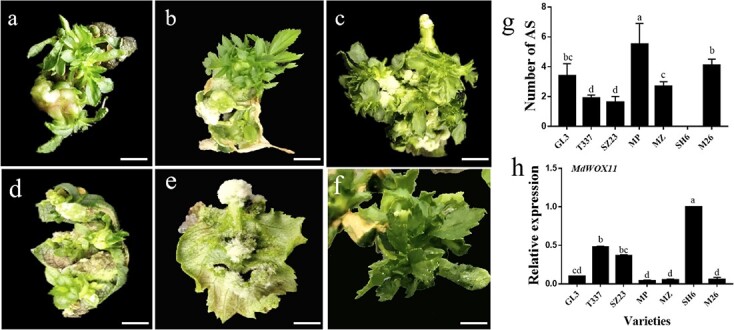
Morphological observations of the regeneration of adventitious shoots (AS) in six apple rootstocks. The hormone combination was TDZ 2 mg·L−1 and NAA 0.5 mg·L−1. a: T337; b: SZ23; c: Malus prunifolia (MP); d: Malus zumi (MZ); e: SH6; f: M26. Bar = 0.5 cm. g: the number of AS after 10 weeks of culture on leaves of six apple rootstocks and GL-3. h: Analysis of MdWOX11 expression in leaves of six apple rootstocks and GL-3. Values represent the mean ± SE of three biological replicates; letters indicate significant differences between means (P < 0.05).
AS regeneration in GL-3 and MdWOX11 transgenic plants
To analyze the function of MdWOX11 during AS formation, we obtained MdWOX11-OE and MdWOX11-RNAi transgenic plants by an Agrobacterium-mediated method. MdWOX11 expression in three OE and three RNAi lines is shown in Figure S1; interference with MdWOX11 inhibited the expression of MdWOX11 but not of MdWOX3, MdWOX8, and MdWOX9. MdWOX11OE-23# and MdWOX11RNAi-10# were selected for AS induction. The expression of MdWOX11 was higher in MdWOX11-OE transgenic plants than in GL-3 and MdWOX11-RNAi transgenic plants during AS formation (Figure S1). The AS regeneration phenotypes of GL-3 and the MdWOX11 transgenic lines are shown in Figure 2. When leaves from stem cuttings were cultured on AS induction medium for 10 weeks, GL-3 and MdWOX11 transgenic plants differed in their AS regeneration ability. The average number of regenerated AS per leaf and the AS increment coefficient for MdWOX11-RNAi transgenic plants were clearly higher than those for GL-3. However, no significant difference in AS regenerative efficiency was noted between them. By contrast, the AS regeneration ability of MdWOX11-OE transgenic lines was significantly lower than that of GL-3 and MdWOX11-RNAi plants. The average number of regenerated AS in MdWOX11-OE transgenic plants was lower than that in GL-3 and MdWOX11-RNAi plants. The AS regeneration frequency and AS increment coefficient were also significantly lower in MdWOX11-OE. These results indicated that MdWOX11 inhibited AS formation.
Figure 2.

Regeneration of adventitious shoots (AS) in GL-3 and the MdWOX11 transgenic plants after 10 weeks of culture. The phenotype of AS in a: GL-3; b: MdWOX11-OE; and c: MdWOX11-RNAi. Bar = 1 cm. d: AS increment coefficient; values represent the mean ± SE (n = 108). e: Number of AS per explant; values represent the mean ± SE (n = 9). f: AS regenerative efficiency; values represent the mean ± SE (n = 9). Significant differences were determined using the Student’s t test.
We next observed the anatomical and morphological features of AS formation in the leaves of wild-type GL-3 and MdWOX11 transgenic plants. The leaves were significantly enlarged after 3–7 days of AS-inducing culture, and a small amount of callus was visible. The callus became larger at approximately 2 weeks. At 21 d, most GL-3 and MdWOX11-RNAi transgenic leaves showed obvious white AS, but MdWOX11-OE leaves did not show AS formation at 21 d (Figure 3). Based on these initial observations, we chose to sample leaves for hormone content measurements and RNA-seq at three specific times of AS development: 0 d (T1), 15 d (T2), and 21 d (T3).
Figure 3.
Phenotypic and anatomical observations during the formation of adventitious shoots (AS) in the leaves of GL-3 and MdWOX11 transgenic plants (MdWOX11-OE and MdWOX11-RNAi) at 0, 7, 15, and 21 d. In the photographs, bar = 0.1 cm. In micrographs, the red arrow indicates the AS primordium.
Hormone levels and hormone ratios during AS formation in GL-3 and MdWOX11 transgenic plants
Samples were taken at three stages of AS development in GL-3 and MdWOX11 transgenic plants, and hormone levels were measured (Figure 4). The trends in hormone content differed between the two types of transgenic plants at different stages. At T1, zeatin riboside (ZR), IAA, GA, and ABA content were lower in GL-3 than in MdWOX11 transgenic lines, and ZR and ABA content were higher in MdWOX11-RNAi than in MdWOX11-OE transgenic lines. By contrast, IAA and GA content were lower in MdWOX11-RNAi than in MdWOX11-OE transgenic lines. At T2 and T3, IAA and GA content were ranked MdWOX11-RNAi > MdWOX11-OE > GL-3. The ZR, GA, and ABA contents decreased significantly in MdWOX11 transgenic plants from T1 to T2, and the contents of IAA and ABA rose from T2 to T3. The IAA/ZR, IAA/GA, ABA/IAA, and ABA/GA ratios were also calculated during AS formation. At T1, the IAA/ZR and IAA/GA ratios were lower in MdWOX11-OE than in MdWOX11-RNAi transgenic lines, and the ABA/IAA and ABA/GA ratios were higher in MdWOX11-RNAi than in MdWOX11-OE transgenic lines. At T2 and T3, the IAA/ZR, ABA/IAA, and ABA/GA ratios were higher in MdWOX11-RNAi than in MdWOX11-OE transgenic lines, but the IAA/GA ratio was lower in MdWOX11-RNAi. Moreover, the IAA/ZR, ABA/IAA, and ABA/GA ratios increased gradually during AS formation in MdWOX11-RNAi, and the IAA/ZR and IAA/GA ratios decreased from T1 to T2 in MdWOX11-OE (Figure 4).
Figure 4.
The contents and ratios of ZR, IAA, GA1 + 3, ABA, IAA/ZR, IAA/GA, ABA/IAA, and ABA/GA in GL-3 and MdWOX11 transgenic plants at different stages of AS development. Values represent the mean ± SE of three biological replicates, and letters indicate significant differences between means (P < 0.05).
Analysis of RNA-seq data and identification of DEGs
To identify genes specifically associated with AS formation, GL-3 and the transgenic lines were sampled at T1, T2, and T3 for RNA-seq analysis. A Venn diagram was constructed to show differentially expressed genes (DEGs) in MdWOX11-OE and MdWOX11-RNAi plants relative to GL-3 at three stages of AS development. At T1, there were 2858 and 2442 DEGs between GL-3 and MdWOX11-OE and MdWOX11-RNAi, respectively; these numbers were 2909 and 710 at T2, and 2079 and 1492 at T3 (Figure 5). In total, there were 3832, 3373, and 3311 DEGs between GL-3 and the transgenic lines at T1, T2, and T3 (8066 DEGs across all AS developmental stages). We performed enrichment analysis of the DEGs, and the results are shown in Supplemental Figure S2. A total of 138 enriched KEGG pathways were identified in the DEGs; these included plant hormone signal transduction, enzyme signal pathways, sugar metabolism–related pathways, and others. GO enrichment analysis of DEGs was also performed, and the results are presented in Supplemental Figure S3.
Figure 5.

Differentially expressed genes (DEGs) between GL-3 and MdWOX11 transgenic plants during AS regeneration. GL-3, MdWOX11-OE, and MdWOX11-RNAi leaves were cultured in shoot induction medium for 0 d (T1), 15 d (T2), and 21 d (T3).
Cluster analysis of the expression profiles of hormone-related genes (IAA, CK, GA, and ABA) in MdWOX11 transgenic plants during AS formation
Hormone–related genes were analyzed in the MdWOX11 transgenic plants during AS formation. Expression levels of the IAA transport–related genes MdAUX1, MdLAX2, and MdPIN5 were higher at T1 than at T2 and T3, and their expression levels were higher in MdWOX11-OE transgenic lines at T1. Expression levels of the IAA signal transduction–related genes MdIAA14 and MdARF1 were higher during T2 and T3, and their expression levels were higher in MdWOX11-OE transgenic lines than in GL-3 and MdWOX11-RNAi. Expression of MdARF1 was highest in MdWOX11-RNAi transgenic lines at T2 and T3, and its expression was more than five times higher in MdWOX11-RNAi transgenic lines than in GL-3 plants (Figure 6). Expression levels of the CK signal transduction–related genes MdAHP1 and MdARR16 were higher at T3; levels of MdARR9, MdCKX5, and MdIPT1 were higher at T2 and T3; and levels of MdAHP1, MdAHK1, and MdARR16 rose gradually during AS formation in GL-3. At T2 and T3, expression levels of MdAHP1 and MdIPT1 were higher in GL-3 plants than in MdWOX11-RNAi and MdWOX11-OE transgenic lines, and expression of MdCKX5 was higher in MdWOX11-OE transgenic lines than in MdWOX11-RNAi and GL-3 (Figure 7). Expression levels of the GA-related genes MdGAI and MdRGL2 were higher in MdWOX11-OE transgenic lines than in GL-3 and MdWOX11-RNAi at T1 and T2. At T3, their expression levels were highest in GL-3. Expression levels of the ABA-related genes MdABG3 and MdABI2 were higher in MdWOX11-RNAi transgenic lines than in GL-3 and MdWOX11-OE during AS formation (Figure 8).
Figure 6.

Differential expression of genes related to auxin transport and signal transduction at different stages of AS development in GL-3 and MdWOX11 transgenic plants. a: Heatmap of log2(FPKM) values for auxin-related genes. b: Relative expression of genes related to auxin transport and signal transduction at different stages of AS development in GL-3 and MdWOX11 transgenic plants. Values represent the mean ± SE of three biological replicates, and letters indicate significant differences between means (P < 0.05).
Figure 7.

Differential expression of CK-related genes at different stages of AS development in GL-3 and MdWOX11 transgenic plants. a: Heatmap of log2(FPKM) values for CK-related genes. b: Relative expression of CK-related genes at different stages of AS development in GL-3 and MdWOX11 transgenic plants. Values represent the mean ± SE of three biological replicates, and letters indicate significant differences between means (P < 0.05).
Figure 8.

Differential expression of ABA- and GA-related genes at different stages of AS development in GL-3 and MdWOX11 transgenic plants. a: Heatmap of log2(FPKM) values for ABA- and GA-related genes. b: Relative expression of ABA- and GA-related genes at different stages of AS development in GL-3 and MdWOX11 transgenic plants. Values represent the mean ± SE of three biological replicates, and letters indicate significant differences between means (P < 0.05).
Cluster analysis of the expression profiles of shoot development–related genes in MdWOX11 transgenic plants during AS formation
Shoot development–related genes were analyzed in the MdWOX11 transgenic plants during AS formation. The expression of most shoot development–related genes increased with AS production. At T1, the expression levels of MdLBD25, MdTCP9, MdTCP14, MdWOX4, MdDRN, MdBUM, and MdABS2 were higher in GL-3 plants than in MdWOX11-RNAi and MdWOX11-OE transgenic plants. The expression levels of MdLBD25, MdTCP9, MdDRN, MdSYP22, MdBUM, and MdABS2 rose gradually as AS formation progressed in GL-3. At T3 (the AS emergence stage), the expression levels of MdLBD25, MdTCP9, MdTCP14, MdWOX4, and MdSYP22 were lower in MdWOX11-RNAi than in GL-3 and MdWOX11-OE transgenic lines, whereas the expression levels of MdDRN, MdSYP22, MdBUM, and MdABS2 were higher in GL-3 than in MdWOX11-RNAi and MdWOX11-OE (Figure 9).
Figure 9.

Differential expression of shoot development–related genes at different stages of AS development in GL-3 and MdWOX11 transgenic plants. a: Heatmap of log2(FPKM) values for AS development–related genes. b: Relative expression of shoot development–related genes at different stages of AS development in GL-3 and MdWOX11 transgenic plants. Values represent the mean ± SE of three biological replicates, and letters indicate significant differences between means (P < 0.05).
To identify genes that act downstream of MdWOX11, we analyzed the promoters of the hormone- and shoot development–related genes above. The MdCKX5 promoter contained the largest number [3] of WOX-binding elements (TTAATGG), and a yeast one-hybrid (Y1H) assay, dual-luciferase assays, and ChIP-qPCR were performed to verify the binding of MdWOX11 to the MdCKX5 promoter. A Y1H assay showed that MdWOX11 bound to the promoter of MdCKX5 at a binding site 51–636 bp before the initiation codon (Figure 10a), and this sequence is shown in Figure S4. We next performed transient dual-luciferase assays using MdWOX11 as the effector and the Luc gene under the control of the MdCKX5 promoter as the reporter to determine whether MdWOX11 regulates MdCKX5 expression. The Luc/Ren activity was significantly enhanced by MdWOX11 (Figure 10b). MdWOX11-OE transgenic leaves during AS development were used for ChIP-qPCR, and the results were consistent with the Y1H results. MdWOX11 bound to the promoter of MdCKX5 (P4); fragments P1, P2, and P4 contained WOX-box binding elements, whereas P3 did not (Figure 10c).
Figure 10.
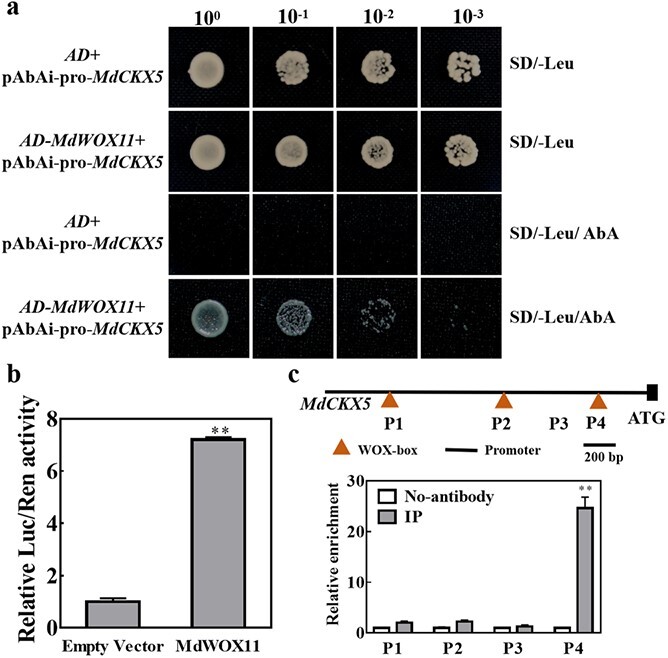
Protein–DNA interactions between MdWOX11 and the MdCKX5 promoter. a: Yeast one-hybrid assays in which the promoter sequence of MdCKX5 was cloned and inserted into the pAbAi vector to generate pAbAi-pro-MdCKX5 and the open reading frame of MdWOX11 was cloned and inserted into the pGADT7 vector to generate AD-MdWOX11. AD-Empty indicates the empty pGADT7 vector. b: Dual-luciferase assay results confirmed that MdWOX11 enhances MdCKX5 promoter activity. Values represent the mean ± SE of three biological replicates, and significant differences were determined using t-tests (**P < 0.01). c: ChIP-qPCR analysis of the association of MdWOX11 with promoter fragments of MdCKX5 using an anti-MdWOX11 antibody; no-antibody indicates the negative control. Values represent the mean ± SE of three biological replicates, and significant differences were determined using the Student’s t test (**P < 0.01).
Discussion
Adventitious shoots (AS) are buds that grow from stem internodes, roots, leaves, or callus through in vitro culture; they differ from terminal, axillary, and accessory buds. AS regeneration is a display of plant totipotency. Some plants may also produce AS near a wound after injury, and the production of AS is a plant response to signals from external stimuli [31]. In this study, injury to the transverse vein served as a signal in leaves. Based on an initial assessment of AS developmental phenotypes through time, we collected samples at three stages of AS development: T1 is the starting point of AS formation, T2 is the stage of callus formation, and T3 is the stage at which green shoot points appeared around the callus. AS formation and regeneration can occur in two ways: the direct proliferation of AS or the indirect proliferation of AS from callus. In this study, most AS formation in apple occurred by indirect proliferation from callus.
Many factors influence the occurrence of AS, and genotype is one of the most important [32]. In this study, we compared the AS regeneration ability of explants and found that SH6 did not produce AS, whereas M26 and MP had a high AS formation ability (Figure 1). Previous research found that the regeneration ability of the leaf blade and petiole was strongly associated with genotype [33]. Hormones are another factor that influences the occurrence of AS. AS regeneration in vitro requires the combined action of IAA and CK [34]. CK is commonly used to induce AS formation. The effects of exogenous IAA content are shown in Table S1, and an appropriate CK/IAA ratio was conducive to AS formation.
At the level of gene regulation, most research on WOX11 function has focused on its regulation of adventitious root formation [35]; little research has been performed on its role in AS formation. Expression analyses of different tissues showed that the expression of MdWOX11 was lower in leaves than in roots (Figure S1a), and the functions and regulatory mechanisms of MdWOX11 may therefore be different between roots and leaves. Our expression analysis suggested that MdWOX11 was a negative regulator of AS formation in apple (Figure 1h). GL-3, as a transgenic apple material, has a strong AS regeneration ability. Analysis of MdWOX11 transgenic lines showed that MdWOX11 inhibited AS formation (Figure 2). This is the first study in which MdWOX11 transgenic lines have been used to investigate the role of MdWOX11 in the AS formation of apple, and MdWOX11 clearly plays a key role in increasing the rate of apple leaf regeneration.
We next asked how MdWOX11 influences hormone levels and hormone-related gene expression to regulate AS formation. We found differences in the endogenous hormone contents of MdWOX11 transgenic plants at different stages of AS formation (Figure 4). MdWOX11 transgenesis affected the distribution of endogenous hormones and thereby affected the expression of hormone-related genes. Overexpression of MdWOX11 increased IAA contents and the expression of IAA transport–related genes at T1 (Figure 4 and 5). Studies on the IAA-responsive promoter DR5 found that the IAA response was significantly promoted in the callus induction stage [12]. In the early stage of AS regeneration, IAA-related genes such as AUX/IAA are upregulated in CIM medium [36]. In this study, the expression levels of the IAA signaling–related genes MdIAA14 and ARF1 were upregulated on CIM medium (Figure 6). Furthermore, CK-related genes play an essential role in the regulation of AS formation. Studies have shown that ARR15 and ARR16 expression increases during AS regeneration, and an increase in the expression of ARR15 and ARR16 was detected at the AS regeneration stage [36]. The expression of CK-regulating genes is also upregulated in SIM culture [37]. In this study, the expression of ARR9 and ARR16 was upregulated in SIM medium during the period of AS emergence (Figure 7). The overexpression of the AHP2 gene in Arabidopsis enhanced the effect of CK on roots and hypocotyls [38], suggesting that CK regulates the process of callus conversion to AS formation. Our results showed that MdAHP1 expression was continuously upregulated in GL-3 and MdWOX11-RNAi plants but downregulated in MdWOX11-OE transgenic plants (Figure 7). Research has shown that even on medium without CK, plants that overexpressed IPTs underwent spontaneous healing through the production of AS on injured tissue [39]. In our study, the expression of IPT1 genes was higher in GL-3 than in MdWOX11-OE transgenic plants (Figure 7). The expression patterns of CK-related genes identified in this study indicated that they play a vital role in callus formation, AS formation, and AS regeneration. The process of AS regeneration is regulated not only by CK but also by hormone interactions. For example, GA is a negative regulatory factor in AS formation [10]. In our study, RNAi of MdWOX11 influenced GA-related gene expression (Figure 8), providing further evidence that GA is a negative regulator of AS formation.
Among the genes that regulate shoot development, reports have shown that TCP3 inhibits shoot formation [40], WUS and WOX5 participate in shoot and root development, respectively [41], and WOX2 plays an important role in shoot apical buds [42]. Overexpression of WOX4 increased the number of shoot primordia structures induced by wounding and plant hormones [43]. The transcription factor gene LBD16 is also related to AS regeneration [44]. Here, we documented changes in the expression levels of several transcription factor genes from different families, such as the TCP, LBD, and WOX families. Their expression was lower in MdWOX11-RNAi transgenic plants during the period of AS emergence, and these genes may therefore act downstream of MdWOX11 to regulate AS formation. In addition, MdDRN, MdSYP22, MdBUM, and MdABS2 are related to shoot formation, and they showed differential expression in the MdWOX11 transgenic plants, suggesting that they act as positive regulatory factors during AS formation (Figure 9).
Y1H and dual-luciferase assays and ChIP-qPCR showed that MdCKX5 acts downstream of MdWOX11 to regulate AS formation (Figure 10). This is the first study to show that WOX11 regulates CK oxidase/dehydrogenase–related genes. Previous studies have shown that WOX11 regulates the expression of CK homeostasis–related genes in rice [45]. Some reports have shown that a decrease in endogenous CK content is directly related to an increase in CKX activity [26]. Centaurium erythraea lines that overexpressed AtCKX1 and AtCKX2 were characterized by a decline in the average number of spontaneously regenerated shoots [46], and AtCKX transgenes affected CK metabolism in transgenic C. erythraea plants [47]. OsGRF4 regulated two CK dehydrogenase precursor genes (CKX5 and CKX1), resulting in increased CK levels [48]. Therefore, we speculate that MdWOX11 inhibited AS formation by activating CK degradation, partly through MdCKX5 and perhaps through other MdCKXs. In MdWOX11-OE transgenic plants, MdWOX11 binds to the promoter of MdCKX5 and induces its expression; MdCKX5 then promotes the degradation of CK, leaf CK levels decline, and low expression of CK response–related genes inhibits AS formation (Figure 11). In MdWOX11-RNAi transgenic plants, transcription of MdCKX5 is inhibited by low expression of MdWOX11, low MdCKX5 levels limit CK degradation, and high CK content induces the expression of CK response–related genes, leading to AS production (Figure 11). Apple encodes 1500 transcription factors, and bud regeneration is a very complex process. Therefore, a large number of transcription factors and the interactions among physiological signals during callus formation and AS regeneration require further exploration in order to fully describe their underlying molecular mechanisms.
Figure 11.

A hypothetical model of MdWOX11 regulating the transcription of MdCKX5 to mediate AS formation in MdWOX11 transgenic apple.
Conclusions
Genotype and CK/IAA ratio were the key factors that affected AS formation ability in apple leaves. The expression of MdWOX11 was negatively correlated with AS formation ability, and MdWOX11 overexpression inhibited AS formation. Y1H and dual-luciferase reporter assays showed that MdCKX5 acts downstream of MdWOX11 to control AS formation.
Methods
Materials and growth conditions
The tissue-cultured materials originated from buds collected from adult-phase materials in March. Tissue culture cuttings of M. prunifolia (MP), M. zumi (MZ), M16 × M9 (M26), Malus honanensis Rehd. × Ralls Janet (SH6), SZ23, and M9-T337 (T337) were grown at the Northwest Agriculture and Forestry University, Yangling (108°04′ E, 34°16′ N), China under tissue culture conditions and used as cuttings for AS formation. MP, MZ, M26, SH6, SZ23, and T337 were cultured on MS medium with 0.1 mg·L−1 indole-3-butyric acid (IBA) and 0.6 mg·L−1 6-benzylaminopurine (6-BA) for 28 days, and fully unfolded young leaves were excised from cuttings for use in regeneration. Cuts were made across the leaf edges and veins to induce AS. A regeneration medium with different proportions of NAA (0.1, 0.5, and 1.0 mg·L−1) was designed, and the medium was formulated as MS basal medium with 2.0 mg·L−1 TDZ and adjusted to pH 5.8 before sterilization.
Genetic transformation and growth of transformed apple plants
35S:MdWOX11-OE and 35S:MdWOX11-RNAi transgenic apple plants were produced in the “GL-3” genetic background [49]. The sequence for MdWOX11 interference is provided in Figure S4. The resulting constructs were transferred into Agrobacterium tumefaciens (strain EHA105), and Agrobacterium-mediated transformation was used to obtain transgenic apple lines as described previously [50]. Microcuttings from MdWOX11 transformed lines were harvested for analysis of gene expression. AS induction from leaves of transformed plants was performed as described above. GL-3 was obtained from Prof. Zhihong Zhang (Shenyang Agricultural University, Shenyang, Liaoning). GL-3, MdWOX11 overexpression (35S:MdWOX11-OE), and MdWOX11 RNA interference (35S:MdWOX11-RNAi) transgenic apple plants were cultured on MS medium with 0.2 mg·L−1 IBA and 0.3 mg·L−1 6-BA. AS regeneration medium contained 2.0 mg·L−1 TDZ and 0.5 mg·L−1 NAA. After 3 weeks of dark treatment, the plants were transferred to light culture, and the new medium was replaced once every 4 weeks for 10 weeks of culture.
Morphological measurements and anatomical observations
A number of morphological parameters were calculated, including the regenerative efficiency, number of shoots per leaf, and the AS increment coefficient. A total of 180 leaves were analyzed, 60 from each group, and leaves were harvested at 0, 7, 15, and 21 d. AS regenerative efficiency (%) was calculated as (number of explants with regenerated AS/total number of explants) × 100%. The average number of AS per explant was calculated as (total number of AS regenerated/number of explants that regenerated AS). The AS increment coefficient was calculated as (total number of AS regenerated/total number of explants inoculated). The collected samples were immediately immersed in liquid nitrogen and stored at −80°C for hormone and RT-qPCR analysis. Anatomical observations were performed using previously described protocols [51–53].
Hormone extraction and measurement
The 0.1-mg samples for hormone extraction were harvested at 0 d (T1), 15 d (T2), and 21 d (T3) from GL-3 and MdWOX11 transgenic plants. Hormones were purified and extracted from the harvested samples by a previously described procedure [54]. Three biological replicates were used for each sample and time point. The enzyme-linked immunosorbent assay (ELISA) technique was used to detect and analyze hormones [54].
RNA sequencing and DEG identification
A total of 27 RNA samples from GL-3 and MdWOX11 transgenic apple leaves at three AS developmental stages (T1, T2, and T3) were sent to Shanghai Meiji Biomedical Technology for RNA-sequencing and transcriptome assembly using previously described protocols [55]. Fragments per kilobase of transcript per million mapped reads (FPKM) values were used to calculate transcript abundance in GL-3 and MdWOX11 transgenic plants [56]. A summary of the sequencing statistics for reads and bases obtained in each sample and statistics for the mapping of clean reads from each sample to the reference genome are given in Supplemental Tables S2 and S3. The significance of differentially expressed genes (DEGs) was analyzed using a published method [57]. The biological processes associated with each gene in each sample were evaluated using a Gene Ontology (GO) analysis (http://www.geneontology.org/) [58]. KEGG pathways with corrected P-values <0.01 were considered to be significantly enriched (http://www.genome.jp/kegg/).
Venn diagrams of DEGs and expression profile analyses
DEGs from the 27 leaf libraries were visualized using Venn diagrams [59,60] with VENNTURE software (http://www.irp.nia.nih.gov/branches/lci/nia_bioinformatics_software.html). A cluster analysis was performed with Multiple Experiment Viewer software (MEV4.2) (http://mev.tm4.org/) based on the FPKM values of the genes to generate hierarchical clustering heatmaps.
Extraction of RNA and synthesis of cDNA
Total RNA was extracted using a CTAB-based method [61], and total RNA integrity was verified with 2% agarose gels. cDNA was synthesized using the Prime Script RT Reagent Kit with gDNA Eraser (TaKaRa Bio, Shiga, Japan).
RT-qPCR analyses
The expression of MdWOX11 was analyzed in six apple rootstocks and GL-3. The expressions of genes related to CK, IAA, GA, ABA, and shoot development were analyzed by RT-qPCR in the GL-3 and MdWOX11 transgenic lines during AS formation. Primers were designed as described previously [62], and gene-specific primers are listed in Supplemental Table S4. RT-qPCR assays were performed as described previously [63], and the apple ACTIN gene was used for normalization. Three biological replicates and three technical replicates were tested for each sample. Relative expression of the analyzed genes was calculated by the 2−ΔΔCt method [64].
Y1H and dual-luciferase reporter assays
The Matchmaker Gold Yeast One-Hybrid System (Clontech, Mountain View, CA, USA) was used for the yeast one-hybrid assays. The CDS of MdWOX11 was cloned and inserted into the pGADT7 vector (AD-MdWOX11), and the promoter sequence of MdCKX5 (pro-MdCKX5) was inserted into the pAbAi vector (pAbAi-pro-MdCKX5). pAbAi-pro-MdCKX5 was transformed into yeast cells, which were plated on SD/−Ura medium supplemented with Aureobasidin A (AbA) to determine the minimal inhibitory concentration of AbA, 500 ng/ml. The AD-MdWOX11 vector was inserted into yeast cells transformed with pAbAi-pro-MdCKX5 and selected on SD/−Leu/AbA medium. For dual-luciferase assays, the pro-MdCKX5 sequence was inserted into the pGreen II 0800-LUC vector, and the CDS of MdWOX11 was cloned and inserted into the pGreenII 62-SK vector (MdWOX11-SK). The empty pGreenII-62-SK vector was used as the negative control. The constructs were transformed into Agrobacterium strain GV3101 and co-transformed into N. benthamiana leaves. After 3 d of culture in the dark, LUC and REN activity were quantified by the dual-luciferase reporter assay system (Promega, E1910). At least six biological replications were performed for each co-transformation, and the ratios of LUC to REN were calculated for treatments and controls to assess the binding activity of MdWOX11 to pro-MdCKX5.
ChIP-qPCR
Leaves of MdWOX11-OE transgenic plants during AS formation were used to perform ChIP-qPCR. The ChIP assay was performed with anti-MdWOX11 polyclonal antibody produced from rabbit (GenScript, Nanjing, China); no antibody served as the negative control. The primers used for ChIP-qPCR are listed in Supplemental Table S4, and three biological replicates were used for each sample.
Statistical analysis
The significance of genotype and time point effects were determined using Analysis of Variance (ANOVA), and significant differences between means were determined at the P < 0.05 level using SPSS 11.5 software (SPSS, Chicago, IL, USA). Figures were constructed using SigmaPlot 12.0 (Systat Software, Inc.).
Abbreviations
AS, adventitious shoot; CIM, callus induction medium; SIM, shoot induction medium; SAM, shoot apical meristem; ARF, auxin response factor; RNA-seq, RNA sequencing; TDZ, thidiazuron; NAA, 1-naphthaleneacetic acid; MP, M. prunifolia; MZ, M. zumi; IAA, auxin; CK, cytokinin; GA, gibberellins; ABA, abscisic acid; ZR, zeatin riboside; WOX5, WUSCHEL-RELATED HOMEOBOX GENE 5; WOX11, WUSCHEL-RELATED HOMEOBOX GENE 11; IBA, indole-3-butyric acid; 6-BA, 6-benzylaminopurine; ELISA, enzyme-linked immunosorbent assay; LBD16, LATERAL ORGAN BOUNDARIES DOMAIN16; DEG, differentially expressed gene;Y1H, yeast one-hybrid; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; ANOVA, Analysis of Variance.
Acknowledgments
This work was financially supported by the National Key Research and Development Project (2018YFD1000101, 2019YFD1001803), the Key Research and Development Project in the Shaanxi Province of China (2019TSLNY02-04), Sub-topics of Major Scientific and Technological Project in Shaanxi Province (2020zdzx03-01-04), Tang Scholar by Cyrus Tang Foundation and Northwest Agriculture and Forestry University, the China Apple Research System (CARS-27), and the China Postdoctoral Science Foundation (2020M683584). We would like to thank Prof. Zhihong Zhang (Shenyang Agricultural University, Shenyang, Liaoning) for providing tissue-cultured “GL-3” plants.
Author contributions
D.Z., J.M., and D.M. designed the research study. D.M. and J.M. performed the research. J.M., D.M., C.N., X.M., K.L., S.C., and X.L. analyzed the data. J.M., D.Z., and M.T. wrote the paper. All authors approved the manuscript.
Data availability
All relevant data are provided within the paper and its supplementary files.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
References
- 1. Xu L, Huang H. Genetic and epigenetic controls of plant regeneration. Curr Top Dev Biol. 2014;108:1–33. [DOI] [PubMed] [Google Scholar]
- 2. Shin J, Bae S, De Seo PJ. Novo shoot organogenesis during plant regeneration. J Exp Bot. 2019;71:63–72. [DOI] [PubMed] [Google Scholar]
- 3. Lall S, Nettleton D, DeCook R et al. Quantitative trait loci associated with adventitious shoot formation in tissue culture and the program of shoot development in Arabidopsis. Genetics. 2004;167:1883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagaoka OM. Shoot regeneration of tomato (Lycopersicon esculentum mill.) in tissue culture using several kinds of supporting materials. Plant Sci. 1995;108:93–100. [Google Scholar]
- 5. Cédric F, Annick BD, Scutt CP, Ferdinand M. Evolution of the ARF gene family in land plants: old domains, new tricks. Molecular Biology & Evolution. 2012;30:45–56. [DOI] [PubMed] [Google Scholar]
- 6. Fasolo F. Adventitions shoot formation on excised leaves of in vitro grown shoots of apple cultivars. Plant Cell Tissue Organ Cult. 1989;16:75–87. [Google Scholar]
- 7. Skoog F, CO, M. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. ChemInform. 1957;11:118–30. [PubMed] [Google Scholar]
- 8. Iwase A, Harashima H, Ikeuchi M et al. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell. 2017;29:54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Werner T, Motyka V, Miroslav S et al. Regulation of plant growth by cytokinin. Proceedings of the National Academy of ences. 2001;98:10487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ezura H, Harberd NP. Endogenous gibberellin levels influence in-vitro shoot regeneration in Arabidopsis thaliana (L.). Heynh Planta. 1995;197:301–5. [DOI] [PubMed] [Google Scholar]
- 11. Paulraj S, Lopez-Villalobos A, Yeung EC. Abscisic acid promotes shoot regeneration in Arabidopsis zygotic embryo explants. In Vitro Cellular & Developmental Biology - Plant. 2014;50:627–37. [Google Scholar]
- 12. Gordon SP, Heisler MG, Reddy GV et al. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 2007;134:3539–48. [DOI] [PubMed] [Google Scholar]
- 13. Pernisova M, Grochova M, Konecny T et al. Cytokinin signalling regulates organ identity via the AHK4 receptor in Arabidopsis. Development. 2018;145:556–66. [DOI] [PubMed] [Google Scholar]
- 14. Zhao Y, Hu Y, Dai M et al. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009;21:736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Sheng L, Yingqiang X et al. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014;26:1081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng S, Tan F, Lu Y et al. WOX11 recruits a histone H3K27me3 demethylase to promote gene expression during shoot development in rice. Nucleic Acids Res. 2018;46:2356–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Hu X, Qin P et al. The WOX11-LBD16 pathway promotes pluripotency acquisition in callus cells during de novo shoot regeneration in tissue culture. Plant & cell physiology. 2018;59:734–43. [DOI] [PubMed] [Google Scholar]
- 18. Ckurshumova W, Smirnova T, Marcos D et al. Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytol. 2015;204:556–66. [DOI] [PubMed] [Google Scholar]
- 19. Qiao M, Xiang F. A set of Arabidopsis thaliana miRNAs involve shoot regeneration in vitro. Plant Signal Behav. 2013;8:e23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiao M, Zhao ZJ, Xiang FN. Arabidopsis thaliana in vitro shoot regeneration is impaired by silencing of TIR1. Biol Plant. 2012;56:409–14. [Google Scholar]
- 21. Zhao X, Su YH, Zhang CL et al. Differences in capacities of in vitro organ regeneration between two Arabidopsis ecotypes Wassilewskija and Columbia. Plant Cell Tissue & Organ Culture. 2013;112:65–74. [Google Scholar]
- 22. Zhao D, Wang Y, Feng C et al. Overexpression of MsGH3.5 inhibits shoot and root development through the auxin and cytokinin pathways in apple plants. Plant J. 2020;103:166–83. [DOI] [PubMed] [Google Scholar]
- 23. Inoue T, Higuchi M, Hashimoto Y et al. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–3. [DOI] [PubMed] [Google Scholar]
- 24. Spíchal L, Rakova NY, Riefler M et al. Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant & Cell Physiology. 2004;45:1299–305. [DOI] [PubMed] [Google Scholar]
- 25. Bartrina I, Otto E, Strnad M et al. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell. 2011;23:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang S, Yu H, Goh C. Functional characterisation of a cytokinin oxidase gene DSCKX1 in dendrobium orchid. Plant Mol Biol Report. 2003;51:237–48. [DOI] [PubMed] [Google Scholar]
- 27. Buechel S, Leibfried A, JPC T et al. Role of A-type ARABIDOPSIS RESPONSE REGULATORS in meristem maintenance and regeneration. Eur J Cell Biol. 2010;89:279–84. [DOI] [PubMed] [Google Scholar]
- 28. Hwang I, Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. 2001;413:383–9. [DOI] [PubMed] [Google Scholar]
- 29. Kai Ishida TY, Yokoyama A, Mizuno T. Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant & Cell Physiology. 2008;49:47. [DOI] [PubMed] [Google Scholar]
- 30. Meng WJ, Cheng ZJ, Sang YL et al. Type-B ARABIDOPSIS RESPONSE REGULATORs is critical to the specification of shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017;29:1357–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25:3159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu & L., H. Adventitious shoot regeneration of two dwarfing pear rootstocks and the development of a transformation protocol. Journal of Pomology & Horticultural ence. 2000;75:745–52. [Google Scholar]
- 33. Lu YF, Zhang LR, Wang YR, Yao YC. Establishment of an efficient in vitro plantlet regeneration system from leaf explants of ornamental crabapple (malus spp.). Journal of Pomology & Horticultural ence. 2015;90:585–92. [Google Scholar]
- 34. Tezuka T, Harada M, Johkan M et al. Effects of auxin and cytokinin on in vivo adventitious shoot regeneration from decapitated tomato plants. Hortscience A Publication of the American Society for Horticultural Science. 2011;46:1661–5. [Google Scholar]
- 35. Mao J, Niu C, Li K et al. Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol. 2020;20:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Che P, Lall S, Nettleton D et al. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006;141:620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Che P, Gingerich DJ, Lall S et al. Global and hormone-induced gene expression changes during shoot development in Arabidopsis. Plant Cell. 2002;14:2771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomomi S, Miwa K, Ishikawa K et al. The Arabidopsis sensor his-kinase, AHK4, can respond to cytokinins. Plant & Cell Physiology. 2001;42:107–13. [DOI] [PubMed] [Google Scholar]
- 39. Tatsuo K. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant & Cell Physiology. 2001;42:677–85. [DOI] [PubMed] [Google Scholar]
- 40. Koyama T, Furutani M, Tasaka M et al. TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell. 2007;19:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nardmann J, Reisewitz P, Werr W. Discrete shoot and root stem cell-promoting WUS/WOX5 functions are an evolutionary innovation of angiosperms. Mol Biol Evol. 2009;26:1745–55. [DOI] [PubMed] [Google Scholar]
- 42. Ravindra B, Malabadi K, Nataraja SV, Kumar & Mulgund, G.S. Evidence of WUSCHEL (WOX2) gene expression during induction of somatic embryogenesis from apical shoot buds of mature trees of Pinus roxburghii. Research. Plant Biol. 2011;1:7–12. [Google Scholar]
- 43. Dai R, Jin H-P, Wang Z et al. Cloning and characterization of WOX4 gene from Vitis vinifera L. involved in stem cell regulation. Agric Sci China. 2011;10:1861–71. [Google Scholar]
- 44. Riechmann JL, Heard J, Martin G et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105. [DOI] [PubMed] [Google Scholar]
- 45. Jiang W, Zhou S, Zhang Q et al. Transcriptional regulatory network of WOX11 is involved in the control of crown root development, cytokinin signals, and redox in rice. J Exp Bot. 2017;68:2787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trifunović M, Cingel A, Simonović A et al. Overexpression of Arabidopsis cytokinin oxidase/dehydrogenase genes AtCKX1 and AtCKX2 in transgenic Centaurium erythraea Rafn. Plant Cell, Tissue and Organ Culture (PCTOC). 2013;115:139–50. [Google Scholar]
- 47. Trifunović M, Motyka V, Cingel A et al. Changes in cytokinin content and altered cytokinin homeostasis in AtCKX1 and AtCKX2-overexpressing centaury (Centaurium erythraea Rafn.) plants grown in vitro. Plant Cell Tissue Organ Cult. 2015;120:767–77. [Google Scholar]
- 48. Sun P, Zhang W, Wang Y et al. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J Integr Plant Biol. 2006;58:836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dai H, Li W, Mao W et al. Development of an efficient regeneration and agrobacterium-mediated transformation system in crab apple (Malus micromalus) using cotyledons as explants. In Vitro Cellular & Developmental Biology - Plant. 2013;50:1–8. [Google Scholar]
- 50. Sedira M, Holefors A, Welander M. Protocol for transformation of the apple rootstock Jork 9 with the rol B gene and its influence on rooting. Plant Cell Report. 2001;20:517–24. [Google Scholar]
- 51. Naija S, Elloumi N, Jbir N et al. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM106 cultured in vitro. Comptes Rendus: Biologies. 2008;331:518–25. [DOI] [PubMed] [Google Scholar]
- 52. Qiyun XU, Chai F, Xincheng AN, Han S. Production method for paraffin section of invasive species of bemisia tabaci. Plant Diseases and Pests. 2012;3:46–8. [Google Scholar]
- 53. Yang JP. Improvement of traditional paraffin section preparation methods. J Biol. 2006;1:47–8. [Google Scholar]
- 54. Dobrev PI, Kamínek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A. 2002;950:21–9. [DOI] [PubMed] [Google Scholar]
- 55. Xing L, Zhang D, Li Y-M et al. Transcription profiles reveal sugar and hormone signaling pathways mediating flower induction in apple (Malus domestica Borkh.). Plant & Cell Physiology. 2015;56:2052–68. [DOI] [PubMed] [Google Scholar]
- 56. Yekutieli D, Y, B. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–88. [Google Scholar]
- 57. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11. https://genomebiology.com/2010/11/10/R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Du Z, Zhou X, Ling Y et al. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Botton A, Eccher G, Forcato C et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011;155:185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martin B, Chadwick W, Yi T et al. Vennture–a novel venn diagram investigational tool for multiple pharmacological dataset analysis. PLoS One. 2012;7:e36911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gambino G, Perrone I, Gribaudo I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal. 2008;19:520–5. [DOI] [PubMed] [Google Scholar]
- 62. Mao J, Zhang D, Meng Y et al. Inhibition of adventitious root development in apple rootstocks by cytokinin is based on its suppression of adventitious root primordia formation. Physiologia_Plantarum. 2018;166:663–76. [DOI] [PubMed] [Google Scholar]
- 63. Li G, Ma J, Tan M et al. Transcriptome analysis reveals the effects of sugar metabolism and auxin and cytokinin signaling pathways on root growth and development of grafted apple. BMC Genomics. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are provided within the paper and its supplementary files.




