Abstract
The recent pandemic, Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has devastated humanity and is continuing to threaten us. Due to the high transmissibility of this pathogen, researchers are still trying to cope with the treatment and prevention of this disease. Few of them were successful in finding cure for COVID-19 by including repurposed drugs in the treatment. In such pandemic situations, when it is nearly impossible to design and implement a new drug target, previously designed antiviral drugs could help against novel viruses, referred to as drug repurposing/redirecting/repositioning or re-profiling. This review describes the current landscape of the repurposing of antiviral drugs for COVID-19 and the impact of these drugs on our nervous system. In some cases, specific antiviral therapy has been notably associated with neurological toxicity, characterized by peripheral neuropathy, neurocognitive and neuropsychiatric effects within the central nervous system (CNS).
Keywords: COVID-19, SARS-CoV-2, Antiviral therapy, Repurposing drugs, Neuropsychiatric effects
Abbreviations
- ACE
Angiotensin-converting enzyme
- ALT
alanine transaminase
- AST
aspartate transaminase
- BBB
blood-brain barrier
- CC
cytotoxic concentration
- CDC
Centers for Disease Control and Prevention
- CES
carboxylesterase
- CNS
central nervous system
- CoV
Coronavirus
- COVID-19
Coronavirus Diseases 2019
- DDIs
drug-drug interactions
- DRV
Darunavir
- EC
effective concentration
- EMA
European Medicines Agency
- FAERS
Food and Drug Administration Adverse Event Reporting System
- FDA
Food and Drug Administration
- FMO
flavin-containing monooxygenase
- GI
gastrointestinal; gp-glycoprotein
- HA
hemagglutinin
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- ICMR
Indian Council of Medical Research
- ICTV
International Committee on Taxonomy of Viruses
- IFN
interferon
- LPV/RTV
Lopinavir/ritonavir
- MERS
Middle East Respiratory Syndrome
- mRNA
messenger RNA
- MRP
multidrug resistance protein
- NA
neuraminidase
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- NIV
National Institute of Virology
- NPAE
neuropsychiatric adverse events
- OAT
organic anion transporter
- OC
oseltamivir carboxylate
- OP
oseltamivir phosphate
- PNS
peripheral nervous system
- RdRp-
RNA-dependent RNA polymerase
- RDV
Remdesivir
- RNA
ribonucleic acid
- RSV
respiratory syncytial virus
- SARS
Severe Acute Respiratory Syndrome
- SLC
solute carrier
- WHO
World Health Organization
1. Introduction
Infectious diseases caused by viruses cause high morbidity and mortality throughout the world and mostly affect developing countries. At present, there are >200 known species of virus, capable of causing human infections. On average, three to four emerging or re-emerging viruses are reported annually and more than half either originate and/or are directly transmitted from animals [1,2]. As per the World Health Organization (WHO), 60% of the agents accepted as human pathogens find their source in the animal kingdom. In the past three decades, 75% of new human pathogens detected, have the potential to cross the animal human interface [3]. Most of the epidemic and pandemic outbreaks in the 21st century, including Influenza A (H1N1, H5N1), SARS (severe acute respiratory syndrome), MERS (Middle East respiratory syndrome), Ebola, Nipah and the recent COVID-19, have animal origins.
The recent pandemic, Coronavirus Disease 2019 (COVID-19) that has been caused by a 2019-Novel coronavirus (2019-nCoV) was named as severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) [4]. SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA virus from the family Coronaviridae (genus Betacoronavirus). This virus is a close phylogenetic relative of the SARS virus, which was responsible for the SARS outbreaks during 2002–2004, and the MERS virus, which has been responsible for MERS outbreaks since 2012 [[5], [6], [7]]. Initially, the SARS-CoV-2 outbreak began in Wuhan, China and consequently started spreading all across the world. According to the COVID-19 global case dashboard published by the WHO, as of December 13, 2021, this virus has infected around 270 million people and resulted in more than five million deaths [8].
Over the past three decades, outbreaks resulting from emerging or re-emerging species of viruses have led to the discovery of many antiviral targets and consequently, to the development of several antiviral agents. The initial stages of such outbreaks are particularly challenging due to the urgency of finding an effective treatment plan to curb the spread of infection and developing an efficient therapy. The need for new antiviral drugs in the treatment of chronic viral diseases motivates researchers to find newer targets and mechanisms for the development of new antivirals. However, it should be noted that the process of drug development is tedious and requires several years to reach its intended beneficiaries. Even if a suitable drug candidate is developed, the large-scale production in a short time is usually challenging [9]. To overcome this situation, previously designed antiviral drugs are tested against novel viruses, referred to as drug repurposing/redirecting/repositioning or re-profiling. Side-effects ranging from mild to severe are common with consumption of any drugs, not the least with antiviral agents. The vast majority of the commercially available drugs may have mild side effects, such as allergic reactions, nausea and some gastrointestinal symptoms that are most often self-limiting and/or might require minimal medical management. However, in some cases, specific antiviral therapy has been notably associated with neurological toxicity, characterized by peripheral neuropathy, neurocognitive and neuropsychiatric effects within the central nervous system (CNS) [10]. For instance, oseltamivir, an antiviral agent, has been extensively documented to be associated with neurological toxicity [[11], [12], [13]]. Similar effects have also been observed in the case of COVID-19 infection where psychiatrists are being consulted to assist with the treatment of infected individuals, some of whom display challenging neuropsychiatric comorbidities [14].
In this review, we will first highlight the current landscape of the repurposing of antiviral drugs for COVID-19. Further, we will discuss some of the studies which have highlighted the neuropsychiatric effects caused as a result of treatment by certain antiviral drugs. Finally, given the vast amount of data available on clinical studies and the associated adverse effects for oseltamivir, we will discuss studies involving this drug and their reported consequential neuropsychiatric effects in detail.
2. Methods
The literature in this area is limited and a non-systematic narrative review was therefore undertaken. We used a structured PubMed search using the following search terms, ((“Antiviral Agents"[Mesh]) AND (“Antiviral Agents/administration and dosage"[Mesh] OR “Antiviral Agents/adverse effects"[Mesh] OR “Antiviral Agents/classification"[Mesh] OR “Antiviral Agents/poisoning"[Mesh] OR “Antiviral Agents/therapeutic use"[Mesh])) AND “Respiratory Tract Infections/virology"[Mesh] for articles in all languages from the year 2010–2021 (2,078 results).
We selected the following antiviral drugs for COVID-19 which are most commonly used in healthcare settings and some of them are known to cause neuropsychiatric effects; Lopinavir and ritonavir, oseltamivir, zanamivir, peramivir, remdesivir, ribavirin, darunavir, arbidol, favipiravir, and molnupiravir.
3. Repurposing of COVID-19 antiviral drugs
The drug repurposing approach suggestively reduces the cost and timeline of drug discovery for novel pathogens [15]. One of the important advantages of drug repurposing over the traditional drug development process is that the repurposed drug offers a considerable amount of existing data through a significant number of studies, the safety of that specific drug is known, and the risk of failure is reduced [16]. Several in vitro, preclinical and clinical trials have demonstrated the anti-COVID efficacy of known repurposed drugs [17]. For instance, in October 2020, the US Food and Drug Administration (FDA) authorized the administration of Remdesivir for the treatment of hospitalized COVID-19 patients. However, at present, the drug is no longer recommended by the WHO [18]. Remdesivir was previously under development for the treatment of Ebola virus disease in 2014–2016 [19].
4. Repurposed drugs and their potential side effects on CNS
COVID-19 patients are currently receiving a variety of supportive treatments, which are determined by their symptoms [20]. However, many clinical professionals prescribe medications or drugs which are previously being used for specific diseases, including antiviral drugs, anti-parasitic drugs, or immunotherapeutics. Given the continuing course of the COVID-19 pandemic, we are focusing this review on reporting the potential CNS side effects of the antiviral drugs that are currently and/or those that were repurposed during this ongoing pandemic for the treatment of COVID-19.
4.1. Lopinavir and ritonavir
Lopinavir/ritonavir (LPV/RTV) is a combination drug of a nucleoside analogue and a protease inhibitor used for the treatment of human immunodeficiency virus (HIV) type 1, approved by the FDA in 2000 [21,22]. There are many antiretrovirals (protease inhibitors), currently used against coronaviruses for treatment such as lopinavir, darunavir, and atazanavir [23]. LPV/RTV treatment is common and found to be effective against MERS-CoV and SARS-CoV, and it subsequently went into a clinical trial for treatment against SARS-CoV-2. In an in vitro study by Kang et al., LPV/RTV was found to be effective against SARS-CoV-2 [24] Initially, LPV/RTV was known to suppress 3-chymotrypsin-like protease which is known to be present in SARS and MERS coronavirus [25]. Whether LPV/RTV efficiently blocks the papain-like protease and 3-chymotrypsin-like protease in the SARS-CoV-2 virus remains debatable [25]. In combination therapy of LPV/RTV for the treatment of HIV infection, LPV is the main antiviral agent, whereas RTV acts as a pharmacokinetic promoter by inhibiting cytochrome P450-3A4 enzymes which leads to increased LPV plasma concentrations [26]. The University of Oxford is conducting a randomised trial for SARS-CoV-2 involving orally administered doses of LPV/RTV (LPV-400 mg and RTV-100 mg), twice a day for 10 days [27]. Liu et al. reported 51 positive COVID-19 patients who underwent treatment with interferon, LPV, RTV, corticosteroids, and conventional Chinese medicine, where 50 patients out of the 51 recovered successfully and were discharged from the hospital [28]. However, in July 2020, the WHO recommended discontinuing the use of LPV/RTV as there was no reduction in the mortality rates of COVID-19 hospitalized patients [29].
LPV/RTV drugs have prominent adverse effects including prolonged QT intervals [30], nausea, vomiting, and diarrhoea [31]. Although there are very few case reports or trials explaining the neuropsychiatric side effects of this combination drug therapy, possible neuropsychiatric side effects include abnormal dreams, agitation, anxiety, confusion, and emotional lability [22].
4.2. Oseltamivir
Oseltamivir has been commonly used in treating Influenza A and B infections. It was approved by the FDA in 2000 [32]. It acts as a competitive inhibitor of the viral neuraminidase restricting the release of viral particles from the host cells. A study by Zhang et al. suggests that the active site of the spike (S) 1 protein of SARS is similar to that of neuraminidase in influenza. Neuraminidase inhibitors may therefore be useful for the treatment of SARS-CoV [33]. Several clinical trials are still investigating the efficacy of oseltamivir in treating SARS-CoV-2 infection. Few in vitro and in vivo studies have reported that treatment with oseltamivir might not have a beneficial effect on the outcomes among patients with SARS-CoV-2. A study by Tan et al. suggests oseltamivir to not be effective for treating patients, and it should be prescribed only for treating influenza [34]. Wang et al. and Choy et al. in their studies also report oseltamivir to be ineffective at inhibiting SARS-CoV-2 in vitro [35,36]. There are ongoing clinical trials where oseltamivir is used in combination with chloroquine, favipiravir, antibiotics (azithromycin), and corticosteroids [37,38]. An ongoing phase 3 clinical trial in Pakistan [39] is evaluating the effectiveness of oseltamivir (75 mg orally twice a day for 5 days) alone or in combination with hydroxychloroquine (200 mg orally 8hr thrice a day for 5 days) and azithromycin (500 mg orally on day 1, followed by 250 mg orally twice a day on days 2–5).
Oseltamivir is ingested in the form of an oral prodrug (oseltamivir phosphate) and it is quickly converted by the hepatic esterases into its active form, that is oseltamivir carboxylate [40]. It is observed that neither oseltamivir nor its metabolite is a substrate for or inhibitor of cytochrome P450 isoforms [41,42]. Davies et al. [42] concluded that oseltamivir is suitable for mostly all age groups and has a predictable linear pharmacokinetic profile. Oseltamivir is generally well tolerated but adverse effects in adults include gastrointestinal disturbances, headache, insomnia, vertigo, bronchitis, and hypersensitive reactions. Few Japanese studies have reported acute onset of neuropsychiatric manifestations and sudden deaths in the children and adolescent population [[11], [12], [13]]. The details of oseltamivir are explained further in the following section.
4.3. Zanamivir
Zanamivir is a competitive inhibitor of viral neuraminidase [43]. It is used in the treatment of Influenza caused by Influenza A and Influenza B viruses [44]. It was approved for use by the FDA in 2006. A 2020 publication by Hall et al. [45] showed Zanamivir as a potential candidate for COVID-19 therapy due to its action as a 3CLPRO main proteinase inhibitor, along with Indinavir, Saquinavir, and Remdesivir. However, multiple in vitro studies have shown Zanamivir to possess no substantial anti-SARS-CoV-2 activity [36].
4.4. Peramivir
Peramivir is a transition state analogue inhibitor of viral neuraminidase, which prevents the production of viral particles. It was tested during the 2009–2010H1N1 epidemic where it failed to show any benefit as compared to placebo. It was approved in 2014 for the treatment of uncomplicated influenza, with limited use among severely ill patients [46]. Peramivir, along with related neuraminidase inhibitors, is not recommended for use against COVID-19 as the SARS-CoV-2 virus uses mechanisms other than the ones involving neuraminidase to facilitate its way out of the infected cell [23,47].
4.5. Remdesivir
Remdesivir (RDV), a broad-spectrum antiviral which was developed by Gilead Sciences Inc. in 2017, as a potential antiviral agent for Ebola virus infection [48,49]. On October 22, 2020, it was approved by the FDA to treat COVID-19 patients [50]. RDV is an adenosine triphosphate nucleoside analogue. It metabolizes into its active form, GS-441524 and interferes with viral RNA polymerase and inhibits viral nucleotide synthesis [51]. In vivo and in vitro studies show that RDV is active against several virus families: filoviridae (Ebola virus), paramyxoviridae (Respiratory Syncytial Virus (RSV), Nipah virus), and coronaviridae (MERS-CoV, SARS CoV, and SARS-CoV-2) [[51], [52], [53], [54]]. Another active metabolite of RDV, GS-5734 showed evidence in animal cell cultures, that it can block the replication of both, MERS-CoV and SARS-CoV [53]. A study by Holshue et al. in the USA reported that RDV in intravenous (IV) form, when administered to a COVID-19 patient, showed promising results [55]. Several clinical trials have been initiated to assess the safety and efficacy of RDV in COVID-19 patients. During the primary stages of the COVID-19 pandemic in China, a randomized, placebo-controlled, double-blinded, multicentric clinical trial was launched in February 2020, they recruited an experimental group of 308 patients and a placebo group of 452 patients. A preliminary dose of 200 mg of RDV was given to the experimental group, followed by a dose of 100 mg for 9 consecutive days via intravenous infusion. The results of this trial were expected by April 2020 but, unfortunately, the trial was terminated and the results of this study remain unknown [56]. A recent trial completed by the National Institute of Allergy and Infectious Diseases (NIAID) in December 2020, reported that RDV showed promising results compared to placebo and the recovery time among adults was substantially shortened [57].
Researchers are still trying to understand the pharmacokinetic profile of RDV, however, an anabolic intracellular kinase plays a key role in the metabolism of RDV [19]. According to the FDA, data related to the toxicity and adverse effects is limited as the drug is still being tested in several clinical trials, but so far it is known to cause nausea, increased ALT and AST levels, and hypersensitivity in some individuals [58].
4.6. Ribavirin
Ribavirin (RBV), a purine nucleoside analogue, was approved as a broad-spectrum antiviral drug by the FDA in 2003 [59]. It is mainly used for treating chronic Hepatitis C virus (HCV) infection, originally being approved for the treatment of severe RSV infection in children [60]. For chronic HCV treatment, it is given in combination with peginterferon-alfa. Apart from RSV and HCV, it has demonstrated activity against hepatitis B virus (HBV), Lassa fever [61], Influenza A and B [62], SARS-CoV [63], and MERS-CoV [64]. The binding activity of RBV triphosphate within the nucleotide-binding pocket of the enzyme inhibits the synthesis of viral mRNA polymerase, which eventually results in the reduction of the viral load [65]. RBV inhibits mRNA capping which induces mutation in the viral replication. This mechanism of action makes RBV a potential candidate in the treatment of SARS-CoV-2 [66]. Several clinical trials are being conducted to check the efficiency of RBV in COVID-19 patients [[67], [68], [69], [70]]. In a retrospective cohort study involving severe COVID-19 patients, Tong et al., reported that ribavirin was well tolerated by the patients and that there were no significant adverse effects observed [71]. Contrary to this, previous studies suggested that RBV is known to cause severe adverse effects, it is teratogenic and known to affect the nervous system causing depression, suicidal thoughts, insomnia and dyspnoea [40].
One way through which RBV is metabolized is a reversible phosphorylation pathway in nucleated cells, the alternative being a degradative pathway involving deribosylation and amide hydrolysis which results in a triazole carboxylic acid metabolite [72]. In vitro studies have reported that ribavirin alone is not a substrate of CYP450 enzymes [73]. However, Simeprevir (a combination of ribavirin and peginterferon-alfa) is metabolized by the cytochrome P4503A4 (CYP3A4) pathway [74]. In COVID-19 patients, RBV with a dose of 500 mg, 2–3 times a day in combination with LPV/RTV or IFN-ɑ is given for ten days [75].
4.7. Darunavir
Darunavir (DRV) is a protease inhibitor that has been extensively used for the treatment of HIV-1 in combination with ritonavir. It was approved by the FDA in 2016 for treating HIV positive pregnant women [76]. Currently, it is being tested against SARS-CoV-2 infection, several clinical trials are underway and some of them are labelled as completed. Cell culture studies demonstrated that DRV tends to inhibit the replication of SARS-Cov-2 virus particles at a concentration of 300 μM [77]. A clinical trial in Qatar [78] is under process for studying the efficacy and safety outcomes of DRV/Cobicistat (cobicistat is a CYP3A4 inhibitor) with a dosage regimen of 800mg/150 mg, one tablet once daily. The studies by De Meyer et al. [79]and Chen et al. [80]suggest that DRV and cobicistat showed no antiviral activity against SARS-CoV-2.
CYP3A is a common metabolizing agent of DRV, and this metabolism is extensive in individuals who have not received any booster (ritonavir for example). The metabolism involved is primarily via isobutyl aliphatic hydroxylation, carbamate hydrolysis, and aniline aromatic hydroxylation. It may also occur via benzylic aromatic hydroxylation and glucuronidation [81]. The most reported adverse reactions to DRV include diarrhoea, nausea, rash, headache, abdominal pain, and vomiting [82].
4.8. Umifenovir
Umifenovir or Arbidol is a broad-spectrum antiviral drug and is currently licensed in Russia and China for the prophylaxis and treatment of influenza. But it is not yet approved by the FDA. It inhibits the fusion of the viral envelope with the host cell membrane, thereby blocking the entry of the virus into host cells and preventing viral infection and replication [83]. Its efficacy has been reported against several viruses; flaviviruses, foot and mouth disease, Lassa virus, herpes simplex virus and the Ebola virus [[84], [85], [86]]. Arbidol acts by binding to the hemagglutinin (HA) protein of the influenza virus. Comparative protein sequence analysis has demonstrated that a short region of the S2 domain of SARS-CoV-2 spike glycoprotein resembles influenza A H3N2 HA protein [87]. Hence, it is currently being studied as a treatment for COVID-19 [88]. Some recent small scale clinical studies with smaller sample sizes have drawn controversial conclusions about the efficacy of umifenovir for COVID-19. Wang et al. reported that treatment with umifenovir showed a high recovery rate and decreased mortality rate in their cohort [89]. However, Lian et al. reported no improved outcomes associated with umifenovir treatment [90].
The pharmacokinetics profile of umifenovir in in vitro studies explained that CYP3A4 is the major enzyme involved in metabolism, whereas other enzymes from P450 and a few flavin-containing monooxygenase (FMO) enzymes have minor contributions in metabolism pathways [91]. The detailed data on the adverse effects of umifenovir is not yet available.
4.9. Favipiravir
Favipiravir (Avigan) is a purine base analogue with a pyrazine carboxamide structure and was developed in 2014 in Japan as a treatment for the influenza strains that are resistant to neuraminidase inhibitors [92]. It has also been used against other RNA viruses like Ebola and norovirus [93]. Favipiravir targets RNA-dependent RNA polymerase (RdRp) enzymes, that interfere with the transcription and replication processes of viral genomes [94]. Due to its mechanism of action, researchers are using favipiravir against SARS-CoV-2, targeting its RdRp gene. Initially, favipiravir was used in Wuhan. As the pandemic spread to the rest of the world, it received approval for emergency use in several countries. Shannon et al. concluded that nucleoside analogues (favipiravir) are promising drugs for COVID-19 treatment [95]. Favipiravir is being evaluated in combination with other antivirals such as umifenovir to see whether these drugs interact in a complementary or collaborative manner [96]. Favipiravir showed efficacy in in vitro studies with half-maximal effective concentration (EC50) of 61.88 μM and half cytotoxic concentration (CC50) of around 400 μM, indicating that high concentration is required for effective treatment [97]. Both dose-dependent and time-dependent pharmacokinetics are exhibited by favipiravir. It is not metabolized by the cytochrome P450 system but has been shown to inhibit one of its components (CYP2C8) [98,99]. During the last few months, many clinical trials are being conducted in several countries for testing favipiravir against SARS-CoV-2. In China, a prospective open-label multicentric trial conducted by Chen et al. [100]compared two treatment arms; conventional therapy in combination with umifenovir (200 mg thrice a day) or favipiravir (1600 mg twice daily followed by 600 mg twice daily) for 7 days (extendable to 10 days). The recovery rate of both groups did not differ significantly. Posthoc analysis illustrated that favipiravir-treated patients showed clinical improvement on day seven. Another study from China [101] found that favipiravir had an enhanced antiviral action compared to LPV/RTV.
The adverse effects pertaining to this drug include increased uric acid level, diarrhoea, neutropenia, increased liver transaminases, teratogenicity, QT prolongation and possible psychiatric symptoms [102].
4.10. Amantadine
Amanatdine, an ion channel blocker was introduced in the 1960's as an antiviral drug against influenza A and accidently it showed activity against Parkinsonian (Parkinson's disease). The FDA licensed the use of amantadine in 1976 for the treatment and prophylaxis of influenza A [103,104]. The antiviral activity of amantadine prevents the release of viral nucleic acid into the host cell by interfering with the viral M2 protein [105]. Similarly, researchers are claiming that amantadine may interrupt the E-channel of coronavirus and thus prevent the release of the viral nucleic acids [106,107]. A retrospective cohort study evaluated amantadine among COVID-19 patients and did not find any significant effect in the treatment [108]. However, an in vitro study by Fink et al., reported that amantadine inhibits the replication of SARS-CoV-2 in Vero E6 cells [109]. A hospital-based cohort study represented the antiviral effect of amantadine in COVID-19 patients with known comorbidities (Parkinson's disease and multiple sclerosis) [110]. Clinical trials of amantadine against SARS-CoV-2 are under process at Copenhagen University Hospital [111] and Noblewell [112].
The most common adverse effects concerning to this drug include hypersensitivity, nausea, dizziness, insomnia [113]. Other complications include cardiotoxicity, CNS toxicity (seizures, blurred vision, suicidal thoughts), renal and respiratory toxicity [105].
4.11. Molnupiravir
Molnupiravir (EIDD-2801 or MK-4482) is a prodrug of β-D-N4-hydroxycytidine (NHC), which is a ribonucleoside analogue and acts as a broad-spectrum antiviral against HCV, Ebola virus, coronaviruses, RSV and influenza viruses [114,115]. Molnupiravir has been shown to have antiviral effects against SARS-CoV-2 in both in vitro as well as in vivo (animal models) studies [[116], [117], [118]]. Studies suggest, the levels of viral RNA are lowered once this particular medicine is used. The drug was successful in completing the first two phases of clinical studies and is currently in Phase 3 clinical trial [119,120]. There were no possible investigations of neuropsychiatric effects of this drug, and the adverse events included headache and diarrhoea in a few patients [121].
List of experimental evidence for efficacies of above mentioned drugs is summarised in Table 1 .
Table 1.
Comparison of in vitro and in vivo efficacies of existing antiviral agents against SARS CoV-2 from the published literature.a.
| Drug | In-vivo study and trial result | In-vitro study result |
|---|---|---|
| Lopinavir/ritonavir** | Not efficient [122] Trial results awaited [27] |
Anti-SARS CoV-2 activity reported at concentration of LPV- 7 μg/mL and RTV- 1.75 μg/mL [24] |
| Oseltamivir** | Not efficient [34] Trial results awaited [39] |
Not efficient [35,36] |
| Zanamivir | Anti-SARS CoV-2 activity reported [45] | Not efficient [36] |
| Peramivir | Not efficient [23,47] | Not efficient [23,47] |
| Remdesivir | Anti-SARS CoV-2 activity reported [55,57] | Anti-SARS CoV-2 activity was reported in Vero E6 cells at 1.76 μM concentration [97] |
| Ribavirin** | Anti-SARS CoV-2 activity reported [71] Trial results awaited [[67], [68], [69], [70]] |
NA |
| Darunavir** | Not efficient [79,80] Trial results awaited [78] |
Anti-SARS CoV-2 activity at a concentration of 300 μM [77] |
| Umifenovir*** | Anti-SARS CoV-2 activity reported [89] | Anti-SARS CoV-2 activity at a concentration of 21–36 μM [123] |
| Favipiravir* | Anti-SARS CoV-2 activity reported [100,101] | Anti-SARS CoV-2 activity at a concentration of around 400 μM [97] |
| Amantadine** | Anti-SARS CoV-2 activity reported [110] Trial results awaited [111,112] |
Anti-SARS CoV-2 activity at a concentration of 83–119 μM [109] |
| Molnupiravir** | Anti-SARS CoV-2 activity reported [117,118] Trial results awaited [119,120] |
Anti-SARS CoV-2 activity at a concentration of 3.4 μM and 5.4 μM [117]. |
* trials completed; ** trials underway still; *** trials contradictory.
5. Neuropsychiatric side effects and drug-drug interactions of potent antivirals used to treat COVID-19
Emerging reports from patients suffering from COVID-19 note the effect of the virus on multiple organs and associated vasculature, especially the vasculature around the brain [124]. Particularly concerning the central nervous system (CNS), stroke, delirium, lethal clot formation, and rare cases of encephalitis are reported. Effects in the peripheral nervous system (PNS) are also reported in the form of loss of taste, smell, vision, and neuropathic pain [125]. In-vitro studies suggest that the SARS-CoV-2 viral spike protein could have significant effects on the integrity of the blood-brain barrier by interacting with vascular Angiotensin-converting enzyme 2 (ACE2), which is upregulated in cases of dementia [126], conditions that are largely seen in the ageing population. The spike protein has also been shown to elicit an inflammatory response in brain endothelial cells, which could contribute towards altered integrity of the blood-brain barrier (BBB) [127]. This could, in a clinical setting, increase the permeability of the CNS with respect to antiviral agents. This carries the potential of emergence of deleterious effects from antivirals, or the worsening of known effects in the CNS. It is therefore also imperative for healthcare workers and drug researchers to be knowledgeable with respect to the mechanisms of antiviral treatments, neuropsychiatric effects, and the possible drug-drug interactions (DDIs) with psychotropic medication. The brief summary of the proposed antiviral drugs for COVID-19 was explained in an earlier section of this review, but to understand the neuropsychiatric side effects and drug-drug interactions, refer to Table 2 . Drug interactions with common therapeutics used in COVID-19 treatment are explained. Therapeutic recommendations from National Institutes of Health (NIH) [128] include: Dexamethasone (corticosteroid), Remdesivir (antiviral), antithrombotic drugs (e.g. Warfarin), Chloroquine or Hydroxychloroquine (antimalarial drugs) with or without Azithromycin (antibiotic), Lopinavir/Ritonavir (antiviral), and Ivermectin (antiparasitic drug), tocilizumab or baricitinib (immunosuppressive drug). Among these recommendations, the use of chloroquine or hydroxychloroquine with/without azithromycin has gained significant attention and currently remains debatable.
Table 2.
Antivirals with their associated neuropsychiatric effects and drug-drug interactions (DDIs).
| Proposed antiviral treatment | Contraindications | Common Adverse effects | Neuropsychiatric side effects | Drug-drug interactions | Sources |
|---|---|---|---|---|---|
| LPV/RTV | Hypersensitivity | Diarrhoea, vomiting, nausea, hypercholesterolemia, hypertriglyceridemia, pancreatitis | – | Drugs requiring high CYP3A dependence for clearance, CYP3A inducers | [22] |
| Oseltamivir | Hypersensitivity | Nausea, vomiting, diarrhoea, abdominal pain | Increased risk of confusion, abnormal behaviour, delirium, delusion, hallucination | Not a substrate nor does it affect CYP450 isoenzymes | [[11], [12], [13]]. |
| Zanamivir | Hypersensitivity | Sinusitis, bronchitis, dyspnea, fever or chills, diarrhoea, arthralgia, and articular rheumatism | Headache and dizziness in adults Seizures, confusion, abnormal behaviour among the pediatric age group |
Not a substrate nor does it affect CYP450 isoenzymes | [129] |
| Peramivir | Hypersensitivity | Skin reactions | Depression, hallucinations, confusion, delirium, restlessness, abnormal behaviour, anxiety, and nightmares | Can alter the efficacy of live attenuated influenza vaccines. | [[130], [131], [132]] |
| Remdesivir | Hypersensitivity | Increased transaminase levels | – | Chloroquine/Hydroxychloroquine co-administration may reduce the anti-viral activity | [58,133] |
| Ribavirin | Monotherapy: Hypersensitivity, pregnancy, haemoglobinopathies heart disease Combination therapy with pegylated interferon alfa-2a: autoimmune hepatitis, hepatic decompensation, pancreatitis, organ transplant |
Monotherapy: Itching and rash Combination therapy with pegylated interferon alfa-2a: autoimmune and infectious disorders, suppression of bone marrow function, haemolytic anaemia, diabetes, pulmonary dysfunction, pancreatitis, dyspnoea, pulmonary infiltrates, sarcoidosis, pneumonitis | Depression, suicidal ideation, dizziness, confusion, somnolence, insomnia | does not inhibit CYP450 enzymes.
|
[134,135] |
| Darunavir | Acute/cytolytic hepatitis, skin reactions, sulfonamide allergy | – | – | Alfuzosin, dihydroergotamine, ergonovine, ergotamine, methylergonovine, cisapride, pimozide, oral midazolam, triazolam, St. John's Wort, lovastatin, simvastatin, rifampin and sildenafil (for treatment of pulmonary arterial hypertension) | [82] |
| Favipiravir | Pregnancy | Hyperuricemia, diarrhoea, reduced neutrophil count | Abnormal behaviour, anxiety, insomnia, so on (Very rare) | Pyrazinamide, Pyrazinamide, Theophylline, Famciclovir, sulindac, Acyclovir | [102,136] |
| Amantadine | Hypersensitivity, Pregnancy | Cardiotoxicity, arrhythmias, tachycardia, Acute respiratory failure, pulmonary edema, hypersexuality, elevated serum creatinine, alkaline phosphatase, bilirubin, etc. | Delirium, seizures, hypokinesia, delusions, aggressive behaviour, blurred vision, depression, agitation, hallucinations, etc. | Anticholinergic drugs, Acetaminophen Can alter the efficacy of live attenuated influenza vaccines. |
[105,137,138] |
6. Lessons from oseltamivir
Oseltamivir (Tamiflu) was approved by the US FDA in 1999 to treat influenza within the first 48 h of the onset of symptoms [139]. The drug was also approved by the European Medicines Agency in 2002. The drug was generally described as safe with less than a 1% rate of discontinuation due to adverse effects in patients, based on two trials with 849 enrolled patients [140]. Based on these reports, concerns of an outbreak of avian flu, fuelled by the 2009–2010 H1N1 pandemic, countries around the world stockpiled enormous quantities of oseltamivir for their respective healthcare systems [141]. In 2010, in the wake of the H1N1 pandemic, WHO added Oseltamivir to the list of essential medications, cementing its place among the essential drug inventories, around the world [142]. However, several shortcomings in reporting around the safety of oseltamivir at individual patient levels from agencies such as the EMA, CDC and WHO were highlighted later in great detail [142].
A large-scale analysis of data from the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) revealed reports of neuropsychiatric adverse events (NPAE), described as ‘abnormal behaviour’, ‘hallucination’, and ‘convulsion’ associated with oseltamivir treatment [143]. Occurrence of ‘abnormal behaviour’ among male patients within the age range of 10–19 years was reported. It was also found that 59.4% of the adverse events were observed among people under the age of 16. This was attributed to the higher use of the drug in Japan among the paediatric population as well as a few other temporal reasons. In 2018, Kang et al. [144], reported a large case-crossover research from Korea which found a 1.2–8 times greater incidence of NPAEs 14 days following oseltamivir exposure compared to controls. The association was found to be consistent when the data was controlled for seasonality, age, sex, the Charlson comorbidity index, or the presence of Influenza related high-risk complications, indicating the contribution of oseltamivir to the NPAEs. These aforementioned findings fall in line with other studies and case reports from around the world, which demonstrates the increased risk of NPAEs, associated with the use of Oseltamivir. Suzuki et al. [145] demonstrated that oseltamivir sialylated a serum glycolipid that induced jump down behaviour via stimulation of D2 receptors in the brain. This effect on the dopaminergic system may be associated with ‘abnormal behaviours’. Additionally, the presence of an inflammatory state associated with Influenza has been shown to increase the level of IL-6 expression, leading to the increase in the relative concentration of unmodified oseltamivir by inhibiting its carboxylation in the liver [146]. This prodrug usually has limited penetration across the BBB (Fig. 1 ) due to the presence of P-glycoprotein (P-gp), as demonstrated in murine models. However, the infection and inflammation also alter the activity of the P-gp at the BBB [147]. The combined effect of these phenomena could lead to an increase in the concentration of unmodified oseltamivir in the brain as well as the serum, which may cause a sudden onset of NPAEs within 24 h of the administration of the drug [148]. The lack of expository data analysis, and the joint action of the infection as well as the drug molecule, provide an exemplary blueprint against the haphazard use of drugs in the current as well as future pandemic situations.
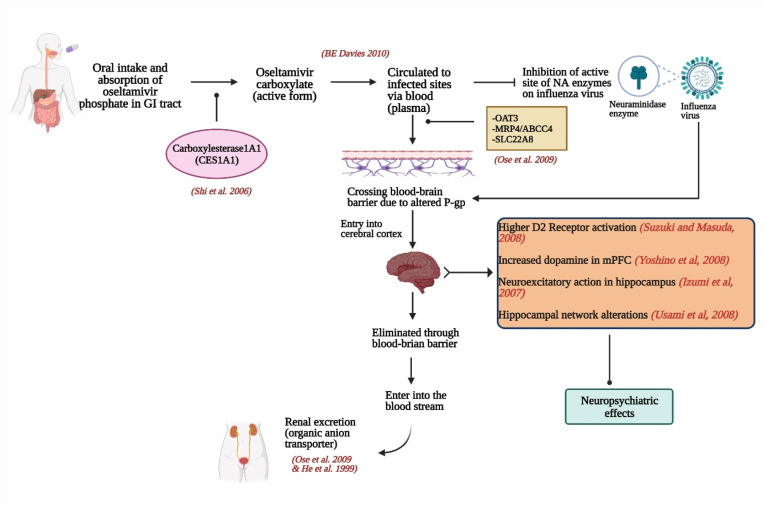
Fig. 1.
Probable mechanism of Oseltamivir causing NPAEs Oseltamivir is ingested in the form of an oral prodrug (oseltamivir phosphate; OP), which is then absorbed in the gastrointestinal (GI) tract and metabolized to the active form, oseltamivir carboxylate (OC) by hepatic esterases-carboxylesterase1A1 (CES1A1) [42]. OC is then circulated in the blood and is distributed to the infected sites via plasma to inhibit the neuraminidase (NA) enzyme present in the influenza virus. The distribution of oseltamivir in the brain is attributable to the active efflux at the BBB via the transporter proteins- OAT3 (organic anion transporter 3), MRP4 (multidrug resistance protein 4) and SLC22A8 (solute carrier family 22 member 8) [149]. The efflux takes place because of alteration in the P-gp gene [147]. The function of P-gp is to guard the BBB by not allowing the drug to cross the BBB [149]. The drug then enters the cerebral cortex where the neuropsychiatric effects are observed. Eventually, oseltamivir is eliminated through BBB with the help of MRP4 and OAT3 in the bloodstream. And finally, the drug is excreted from the body via renal excretion by organic anion transporter proteins [150].
7. Conclusion
In this review, we compiled and discussed the in vitro and in vivo efficacies of various antiviral agents against the SARS-CoV-2 virus. We also emphasized the reported drug-drug interactions and neuropsychiatric effects of the available antiviral agents. A large section of the literature on antivirals focuses on studies performed on murine models, and the effects of antivirals on the human brain must be thoroughly investigated in order to establish reliable treatment regimens for COVID-19 and other emerging and re-emerging viral infections. Optimizing the use of antiviral agents either as mono or in combination therapy after careful consideration of the potential adverse side-effects is warranted.
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Madhura Punekar: Writing – original draft, Conceptualization. Manas Kshirsagar: Writing – original draft, Conceptualization. Chaitanya Tellapragada: Writing – review & editing. Kanchankumar Patil: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Woolhouse M., Gaunt E. Ecological origins of novel human pathogens. Crit. Rev. Microbiol. 2007 doi: 10.1080/10408410701647560. [DOI] [PubMed] [Google Scholar]
- 2.Woolhouse M., Scott F., Hudson Z., et al. Human viruses: discovery and emeraence. Philos Trans R Soc B Biol Sci. 2012;367:2864–2871. doi: 10.1098/rstb.2011.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO EMRO | Zoonotic disease: emerging public health threats in the Region | RC61 | À propos de l’OMS.
- 4.Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Research. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N., Shi X., Jiang L., et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong N.S., Zheng B.J., Li Y.M., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard.
- 9.Ashburn T.T., Thor K.B. Nature Publishing Group; 2004. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. [DOI] [PubMed] [Google Scholar]
- 10.Abers M.S., Shandera W.X., Kass J.S. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs. 2014;28:131–145. doi: 10.1007/s40263-013-0132-4. [DOI] [PubMed] [Google Scholar]
- 11.Sugaya N. Widespread use of neuraminidase inhibitors in Japan. J. Infect. Chemother Off. J. Jpn Soc. Chemother. 2011;17:595–601. doi: 10.1007/s10156-011-0288-0. [DOI] [PubMed] [Google Scholar]
- 12.Ono H., Okamura M., Fukushima A. [Similarity of clinically significant neuropsychiatric adverse reactions listed in package inserts between the anti-influenza drugs oseltamivir and amantadine (possibility attributable to common pharmacological effects)] Yakugaku Zasshi. 2018;138:1201–1215. doi: 10.1248/yakushi.18-00022. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell S.R.J. Tamiflu and neuropsychiatric disturbance in adolescents. BMJ. 2007;334:1232–1233. doi: 10.1136/bmj.39240.497025.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilbul M., Paparone P., Kim A.M., et al. Psychopharmacology of COVID-19. Psychosomatics. 2020;61:411–427. doi: 10.1016/j.psym.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng F., Murray J.L., Rubin D.H. Elsevier Ltd; 2016. Drug Repurposing: New Treatments for Zika Virus Infection. [DOI] [PubMed] [Google Scholar]
- 16.García-Serradilla M., Risco C., Pacheco B. Elsevier B.V; 2019. Drug Repurposing for New, Efficient, Broad Spectrum Antivirals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senanayake S.L. Drug repurposing strategies for COVID-19. Future Drug Discov. 2020;2 doi: 10.4155/fdd-2020-0010. [DOI] [Google Scholar]
- 18.WHO recommends against the use of remdesivir in COVID-19 patients. https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-19-patients Accessed.
- 19.Warren T.K., Jordan R., Lo M.K., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonhospitalized adults: therapeutic management. In: COVID-19 Treat. Guidel. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/. Accessed 25 Mar 2022.
- 21.Croxtall J.D., Perry C.M. Lopinavir/Ritonavir: a review of its use in the management of HIV-1 infection. Drugs. 2010;70:1885–1915. doi: 10.2165/11204950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Drug Approval Package: Kaletra (Lopinavir/Ritonavir) NDA #21-226 & 21-251.
- 23.Frediansyah A., Tiwari R., Sharun K., et al. Antivirals for COVID-19: a critical review. Clin. Epidemiol. Glob. Health. 2021;9:90–98. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang C.K., Seong M.-W., Choi S.-J., et al. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J. Intern. Med. (Engl. Ed.) 2020;35:728–787. doi: 10.3904/kjim.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronaviruses — drug discovery and therapeutic options | nature reviews drug discovery. https://www.nature.com/articles/nrd.2015.37 [DOI] [PMC free article] [PubMed]
- 26.Li F., Lu J., Ma X. CYP3A4-mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab. Dispos. Biol. Fate Chem. 2012;40:18–24. doi: 10.1124/dmd.111.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.University of Oxford . 2020. Randomised Evaluation of COVID-19 Therapy. clinicaltrials.gov. [Google Scholar]
- 28.Liu L., Gao J.-Y., Hu W., et al. 2020. Clinical Characteristics of 51 Patients Discharged from Hospital with COVID-19 in Chongqing,China. medRxiv 2020.02.20.20025536. [DOI] [Google Scholar]
- 29.“Solidarity” clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- 30.Kalil A.C. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020;323:1897–1898. doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 31.Bishara D., Kalafatis C., Taylor D. Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents. Ther Adv Psychopharmacol. 2020;10 doi: 10.1177/2045125320935306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drug approval package: tamiflu (oseltamivir phosphate) NDA #021246. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-246_Tamiflu.cfm
- 33.Zhang X.W., Yap Y.L. The 3D structure analysis of SARS-CoV S1 protein reveals a link to influenza virus neuraminidase and implications for drug and antibody discovery. THEOCHEM. 2004;681:137–141. doi: 10.1016/j.theochem.2004.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan Q., Duan L., Ma Y., et al. Is oseltamivir suitable for fighting against COVID-19: in silico assessment, in vitro and retrospective study. Bioorg. Chem. 2020;104:104257. doi: 10.1016/j.bioorg.2020.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Cao R., Zhang H., et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:28. doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Públic. 2020;44 doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu R., Wang L., Kuo H.-C.D., et al. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azhar S. 2020. Pakistan Randomized and Observational Trial to Evaluate Coronavirus Treatment. clinicaltrials.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilal-Dandan R., Knollmann B., Brunton L. thirteenth ed. McGraw-Hill Education; 2017. Goodman and Gilman's the Pharmacological Basis of Therapeutics. [Google Scholar]
- 41.Oseltamivir. https://go.drugbank.com/drugs/DB00198
- 42.Davies B.E. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 2010;65 doi: 10.1093/jac/dkq015. ii5–ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods J.M., Bethell R.C., Coates J.A., et al. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob. Agents Chemother. 1993;37:1473–1479. doi: 10.1128/AAC.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn C.J., Goa K.L. Zanamivir: a review of its use in influenza. Drugs. 1999;58:761–784. doi: 10.2165/00003495-199958040-00016. [DOI] [PubMed] [Google Scholar]
- 45.Hall D.C., Ji H.-F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Trav. Med. Infect. Dis. 2020;35:101646. doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rome B.N., Avorn J. Drug evaluation during the covid-19 pandemic. N. Engl. J. Med. 2020;382:2282–2284. doi: 10.1056/NEJMp2009457. [DOI] [PubMed] [Google Scholar]
- 47.Li H., Wang Y.M., Xu J.Y., Cao B. [Potential antiviral therapeutics for 2019 novel coronavirus] Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin J Tuberc Respir Dis. 2020;43:E002. doi: 10.3760/cma.j.issn.1001-0939.2020.0002. [DOI] [PubMed] [Google Scholar]
- 48.Hoenen T., Groseth A., Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat. Rev. Microbiol. 2019;17:593–606. doi: 10.1038/s41579-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 49.Siegel D., Hui H.C., Doerffler E., et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 50.Remdesivir FDA. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=214787
- 51.Neupane K, Ahmed Z, Pervez H, et al Potential Treatment Options for COVID-19: A Comprehensive Review of Global Pharmacological Development Efforts. Cureus 12:. 10.7759/cureus.8845. [DOI] [PMC free article] [PubMed]
- 52.Lo M.K., Feldmann F., Gary J.M., et al. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo M.K., Jordan R., Arvey A., et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheahan T.P., Sims A.C., Graham R.L., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.A trial of remdesivir in adults with mild and moderate COVID-19 - No study results posted - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT04252664
- 57.National Institute of Allergy and Infectious Diseases (NIAID) 2020. A Multicenter, Adaptive, Randomized Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalized Adults. clinicaltrials.gov. [Google Scholar]
- 58.VEKLURY® (Remdesivir) FDA.
- 59.Drug Approval Package: Rebetol (Ribavirin) NDA #021546.
- 60.Krilov L.R. Respiratory syncytial virus: update on infection, treatment, and prevention. Curr Infect Rep. 2001;3:242–246. doi: 10.1007/s11908-001-0026-3. [DOI] [PubMed] [Google Scholar]
- 61.Huggins J.W. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev. Infect. Dis. 1989;11(Suppl 4):S750–S761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 62.Van Voris L.P., Newell P.M. Antivirals for the chemoprophylaxis and treatment of influenza. Semin. Respir. Infect. 1992;7:61–70. [PubMed] [Google Scholar]
- 63.Saijo M., Morikawa S., Fukushi S., et al. Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antivir. Res. 2005;66:159–163. doi: 10.1016/j.antiviral.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chong Y.P., Song J.Y., Seo Y.B., et al. Antiviral treatment guidelines for Middle East respiratory syndrome. Infect Chemother. 2015;47:212–222. doi: 10.3947/ic.2015.47.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Te H.S., Randall G., Jensen D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. N. 2007;3:218–225. [PMC free article] [PubMed] [Google Scholar]
- 66.Ölschläger S., Neyts J., Günther S. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antivir. Res. 2011;91:89–93. doi: 10.1016/j.antiviral.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Bausch Health Americas, Inc . 2021. An Open-Label Study to Evaluate the Safety and Efficacy of VIRAZOLE® (RIBAVIRIN for INHALATION SOLUTION, USP) in Hospitalized Adult Participants with Respiratory Distress Due to COVID-19. clinicaltrials.Gov. [Google Scholar]
- 68.The University of Hong Kong . 2020. An Open-Label Randomised Controlled Trial on IFN Beta-1b and Ribavirin Combination, as Treatment for Covid-19 Infection. clinicaltrials.gov. [Google Scholar]
- 69.Elalfy H. 2021. Effect of a Combination of Nitazoxanide, Ribavirin and Ivermectin Plus Zinc Supplement on the Clearance of COVID-19: Extension Study. clinicaltrials.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venter P.F. 2021. A Randomized, Double-Blind, Study Comparing the Efficacy, Safety, and Tolerability of Oral Administration of Ribavirin (RBV) and Nitazoxamide (NTZ)Versus Placebo in SARS-CoV-2 Virus Infected Participants (DuACT) clinicaltrials.gov. [Google Scholar]
- 71.Tong S., Su Y., Yu Y., et al. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int. J. Antimicrob. Agents. 2020;56:106114. doi: 10.1016/j.ijantimicag.2020.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montvale N., TP . Thomson PDR; Montvale, NJ: 2006. Physicians' Desk Reference 2006. [Google Scholar]
- 73.PubChem Ribavirin.
- 74.Ahmed A., Lutchman G.A., Kwo P.Y. Drug‐drug interactions in hepatitis C virus treatment: do they really matter? Clin. Liver Dis. 2017;10:111. doi: 10.1002/cld.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 76.Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021976
- 77.Pandey A., Nikam A.N., Shreya A.B., et al. Elsevier Inc; 2020. Potential Therapeutic Targets for Combating SARS-CoV-2: Drug Repurposing, Clinical Trials and Recent Advancements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darunavir/cobicistat vs. Lopinavir/ritonavir in COVID-19 pneumonia in Qatar - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04425382
- 79.De Meyer S., Bojkova D., Cinatl J., et al. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J., Xia L., Liu L., et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vermeir M., Lachau-Durand S., Mannens G., et al. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab. Dispos. 2009;37:809–820. doi: 10.1124/dmd.108.024109. [DOI] [PubMed] [Google Scholar]
- 82.FDA Approved Drug Products: Prezista (Darunavir) Oral Tablets/suspension.
- 83.Blaising J., Polyak S.J., Pécheur E.-I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haviernik J., Štefánik M., Fojtíková M., et al. Arbidol (umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. 2018. Viruses 10: [DOI] [PMC free article] [PubMed]
- 85.Hulseberg C.E., Fénéant L., Szymańska-de Wijs K.M., et al. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J. Virol. 2019;93 doi: 10.1128/JVI.02185-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M.-K., Liu Y.-Y., Wei F., et al. Antiviral activity of arbidol hydrochloride against herpes simplex virus I in vitro and in vivo. Int. J. Antimicrob. Agents. 2018;51:98–106. doi: 10.1016/j.ijantimicag.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int. J. Antimicrob. Agents. 2020;56:105998. doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang D., Yu H., Wang T., et al. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z., Chen X., Lu Y., et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 90.Lian N., Xie H., Lin S., et al. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng P., Zhong D., Yu K., et al. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob. Agents Chemother. 2013;57:1743–1755. doi: 10.1128/AAC.02282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 2019;14:3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.PubChem Favipiravir.
- 95.Shannon A., Selisko B., Le N., et al. Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. 2020. bioRxiv. [DOI]
- 96.Glenmark Starts Phase III Favipiravir Combination Trial for Covid-19.
- 97.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madelain V., Nguyen T.H.T., Olivo A., et al. Ebola Virus Infection: a review on the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toyama Chemicals. Summary of Product Characteristics of Avigan.
- 100.Chang Chen, MD, Zhenshun Cheng, MD, Jianyuan Wu, PhD, et al Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. [DOI] [PMC free article] [PubMed]
- 101.Cai Q., Yang M., Liu D., et al. Engineering; 2020. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76:370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jefferson T.O., Demicheli V., Deeks J.J., Rivetti D. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst Rev CD001169. 2000 doi: 10.1002/14651858.CD001169. [DOI] [PubMed] [Google Scholar]
- 104.Crosby N.J., Deane K., Clarke C.E. Amantadine for dyskinesia in Parkinson's disease. Cochrane Database Syst. Rev. 2003 doi: 10.1002/14651858.CD003467. 2003:CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.SYMMETREL® (Amantadine Hydrochloride USP).
- 106.Toft-Bertelsen T.L., Jeppesen M.G., Tzortzini E., et al. Amantadine has potential for the treatment of COVID-19 because it inhibits known and novel ion channels encoded by SARS-CoV-2. Commun. Biol. 2021;4:1347. doi: 10.1038/s42003-021-02866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aranda Abreu G.E., Hernández Aguilar M.E., Herrera Covarrubias D., Rojas Durán F. Amantadine as a drug to mitigate the effects of COVID-19. Med. Hypotheses. 2020;140:109755. doi: 10.1016/j.mehy.2020.109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mancilla-Galindo J., García-Méndez J.Ó., Márquez-Sánchez J., et al. All-cause mortality among patients treated with repurposed antivirals and antibiotics for COVID-19 in Mexico City: a real-world observational study. EXCLI J. 2021;20:199–222. doi: 10.17179/excli2021-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fink K., Nitsche A., Neumann M., et al. Amantadine inhibits SARS-CoV-2 in vitro. Viruses. 2021;13:539. doi: 10.3390/v13040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamel W.A., Kamel M.I., Alhasawi A., et al. Effect of pre-exposure use of amantadine on COVID-19 infection: a hospital-based cohort study in patients with Parkinson's disease or multiple sclerosis. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.704186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amantadine for COVID-19 - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04894617
- 112.Efficacy of amantadine treatment in COVID-19 patients - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04952519
- 113.Bakhati B., Sibi V.M., Mekala A.P., et al. Amantadine-induced cardiac arrest in a patient with COVID-19. Cureus. 2022;14 doi: 10.7759/cureus.21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y., Li P., Solanki K., et al. Viral polymerase binding and broad-spectrum antiviral activity of molnupiravir against human seasonal coronaviruses. Virology. 2021;564:33–38. doi: 10.1016/j.virol.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Şimşek-Yavuz S., Komsuoğlu Çelikyurt F.I. Antiviral treatment of COVID-19: an update. Turk. J. Med. Sci. 2021 doi: 10.3906/sag-2106-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wahl A., Gralinski L.E., Johnson C.E., et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6:11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheahan T.P., Sims A.C., Zhou S., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Painter G.R., Natchus M.G., Cohen O., et al. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr Opin Virol. 2021;50:17–22. doi: 10.1016/j.coviro.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Merck Sharp & Dohme Corp . 2021. A Phase 2/3, Randomized, Placebo-Controlled, Double-Blind Clinical Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of MK-4482 in Non-hospitalized Adults with COVID-19. clinicaltrials.Gov. [Google Scholar]
- 121.Painter W.P., Holman W., Bush J.A., et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02428-20. e02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao B., Wang Y., Wen D., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leneva I., Kartashova N., Poromov A., et al. Antiviral activity of umifenovir in vitro against a broad spectrum of coronaviruses, including the novel SARS-CoV-2 virus. Viruses. 2021;13:1665. doi: 10.3390/v13081665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. 2020 doi: 10.1101/2020.05.18.20099960. medRxiv 2020.05.18.20099960. [DOI] [Google Scholar]
- 125.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77:683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ding Q., Shults N.V., Harris B.T., Suzuki Y.J. Angiotensin-converting enzyme 2 (ACE2) is upregulated in Alzheimer's disease brain. 2020. bioRxiv. [DOI] [PMC free article] [PubMed]
- 127.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antiviral Therapy.
- 129.Zanamivir FDA Label.
- 130.Nakamura S., Miyazaki T., Izumikawa K., et al. Efficacy and safety of intravenous peramivir compared with oseltamivir in high-risk patients infected with influenza A and B viruses: a multicenter randomized controlled study. Open Forum Infect. Dis. 2017;4 doi: 10.1093/ofid/ofx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shetty A.K., Peek L.A. Peramivir for the treatment of influenza. Expert Rev. Anti Infect. Ther. 2012;10:123–143. doi: 10.1586/eri.11.174. [DOI] [PubMed] [Google Scholar]
- 132.RAPIVABTM (Peramivir Injection).
- 133.Gulati G., Kelly B.D. Does remdesivir have any neuropsychiatric adverse effects? Ir. J. Psychol. Med. 2020:1–2. doi: 10.1017/ipm.2020.67. [DOI] [PubMed] [Google Scholar]
- 134.Nyström K., Waldenström J., Tang K.-W., Lagging M. Ribavirin: pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019;14:153–160. doi: 10.2217/fvl-2018-0166. [DOI] [Google Scholar]
- 135.Full article: treatment of hepatitis C virus infection for adults and children: updated Swedish consensus guidelines. 2017. https://www.tandfonline.com/doi/full/10.1080/23744235.2018.1445281 [DOI] [PubMed]
- 136.Report on the Deliberation Results- Avigan.
- 137.Chang C., Ramphul K. StatPearls. StatPearls Publishing. 2022. Amantadine. Treasure Island (FL) [Google Scholar]
- 138.Elsevier – drug monograph │ amantadine. https://elsevier.health/en-US/preview/amantadine#interactions
- 139.Ebell M.H. WHO downgrades status of oseltamivir. BMJ. 2017;358:j3266. doi: 10.1136/bmj.j3266. [DOI] [PubMed] [Google Scholar]
- 140.Anonymous . Eur. Med. Agency. 2018. Tamiflu.https://www.ema.europa.eu/en/medicines/human/EPAR/tamiflu [Google Scholar]
- 141.Tamiflu: “a nice little earner” | the BMJ. https://www.bmj.com/content/348/bmj.g2524 [DOI] [PubMed]
- 142.World Health Organization. WHO Model List of Essential Medicines. 16th list (updated).
- 143.Ueda N., Umetsu R., Abe J., et al. Analysis of neuropsychiatric adverse events in patients treated with oseltamivir in spontaneous adverse event reports. Biol. Pharm. Bull. 2015;38:1638–1644. doi: 10.1248/bpb.b15-00253. [DOI] [PubMed] [Google Scholar]
- 144.Kang H.-R., Lee E.-K., Kim W.J., Shin J.-Y. Risk of neuropsychiatric adverse events associated with the use of oseltamivir: a nationwide population-based case-crossover study. J. Antimicrob. Chemother. 2019;74:453–461. doi: 10.1093/jac/dky445. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki M., Masuda Y. Effect of a neuraminidase inhibitor (oseltamivir) on mouse jump-down behavior via stimulation of dopamine receptors. Biomed. Res. Tokyo Jpn. 2008;29:233–238. doi: 10.2220/biomedres.29.233. [DOI] [PubMed] [Google Scholar]
- 146.Hama R. The mechanisms of delayed onset type adverse reactions to oseltamivir. Inf. Disp. 2016;48:651–660. doi: 10.1080/23744235.2016.1189592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo | PNAS. https://www.pnas.org/content/97/7/3473 [DOI] [PMC free article] [PubMed]
- 148.Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir. Drug Saf. 2008;31(12):1097–1114. doi: 10.2165/0002018-200831120-00006. [DOI] [PubMed] [Google Scholar]
- 149.Ose A., Kusuhara H., Yamatsugu K., et al. P-glycoprotein restricts the penetration of oseltamivir across the blood-brain barrier. Drug Metab. Dispos. Biol. Fate Chem. 2008;36:427–434. doi: 10.1124/dmd.107.018556. [DOI] [PubMed] [Google Scholar]
- 150.Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802 - PubMed. https://pubmed.ncbi.nlm.nih.gov/10628898/ [DOI] [PubMed]