Abstract
Gingival repigmentation is an inevitable hindrance among different procedures accepted for gingival depigmentation. To overcome this, there is a need for the procedure that can delay the duration of reappearance of pigmentation. A number of studies using herbal extracts with antioxidant property shown to have anti melanogenic effect. In the present in - vitro study, we investigated the effect of banana stem and flower extracts on melanocytes, as Banana stem and flower are rich in polyphenols and flavonoids, which are potent antioxidants. The melanocytes were exposed to ethanolic extract of banana stem and flower at 2 concentrations (100 μgm, 150 μgm) for 72 h. The cellular melanin contents were measured using Bradford assay which depicted the reduction in the melanin content and Resazurin assay was used for assessment of cell viability showed no significant cytotoxic effect of banana stem and flower on the cells. The cells exposed to higher concentration (150 μgm) of banana stem and flower showed significant reduction in melanin content. Flower extract showed better reduction in the melanin content. Based on these results both banana flower and stem can be tried as potent gingival depigmenting agent.
1. Introduction
“Smile is the prettiest thing you can wear”, along with white esthetics pink esthetics also contribute to the harmony of gratifying smiles. The color of the gingiva is coral pink, which will be influenced by the thickness of the gingival epithelium, vascularization, degree of keratinization and presence of the pigments. These pigments can be exogenous or endogenous in origin. Although, melanin is the most commonly seen endogenous pigment of neurocrestal origin, other pigments like Carotene, Reduced hemoglobin and Oxyhemoglobin can also lead to gingival pigmentation.1Melanin pigment appears as early as 3 h after birth in the gingiva and is the only sign of pigmentation on the body.2 The occurrence of pigmentation can range from physiologic reasons (e.g. racial pigmentation) to manifestations of systemic illnesses (e.g. Addison's disease) to malignant neoplasms (e.g. melanoma and Kaposi's sarcoma).3 Gingival hyperpigmentation is generally not bothersome until the patient exhibits a high smile line and/or disparity with skin complexion. This affects a person's emotional well-being and can contribute to a decrease in self-esteem; forms the main reason for seeking treatment.4
Several procedures have been used for the treatment of gingival hyperpigmentation, such as the Scalpel technique, Bur abrasion, Electro-surgery, Cryosurgery, Radiosurgery, Lasers. Furthermore, free gingival graft and acellular dermal matrix have been used to mask gingival pigmentation.5 Each of these has its advantages and limitations. The most common shortcoming of all these procedures is re-pigmentation, which can be as early as 33 days.2Thus, the depigmentation procedures are challenging for the clinician.
To overcome these withering effects and to prolong the duration of recurrence, natural or plant-based products have been tried and tested for the treatment of gingival hyperpigmentation. Studies have shown that the use of cinnamon, ascorbic acid and curcumin exhibiting antioxidant properties helped in the scavenging of reactive oxygen inhibiting the melanin formation by preventing reduction of the dopamine.6, 7, 8 This led us to explore the most common, easily feasible natural or plant extracts having antioxidant properties.
Banana is one of the most widely consumed fruits and all parts of the plant, including flower, fruit, peel, and stem, are shown to have medicinal benefits. This plant is known to have antimicrobial, anti-inflammatory, anti-neoplastic, hepato-protective and antioxidant properties due to the presence of polyphenols such as flavonoids, acid lycopene, beta carotene, tannin and saponin.9 Thus, this in vitro study was conducted to determine whether banana stem and flower could inhibit melanin synthesis and its effect on cell viability.
2. Materials and methods
2.1. Preparations of the banana stem and flower extract
Commercially available banana stem and flower powders were procured from Neotea herbal products, which are approved by FSSAI (Food Safety and Standards Authority of India). These powders were prepared from dehumidified air dried with hammer mill grinding. Using these commercially available powders ethanolic extract was prepared by maceration technique. Ethanol and water were mixed in a 1:1 ratio. 50 μg powders were dissolved in 1 ml of the ethanol and water solution. The extract was centrifuged at full speed for 30 s and filtered in 0.22 μM filter. The obtained extract was approximated at a concentration of 100 μg/ml 2 μl of the solution was added for every 1 ml of culture medium.
2.2. Preparation of melanocytes cell culture
Human melanocyte cells were used in the present study. 12 cell plates of approximately 2 X 105 human melanocyte cells were seeded for 4 days. After 4 days the cells were made 85–90% confluent. Before the experiment, the culture medium was changed once and 500 μl of the extract was added to each well. After adding the extract the cells were incubated for 72 h. Cells were harvested and stored at 4 °C.
2.3. Experimental procedure
100 μgm and 150 μgm of banana stem and flower extracts were used in the experiment. After incubation of the cells, they were trypsinized to dissociate the cells from the culture plate and the extract was added. The cells with the extract were transferred to the Eppendorf and centrifuged at 1800 rpm for 4 min. Cells were then washed with phosphate-buffered saline (PBS).
3. Assays
3.1. Estimation of melanin pigments (Bradford assay)
Melanin pigment was assessed using the Bradford assay, which is a colorimetric protein assay based on an absorbance shift of the dye Coomassie Brilliant glue G-250. The dye forms a complex with the proteins, and the amount of the complex present in the solution is a measure of the protein concentration.
Melanin pigments were estimated by lysing cells with sonication buffer and then pelleted, washed once with 1:1 ethanol and diethyl ether mixture, the pellet was air-dried, solubilized in a buffer (2 mM NaOH, 20% DMSO) at 60 °C for 30 min and then melanin absorbance was measured at 492 nm (Tecan). The results were normalized to protein concentration and then plotted as graphs.10
3.2. Resazurin dye test for cytotoxicity
The Resazurin assay, cell viability and proliferation indicator, works by conversion of resazurin, a non-fluorescent indicator dye to resorufin, a highly fluorescent dye via reduction reactions of metabolically active cells. The amount of fluorescence produced is proportional to the number of living cells. In the present study, melanocytes treated with banana stem and flower were exposed to resazurin dye and then the samples were analyzed spectrophotometrically (λEx/λEm:530/580 nm) and also observed under a fluorescent microscope to evaluate the tyrosinase activity.11
4. Results
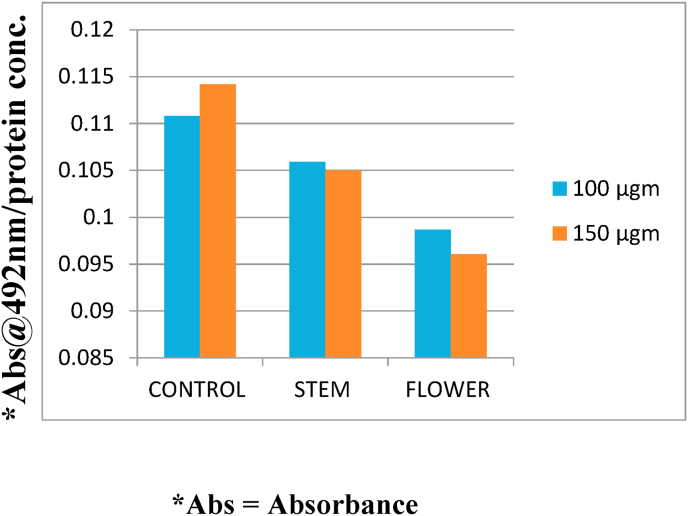
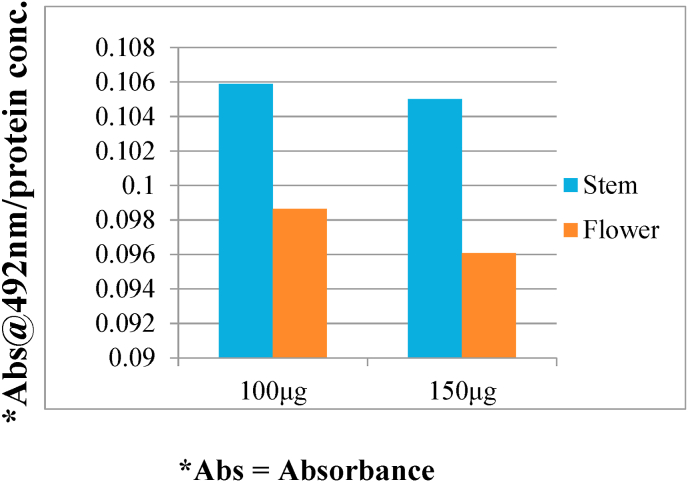
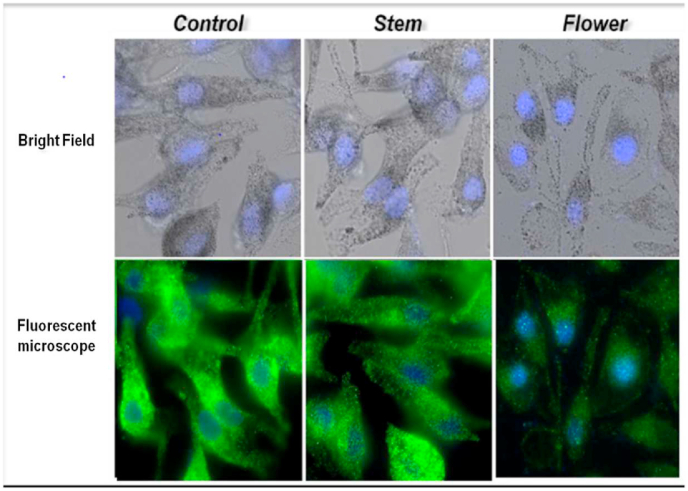
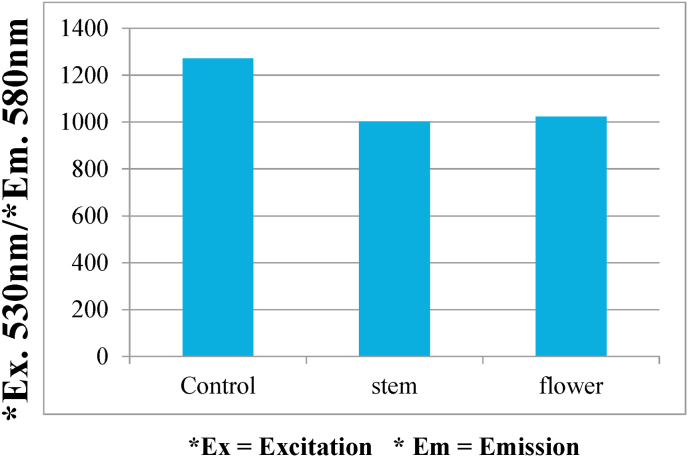
To determine the effect of banana stem and flower on melanin synthesis, the melanocytes were exposed to ethanolic extract of banana stem and flower at 2 concentrations (100 μgm, 150 μgm) for 72 h. The cellular melanin contents were then measured. Fig. 1 shows that the melanin content of the cells treated with both banana stem and flower was reduced compared to control. However significant reduction was seen in the cells treated with flower. Fig. 2 shows that the cells exposed to higher concentration (150 μgm) of banana stem and flower depicted reduced melanin content compared to lower concentration (100 μgm). To determine the effect of banana stem and flower on cell viability, melanocytes treated with 100 μgm of banana stem and flower extract were exposed to resazurin dye and assessed using a fluorescent microscope and spectrophotometer. Fig. 3 shows a fluorescent image of the cells and it can be observed that these extracts have no significant cytotoxic effect on the cells. Fig. 4 showed the relevant results when analyzed spectrophotometrically.
Fig. 1.
Comparison of Melanin absorbance at 492 nm for 100 μgm and 150 μgm of banana stem and flower extracts. Data are expressed as amount of melanin protein absorbed by spectrophotometry.
Fig. 2.
Comparison of the effect of ethanolic extract of banana stem and flower on melanocytes. Data are expressed as amount of melanin protein absorbed by spectrophotometry at 492 nm.
Fig. 3.
Bright field and fluorescent microscopic image melanocytes treated with control, stem and flower. Banana flower showed reduced green fluorescence illustrating decline in number of melanocytes.
Fig. 4.
Comparison of the effect of the banana stem and flower on cell viability using Resazurin Assay test. Data are expressed based on the spectrometric analysis with the excitation at 530 nm and emission at 580 nm.
5. Discussion
Melanocytes are the dendritic cells situated in the basal and spinous layers of the epithelium which are synthesized in the form of granules and moved to keratinocytes present in the suprabasal layer. The degree of melanin synthesis is directly correlated to the activity of the tyrosinase enzyme, a membrane protein that helps in the oxidation of DOPA to DOPA quinone, a rate-limiting step in the melanin synthesis. Hence any uncontrolled activity of tyrosinase leads to hyperpigmentation.12 In the oral cavity, gingiva is the first and most frequently pigmented structure and this becomes bothersome when the patient presents with “A gummy smile”.13
Different techniques have been tried for the treatment of gingival hyperpigmentation, however, they failed to bear the cost of a long-lasting remedy. It has been documented that the repigmentation is bound to occur regardless of treatment rendered, and it varies from person to person and different surgical procedures accomplished.14,15Although the mechanism of gingival repigmentation is not clearly understood; it is explained by migration theory which states that active melanocytes from the adjacent pigmented tissues migrate to the treated areas, resulting in repigmentation16 or it could be due to leftover melanocytes at the surgical site which might get activated and twitches synthesizing melanin.17
To defeat these issues, there is a requirement for a product that can be applied over and again with no damage. Topical application of ascorbic acid weekly after the gingival depigmentation procedure has shown satisfactory results in delaying the repigmentation.18 Similarly in the double-masked, placebo-controlled clinical trial, four weeks of ascorbic acid topical application on gingiva showed the significant relative change in gingival pigmentation.7
The potential inhibitory activity of curcumin was assessed on the melanocyte-stimulating hormone (MSH)-stimulated melanogenesis signal pathway on B16F10 melanoma cells, the results of which revealed that curcumin inhibited melanin synthesis by suppressing the cellular melanin contents and the tyrosinase activity.8 A study using Cinnamomum cassia Presl on B16 melanoma cells revealed low cytotoxicity and high depigmenting activity, which was credited to its anti-oxidative property.6 Likewise, in-vitro and in-vivo evaluation of the depigmenting activity of raspberry ketone was also tried, which revealed promising results in inhibiting melanogenesis.19
Many studies on the banana stem and flower extract have proved that they are rich in bioactive compounds including phenolics, flavonoids, vitamin E, saponin, alkaloids, oxalate and tannin and also have potent antioxidant and/or free radical scavenging activity. 20,21Taking all these studies into thought, it proves that banana is a natural antioxidant and also considering few other enumerated studies where most common antioxidants (ascorbic acid, curcumin)7,8 have demonstrated anti-melanogenic activity, we hypothesized that banana stem and flower could have a depigmenting effect on melanocytes.
In the present study, the melanocytes were exposed to ethanolic extract of banana stem and flower at 2 concentrations (100 μgm, 150 μgm) for 72 h. The cellular melanin content was measured using the Bradford assay, which depicted the reduction in the melanin content of the cells treated with both banana stem and flower. The depigmenting effect of banana flower and stem is seen in a dose-dependent manner, also flower showed a better reduction, i.e. the cells exposed to higher concentration (150 μgm) of banana stem and flower showed a significant reduction in melanin content compared to lower concentration (100 μgm). Also, the resazurin dye test, test for cell viability revealed that there is no significant cytotoxic effect of banana stem and flower on the cells.
There are scares of literature accessing the anti-melanogenic properties using various parts of the banana plant (peel, flower, stem, etc). A study was done by Phacharapiyangkul et al. using Musa sapientum Linn. (Banana) peel ethanol extracts (MPE) on melanocyte-stimulating hormone (MSH), and the results anti-melanogenic action was attributed to AKT pathways, reducing microphthalmia-associated transcription factor expression and tyrosinase enzyme family production. Another study investigated the effect of Sucrier banana peel extracts on B16F10 mouse melanoma cells. It inhibited melanogenesis process through p38 signaling pathway. The extract decreased the expression of melanogenesis relate protein as a microphthalmia-associated transcription factor (MITF) and tyrosinase protein after 24 h of incubation with α-melanocyte-stimulating hormones (MSH) stimulating.22,23
Heravi et al., on the other hand, examined the depigmenting effects of banana peel (Musa sapientum pericarp) and spinach leaves (Spinacia oleracea folium) and determined that spinach leaves had a greater inhibitory impact and a lower cytotoxic effect than banana peel.24
The promising depigmenting effect of banana stem and flower can be ascribed to its antioxidant property due to the presence of flavonoids. Research on molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma explained that flavonoids inhibit melanogenesis by their action on transcriptional factor MiTF and/or the melanogenesis enzymes tyrosinase, DCT2, or TYRP-1. It acts by inhibiting cell proliferation, invasion and also by inducing cell apoptosis.9 Thus flavonoids acts on melanocytes by multiple mechanisms that are via ROS-scavenging, immune-modulation, cell cycle regulation and epigenetic modification, etc. Also in our study, flower extract showed a more depigmenting effect than stem extract which could be due to its higher flavonoid content than stem.20,21 Study on the total antioxidant assay of banana showed that, ethanolic extract has a maximum concentration of antioxidants as compared to other solvents.25 Therefore, ethanolic extracts were used in our study. The promising depigmenting effect of the extract in this present study can be credited to the antioxidant property of both banana flower and stem owed to presence of flavonoids with the additional benefit of ethanol as solvent.
6. Strength and weakness of the study
As there is a revolutionary change in the cosmetic industry from chemical to herbal products, there will be a good acceptance by the patient to the natural products like banana stem and flower. Banana plant and its components are proved to have antioxidant, antibacterial and anti-inflammatory property and the use of such products yield fewer allergic reactions.
Limitations of the study: Only the Bradford test was employed to quantify melanin pigmentation in this study; however, different assays can be used to measure melanin pigmentation. In future clinical investigations must be undertaken to emphasize the acquired results.
7. Conclusion
This study results showed that both banana flower and stem extract revealed low cytotoxicity along with de-pigmenting effect however the flower showed better effect as compared with stem and control. However, further studies have to be piloted with standard tyrosinase inhibitors and exposing melanocytes for longer periods to validate these results. In future in vivo studies can be conducted to evaluate the effectiveness of the banana flower and stem extract clinically, which may lead to the development of a revolutionary non-invasive treatment in delaying repigmentation after surgical gingival depigmentation procedures – “A herbal Gingival brightening cream”.
Contributor Information
Sowmya N K, Email: drsowmyamds@gmail.com.
Goriparthi Neeharika Sree, Email: dr.neeharika91@gmail.com.
Pooja Patil, Email: poojavpatil30@gmail.com.
D.S. Mehta, Email: dsmehta2010@gmail.com.
References
- 1.Malhotra S., Sharma N., Basavaraj P. Gingival esthetics by depigmentation. J Periodontal Med Clin Pract. 2014;1:79–84. [Google Scholar]
- 2.Dummett C.O. Physiologic pigmentation of the oral and cutaneous tissues in the Negro. J Dent Res. 1946;25:421. doi: 10.1177/00220345460250060201. [DOI] [PubMed] [Google Scholar]
- 3.Sreeja C., Ramakrishnan K., Vijayalakshmi D., Devi M., Aesha I., Vijayabanu B. Oral pigmentation: a review. J Pharm Bio allied Sci. 2015;7:403–408. doi: 10.4103/0975-7406.163471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dummett C.O. Mental attitudes toward oral pigmentations. Military Medicine. 1969;134:119–123. [PubMed] [Google Scholar]
- 5.Steigmann S. Treatment of melanin-pigmented gingiva and oral mucosa by CO2 laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:14–15. doi: 10.1067/moe.2000.106396. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen D.H., Nguyen D.T., La L.H., et al. Depigmenting effect of Cinnamomum cassia Presl in B16F10 melanoma cells. Korean J Chem Eng. 2007;24:827–830. [Google Scholar]
- 7.Shimada Y., Tai H., Tanaka A., et al. Effects of ascorbic acid on gingival melanin pigmentation in vitro and in vivo. J Periodontol. 2009;80:317–323. doi: 10.1902/jop.2009.080409. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.H., Jang J.Y., Park C., Kim B.W., Choi Y.H., Choi B.T. Curcumin suppresses alpha-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int J Mol Med. 2010;26:101–106. [PubMed] [Google Scholar]
- 9.Liu-Smith F., Meyskens F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res. 2016;60:1264–1274. doi: 10.1002/mnfr.201500822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ninfa A.J., Ballou D.P., Benore M. second ed. John Wiley & Sons; 2009. Fundamental Laboratory Approaches for Biochemistry and Biotechnology. [Google Scholar]
- 11.Walzl A., Unger C., Kramer N., et al. The resazurin reduction assay can distinguish cytotoxic from cytostatic compounds in spheroid screening assays. J Biomol Screen. 2014;19:1047–1059. doi: 10.1177/1087057114532352. [DOI] [PubMed] [Google Scholar]
- 12.Hedin C.A., Larsson A. Large melanosome complexes in the human gingival epithelium. J Periodontol Res. 1987;22:10813–10815. doi: 10.1111/j.1600-0765.1987.tb01548.x. [DOI] [PubMed] [Google Scholar]
- 13.Plonka P.M., Passeron T., Brenner M., et al. What are melanocytes really doing all day long? Exp Dermatol. 2009;18:799–819. doi: 10.1111/j.1600-0625.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergamaschi O., Kon S., Doine A.I., Ruben M.P. Melanin repigmentation after gingivectomy: a 5-year clinical and transmission electron microscopic study in humans. Int J Periodontics Restorative Dent. 1993;13:85–92. [PubMed] [Google Scholar]
- 15.Prasad D., Sunil S., Mishra R., Sheshadri P. Treatment of gingival pigmentation: a case series. Indian J Dent Res. 2005;16:171–176. doi: 10.4103/0970-9290.29901. [DOI] [PubMed] [Google Scholar]
- 16.Perlmutter S., Tal H. Repigmentation of the gingiva following surgical injury. J Periodontol. 1986;57:48–50. doi: 10.1902/jop.1986.57.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Ginwalla T.M., Gomes B.C., Varma B.R. Surgical removal of gingival pigmentation. J Indian Dent Assoc. 1966;38:147–150. [PubMed] [Google Scholar]
- 18.Sheel V., Purwar P., Dixit J., Rai P. Ancillary role of vitamin C in pink aesthetics. BMJ Case Rep. 2015;8:1–4. doi: 10.1136/bcr-2014-208559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C.H., Ding H.Y., Kuo S.Y., Chin L.W., Wu J.Y., Chang T.S. Evaluation of in vitro and in vivo depigmenting activity of raspberry ketone from Rheum Officinale. Int J Mol Sci. 2011;12:4819–4835. doi: 10.3390/ijms12084819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apriasari M.L., Suhartono E. Bioactive compound and antioxidant activity of methanol extract Mauli bananas (Musa sp) stem. Int J Biosci Biochem Bioinforma. 2014;4:110–119. [Google Scholar]
- 21.Zhan-Wu Sheng, Ma Wei-Hong, Gao Jin-He, et al. Antioxidant properties of banana flower of two cultivars in China using 2, 2-diphenyl-1-picrylhydrazyl (DPPH,) reducing power, 2 2’-azinobis-(3-ethylbenzthiazoline-6-sulphonate (ABTS) and inhibition of lipid peroxidation assays. Afr J Biotechnol. 2011;10:4470–4477. [Google Scholar]
- 22.Phacharapiyangkul N., Thirapanmethee K., Sa-ngiamsuntorn K., Panich U., Lee C.-H., Chomnawang M.T. The ethanol extract of Musa sapientum Linn. Peel inhibits melanogenesis through AKT signaling pathway. Cosmetics. 2021;8:70–79. [Google Scholar]
- 23.Phacharapiyangkul N., Thirapanmethee K., Sa-ngiamsuntorn K., Panich U., Lee C.H., Chomnawang M.T. Effect of sucrier banana peel extracts on inhibition of melanogenesis through the ERK signaling pathway. Int J Med Sci. 2019;16:602–606. doi: 10.7150/ijms.32137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heravi G., Khazaeli P., Mehrabani M., Rahimi H., Nematolahi N. Cream formulation and in vitro evaluation of depigmenting and cytotoxic effect of Musa sapientum pericarp and Spinacia oleracea folium. Res Pharm Sci. 2021;7:5–15. [Google Scholar]
- 25.Priya R.K., Srivastava S., Singh K.K., Mathad C., Thind P.S. Study of antioxidant and antimicrobial properties, phytochemical screening and analysis of sap extracted from banana (Musa acuminata) pseudostem. Int J Adv Biotechnol Res. 2014;5:649–658. [Google Scholar]