Abstract
Background:
Assimilating diagnostic radiology education into undergraduate medical education remains a challenge. This challenge places a greater emphasis in surgical residency to ensure this education occurs. The objective of this study is to determine whether a 3D-reconstruction may improve surgical residents’ assessment of resectability of pancreatic lesions
Methods:
Four cases were identified of patients with a pancreatic lesion; high-quality, triphasic abdominal CT scans were obtained and evaluated to ensure sufficient resolution and slice thickness. The images then were used for 3D-reconstruction of the cases.
Results:
PGY3–5 residents had a statistically-significant higher percentage of correct answers on objective questionnaire items using CT in conjunction with 3D-reconstruction software versus CT only. PGY1–2 residents had a higher percentage of correct answers using 3D-reconstruction software, but the difference was not statistically significant. statistically significant.
Conclusions:
3D-reconstruction software could be a viable tool to augment radiology education within a surgery residency, especially in CT interpretation, but there appears to be a minimum threshold of knowledge needed for meaningful improvement; therefore, this software may be more useful for PGY3–5 residents than PGY1–2 residents.
Keywords: resectability, reconstruction, software, surgery, radiology, education
Introduction
Diagnostic radiology is closely integrated into the field of surgery, and it is important that surgeons be competent in ordering and interpreting radiologic tests for patients to aid in operating. However, studies have reported a low instance of diagnostic radiology education (1, 2), which contradicts the recognition of its value in training future physicians (3). One possible way to enhance radiology education during surgical residency is to implement 3D reconstruction software to augment the interpretation of CT scans. Software from companies such as MeVis Medical Solutions can construct a 3D image from high quality, triphasic CT scans to aid in visualization of an area before surgery. One application of this software is in surgical oncology; abdominal CT scans are used to assess resectability of a pancreatic lesion by examining the involvement in surrounding vasculature and the lesion’s shape and margins. 3D software can help residents and surgeons to view the tumor in a more recognizable form and aid in accurately assessing resectability before surgery is ever performed. The purpose of this study is to assess whether a 3D reconstruction software for abdominal CT scans for pancreatic lesions will lead to more accurate assessments in PGY1–5 surgical residents and if there is a group of residents that would benefit more from the software.
Materials and methods
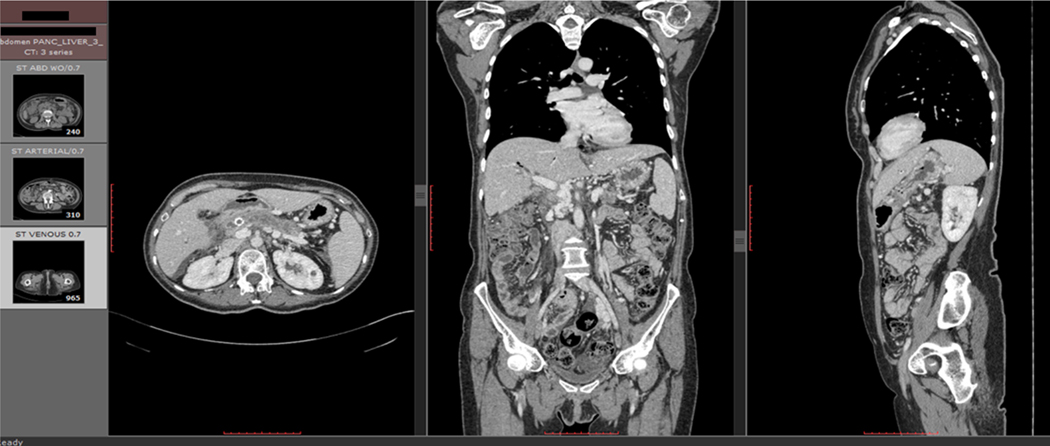
First, triple phase CT liver scans were obtained for 4 patients with pancreatic lesions (Fig. 1a). The CT scans were assessed for quality to ensure sufficient resolution and slice thickness for this assessment. This quality assessment is described in Appendix 1.
Figure 1.
Figure 1a: Triple Phase CT Scan. Triple phase CT scans were obtained for the livers of each patient. Scans included axial, coronal, and sagittal views of the abdomen in all three phases.
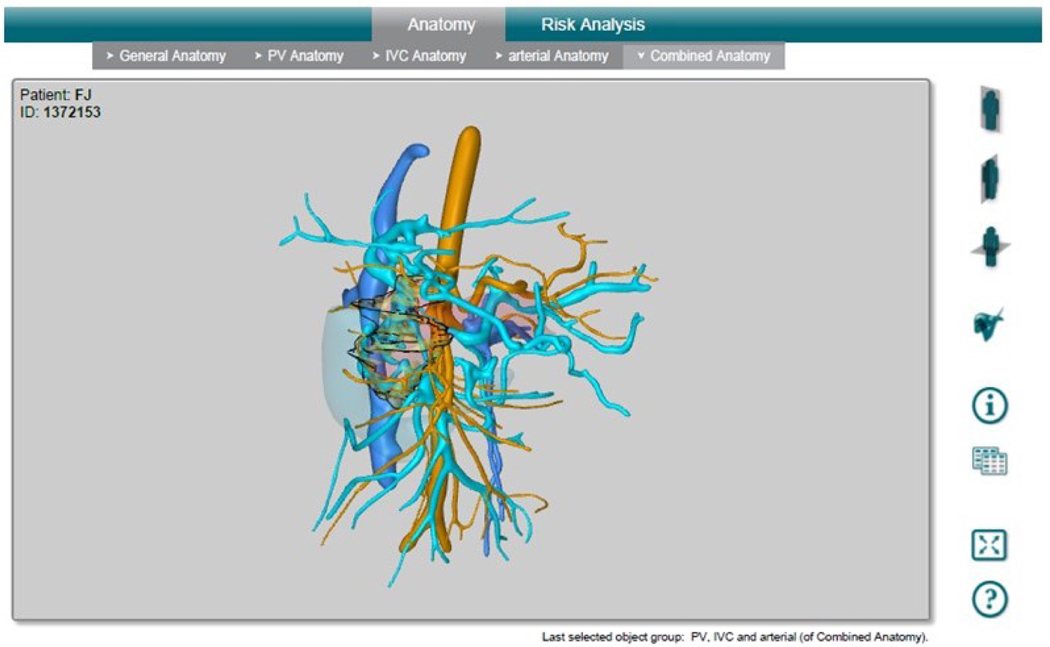
Figure 1b: MeVis 3D Reconstruction Software Interface. The software reconstructs arterial and venous vasculature, duodenum, the pancreas, and lesion. Arterial vasculature is shown in orange; venous vasculature is shown in blue; the duodenum is shown as a transparent blue solid volume; the pancreas is shown as a transparent pink solid volume; the lesion is shown as a transparent yellow solid volume in the reconstruction.
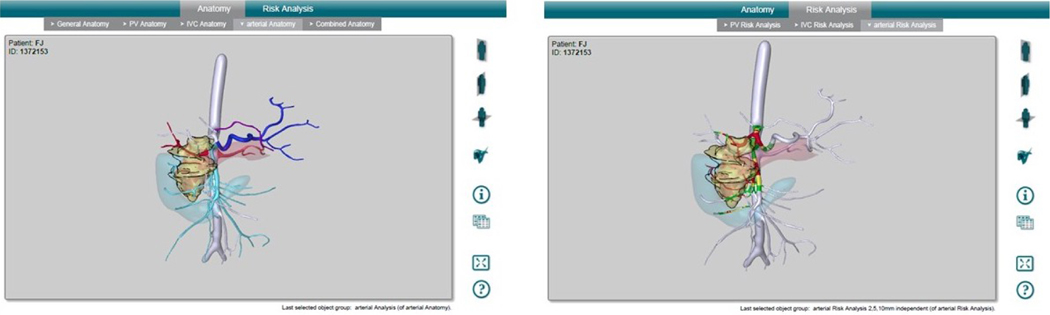
Figure 1c: MeVis 3D Reconstruction Interface without Risk Analysis (Left) and with Risk Analysis (Right). Vasculature is color coded based on distance from the lesion to aid the user in making a risk analysis of damage of the vasculature during a resection of the lesion. White vessels are more than 10 mm away from the lesion; green vessels are between 5–10 mm away from the lesion; yellow vessels are between 2–5 mm away from the lesion; red vessels are less than 2 mm away from the lesion, contacting the lesion, or encased within the lesion.
CT scans were then sent to MeVis Medical Solutions Ag, Distant Services Unit to be reconstructed into a 3D pdf file. The 3D pdf file includes reconstruction of the pancreas and lesion, portal vein vasculature reconstruction, inferior vena cava vasculature reconstruction, and arterial vasculature reconstruction (Fig. 1b), as well as a risk assessment for all vasculature which includes color coding the vessels based on how close each segment is to the lesion (Fig. 1c).
Surgical residents at the University of Louisville were contacted, and those who agreed to participate assessed the resectability of cases using either triphasic CT alone or triphasic CT in conjunction with the 3D reconstruction file from MeVis. Residents ranged from PGY1–5. The assessment was conducted via filling out a questionnaire, which is contained in Appendix 2. First, residents were asked to fill out the questionnaire using CT only; then, they filled out the questionnaire on the same case using both CT and the 3D reconstruction program. If time permitted, residents would go through an additional case in the same manner.
Dr. Robert Martin assessed each of the cases to determine the correct values for question 1 (T staging of the cancer), question 2 (risk of invasion and R1 resection for vessels), and question 3 (risk of R1 resection for margins of resection), and the residents’ answers were compared to the true values determined by Dr. Martin. Questions 4–5 were subjective questions on confidence of resectability of the tumor and obtaining R0 (margin negative) resection for all surgical margins described in question 3 (pancreatic neck transection, SMV portal vein groove, SMA retroperitoneum, and posterior along the IVC anterior surface), so these questions were used to determine whether confidence in resectability changed using CT only versus using CT in conjunction with the 3D reconstruction program.
Results
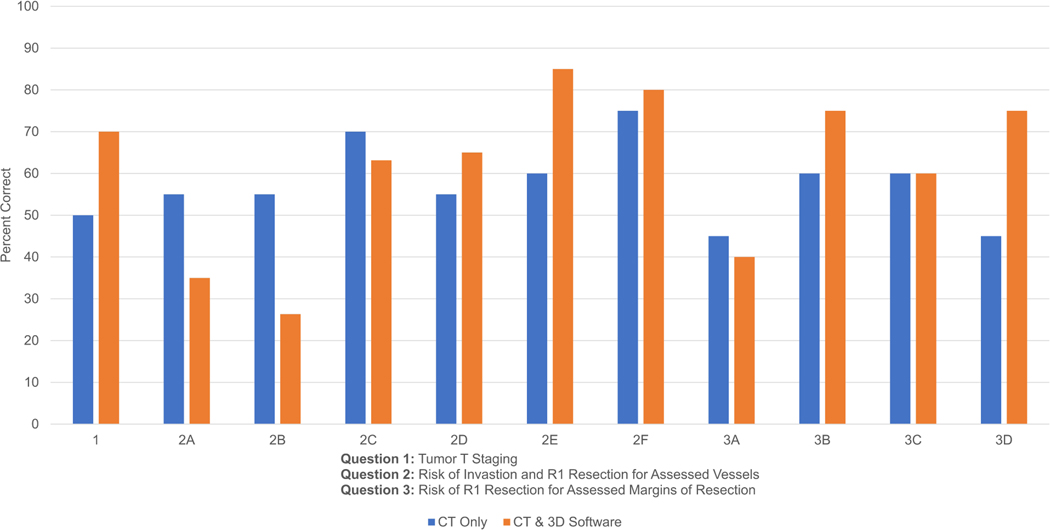
A total of (10) PGY1, (10) PGY2, (10) PGY3, (10) PGY4, and (8) PGY5 residents completed the assessment. The PGY1 and PGY2 residents were grouped together for assessment, and the PGY3, PGY4, and PGY5 residents were grouped together for assessment. The questionnaire consists of five questions: questions 1–3 have objective answers that assess staging of the tumor (T1-T4), its involvement with six major vessels near the pancreas (portal vein, splenic vein, SMV, SMA, hepatic artery, and splenic artery), and four surgical margins of resection (pancreatic neck transection, SMV portal vein groove, SMA retroperitoneum, and posterior along the IVC anterior surface). Questions 4–5 have subjective answers that assess confidence about resectability and specifically the ability to obtain R0 resection for all surgical margins discussed in question 3. Questions 1–3 were assessed for correctness since they are objective, and questions 4–5 were assessed as comparison of confidence before and after using the 3D software.
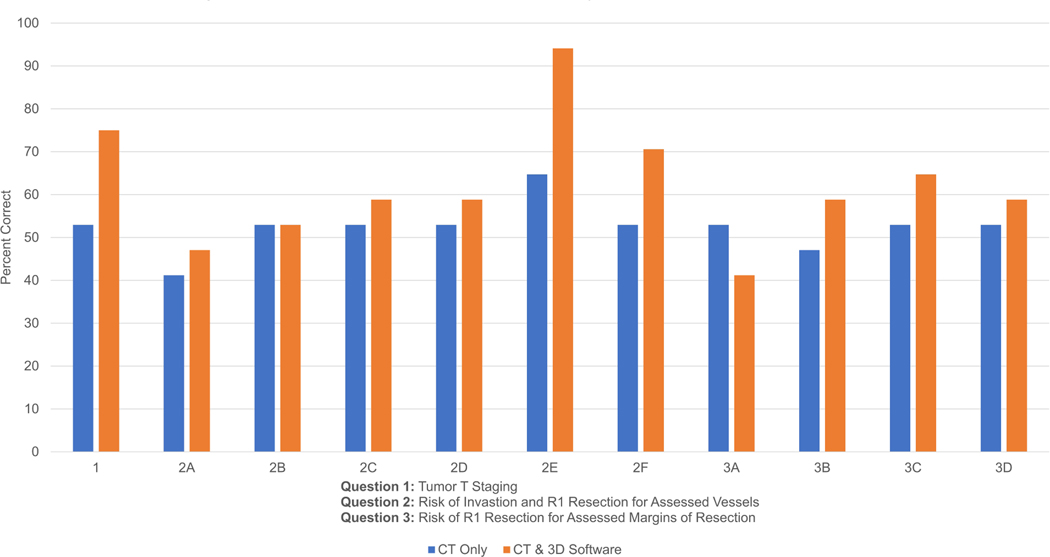
In questions 1–3, PGY1–2 residents overall scored 57.3% using CT only, compared to 61.3% overall using CT with the 3D software. On individual questions, residents scored higher on 6 out of 11 questions using CT with 3D software, higher on 4 out of 11 questions using CT scan only, and the same on one question (Fig. 2a).
Figure 2.
Figure 2a: PGY1–2 Results: CT Only vs. CT & 3D Software. PGY1–2 residents scored 57.3% overall using CT only while scoring 61.3% overall using CT with the 3D software.
Figure 2b: PGY3–5 Results: CT Only vs. CT & 3D Software. PGY3–5 residents scored 52.4% overall using CT only while scoring 61.9% overall using CT with the 3D software.
In questions 1–3, PGY3–5 residents overall scored 52.4% using CT only, compared to 61.9% overall using CT with the 3D software. On individual questions, residents scored higher on 9 out of 11 questions using CT with the 3D software, higher on 1 out of 11 questions using CT scan only, and the same on one question (Fig. 2b).
Questions 4–5 assessed degree of confidence in resectability of the tumor and confidence in obtaining R0 resection for all of the surgical margins in question 3 (pancreatic neck transection, SMV portal vein groove, SMA retroperitoneum, and posterior along the IVC anterior surface). Changes in residents’ responses between CT only compared to CT with 3D reconstruction software were examined to determine whether residents’ confidence in resectability was different with CT only compared to CT with 3D reconstruction. An increase in confidence is defined as going from a more moderate level of confidence to a more extreme level of confidence in resectability, regardless of whether the resident thought that the case was more or less resectable. Essentially, confidence was assessed on a scale from 1–5, where 3 indicated uncertainty, 1 was the highest level of confidence that the case was resectable, and 5 was the highest level of confidence that the case was not resectable; any response that deviated farther from uncertainty and the middle of the scale (3) compared to the original response is defined as an increase in confidence.
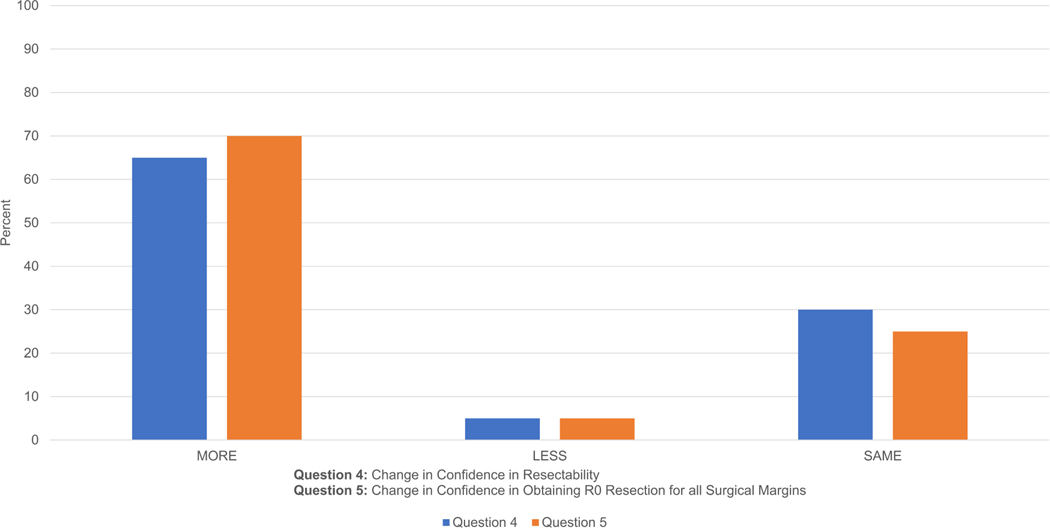
For question 4 (confidence in resectability), 20 cases were examined by PGY1–2 residents; 65% responded with a higher degree of confidence using CT with 3D reconstruction software versus CT alone; 5% responded with a lesser degree confidence, and 30% responded with the same degree of confidence. For question 5 (obtaining R0 resection for all surgical margins), 70% responded with a higher degree of confidence using CT with 3D reconstruction software versus CT alone, 5% responded with a lesser degree of confidence, and 25% responded with the same degree of confidence (Fig. 3a).
Figure 3.
Figure 3a: PGY1–2 Results: Questions 4–5 Confidence Level. On both questions, a majority of residents were more confident using the CT and 3D software together compared to using CT alone.
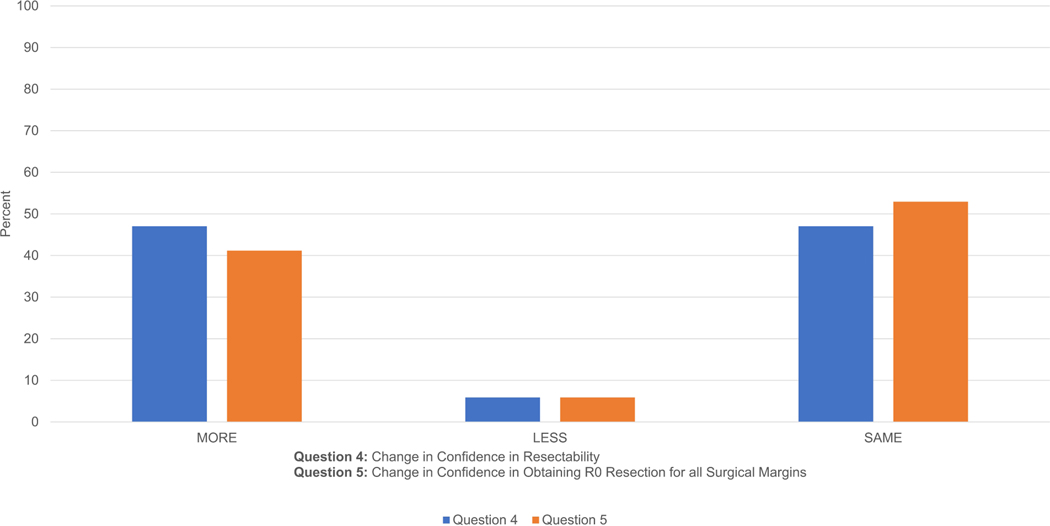
Figure 3b: PGY3–5 Results: Questions 4–5 Confidence Level. On both questions, a majority of residents were at least at the same degree of confidence using CT with 3D software compared to using CT alone; only 6% were less confident.
For question 4 (confidence in resectability), 17 cases were examined by PGY3–5 residents; 47% responded with a higher degree of confidence using CT with 3D reconstruction software versus CT alone, 6% responded with a lesser degree of confidence, and 47% responded with the same degree of confidence. For question 5 (obtaining R0 resection for all surgical margins), 41% responded with a higher degree of confidence using CT with 3D reconstruction versus CT alone, 6% responded with a lesser degree of confidence, and 53% responded with the same degree of confidence (Fig. 3b).
Discussion
On questions 1–3, which were objective and assessed knowledge of T staging of the tumor, its involvement with six major vessels near the pancreas, and four surgical margins of resection, PGY1–2 residents scored higher overall using the 3D software (61.3%) compared to CT scan alone (57.3%). However, using a paired t-test, P=0.48 at a 95% confidence limit, so this difference is not considered statistically significant. PGY3–5 residents also scored higher overall using the 3D software (61.9%) compared to CT scan alone (52.4%). Using a paired t-test, P=0.017 at a 95% confidence level, so this difference is considered statistically significant. PGY3–5 residents have more knowledge and surgical experience in assessing resectability through CT scans, whereas it is possible that PGY1–2 residents have not had enough experience for this software to make a significant difference yet in their ability to answer the questions correctly. Therefore, it seems that this 3D model would have a more significant impact in improving PGY3–5 residents’ assessments of the tumor and its resectability.
Questions 4 and 5 were subjective in nature and assessed confidence in resection of the tumor as well as confidence in attaining R0 for the surgical margins in question 3. A majority of PGY1–2 residents responded with a higher degree of confidence on both questions when using the 3D software compared to using CT scan alone; it seems the PGY1–2 residents feel more confident in assessing resectability and the ability to obtain R0 resection with the 3D software, even though their scores didn’t improve significantly on the objective questions as stated above. Similarly, more PGY35 residents also responded with a higher degree of confidence than those who responded with a lower degree of confidence. Therefore, it seems that using the 3D software makes the residents feel more confident about resectability and the ability to obtain R0 resections R0 resection versus only viewing the case as a CT scan without a 3D model.
Similar software has already been implemented in the field of surgery in various ways with some success. There are many examples of using 3D reconstruction software to aid in preoperative planning for tumor staging and anatomy in hilar cholangiocarcinomas (4, 5), biliary cancers (6), pancreatic tumors (7, 8), local resection of hepatocellular carcinoma (9), and pediatric solid tumors (10). Similar software has also been used as a preoperative risk analysis tool for hepatic resection (11). 3D reconstruction software can be especially useful to assess whether a patient’s case is complex or atypical and if so, aid in preoperative planning; this has been shown useful during hepatic resection in patients with unconventional resection planes (12) and hepatic artery variance (13). One study compared CT angiography, which is used to assess resectability in pancreatic tumors, to a 3D reconstructed model from CT images and found the 3D model to have higher PPV, NPV, sensitivity, specificity, and overall accuracy (14). Another study compared 3D models to 2D MRI’s, and a majority of surgeons surveyed found the 3D model easier to use than the 2D models; the models could potentially increase accuracy, and decrease preoperative planning (15). 3D reconstruction software and modeling has been used even earlier in medical education to supplement clinical anatomy to medical students for hepatic anatomy (16, 17).
Implementing this technology would require further training for surgeons to use effectively; one study found that after one day of training, a majority of surgeons in the study assessed the software positively, but a majority also said that more than one day of training would be needed for formal training to make the 3D models even more useful (18). The technology is also evolving; there are many different open source programs with different interfaces, so a challenge would be to develop a more standardized and centralized 3D model with similar risk assessment.
3D reconstruction technology continues to evolve and improve to become more useful and intuitive to surgeons. There is an example of improving how the 3D models are visualized using cinematic volume rendering, which can be used to simulate camera effects such as shadows and lighting to make the model look cadaver-like and more realistic (19). Some surgeons have used the 3D models as a form of augmented reality during surgery to assist in real time during pancreatico-duodenectomy (20) and other operations such as cholecystectomy, distal pancreatic resection, and robotic liver resection (21). One study even used a autostereoscopic headset for live updates with integrated video, with the assertion that this technology has a future place in telemedicine, surgical planning, and navigation (22); this could have a place in resident training in the future, with residents seeing more cases and rarer procedures without needing to being present the operating room.
Conclusions
The 3D reconstruction software used by MeVis to turn high quality CT scans into a 3D model with risk assessment of vasculature involvement in a pancreatic lesion could be a viable educational tool to assess the possibility of surgical resection, especially in higher-level surgical residents (PGY3–5). It seems that background knowledge and competency is needed for this tool to be useful and to show a meaningful improvement in resectability assessment. Using a 3D model could also improve confidence in assessing resectability and obtaining R0 resection. In the field of surgery, it is increasingly important for surgeons to not only be competent in surgical procedures but also in other aspects of medicine like radiology and being able to perform many different procedures. 3D reconstruction software, such as the one being evaluated in this study, will be an important tool in the future of surgery to aid surgeons in giving the best care possible for a wide variety of patient cases. This software has already been implemented elsewhere in the field of surgery with some success, and with an appropriate threshold of expertise to make these models useful, there is potential to improve patient care and minimize adverse surgical outcomes.
Supplementary Material
Highlights.
Surgical residency radiology training is needed to make up for inadequate training of undergraduates
High-resolution CT scans can be processed by software to produce 3-D imaging
3D software makes the residents feel more confident about resectability and the ability to obtain R0 resections R0 resection versus only viewing the case as a CT scan
ACKNOWLEDGEMENTS
NCI R25 Grant, University of Louisville Cancer Education Program (R25-CA134283)
Funding:
This work was supported by the National Institutes of Health (R25-CA134283).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saha A, Roland RA, Hartman MS, Daffner RH. Radiology medical student education: an outcome-based survey of PGY-1 residents. Acad Radiol. 2013. Mar;20(3):284–9. PubMed PMID: 23452472. [DOI] [PubMed] [Google Scholar]
- 2.Oancea L, Gunderman R, Carrico C, Straus C. Teaching radiology in medical school: an association for medical student educators in radiology survey. Acad Radiol. 2013. Feb;20(2):255–6. PubMed PMID: 23395243. [DOI] [PubMed] [Google Scholar]
- 3.Webb EM, Naeger DM, McNulty NJ, Straus CM. Needs Assessment for Standardized Medical Student Imaging Education: Review of the Literature and a Survey of Deans and Chairs. Acad Radiol. 2015. Oct;22(10):1214–20. PubMed PMID: 26259548. [DOI] [PubMed] [Google Scholar]
- 4.Endo I, Shimada H, Sugita M, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery. 2007. Nov;142(5):666–75. PubMed PMID: 17981186. [DOI] [PubMed] [Google Scholar]
- 5.Qu J, Fung A, Kelly P, et al. Visualising a rare and complex case of advanced hilar cholangiocarcinoma. J Vis Commun Med. 2017. Jan;40(1):26–31. PubMed PMID: 28290711. Epub 2017/03/16. [DOI] [PubMed] [Google Scholar]
- 6.Okuda Y, Taura K, Seo S, et al. Usefulness of operative planning based on 3-dimensional CT cholangiography for biliary malignancies. Surgery. 2015. Nov;158(5):1261–71. PubMed PMID: 26054319. Epub 2015/06/10. [DOI] [PubMed] [Google Scholar]
- 7.Fang CH, Kong D, Wang X, et al. Three-dimensional reconstruction of the peripancreatic vascular system based on computed tomographic angiography images and its clinical application in the surgical management of pancreatic tumors. Pancreas. 2014. Apr;43(3):389–95. PubMed PMID: 24622068. [DOI] [PubMed] [Google Scholar]
- 8.Wen Z, Yao F, Wang Y. 64-Slice spiral computed tomography and three-dimensional reconstruction in the diagnosis of cystic pancreatic tumors. Exp Ther Med. 2016. Apr;11(4):1506–12. PubMed PMID: 27073473. Pubmed Central PMCID: PMC4812263. Epub 2016/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang Y, Peitgen HO, et al. Precise local resection for hepatocellular carcinoma based on tumor-surrounding vascular anatomy revealed by 3D analysis. Dig Surg. 2012;29(2):99–106. PubMed PMID: 22441716. Epub 2012/03/24. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs J, Warmann SW, Szavay P, et al. Three-dimensional visualization and virtual simulation of resections in pediatric solid tumors. J Pediatr Surg. 2005. Feb;40(2):364–70. PubMed PMID: 15750930. [DOI] [PubMed] [Google Scholar]
- 11.Lang H, Radtke A, Hindennach M, et al. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg. 2005. Jul;140(7):629–38; discussion 38. PubMed PMID: 16027326. [DOI] [PubMed] [Google Scholar]
- 12.Radtke A, Sotiropoulos GC, Molmenti EP, et al. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg. 2010. Nov;252(5):876–83. PubMed PMID: 21037445. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Fang CH, Fan YF, et al. To assess the benefits of medical image three-dimensional visualization system assisted pancreaticoduodenctomy for patients with hepatic artery variance. Int J Med Robot. 2014. Dec;10(4):410–7. PubMed PMID: 24711375. Epub 2014/04/09. [DOI] [PubMed] [Google Scholar]
- 14.Fang CH, Zhu W, Wang H, et al. A new approach for evaluating the resectability of pancreatic and periampullary neoplasms. Pancreatology. 2012. Jul-Aug;12(4):364–71. PubMed PMID: 22898639. Epub 2012/08/18. [DOI] [PubMed] [Google Scholar]
- 15.Yeo CT, MacDonald A, Ungi T, et al. Utility of 3D Reconstruction of 2D Liver Computed Tomography/Magnetic Resonance Images as a Surgical Planning Tool for Residents in Liver Resection Surgery. J Surg Educ. 2017. Aug 16. PubMed PMID: 28822820. Epub 2017/08/22. [DOI] [PubMed] [Google Scholar]
- 16.Jurgaitis J, Paskonis M, Pivoriunas J, et al. The comparison of 2-dimensional with 3-dimensional hepatic visualization in the clinical hepatic anatomy education. Medicina (Kaunas). 2008;44(6):428–38. PubMed PMID: 18660637. Epub 2008/07/29. [PubMed] [Google Scholar]
- 17.Kong X, Nie L, Zhang H, et al. Do Three-dimensional Visualization and Threedimensional Printing Improve Hepatic Segment Anatomy Teaching? A Randomized Controlled Study. J Surg Educ. 2016. Mar-Apr;73(2):264–9. PubMed PMID: 26868314. Epub 2016/02/13. [DOI] [PubMed] [Google Scholar]
- 18.Ganry L, Hersant B, Bosc R, et al. Study of medical education in 3D surgical modeling by surgeons with free open-source software: Example of mandibular reconstruction with fibula free flap and creation of its surgical guides. J Stomatol Oral Maxillofac Surg. 2018. Feb 27. PubMed PMID: 29499364. Epub 2018/03/03. [DOI] [PubMed] [Google Scholar]
- 19.Glemser PA, Engel K, Simons D, et al. A New Approach for Photorealistic Visualization of Rendered Computed Tomography Images. World Neurosurg. 2018. Mar 7. PubMed PMID: 29524708. Epub 2018/03/11. [DOI] [PubMed] [Google Scholar]
- 20.Marzano E, Piardi T, Soler L, et al. Augmented reality-guided artery-first pancreatico-duodenectomy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013. Nov;17(11):1980–3. PubMed PMID: 23943389. [DOI] [PubMed] [Google Scholar]
- 21.Volonte F, Pugin F, Bucher P, et al. Augmented reality and image overlay navigation with OsiriX in laparoscopic and robotic surgery: not only a matter of fashion. J Hepatobiliary Pancreat Sci. 2011. Jul;18(4):506–9. PubMed PMID: 21487758. [DOI] [PubMed] [Google Scholar]
- 22.Fan Z, Weng Y, Chen G, Liao H. 3D interactive surgical visualization system using mobile spatial information acquisition and autostereoscopic display. J Biomed Inform. 2017. Jul;71:154–64. PubMed PMID: 28533140. Epub 2017/05/24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.