Abstract
Hunter syndrome is a neurodegenerative lysosomal storage disorder with limited treatment options to halt the progressive neurocognitive decline. Whilst Intravenous enzyme replacement therapy (ERT) does not cross the blood brain barrier; Intrathecal ERT, in clinical studies, did not demonstrate significant effect on cognition, despite having better CNS delivery. Hematopoietic stem cell transplantation (HSCT) has the potential to treat CNS disease. We reviewed the literature and outline our experience of treating two siblings with severe Hunter syndrome: ‘Sibling A' with intravenous and intrathecal ERT and ‘Sibling B' with Early HSCT. A literature review identified 8 articles reporting on the comparative efficacy of both treatments. Our clinical outcomes indicate that Sibling B performed better than Sibling A in relation to early developmental milestones as well as neurocognition, activities of daily living, quality of life and neurophysiological outcomes in mid childhood. Sibling A's developmental trajectory fell within the extremely low range and Sibling B's development trajectory fell within the low-average to average range. This suggests HSCT had a disease modifying effect and highlights the efficacy of early HSCT in moderating the CNS progression in Hunter syndrome. Long term follow up is needed to elucidate the efficacy of HSCT on neurological progression.
Keywords: Hunter syndrome, Mucopolysaccharidosis II, Enzyme replacement therapy(ERT), Neurocognition, Activities of daily living/ADL, Quality of life, Severe Hunter syndrome, Haematopoietic stem cell transplantation(HSCT)
1. Introduction
Hunter syndrome or Mucopolysaccharidosis II, is an X-linked, progressive lysosomal storage disease in which a deficiency of the lysosomal enzyme, Iduronate-2-sulfatase (I2S) leads to multi organ accumulation of the glycosaminoglycans (GAGS) dermatan and heparan sulphate and the resultant clinical sequelae. Phenotypical spectrum is varied with significant neurological impairment and regression noted in the severe phenotypes which represent nearly two thirds of the patients with Hunter syndrome [1]. Untreated, these patients do not survive beyond the second decade of life. Current treatment options are limited. Enzyme replacement therapy (ERT) has become the mainstay of treatment and has proven efficacy in reducing the GAG levels, improving visceral organ function and survival. Intravenous ERT is however, expensive, needs weekly administration and does not cross the blood brain barrier and is therefore ineffective in arresting cognitive decline. Theoretically, intrathecal ERT should improve CNS delivery of the drug, however the result of an open label phase I/II study did not demonstrate a significant improvement in cognition [2]. Successful Haematopoietic stem cell transplantation (HSCT) enables donor stem cells to migrate across the blood brain barrier and has the potential to treat central nervous system disease (CNS) if initiated early. We hereby present a comparative report of early HSCT versus ERT in two siblings with severe Hunter syndrome. We also present a literature review on the efficacy of these two treatment strategies in severe Hunter syndrome.
2. Aims
-
1)
To report our experience of early HSCT as compared to ERT on neurological progression in siblings with severe Hunter syndrome.
-
2)
To report findings from a literature review of the two treatment strategies in severe Hunter syndrome.
3. Outcome measures
Primary outcome measures:
-
a)
Initial developmental assessments in infancy and regular neurocognitive follow-up assessments
-
b)
Monitoring Activities of Daily Living (ADL's)
-
c)
Parental report of Paediatric Quality of Life (QoL)
Secondary outcome measures:
-
a)
Neuro imaging findings.
-
b)
Neurophysiology.
-
c)
Cardiovascular structural and functional changes.
-
d)
Urinary Glycosaminoglycans.
-
e)
Growth.
4. Methodology
4.1. Case report
Subjects and setting: Two pre-identified siblings (A and B) with genetically confirmed Hunter syndrome, predictive of severe neurological phenotype, at a single tertiary center.
Data collection: Patient records were retrospectively reviewed and information on clinical presentation, genotype, clinical intervention, primary and secondary outcome measures were gathered. Quality of life scores were measured using the Paediatric Quality of Life (PEDS-QoL) questionnaire.
Ethics: Clinical interventions and monitoring were part of standard clinical care and hence ethical approval was not required for the study. Parental consent was sought for publication.
4.2. Literature review
Preliminary search of Medline, Embase and Cochrane library was undertaken using different combinations of the following keywords: Hunters syndrome, Mucopolysaccharidosis type II, Bone marrow transplantation, enzyme replacement therapy (ERT), haematopoietic stem cell transplantation (HSCT). Idursulfase, ERT for Hunter syndrome has been available since 2006 and hence our search strategy was to capture all the articles comparing the efficacy of the ERT and HSCT published during the time period 2006–2021.
Articles comparing the efficacy of HSCT and ERT in Hunter syndrome were studied and data related to our primary or secondary outcome measures, where available, was collected.
5. Results
5.1. Case report
Patient characteristics
Index case, sibling A, was diagnosed with Hunter syndrome at the age of 2 years. Sibling B was diagnosed soon after birth. Both siblings were confirmed to be hemizygous for the pathogenic mutation G224E in the IDS which predicts the severe phenotype [3].
Clinical interventions
Sibling A: Intravenous and Intrathecal ERT.
At 25 months of age, Sibling A was commenced on weekly intravenous Idursulfase and at 36 months a monthly intrathecal Idursulfase (HGT-HIT-094) at a dose of 10 mg. At 9 years of age he continues on both intravenous and intrathecal ERT.
Sibling B: Early HSCT.
At 6 weeks of age, Sibling B received early HSCT. This was in view of the potential CNS involvement. He did not receive ERT prior to the transplant period.
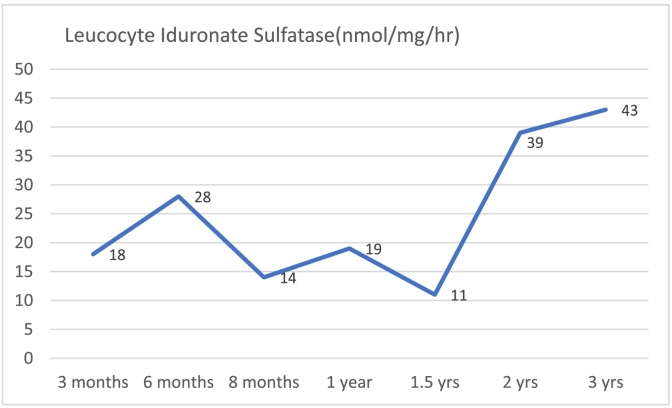
He received unrelated cord blood stem cell (6/6) transplant from a 3 year old female donor with O + ve blood group and was EBV and CMV negative. Sibling B was conditioned using a reduced intensity conditioning regime including Fludarabine, Busulfan and ATG(anti-thymocyte antigen. Stem cell transplant was successful with full donor chimerism and normal white cell enzyme levels, 3 months post-transplant and being maintained over time (Graph 1 showing white cell enzymes). Post-transplant course was complicated by severe skin, gut and ocular graft versus host disease. He lost sight in one eye due to severe graft vs host disease and received ocular allogeneic stem cell therapy. He needed enteral feeding support via gastrostomy for gut GVHD for a period of 12 months post-transplant. He also developed bronchiectasis and bronchiolitis obliterans, two years post-transplant, and continues to receive prophylactic antibiotics and chest physiotherapy.
Graph 1.
White cell enzyme levels with time
Outcome measures:
Primary Outcome measures:
-
a)
Neurocognitive performance
Initial Developmental Assessment.
Both siblings had regular assessments to monitor the efficacy of the two treatments. Initial developmental assessment was undertaken during infancy using Bayley Scales of Infant and Toddler Development (at 27 and 28 months respectively – see Table 1).
Table 1.
Initial developmental assessment – Bayley Scales of Infant and Toddler Development.
| Domain | Composite Score | Sibling A - Age 27 Months |
Sibling B – Age 28 months |
|||||
|---|---|---|---|---|---|---|---|---|
| Age Equivalent | Percentile Rank | Qualitative Descriptor | Composite Score | Age Equivalent | Percentile Rank | Qualitative Descriptor | ||
| Language | 56 | – | 0.2 | Extremely Low | 83 | 13 | Low Average | |
| Receptive | 10 months | 24 months | ||||||
| Expressive | 9 months | 16 months | ||||||
| Motor | 85 | – | 16 | Low Average | 97 | 42 | Average | |
| Fine | 21 months | 28 months | ||||||
| Gross | 26 months | 20 months | ||||||
| Cognitive | 90 | – | 25 | Average | 100 | 50 | Average | |
At initial developmental assessment: Sibling B's scores were all higher than Sibling A's scores across all of the developmental domains. Nevertheless, both Sibling A and Sibling B's scores all fell within the broader average ranges with the exception of Sibling A's language scores which fell within the extremely low ranges.
Follow-up assessment.
Follow-up assessments were completed during childhood, using the Wechsler Pre-School and Primary Scale of Intelligence (WPPSI-IV) and the Wechsler Intelligence Scale for Children (WISC-V). Only a limited range of assessments could be completed by both siblings: the Visual Spatial Index (VSI); a measure of Processing Speed (Bug Search) and a measure of Language (Receptive Vocabulary), therefore, only these outcomes have been reported for both Sibling A and Sibling B.
Sibling A received assessments at 7 years 4 months and 9 years 9 months of age (see Table 2) and Sibling B received assessments at 4 years 6 months and 6 years 6 months (Table 3).
Table 2.
Sibling A: follow-up assessments.
| Index/Subtest | 7 years and 4 months (WPPSI-IV) |
9 years and 3 months (WPPSI-IV and WISC-V) |
||||||
|---|---|---|---|---|---|---|---|---|
| Index/Scaleds Score | Age Equivalent | Percentile Rank | Qualitative Descriptor | Composite Score | Age Equivalent | Percentile Rank | Qualitative Descriptor | |
| Visual Spatial | 66 (WPPSI-IV) | – | 1st | Extremely Low | 64 (WISC-V) | – | 1st | Extremely Low |
| Block Design (WPPSI-IV) | 4 | 4:1 | 2nd | Very Low | N/A | 4:7 | ||
| Block Design (WISC-V) | 4 | 2nd | Very Low | |||||
| Object Assembly (WPPSI-IV) | 4 | 4:4 | 2nd | Very Low | 6:6 | |||
| Visual Puzzles (WISC-V) | 3 | 1st | Extremely Low | |||||
| Processing Speed Index | ||||||||
| Bug Search | 6 | 4:7 | 5:1 | Low Average | 5:1 | |||
| Language Index | ||||||||
| Receptive Vocabulary | 1 | <2:7 | 0.1 | Extremely Low | 2:7 | |||
Table 3.
Sibling B: follow-up assessments.
| Index/Subtest | 4 years and 6 months (WPPSI-IV) |
6 years and 6 months (WPPSI-IV and WISC-V) |
||||||
|---|---|---|---|---|---|---|---|---|
| Index/Scaled Score | Age Equivalent | Percentile Rank | Qualitative Descriptor | Composite Score | Age Equivalent | Percentile Rank | Qualitative Descriptor | |
| Visual Spatial | 100 | – | 50 | Average | 88 | – | 21 | Low Average |
| Block Design (WPPSI-IV) | 10 | 4:7 | 50th | Average | 8 | 5:4 | 25th | Average |
| Object Assembly (WPPSI-IV) | 10 | 4:4 | 50th | Average | 8 | 5:7 | 25th | Average |
| Processing Speed Index | ||||||||
| Bug Search | 6 | <4:1 | 9th | Low Average | 6 | 4:10 | 9th | Low Average |
| Vocabulary Acquisition | ||||||||
| Receptive Vocabulary | 15 | 5:10 | 95th | Superior | ||||
A comparison of Sibling A and Sibling B's WPPSI-V scores at 7 years 4 months (Sibling A) and 6 years 6 months (Sibling B) is presented in Table 4. These scores represent assessments at a similar age, and both utilise the WPPSI-IV, which provides an opportunity for direct comparison.
Table 4.
Sibling A and Sibling B: comparison of follow-up assessments.
| Index/Subtest | Index/Scaled Score | Sibling A – 7 years and 4 months |
Sibling B – Age 6 years and 6 months |
|||||
|---|---|---|---|---|---|---|---|---|
| Age Equivalent | Percentile Rank | Qualitative Descriptor | Composite Score | Age Equivalent | Percentile Rank | Qualitative Descriptor | ||
| Visual Spatial | 66 | – | 1st | Extremely Low | 88 | – | 21st | Low Average |
| Block Design | 4 | 4:1 | 2nd | Very Low | 8 | 5:4 months | 25th | Average |
| Object Assembly | 4 | 4:4 | 2nd | Very Low | 8 | 5:7 months | 25th | Average |
| Processing Speed | ||||||||
| Bug Search | 6 | 5:1 | 9th | Low average | 6 | 4:10 months | 9th | Low Average |
Sibling A's visual spatial abilities have consistently fallen within the extremely low range, however, he continues to make progress in all areas as reflected by the increase in his WPPSI-IV ‘age equivalent’ scores.
Sibling B's visual spatial abilities have consistently fallen within the broader average ranges, and whilst he continues to make progress in all areas (as reflected by his age equivalent scores), there has been a slight reduction in his standardized scaled scores and index scores between age 4 and 6 years.
Sibling B's visual spatial abilities were significantly better than Sibling A's visual spatial abilities when they were assessed at 6 and 7 years of age. Their processing speed abilities were, however, comparable.
Overall Comparisons.
In infancy, both Sibling A and Sibling B achieved scores within the average ranges on the ‘cognitive scale’ (at 27 and 28 months respectively), albeit Sibling B's scores were higher than Sibling A's.
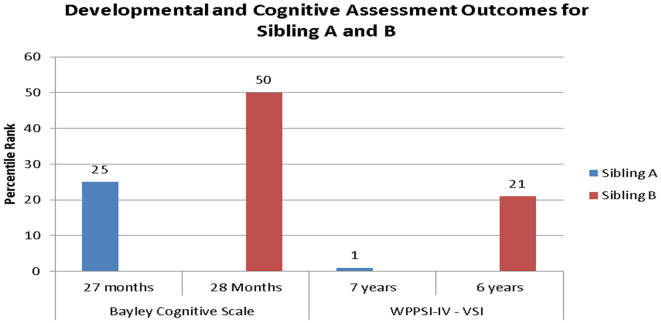
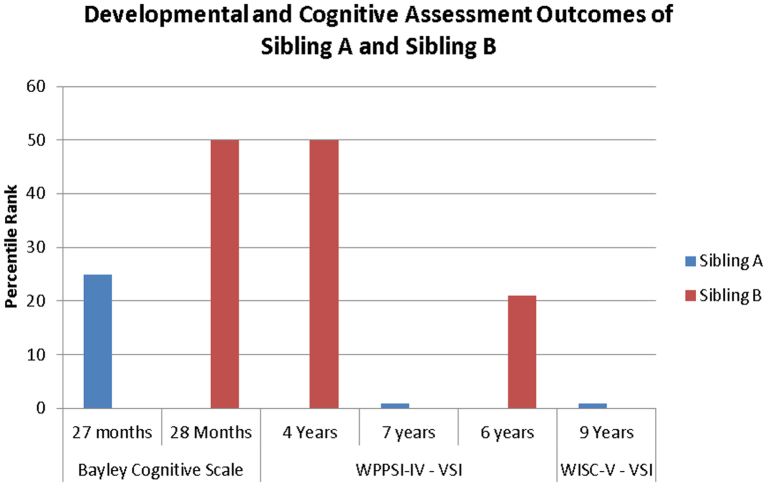
In childhood, Sibling B's visual spatial scores were significantly better than Sibling A's, whereby Sibling B's scores fell within the low average ranges, and Sibling A's fell within the extremely low ranges (see Fig. 1, Fig. 2).
-
b)
Activities of Daily Living (ADL's)
Fig. 1.
Initial and Follow-up Assessments for Sibling A and Sibling B: Direct Comparisons.
Fig. 2.
Initial and Follow-Up Assessments for Sibling A and Sibling B: All assessments.
Sibling A, Sibling B and their parents reported on their ADL's during their routine medical reviews. This included reports of: education; social activities; physical activities and self-care. Their medical records were reviewed and their responses collated as follows:
Sibling A: needs help with most ADL's. He doesn't have normal range of movements at his joints. He attends special needs school.
Sibling B is independent and does not need any help with ADL's. He enjoys gymnastics and has normal range of movements at his joints. He attends mainstream school.
-
c)
Quality of life scores:The Paediatric Quality of Life Inventory (Peds-QL) is a brief measure of health-related quality of life (HR-QoL) in children and young people. It assesses four domains of HR-QoL: physical, emotional, social and school functioning.
Questionnaires were completed by both parents and their responses indicate that Sibling B's Physical and Psychosocial HR-QoL is better than Sibling A's (whereby scores are out of 100, and higher scores are indicative of better HR-QoL) (see Table 5).
Table 5.
Paediatric quality of life (PEDQ-OL) outcomes.
| Sibling A | Sibling B | |
|---|---|---|
| Physical Health QoL | 50 | 93.75 |
| Psychosocial QoL | 48.33 | 83.3 |
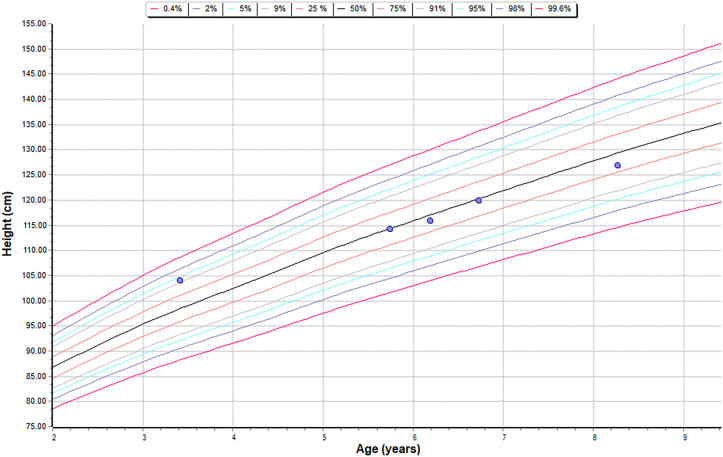
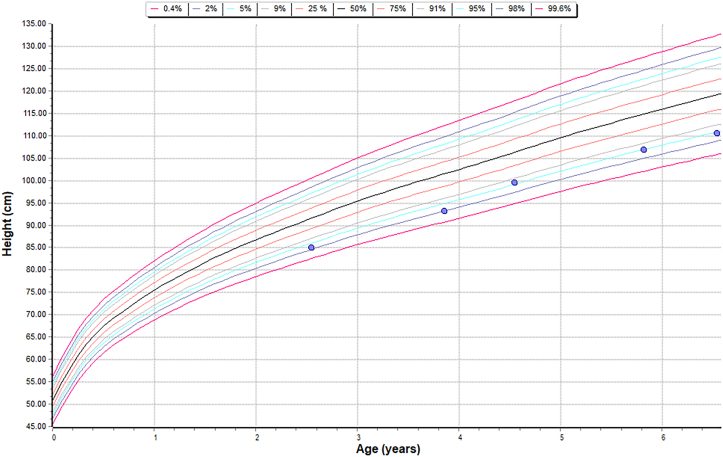
Height chart – sibling A – on Intravenous and Intrathecal ERT.
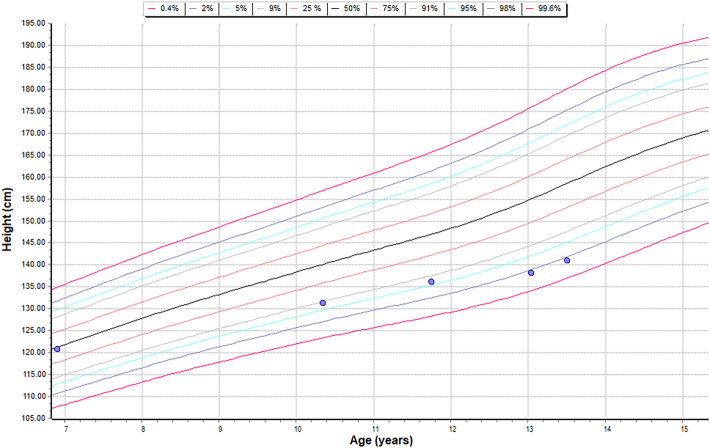
Height chart – sibling B – HSCT.
Height chart – patient on Intravenous ERT.
Comparison of linear growth data of both siblings and a patient who is on just intravenous ERT to understand the effect of intervention on the linear growth.
5.2. Literature review
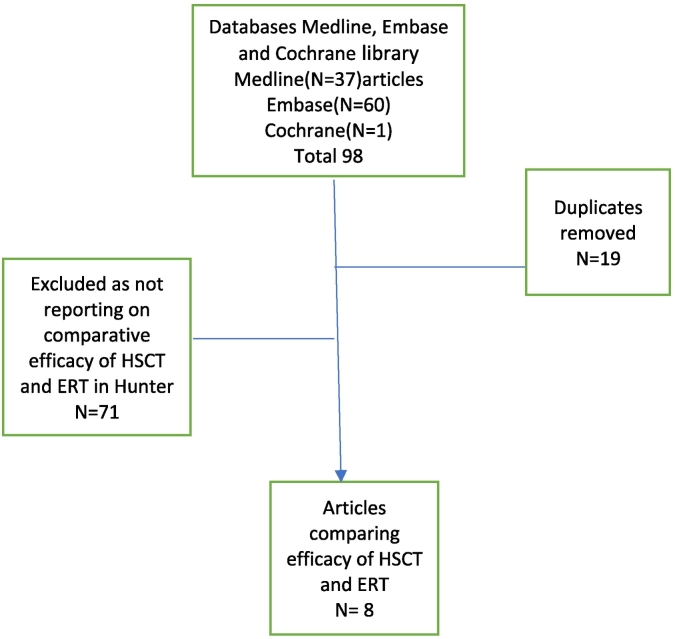
Our search identified 98 articles, out of which 90 were excluded and only 8 articles reporting the comparative efficacy of HSCT and ERT were included.
Literature search - Comparative efficacy of Haematopoietic Stem Cell Transplantation Vs Enzyme Replacement Therapy in Hunter Syndrome, (November 2021), performed by Derick Yates, Birmingham Women's and Children's NHS Trust Library and Knowledge service.
Summary of patient characteristics and clinical interventions for all the eight studies included in the review( Table 7 ):
Table 7.
Summary of the literature review.
| Author&Date | Purpose | Methods | Results | Comments |
|---|---|---|---|---|
| Patel et al. 2014 [4] | To study the efficacy of ERT and HSCT on growth and clinical data | Data was obtained from 44 Japanese male patients with MPS II. Age range: 9 months – 18 years Phenotype: 35 severe and 9 attenuated 26 patients - ERT, 12 patients - HSCT 6 - both ERT and HSCT |
MPS II patients, who had been treated with either ERT or HSCT, had increased height and weight when compared to untreated patients. HSCT and ERT were equally effective in restoring growth of MPS II patients. | Study reported effect of both treatments on growth in severe phenotypes but no data available on either of the primary or secondary outcome measures |
| Tanjuakio et al. 2015 [5] | To assess the clinical phenotype and therapeutic efficacy of ERT and HSCT in patients with Hunter syndrome. | A questionnaire of activities of daily living(ADL) with 3 domains: “movement,” “movement with cognition,” and “cognition.” Age range:4–49 years Subjects: 51- severe phenotype 23- attenuated phenotype Early ERT:20 Late ERT:25 Early HSCT: 10 with severe phenotype Late HSCT: 8 with severe phenotype |
HSCT provides a higher ADL score than early ERT, and there was a significant difference in ADL scores between late ERT and HSCT groups. ADL scores of patients treated with HSCT were not statistically different from those treated with early ERT, average scores were higher for the HSCT treated patients for each domain and each age group. While “Movement” and “Movement with Cognition” scores appear to be stable across age groups for HSCT treated patients, cognition scores remain low for HSCT treated patients. The questionnaire showed the benefits of early treatment, with HSCT showing better results compared to ERT, although the differences were not statistically significant. |
Study included children with severe phenotype and reported treatment efficacy on ADL |
| Kubakski et al. 2017 [6] | To assess the efficacy of HSCT and ERT on somatic features, GAG levels, activities of daily living and cranial MRI findings Mean age at HSCT was 5.5 years (2 to 21.4 years) in new cases and 5.5 years (10 months to 19.8 years) in published cases. |
146 HSCT patients (27 new and 119 published cases) 51 ERT and 15 untreated cases. Data collected on Glycosaminoglycan (GAG) levels MRI findings were investigated in 13 treated patients (6 ERT and 7 HSCT). Impact of HSCT and ERT on activities of daily living |
HSCT patients showed greater improvement in somatic features, joint movements and activities of daily living, compared to ERT patients. GAG levels in blood were significantly reduced by ERT and levels were even lower after HSCT. HSCT patients showed either improvement or no progression of abnormal findings on brain MRI while abnormal findings became more extensive after ERT. Graft-versus-host disease occurred in 8 (9%) out of 85 published cases and 9 (8%) cases died due to transplant-associated complications. |
Treatment efficacy on ADL,MRI changes and GAG levels studied |
| Haiyan Nan et al. 2020 [7] | Evaluated the pros and cons of HSCT and ERT in MPS I and MPS II | Review article comparing the efficacy of both treatments | Authors eluded that the difference effect of HSCT in MPS I and MPS II may be explained by the different times of diagnosis with earlier diagnosis in children with MPS I compared to MPS II. For both conditions, ERT improves survival and alleviates visceral manifestations of both conditions but is ineffective in controlling neurological progression. | Limitations: review article |
| Tansek et al. 2021 [8] | Case series of three unrelated patients with MPS II | Case 1: diagnosed at 35 months – untreated Case 2: diagnosed at 26 months – ERT Case 3: diagnosed at 14 months - HSCT |
HSCT improved both visceral and neurocognitive outcomes compared to ERT | Similar to our study in reporting comparative efficacy of both treatments on patients with severe phenotype |
| Bivina, L. et al. [9] | Case series of three siblings with Hunter syndrome | Case 1: Oldest sibling was diagnosed at age six years and received ERT for four years. Case 2: Second sibling was diagnosed at age two and a half years and has continued to receive ERT since then. Case 3 The youngest brother was diagnosed prenatally, received ERT starting at four months of age, and underwent a mismatched unrelated umbilical cord blood transplant at ten months of age. |
Case 3: The progression of his neurological disease has been slowed compared to both his siblings and he made developmental progress | Similar to our study in reporting comparative efficacy of both treatments on siblings with severe phenotype |
| Tanaka, A., et al. 2014 [10] | Neurocognitive assessments of patients with severe MPS II on ERT or HSCT. No of patients on ERT n = 24 No of patients on HSCT n = 23 Two groups 1)Severe/Type C with missense mutations HSCT – n = 12 ERT – n = 9 2)More severe/Type D with null mutations HSCT – n = 13 ERT – n = 10 |
Development Quotient records of all MPSII patients on ERT and HSCT were collected. | Patients with ERT developed slowly until age 5 and then deteriorated, which was similar to natural history. HSCT group: 5/23patients did not show deterioration even after age 5. 3/5: showed cognitive development and 2/5 showed stabilization in developmental age. All of them were Type C and four of them received HSCT at age 2. The values of DQ of these patients at baseline of HSCT were 50–80, and none of them had either cortical atrophy or hydrocephalus |
Study reported comparative efficacy of both treatments on neurocognitive decline in severe Hunter |
| Tomita K et al. | To investigate the effect on activities of daily living with symptomatic progression in patients with mucopolysaccharidosis type II (MPS II) Case series of 28 patients |
Clinical data were retrospectively collected from the medical records of 28 patients with MPS II between October 2007 and August 2019. Activities of daily living were assessed over time using a 5-point scale (from stage 1, indicating independent, to stage 5, indicating total assistance + medical care); N = 8 attenuated types N = 20 are severe types N = 20 underwent enzyme replacement therapy (ERT) alone N = 5 underwent hematopoietic stem cell transplantation (HSCT) alone N = 3 underwent both treatments |
In severe type, the activity deteriorated regardless of the stage at which ERT was initiated. The activity declined slower in patients who received HSCT at an early stage. |
Study assessed the efficacy of treatments on activities of daily living |
We included eight articles reporting on the comparative efficacy of HSCT and ERT. Each individual study characteristics, results and limitations outlined in the table below.
Our search did not identify any article that reported on the efficacy of both the treatment strategies on all our outcome measures.
6. Discussion
Siblings A and B were confirmed to be hemizygous for the mutation G224E mutation in the IDS which has previously been reported in the literature as being pathogenic and associated with a severe phenotype [3]. We have been able to report the comparative efficacy of two different treatments on disease progression in two siblings at similar chronological age for most outcome measures. ERT for sibling A showed beneficial effect in terms of reduction in quantitative GAG levels, mobility and growth (Table 6). He is also on intrathecal ERT which did not fully arrest the cognitive decline as evident from his neurocognitive assessments, ADL's and QoL scores. This could have been possibly due to the timing of initiation of intrathecal ERT which was started when he was 3 years old. Whilst the Bayley Scales and the WPPSI-IV are not directly comparable, it appears that Sibling A's developmental trajectory altered from being within the average ranges in infancy to extremely low ranges in middle-childhood. In childhood, his visual spatial abilities consistently fell within the extremely low range, however an increase in his WPPSI-IV ‘age equivalent’ scores between assessments reflects continued progress, which could be attributed to the effect of intrathecal ERT. Sibling B's developmental trajectory has remained relatively stable, and he continues to maintain his scores within the average ranges.Our study is unique as it involves both intrathecal and intravenous ERT, although we cannot make a valid comparison of intrathecal ERT and HSCT.
Table 6.
Secondary outcome measures
| Sibling A | Sibling B | |
|---|---|---|
| Neuro imaging findings | At 25 months of age Slightly delayed myelination for age. Bilateral perivascular spaces prominent posteriorly than anteriorly. Mild white matter hyper intensity in bilateral trigones more prominent than usual for age. |
At 20 months of age There are areas of subcortical white matter that have not yet myelinated. More focal regions of high FLAIR signal are seen in the peritrigonal white matter. The corpus callosum is thinner than normally seen. There are prominent perivascular spaces seen but these are not as numerous or as large as seen in his sibling. |
| Neurophysiology | Showed physical signs and symptoms of carpal tunnel syndrome including trigger finger and biting hands. Nerve conduction studies at two years of age confirmed severe bilateral median entrapment neuropathies at the carpal tunnel. |
Nerve conduction studies at 12 months of age showed no evidence of entrapment neuropathies and no physical signs or symptoms of carpal tunnel syndrome. |
| Cardiovascular | Sibling A (Chronological age of 25 months) has good cardiac function and normal heart structure. LV dimension 37 mm diameter with a shortening fraction of 30%. Exhibits mild dysplasia in the mitral and aortic valves which is a consistent feature with young people with this pathology, there was no aortic regurgitation noted. | Sibling B (chronological age of 31 months) shows good biventricular function. There was no mitral stenosis or regurgitation and mild degree of mitral valve prolapse. The aortic valve looked normal with the aortic stenosis or regurgitation, LV shortening fraction of 33.33%. |
| Urinary Glycosaminoglycans | At diagnosis 62.3 mg/mmol Creatinine (0–37.6) At the age of 4 years 8 months 16.8 mg/mmol Creatinine(0.0–16.6) |
At the age of 7 months 25.7 mg/mmol Creatinine(0.0–58.2) |
| Growth | Sibling A was on 95th centile for his height, initially but has fallen off to less than average centile over the last few years | Sibling B maintained his linear growth along the 5th centile |
HSCT in sibling B with a predicted severe phenotype, certainly demonstrated a disease modifying effect on all manifestations of the condition.This is reflected in all primary and secondary outcome measures in him compared to his brother at a similar age. He is fully independent with normal range of movements, enjoys gymnastics and scored better in all parent reported QoL measures. Even with HSCT, however, brain appearance is not entirely normal which may represent an incomplete treatment effect and/or the effect of disease onset prior to adequate CNS stem cell engraftment including prenatal disease. HSCT certainly slowed but not fully arrested the cognitive decline which is evident in sibling B's visual spatial scores reducing slightly between 4.5 years and 6.5 years of age. We certainly need a long term follow up study to elucidate and fully understand the benefits of HSCT for sibling B. We envisage to undertake his assessment when he is 9 years old so that we can compare that to the neurocognitive assessment undertaken for sibling A at a similar age. HSCT is not without limitations and can be associated with morbidity and mortality. Sibling B had severe skin, gut and ocular graft versus host disease and other transplant associated complications like bronchiectasis necessitating long term follow up and intervention.
HSCT is a potentially underutilised treatment strategy and the experience of HSCT for severe phenotypical Hunter syndrome is limited as evident in our literature review.
Barth et al. undertook a critical analysis of the published literature reporting HSCT outcomes in Hunter syndrome till 2017 [12]. Their study reported high rates of transplant associated morbidity and graft failure in stem cell transplants for MPSII, prior to 2005. Poor patient selection criteria and toxicity of the conditioning regimes were identified as possible contributors. Post transplantation outcomes improved since 2012 when conditioning regimes were altered to incorporate less toxic agents. Their analysis certainly recommended early use of HSCT in Hunter syndrome. A nationwide retrospective study of HSCT in MPSII patients in Japan, showed stabilization of brain atrophy and reduction in cardiac valvular regurgitation in transplanted patients [13]. Study results supported the early consideration of HSCT in MPSII patients before the appearance of neurological signs and symptoms. Treatment outcomes are better when transplanted before the manifestation of atrophic changes on brain imaging or valvular regurgitation on echocardiogram. The study of ADL from transplanted patient records showed that HSCT-treated patients maintained almost the same levels of speech ability and gait as at baseline or an improvement in most patients [8,11]. HSCT if successful has the advantage of provision of enzyme therapy with one off administration and is superior to ERT in terms of stabilising the CNS disease progression, cost effectiveness and quality of life scores.
Several factors influence the efficacy of therapeutic interventions like intrathecal ERT or HSCT. Timing of the intervention in relation to the onset of neurological involvement and severity of the disease phenotype, play a crucial role in determining the neurocognitive outcome [14]. HSCT with its proven efficacy in halting the neurocognitive decline was successful in sibling B, with a predicted severe phenotype, in view of pre symptomatic diagnosis. This is our first case of HSCT in a sibling with predicted severe phenotypical Hunter syndrome. Our experience highlights the advantage of early diagnosis either through antenatal testing or through new born screening programmes available in some countries. Pre symptomatic diagnosis allows to utilise therapeutic modalities like gene therapy, intrathecal ERT, HSCT or fusion proteins but the choice of intervention is best made tailored to the individual case.
6.1. Conclusion
Results of review of our experience and of the literature, support the use of early HSCT as a treatment strategy that can positively impact on the neurological disease progression in patients with severe Hunter syndrome. HSCT is an effective therapeutic strategy for early stage treatment of MPS II such as for children diagnosed on pre symptomatic testing or through new born screening programmes [15]. Further research is, however, needed to determine how long after birth, HSCT remains effective for, in children with Hunter syndrome. There are new therapies on the horizon such as gene therapy, fusion proteins to cross the blood brain barrier and chaperone therapy for which clinical trials are currently in progress [16]. Early diagnosis and initiation of the treatment are imperative to maximise therapeutic outcomes.
Funding
No external funding for all assessments which were done as a part of standard clinical care.
Declaration of Competing Interest
None.
Acknowledgements
Ms. Sarah Dowden, Biochemist at Birmingham Women's and Children's Hospital, for provision of laboratory data.
Mr. Derick Yates, Librarian, Birmingham Women's and Children's NHS Trust Library and Knowledge service,for undertaking literature search.
Multidisciplinary team, Paediatric Inherited Metabolic Disorders, Royal Manchester Children's Hospital, for ongoing provision of clinical care for sibling A.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2022.100881.
Appendix A. Supplementary data
Search strategy for literature search
References
- 1.Ficicioglu C., et al. Intrafamilial Variability in the Clinical Manifestations of Mucopolysaccharidosis Type II: Data from the Hunter Outcome Survey (HOS) Am J Med Genet A. 2018:301–310. doi: 10.1002/ajmg.a.38551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muenzer J., Hendriksz C., Fan Z., et al. A phase I/II study of intrathecal Idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med. 2016;18(1):73–81. doi: 10.1038/gim.2015.36. [DOI] [PubMed] [Google Scholar]
- 3.Karsten S., et al. Mutational spectrum of the iduronate-2-sulfatase (IDS) gene in 36 unrelated Russian MPS II patients. Hum. Genet. 1998;Dec;103(6):732–735. doi: 10.1007/s004390050901. [DOI] [PubMed] [Google Scholar]
- 4.Patel P., et al. Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with hunter syndrome. Mol. Genet. Metab. Rep. 2014;1(1):184–196. doi: 10.1016/j.ymgmr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanjuakio J., et al. Activities of daily living in patients with hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol. Genet. Metab. 2015;114(2):161–169. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubaski F., et al. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis type II. Mol. Genet. Metab. 2017;120(1–2):S77. [Google Scholar]
- 7.Nan H., et al. Mucopolysaccharidoses I and II: brief review of therapeutic options and supportive/palliative therapies. Biomed. Res. Int. 2020;2020:2408402. doi: 10.1155/2020/2408402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemencic S., et al. Therapy-type related long-term outcomes in mucopolysaccaridosis type II (hunter syndrome) - case series. Mol. Genet. Metab. Rep. 2021;28 doi: 10.1016/j.ymgmr.2021.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bivina L., Boyadjiev S.A. Mucopolysaccharidosis type II (MPS II): case report of three affected siblings. Mol. Genet. Metab. 2014;111(2):S27. [Google Scholar]
- 10.Tanaka A., et al. Genotype of mucopolysaccharidosis type II severe form and the efficacy of enzyme replacement therapy or hematopoietic stem cell transplantation on cognitive function. Mol. Genet. Metab. 2015;114(2):S111–S112. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita K., et al. Real world long-term outcomes in patients with mucopolysaccharidosis type II: a retrospective cohort study. Mol. Genet. Metab. Rep. 2021;29 doi: 10.1016/j.ymgmr.2021.100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barth A.L., Horovitz D.D.G. Hematopoietic stem cell transplantation in Mucopolysaccharidosis type II: a literature review and critical analysis. J. Inborn Errors Metab. Screen. 2018;6 [Google Scholar]
- 13.Tanaka, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan – compares ERT and HSCT. Mol. Genet. Metab. 2012;107(3):513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Grant, et al. Timing is everything: Clinical courses of Hunter syndrome associated with age at initiation of therapy in a sibling pair. MGM Rep. 2022;2(30):100845. doi: 10.1016/j.ymgmr.2022.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruijter G.J.G., et al. New born screening for hunter disease: a small-scale feasibility study. JIMD Rep. 2014;14:23–27. doi: 10.1007/8904_2013_279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuyama, et al. A phase 2/3 trial of Pabinafusp Alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther. 2020;29(2):671–679. doi: 10.1016/j.ymthe.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy for literature search