Abstract
Introduction
Post-operative delirium (POD) is associated with increased morbidity and mortality rates in older patients. Neuroinflammation, the activation of the intrinsic immune system of the brain, seems to be one of the mechanisms behind the development of POD. The aim of this study was to explore the association between the perioperative inflammatory response and the development of POD in a cohort of older oncological patients in need for surgery.
Methods
In this prospective cohort study, patients 65 years and older in need for oncologic surgery were included. Inflammatory markers C-reactive protein (CRP), interleukin-1 beta (IL-1β), IL-6, IL10 and Neutrophil gelatinase-associated lipocalin (NGAL) were measured in plasma samples pre- and post-operatively. Delirium Observation Screening Scale (DOS) was used as screening instrument for POD in the first week after surgery. In case of positive screening, diagnosis of POD was assessed by a clinician.
Results
Between 2010 and 2016, plasma samples of 311 patients with median age of 72 years (range 65–89) were collected. A total of 38 (12%) patients developed POD in the first week after surgery. The perioperative increase in plasma levels of IL-10 and NGAL were associated with POD in multivariate logistic regression analysis (OR 1.33 [1.09–1.63] P = 0.005 and OR 1.30 [1.03–1.64], P = 0.026, respectively). The biomarkers CRP, IL-1β and IL-6 were not significantly associated with POD.
Conclusions
Increased surgery-evoked inflammatory responses of IL-10 and NGAL are associated with the development of POD in older oncological patients. The outcomes of this study contribute to understanding the aetiology of neuroinflammation and the development of POD.
Keywords: Post-operative delirium, inflammatory response, neuroinflammation, older patients, cancer, older people
Key Points
The incidence of post-operative delirium was 12,2% in a population of older oncologic patients.
The perioperative increases in plasma levels of IL-10 and Neutrophil gelatinase-associated lipocalin (NGAL) were associated with the occurrence of post-operative delirium.
The surgery-evoked inflammatory response can induce neuroinflammation and post-operative delirium.
Introduction
Globally, the population of people aged 65 years and over is growing [1]. As cancer is a disease affecting mainly older people, the number of older patients in need for oncologic surgery is increasing. Post-operative delirium (POD) is an acute confusion disorder characterised by an altered level of consciousness, inattention and disorganised thinking. POD affects cognitive performance, mainly in older patients [2, 3]. The incidence of POD varies between 10 and 44%, depending on population, timing of post-operative assessment, type of surgical procedure and criteria used for diagnosis [4, 5]. POD is associated with high morbidity and mortality rates, a decline in activities of daily living and a decreased quality of life and is therefore highly relevant in older patients [2, 6]. Incidence of and risk factors for the development of POD-like mild cognitive impairment have been extensively investigated across different surgical populations. The underlying mechanisms however have not yet been fully identified [3].
Activation of the intrinsic immune system of the brain has been postulated as an important mechanism behind POD [7]. This neuroinflammation can be induced by either systemic illness or surgical trauma that activates a systemic inflammatory reaction [8], and can lead to neuronal dysfunction and even neuronal death [9]. Older people with cancer can have chronic increased expression of inflammatory mediators as a result of remodelling of the immune system and also induced by the presence of the tumour. A senescent immune system is characterised by a decline in reliability and efficiency of the immune response to new antigens [10]. This leads to chronic increased levels of inflammatory cytokines. Inflammatory markers that reflect the inflammatory response to surgery include C-reactive protein (CRP), Interleukin-1β (IL-1β), IL-6, IL-10 and Neutrophil gelatinase-associated lipocalin (NGAL) [11, 12]. CRP is an acute phase protein produced in response to especially IL-6. IL-1β and IL-6 are pro-inflammatory cytokines involved in the upregulation of the inflammatory response, and IL-10 can have both a pro- and anti-inflammatory role in the response to injury [13, 14]. NGAL has an important role in modulating the inflammatory response [15]. The expression of NGAL is also upregulated in acute kidney failure and in several cancers [15].
We expect that older patients with a senescent immune system are prone to develop an increased systemic inflammatory response to surgery, and are more at risk for neuroinflammation and development of POD. Whether the inflammatory response evoked by surgery itself is associated with the development of POD in the specific group of older cancer patients is unknown. The aim of the current study was to explore the association of the surgery-evoked inflammatory response and POD in a cohort of older oncologic patients.
Methods
The PICNIC and PICNIC-B-HAPPY cohort
This study combined data from the prospective cohort studies ‘PICNIC’ (Post-operative Cognitive dysfunctioN In older Cancer patients) and ‘PICNIC-B-HAPPY’ (Predicting Post-operative Outcome in older Surgical Cancer Patients: Biomarkers and Handgrip Strength as Predictors of Post-operative Outcome in older patients), both conducted at the University Medical Center Groningen (UMCG), the Netherlands [16–21]. These studies were prospectively registered in the Dutch Clinical Trial Database at www.trialregister.nl (NL4219 and NL4441), following approval by the Medical Ethical Committee of the UMCG. Data collection was conducted according to the Declaration of Helsinki. Patients were enrolled between July 2010 and December 2016 after written informed consent.
Patients and clinical data collection
For both cohort studies, patients 65 years and older with an indication for elective surgery for a solid malignant tumour were included. In cases where histological examination revealed a benign tumour, patients were excluded from analysis. Patients were also excluded from the subsequent analysis in case of an incomplete set of inflammatory markers determined pre- and post-operatively.
Outcome—delirium analysis
The primary outcome was the development of POD within 7 days post-operatively during hospital stay. The secondary outcome is the relation between the perioperative inflammatory response and POD.
The Delirium Observation Screening scale (DOS) as screening instrument for delirium was filled out every shift by a registered nurse up to the 7th post-operative day (Figure 1). The DOS scale is a 13 item observation rated screening scale, designed for the early recognition of delirium based on observations by the nurses three times a day. Positive screening comprises a score ≥3 as average on a day. POD was diagnosed during hospital admission by ward physicians, geriatricians or psychiatrists and registered in the Electronic Medical Record (EMR). For more than 10 years, the DOS scale is a frequently used and accurate screening tool for early recognition of delirium with a sensitivity of 90% and a specificity of 92% [22, 23].
Figure 1.
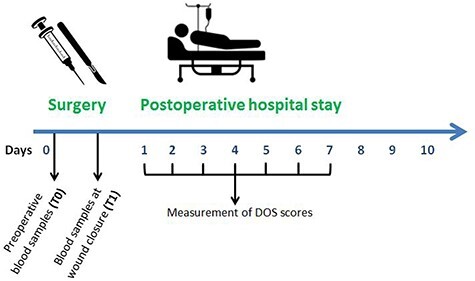
Schematic overview study.
Blood sampling and biochemical analyses
Blood samples were collected: (1) preoperatively before anaesthesia induction (T0), and (2) at wound closure (T1) (see Figure 1). After blood samples were centrifuged at 2600 G for 10 min, plasma was removed and stored at −80°C. The following inflammatory biomarkers were assessed in plasma; CRP, IL-1β, IL-6, IL-10 and NGAL.
Analyses were performed in batches by HaemoScan® (Groningen, The Netherlands) using sandwich Enzyme-Linked Immuno Sorbent Assay (ELISA) for interleukins, developed by BioLegend (San Diego, CA) and high sensitivity CRP ELISA (Dakopatts, Glostrup, Denmark) for CRP. NGAL was determined in plasma by means of ELISA (R&D systems, Minneapolis, MN).
Clinical data
Clinical data such as age, sex, tumour type, disease stage, comorbidities according to the Charlson Comorbidity Index (CCI), frailty according to the Groningen Frailty Indicator (GFI), overall cognition according to the Mini–Mental State Examination (MMSE), neo-adjuvant treatment and surgical characteristics were prospectively collected. Duration of anaesthesia and blood loss were measured perioperatively. Complications following surgery were collected up to 30 days post-operatively, defined according to the Clavien Dindo Classification.
Data analysis and statistics
As a measurement of the surgery-evoked inflammatory response, preoperative results of the inflammatory factor assays (T0) were subtracted from the post-operative outcomes (T1) (ΔCRP, ΔIL-1β, ΔIL-6, ΔIL-10 and Δ-NGAL). Data were described in percentages, medians and ranges. Data were presented stratified by the occurrence of POD yes or no within the first week after surgery. Differences in plasma levels between patients with and without POD were tested with the Mann–Whitney U test. Univariate and multivariate logistic regression analysis were performed and Odds ratios (ORs) and the corresponding 95% confidence intervals (95%CIs) were estimated. With logistic regression analysis, the relation between the occurrence of POD and the perioperative inflammatory response as reflected by CRP, IL-1β, IL-6, IL-10 and NGAL was examined. The variables age, sex, comorbidities according to CCI, frailty according to GFI, MMSE score, type of surgery and anaesthesia duration were used as possible confounders. To deal with potential effects of differences regarding the invasiveness of the surgical intervention, we included anaesthesia duration in all multivariable models, as anaesthesia duration is an indicator for the extent of surgery. In addition, a sensitivity analysis was performed including only patients that underwent intra-abdominal surgery to evaluate the effect of the surgery-evoked inflammatory response in major surgery. P values <0.05 were considered to be statistically significant. Data analysis was performed using IBM SPSS version 23 (IBM Corporation, Armonk, NY).
Results
Of the 525 patients included in the two cohort studies, 15 patients were incorrectly included, for example in case of cancellation of surgery, and 30 patients withdrew their consent before surgery. In addition, 169 patients without malignancy, with incomplete blood sampling or aged younger than 65 years were excluded. A total of 311 patients were included in the current analysis (Figure 2). The median age was 72 years (range 65–89) and 168 (54%) patients were male (Table 1). The majority of patients was diagnosed with a carcinoma (71%). Of all included patients, 38 (12.2%) developed POD in the first 7 days after surgery, of whom 82% were male and 87% underwent a laparotomy. Table A1 in the appendix shows a stratification of all patient, tumour and surgical characteristics for female and male patients. Females were more often frail and males had higher comorbidity and complication rates. No significant differences according the extent of surgery were found between males and females.
Figure 2.
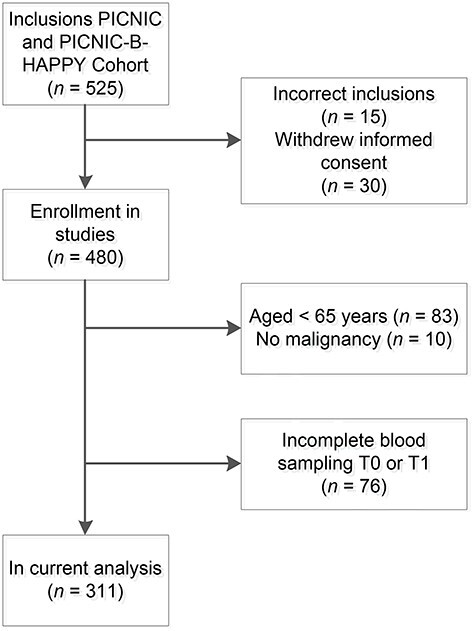
Consort diagram of included and excluded patients.
Table 1.
Patient, tumour and surgical characteristics for all patients and patients with post-operative delirium (POD) within 7 days post-operatively during hospital stay (n = 311)
| Patient characteristics | All patients (n = 311) | No POD (n = 273) | POD (n = 38) | P a |
|---|---|---|---|---|
| Median age (IQR) | 72 (68–72) | 71 (68–77) | 73 (68–80) | 0.557 |
| Sex; n (%) | 0.001 | |||
| Female | 143 (46) | 136 (50) | 7 (18) | |
| Male | 168 (54) | 137 (50) | 31 (82) | |
| CCI; n (%) | 0.029 | |||
| ≤3 | 142 (46) | 131 (48) | 11 (29) | |
| >3 | 169 (54) | 142 (52) | 27 (71) | |
| GFI; n (%) | 0.323 | |||
| <4 | 216 (69) | 187 (68) | 29 (76) | |
| ≥4 | 95 (31) | 86 (32) | 9 (24) | |
| MMSE; n (%) | 0.804 | |||
| ≤26 | 37 (12) | 32 (12) | 5 (13) | |
| >26 | 274 (88) | 241 (88) | 33 (87) | |
| Tumour characteristics | ||||
| Tumour location; n (%) | 0.698 | |||
| Colorectal | 94 (30) | 74 (27) | 20 (52) | |
| Gastric | 20 (6) | 16 (6) | 4 (10) | |
| Oesophageal | 23 (7) | 18 (7) | 5 (13) | |
| Small bowel | 9 (3) | 8 (3) | 1 (3) | |
| Pancreaticobiliary | 22 (7) | 18 (7) | 4 (10) | |
| Breast | 2 (1) | 1 (0) | 1 (3) | |
| Thyroid | 13 (4) | 12 (4) | 1 (3) | |
| Gynaecological | 51 (17) | 50 (18) | 1 (3) | |
| Skin (incl. melanoma) | 42 (14) | 42 (15) | ||
| Soft tissue (incl. sarcoma) | 35 (11) | 34 (13) | 1 (3) | |
| Tumour type; n (%) | 0.292 | |||
| Carcinoma | 220 (71) | 185 (68) | 35 (92) | |
| Sarcoma | 31 (10) | 30 (11) | 1 (3) | |
| Melanoma | 35 (11) | 35 (13) | 0 (0) | |
| Other Malignancy | 25 (8) | 23 (8) | 2 (5) | |
| Disease stage; n (%) | 0.177 | |||
| I | 75 (24) | 61 (22) | 14 (37) | |
| II | 79 (25) | 70 (26) | 9 (24) | |
| III | 86 (28) | 80 (29) | 6 (15) | |
| IV | 71 (23) | 62 (23) | 9 (24) | |
| Neoadjuvant therapy; n (%) | 0.112 | |||
| None | 232 (72) | 202 (74) | 21 (55) | |
| Chemotherapy | 30 (10) | 25 (9) | 5 (13) | |
| Radiotherapy | 17 (5) | 14 (5) | 3 (8) | |
| Chemoradiotherapy | 41 (13) | 32 (12) | 9 (24) | |
| Surgical characteristics | ||||
| Type of surgery; n (%) | 0.007 | |||
| Resection by laparotomy | 174 (56) | 141 (52) | 33 (87) | |
| Resection by laparoscopy | 35 (11) | 33 (12) | 2 (5) | |
| Lymph node dissection | 31 (10) | 29 (10) | 2 (5) | |
| Superficial/Extremities | 71 (23) | 70 (26) | 1 (3) | |
| Type of anaesthesia; n (%) | 0.495 | |||
| Regional | 10 (3) | 10 (4) | 0 (0) | |
| General | 146 (47) | 131 (48) | 15 (39) | |
| Regional + General | 155 (50) | 132 (48) | 23 (61) | |
| Duration of anaesthesia; n (%) | <0.001 | |||
| ≤180 min | 137 (44) | 132 (48) | 5 (13) | |
| >180 min | 174 (56) | 141 (52) | 33 (87) | |
| Median blood loss in millilitres (IQR) | 100 (10–450) | 100 (0–400) | 550 (138–900) | 0.001 |
| Complications; n (%) b | <0.001 | |||
| Clavien Dindo score <3 | 269 (86) | 244 (89) | 25 (66) | |
| Clavien Dindo score ≥3 | 42 (14) | 29 (11) | 13 (34) | |
Variables are denoted as percentages or as Median (Interquartile range (IQR)).
CCI Charlson Comorbidity index, GFI Groningen Frailty Index, MMSE Mini–Mental State Examination.
aUnivariate logistic regression analysis was performed with POD as outcome measurement. Bold values are considered significant (P < 0.05).
bPOD is not included in these complication-scores.
Inflammatory markers
Figure 3 shows the differences in the inflammatory response ∆T1-T0 (the difference between plasma levels at wound closure and preoperative plasma levels) of CRP, IL-1β, IL-6, IL-10 and NGAL for patients with and without POD. For IL-6, IL-10 and NGAL, there was a significantly higher perioperative inflammatory response for patients with POD. An overview of the differences in preoperative plasma levels (T0) and the inflammatory response (∆T1-T0) for all included patients and patients with and without POD is shown in the appendix (Table A2).
Figure 3.

This figure depicts the perioperative inflammatory response ∆ (T1-T0) in plasma levels (median, interquartile range and range) of CRP, IL-1β, IL-6, IL-10 and NGAL for patients with and without post-operative delirium (POD). *Plasma levels of inflammatory markers with an asterisk were significantly higher in patients with POD.
Multivariate logistic regression analysis was used to explore the independent prognostic effect of the inflammatory markers IL-6, IL-10 and NGAL (Table 2). Results show that the perioperative inflammatory responses of IL-10 (OR 1.33 [1.09–1.63] P = 0.005) and NGAL (OR 1.30 [1.03–1.64], P = 0.026) when adjusted for confounders were significantly associated with the development of POD. Also male gender, severe comorbidities and intracavitary surgery were significantly associated with the development of POD (Table 2).
Table 2.
Factors associated with post-operative delirium (POD), multivariable logistic regression analysis with a model for IL-6, IL-10 and NGAL (n = 311)
| POD (yes vs no) (n = 38 vs n = 273) | |||
|---|---|---|---|
| Model for IL-6 | Model for IL-10 | Model for NGAL | |
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| ∆ (T1-T0) Biomarkers a | |||
| IL-6 | 1.00 (0.95–1.05) | - | - |
| IL-10 | - | 1.33 (1.09–1.63)** | - |
| NGAL | - | - | 1.30 (1.03–1.64)* |
| Age (years) | 1.03 (0.97–1.10) | 1.04 (0.97–1.11) | 1.03 (0.96–1.11) |
| Sex | |||
| Female | 1 | 1 | 1 |
| Male | 3.89 (1.58–9.55)** | 4.45 (1.72–11.53)** | 4.83 (1.79–13.06)** |
| CCI | |||
| ≤3 | 1 | 1 | 1 |
| >3 | 2.11 (0.95–4.66) | 2.61 (1.12–6.10)* | 2.48 (0.99–6.21) |
| GFI | |||
| <4 | 1 | 1 | 1 |
| ≥4 | 0.76 (0.32–1.79) | 0.82 (0.34–1.96) | 0.59 (0.23–1.56) |
| MMSE | |||
| >26 | 1 | 1 | 1 |
| ≤26 | 1.17 (0.38–3.60) | 1.14 (0.35–3.77) | 0.98 (0.25–3.80) |
| Type of Surgery | |||
| Superficial | 1 | 1 | 1 |
| Intra-abdominal/thoracic | 5.62 (1.23–25.61)* | 4.96 (1.07–22.94)* | 5.85 (1.19–28.65)* |
| Duration of anaesthesia | |||
| ≤180 min | 1 | 1 | 1 |
| >180 min | 3.93 (1.34–11.53)* | 2.82 (0.97–8.27) | 2.42 (0.78–7.53) |
* P < 0.05, **P < 0.01, ***P < 0.001 (bold values are considered significant).
OR Odds Ratio, CI 95% confidence interval, CCI Charlson comorbidity score, GFI Groningen Frailty Indicator score, MMSE Mini–Mental State Examination, CRP C-reactive protein, IL interleukin, NGAL Neutrophil gelatinase-associated lipocalin.
aPlasma levels of the delta biomarkers were divided by a factor 100 to improve readability of OR’s and 95% CI’s.
Results of a sensitivity analysis including only patients that underwent intra-abdominal surgery also show that the perioperative inflammatory responses of IL-10 (OR 1.33 [1.08–1.63] P = 0.006) and NGAL (OR 1.34 [1.05–1.71], P = 0.020) when adjusted for confounders were significantly associated with POD (Appendix, Table A3). However, an extra sensitivity analysis that included perioperative blood loss as a confounder showed that the perioperative inflammatory response of NGAL was no longer significantly associated with POD (OR 1.25 [0.97–1.62] P = 0.092). Severe blood loss (≥500 ml) was significantly associated with POD. (Appendix, Table A4).
Discussion
In this prospective cohort study, the incidence of POD within 7 days after surgery was 12.2%, which is in line with the literature [4, 5]. The increase in plasma levels of the inflammatory biomarkers IL-10 and NGAL following surgery was significantly associated with the development of POD in older oncological patients. For the specific group of patients that underwent intra-abdominal surgery, the surgery-evoked increase in plasma levels of IL-10 and NGAL were also significantly associated with POD.
Although in the current literature there is little evidence of an association between plasma levels of IL-10 and cognitive symptoms, different kinds of associations have been described. Xin et al. [24] found that patients with POD had a decrease in IL-10 plasma levels between anaesthesia induction and wound closure. Magaki et al. [25] found increased plasma levels of IL-10 in patients with mild cognitive impairment. Other studies did not show an association between IL-10 and cognitive decline [26, 27]. It is known that IL-10 plasma levels peak between 2 and 6 h after injury, which makes it likely that our measurements of IL-10 at wound closure after surgery are detected in this phase of the cytokine response [13]. The timing of plasma level measurements could be part of the explanation that a relationship for the perioperative inflammatory response was found in IL-10, and not in IL-1β which peaks more early. In addition to functioning as an immunosuppressive agent, IL-10 can also promote inflammation by supporting B-cell and CD8+ T-cell activation. The mechanisms that determine if IL-10 has an immune suppressive function or an immune stimulating function are still largely unknown [28, 29]. The activation of the inflammatory cascade, including the release of IL-10, might contribute to neuroinflammation as underlying process in the development of POD.
Several earlier studies found a relationship between plasma levels of NGAL and cognitive and mental dysfunction, such as depression and Alzheimer’s disease [30, 31]. NGAL is a well-known biomarker for renal injury [32]. There is evidence that NGAL also plays an important role in neuroinflammation and might be a potential biomarker for conditions with neuronal damage [33]. NGAL is an acute phase protein released from different cell types as a result of a systemic inflammatory reaction. The release of NGAL in the brain leads to the activation of microglia and astrocytes, neutrophil infiltration and the production of inflammatory cytokines. NGAL also causes an increased permeability of the blood–brain barrier, which can facilitate entry of inflammatory mediators into the brain and thereby exaggerating neuroinflammation [34]. A rat model for post-operative cognitive decline showed increased NGAL concentrations after cardiac and abdominal surgery [35]. Despite the promising results of earlier studies on NGAL and cognitive decline, this is the first study that has examined the association between surgery-evoked inflammatory response in NGAL and the development of POD after surgery in a large group of older cancer patients.
Our current study shows that specifically the surgery-evoked increase in plasma levels of NGAL and IL-10 is associated with POD. This suggest that the inflammatory response in older oncologic patients caused by surgery itself can induce neuroinflammation and POD. These findings underscore the hypothesis that an increased inflammatory response to surgical trauma, especially in major surgery with prolonged duration, can lead to neuroinflammation and the clinical manifestation of POD.
Why the inflammatory markers IL-10 and NGAL were found to be associated with POD, while CRP, IL-1β and IL-6 were not, is not fully understood. In the current study, increased plasma levels of IL-6 were associated with POD. However, in multivariate logistic regression analysis this biomarker did not show a significant relationship with POD. Several earlier studies described a relation between increased post-operative IL-6 levels and POD for different types of surgery [36, 37]. In contrast to the current study, measurements of IL-6 took place at the first or second post-operative day instead of the moment of wound closure in the current study. It is known that the maximum plasma level of Il-6 after surgery is reached between 3 h and 1 day after skin closure. [38] This raises the possibility that in our study the maximum IL-6 plasma levels were not measured. IL-1β has a very short half-life and the inflammatory activity of IL-1β is challenging to measure in plasma [39]. There is some evidence for an association of IL-1β levels with POD, but the results of these studies did not specifically represent the inflammatory response caused by surgery itself. Cape et al. [40] found a relation between increased IL-1β, measured at the third and fourth post-operative day in cerebrospinal fluid, and POD in patients who had hip surgery. Wanderlind et al. [41] found an association between elevated IL-1β and POD in patients during ICU admission. Several studies described a relationship between elevated CRP levels and POD. Kotfis et al. [42] found a relationship between elevated plasma CRP levels at the fifth post-operative day and POD. However, no relationship was found between POD and plasma CRP levels measured directly after surgery. A meta-analysis from Liu et al. [43] also showed significant increases in plasma CRP levels in patients with POD. In these studies, CRP was measured at different moments in time and different screening methods for POD were used.
Our results show that severe blood loss was associated with POD in patients that underwent intra-abdominal surgery. These outcomes can support the theory that hypo perfusion can influence the development of POD. However, perioperative blood loss does not fully represent intraoperative hypo perfusion. Intraoperative blood pressure, mean arterial pressure and the intraoperative use of inotropes and vasopressors could be a better proxy of hypo perfusion.
It is known that major surgery is a risk factor for the development of POD [44]. It is likely that major surgery and prolonged anaesthesia duration both lead to increased neuroinflammation. POD was more seen in males, which is in line with the literature [45]. It is likely that men are more at risk for neuroinflammation because of a more active immune system [46]. In our current study, males had higher comorbidity and complications rates and females were more often frail. Frail patients and patients with comorbidities or pre-existent cognitive decline are claimed to be more at risk for POD [47]. In contrast to the literature, no relation was found between frailty and POD, nor with comorbidities and overall cognition and POD. An explanation could be that the frailest patients with severe comorbidity and patients with pre-existent cognitive decline are more likely to be unfit for surgical treatment [48]. In the current study, only 24% of included patients with POD were frail, compared with 28–42% considered as frail in the older oncologic population [49, 50].
Evaluation of the study
This is the first study describing the perioperative inflammatory response in relationship to the development of POD in a large heterogeneous cohort of older cancer patients. Most of the inflammatory markers have not been studied before in older cancer patients perioperatively.
This study shows that the perioperative inflammatory response in IL-10 and NGAL following surgery itself in older patients is associated with POD. Since NGAL is a well-known marker for renal injury and is released due to renal hypo perfusion, it would be interesting to know if cerebral damage due to hypo perfusion could also lead to elevated levels of NGAL and POD. We found that patients with POD had more severe complications compared with patients without POD (34% vs. 11%, respectively). It would be interesting to evaluate if the onset of POD is related with surgery itself, with the onset of post-operative (inflammatory) complications or with both.
Further perspectives and clinical implications
The outcomes of this study contribute to our insight in the aetiology of neuroinflammation after surgery. Further evaluation of the perioperative inflammatory response in relation with the development of POD and other manifestations of post-operative cognitive decline is necessary for detecting the underlying mechanism. Analysing other types of surgery would be interesting to investigate if the perioperative inflammatory response is also related to POD in non-cancer patients. It would be interesting to evaluate if hypo perfusion, measured by intraoperative blood pressure, is related with the development of POD. Further research is needed to determine if intraoperative hypo perfusion can cause POD by inflammation or by another mechanism. Further research is needed to determine if preventive measurements like anti-inflammatory drugs could be beneficial in preoperative treatment of older patients who are at increased risk for POD.
Conclusions
Our data show an association between the perioperative inflammatory response in IL-10 and NGAL, and the development of POD delirium during hospital admission in older cancer patients. The outcomes of this study could be helpful in understanding the aetiology of neuroinflammation and support the theory that neuroinflammation can be caused by surgery itself. The relationship between hypo perfusion and POD needs further evaluation. For better understanding of the relation between the perioperative inflammatory response and post-operative cognitive decline, further prospective research is necessary to validate our findings.
Supplementary Material
Acknowledgements
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.
Contributor Information
Baukje Brattinga, University of Groningen, University Medical Center Groningen, Department of Surgery, 9700 RB Groningen, The Netherlands.
Matthijs Plas, University of Groningen, University Medical Center Groningen, Department of Surgery, 9700 RB Groningen, The Netherlands.
Jacoba M Spikman, University of Groningen, University Medical Center Groningen, Department of Neurology, 9700 RB Groningen, The Netherlands.
Abraham Rutgers, University of Groningen, University Medical Center Groningen, Department of Rheumatology and Clinical Immunology, 9700 RB Groningen, The Netherlands.
Jacco J de Haan, University of Groningen, University Medical Center Groningen, Department of Medical Oncology, 9700 RB Groningen, The Netherlands.
Anthony R Absalom, University of Groningen, University Medical Center Groningen, Department of Anesthesiology, 9700 RB Groningen, The Netherlands.
Hanneke van der Wal-Huisman, University of Groningen, University Medical Center Groningen, Department of Surgery, 9700 RB Groningen, The Netherlands.
Geertruida H de Bock, University of Groningen, University Medical Center Groningen, Department of Epidemiology, 9700 RB Groningen, The Netherlands.
Barbara L van Leeuwen, University of Groningen, University Medical Center Groningen, Department of Surgery, 9700 RB Groningen, The Netherlands.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
None.
References
- 1. Pilleron S, Sarfati D, Janssen-Heijnen M et al. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer 2019; 144: 49–58. [DOI] [PubMed] [Google Scholar]
- 2. Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin 2015; 33: 505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skvarc DR, Berk M, Byrne LK et al. Post-operative cognitive dysfunction: An exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev 2018; 84: 116–33. [DOI] [PubMed] [Google Scholar]
- 4. Shoair O, Grasso M II, Lahaye L et al. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study. J Anaesthesiol Clin Pharmacol 2015; 31: 30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson TN, Raeburn CD, Tran ZV et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009; 249: 173–8. [DOI] [PubMed] [Google Scholar]
- 6. Shi Z, Mei X, Li C et al. Postoperative delirium is associated with long-term decline in activities of daily living. Anesthesiology 2019; 131: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ni Choileain N, Redmond HP. Cell response to surgery. Arch Surg 2006; 141: 1132–40. [DOI] [PubMed] [Google Scholar]
- 8. Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg 2019; 128: 781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science 2016; 353: 777–83. [DOI] [PubMed] [Google Scholar]
- 10. De Martinis M, Franceschi C, Monti D et al. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 2005; 579: 2035–9. [DOI] [PubMed] [Google Scholar]
- 11. Gouweleeuw L, Hovens IB, van Leeuwen BL et al. Neutrophil gelatinase-associated lipocalin and microglial activity are associated with distinct postoperative behavioral changes in rats. Behav Brain Res 2017; 319: 104–9. [DOI] [PubMed] [Google Scholar]
- 12. Lu C-H, Lee S-H, Liu K-H et al. Older age impacts on survival outcome in patients receiving curative surgery for solid cancer. Asian J Surg 2018; 41: 333–40. [DOI] [PubMed] [Google Scholar]
- 13. Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery 2000; 127: 117–26. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakraborty S, Kaur S, Guha S et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta Rev Cancer 2012; 1826: 129–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plas M, Rotteveel E, Izaks GJ et al. Cognitive decline after major oncological surgery in the elderly. Eur J Cancer 2017; 86: 394–402. [DOI] [PubMed] [Google Scholar]
- 17. Weerink LBM, van Leeuwen BL, Gernaat SAM et al. Vitamin status and the development of postoperative cognitive decline in elderly surgical oncologic patients. Ann Surg Oncol 2018; 25: 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du J, Plas M, Absalom AR et al. The association of preoperative anxiety and depression with neurocognitive disorder following oncological surgery. J Surg Oncol 2020; 121: 676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plas M, de Haan JJ, van der Wal-Huisman H et al. The systemic impact of a surgical procedure in older oncological patients. Eur J Surg Oncol 2019; 45: 1403–9. [DOI] [PubMed] [Google Scholar]
- 20. Plas M, Rutgers A, van der Wal-Huisman H et al. The association between the inflammatory response to surgery and postoperative complications in older patients with cancer; a prospective prognostic factor study. J Geriatr Oncol 2020; 11: 873–9. [DOI] [PubMed] [Google Scholar]
- 21. Brattinga B, Rutgers A, De Haan JJ et al. Preoperative inflammatory markers as a predictor of three-year overall survival in older cancer patients undergoing oncologic surgery. Cancers 2021; 13: 1824. 10.3390/cancers13081824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park J, Jeong E, Lee J. The delirium observation screening scale: a systematic review and meta-analysis of diagnostic test accuracy. Clin Nurs Res 2020; 30: 464–73. [DOI] [PubMed] [Google Scholar]
- 23. Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The delirium observation screening scale: a screening instrument for delirium. Res Theory Nurs Pr 2003; 17: 31–50. [DOI] [PubMed] [Google Scholar]
- 24. Xin X, Chen J, Hua W et al. Intraoperative dexmedetomidine for prevention of postoperative delirium in elderly patients with mild cognitive impairment. Int J Geriatr Psychiatry 2021; 36: 143–51. [DOI] [PubMed] [Google Scholar]
- 25. Magaki S, Mueller C, Dickson C et al. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol 2007; 42: 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wennberg AMV, Hagen CE, Machulda MM et al. The cross-sectional and longitudinal associations between IL-6, IL-10, and TNFα and cognitive outcomes in the Mayo Clinic study of aging. Journals Gerontol Ser A 2019; 74: 1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chi GC, Fitzpatrick AL, Sharma M et al. Inflammatory biomarkers predict domain-specific cognitive decline in older adults. Journals Gerontol Ser A 2017; 72: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bedke T, Muscate F, Soukou S et al. IL-10-producing T cells and their dual functions. Semin Immunol 2019; 44: 101335. [DOI] [PubMed] [Google Scholar]
- 29. Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 2005; 78: 1043–51. [DOI] [PubMed] [Google Scholar]
- 30. Naudé PJW, Nyakas C, Eiden L et al. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer’s disease. FASEB J 2012; 26: 2811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naudé PJW, Eisel ULM, Comijs HC et al. Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. J Psychosom Res 2013; 75: 444–50. [DOI] [PubMed] [Google Scholar]
- 32. Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest 2008; 68: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee S, Kim J-H, Kim J-H et al. Lipocalin-2 is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem 2011; 286: 43855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suk K. Lipocalin-2 as a therapeutic target for brain injury: An astrocentric perspective. Prog Neurobiol 2016; 144: 158–72. [DOI] [PubMed] [Google Scholar]
- 35. Hovens IB, van Leeuwen BL, Mariani MA et al. Postoperative cognitive dysfunction and neuroinflammation; cardiac surgery and abdominal surgery are not the same. Brain Behav Immun 2016; 54: 178–93. [DOI] [PubMed] [Google Scholar]
- 36. Vasunilashorn SM, Ngo L, Inouye SK et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. Journals Gerontol Ser A 2015; 70: 1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu P, Li Y-W, Wang X-S et al. High serum interleukin-6 level is associated with increased risk of delirium in elderly patients after noncardiac surgery: a prospective cohort study. Chin Med J (Engl) 2013; 126: 3621–7. [PubMed] [Google Scholar]
- 38. Sakamoto K, Arakawa H, Mita S et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine 1994; 6: 181–6. [DOI] [PubMed] [Google Scholar]
- 39. Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 2011; 22: 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cape E, Hall RJ, van Munster BC et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res 2014; 77: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wanderlind MLZ, Gonçalves R, Tomasi CD et al. Association of neurogranin with delirium among critically ill patients. Biomark Med 2020; 14: 1613–7. [DOI] [PubMed] [Google Scholar]
- 42. Kotfis K, Ślozowska J, Safranow K et al. The practical use of white cell inflammatory biomarkers in prediction of postoperative delirium after cardiac surgery. Brain Sci 2019; 9: 308. 10.3390/brainsci9110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu X, Yu Y, Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): a meta-analysis of observational studies. PLoS One 2018; 13: e0195659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He Z, Cheng H, Wu H et al. Risk factors for postoperative delirium in patients undergoing microvascular decompression. PLoS One 2019; 14: e0215374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med 2017; 377: 1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischer J, Jung N, Robinson N et al. Sex differences in immune responses to infectious diseases. Infection 2015; 43: 399–403. [DOI] [PubMed] [Google Scholar]
- 47. Inouye SK, Robinson T, Blaum C et al. Postoperative delirium in older adults: best practice statement from the American geriatrics society. J Am Coll Surg 2015; 220: 136–148.e1. [DOI] [PubMed] [Google Scholar]
- 48. Mody L, Miller DK, McGloin JM et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc 2008; 56: 2340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Handforth C, Clegg A, Young C et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015; 26: 1091–101. [DOI] [PubMed] [Google Scholar]
- 50. Tan K-Y, Kawamura YJ, Tokomitsu A et al. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg 2012; 204: 139–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


