Abstract
Recently approved migraine preventive therapies facilitate rapid control of migraine activity, potentially improving patients’ lives and minimizing the societal burden of migraine. This review synthesizes available evidence on rates and timing of early onset of migraine prevention and identifies patient-level outcomes related to early onset prevention. This evidence-based scoping review identified all available clinical trial evidence regarding the early onset of prevention of migraine, under the hypothesis ‘Patients with migraine (episodic or chronic) report additional benefits when receiving an approved migraine preventive treatment that demonstrates an early onset of prevention’. Early onset of prevention was defined as migraine preventive benefits within 30 days post-administration. PubMed, EMBASE, and CINAHL were searched for publications between 1988 and 2020. Overall, 16 publications described 18 studies. All studies were conducted in approved treatments [four anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies and one chemodenervation agent] in patients with episodic/chronic migraine; no publications were identified for traditional oral agents for early migraine prevention. Compared to placebo, erenumab (three studies) reduced weekly migraine days within 1 week; fremanezumab (six studies) increased reports of no headache of at least moderate severity on Day 1 and significantly reduced migraine frequency within 1 week; galcanezumab (three studies) significantly reduced the mean number of patients with migraine beginning Day 1 and each day of the first week; eptinezumab (four studies) significantly reduced migraine attack likelihood on Day 1 by > 50% versus baseline; and onabotulinumtoxinA (two studies) reduced headache and migraine days within 1 week. Four publications described function, disability, and quality of life improvements as early as Week 4; none reported cost–benefit. Anti-CGRP monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) and a chemodenervation agent (onabotulinumtoxinA) provide clinically relevant benefits during the first treatment week. Literature describing clinically relevant benefits regarding early onset of prevention in patients with migraine is limited.
Keywords: clinical benefits, early onset, migraine, prevention
Introduction
Migraine prevention is an important component of overall migraine management; it is recommended not only to reduce migraine attack frequency, severity, duration, and related disability but also to improve responsiveness to acute medications (and avoid escalation in use and reduce reliance on them), to improve health-related quality of life, and to reduce headache-associated distress, psychological symptoms, and overall costs. 1 Preventive therapies are recommended for a broad segment of patients with migraine, including those who experience frequent and/or disabling attacks and those who cannot use, do not use, or use more than the recommended dosage of acute therapies (Table 1).1,2 However, until 2018, treatments for the pharmacologic prevention of migraine were primarily oral medications initially developed for other therapeutic uses. Some of these have established efficacy in migraine prophylaxis, but many other off-label treatments are used based on clinical experience alone rather than supportive evidence.
Table 1.
International Classification of Headache Disorders, third edition, criteria for migraine, and chronic migraine.
| Migraine |
| (A) At least five attacks fulfilling criteria B–D |
| (B) Headache attacks lasting 4–72 h (when untreated or unsuccessfully treated) |
| (C) Headache has at least two of the following four characteristics: |
| 1. Unilateral location |
| 2. Pulsating quality |
| 3. Moderate or severe pain intensity |
| 4. Aggravation by or causing avoidance of routine physical activity (e.g., walking or climbing stairs) |
| (D) During headache at least one of the following: |
| 1. Nausea and/or vomiting |
| 2. Photophobia and phonophobia |
| (E) Not better accounted for by another diagnosis |
| Chronic migraine |
| (A) Migraine-like or tension-type-like headache on > 15 days/month for > 3 months that fulfill criteria B and C |
| (B) Occurring in a patient who has had at least five attacks fulfilling criteria B–D for migraine without aura and/or criteria B and C for migraine with aura |
| (C) On ⩾ 8 days/month for > 3 months, fulfilling any of the following: |
| 1. Criteria C and D for migraine without aura |
| 2. Criteria B and C for migraine with aura |
| 3. Believed by the patient to be migraine at onset and relieved by a triptan or ergot derivative |
| (D) Not better accounted for by another diagnosis |
ICHD-3, International Classification of Headache Disorders, third edition.
Reproduced with permission of International Headache Society. 2
Beta-blockers, tricyclic antidepressants, and antiepileptic medications have long been used for the preventive treatment of migraine and have been recommended in clinical guidelines; however, they are limited in overall preventive effectiveness.1,3 Titration typically takes at least 2, and often, up to 6 months to determine efficacy in a given patient due to pharmacokinetic profiles and individualized dosing requirements;3–5 this leaves patients exposed to high levels of migraine activity for an undesirable amount of time before optimal effect is achieved. An additional challenge is that adherence and persistence with traditional migraine preventive therapies is low;6,7 this is not surprising given that patients with migraine consider speed of onset as one of the most important attributes of preventive treatment (second only to efficacy) 8 , and they often make decisions about switching or discontinuing therapy early in the course of treatment.6,7 Side effects are also commonly cited as a reason for the premature discontinuation of preventive therapies. 9
OnabotulinumtoxinA was added to the armamentarium for the preventive treatment of chronic migraine in 2010, 10 having demonstrated the ability to reduce headache frequency in a more timely fashion in patients with chronic migraine, including those with medication overuse.11,12 Maintenance therapy is administered every 12 weeks, and continued use has been associated with ‘wearing off’ of benefit before 12 weeks in some patients.13,14
The more recent introduction of therapies targeting calcitonin gene-related peptide (CGRP) has garnered much interest in the headache community, as these agents have not only consistently demonstrated early onset but have also exhibited sustained reduction of disease activity, thus offering great potential to improve the lives of patients and to minimize the burden of migraine on healthcare systems and society. The primary objective of this literature review is to identify and provide a synthesis of all available clinical trial evidence related to the rates and timing of the early onset of prevention in patients with migraine. A secondary objective is to identify any specific benefits of this early onset of migraine in terms of cost–benefit and patient-level outcomes.
Materials and methods
To evaluate the full impact of an early onset of preventive benefits, an evidence-based, hypothesis-driven, scoping literature review was undertaken to identify all available evidence related to an early onset of prevention in patients with episodic or chronic migraine. The hypothesis of ‘Patients with migraine (episodic or chronic) report additional benefits when receiving an approved migraine preventive treatment that demonstrates an early onset of prevention’ was utilized to form the basis of the literature search, with the term ‘early onset of prevention’ defined as the demonstration of preventive benefits within 1 month (30 days) of the initiation of treatment. The search was conducted across multiple electronic literature databases (PubMed, EMBASE, and CINAHL) from 1988 (based on the establishment of the International Classification of Headache Disorders, first edition, diagnostic criteria) 15 to 20 September 2020.
The literature search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 16 with the hierarchy of evidence for the analysis of identified publications based on the modified selection criteria of Guyatt et al. 17 and Greenhalgh 18 . Grading of evidence was based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to assess the certainty and strength of clinical evidence. 19 The literature search strategy was developed using a combination of Medical Subject Heading (MeSH) terms and keywords, with PubMed used as the primary literature database to structure the following search criteria:
-
Research Hypothesis
‘Patients with migraine (episodic or chronic) report additional benefits when receiving an approved migraine treatment that demonstrates an early onset of prevention’
-
Patient population (search terms used)
Adult (> 18 years); Young Adult (19‒24 years); Adult (19–44 years); Aged (> 65 years); Middle Aged (45–64 years); Middle Aged; Aged (> 45 years); 80 and above (> 80 years)
Migraine; Migraine (Episodic); Migraine (Chronic); Migraine (High-Frequency Episodic); Transformed Migraine; Medication Overuse; Medication Overuse Headache
-
Therapeutic Indication
Migraine Prevention; Migraine Preventive Therapy; Migraine Prophylaxis
-
Approval
Approved by the United States Food and Drug Administration 20
-
Outcomes
Change in Migraine Frequency
Change in Migraine Severity
Change in Migraine Duration
Change in Migraine Symptomatology
Change in Patient-Reported Outcomes
Change in Health-Related Quality of Life
-
Time Course
Day 1; Week 1; Day 7; Week 4; Day 28; Month 1; Day 30
-
Journal Type
Peer-reviewed
-
Language
English
-
Types of Evidence
Clinical Study; Clinical Trial; Randomized Controlled Trial; Multicenter Study; Observational Study; Meta-analysis; Systematic Review
-
Literature Type
Full Text; Free Full Text; Open Access
-
Time Period
1988–20 September 2020
-
Hierarchy of Evidence
Systematic Reviews; Meta-Analyses; Randomized Clinical Trials (RCTs) with definitive results; RCTs with non-definitive results; Cohort Studies
-
GRADE Level
High; Moderate
The search was conducted on 21–24 September 2020 to identify available scientific literature in support of the hypothesis and evidence that may support the null hypothesis (i.e. no additional benefits). Two independent medical researchers conducted the search under the guidance of the authors and extracted information from the articles, first by reviewing titles and abstracts and then by reviewing the full-text articles. Inter-rater reliability was performed through the calculation of percentage agreement. A third-party reviewer (C.G.) was available to resolve and reconcile any disagreements. Relevant information regarding (1) study type, (2) number of patients and type of interventions used in the study, and (3) outcomes and parameters was recorded. Multiple publications from a unique study were included, and publications based on secondary, exploratory, or post hoc analyses. The National Clinical Trial (NCT) study number was recorded for all identified journal articles and used as a reference to recognize duplicate publications. Clinical trial records for each identified journal article were then examined on https://clinicaltrials.gov to identify any missing baseline characteristics, demographics, or patient numbers. Evidence from systematic literature reviews and meta-analyses was broken down into individual clinical trials, with the publications excluded once studies were identified.
Results
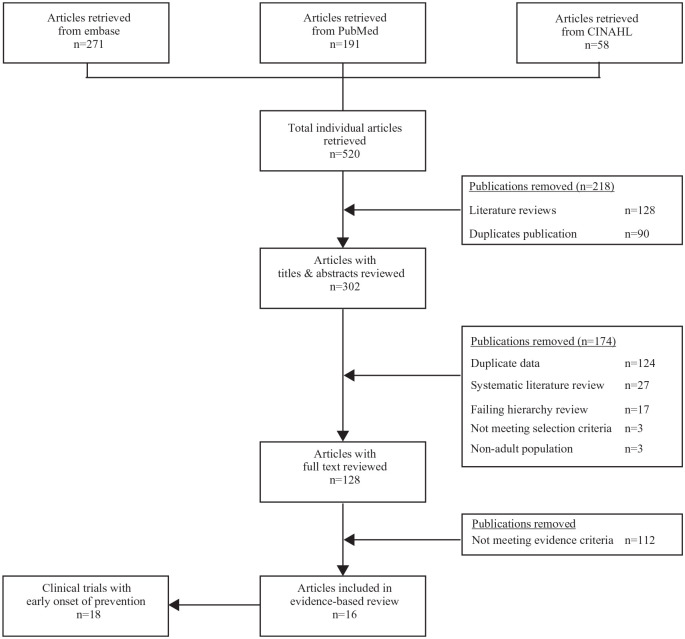
The PRISMA flowchart of the evidence-based literature search is shown in Figure 1. The initial search yielded 520 potentially relevant publications across all databases, with 90 articles excluded for being duplicate publications across the searched databases. A further 128 literature review articles were excluded. After screening the titles and abstracts of the remaining 302 articles, an additional 174 were excluded for not meeting the selection criteria [e.g. including pediatric patients (n = 3), being duplicate publications (e.g. a conference abstract being published as a full-text article), containing duplicate data presentations, or not containing specific data related to the early onset of prevention]. A total of 27 systematic literature reviews were captured as part of the literature search, which were first assessed for any additional clinical trial evidence not already captured in the search and then excluded after new evidence was identified (none found). Full-text review of the remaining 128 articles excluded 17 for failing to meet the predefined hierarchy criteria (i.e. open-label design). After screening and title/abstract review, 128 articles were then subject to full-text review in relation to the predefined scoping hypothesis; of these, 112 were excluded due to not meeting evidence criteria. No active-controlled trials or observational studies were identified during the literature search process.
Figure 1.
Evidence-based literature search flow chart.
In total, 16 peer-reviewed articles were identified for inclusion in this evidence-based scoping review, which reported clinical benefits associated with the early onset of prevention from 18 randomized, double-blind, placebo-controlled clinical trials in patients with either episodic or chronic migraine (Table 2). Evidence was found for the four approved anti-CGRP monoclonal antibodies (mAbs) [erenumab (n = 3), fremanezumab (n = 6), galcanezumab (n = 3), and eptinezumab (n = 4)] in patients with episodic and chronic migraine and for the chemodenervation agent [onabotulinumtoxinA (n = 2)] in patients with chronic migraine. No evidence for an early onset of prevention was identified for any oral preventive agent currently approved or recommended for the prevention of migraine.
Table 2.
Studies of migraine preventive therapies reporting an early onset of preventive effect*: clinical efficacy endpoints.
| Study | Study design | Population | Dose | Early onset timepoint | Onset of preventive effect | GRADE Ranking † |
|---|---|---|---|---|---|---|
| Erenumab | ||||||
| NCT02456740 (STRIVE) and NCT0206641521 | Post hoc analysis of two R, DB, PC studies (NCT02456740: Phase II; NCT02066415: Phase III) | Adults with EM (N = 955) and CM (N = 667) | 70 or 140 mg monthly | EM Week 1 |
Nominally significant reductions in WMD versus placebo as early as Week 1 (not adjusted for multiple comparisons) LSM change from baseline (95%CI) Week 1 Placebo: −0.1 (−0.3, 0.0) Erenumab 70 mg: −0.3 (−0.5, −0.2); p = 0.130 Erenumab 140 mg: −0.6 (−0.7, −0.4); p < 0.001 Week 4 Placebo: −0.4 (−0.5, −0.2) Erenumab 70 mg: −0.6 (−0.8, −0.5); p = 0.029 Erenumab 140 mg −0.6 (−0.8, −0.5); p = 0.019 |
High |
|
CM
Week 1 |
Nominally significant reductions in WMD versus placebo for both doses at Week 1 and sustained through Week 4 Week 1 Placebo: −0.5 (−0.8, −0.3) Erenumab 70 mg: −0.9 (−1.2, −0.7); p = 0.047 Erenumab 140 mg: −0.8 (−1.1,−0.5); p = 0.18 Week 4 Placebo: −0.8 (−1.0, −0.6) Erenumab 70 mg: −1.5 (−1.7, −1.2); p < 0.001 Erenumab 140 mg: −1.4 (−1.6, −1.1); p = 0.002 |
|||||
| NCT02483585 (ARISE) 22 | Phase III, R, DB, PC | 577 adults (18–65 years) with EM | 70 mg monthly | Month 1 | Primary outcome: LSM change from baseline in MMD at Month 3, −2.9 days versus −1.8 days (p < 0.001) Exploratory: Reduction in MMD achieved nominal significance (not adjusted for multiple comparisons) at Month 1 (p < 0.001 versus placebo) |
Moderate |
| Fremanezumab | ||||||
| NCT02629861 (HALO EM)23,24 | Phase III, R, DB, PC | 875 adults (18–70 years) with EM | Monthly (225 mg) Quarterly (675 mg at Month 1, placebo at Months 2 and 3), placebo | Day 1 | Both regimens reduced mean weekly headache days at Week 1 versus placebo (monthly, −0.9 versus −0.3; quarterly, −0.8 versus −0.3; both p < 0.0001) (mixed-effect model for repeated measures) More patients reported no migraine by the next day following the first injection [monthly, 79.4%; quarterly, 79.2% versus placebo, 66.6% (p = 0.0002 and 0.0004 versus placebo, respectively)] (post hoc analysis) |
High |
| NCT02621931 (HALO CM)25,26 | Phase III, R, DB, PC | 1130 adults (18–70 years) with CM | Monthly (675 mg followed by 225 mg at Months 2 and 3) or quarterly (675 mg at Month 1, followed by placebo injections at Months 2 and 3) | Day 1 | Reduced mean weekly headache days at Week 1 versus placebo (−1.1 versus −0.5; p < 0.0001) (mixed-effect model for repeated measures) More patients reported no headache of at least moderate severity by the next day following the first injection (fremanezumab, 69%; placebo, 61%; p = 0.0036) (post hoc analysis) |
High |
| NCT02025556 (HFEM) 27 | Post hoc analysis of a 16-week, phase II, R, DB, PC study | 297 adults (18–65 years) with high-frequency EM | 225 or 675 mg monthly | Week 1 | Significantly reduced migraine frequency compared to placebo within the first week of therapy; LSM differences versus placebo at Week 1 were −0.93 WMD and −1.02 WMD, both p < 0.0001 The benefit was maintained for the second and third weeks of therapy (p < 0.001); weekly mean headache days and headache hours were also reduced (p < 0.01) |
Moderate |
| NCT02021773 28 | Phase IIb, R, DB, PC (post hoc analysis) |
261 adults (18–65 years) with CM | 900 mg (225 mg monthly × 4) or 675/225 (675 Month 1 then 225/month × 3) | Days 3–7 | 900 mg: reduced mean headache hours from baseline to Day 3 (−3.08 versus +0.36 for placebo, p = 0.0331) 675/225: reduced mean headache hours from baseline to Day 7 (−7.28 versus −1.59, p = 0.0486) |
Moderate |
| Galcanezumab | ||||||
| NCT01625988(ART-01) 29 | Post hoc analysis of a Phase II, R, DB, PC study | 217 adults (18–65 years) with EM | 150 mg every 2 weeks | Week 1 | Reduced weekly mean headache days from baseline at Week 1 (−0.89) versus placebo (−0.53; p = 0.018) Greater proportion of patients experienced ⩾ 50% reduction in number of weekly mean headache days at Week 1 (62% versus 42%; p < 0.05) |
Moderate |
| NCT02614183 (EVOLVE-1) and NCT02614196 (EVOLVE-2) 30 | Post hoc analysis of two Phase III, R, DB, PC studies | 1773 adults (18–65 years) with EM | 120 mg monthly with loading dose of 240 mg administered the first month | Day 1 | Reduced mean headache days at Week 1 (OR 2.71 and 2.88 for EVOLVE-1 and EVOLVE-2 respectively; both p < 0.001) (repeated measures ordinal logistic regression) The mean number of patients with migraine headaches each day of Week 1 was significantly lower with galcanezumab versus placebo beginning Day 1 post-injection (EVOLVE-1, p = 0.002; EVOLVE-2, p = 0.038) |
High |
| Eptinezumab | ||||||
| NCT0177252431 | Phase II, R, DB, PC | 174 adults (18–55 years) with EM |
1000 mg, placebo | Month 1 | Reduced mean MMD between baseline and Week 1–4 versus placebo (−5.6 versus −3.9, p = 0.0007) | Moderate |
| NCT0227517732 | Phase IIb, R, DB, PC (post hoc analysis) |
616 adults (18–55 years) with CM | 10, 30, 100, 300 mg, placebo | Day 1 | Eptinezumab 100 and 300 mg reduced the likelihood of a migraine attack more than placebo in the 24 h post-infusion versus baseline Migraine attack on Day 1: Eptinezumab 100 mg, 29.3%; eptinezumab 300 mg, 26.3%; placebo, 48.7% versus migraine occurrence on any given day during 28-day screening period: Eptinezumab 100 mg, 60.4%; eptinezumab 300 mg, 59.1%; placebo, 58.7% |
Moderate |
| NCT02559895 (PROMISE-1) 33 | Phase III, R, DB, PC | 888 adults (18–75 years) with EM | 30, 100, 300, placebo | Day 1 | Eptinezumab 100 and 300 mg reduced the likelihood of a migraine attack in the 24 h post-infusion 50% versus baseline and significantly more than placebo Overall baseline prevalence, 30.7% Day 1 prevalence: Eptinezumab 100 mg, 14.8%; eptinezumab 300 mg, 13.9%; placebo, 22.5% (p = 0.0312 and p = 0.0159 versus placebo, respectively) |
High |
| NCT02974153 (PROMISE-2) 34 | Phase III, R, DB, PC | 1072 adults (18–65 years) with CM | 100, 300 mg, placebo | Day 1 | Eptinezumab 100 and 300 mg reduced the likelihood of a migraine attack in the 24 h post-infusion 50% versus baseline and significantly more than placebo Overall baseline prevalence, 58%; Day 1 prevalence: Eptinezumab 100 mg, 28.6%; eptinezumab 300 mg, 27.8%; placebo, 42.3% (both p < 0.001 versus placebo) |
High |
| OnabotulinumtoxinA | ||||||
| NCT00156910 (PREEMPT 1) and NCT00168428 (PREEMPT 2) 12 | Phase III, R, DB, PC | 1384 adults (18–65 years) with CM | 155–195 U every 12 weeks | Week 1 | Reduced headache and migraine days versus placebo compared to Week 4 of baseline (−0.9 versus −0.7 headache days/week, p = 0.046; −1.0 versus −0.7 migraine days/week, p = 0.031) | Moderate |
| Week 3 | Reduced headache and migraine days versus placebo compared to Week 4 of baseline (−1.6 versus −1.1 headache days/week, p < 0.001; −1.6 versus 1.1 migraine days/week, p < 0.001) |
CI, confidence interval; CM, chronic migraine; DB, double-blind; EM, episodic migraine; LSM, least-squares mean; MMD, monthly migraine days; OR, odds ratio; PC, placebo-controlled; R, randomized; WMD, weekly migraine days.
Early was defined as the demonstration of preventive benefits within 1 month (30 days) post-initiation.
Grading criteria based on the GRADE methodology of assessing the certainty in evidence and the strength of recommendations. 19
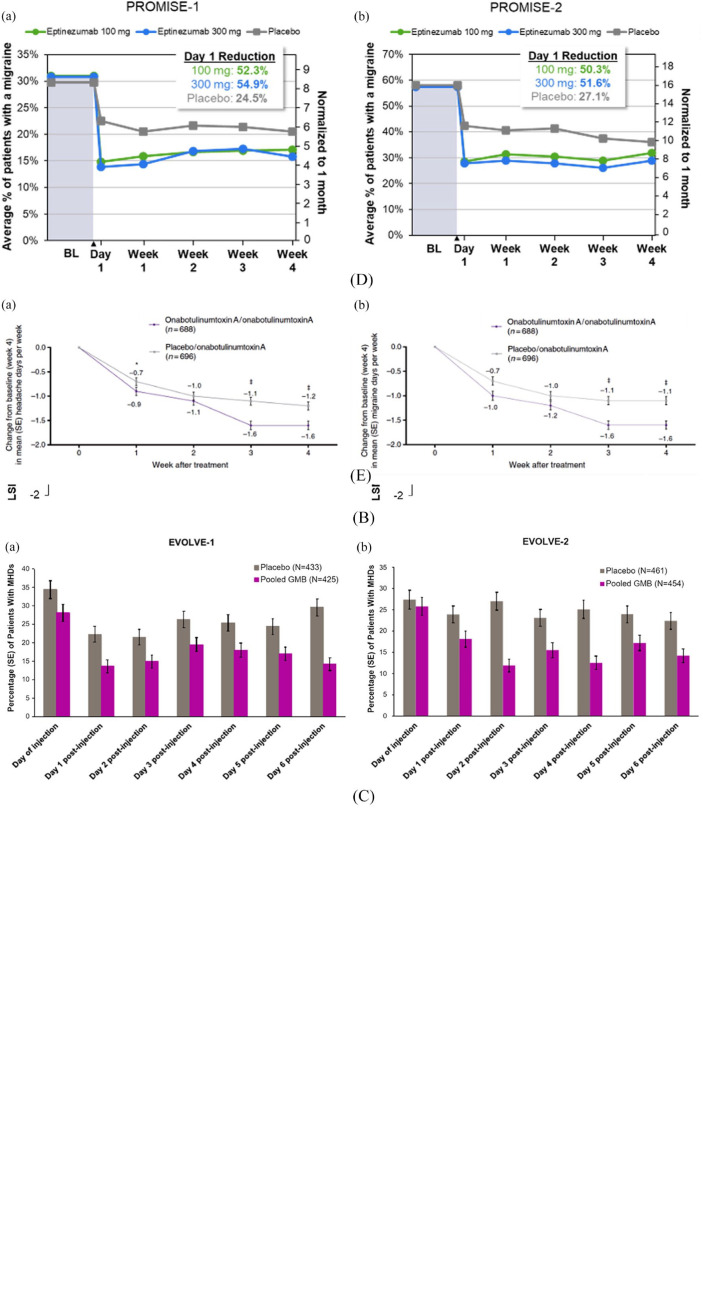
The evidence supporting early onset of prevention was generated across clinical trial populations that included patients with a diagnosis of either episodic or chronic migraine, supporting the robustness of the data across a wide patient type (Figure 2). For patients with migraine treated with eptinezumab in the PROMISE-1 33 or PROMISE-2 34 studies, the early preventive benefits were observed as early as Day 1 post-treatment administration in a post hoc analysis, where eptinezumab 100 and 300 mg reduced the likelihood of a migraine attack in the 24 h post-infusion by > 50% versus baseline and significantly more than placebo. 35 Similar results were observed in the post hoc analysis of the EVOLVE-1 36 and EVOLVE-2 37 studies for galcanezumab, 30 where the mean number of patients with headaches due to migraine each day of Week 1 was significantly lower with galcanezumab compared to placebo beginning Day 1 post-injection. In the fremanezumab clinical trials, more patients reported no headache of at least moderate severity by the next day following the first injection across multiple trials, 25 with a significant reduction in migraine frequency compared to placebo within the first week of therapy.23,24,26,28,38 In patients treated with erenumab, there was a nominally significant reduction in weekly migraine days as early as Week 1 in the pooled post hoc analysis of patients with episodic or chronic migraine.21,22 In the pooled analysis of the PREEMPT clinical trials39,40 for onabotulinumtoxinA in patients with chronic migraine, 12 there was a reduction in headache and migraine days as early as Week 1 when compared to placebo at the end of Week 4 post-treatment. Across all identified studies, the early onset of prevention was also durable in nature, lasting through at least 12 weeks of treatment.
Figure 2.
Clinically relevant benefits of an early onset of prevention: reduction in headache and migraine days. (A) Change from Baseline in weekly migraine days during the first month of erenumab in (a) episodic and (b) chronic migraine; (B) Change from baseline in (a) weekly headache days and (b) weekly migraine days during the first month of fremanezumab in chronic migraine; (C) Patients with headache each day (a) in EVOLVE-1 and (b) EVOLVE-2 during the first week of galcanezumab in episodic migraine; (D) Change from baseline in weekly migraine days during the first day and month of eptinezumab in (a) episodic and (b) chronic migraine; (E) Change from baseline in (a) mean headache days and (b) mean migraine days during the first month of onabotulinumtoxinA in chronic migraine.
All figures reprinted with permission: (A) from Schwedt et al.; 21 (B) from Winner et al.; 26 (C) from Detke et al.; 30 (D) from Dodick et al.; 35 and (E) from Dodick et al. 12
In addition to these clinical benefits associated with an early onset of prevention, patient-reported outcome measures (PROMs) were identified in four publications that outline the results of treatment on six specific PROMs across four clinical trials. These included improvements in 36-item Short-Form Health Survey (SF-36) bodily pain, role-physical, and social functioning domains beginning as early as Week 4 with eptinezumab and improved 6-item Headache Impact Test (HIT-6) total scores, Migraine-Specific Quality-of-life (MSQ), -Role Function Preventive (RFP), -Role Function Restrictive (RFR), and -Emotional Function (EF) scores, and Migraine Disability Assessment (MIDAS) scores at Week 4 with fremanezumab. The full scoping evaluation of the benefits associated with the early onset of prevention in patients with migraine are detailed in Table 3. As a result of the strength of identified evidence (based on the GRADE criteria), the null hypothesis was rejected. Furthermore, no evidence was identified in any publication to support the null hypothesis that patients with migraine do not benefit from an early onset of prevention.
Table 3.
Studies of migraine preventive therapies reporting an early onset of preventive effect*: clinical efficacy endpoints: impact on function, disability, and quality of life.
| Study | Study design | Population | Dose | Impact |
|---|---|---|---|---|
| Fremanezumab | ||||
| NCT02621931 (HALO-CM)38,41,42 |
Phase III, R, DB, PC | 1130 adults (18–70 years) with CM | Quarterly (675 mg) or monthly (675/225/225 mg) | • Percentage of patients reporting PGIC ⩾ 5 at Week 4 was 53% and 54% for quarterly and monthly fremanezumab administration, respectively (versus 31% for placebo, p < 0.0001 for both comparisons) • Improved HIT-6 total scores 4 weeks after the final dose more than placebo [−6.4, −6.8 versus −4.5 (both p < 0.001 versus placebo)] in the total population • Improved MSQ-RFP, -RFR, and -EF scores more than placebo at Week 4 in the total population (all p < 0.0001) ○ MSQ-RFR score: 19.1, 19.4 versus 12.0 (quarterly, monthly, placebo, respectively) ○ MSQ-RFP score: 15.3, 15.8 versus 9.4 ○ MSQ-EF score: 19.1, 19.5 versus 12.1 |
| NCT02629861 (HALO EM) 24 |
Phase III, DB, R, DB, PC | 875 adults (18–70 years) with EM | Monthly (225 mg) and quarterly (675 mg) | • Improved MIDAS scores at Week 4 ○ −24.6, −23.0 versus −17.5 (p < 0.001 and p = 0.002, respectively) |
| Eptinezumab | ||||
| NCT0177252431 | Phase II, R, DB, PC | 163 adults (18–55 years) with EM | 1000 mg | • Improved HIT-6 total score by −10.2 at Week 4 [versus −5.8 with placebo; difference (95% CI), −4.4 (−7.2 to −1.6)] • Improved MSQ-RFP score by 29.3 at Week 4 [versus 19.9 with placebo; difference (95% CI), 9.4 (1.8 to 17.0)] • Improved MSQ-RFR score by 21.1 at Week 4 [versus 16.3 with placebo; difference (95% CI), 4.8 (−2.6 to 12.2)] • Improved MSQ-EF score by 25.1 at Week 4 [versus 19.4 with placebo; difference (95% CI), 5.7 (−3.1 to 14.5)] |
| NCT02559895 (PROMISE-1) 43 |
Phase III, R, DB, PC | 888 adults (18–75 years) with EM | 30, 100, and 300 mg | • Improved SF-36 domains that were impaired or below normative values at baseline (bodily pain, role-physical, and social functioning), beginning as early as Week 4 ○ Bodily pain: Improvements ranged from 3.8 to 4.1 (eptinezumab) versus 2.3 (placebo) at Week 4 ○ Role-physical: Improvements ranged from 2.1 to 2.6 (eptinezumab) versus 1.5 (placebo) at Week 4 ○ Social functioning: Improvements ranged from 1.7 to 3.0 (eptinezumab) versus 1.8 (placebo) versus placebo |
CI, confidence interval; chronic migraine; DB, double-blind; EM, episodic migraine; HIT-6, 6-item Headache Impact Test; MIDAS, Migraine Disability Assessment; MSQ-EF, Migraine-Specific Quality-of-Life Questionnaire–Emotional Function; MSQ-RFP, Migraine-Specific Quality-of-Life Questionnaire–Role Function Preventive; MSQ-RFR, Migraine-Specific Quality-of-Life Questionnaire–Role Function Restrictive; PC, placebo controlled; R, randomized; WMD, weekly migraine day
Early was defined as the demonstration of preventive benefits within 1 month (30 days) post-initiation.
Discussion
The results of this evidence-based scoping literature analysis identified clinically and statistically significant evidence that supports the hypothesis that patients with migraine benefit from an early onset of prevention with currently approved anti-CGRP mAbs and onabotulinumtoxinA. Supporting patient-reported outcome evidence was also identified, but only from a limited number of identified clinical trials. The consistency of evidence from the newer therapeutic agents utilized for migraine prevention and the overall high grade of evidence across all clinical trial publications are indirect indicators that migraine can not only be prevented to some degree of confidence across patients but can also be achieved in a realistic time frame that addresses concerns of the patient regarding the onset of the prevention of future migraine attacks.
This relationship between early improvement and overall treatment benefits is not unique to the treatment of migraine. Rapid improvement in other pain disorders improved the patients’ confidence in the treatment chosen, which likely improves adherence and persistence with the medication in question. Rapid improvement also strongly correlates with good long-term outcomes in multiple pain disorders, such as post-operative pain 44 and other chronic pain disorders, such as fibromyalgia. 45
The evidence in this scoping review regarding the clinical benefits of an early onset of migraine prevention was identified in 16 peer-reviewed publications, which encompassed two studies of onabotulinumtoxinA in chronic migraine, 12 and the phase II and phase III studies for the anti-CGRP mAbs erenumab,21,22 fremanezumab,23–28,38 galcanezumab,29,30 and eptinezumab,31–34 which were investigated in patients with either episodic or chronic migraine. Clinical benefits associated with an early onset of prevention were identified as early as 1-day post-administration, based on the numeric reduction in headache/migraine days, headache/migraine hours, or headache/migraine attacks. While not a mandated clinical trial endpoint for the regulatory approval of preventive agents for migraine, the inclusion of clinical trial endpoints that enables an early evaluation of prevention (potentially as early as 24 h post-initiation of treatment) provides a greater insight of the cumulative benefits of these newer compounds. As seen in the recently published RELIEF study, when initiated during a migraine attack, eptinezumab demonstrated clinical efficacy within 2 h of administration. 46 In addition, the identified clinical evidence could be viewed as reflective of the pharmacokinetic profiles of the anti-CGRP mAbs, where Cmax and Tmax are achieved in a matter of minutes 47 to days48–50 compared to weeks, and often months, the time-frame profile characteristic of the older oral preventive agents. While further clinical studies are required, there may be a correlation between the early onset of migraine prevention and the rapid onset of Cmax and Tmax with the anti-CGRP mAbs, as hypothesized by Baker et al. 47
Patient-reported outcomes evidence in support of an early onset of preventive benefit was limited, potentially due to the lack of necessity for including these instruments in clinical registration studies. A total of four publications described improvement in function, disability, and quality of life as early as 4 weeks after initiation as measured using established PROMs. Specific evidence supporting an early onset of preventive benefits on PROMs was identified for eptinezumab on the HIT-6, 31 MSQ, 31 and SF-36, 43 and for fremanezumab on the HIT-6, 38 MSQ, 41 Patient Global Impression of Change (PGIC), 42 and MIDAS. 24
Limitations
This evidence-based, hypothesis-driven, scoping literature review has several limitations that may impact the overall weight of evidence identified. The early onset of prevention was not a predefined endpoint in any of the identified studies, with the identified evidence generated from secondary or post hoc analyses of phase II or phase III clinical trials. The impact of an early onset of prevention was sparingly reported through global PROMs, limiting the patient perspective. No analysis based on migraine disease severity, duration, or symptomatology was identified. Furthermore, evidence presented in congress abstracts or presentations was excluded, potentially limiting the identification of evidence from traditional oral formulations or investigational compounds. In addition, the search was limited to therapies approved by the US Food and Drug Administration.
Conclusion
To the authors’ knowledge, this is the first scoping review of the evidence related to clinical and patient-reported benefits associated with an early onset of prevention in patients with migraine. While clinical and regulatory guidance documents typically evaluate the preventive benefit of treatment after a minimum of 12 weeks of therapy, newer preventive therapies, such as the anti-CGRP mAbs (erenumab, fremanezumab, galcanezumab, and eptinezumab), and the chemodenervation agent onabotulinumtoxinA, provide clinically relevant benefits by the end of the first week, with benefits sometimes reported as early as the first-day post-administration. Although the definition of an ‘early onset’ could not be consistently measured across clinical trials (Day 1 versus Day 7), clinical endpoints varied across studies, and there was limited evidence related to patient-reported outcomes; the overall strength of the data across patients with episodic and chronic migraine suggests that a new threshold in clinical effectiveness for migraine preventive treatments may be achievable. Further studies with improved study designs, standardized outcome definitions, and more rigorous methodologies are warranted to fully evaluate the clinically relevant benefits associated with an early onset of prevention in patients with migraine.
Acknowledgments
The authors thank Dr. Anirban Basu for his participation in the advisory board and input on this work. The authors also thank Philip Sjostedt, BPharm, MPH, of The Medicine Group, LLC (New Hope, PA, United States) for providing medical writing support, which was funded by Lundbeck LLC (Deerfield, IL, USA) and in accordance with Good Publication Practice guidelines. The authors have authorized the submission of this manuscript by The Medicine Group on their behalf and have approved any statements and declarations.
Footnotes
Ethical approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Christopher Gottschalk: Conceptualization; Writing – review & editing.
Dawn C. Buse: Conceptualization; Writing – review & editing.
Michael J. Marmura: Conceptualization; Writing – review & editing.
Bradley Torphy: Conceptualization; Writing – review & editing.
Jelena M. Pavlovic: Conceptualization; Writing – review & editing.
Paula K. Dumas: Conceptualization; Writing – review & editing.
Nim Lalvani: Conceptualization; Writing – review & editing.
Andrew Blumenfeld: Conceptualization; Writing – review & editing.
ORCID iD: Christopher Gottschalk  https://orcid.org/0000-0002-1105-6910
https://orcid.org/0000-0002-1105-6910
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This scoping literature review was sponsored and funded by H. Lundbeck A/S and Lundbeck Seattle BioPharmaceuticals, Inc. All authors prepared, reviewed, and approved the article, and made the decision to submit the article for publication. Editorial support for the development of this article was funded by H. Lundbeck A/S.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.G. has been a paid consultant for Alder/Lundbeck, Biohaven, Amgen/Novartis, Theranica, Axsome, Upsher Smith, Spherix Global Insights, and Vorso and has been a past member of speaker bureaus for Amgen/Novartis, Allergan/AbbVie, Biohaven, Lilly, Theranica, and Upsher Smith. He served as an Associate Editor of Headache until 2020 and is a board member of the Headache Cooperative of New England (HCNE). D.C.B. has received grant support from the Food and Drug Administration and the National Headache Foundation and grant support and honoraria from Allergan, Amgen, Lilly, Lundbeck, and Teva. She serves on the editorial board of Current Pain and Headache Reports. M.J.M. has received compensation for consultation from Lundbeck and Theranica. He has participated in speaker bureaus for Lilly and Amgen/Novartis. He has received salary support for serving as principal investigator from Teva, GammaCore, and Allergan/AbbVie. He has received payments for authorship or royalties from Demos Medical, Cambridge University Press, and MedLink.
B.T. has received compensation for consulting from Amgen, Novartis, Biohaven, Lilly, Lundbeck, Teva, and Theranica. He has participated in speaker bureaus with Allergan/AbbVie, Amgen, Biohaven, Lilly, Lundbeck, and Teva, and he has received financial compensation for serving as principal investigator with Amgen and Theranica. J.M.P. has received grant support from the National Institutes of Health and compensation for consulting from Alder/Lundbeck, Allergan/AbbVie, Amgen/Novartis, and Biohaven. P.K.D. has received grant support from Amgen/Novartis, Allergan/AbbVie, and Lilly. She serves as Editor in Chief of Migraine Again, Editor at Large of Everyday Health, and Co-Producer/Co-Owner of Migraine World Summit, which is supported by Lundbeck, Impel, Lilly, Allergan/AbbVie, Axon Optics, and Teva. N.L. has no compensation or conflicts to report. All funding or sponsorships are directed to the American Migraine Foundation. A.B. serves as a consultant and/or a promotional speaker for Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Lundbeck, Novartis, Promius, Supernus, Teva, and Theranica.
Availability of data and materials: Not applicable.
Contributor Information
Christopher Gottschalk, Division of General Neurology, Neurology, Yale School of Medicine, Yale Physicians Building, 800 Howard Avenue, Ste Lower Level, New Haven, CT 06519, USA.
Dawn C. Buse, Montefiore Headache Center, Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, USA
Michael J. Marmura, Jefferson Headache Center, Thomas Jefferson University, Philadelphia, PA, USA
Bradley Torphy, Chicago Headache Center and Research Institute, Chicago, IL, USA.
Jelena M. Pavlovic, Montefiore Headache Center, Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, USA
Paula K. Dumas, World Health Education Foundation, Irvine, CA, USA
Nim Lalvani, American Migraine Foundation, Mount Royal, NJ, USA.
Andrew Blumenfeld, Headache Center of Southern California, The Neurology Center of Southern California, Carlsbad, CA, USA.
References
- 1. Ailani J, Burch RC, Robbins MS, et al. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache 2021; 61: 1021–1039. [DOI] [PubMed] [Google Scholar]
- 2. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 3. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78: 1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silberstein SD. Preventive migraine treatment. Continuum (Minneapolis, Minn) 2015; 21: 973–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar A, Kadian R. Headache, migraine prophylaxis. Treasure Island, FL: StatPearls Publishing, 2018. [PubMed] [Google Scholar]
- 6. Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 2014; 20: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia 2017; 37: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peres MF, Silberstein S, Moreira F, et al. Patients’ preference for migraine preventive therapy. Headache 2007; 47: 540–545. [DOI] [PubMed] [Google Scholar]
- 9. Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache 2013; 53: 644–655. [DOI] [PubMed] [Google Scholar]
- 10. Botox [package insert]. Madison, NJ: Allergan, 2021. [Google Scholar]
- 11. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open 2019; 9: e027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dodick DW, Silberstein SD, Lipton RB, et al. Early onset of effect of onabotulinumtoxinA for chronic migraine treatment: analysis of PREEMPT data. Cephalalgia 2019; 39: 945–956. [DOI] [PubMed] [Google Scholar]
- 13. Masters-Israilov A, Robbins MS. OnabotulinumtoxinA wear-off phenomenon in the treatment of chronic migraine. Headache 2019; 59: 1753–1761. [DOI] [PubMed] [Google Scholar]
- 14. Zidan A, Roe C, Burke D, et al. OnabotulinumtoxinA wear-off in chronic migraine, observational cohort study. J Clin Neurosci 2019; 69: 237–240. [DOI] [PubMed] [Google Scholar]
- 15. ICDH-1. Diagnostic criteria, https://ichd-3.org/evolution-of-ihs-classification-1-3/ (accessed 14 June 2021).
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt GH, Sackett DL, Sinclair JC, et al. Users’ guides to the medical literature. IX. A method for grading health care recommendations. Evidence-Based Medicine Working Group. JAMA 1995; 274: 1800–1804. [DOI] [PubMed] [Google Scholar]
- 18. Greenhalgh T. How to read a paper. Getting your bearings (deciding what the paper is about). BMJ 1997; 315: 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schünemann HJ, Best D, Vist G, et al. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. CMAJ 2003; 169: 677–680. [PMC free article] [PubMed] [Google Scholar]
- 20. Food Drug Administration. Drugs@FDA: FDA-Approved Drugs, https://www.accessdata.fda.gov/scripts/cder/daf/
- 21. Schwedt T, Reuter U, Tepper S, et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain 2018; 19: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018; 38: 1026–1037. [DOI] [PubMed] [Google Scholar]
- 23. Brandes J, Yeung PP, Aycardi E, et al. Early onset of action with fremanezumab versus placebo for the preventive treatment of episodic migraine (P4.107). Neurology 2018; 90: P4107. [Google Scholar]
- 24. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 2018; 319: 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeung PP, Aycardi E, Bigal M, et al. Early onset of action with fremanezumab versus placebo for the preventive treatment of chronic migraine (P4.102). Neurology 2018; 90: P4102. [Google Scholar]
- 26. Winner PK, Spierings ELH, Yeung PP, et al. Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache 2019; 59: 1743–1752. [DOI] [PubMed] [Google Scholar]
- 27. Silberstein SD, Rapoport AM, Loupe PS, et al. The effect of beginning treatment with fremanezumab on headache and associated symptoms in the randomized phase 2 study of high frequency episodic migraine: post-hoc analyses on the first 3 weeks of treatment. Headache 2019; 59: 383–393. [DOI] [PubMed] [Google Scholar]
- 28. Bigal ME, Dodick DW, Krymchantowski AV, et al. TEV-48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology 2016; 87: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goadsby PJ, Dodick DW, Martinez JM, et al. Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: a post-hoc analysis. J Neurol Neurosurg Psychiatry 2019; 90: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Detke HC, Millen BA, Zhang Q, et al. Rapid onset of effect of galcanezumab for the prevention of episodic migraine: analysis of the EVOLVE studies. Headache 2020; 60: 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 32. Dodick DW, Lipton RB, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: a randomized phase 2b clinical trial. Cephalalgia 2019; 39: 1075–1085. [DOI] [PubMed] [Google Scholar]
- 33. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia 2020; 40: 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 2020; 94: e1365–e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dodick DW, Gottschalk C, Cady R, et al. Eptinezumab demonstrated efficacy in sustained prevention of episodic and chronic migraine beginning on Day 1 after dosing. Headache 2020; 60: 2220–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurology 2018; 75: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia 2018; 38: 1442–1454. [DOI] [PubMed] [Google Scholar]
- 38. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 2017; 377: 2113–2122. [DOI] [PubMed] [Google Scholar]
- 39. Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010; 30: 793–803. [DOI] [PubMed] [Google Scholar]
- 40. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010; 30: 804–814. [DOI] [PubMed] [Google Scholar]
- 41. Silberstein SD, Cohen JM, Seminerio MJ, et al. The impact of fremanezumab on medication overuse in patients with chronic migraine: subgroup analysis of the HALO CM study. J Headache Pain 2020; 21: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lipton RB, Cohen JM, Gandhi SK, et al. Effect of fremanezumab on quality of life and productivity in patients with chronic migraine. Neurology 2020; 95: e878–e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith TR, Janelidze M, Chakhava G, et al. Eptinezumab for the prevention of episodic migraine: sustained effect through 1 year of treatment in the PROMISE-1 study. Clin Ther 2020; 42: 2254–2265.e2253. [DOI] [PubMed] [Google Scholar]
- 44. Blichfeldt-Eckhardt MR. From acute to chronic postsurgical pain: the significance of the acute pain response. Dan Med J 2018; 65: B5326. [PubMed] [Google Scholar]
- 45. Wang F, Ruberg SJ, Gaynor PJ, et al. Early improvement in pain predicts pain response at endpoint in patients with fibromyalgia. J Pain 2011; 12: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 46. Winner PK, McAllister P, Chakhava G, et al. Effects of intravenous eptinezumab vs placebo on headache pain and most bothersome symptom when initiated during a migraine attack: a randomized clinical trial. JAMA 2021; 325: 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baker B, Schaeffler B, Beliveau M, et al. Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine. Pharmacol Res Perspect 2020; 8: e00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vu T, Ma P, Chen JS, et al. Pharmacokinetic-pharmacodynamic relationship of erenumab (AMG 334) and capsaicin-induced dermal blood flow in healthy and migraine subjects. Pharm Res 2017; 34: 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fiedler-Kelly JB, Cohen-Barak O, Morris DN, et al. Population pharmacokinetic modelling and simulation of fremanezumab in healthy subjects and patients with migraine. Br J Clin Pharmacol 2019; 85: 2721–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Monteith D, Collins EC, Vandermeulen C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the CGRP binding monoclonal antibody LY2951742 (galcanezumab) in healthy volunteers. Front Pharmacol 2017; 8: 740. [DOI] [PMC free article] [PubMed] [Google Scholar]