Abstract
Nanotechnology in modern material science is a research hot spot due to its ability to provide novel applications in the field of dentistry. Zinc Oxide Nanoparticles (ZnO NPs) are metal oxide nanoparticles that open new opportunities for biomedical applications that range from diagnosis to treatment. The domains of these nanoparticles are wide and diverse and include the effects brought about due to the anti-microbial, regenerative, and mechanical properties. The applications include enhancing the anti-bacterial properties of existing restorative materials, as an anti-sensitivity agent in toothpastes, as an anti-microbial and anti-fungal agent against pathogenic oral microflora, as a dental implant coating, to improve the anti-fungal effect of denture bases in rehabilitative dentistry, remineralizing cervical dentinal lesions, increasing the stability of local drug delivery agents and other applications.
Keywords: zinc oxide nanoparticles, biomedical application, nanodentistry, dental applications, restorative material
1 Introduction
Nanotechnology, wherein matter is manipulated on a molecular scale, has revolutionized modern dentistry. “Nanodentistry” is the amalgamation of nanotechnology and dentistry and provides the scope for the formulation of innovative materials that can have many potential applications in clinical practice. The nano size confers a larger surface area, allows the controlled synthesis and is also capable of altering the desired physical and chemical properties that enables them for unique interactions with biomolecules. They also have a higher percentage of surface atoms, which maximized their ability due to an increase in surface reactivity (Rasmussen et al., 2010).
Zinc is an essential trace element which is found in the muscle, bone, skin and also in the hard tissues of the tooth. Zinc Oxide Nanoparticle (ZnO NP) is a white colored odorless powder and has a molecular weight of 81.38 g/mol. FDA considers it as a generally recognized as safe (GRAS) substance. Its extensive applications in dentistry are credited to the unique optical, magnetic, morphological, electrical, catalytic, mechanical, and photochemical properties which can be easily altered as per the requirements: by modifying the size, doping with supplementary compounds, or adjusting the conditions of synthesis. As the size of the particles decrease, the desirable characteristics improve (Baek et al., 2012).
In the present, ZnO NPs are being investigated as associates of anti-microbial agents which are one of the most important reasons for its use. A recent theory that explains this is the “Trojan Horse effect”, which states that the acidic lysosomal environment promotes nanoparticle degradation, that in turn brings about conversion of core metals to ions and the release of substances that are toxic and in turn interrupt cell reproduction. Other mechanisms of their anti-microbial action are by locally changing the microenvironments near the microbes and by producing reactive oxygen species (ROS) or by increasing solubility of these nanoparticles. This can induce interplay with -SH group of the enzymes in the microbes and cause malfunction of organelles causing denaturation of the proteins and resulting in damage to DNA. This in turn alters the DNA replication of the microorganisms. Another possible anti-microbial mechanismis by the release of H2O2 (Şuhani et al., 2018) and by the displacement of Magnesium ions which interferes with the metabolism of the bacteria. The enhanced effect against microbes is attributed to the increased ratio of surface/volume. Hence, the incorporation of ZnO NPs in dental restorative materials, luting materials, tissue conditioners, intracanal medicaments, irrigants, adhesives and other materials can have beneficial anti-microbial effects.
Further research is also being done on this nanoparticle, due to the unlimited fields of application such as regarding its anti-inflammatory activity in response to pathogens, its anti-demineralizing and remineralizing effect on the hard tissues of the tooth, its potential as an anti-cancer agent and many others (Carrouel et al., 2020; Wiesmann et al., 2020). ZnO NPs hence have widespread applications in the field of restorative dentistry, endodontics, regenerative endodontics, prosthetic dentistry, orthodontics, preventive dentistry, implantology and periodontology (Moradpoor et al., 2021). Although ZnO NPs are considered to be a biologically safe material that does not exhibit cell toxicity, however, further research into the regulatory and safety concerns in oral care products on long term use must be discussed, questioned and further researched upon. Majority of the research regarding these NPs are limited to in-vitro studies and few animal studies. Therefore, further investigations and clinical trials must be carried out in order to utilize it to its full potential.
2 Applications of Zinc Oxide Nanoparticles in Dentistry
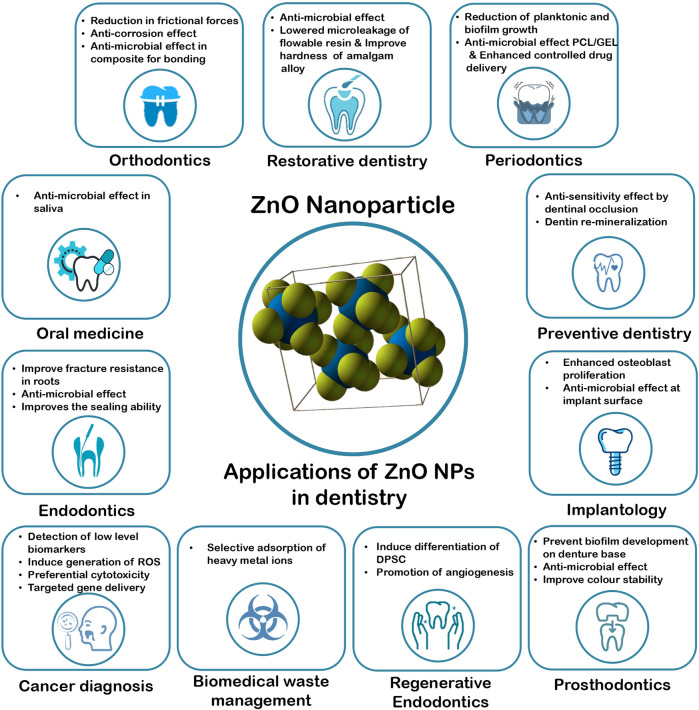
Zinc Oxide Nanoparticles have a wide range of applications in the various branches of dentistry, such as in the field of restorative dentistry, endodontics, regenerative endodontics, periodontics, prosthodontics, orthodontics, oral medicine, cancer diagnosis, dental implantology, preventive dentistry and biomedical waste management. The research performed using these nanoparticles are summarized in Table1 and Figure 1.
TABLE 1.
Studies focussing on the Applications of ZnO NPs in dentistry.
| Sl No. | Modification | Effect | Author |
|---|---|---|---|
| 1 | Dental resin composite containing ZnO NP | Inhibition of adhesion of S. mutans | Wang et al. (2019) |
| 2 | Flowable resin composite with ZnO NP | Decreased microleakage | Teymoornezhad et al. (2016) |
| 3 | Flowable resin composite with ZnO NP | Decreased microleakage | Hojati et al. (2013) |
| 4 | ZnO NP + resin-based dental composites | Anti-bacterial activity against S. sobrinus | Aydin Sevinç and Hanley, 2010) |
| 5 | ZnO NP | Anti-bacterial activity against S. mutans and Lactobacillus | Kasraei et al. (2014) |
| 6 | ZnO NP and GIC | Anti-bacterial activity against S. mutans | Vanajassun et al. (2014) |
| 7 | ZnO NP added into dental adhesive systems | Improved anti-microbial properties | Saffarpour et al. (2016) |
| 8 | ZnO NP added into dental adhesive systems | Improved anti-microbial properties | Jowkar et al. (2018) |
| 9 | ZnO NP added into dental adhesive systems | Improved anti-microbial properties | Gutiérrez et al. (2019) |
| 10 | ZnO NP used as interim cements | Anti-bacterial activity against S. mutans | Andrade et al. (2018) |
| 11 | ZnO NP | Anti-bacterial activity against E. coli | Liu et al. (2009) |
| 12 | Combination of ZnO NP and EDTA solution as irrigant | Enhanced fracture resistance of roots | Jowkar et al. (2020) |
| 13 | Addition of ZnO NP, calcium hydroxide NPs and chlorhexidine as intracanal medicament | Anti-bacterial activity against E. faecalis | Aguiar et al. (2015) |
| 14 | ZnO NP as sealer | Enhanced sealing with remineralization of radicular dentin | Toledano et al. (2020) |
| 15 | ZnO NP as nanosealer | Reduction in apical microleakage | Javidi et al. (2014) |
| 16 | ZnO NP | Improved penetration depth into dentinal tubules | Elkateb et al. (2015) |
| 17 | ZnONP added into gutta percha cones | Enhanced anti-bacterial activity against S. aureus and E. faecalis, excellent hermetic seal | Alves et al. (2018) |
| 18 | ZnO NP in Zinc-Bioglass | Induce differentiation of hDPSCs | Huang et al. (2017) |
| 19 | ZnO NP in Zinc-Bioglass and Calcium Phosphate Cement | Promotion of odontogenic differentiation and angiogenesis | Zhang et al. (2015) |
| 20 | ZnO NP containing composite membranes of PCL/GEL | Anti-bacterial activity against S. aureus | ZnO NP + composite membranes of PCL/GEL |
| 21 | ZnO NP containingelectrospun membranes of PCL/GEL | Anti-bacterial activity against P. gingivalis and F. nucleatum | ZnO NP + electrospun membranes of PCL/GEL |
| 22 | ZnO NP and serum albumin microspheres with minocycline | Enhanced anti-microbial spectrum, pH-responsiveness, sustained release, tissue-repairing and adhesion and enhanced controlled drug delivery | ZnO NP + serum albumin microspheres containing minocycline |
| 23 | ZnO NP added into PMMA in denture bases | Anti-fungal effect against C. albicans | Cierech et al. (2019) |
| 24 | ZnO NP incorporated into Auto-polymerized acrylic resins | Improvement of flexural strength | Kati. (2019) |
| 25 | ZnO NP added into Tissue conditioner | Anti-fungal effect | Homsiang et al. (2020) |
| 26 | Combination of ZnONP, chitosan and Ag NP | Anti-microbial effect against C. albicans, S. mutans, P. aeruginosa and E. faecalis | Mousavi et al. (2020) |
| 27 | ZnO NP in silicone prosthesis | Improved colour stability | Charoenkijkajorn and Sanohkan. (2020) |
| 28 | ZnO NP coating in NiTi wires | Reduction in the frictional forces, enhanced corrosion resistance and anti-bacterial effect against S. mutans | Kachoei et al. (2016) |
| 29 | ZnO NP and chitosan NPs in resin-based dental composite bonding agents | Anti-bacterial activity against S. mutans, S. sanguis and L. acidophilus | Mirhashemi et al. (2013 |
| 30 | ZnO NP | Anti-fungal effect against C. albicans (oral thrush) | Abd et al. (2015) |
| 31 | ZnO NPs and Ag NPs | Enhanced anti-bacterial effect in human and artificial saliva | Pokrowiecki et al. (2019) |
| 32 | ZnO NPs | Anti-fungal effect on Aspergillus niger and C. albicans | Pillai et al. (2020) |
| 33 | ZnO NPs | Enhancedsensitivity to E. coli, P. aeruginosa and Methicillin Resistant S. aureus | Ali et al. (2016) |
| 34 | Combination of ZnO NPs, Quercetin, Ceftriaxone, Ampicillin, Naringin and Amphotericin B | Enhanced drug efficacy against Methicillin resistant S. aureus, S. pneumoniae and S. pyogenes, E. coli, S. marcescens and P. aeruginosa | Akbar et al. (2021) |
| 35 | ZnO NPs | Detect low-level expression of biomarkers, preferential cytotoxicity against cancer cells, induce ROS. | Rasmussen et al. (2010) |
| 36 | ZnO NPs and PEG | Enhanced anti-bacterial activity against E. coleus and S. aureus, low concentration cytotoxic action against cancer cells | Nair et al. (2008) |
| 37 | ZnO NPs and daunorubicin for UV irradiation | Synergistic cytotoxic effects on leukemic cells | Guo et al. (2008) |
| 38 | ZnO NPs | Dental wastewater purification treatment | Gu et al. (2020) |
| 39 | ZnO NPs | Purification of wastewater | Spoială et al. (2021) |
| 40 | ZnO NPs on implant surface | Enhanced proliferation of osteoblasts, anti-bacterial effect against S. aureus | Memarzadeh et al. (2015) |
| 41 | ZnO NPs on implant surface | Anti-bacterial effect against S. mutans | Abdulkareem et al. (2015) |
| 42 | ZnO NPs on implant surface | Anti-bacterial effect against S. mutans | Kulshrestha et al. (2014) |
| 43 | ZnO NPs incorporated into dentifrice | Inhibition of dentin demineralization and enhanced anti-microbial effects | Khan et al. (2020) |
| 44 | ZnO NPs nanogels | Promote dentin mineralization | Toledano et al. (2019) |
| 45 | ZnO NPs | Dentinal tubule occlusion | Toledano-Osorio et al. (2018) |
FIGURE 1.
Applications of ZnO NPs in Dentistry.
2.1 Restorative Dentistry
ZnO NPs have been found to improve the mechanical and anti-bacterial properties of dental restorative materials. According to a study by Wang et al., it was reported that when ZnO NPs were incorporated in dental resin composites, there was inhibition in the growth and adhesion of S. mutans, and in small amounts did not affect the mechanical properties. This is extremely beneficial in not only in the prevention of secondary caries but also in the interception of bulk fracture of the material (Wang et al., 2019). Similarly, in a study done by Teymoornezhad et al., it was reported that incorporation of 3% ZnO NPs on flowable resin composite lowered the microleakage (Teymoornezhad et al., 2016). A comparable outcome was reported in the study by Hojati et al. (2013), on flowable resin composite (Tavassoli Hojati et al., 2013). When 10% ZnO NPs was added to resin-based dental composites, it showed anti-bacterial effectiveness against S. Sobrinus (Aydin Sevinç and Hanley 2010). These NPs were also found to exhibit anti-bacterial activity against S. mutans and Lactobacillus (Kasraei et al., 2014). ZnO NPs when incorporated in Glass Ionomer Cement (GIC) was also found to significantly improve the anti-bacterial properties against S. mutans without altering the mechanical properties (Vanajassun et al., 2014).
In various studies, it has been reported that incorporation of ZnO NPs into dental adhesive systems significantly improved the anti-microbial properties without affecting the bond strength adversely (Saffarpour et al., 2016; Jowkar et al., 2018; Gutiérrez et al., 2019). Zinc particles have proven to produce a strong bond at the interface of the dentin and resin by bringing about a decrease in the degeneration of collagen. The hardness of amalgam alloy was found to increase in proportion with the percentage loading of ZnO NPs (Yahya et al., 2013).
ZnO NPs when added to interim cements also exhibited anti-bacterial activity against S. mutans (Andrade et al., 2018). ZnO NPs were found to alter the lipid and protein contents of the cell membranes of E. coli, which caused distortion leading to leakage of cellular components, ultimately resulting in death (Liu et al., 2009). These properties are extremely beneficial in preventing the occurrence of secondary caries.
However, it was reported that the addition of 1% and 2% by weight of ZnO NPs into GIC did not exhibit anti-microbial activity against strains of S. mutans. This might be attributed to the inherent anti-bacterial property of the cement (Garcia et al., 2017). The incorporation of nano-spherical and nano-flower ZnO NPs to GIC was found to decrease the surface hardness, without affecting the flexural strength while incorporation of nano-rod ZnO NPs had no effect on the mechanical properties (Panahandeh et al., 2018). In another study done by Wang et al., it was reported that with the increase in the quantity of ZnO NPs, there was a decrease in the mechanical properties of dental composite resins, with the exception of flexural strength, which may be attributed to the agglomeration of the nanoparticles (Wang et al., 2019). In a systematic review by Arun et al., on the anti-bacterial properties of composite material incorporated with ZnO NPs, it was concluded that the material is unlikely to present a clinical advantage due to the short lifetime of anti-bacterial properties and the poor results against multi-species biofilms (Arun et al., 2021).
2.2 Endodontics
The applications of ZnO NPs in endodontics are diverse. In a study by Jowkar et al., When incorporated in EDTA solution for irrigation, the fracture resistance of the roots was enhanced (Jowkar et al., 2020a). In a study done by Aguiar et al., it was reported that these NPs promoted alkalinization and action against E. faecalis when used as an intracanal medicament along with calcium hydroxide NPs and chlorhexidine (Aguiar et al., 2015). ZnO NPs when used as an sealer after endodontic therapy was found to exhibit excellent sealing efficacy along with remineralization of the radicular dentin thereby strengthening the tooth (Toledano et al., 2020). It was also reported that ZnO NPs brought about a reduction in the apical microleakage when used as a nano-sealer in endodontics (Javidi et al., 2014). It also significantly improved the penetration depth into the dentinal tubules (Elkateb et al., 2015). Pristine gutta percha cones that were pre-treated argon plasma treatment and coated with ZnO NPs were found to exhibit antibacterial activity against S. aureus and E. fecalis which provides an excellent hermetic seal thereby reducing chances of reinfection and subsequent endodontic failure (Alves et al., 2018).
However, a study done by Jowkar et al., showed that push-out bond strength of the fiber posts did not improve on the addition of ZnO NPs (Jowkar et al., 2020b). When incorporated into Portland cement (PC) along with ZrO2, it was found not to impede with the anti-biofilm activity and to provide radiopacity to the cement. Also, the presence of ZnO NPs significantly reduced the compressive strength of the material (Guerreiro-Tanomaru et al., 2014).
2.3 Regenerative Endodontics
Incorporation of these NPs along with SiO2, Na2O, CaO and P2O5 to formulate Zinc-Bioglass, was reported to induce the differentiation of human Dental Pulp Stem Cells (hDPSCs) by bringing about an increase in the ALP activity (Huang et al., 2017). Similarly, it was reported that Zinc-Bioglass when incorporated with Calcium Phosphate Cement brought about odontogenic differentiation and also promoted angiogenesis by activating the Wnt, integrin, NF-kB, and MAPK pathways (Zhang et al., 2015). These play a pivotal role in the regeneration of the dentin-pulp tissues.
2.4 Periodontics
In the field of periodontal regeneration using guided tissue regeneration, the loading of ZnO NPs into composite membranes of polycaprolactone (PCL) and gelatin (GEL) which were electrospun, brought about reduction in the planktonic and the biofilm growth of the S. aureus significantly. These local anti-bacterial properties brought about enhancement in the clinical prognosis of treatments (Prado-Prone et al., 2020). Similarly, when ZnO NPs were incorporated in electrospun membranes made of PCL and PCL/GEL, it showed anti-bacterial activity against P. gingivalis and F. nucleatum species which in turn brought about an enhanced and better predictable periodontal regeneration (Münchow et al., 2015). ZnO NPs and serum albumin microspheres containing minocycline when incorporated in a Carbopol hydrogel exhibited enhancement of properties such as the anti-microbial spectrum, pH-responsiveness, sustained release, tissue-repairing and adhesion, and also enhanced controlled drug delivery that can increase stability of the drug (Mou et al., 2019).
2.5 Prosthodontics
The incorporation of ZnO NPs into the PMMA in denture bases was found to prevent biofilm development by C. albicans without exerting a cytotoxic effect on the host cells. Further research can advocate its application as a novel denture base material (Cierech et al., 2019). ZnO NPs in concentrations of 1wt% and 2wt% when incorporated in auto-polymerized acrylic resins was found to improve the flexural strength significantly (Kati 2019). In a study wherein 15 wt% ZnO NPs were incorporated into the tissue conditioner was also found to exhibit an anti-fungal effect (Homsiang et al., 2020). In another study, it was assessed that ZnO NPs along with chitosan and Silver NPs in the concentration of 2.5% inhibited the growth of C. albicans, and at a concentration of 5% inhibited the growth of S. mutans, P. aeruginosa and E. faecalis (Mousavi et al., 2020). The incorporation of 1.5% of ZnO NPs was found to improve the colour stability of silicone prosthesis (Charoenkijkajorn and Sanohkan 2020).
2.6 Orthodontics
Nanoparticles have been used in orthodontics to improve the quality of orthodontic treatment either in the form of nano-coated archwires, orthodontic adhesives, and orthodontic brackets (Tahmasbi et al., 2019; Moradpoor et al., 2021). The zinc oxide nanoparticles coated orthodontic appliances minimise bacterial adhesion and enamel demineralization due to its antimicrobial and remineralization potential. Even attempts are made to add ZNO NPs into both orthodontic attachments and bonding materials since they provide a platform for bacterial attachment (Jatania and Shivalinga 2014; Riad et al., 2015; Reddy et al., 2016; Tahmasbi et al., 2019).
It was reported that coating of the NiTi wires with ZnO NPs brought about reduction in the frictional forces by 21% and exhibited anti-bacterial activity against S. mutans. It was also reported that ZnO NPs exhibited anti-corrosion effect that enhanced the corrosion resistance propertiesin the orthodontic wires (Kachoei et al., 2016). When a mixture of 10% weight each of ZnO NPs and chitosan NPs was incorporated into are resin-based dental composite bonding agents for the placement of brackets, it exhibited anti-bacterial activity against S. mutans, S. sanguis and L. acidophilus. This can significantly bring about reduction in the incidence of white-spot lesions during orthodontic therapy (Mirhashemi et al., 2013). Another study investigated that ZNO and CuO NPs coated orthodontic brackets showed better antibacterial activity against S. mutans, thus reducing the incidence of dental caries (Ramazanzadeh et al., 2015). It has been reported that when both orthodontic wires and brackets were coated with ZnO NPs the antibacterial potential against S. mutans was enhanced and reduced the frictional forces of coated wires (Behroozian et al., 2016). Similarly the stainless steel wires and orthodontic brackets coated with chitosan NPs or ZnO NPs reduced the friction between orthodontic brackets and a Stainless steel wire thus enhances the anchorage control and root resorption risk (Elhelbawy and Ellaithy 2021). Europium ions doped ZnO NPs were incorporated has orthodontic nanoadhesive enhanced the visibility of material for thorough removal of orthodontic adhesive after completion of treatment (Yamagata et al., 2012). It has been reported that orthodontic adhesive with less titanium dioxide, zinc oxide, and silver NPs causes bracket failure because the combination reduces shear bond strength (Reddy et al., 2016). The addition of ZnO to a light cure resin modified GIC as an orthodontic bonding agent improved the original compound’s antimicrobial, physical, and flexural properties (Nuri Sari et al., 2015). Hence ZnO NPs have the potential to be widely used in orthodontic applications to improve treatment outcomes, including increased strength of materials and reduced bacterial count around the orthodontic appliance.
2.7 Oral Medicine
ZnO NPs have an inhibitory effect on C. albicans in saliva and hence can be used in the treatment of oral thrush, starting from a concentration of 0.05 mg/ml. It was also reported that ZnO NPs along with Silver NPs exhibited enhanced anti-bacterial effect in human and artificial saliva, which can have widespread applications in clinical scenarios (Pokrowiecki et al., 2019). ZnO NPs which are biosynthesized from Beta vulgaris was found to exhibit anti-fungal effect on pathogens such as Aspergillus niger and C. albicans (Pillai et al., 2020). ZnO NPs that are synthesized from Aloe vera leaf extract have been demonstrated to exhibit pronounced sensitivity to E. coli, P. aeruginosa and Methicillin Resistant S. aureus, and hence can be considered as a promising candidate for nano-antibiotics, which deals with the enhancement of the effect against the bacterial strains that are resilient to conventional antibiotics (Ali et al., 2016). These NPs when conjugated with drugs such as Quercetin, Ceftriaxone, Ampicillin, Naringin and Amphotericin B showed enhanced drug efficacy against Methicillin resistant S. aureus, S. pneumoniae, S. pyogenes, E. coli, Serratia marcescens and P. aeruginosa. Hence, they provide a propitious approach in the combat against disease resistant pathogens (Akbar et al., 2021).
2.8 Cancer Diagnosis
ZnO NPs can be implicated in the diagnosis of cancers as it is proven to detect low-level expression of biomarkers which are used for early cancer detection. In vitro, it exhibits an inherent preferential cytotoxicity against cancer cells. It also possesses the ability to induce the generation of Reactive Oxygen Species (ROS) due to its semiconductor properties, as the electrons within the NPs can react with O2 or hydroxyl ions or water after migrating to the surface to form superoxide and hydroxyl radicals (Manthe et al., 2010). These can set about cell death when the anti-oxidative capacity of the cell is exceeded. Research is being carried out on the utilization of ZnO NPs for gene silencing and targeted gene delivery, which can be utilized to combat cancer (Rasmussen et al., 2010). ZnO NPs that were coated with polyethylene glycol (PEG) were found to exhibit enhanced anti-bacterial activity against E. coleus and S. aureus by bringing about damage to the cell membrane. They were also found to exhibit a low concentration threshold for cytotoxic action, with a which is due to the upregulation of the Fas ligand on the cell membrane which brings about apoptosis of the cancer cells (Nair et al., 2008). ZnO NPs also exhibits an efficient role in non-surgical tumor ablation method used in cancer therapy. It was demonstrated that ZNO NPs when combined with anti-cancer drug daunorubicin, along with Ultra Violet irradiation, exhibited synergistic cytotoxic effects on the leukemic cells (Guo et al., 2008).
2.9 Biomedical Waste Management
The ZnO NPs are found to selectively remove heavy metal ions such as Chromium by adsorption by virtue of their hydroxyl ions. It can therefore be used in dental waste water purification treatment as a green pollutant-diminishing strategy (Gu et al., 2020). Other studies have proven the efficacy of ZnO NPs which can be used in nano-composite membranes used for the purification of water. This is due to its antibacterial activity against S. aureus and the favourable photocatalytic activity, which enhances the adsorption of organic pollutants, pesticides and microbes that are found in the wastewaterrendering it safe (Spoială et al., 2021).
2.10 Implantology
Chemical modifications of dental implant surfaces with ZNO NPs, which are effective antimicrobial agents, have been carried out in order to reduce the risk of dental implant failure and improve osteointegration. On coating the implant surface with ZnO NPs, the underlying osteoblast cells exhibited an enhanced proliferation after 5 and 10 days. They also exhibited anti-microbial properties against S. aureus. These properties are useful to promote bone growth and in the inhibition of infection at the implant site (Memarzadeh et al., 2015). Similar results were reported in studies by Abdulkareem et al. and Kulshrestha et al., on the effect of ZnO NPs against S. mutans biofilm on dental implant surfaces (Kulshrestha et al., 2014; Abdulkareem et al., 2015). According to the findings, ZNO bio-functionalized thin films containing DMP1 peptides can improve the physicochemical, osteogenic, apatite nucleation and corrosion resistance properties of this material suggesting promising applications in dental implant (Trino et al., 2018).
Titania (Ti)-zinc (Zn)-oxide nanocomposite-(nC) thin films were co-sputtered to strengthen the cohesiveness of metallic fixtures with bone. The developed thin film also exhibited strong antibacterial activity against S. aureus and E. coli (Goel et al., 2019). Modified titanium implant materials developed using N-halamine and ZnO nanoparticles demonstrated remarkable antibacterial activity against P. aeruginosa, E. coli, and S. aureus without using antibiotics (Li et al., 2017). Titanium implants with coatings of Poly (lactic-co-glycolic acid)/Silver/ZnO nanorods demonstrated long-lasting antibacterial activity against S. aureus and E. coli, as well as excellent cytocompatibility and biocompatibility (Xiang et al., 2017).
2.11 Preventive Dentistry
ZnO NPs incorporated in a dentifrice was found to cause dentinal tubule occlusion. These can also be incorporated as preservatives in dentifrices as it not only brings about inhibition of dentin demineralization but also exhibits enhanced anti-microbial effects (Khan et al., 2020). Its incorporation and in nanogels and application on eroded cervical dentin, was found to promote dentin mineralization (Toledano et al., 2019). These can be utilized in achieving an anti-sensitivity effect. Studies have shown that dentin which is treated with ZnO NPs exhibited greater ability to produce dentinal tubule occlusion which makes it an effective agent in the treatment of dentinal hypersensitivity 67]. The ZnO NPs treated dentin was found to have higher levels of proteoglycans that act as bonding agents between the HAp crystals and collagen network. Further, they enhance the release small integrin-binding ligand N-linked glycoproteins and small leucine-rich proteoglycans from dentin through Matrix Metalloproteinase-3 activity. These proteins take part in the mineralization of dentin, and the immobilized phosphorylated proteins induce formation of mineral. Zinc NPs also reduces the collagen degradation which is mediated by Matrix Metalloproteinase-3 in dentin that is partially demineralized and hence promotes dentin re-mineralization (Toledano-Osorio et al., 2018; Toledano et al., 2019).
3 Conclusion
Nano-dentistry has opened a new standpoint for revolution in oral care and portrays a growing field with the capability to address the new and improved applications in dentistry. ZnO NPs have a broad spectrum of applications the various fields of dentistry such as restorative dentistry, endodontics, regenerative endodontics, periodontology, prosthodontics, orthodontics, implantology, preventive dentistry among other fields. The use of ZnO NPs represents a broadening horizon for the diagnosis, treatment, and prevention of various oral conditions, and in enhancing the characteristics of existing dental materials. It is hence crucial to strengthen the symbiosis between cliniciansand materials scientists asnano-dentistry is still technologydriven, with many roadblocks ahead. However, most of the research is still in the development pipeline and for realizing the complete in vivo potential in dentistry, further research that focus on its clinical implications should be carried out.
Acknowledgments
The authors thank MS Ramaiah University of Applied Sciences for the support.
Author Contributions
PC: Conceptualization, Resources, Data Curation, Original Draft Preparation, Supervision. GS: Conceptualization, Resources, Data Curation, Original Draft Preparation. SV: Original Draft Preparation, Review & Editing, Visualization. DA: Original Draft Preparation, Review & Editing, Visualization. AA: Review & Editing, Visualization, Supervision. BZ: Review & Editing, Visualization, Supervision. NA: Review & Editing, Visualization, Supervision. SP: Original Draft Preparation, Review & Editing, Visualization, Supervision. All authors agree to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdulkareem E. H., Memarzadeh K., Allaker R. P., Huang J., Pratten J., Spratt D. (2015). Anti-biofilm Activity of Zinc Oxide and Hydroxyapatite Nanoparticles as Dental Implant Coating Materials. J. Dent. 43, 1462–1469. 10.1016/j.jdent.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Aguiar A. S., Guerreiro-Tanomaru J. M., Faria G., Leonardo R. T., Tanomaru-Filho M. (2015). J. Contemp. Dent. P. R. 16, 624–629. [DOI] [PubMed] [Google Scholar]
- Akbar N., Aslam Z., Siddiqui R., Shah M. R., Khan N. A. (2021). Zinc Oxide Nanoparticles Conjugated with Clinically-Approved Medicines as Potential Antibacterial Molecules. Amb. Expr. 11, 104. 10.1186/s13568-021-01261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K., Dwivedi S., Azam A., Saquib Q., Al-Said M. S., Alkhedhairy A. A., et al. (2016). Aloe Vera Extract Functionalized Zinc Oxide Nanoparticles as Nanoantibiotics against Multi-Drug Resistant Clinical Bacterial Isolates. J. Colloid Interface Sci. 472, 145–156. 10.1016/j.jcis.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Alves M. J., Grenho L., Lopes C., Borges J., Vaz F., Vaz I. P., et al. (2018). Antibacterial Effect and Biocompatibility of a Novel Nanostructured ZnO-Coated Gutta-Percha Cone for Improved Endodontic Treatment. Mater. Sci. Eng. C 92, 840–848. 10.1016/j.msec.2018.07.045 [DOI] [PubMed] [Google Scholar]
- Andrade V., Martínez A., Rojas N., Bello-Toledo H., Flores P., Sánchez-Sanhueza G., et al. (2018). J. Prosthet. Dent. 119, 862–e1. 10.1016/j.prosdent.2017.09.015 [DOI] [PubMed] [Google Scholar]
- Arun D., Adikari Mudiyanselage D., Gulam Mohamed R., Liddell M., Monsur Hassan N. M., Sharma D. (2021). Materials 14, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin Sevinç B., Hanley L. (2010). J. Biomed. Mat. Res. B Appl. Biomater. 94, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M., Chung H.-E., Yu J., Lee J.-A., Kim T.-H., Oh J.-M., et al. (2012). Int. J. Nanomedicine 7, 3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozian A., Kachoei M., Khatamian M., Divband B. (2016). The Effect of ZnO Nanoparticle Coating on the Frictionalresistance between Orthodontic Wires and Ceramic Brackets. J. Dent. Res. Dent. Clin. Dent. Prospects 10 (2), 106–111. 10.15171/joddd.2016.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Viennot S., Ottolenghi L., Gaillard C., Bourgeois D. (2020). Nanoparticles as Anti-microbial, Anti-inflammatory, and Remineralizing Agents in Oral Care Cosmetics: a Review of the Current Situation. Nanomaterials 10 (1), 140. 10.3390/nano10010140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenkijkajorn D., Sanohkan S. (2020). The Effect of Nano Zinc Oxide Particles on Color Stability of MDX4-4210 Silicone Prostheses. Eur. J. Dent. 14, 525–532. 10.1055/s-0040-1713058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cierech M., Wojnarowicz J., Kolenda A., Krawczyk-Balska A., Prochwicz E., Woźniak B., et al. (2019). Zinc Oxide Nanoparticles Cytotoxicity and Release from Newly Formed PMMA-ZnO Nanocomposites Designed for Denture Bases. Nanomaterials 9, 1318. 10.3390/nano9091318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhelbawy N., Ellaithy M. (2021). Comparative Evaluation of Stainless-Steel Wires and Brackets Coated with Nanoparticles of Chitosan or Zinc Oxide upon Friction: An In Vitro Study. Int. Orthod. 19 (2), 274–280. 10.1016/j.ortho.2021.01.009 [DOI] [PubMed] [Google Scholar]
- Elkateb W., Massoud A., Mokhless N. (2015). Trans. Shalaby, 11. [Google Scholar]
- Garcia P. P. N. S., Cardia M. F. B., Francisconi R. S., Dovigo L. N., Spolidório D. M. P., de Souza Rastelli A. N., et al. (2017). Antibacterial Activity of Glass Ionomer Cement Modified by Zinc Oxide Nanoparticles. Microsc. Res. Tech. 80, 456–461. 10.1002/jemt.22814 [DOI] [PubMed] [Google Scholar]
- Goel S., Dubey P., Ray S., Jayaganthan R., Pant A. B., Chandra R. (2019). Co-sputtered Antibacterial and Biocompatible Nanocomposite Titania-Zinc Oxide Thin Films on Si Substrates for Dental Implant Applications. Mater. Technol. 34 (1), 32–42. 10.1080/10667857.2018.1488924 [DOI] [Google Scholar]
- Gu M., Hao L., Wang Y., Li X., Chen Y., Li W., et al. (2020). The Selective Heavy Metal Ions Adsorption of Zinc Oxide Nanoparticles from Dental Wastewater. Chem. Phys. 534, 110750. 10.1016/j.chemphys.2020.110750 [DOI] [Google Scholar]
- Guerreiro-Tanomaru J. M., Trindade-Junior A., Cesar Costa B., da Silva G. F., DrullisCifali L., Basso Bernardi M. I., et al. (2014). Sci. World J. 2014. 10.1155/2014/975213 [DOI] [Google Scholar]
- Guo D., Wu C., Jiang H., Li Q., Wang X., Chen B. (2008). Synergistic Cytotoxic Effect of Different Sized ZnO Nanoparticles and Daunorubicin against Leukemia Cancer Cells under UV Irradiation. J. Photochem. Photobiol. B Biol. 93, 119–126. 10.1016/j.jphotobiol.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Gutiérrez M. F., Alegría-Acevedo L. F., Méndez-Bauer L., Bermudez J., Dávila-Sánchez A., Buvinic S., et al. (2019). J. Dent. 82, 45–55. [DOI] [PubMed] [Google Scholar]
- Homsiang W., Kamonkhantikul K., Arksornnukit M., Takahashi H. (2020). Dent. Mat. J. [DOI] [PubMed] [Google Scholar]
- Huang M., Hill R. G., Rawlinson S. C. F. (2017). Zinc Bioglasses Regulate Mineralization in Human Dental Pulp Stem Cells. Dent. Mater. 33, 543–552. 10.1016/j.dental.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Jatania A., Shivalinga B. M. (2014). An In Vitro Study to Evaluate the Effects of Addition of Zinc Oxide to an Orthodontic Bonding Agent. Eur. J. Dent. 08 (01), 112–117. 10.4103/1305-7456.126262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javidi M., Zarei M., Naghavi N., Mortazavi M., Nejat A. H. (2014). Contemp. Clin. Dent. 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowkar Z., Farpour N., Koohpeima F., Mokhtari M. J., Shafiei F. (2018). J. Contemp. Dent. P. R. 19, 1404–1411. [PubMed] [Google Scholar]
- Jowkar Z., Hamidi S. A., Shafiei F., Ghahramani Y. (2020). The Effect of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Final Irrigation Solutions on the Fracture Resistance of Root-Filled Teeth. Ccide Vol. 12, 141–148. 10.2147/ccide.s253251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowkar Z., Omidi Y., Shafiei F. (2020). J. Clin. Exp. Dent. 12, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachoei M., Nourian A., Divband B., Kachoei Z., Shirazi S. (2016). Zinc-oxide Nanocoating for Improvement of the Antibacterial and Frictional Behavior of Nickel-Titanium Alloy. Nanomedicine 11, 2511–2527. 10.2217/nnm-2016-0171 [DOI] [PubMed] [Google Scholar]
- Kasraei S., Sami L., Hendi S., AliKhani M.-Y., Rezaei-Soufi L., Khamverdi Z. (2014). Antibacterial Properties of Composite Resins Incorporating Silver and Zinc Oxide Nanoparticles onStreptococcus mutansandLactobacillus. Restor. Dent. Endod. 39, 109–114. 10.5395/rde.2014.39.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kati F. A. (2019). Effect of the Incorporation of Zinc Oxide Nanoparticles on the Flexural Strength of Auto- Polymerized Acrylic Resins. J. Oral Res. 8, 37–41. 10.17126/joralres.2019.010 [DOI] [Google Scholar]
- Khan A. S., Farooq I., Alakrawi K. M., Khalid H., Saadi O. W., Hakeem A. S. (2020). Dentin Tubule Occlusion Potential of Novel Dentifrices Having Fluoride Containing Bioactive Glass and Zinc Oxide Nanoparticles. Med. Princ. Pract. 29, 338–346. 10.1159/000503706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshrestha S., Khan S., Meena R., Singh B. R., Khan A. U. (2014). A Graphene/zinc Oxide Nanocomposite Film Protects Dental Implant Surfaces against cariogenicStreptococcus Mutans. Biofouling 30, 1281–1294. 10.1080/08927014.2014.983093 [DOI] [PubMed] [Google Scholar]
- Li Y., Liu X., Tan L., Cui Z., Yang X., Yeung K. W. K., et al. (2017). Construction of N-Halamine Labeled Silica/zinc Oxide Hybrid Nanoparticles for Enhancing Antibacterial Ability of Ti Implants. Mater. Sci. Eng. C 76, 50–58. 10.1016/j.msec.2017.02.160 [DOI] [PubMed] [Google Scholar]
- Liu Y., He L., Mustapha A., Li H., Hu Z. Q., Lin M. (2009). Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 107, 1193–1201. 10.1111/j.1365-2672.2009.04303.x [DOI] [PubMed] [Google Scholar]
- Manthe R. L., Foy S. P., Krishnamurthy N., Sharma B., Labhasetwar V. (2010). Tumor Ablation and Nanotechnology. Mol. Pharm. 7, 1880–1898. 10.1021/mp1001944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarzadeh K., Sharili A. S., Huang J., Rawlinson S. C. F., Allaker R. P. (2015). Nanoparticulate Zinc Oxide as a Coating Material for Orthopedic and Dental Implants. J. Biomed. Mat. Res. 103, 981–989. 10.1002/jbm.a.35241 [DOI] [PubMed] [Google Scholar]
- Mirhashemi A., Bahador A., Kassaee M., Daryakenari G., Ahmad-Akhoundi M., Sodagar A. (2013). J. Med. Bacteriol. 2, 1–10. [Google Scholar]
- Moradpoor H., Safaei M., Mozaffari H. R., Sharifi R., Imani M. M., Golshah A., et al. (2021). An Overview of Recent Progress in Dental Applications of Zinc Oxide Nanoparticles. RSC Adv. 11 (34), 21189–21206. 10.1039/d0ra10789a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou J., Liu Z., Liu J., Lu J., Zhu W., Pei D. (2019). Hydrogel Containing Minocycline and Zinc Oxide-Loaded Serum Albumin Nanopartical for Periodontitis Application: Preparation, Characterization and Evaluation. Drug Deliv. 26, 179–187. 10.1080/10717544.2019.1571121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S. A., Ghotaslou R., Khorramdel A., Akbarzadeh A., Aeinfar A. (2020). Ir. J. Med. Sci. [DOI] [PubMed] [Google Scholar]
- Münchow E. A., Albuquerque M. T. P., Zero B., Kamocki K., Piva E., Gregory R. L., et al. (2015). Dent. Mat. 31, 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Sasidharan A., Divya Rani V. V., Menon D., Nair S., Manzoor K., et al. (2008). Role of Size Scale of ZnO Nanoparticles and Microparticles on Toxicity toward Bacteria and Osteoblast Cancer Cells. J. Mater Sci. Mater Med. 20, 235–241. 10.1007/s10856-008-3548-5 [DOI] [PubMed] [Google Scholar]
- Nuri Sari M., Rahmani N., Araghbidi Kashani M., Eslami Amirabadi G., Akbari Sari A., Seyedtabaii E. (2015). Effect of Incorporation of Nano-Hydroxyapatite and Nano-Zinc Oxide in Resin Modified Glass Ionomer Cement on Metal Bracket Debonding. J. Islamic Dent. Assoc. Iran 27 (2), 70–76. [Google Scholar]
- Panahandeh N., Torabzadeh H., Aghaee M., Hasani E., Safa S. (2018). J. Conserv. Dent. JCD 21, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A. M., Sivasankarapillai V. S., Rahdar A., Joseph J., Sadeghfar F., Anuf A R., et al. (2020). Green Synthesis and Characterization of Zinc Oxide Nanoparticles with Antibacterial and Antifungal Activity. J. Mol. Struct. 1211, 128107. 10.1016/j.molstruc.2020.128107 [DOI] [Google Scholar]
- Pokrowiecki R., Wojnarowicz J., Zareba T., Koltsov I., Lojkowski W., Tyski S., et al. (2019). Nanoparticles and Human Saliva: A Step towards Drug Delivery Systems for Dental and Craniofacial Biomaterials. Ijn 14, 9235–9257. 10.2147/ijn.s221608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Prone G., Silva-Bermudez P., Bazzar M., Focarete M. L., Rodil S. E., Vidal-Gutiérrez X., et al. (2020). Antibacterial Composite Membranes of Polycaprolactone/gelatin Loaded with Zinc Oxide Nanoparticles for Guided Tissue Regeneration. Biomed. Mat. 15, 035006. 10.1088/1748-605x/ab70ef [DOI] [PubMed] [Google Scholar]
- Ramazanzadeh B., Jahanbin A., Yaghoubi M., Shahtahmassbi N., Ghazvini K., Shakeri M., et al. (2015). Comparison of Antibacterial Effects of ZnO and CuO Nanoparticles Coated Brackets against Streptococcus Mutans. J. Dent. (Shiraz) 16 (3), 200–205. [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. W., Martinez E., Louka P., Wingett D. G. (2010). Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 7, 1063–1077. 10.1517/17425247.2010.502560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. K., Kambalyal P. B., Patil S. R., Vankhre M., Khan M. Y., Kumar T. R. (2016). Comparative Evaluation and Influence on Shear Bond Strength of Incorporating Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles in Orthodontic Adhesive. J. Orthod. Sci. 5 (4), 127–131. 10.4103/2278-0203.192115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M., Harhash A. Y., Elhiny O. A., Salem G. A. (2015). Evaluation of the Shear Bond Strength of Orthodontic Adhesive System Containing Antimicrobial Silver Nano Particles on Bonding of Metal Brackets to Enamel. Life Sci. J. 12 (12), 27–34. [Google Scholar]
- Saffarpour M., Rahmani M., Tahriri M., Peymani A. (2016). Antimicrobial and Bond Strength Properties of a Dental Adhesive Containing Zinc Oxide Nanoparticles. Braz. J. Oral Sci. 15, 66–69. 10.20396/bjos.v15i1.8647127 [DOI] [Google Scholar]
- Spoială A., Ilie C.-I., Trușcă R.-D., Oprea O.-C., Surdu V.-A., ȘtefanVasile B., et al. (2021). Mat. Basel Switz. 14, 4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şuhani M. F., Băciuţ G., Băciuţ M., Şuhani R., Bran S. (2018). Clujul Med. 91, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasbi S., Mohamadian F., Hosseini S., Eftekhar L. (2019). A Review on the Applications of Nanotechnology in Orthodontics. Nanomedicine J. 6 (1), 11–18. [Google Scholar]
- Tavassoli Hojati S., Alaghemand H., Hamze F., Ahmadian Babaki F., Rajab-Nia R., Rezvani M. B., et al. (2013). Antibacterial, Physical and Mechanical Properties of Flowable Resin Composites Containing Zinc Oxide Nanoparticles. Dent. Mater. 29, 495–505. 10.1016/j.dental.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Teymoornezhad K., Alaghehmand H., Daryakenari G., Khafri S., Tabari M. (2016). Evaluating the Microshear Bond Strength and Microleakage of Flowable Composites Containing Zinc Oxide Nano-Particles. Electron. Physician 8, 3289–3295. 10.19082/3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano M., Cabello I., Osorio E., Aguilera F. S., Medina-Castillo A. L., Toledano-Osorio M., et al. (2019). Zn-containing Polymer Nanogels Promote Cervical Dentin Remineralization. Clin. Oral Invest. 23, 1197–1208. 10.1007/s00784-018-2548-1 [DOI] [PubMed] [Google Scholar]
- Toledano M., Osorio E., Aguilera F. S., Muñoz-Soto E., Toledano-Osorio M., López-López M. T., et al. (2020). Polymeric Nanoparticles for Endodontic Therapy. J. Mech. Behav. Biomed. Mater. 103, 103606. 10.1016/j.jmbbm.2019.103606 [DOI] [PubMed] [Google Scholar]
- Toledano-Osorio M., Osorio E., Aguilera F. S., Luis Medina-Castillo A., Toledano M., Osorio R. (2018). Improved Reactive Nanoparticles to Treat Dentin Hypersensitivity. Acta Biomater. 72, 371–380. 10.1016/j.actbio.2018.03.033 [DOI] [PubMed] [Google Scholar]
- Trino L. D., Albano L. G. S., Bronze-Uhle E. S., George A., Mathew M. T., Lisboa-Filho P. N. (2018). Physicochemical, Osteogenic and Corrosion Properties of Bio-Functionalized ZnO Thin Films: Potential Material for Biomedical Applications. Ceram. Int. 44 (17), 21004–21014. 10.1016/j.ceramint.2018.08.136 [DOI] [Google Scholar]
- Vanajassun P. P., Nivedhitha M. S., Nishad N. T., Soman D. (2014). Adv. Hum. Biol. 4, 31. [Google Scholar]
- Wang Y., Hua H., Li W., Wang R., Jiang X., Zhu M. (2019). Strong Antibacterial Dental Resin Composites Containing Cellulose Nanocrystal/zinc Oxide Nanohybrids. J. Dent. 80, 23–29. 10.1016/j.jdent.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Wiesmann N., Tremel W., Brieger J. (2020). Zinc Oxide Nanoparticles for Therapeutic Purposes in Cancer Medicine. J. Mat. Chem. B 8 (23), 4973–4989. 10.1039/d0tb00739k [DOI] [PubMed] [Google Scholar]
- Xiang Y., Li J., Liu X., Cui Z., Yang X., Yeung K. W. K., et al. (2017). Construction of Poly(lactic-Co-Glycolic acid)/ZnO nanorods/Ag Nanoparticles Hybrid Coating on Ti Implants for Enhanced Antibacterial Activity and Biocompatibility. Mater. Sci. Eng. C 79, 629–637. 10.1016/j.msec.2017.05.115 [DOI] [PubMed] [Google Scholar]
- Yahya N., Puspitasari P., Latiff N. R. A. (2013). Hardness Improvement of Dental Amalgam Using Zinc Oxide and Aluminum Oxide Nanoparticles. Charact. Dev. Biosyst. Biomater., 9–32. 10.1007/978-3-642-31470-4_2 [DOI] [Google Scholar]
- Yamagata S., Hamba Y., Nakanishi K., Abe S., Akasaka T., Ushijima N., et al. (2012). Introduction of Rare-Earth-Element-Containing ZnO Nanoparticles into Orthodontic Adhesives. Nano Biomed. 4 (1), 11–17. [Google Scholar]
- Zhang J., Park Y.-D., Bae W.-J., El-Fiqi A., Shin S.-H., Lee E.-J., et al. (2015). Effects of Bioactive Cements Incorporating Zinc-Bioglass Nanoparticles on Odontogenic and Angiogenic Potential of Human Dental Pulp Cells. J. Biomater. Appl. 29, 954–964. 10.1177/0885328214550896 [DOI] [PubMed] [Google Scholar]