Abstract
Coronavirus disease 2019 (COVID-19) has made a global impact on the daily lives of humanity, devastating health systems, and cataclysmically affecting the world’s economy. Currently, the Standard Public Health Protective practices consist of but are not limited to wearing masks, social distancing, isolating sick and exposed people, and contact tracing. Scientists around the globe undertook swift scientific efforts to develop safe and effective therapeutics and vaccines to combat COVID-19. Presently, as of mid-March 2022, 57.05% of the world population have been fully vaccinated, and 65.3% of the United States of America’s (USA) total population have been fully vaccinated while 76.7% have received at least one dose of the vaccine. This article explores the various vaccines created through modern science and technology, including their safety, efficacy, and mechanism of action. Although the vaccines produced are up to 95.0% efficacious, their efficacy wanes over time, underscoring the need for booster doses. Also, vaccination has not been able to prevent “breakthrough” infections. The limitations of the SARS-CoV-2 vaccines indicate that further measures are required to ensure a firm control of the COVID-19 pandemic. Therefore, the Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the use of certain therapeutic agents because they have shown remarkable clinical outcomes. Several therapeutic agents for the treatment of mild-to-moderate COVID-19 include Gilead’s remdesivir, Regeneron’s casirivimab and imdevimab combination, Eli Lilly’s baricitinib and remdesivir combination, Pfizer’s co-packaged nirmatrelvir tablets and ritonavir tablets, and Merck’s molnupiravir capsules. Hence concerted efforts in early and accurate diagnosis, education on the COVID-19 virulence, transmission and preventive measures, global vaccination, and therapeutic agents could bring this COVID-19 pandemic under control across the globe.
Keywords: coronavirus, COVID-19, efficacy, immunization, immunogenicity, safety, SARS-CoV-2, therapeutics, vaccination, vaccines
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the causative agent of the coronavirus disease 2019 (COVID-19), has ravaged the world since 2019 when it was first discovered in Wuhan, China. 1 COVID-19 was officially recognized by the World Health Organization (WHO) as a pandemic in March 2020.2,3 Since then, several COVID-19 vaccines have been authorized or approved by the Food and Drug Administration (FDA) for use in the United States of America (USA), which include the Pfizer-BioNTech COVID-19 vaccine (BNT162b2), Moderna COVID-19 vaccine (mRNA-1273), and Johnson and Johnson’s Janssen COVID-19 vaccine (JNJ-78436735). Despite the introduction of vaccines, the number of confirmed cases and fatalities is still increasing. As of March 21, 2022, the WHO has reported a global total of 469,212,705 confirmed cases and 6,077,252 deaths, of which 78,999,760 confirmed cases and 964,198 deaths are in the USA. 4
The USA experienced a surge in the number of confirmed cases due to the emergence of variants of concern (VOC)–Delta (B.1.617.2) and Omicron (B.1.1.529). 5 According to the Centers for Disease Control and Prevention (CDC’s) “Nowcast” model projection, as of January 2022, the Omicron variant circulating in the USA accounted for 95.4% [CI = 95.0%], while the Delta variant constituted 4.6% [CI = 95.0%]. 6 Figure 1 respectively shows how this circulation is altered several weeks later. 6 Using a like-virus neutralization assay, Omicron was found to evade antibody neutralization by the BNT162b2 messenger ribonucleic acid (RNA) vaccine seen in a study of its effectiveness against Omicron variants. 5 Additional data is needed regarding the effectiveness of the current vaccines against the Omicron variant and its role in preventing hospitalization for COVID-19. Globally, only 57.05% of the population has been fully vaccinated, as of March 21, 2022.4,6 In the USA, since the commencement of the COVID-19 vaccination program on December 14, 2020, 254.8 million people (65.3%) have been fully vaccinated while 76.7% have received at least one dose of the vaccine, as of 16 March 2022. 6
Figure 1.
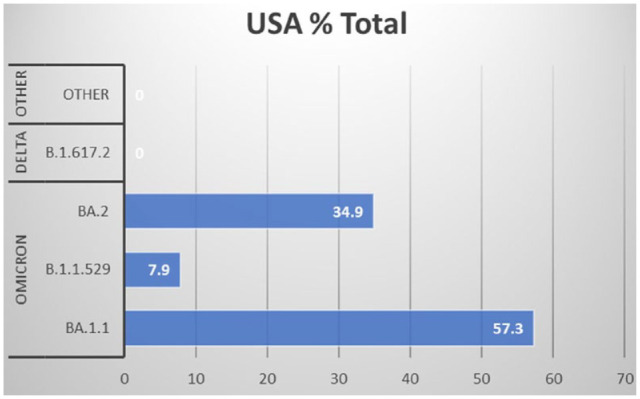
Model of currently circulating variants within the USA.
Note: Data recreated from the CDC as of 13–19 March 2022. 6
The SARS-CoV-2 is a part of the beta coronavirus (CoV) class, and the genomic sequence displays similarities to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle Eastern respiratory syndrome (MERS-CoV) at 79.0% and 51.8%, respectively. 1 Mutation in SARS-CoV-2 typically generate viral variants that change the properties of the virus. Most viruses that have RNA genomes, including the CoV’s, usually mutate at an extremely high rate; however, SARS-CoV-2’s rate of mutation is quite slow because of its “spell-check” mechanism involving an additional protein that corrects major copying errors. This may explain the lack of detection of any VOC during the first wave of COVID-19. Furthermore, vaccine-induced immunity is greater than infection-induced immunity against laboratory-confirmed COVID-19. 7 Hospitalized unvaccinated patients with previous SARS-CoV-2 infection (laboratory-confirmed COVID-19) were compared with patients who were fully vaccinated with an mRNA COVID-19 vaccine, with no history of SARS-CoV-2 infection. 7 It was concluded that vaccine-induced immunity and protection were significantly greater than infection-induced immunity against laboratory-confirmed COVID-19. This underscored the critical need for all eligible persons to be vaccinated against COVID-19 as soon as possible. Despite the high efficacy of the available vaccines, some breakthrough reinfections have been reported, emphasizing the need to expand vaccination programs and the genomic surveillance process to detect emerging variants. Typically, genomic surveillance entails the detection of new variants by isolating and sequencing the genomes of many patient samples to track transmission patterns and incidence of different variants. 7
Currently, some SARS-CoV-2 variants have been described in the literature. 8 For example, the D614G variant originally emerged from Wuhan, China, and spread rapidly around the world. The pharmaceutical companies used this variant’s spike protein sequence to develop the currently available vaccines. 9 The B.1.1.7 variant, which was first detected in the United Kingdom (UK), displayed 18 characteristic mutations indicating greater virulence and transmissibility in a higher percentage of patients than the original D614G variant. The B.1.351 and P1 variants first emerged from Brazil and South Africa, respectively. Further research is ongoing to determine the immune and vaccine protection against the variants. 9 Although there are various variants of CoV’s, they all have commonalities that consist of large, positive-sense, enveloped, and single-stranded RNA viruses with a crown-like structure known as a spike. 1 This implies that the building blocks for the SARS-CoV-2 viruses are being continually exchanged from one variant to another through genetic recombination. Therefore, global collaborative efforts are urgently needed to fully characterize the coronavirus ecosystem, including a viral sampling of multiple bat species and wild and domestic animals that are prone to coronavirus infection. Also, the viral and serological study of humans in direct contact with wildlife and farm animals would be of immense value in timely identification of the emergence of the pandemic and the subsequent prevention and control strategies, including the study of cross-reacting epitopes for vaccine development.
The purpose of this article is to review the advancements and prospects of authorized COVID-19 vaccines available globally with their immunogenicity, safety, and efficacy as well as discuss the investigational therapeutics options for the management of patients with COVID-19.
Understanding how COVID-19 vaccines work
Innate immunity is the body’s first line of defense against pathogens and viruses, and as of today, scientific understanding of the non-specific response to the COVID-19 virus is still unclear.10,11 Initiation of innate immunity begins when pattern recognition receptors (PRRs), a retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and Toll-like receptors (TLRs) are activated. 11 Upon activation of these receptors by viral pathogens, downstream effects will lead to a release of cytokines by infected cells. The types of cytokines released are interferons (IFN) 1 and 3, tumor necrotic factor-alpha (TNF-α), and interleukin (IL) 1, 6, and 18.10,11 All these immune mediators join to facilitate the effects of an adaptive immune response. 10 Studies have shown that if IFN-1 is present early and properly localized, this can limit the effect of CoV infection. 10 Research has also confirmed that SARS-CoV-2 is more sensitive to IFN-1 and 3 pre-treatments in vitro than SARS-CoV-1.10,12 However, the specific IFN gene that is being activated to mediate this protection is still being studied.10,13 Additional data suggest that lymphocyte antigen 6 complex locus E (LY6E) has been shown to interfere with the spike (S) protein of the COVID-19 virus, using angiotensin-converting enzyme 2 (ACE2) as a mechanism for cell entry.10,14 The surface S protein is the primary antigenic target for COVID-19 vaccines. The S antigenic sequence is made up of the less conserved N-terminal S1 component, which contains the receptor-binding domain (RBD) and the conserved C-terminal S2 sequence. 15 It binds to the host cell’s ACE2 receptor and causes membrane fusion. 10 Antibodies that bind to the RBD of the SARS-CoV-2 spike protein can prevent the virus from attaching to the host cell and neutralize it. 10 In the adaptive immune response to SARS-CoV-2, antigen presentation to the immune cells by the antigen presenting cells activates pathogen (virus) specific B-cells and T-cells. 16 The presence of IgGs, IgMs, IgAs, and neutralizing IgGs antibodies in COVID-19 patients signifies a humoral immune response mediated by increased B-cells. 16 IgM antibodies were observed to disappear in the 12th week while the IgG which are viral S-specific and N-specific, were observed to last for a longer period. 16 In COVID-19 patients, the peripheral count of CD4+ and CD8+ T-cells was observed to be reduced greatly. 15
To train the immune system, vaccines introduce weakened or inactive (harmless) parts of the organism in question into the body, which then triggers an immune response. 17 Some vaccines contain the antigen itself while newer ones contain the genetic material necessary for antigen production. To allow the production of long-lasting antibodies and the development of memory cells, some vaccines may need to be administered in multiple doses, weeks or months apart. 17 In doing so, the body is equipped to fight the specific organism, remember it, and rapidly fight it again in the event of future exposures.
Formulation design of COVID-19 vaccines
The three main approaches to designing a vaccine are as follows: (1) using a whole organism, (2) using parts of the organism that triggers an immune response, and/or (3) using the genetic material to provide instructions for making specific proteins, depicted better in Figure 2. 18 Within the whole-microbe approach, vaccines can be classified as inactivated, live-attenuated, or viral vectors. Inactivated vaccines are developed by using chemicals, heat, or radiation to destroy genetic information and deactivate the organism. 18 Even though the pathogen is incapable of infecting cells and replicating, its proteins can stimulate antibody-mediated responses. 19 Live-attenuated vaccines allow for the weakened version of a living organism to grow, replicate, and trigger a strong immune response while causing very mild to no illness. 19 This kind of vaccine mobilizes killer T-cells, helper T-cells, antibody-producing B-cells, and allows for the development of memory cells. 19 Viral vector vaccines work by inserting components of the pathogen into a safe virus and allowing this safe virus to deliver the sub-parts into the body. 18 Both T-cells and B-cells are mobilized to elicit a strong immune response without developing the disease. 20 Viral vector vaccines can be non-replicating and replicating. Non-replicating vector vaccines only produce the vaccine antigen but are unable to make new viral particles. 20 On the contrary, replicating vector vaccines can produce new viral particles which then go on to infect new cells and make the vaccine antigen. 20 The viral vector vaccines that are under development to combat COVID-19 are non-replicating viral vectors. 20
Figure 2.
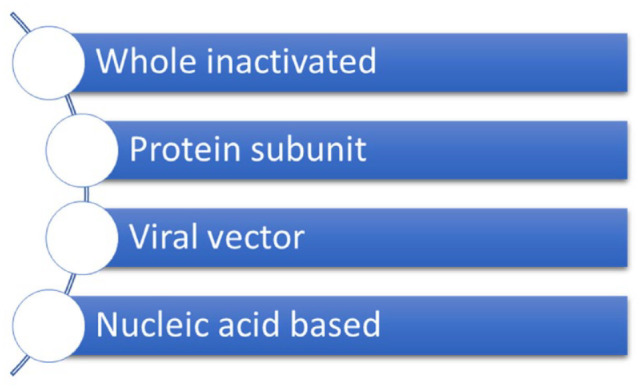
COVID-19 vaccines come in various forms.
The second approach, the subunit vaccine uses only the specific parts of an organism that the immune system needs to identify. 18 These subunits may be proteins or sugars, therefore, only an antibody-mediated immune response is elicited.18,21 The COVID-19 vaccines that are being developed using this approach will utilize protein subunits only. 21
Finally, the genetic approach, also called a nucleic acid vaccine, uses a section of deoxyribonucleic acid (DNA) or RNA to provide instructions for cells to make specific proteins that the immune system needs to detect. 18 Once detected, T-cells and B-cells are mobilized. 22 This is a novel approach that has progressed quickly due to the COVID-19 pandemic. 18 COVID-19 vaccines that have employed this approach, received EUA, and are now being distributed and administered to the population globally include Pfizer-BioNTech and Moderna. 22
In addition to the active component of the vaccine (mRNA), Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines contain other ingredients including lipids or fats that facilitate the penetration of the genetic materials into the cells. 23
For example, some of these ingredients consist of 2[(polyethylene glycol (PEG))-2000]-N, N-di tetradecyl acetamide, plant-derived cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine, and [(4-hydroxybutyl) azanediyl] bis(hexane-6,1-diyl) bis(2-hexyldecanoate). 23 They also include stabilizers such as buffer systems (e.g. dibasic sodium phosphate dihydrate and monobasic potassium phosphate), as well as tonicity adjusting ingredients (e.g. sodium chloride and sucrose). Johnson and Johnson’s Jassen vaccine (JNJ-78436735) contains the recombinant, replication-incompetent Ad26 vector encoding a stabilized variant of the SARS-CoV-2 S protein as the active ingredient which provides the instruction to building harmless protein that initiates the immune response to protect the body from future exposure to COVID-19. EUA COVID-19 vaccines do not contain any preservative (preservative-free), metal, or latex. 23
Immunogenicity, safety, and efficacy of authorized vaccines
Several vaccines have been authorized by Regulatory Agencies across the globe, for the prevention of COVID-19, based on their safety, efficacy, immunogenicity, and tolerability. The extent of protective immunity (efficacy) provided by the coronavirus vaccines depends on the nature of the virus. For example, some respiratory viruses cause systemic infections (e.g. measles, rubella, varicella-zoster, smallpox, etc.) whereas SARS-CoV-2 are non-systemic viruses that have limited contact with the systemic immune system; they primarily infect epithelial cells on mucosal surfaces. Therefore, they elicit suboptimal (incomplete) responses to systemically administered vaccines leading to transient protective immunity and subsequent reinfection. 24
To evaluate the immune responses and vaccine efficacy completely, several criteria have been considered, including the systemic and mucosal correlates of protection after natural coronavirus infection and after vaccination; vaccine approaches that will elicit immunity to multiple viral protein antigens to induce long-term humoral and cellular (memory) immunity; the key humoral and cellular targets that provide sufficiently robust and durable protective immunity against the rapidly evolving coronavirus strains; and the relevant animal models of coronavirus infection and immunity that can be used. Different approaches to vaccine preparation, their respective safety, immunogenicity, and efficacy with specific examples are presented below.
Whole-microbe approach
Sinovac/CoronaVac
The inactivated CoronaVac, formerly known as PiCoVacc, is manufactured by Sinovac. 25 It is a formalin vaccine with 600SU (single-use) inactivated SARS-CoV-2 virus with aluminum hydroxide.25–27 The two doses, each being half-milliliter, are administered in a 28-day interval in the intramuscular area. 27 As of April 1, 2021, over 200 million doses have been delivered with 100 million doses administered globally. 28 The vaccine is safe and well-tolerated in adults aged 18 and older. 29 Side effects of CoronaVac are mild and transient, with injection site pain as the most reported symptom. 29 Although rare, adverse effects pertain to severe allergic reaction symptoms such as difficulty breathing, tachycardia, facial swelling, rash, dizziness, and malaise. 27 Therefore, CoronaVac is not recommended for people who have a history of allergic reactions to the ingredients found in the vaccine. 27 In addition, individuals with uncontrolled or severe chronic disease, manifesting as neurological conditions, who are pregnant, or breastfeeding are advised to consult with their physician. 27 A healthcare consultation is recommended as a precautionary measure for those with thrombocytopenia, hemorrhagic disease, history of convulsions, epilepsy, encephalopathy, or taking immunosuppressive medications. 27 In Phase I and II clinical trials, CoronaVac showed a seroconversion rate above 90.0% which confirmed the vaccine to be immunogenic. 30 In late-stage trials, CoronaVac was shown to have 50.4% effectiveness at preventing mild to severe symptoms of COVID-19, which is significantly lower than the 90.0% efficacies of most vaccines. 31 Furthermore, differences have been seen in clinical trials on its efficacy. The Instituto Butantan trial showed CoronaVac to have an efficacy of above 50.0% after accounting for patients with mild infections in their study trials, but preliminary results originally revealed 78.0%. 32 In Turkey, CoronaVac showed effectiveness of 91.25% and was decreased to 83.5% in the final analysis. 32 Despite variation in effectiveness between regions, the WHO has approved the vaccine for emergency use as it meets the requirements in Mexico, Brazil, Chile, China, Indonesia, Malaysia, and Turkey. 32 With the emergence of new variants, especially those from the UK and South Africa, 33 CoronaVac is considered effective. However, based on preliminary results, it is ineffective against the Brazil P.1 lineage. 34 Sinovac, the manufacturer, is working currently to seek regulatory approval across countries to ensure global accessibility of the vaccine in hopes of managing the spread of new variants.
BBIBP-CorV (China)
BBIBP-CorV, a COVID-19 vaccine, manufactured by Sinopharm, was developed by the Beijing Institute of Biological Products and the China National Pharmaceutical Group (Sinopharm).35,36 It is an inactivated vaccine, consisting of two shots taken at either 14 or 21 days apart and administered intramuscularly. 37 Based on the data released by the Chinese government, the vaccine has overall efficacy of 73.9% in individuals over 18 years of age. 37 However, physician advisement is recommended for individuals who have a history of infection with SARS-CoV-2, experience symptoms of SARS-CoV-2 14 days before vaccination, are pregnant or lactating, are allergic to components in the vaccine, or who have a history of seizures or mental illness. 36 In clinical trials, BBIBP-CorV has shown to be safe, immunogenic, and well-tolerated at all tested dosages (8 μg dose or 4 μg dose) and across age groups (18-59, 60, and older). By day 42, there was an induced humoral response against SARS-CoV-2 and 100.0% seroconversion in all vaccine recipients, showing high neutralizing antibody titers. 36 Fever was a common adverse reaction to the vaccination, along with fatigue and headache. 36
COVAXIN
COVAXIN is an inactivated vaccine produced by Bharat Biotech in partnership with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV). 38 It is a 2-dose regimen with a 28-day interval, given intramuscularly. 38 In Phase I and II trials, COVAXIN showed neutralizing antibody titers with enhanced humoral and cell-mediated immune responses. 38 An interim analysis during the ongoing Phase III clinical trial revealed vaccine efficacy to be 81.0%. 38 Side effects include pain, swelling, redness, itching at the injection site, stiffness, and weakness in the inoculated arm. 39 Other reactions may include body aches, headache, malaise, nausea, and vomiting. 39 Pregnant and nursing mothers, those with high fevers, bleeding disorders, and people with known severe allergies to ingredients in COVAXIN are advised to speak with their physician. 39 Data has not been collected on vaccine safety and efficacy in groups with human immunodeficiency virus (HIV) infection, immunocompromising conditions, or in individuals taking immunosuppressive medications/therapies. 38
Inactivated (Vero Cell)
Inactivated (Vero Cell), was developed by China National Pharmaceutical Group (Sinopharm) through its Wuhan Institute of Biological Products site.40,41 It is an inactivated vaccine, administered intramuscularly in two doses with a 14-day interval having 72.5% efficacy. 41 Individuals who are between 18 to 59 years of age can be vaccinated, except for pregnant and nursing mothers, or those with severe allergies to the contents in Inactivated (Vero Cell) need to consult with their physicians. 42 Based on interim data from Phase I and II clinical trials, the vaccine presented with mild and self-limiting injection site pain with no serious adverse reaction. 42 Inactivated (Vero Cell) demonstrated immunogenicity, a high neutralizing antibody response was seen after 14 days. It is advised that a longer-term assessment of efficacy be conducted in Phase III. 43
CoviVac
CoviVac is developed by the Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of the Russian Academy of Sciences. 32 It is a whole virion, inactivated vaccine, given in two doses with a 14-day interval, intramuscularly.32,44 Researchers at the Chumakov Center have stated that CoviVac is 90.0% effective against COVID-19, with an extensive immune response compared to other Russian-developed vaccines. 44 No adverse reactions have been witnessed in trials. 44 However, it must be noted that Phase III clinical trials was conducted in April 2021. 45 The vaccine is recommended for people between the ages of 18 to 60. 32 CoviVac is contraindicated in pregnant and breastfeeding women, persons with severe allergic reactions, and those with a history of convulsions; therefore, advice from a physician should be sought. 46 Minuscule data is available regarding the immunogenicity of this vaccine from verified sources such as the USA’s CDC or the WHO. 47 Scientists at Chumakov have released information that CoviVac is capable of being effective against the rising variants. 44
RNA nucleic acid and protein subunit approach
Protein subunits consist of a noninfectious part of a COVID-19 protein, which causes the body to build memory cells against it; therefore, increasing immunity against the virus if ever infected in the future. 48 There are two mRNA vaccines which are protein subunit vaccines 48 developed by BioNTech–Pfizer and Moderna that are approved in the USA; four adenovirus vaccines (developed by Oxford–AstraZeneca, Gamaleya, Janssen, and Cansino–Beijing Institute of Biotechnology) approved in the UK, Russia, the USA, and China; and three inactivated vaccines (developed by Sinopharm–China National Institute for Communicable Disease Control and Prevention, Sinopharm–Chinese Academy of Sciences, and Sinovac–China National Institute for Communicable Disease Control and Prevention) first approved in China. 49
Pfizer/BioNTech
Pfizer/BioNTech (BNT162b2) is a lipid nanoparticle (LNP)–formulated, nucleoside-modified RNA vaccine that encodes a prefusion-stabilized, membrane-anchored SARS-CoV-2 full-length S protein.50–53 It was approved by the FDA on August 23, 2021, for persons 16 years and older via two 30 μg doses, 21 days apart from the deltoid muscle, and has been shown to elicit antigen-specific responses against the SARS-CoV-2 virus with a 95.0% efficacy. 54 A booster shot is recommended at least five months after completing the first two shots. It has also received a EUA for children of ages 5 – 15 years, but full approval has not been given for this age group. The side effects of this vaccine are short-term, with local reactions at the injection site that include pain, swelling, or redness. 50 Systemic reactions consist of fatigue, headache, and/or fever with chills. 50 This vaccine is still in the phase of data collection along with its immune response efficacy and durability. 50
Moderna (mRNA-1273)
Moderna (mRNA-1273), is an LNP–encapsulated mRNA vaccine expressing the prefusion-stabilized S glycoprotein of SARS-CoV-2. 54 It is administered as a two-dose injection regimen to the deltoid muscle 28 days apart in the same arm at a volume of 0.5 ml containing 100 μg of mRNA-1273 and is recommended to persons 18 years and older with an efficacy of 94.1%. 54 A booster dose is recommended for people ages 18 years and older at least 5 months after completing the primary vaccination. Retrogenicity, both local and systemic, were the same among age groups 18 to 65 years and older participants >65 years old. 54 Local reactions included erythema, induration, and tenderness at the injection site while systemic reactions consisted of fatigue, myalgia, arthralgia, and headaches. 54 Another adverse symptom reported was hypersensitivity reactions in a small number of participants, however, the safety and immunogenicity are reassuring with the research currently. 54
EpiVacCorona
EpiVacCorona is a synthetic peptide vaccine, which contains a chemically synthesized peptide immunogen of the S protein of SARS-CoV-2. 55 It is then conjugated as a carrier protein to recombinant SARS-CoV-2 protein N and adjuvanted with aluminum hydroxide. 55 The vaccine is administered intramuscularly to the deltoid muscle at a dose of (225 ± 45) μg/0.5ml as a two-dose regimen, to persons 18 to 60 years and spaced 21 days apart. 55 Local symptoms reported were hyperemia, edema, soreness, infiltrate formation, and itching at the injection site, whereas systemic reactions include fever, malaise, headache, and myalgia. 55 Based on trials done, the vaccine was deemed safe with low reactogenicity. 55 Immune responses to coronavirus antigens were reported at 82.1% IgG seroconversion 42 days following the first immunization dose; however, IgM seroconversion amounted to a minimal 5.3% on days 14 and 21 within the first three weeks of vaccination and a further decrease to 1.8% by the thirty-fifth day of vaccination. 55
ZF2001
ZF2001 is another protein subunit vaccine that targets the RBD of the SARS-CoV-2 S protein which focuses on blocking receptor binding, therefore, inducing immunity. 49 ZF2001 is made with an RBD-dimer protein produced in Chinese hamster ovary (CHO) cells adjuvanted with aluminum hydroxide given in a 25 μg three-dose schedule intramuscularly. 49 Local adverse events were mild to moderate with pain, swelling, induration, redness, rash, and pruritus at the injection site, while systemic adverse events within seven days after vaccination included fever, cough, dyspnea, diarrhea, anorexia, nausea, vomiting, arthralgia, arthritis, headache, fatigue, hypersensitivity reactions, irritation, and mental disorders (including anxiety, depression, mania, and insanity). 49 Both local and systemic reactions lasted 3-4 days, and as compared to mRNA-based vaccines or adenovirus-vectored vaccines, fever and fatigue were reported less with ZF2001. 49 While the data from Phase I and II have been deemed safe and efficacious, the safety and immunogenicity are still being reviewed due to the limitations that were presented in the study. 49 One limiting factor was the focus group, which consisted of individuals between the ages of 18 to 59 years old, this excluded children and the elderly thus driving the need for continued research. 49 In addition, most participants are Han Chinese, therefore, diversity in ethnic backgrounds was limited. 49 Moreover, during Phase I of the trial, immunogenicity was tested at day 30 after full vaccination and day 14 after Phase II; therefore, full immunity could not be well assessed during this time frame. 49
Novavax (NVX-CoV2373)
Novavax (NVX-CoV2373) efficacy has been evaluated in Phase II and Phase III trials. 4 The vaccine’s efficacy against mild, moderate, and severe disease was found to be 90.0% in Phase III trials. 4 The Strategic Advisory Group of Experts (SAGE) recommends administering the Novavax (NVX-CoV2373) vaccine in two doses (0.5 ml) intramuscularly for those older than 18 years of age. 4 The two doses should be given three to four weeks apart. 4 Data on the safety and immunogenicity of vaccines for children under the age of 18 are currently being generated; therefore, vaccination of children under the age of 18 is not recommended until such data are sufficiently available and can be further reviewed. 4 There is currently no data on the safety and efficacy of the Novavax (NVX-CoV2373) vaccine in pregnant women. 4 However, based on previous evidence from other protein-based vaccines used during pregnancy, efficacy should be comparable to that of non-pregnant women of a similar age. 4
Nanotechnology-assisted RNA overview
Nanotechnology-enabled vaccines have been positioned as potential solutions to the pandemic outbreak caused by the novel pathogen SARS-CoV-2. 56 RNAs are exceptional bioactive macromolecules that have been identified as key players in regulating a wide range of biochemical pathways. 57 The ability to control cell fate and tissue activities intimately makes RNA-based drugs the most intriguing family of bioactive agents. 57 However, achieving widespread use of RNA therapeutics in humans remains a difficult task due to the instability of naked RNA as well as the presence of biological barriers that prevent RNA from entering cells. 57 Without a doubt, the RNA therapeutics field has expanded dramatically in recent years. 57 Unprecedented industrial and academic collaborations emerged around the world to accelerate vaccine development, resulting in the clinical translation of effective vaccines in less than a year. 56 Today, new requests for the development of RNA-based vaccines have emerged as a result of the COVID-19 pandemic, bringing this technology to the forefront. 57 During the next few decades, the area of RNA therapies is predicted to explode, despite its current limitations, there is space for advancement and improvement. 57
Viral vector approach
Viral vector-based vaccines have been around since the 1970s and differ from most conventional vaccines by using a vector, which is an altered, harmless version of the virus. 58 This modified virus triggers an immune response to recognize the S protein in the case of COVID-19. 58 The viral vector vaccine does not contain the COVID-19 virus and therefore cannot give an individual the disease. 58
JNJ-78436735
The JNJ-78436735 COVID-19 viral vector vaccine is manufactured by Janssen Pharmaceuticals, a company of Johnson and Johnson. 59 Only one shot is required in the muscle of the upper arm and does not contain eggs, preservatives, or latex. 59 The JNJ-78436735 COVID-19 vaccine is recommended for anyone 18 years of age or older and is not recommended for anyone who has known anaphylaxis reactions to any of the ingredients. 59 Common side effects at the site of injection are pain, redness, and swelling. 59 Side effects seen in the rest of the body include fatigue, headache, muscle pain, chills, fever, and nausea. 59 In the rare chance that there is a severe allergic reaction, it will be seen within a few minutes to an hour after receiving the vaccine. 59 The efficacy of this vaccine is 66.3%, with the most protection two weeks post-vaccination. 59 In early April 2021, a Colorado mass vaccination site was shut down due to 11 people having severe side effects from the vaccine. 60 In Wake County, North Carolina the JNJ-78436735 COVID-19 viral vector vaccination was put on hold in April 2021 after 18 people reported reactions due to this vaccine. 60 Upward of 6.8 million doses of this vaccine have been administered within the USA and as of April 12, 2021, it has been reported to monitoring agencies that six women between the ages of 18 and 48, who had received the vaccination reported a rare and severe type of blood clot within 6 to 13 days after inoculation. 61 Johnson and Johnson, the CDC, the FDA, and vaccination sites are closely monitoring all reactions to this vaccine. 60
AZD1222 (ChAdOx1 nCoV-19)
The AZD1222 (ChAdOx1 nCoV-19) vaccine against COVID-19 developed by Oxford University and AstraZeneca is another viral vector vaccine. 62 This vaccine requires two doses given intramuscularly in the deltoid muscle with an interval of eight to 12 weeks. 62 Any individual over the age of 18 and with no history of anaphylaxis to any component of the vaccine is recommended to get the AZD1222 vaccine against COVID-19. 62 If the individual has a severe anaphylactic reaction to the first dose of the vaccine, then the second dose should not be given. 62 The most common side effects of this vaccine include tenderness and pain at the injection site, headache, fatigue, muscle pain, malaise, fever, chills, arthralgia, and nausea. 62 Sparse and infrequent side effects caused by this vaccine are neuroinflammatory conditions, transverse myelitis, trigeminal neuralgia, and multiple sclerosis. 62 In addition, surveillance of AZD1222 has shown several unusual, vaccine-induced immune thrombotic thrombocytopenia (VITT) cases, occurring several days post-inoculation.63,64 Although infrequent, complications from AZD1222 causing thrombotic thrombocytopenia require a thorough investigation to determine the risk to benefit ratio. 64 The efficacy of this vaccine is 63.09%. 62
Sputnik V (Gam-COVID-Vac)
Russia’s Gamaleya National Center of Epidemiology and Microbiology has released a viral vector vaccine for COVID-19 called Sputnik V, also referred to as Gam-COVID-Vac.65,66 Individuals are given two shots of this vaccine intramuscularly, with a 21-day interval between the first and second dose. 65 This vaccine contains a combination of two adenovirus vectors rAd5 given in the first dose and rAd26 which is given in the second dose. 67 This is the only vaccine that uses this approach; other vaccines such as the Oxford–AstraZeneca vaccine, use the same material for both doses. 68 Sputnik V is safe in individuals over 18 years old, and due to very few side effects is not contraindicated in anyone. 68 This vaccine has an efficacy of 91.6%. 65
Convidicea Ad5-nCoV
Convidicea Ad5-nCoV is a viral vector vaccine for COVID-19, made in China by CanSino Biologics Inc. and the Beijing Institute of Biotechnology. 69 Only one shot of this vaccine is required intramuscularly in the arm for anyone over the age of 18 years old. 69 There have not been any anaphylactic side effects reported with this vaccine during Phase III trials with over 40,000 participants. 70 Some common side effects seen with Convidicea are pain and swelling at the injection site, fever, headache, weakness, fatigue, muscle, and joint pain. 69 The efficacy of this vaccine is 65.7% in those who have had no previous infection. 69 The effectiveness can go up to 90.98%, to prevent any serious outcomes caused by COVID-19 and decrease the rate of mortality. 69 Table 1 highlights the COVID-19 vaccines authorized globally, respectively grouped based on the vaccine type: inactivated whole-microbe approach, protein subunit, RNA nucleic acid, and viral vector.
Table 1.
Globally authorized COVID-19 vaccines.
| Manufacturer | Vaccine type | Dose(s) recommended | Efficacy | Safety and reactogenicity | Immunogenicity |
|---|---|---|---|---|---|
| Sinovac/CoronaVac | Inactivated | 2-dose | 50.38% | Safe | Well tolerated and induced humoral responses |
| Sinopharm (Beijing)/BBIBP-CorV | Inactivated | 2-dose | 73.9% | Safe | Immunogenic and induced robust humoral responses rapidly |
| Bharat Biotech/COVAXIN | Inactivated | 2-dose | 81.0% | Safe | Enhanced humoral and cell-mediated immune responses |
| Sinopharm (Wuhan)/Inactivated (Vero Cell) | Inactivated | 2-dose | 72.5% | Safe | Immunogenic; high neutralizing antibody response is seen after 14 days |
| Chumakov/CoviVac | Inactivated | 2-dose | 90.0% + although Phase III clinical trials aren’t expected to start until April 2021 | Safe | The broader immune response that is likely to protect against any variants, compared to the other vaccines developed in Russia |
| Pfizer/BioNTech (BNT162b2) | Protein Subunit | 2-dose | 95.0% | Safe | High neutralizing antibody titers, high antigen specific CD8+, and Th1 type CD4 + T-cell response |
| Moderna (mRNA-1273) | Protein Subunit | 2-dose | 94.1% | Safe | Activates T-cells to facilitate the production of antibody-producing B-cells; response is seen after 14 days |
| EpiVacCorona (Russia) | Protein Subunit | 2-dose | 82.1% | Safe | Induces virus-specific and neutralizing antibodies |
| ZF2001/Anhui Zhifei Longcom Bio | Protein Subunit | 3-dose | - | Safe, based on data populated from Phase I and II of the clinical trial | Full immunity still under investigation |
| Johnson and Johnson/Janssen (JNJ-78436735; Ad26.COV2. s) | Viral Vector | 1-dose | 66.3% | Safe | Well tolerated humoral response with the most protection 2 weeks post vaccine |
| Oxford/AstraZeneca (AZD1222) | Viral Vector | 2-dose | 63.09% | Safe | Well tolerated humoral response |
| Sputnik V/Gam-COVID-Vac (Russia) | Viral Vector | 2-dose | 91.6% | Safe | Well tolerated humoral response |
| Convidicea Ad5-nCoV/(CanSino) | Viral Vector | 1-dose | 65.7% | Safe | Well tolerated humoral response |
Overview of vaccine approaches
Since the pandemic began, developing a safe and effective COVID-19 vaccine has been the main objective worldwide. More than 198 COVID-19 vaccines are being developed worldwide; these include inactivated vaccines, live virus vaccines, recombinant protein vaccines, vectored vaccines, and DNA or RNA vaccines. 29 Before a COVID-19 vaccine can gain approval from WHO and national regulatory agencies, it must go through clinical trials to demonstrate that they meet the global requirements for safety and effectiveness. 71
CoronaVac is an inactivated vaccine made by Sinovac and has desirable immunogenicity, as seen in studies with mice, rats, and non-human primates. 29 It displays an innate immune response by producing vaccine-induced antibodies to SARS-CoV-2 and can stop multiple variants of SARS-CoV-2. 29 BBIBP-CorV by Sinopharm is another inactivated vaccine with a reported 73.9% efficacy in individuals over 18. 37 The vaccine-induced humoral response was deemed safe after favorable results at all tested doses in two age groups, with fever being the most common systemic adverse reaction. 36
By December 11, 2020, the FDA approved a EUA order for the Pfizer–BioNTech mRNA vaccine, allowing healthcare workers to receive the vaccine, with the Moderna (mRNA-1273) mRNA vaccine receiving a EUA status on December 18, 2020. 72 According to a published report, the CDC identifies that Americans above 65 years of age are less likely to be hospitalized than those individuals who have not received either mRNA COVID-19 vaccine.73,74 During the clinical trials, greater reactogenicity was identified after the second dose for both vaccines. 75 However, more patients experienced reactogenicity with the Moderna (mRNA-1273) vaccine compared to the Pfizer-BioNTech vaccine. 75
The JNJ-78436735 (Janssen) COVID-19 viral vector vaccine is a single-dose adenovirus vector. In February 2021, the JNJ-78436735 (Janssen) vaccine was authorized for EUA. 75 However, on April 13, 2021, both the CDC and FDA briefly advised halting its use due to adverse effects and urging patients experiencing severe headaches, abdominal pain, leg pain, and shortness of breath to seek medical attention. 59 Recently, there have been reports of patients experiencing adverse effects such as thrombocytopenia, hemorrhages, and increased clot formation with the Oxford/AstraZeneca COVID vaccine. 76 These adverse effects have led certain parts of Europe and Canada to discontinue the use of the Oxford/AstraZeneca COVID vaccine. 76 Furthermore, during the preclinical safety phase to investigate the toxicity of the Pfizer–BioNTech mRNA vaccine, experiments with rats revealed higher levels of white blood cells and acute-phase reactant proteins. 76 Heparin-binding proteins (HBPs), another kind of acute-phase reactant protein, are also enhanced during the acute phase of an inflammatory response.76–78 Migrating neutrophils prepare HBPs in response to infection and promote vascular permeability, edema, and a pro-inflammatory state.76,78
As suggested by Merchant and colleagues, genetic vaccines may be responsible for initiating an autoimmune response against platelets.76,77 Referring to the condition as vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). 76 The rise in HBPs may also play a role in the elevated levels of anti-PF4/heparin antibodies found in the COVID-19 vaccinated individuals who displayed coagulation disorders in Germany and Austria. 76 Despite the number of adverse clotting events, the European Medicines Agency (EMA) considers it as one of the rarer adverse effects as Germany had reported only 31 cases of cerebral thrombosis out of the 2.7 million doses of AstraZeneca that were given out. 77 Furthermore, a study by Taquet and colleagues assessed that cerebral venous thrombosis (CVT) or portal vein thrombosis (PVT) in the two weeks following diagnosis of COVID-19 was greater than those receiving BNT162 b2 or mRNA-1273 vaccine. 79
Thus far, there is insufficient research to identify how long the vaccines will protect against COVID-19 and when booster doses will be required. 80 However, data regarding innate and cell-mediated immune responses for each of the vaccines are still emerging. 80 According to a pilot trial conducted in England, people who were vaccinated with Pfizer’s (BNT162b2) or AstraZeneca’s (ChAdOx1 nCoV-19) were 40-50% less likely to transmit the virus within their families. 81 Recipients of the vaccine were also seen to develop cellular immune responses, primarily toward CD4+ Th1 cells while CD8+ T-cell responses were minimal. 80
The vaccines currently undergoing clinical trials and in use are still expected to prove effective and safeguard against new strains and variants. 82 This is anticipated due to the humoral and cell-mediated immune response producing a broad variety of immune cells and antibodies. 82 A recent study conducted by Red and colleagues gathered 30 people who recovered from COVID-19 before the variants started appearing to learn if the groups CD8+ T-cells could recognize the three SARS-CoV-2 variants, the UK variant (B.1.1.7), the South African variant (B.1.351), and the Brazil variant (B.1.1.248). 83 As mutated strains of SARS-CoV-2 continue to emerge worldwide, concerns about the vaccine’s efficacy may arise. 82 The B.1.1.7 variant may be 30–50% more infectious than some of the other variants, due to the S protein mutations; research may indicate that the change will not help the virus survive the vaccine. 82 However, during clinical trials, vaccines are less efficacious against the B.1.351 variant than other variants. 82 The findings suggest the possibility of a multi-epitope T-cell response that can identify and interact with the new variants and limit viral escape. 83 While it is still early to determine how the vaccine will affect the spread of the virus, current data suggests that people who are entirely vaccinated are less likely to be asymptomatically infected and thereby less prone to spreading the virus. 84 A study carried out in Kentucky demonstrated that unvaccinated residents had 2.34 times the odds of reinfection compared with those fully vaccinated; thus, suggesting that among individuals with previous SARS-CoV-2 infection, full vaccination provides additional protection against reinfection. 85
WHO and Health Canada have both approved four vaccines against COVID-19 that have met the necessary standards for safety and efficacy: AstraZeneca/Oxford, Johnson and Johnson’s Janssen, Moderna, and Pfizer/BioNTech.86,87 WHO, CDC, and Health Canada strongly recommend individuals take whichever vaccine is made available to them first, even if they have already had COVID-19.86–88 All four COVID-19 vaccines are safe for most people over 18, including those with pre-existing conditions and autoimmune disorders.86–88 Any individuals who have a compromised immune system, or pregnant, have a history of severe allergies, or are severely frail, should discuss their situation with their healthcare provider. 86 Those who are pregnant and are already breastfeeding should continue to do so after vaccination.86–88 After getting vaccinated, as a precaution, recipients should remain at the location where they received the vaccine for at least 15 minutes afterward, in case they develop an unusual reaction, and medical attention needs to be promptly given.86–88 Additional medical attention is required if side effects do not resolve after a few days or if there is pain or redness present at the injection site increasing after 24 hours.86–88 Individuals who have a severe allergic reaction immediately after receiving the first dose of the vaccine should not have any additional doses administered.86–88 Taking painkillers before receiving the vaccination is not recommended because it is unknown how painkillers may affect the vaccine’s efficacy.86–88 Recipients may take painkillers vaccination as needed.86–88 Individuals who are vaccinated must continue to follow COVID-19 safety precautions such as wearing a mask, social distancing, and frequent handwashing.86–88 Even though the vaccine helps prevent severe illness and death, it is still unknown how effective it is in preventing infection and the spread of the virus.86–88
Therapeutic options
It is pivotal to promote the development of effective vaccines for the prevention and control of the virulence and spread of COVID-19 given the rapid transmissibility and spontaneous emergence of the SARS-CoV-2 variants, the severity of the COVID-19 symptoms, and the increased morbidity and mortality rates. It is crucial to explore various therapeutic agents that can block or inhibit the replication and dissemination of the virus or inhibit the inflammatory responses of the antibodies to reduce the cytotoxic effect and immunological pathologies of the virus. Moreover, diagnosis and control of COVID-19 have been quite difficult. Asymptomatic carriers of SARS-CoV-2 who do not show any clinical symptoms can transmit COVID-19 to healthy individuals directly or indirectly by physical contact through coughing or sneezing. Also, it is difficult to control the transmission of the disease from the patients who recovered from COVID-19 to healthy individuals. Discovering new therapeutics for the treatment of COVID-19 is a huge challenge because of the viral genetic recombination of the highly mutable single-stranded RNA CoV genome.89,90 At the inception of the COVID-19 outbreak in China, several therapeutic agents were used to treat patients but were found to be ineffective against the virus. For example, oseltamivir, a potent antiviral agent (neuraminidase inhibitor) that is highly effective against influenza viruses A and B did not show any visible effect against COVID-19 because the virus does not secret neuraminidase. 91 Several patients were also treated with antibiotics including moxifloxacin, ceftriaxone, and azithromycin, as a single drug or in a fixed-dose combination which showed a slight improvement in the clinical outcomes. 91 However, intensive research efforts across the globe have produced therapeutic agents with clinical significance for the treatment of COVID-19. Therefore, a global effort is required to focus on reducing viral transmission using therapeutic strategies to manage the infection.89,92 Therapeutic management may be approached with various techniques which include, but are not limited to supportive care, antivirals, antiparasitic, monoclonal antibodies, corticosteroids/immunosuppressants, and others, respectively depicted in Figure 3.
Figure 3.
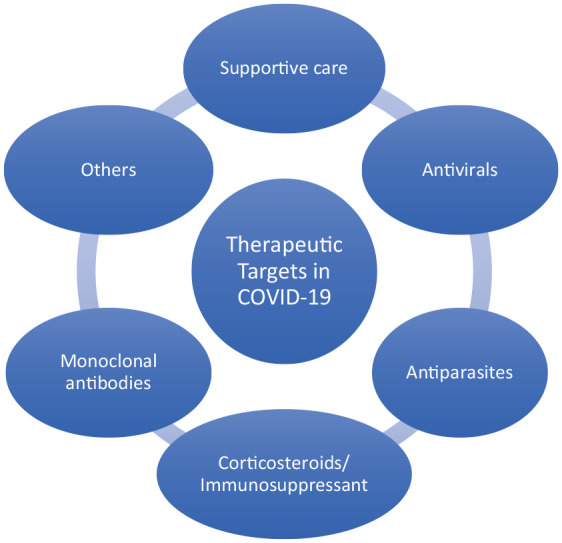
COVID-19 therapeutic approaches.
Note: General overview.
Supplementals
High-dose zinc gluconate and/or ascorbic acid (vitamin C) did not produce compelling evidence in the treatment of symptom duration in patients diagnosed with SARS-CoV-2 infection when compared to standard therapeutic regimens. 93 Whereas catechin, epigallocatechin-3-gallate (EGCG), found in green tea, improves acute lung injury in both pre- and post-COVID-19 disease stages by regulating inflammatory cytokines and inhibiting SARS-CoV-2 proteins. 94 Moreover, vitamin D, an essential hormone, plays a crucial role in adaptive immunity.95,96 It is associated with cell differentiation, proliferation, and maturation, as well as having a direct effect on ACE2 receptors, creating an immune-modulatory effect that may be beneficial in the treatment of SARS-CoV-2.95,97,98 Folic acid (B9), in conjunction with other pharmaceutical agents: azithromycin, hydroxychloroquine, and vitamin C are currently undergoing clinical trials for the management of high-risk COVID-19 patients. 95 As a member of the vitamin B family, folic acid is essential for rapid cell proliferation, as well as the synthesis of pyrimidines, purines, and methionine for DNA, RNA, and protein synthesis. 95
Antivirals/antimalarials
Chloroquine is typically used for the prevention and treatment of malaria or the treatment of rheumatoid arthritis and lupus erythematosus. It also has a wide antiviral effect by increasing the endosomal pH. The RBD of S protein is thought to be a potential target for neutralizing antibodies.99–101 However, it is important to note that the criteria for a EUA for chloroquine phosphate and hydroxychloroquine sulfate are not met, based on the FDA’s ongoing review of the scientific evidence. 102 As a result, on June 15, 2020, the EUA for these two drugs was revoked in the treatment of adults and adolescents hospitalized with COVID-19. 102
Furthermore, the disruption of replication of SARS-CoV-2 is being explored with antivirals, such as remdesivir, ribavirin, lopinavir, ritonavir, favipiravir, and arbidol. 99 EUA has been approved, as of August 2020, for the drug remdesivir in treating all COVID-19 hospitalized adult and pediatric patients, irrespective of disease severity.103-105 Of the drugs disrupting replication, remdesivir showed the most promising results against COVID-19, perhaps because it is a monophosphoramidate prodrug of an adenosine analog. 99 This analog causes the arrest of RNA synthesis by incorporating the viral RNA with the aid of RNA-dependent RNA polymerase.99,101 Similarly, favipiravir and ribavirin are also monophosphoramidate prodrugs, but to the guanine analog, although, unlike remdesivir, these have not produced supportive evidence as effective medications in the treatment of patients with COVID-19.95,99,101
The class of protease inhibitors like lopinavir and ritonavir are synthetic drugs that use an enzyme (coronavirus proteinase 3CLpro or 3 C-like protease) to cleave proteins into smaller fragments that inhibit the growth of SARS-CoV-2, its infectivity, and replication.99,101 Furthermore, camostat mesylate, a potent synthetic serine protease inhibitor with antifibrotic, anti-inflammatory, and potential antiviral properties, blocks cellular entry of SARS-CoV-2 in human lung cells through the blockade of transmembrane protease, serine 2 (TMPRSS2).101,105
Umifenovir has been shown to decrease the incidence of infection by inhibiting the viral fusion of SARS-CoV-2 by impairing conformational changes in the proteins and therefore, increasing membrane rigidity.101,106 In addition, umifenovir inhibits viral entry by slowing clathrin-coated vesicle (CCV) intracellular trafficking through the impaired release of clathrin-coated pits (CCPs) from the plasma membrane, promoting clearance of viral load earlier, improving hospital discharge rate, and decreasing mortality better than those patients receiving lopinavir or ritonavir. 101
On December 22, 2021, the FDA issued a EUA for Pfizer’s co-packaged nirmatrelvir tablets and ritonavir tablets for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients aged 12 years and older weighing at least 40 kg. 107 The FDA has also issued a EUA for Merck’s molnupiravir capsules in the same category, to be used for the treatment of mild-to-moderate COVID-19 in adults 18 years and older, with positive results of direct SARS-CoV-2 viral testing.108,109 These therapeutic agents are not substitutes for vaccines and are not authorized for the pre-exposure or post-exposure prevention of COVID-19.107,109
Antiparasitic
The antiparasitic ivermectin drug, though not FDA approved for the treatment of COVID-19 because of the potential adverse effects, has shown promising data in reducing SARS-CoV-2 in vitro.99,101,110–114 Investigational studies are exploring the possible therapeutic benefits of ivermectin in COVID-19 patients. 113 A Phase III study among patients with dengue virus (DENV) in Thailand had been given ivermectin in a single daily dose. 113 The administered dosage was found safe among this patient population, though it did not produce clinical benefit. 113 Although DENV is different from SARS-CoV-2, perhaps the data obtained may be noteworthy for further consideration in the management of COVID-19. 113
Monoclonal antibodies
Outpatients with COVID-19 who are treated with various monoclonal antibodies have shown positive outcomes.115,116 A type III interferon, such as peginterferon lambda-1, has activity against respiratory pathogens; therefore, promoting viral clearance, shortening the duration of viral shedding, and preventing patient deterioration. 115 Other monoclonal antibodies like tocilizumab and sarilumab inhibit both membrane-bound and soluble IL-6 receptors, particularly in critically ill COVID-19 patients receiving organ support, it improved survival outcomes. 117 However, tocilizumab, from one study, did not result in better clinical outcomes in those patients with severe COVID-19 pneumonia when compared to the placebo effect. 118
Reduction of the viral load has also been proven with the REGN-COV2 antibody cocktail consisting of casirivimab and imdevimab. 119 These two-part noncompeting, neutralizing human IgG1 antibodies target the RBD of the SARS-CoV-2 S protein, preventing entry into human cells through the ACE2 receptor. 119
Corticosteroids/immunosuppressants
Corticosteroids are a group of drugs with immunosuppressant and anti-inflammatory effects that have been shown to inhibit elevated inflammatory responses thus, decreasing the disease severity of COVID-19. 99 Inhalational budesonide, given during the early disease process, has been seen to reduce the overall recovery time. 120 Moreover, low-dose steroids prednisolone and tacrolimus, when used in severe COVID-19 lung injury, have positive clinical effects based on their ability to inhibit pro-inflammatory cytokines that exacerbate deleterious lung pathology. 121 Likewise, in severe COVID-19 patients, promising data using cyclosporine, an immunosuppressant and steroid-sparing agent, has been reported given that the mechanism of action of the drug suppresses lethal inflammation and inhibits cyclophilin enzymes, an enzyme which CoV hijacks to support itself. 92
Interferon-JAK1/2-STAT1 signaling system and NF-κB are principal factors of expression that are induced by SARS-CoV-2; therefore, ruxolitinib, a JAK1/2 inhibitor, can be administered to normalize interferon gene transcripts induced by the virus in lung epithelial cells. 122 When used alone or in combination with remdesivir, an inhibition of C3a protein produced by infected cells was seen further suggesting that JAK inhibitors and drugs that normalize NF-κB may prove to have a clinical effect in severe COVID-19. 122 Together, they have the potential to reduce thrombotic adverse effects and decrease the risks associated with viral replication in severe COVID-19 patients. 122 Furthermore, baricitinib, a Janus-associated kinase (JAK) inhibitor, has anti-inflammatory and antiviral properties that inhibit viral entry into the target host cells. 101 Inhibiting adaptor-related protein 2–associated protein kinase 1 (AP2-AAK1), a regulator of clathrin-dependent endocytosis, improves laboratory and clinical parameters according to a pilot study.101,116
The immunosuppressant, colchicine, acts as a potent anti-inflammatory agent by preventing viral replication through the inhibition of microtubule formation and an inhibitor of IL-1 and IL-6.95,123,124 It also inhibits the expression of E-selectin and reduces neutrophil production of free radicals like superoxide. 125 Furthermore, colchicine is a therapeutic option for patients who have contraindications to other drugs. Moreover, it disrupts inflammasome activation, a direct response seen in COVID-19 patients.124-126
Others
The Clustered Regularly Interspaced Short Palindromic Repeats/Cas13 (CRISPR/Cas13) are a family of enzymes potentially able to inhibit the SARS-CoV-2 replication.99,127 This family of enzymes has been sequenced from many COVID-19 positive patients and is shown to cleave the RNA genome of the virus. 99 In addition to aiding in the amplification of genome assay, direct detection by Cas13 when delivered to infected lungs via an adeno-associated virus vector could target the emerging pathogen. 99
Administering COVID-19 convalescent plasma (CP) for the treatment of hospitalized patients has been issued a EUA as of August 2020. 128 CP is the plasma acquired from those patients who have already survived the infection and have developed an antibody-mediated immunity to SARS-CoV-2.99,121 Initial investigations with CP have proven beneficial; however, additional trials remain necessary for definitive efficacy.99,128 Furthermore, neutralizing antibodies isolated from CP of COVID-19 patients block the binding between the RBD of the S and ACE2 receptor; therefore, further suggesting that this may serve as a promising therapeutic approach. 101
Moreover, stem cells are resistant to viral infection due to their expression of IFN-gamma. 121 Mesenchymal stromal cells (MSCs) may inhibit the release of pro-inflammatory cytokines while mending injured tissue, a critical pathological factor in COVID-19 patients. 121 MSCs exhibit antimicrobial effects which could benefit the patient by reducing the SARS-CoV-2 viral load. 121
Overview of therapeutic approaches
The management of COVID-19 entails supportive care, antivirals, antiparasitic, monoclonal antibodies, corticosteroids, and immunosuppressants. 129 While it may be possible for variants such as the emergent B.1.526 out of New York City, USA to evade vaccine-elicited antibodies, studies suggest that therapeutic antibody therapy may retain its effectiveness and is variant-dependent. 130 The antibody cocktail, REGN10933 showed a 12-fold decrease in titer; however, the combination cocktail with REGN10987 proved to be fully active in neutralizing the S protein substitution of E484 K, a point mutation within the B.1.526 variant. 130 The supplemental appendix will identify current treatment recommendations as suggested by the Infectious Diseases Society of America (IDSA) guideline panel based on the severity of illness and stability of the patient. 129 For the guideline, critical illness is indicated by mechanical ventilation, end-organ damage, and septic shock. 129 Severe illness is indicated by a SpO2 ⩽ 94.0% on room air, with or without the use of supplemental oxygen. 129 Non-severe illness is indicated by a SpO2 > 94.0% not requiring supplemental oxygen. 129
Perspective
Vaccines and therapeutics are essential tools for controlling and combating the COVID-19 pandemic. Achieving and sustaining a strategy to control the virus is seen with faster development and more efficient deployment in medical countermeasures. Increases in breakthrough infections have been attributed to decreasing vaccine-induced immunity and the introduction of SARS-CoV-2 subtypes, necessitating the consideration of vaccination booster doses. 131 Boosters have been found to be safe and effective in increasing SARS-CoV-2-specific neutralizing antibody levels; albeit it is unknown how these doses will alter the global pandemic’s trajectory and herd immunity. 131 When choosing the timing and eligibility for COVID-19 vaccination boosters, the immunology, epidemiology, and equitable vaccine distribution need to be strongly considered. 131 Because of the virus’s novelty, many issues about these vaccinations’ long-term reactogenicity remain unsolved. 132 Additional independent studies on the effectiveness and safety of these vaccinations are highly necessary in order to boost public trust in COVID-19 vaccines and to identify all potential risk factors linked to each vaccine’s adverse responses. 132 These vaccine platforms repurposed therapeutic approaches, and the search for modern technology continues to be investigated and expanded upon.
For example, a self-administered vaccine capable of eliciting long-lasting immunity via sterilizing neutralizing antibodies would be extremely beneficial in combating developing mutant SARS-CoV-2 variants. 133 This may also reduce the chance of vaccinated persons functioning as COVID-19 passive carriers. 133 In one study, the efficacy of an intranasal (IN)-delivered DNA vaccine encoding the S protein of SARS-CoV-2 in immunocompetent BALB/c and C57BL/6 J mice models is explored. 133 The immunological response to IN administration of a SARS-CoV-2-spike DNA vaccine delivered on a modified gold-chitosan nanocarrier demonstrates a robust and persistent increase in antibodies (IgG, IgA, and IgM) and efficient neutralization of pseudoviruses expressing S proteins of distinct SARS-CoV-2 variants (Wuhan, beta, and D614G). 133 Immunophenotyping and histology investigations demonstrate the sequence of events that occur during the detection of SARS-CoV-2 S antigen by local dendritic cells and alveolar macrophages, which prepare the draining lymph nodes and spleen for peak SARS-CoV-2-specific humoral and cellular immune responses. 133 Elevated levels of anti-SARS-CoV-2 IgA in pulmonary mucosa and tissue-resident memory T-cells can effectively block SARS-CoV-2 and its mutations at the point of entry while also providing long-term protection.133,134
Conclusion
COVID-19 has caused a serious global health concern due to its rapid spread, high morbidity, mortality, and economic challenge all over the world. Early diagnosis, effective treatment, and preventive measures are the cornerstones of disease management. This article reviews the safety, efficacy, and immunogenicity of authorized vaccines that are available worldwide, as well as the investigational therapeutics for patients with COVID-19. Although the efficacy of the vaccines varies significantly up to 95.0%, it was observed that a combination of immunity following natural infection with SARS-CoV-2 and vaccine-induced immunity has not been sufficient to prevent the emergence and rapid spread of coronavirus variants such as the Delta (B.1.617.2) and Omicron (B.1.1.529)– ‘variants of concern’. Thus, mutated strains of SARS-CoV-2 continue to emerge worldwide, and concerns about the vaccine’s efficacy are arising. However, current data suggests that people who are completely vaccinated, exhibit mild non-life-threatening symptoms and are less likely to be symptomatically infected and thereby less prone to spreading the virus. Presently, it is not clear whether and how permanent protective immunity can be achieved or if second-generation vaccines with greater immune protection and more durable immunity will be needed. However, the goal at this time remains to increase vaccine production all over the world and get as many people vaccinated as possible. Although the vaccine helps prevent severe illness and death, it is still unknown how effective it is in preventing infection and the spread of the virus, so further studies are needed in this regard. To prevent more deadly waves of COVID-19, vaccines must be accepted by the public. This will need to be coupled with multiple preventive measures such as wearing a mask, social distancing, and frequent hand hygiene, for everyone including those that are vaccinated.
Supplemental Material
Supplemental material, sj-docx-1-tav-10.1177_25151355221097559 for Current advancements and future prospects of COVID-19 vaccines and therapeutics: a narrative review by Adekunle Sanyaolu, Chuku Okorie, Aleksandra Marinkovic, Stephanie Prakash, Martina Williams, Nafees Haider, Jasmine Mangat, Zaheeda Hosein, Vyshnavy Balendra, Abu Fahad Abbasi, Priyank Desai, Isha Jain, Stephen Utulor and Amos Abioye in Therapeutic Advances in Vaccines and Immunotherapy
Acknowledgments
N/A.
Footnotes
Ethical Approval and Consent to participate: N/A.
Consent for publication: N/A.
Author contribution(s): Adekunle Sanyaolu: Conceptualization; Methodology; Resources; Writing – review & editing.
Chuku Okorie: Formal analysis; Supervision; Writing – review & editing.
Aleksandra Marinkovic: Investigation; Project administration; Writing – original draft; Writing – review & editing.
Stephanie Prakash: Investigation; Writing – original draft; Writing – review & editing.
Martina Williams: Investigation; Writing – original draft.
Nafees Haider: Investigation; Writing – original draft.
Jasmine Mangat: Investigation; Writing – original draft.
Zaheeda Hosein: Investigation; Writing – original draft.
Vyshnavy Balendra: Investigation; Writing – original draft.
Abu Fahad Abbasi: Investigation; Writing – original draft.
Priyank Desai: Investigation; Writing – original draft.
Isha Jain: Investigation; Writing – original draft.
Stephen Utulor: Investigation; Writing – original draft.
Amos Abioye: Formal analysis; Writing – review & editing.
ORCID iD: Adekunle Sanyaolu  https://orcid.org/0000-0002-6265-665X
https://orcid.org/0000-0002-6265-665X
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and material: N/A.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Adekunle Sanyaolu, Federal Ministry of Health, Department of Public Health, New Federal Secretariat Complex, Phase III, Ahmadu Bello Way, Central Business District, FCT, Abuja, Nigeria.
Chuku Okorie, Union County College, Plainfield, NJ, USA.
Aleksandra Marinkovic, Saint James School of Medicine, The Quarter, Anguilla.
Stephanie Prakash, Saint James School of Medicine, The Quarter, Anguilla.
Martina Williams, Saint James School of Medicine, The Quarter, Anguilla.
Nafees Haider, All Saints University School of Medicine, Roseau, Dominica.
Jasmine Mangat, Caribbean Medical University School of Medicine, Willemstad, Curacao.
Zaheeda Hosein, Caribbean Medical University School of Medicine, Willemstad, Curacao.
Vyshnavy Balendra, Saint James School of Medicine, The Quarter, Anguilla.
Abu Fahad Abbasi, Loyola University Medical Center, Maywood, IL, USA.
Priyank Desai, American University of Saint Vincent School of Medicine, Kingstown, Saint Vincent, and the Grenadines.
Isha Jain, Windsor University School of Medicine, Cayon, Saint Kitts, and Nevis.
Stephen Utulor, School of Medicine, International University of the Health Sciences, Basseterre, Saint Kitts, and Nevis.
Amos Abioye, Lloyd L. Gregory School of Pharmacy, Palm Beach Atlantic University, West Palm Beach, FL, USA.
References
- 1. Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; coronavirus disease-19). Clin Exp Pediatr 2020; 63: 119–124, https://pubmed.ncbi.nlm.nih.gov/32252141/8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed J 2020; 43: 334–340, https://www.sciencedirect.com/science/article/pii/S2319417020300871 (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreens DM, Daszak P, Taubenberger JK. Escaping Pandora’s box – another novel coronavirus. N Engl J Med 2020; 382: 1293–1295, https://www.nejm.org/doi/full/10.1056/nejmp2002106 (accessed 7 January 2022). [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Coronavirus disease (COVID-19) dashboard, 2022, https://covid19.who.int/table (accessed 22 March 2022). [PubMed]
- 5. Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2022; 386: 494–496, https://pubmed.ncbi.nlm.nih.gov/34965358/ (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. COVID-19, 2022, https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/ (accessed 22 March 2022).
- 7. Bozio CH, Grannis SJ, Naleway AL, et al. Laboratory-confirmed COVID-19 among adults hospitalized with COVID-19-like illness with infection-induced or mRNA vaccine-induced SARS-CoV-2 immunity – nine states, January – September 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1539–1544, https://pubmed.ncbi.nlm.nih.gov/34735425/ (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanyaolu A, Okorie C, Marinkovic A, et al. The emerging SARS-CoV-2 variants of concern. Ther Adv Inf Dis. Epub ahead of print June 2021, https://pubmed.ncbi.nlm.nih.gov/34211709/ (accessed 7 January 2022). [DOI] [PMC free article] [PubMed]
- 9. Wanner M. The emergence of SARS-CoV-2 variants sparks concern. The Jackson Laboratory, 2021, https://www.jax.org/news-and-insights/2021/february/new-coronavirus-variants-spark-concerns (accessed 7 January 2022).
- 10. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity 2020; 52: 910–941, https://www.cell.com/immunity/fulltext/S1074-7613(20)30183-7?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1074761320301837%3Fshowall%3Dtrue (accessed 7 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mortaz E, Tabarsi P, Varahram M, et al. The immune response and immunopathology of COVID-19. Front Immunol 2020; 11: 2037, https://www.frontiersin.org/articles/10.3389/fimmu.2020.02037/full (accessed 7 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036–1045.e9, https://www.sciencedirect.com/science/article/pii/S009286742030489X (accessed 7 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mantlo E, Bukreyeva N, Maruyama J, et al. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res 2020; 179: 104811, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188648/ (accessed 7 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfaender S, Mar KB, Michailidis E, et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. bioRxiv 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7255780/ (accessed 7 April 2021). [DOI] [PMC free article] [PubMed]
- 15. Sindhi R, Ashokkumar C, Spishock B, et al. T-cell and antibody immunity after COVID-19 mRNA vaccines in healthy and immunocompromised subjects: an exploratory study. medRxiv 2021, https://www.medrxiv.org/content/10.1101/2021.05.21.21257442v1.full (accessed 23 March 2022).
- 16. Das A, Roy S, Swarnakar S, et al. Understanding the immunological aspects of SARS-CoV-2 causing COVID-19 pandemic: a therapeutic approach. Clin Immunol 2021; 231: 108804, https://pubmed.ncbi.nlm.nih.gov/34303849/ (accessed 23 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. How do vaccines work, 2020, https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work (accessed 2 April 2021).
- 18. World Health Organization. The different types of COVID-19 vaccines, 2021, https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained (accessed 2 April 2021).
- 19. Gavi, the Vaccine Alliance. What are whole virus vaccines and how could they be used against COVID-19? 2020, https://www.gavi.org/vaccineswork/what-are-whole-virus-vaccines-and-how-could-they-be-used-against-covid-19 (accessed 2 April 2021).
- 20. Gavi, the Vaccine Alliance. What are viral vector-based vaccines and how could they be used against COVID-19? 2020, https://www.gavi.org/vaccineswork/what-are-viral-vector-based-vaccines-and-how-could-they-be-used-against-covid-19 (accessed 2 April 2021).
- 21. Gavi, the Vaccine Alliance. What are protein subunit vaccines and how could they be used against COVID-19? 2020, https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 (accessed 2 April 2021).
- 22. Gavi, the Vaccine Alliance. What are nucleic acid vaccines and how could they be turned against COVID-19? 2020, https://www.gavi.org/vaccineswork/what-are-nucleic-acid-vaccines-and-how-could-they-be-used-against-covid-19 (accessed 2 April 2021).
- 23. Centers for Disease Control and Prevention. COVID-19: Pfizer-BioNTech COVID-19 vaccine (also known as Comirnaty) overview and safety, 2022, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed 7 January 2022).
- 24. Morens DM, Taubenberger JK, Fauci AS. Universal coronavirus vaccines – an urgent need. N Engl J Med 2022; 386: 297–299, https://www.nejm.org/doi/full/10.1056/nejmp2118468 (accessed 5 February 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong Y, Dai T, Wei Y, et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther 2020; 5: 237, https://pubmed.ncbi.nlm.nih.gov/33051445/ (accessed 8 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Regulatory Affairs Professionals Society. COVID-19 vaccine tracker, 2021, https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker (accessed 8 April 2021).
- 27. COVID-19 Vaccination Programme. FAQs: protect yourself and others – get vaccinated. The Government of Hong Kong Special Administrative Region, 2021, https://www.covidvaccine.gov.hk/en/faq#FAQ_B3 (accessed 8 April 2021).
- 28. Reuters. China Sinovac says it reached two billion doses annual capacity for COVID-19 vaccine, 2021, https://www.reuters.com/article/us-health-coronavirus-vaccine-sinovac-idUSKBN2BP07G (accessed 8 April 2021).
- 29. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192, https://www.thelancet.com/article/S1473-3099(20)30843-4/fulltext (accessed 12 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. COVID-19: keeping an eye on COVID VAX – Sinovac biotech – CoronVac. Genetic Engineering & Biological News, 18 May 2020, https://www.genengnews.com/covid-19-candidates/sinovac-biotech/ (accessed 8 April 2021).
- 31. Krammer F. SARS-CoV-2 vaccines in development. Nature 2020; 586: 516–527, https://pubmed.ncbi.nlm.nih.gov/32967006/ (accessed 8 April 2021). [DOI] [PubMed] [Google Scholar]
- 32. Precision Vaccine. Vaccine info: CoronaVac COVID-19 vaccine, 2021, https://www.precisionvaccinations.com/vaccines/coronavac-covid-19-vaccine-sinovac (accessed 9 April 2021).
- 33. NEJM Group. Correspondence: susceptibility of circulating SARS-CoV-2 variants to neutralization, 2021, https://www.nejm.org/doi/metrics/10.1056/NEJMc2103022 (accessed 7 April 2021). [DOI] [PMC free article] [PubMed]
- 34. de Souza WM, Amorim MR, Sesti-Costa R, et al. Levels of SARS-CoV-2 lineage P.1 neutralization by antibodies elicited after natural infection and vaccination. Lancet. Epub ahead of print 1 March 2021, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3793486 (accessed 7 April 2021). [DOI] [PMC free article] [PubMed]
- 35. Corum J, Zimmer C. How the Sinopharm vaccine works. The New York Times, 22 March 2021, https://www.nytimes.com/interactive/2020/health/sinopharm-covid-19-vaccine.html (accessed 6 April 2021).
- 36. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021; 21: 39–51, https://pubmed.ncbi.nlm.nih.gov/33069281/ (accessed 8 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanford Guide. COVID-19, vaccines, prevention. Antimicrobial Therapy, 2021, https://webedition.sanfordguide.com/en/prevention/covid-19-prevention-1/covid-19-prevention-vaccines (accessed 6 April 2021).
- 38. Bharat Biotech. COVAXIN®: India’s first indigenous COVID-19 vaccine, 2021, https://www.bharatbiotech.com/covaxin.html (accessed 9 April 2021).
- 39. Bharat Biotech releases risks, benefits of Covaxin, asks pregnant women to avoid dose. India Today, 19 January 2021, https://www.indiatoday.in/coronavirus-outbreak/vaccine-updates/story/bharat-biotech-covaxin-risks-benefits-asks-pregnant-women-breastfeeding-mothers-to-avoid-1760493-2021-01-19 (accessed 9 April 2021).
- 40. Held S. Two COVID-19 vaccines approved in China in less than 24 hours. BioWorld, 2021, https://www.bioworld.com/articles/504243-two-covid-19-vaccines-approved-in-china-in-less-than-24-hours?v=preview (accessed 9 April 2021).
- 41. Jeong M. Global COVID-19 vaccine summary: side effects. Medical News Today, 22 March 2021, https://www.medicalnewstoday.com/articles/global-covid-19-vaccine-summary-side-effects (accessed 3 April 2021).
- 42. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020; 324: 951–960, https://pubmed.ncbi.nlm.nih.gov/32789505/ (accessed 9 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Institute of Health: Clinical Trials. A study to evaluate the efficacy, safety and immunogenicity of inactivated SARS-CoV-2 vaccines (Vero Cell) in healthy population aged 18 years old and above (COVID-19), 2020, https://clinicaltrials.gov/ct2/show/NCT04510207 (accessed 3 April 2021).
- 44. Ivanova P. Russia approves its third COVID-19 vaccine, CoviVac. Reuters, 2021, https://www.reuters.com/article/us-health-coronavirus-russia-vaccine-idUSKBN2AK07H (accessed 7 April 2021).
- 45. Russia approves CoviVac, its third coronavirus vaccine. Radio Free Europe/Radio Liberty, 21 February 2021, https://www.rferl.org/a/russia-coronavirus-vaccine-covivac/31113697.html (accessed 7 April 2021).
- 46. Inoculation against coronavirus with CoviVac contraindicated during pregnancy. Russian News Agency TASS, 20 February 2021, https://tass.com/society/1258955 (accessed 7 April 2021).
- 47. Abbany Z. Two more Russian vaccines: what we do and don’t know. Deutsche Welle, 9 March 2021, https://www.dw.com/en/two-more-russian-vaccines-what-we-do-and-dont-know/a-56811025 (accessed 7 April 2021).
- 48. Centers for Disease Control and Prevention. Understanding how COVID-19 vaccines work, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html (accessed 4 April 2021). [PubMed]
- 49. Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis 2021; 21: P1107–P1119, https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00127-4/fulltext (accessed 8 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2mRNA COVID-19 vaccine. N Engl J Med 2020; 383: 2603–2615, https://www.nejm.org/doi/10.1056/NEJMoa2034577 (accessed 8 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 2021; 27: 717–726, https://www.nature.com/articles/s41591-021-01294-w (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parry HM, Tut G, Faustini S, et al. BNT162b2 vaccination in people over 80 years of age induces strong humoral immune responses with cross neutralisation of P.1 Brazilian variant. Lancet. Epub ahead of print March 2021, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3816840 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed]
- 53. Garcia-Beltran WF, Lam EC, St. Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021; 184: 2372–2383.e9, https://www.sciencedirect.com/science/article/pii/S0092867421002981 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403–416, https://www.nejm.org/doi/10.1056/NEJMoa2035389 (accessed 8 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ryzhikova AB, Ryzhikov EA, Bogryantseva MP, et al. A single blind, placebo-controlled randomized study of the safety, reactogenicity and immunogenicity of the “EpiVacCorona” vaccine for the prevention of COVID-19, in volunteers aged 18–60 years (Phase I–II). Russ J Infect Immun 2021; 11: 283–296, https://www.iimmun.ru/iimm/article/view/1699/1188 (accessed 8 April 2021). [Google Scholar]
- 56. Lopez-Cantu DO, Wang X, Carrasco-Magallanes H, et al. From bench to the clinic: the path to translation of nanotechnology-enabled mRNA SARS-CoV-2 vaccines. Nanomicro Lett 2022; 14: 41, https://pubmed.ncbi.nlm.nih.gov/34981278/ (accessed 22 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rinoldi C, Zargarian SS, Nakielski P, et al. Nanotechnology-assisted RNA delivery: from nucleic acid therapeutics to COVID-19 vaccines. Small Methods 2021; 5: 2100402, https://onlinelibrary.wiley.com/doi/10.1002/smtd.202100402 (accessed 22 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Centers for Disease Control and Prevention. Understanding viral vector COVID-19 vaccines, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html (accessed 8 April 2021).
- 59. Centers for Disease Control and Prevention. Johnson & Johnson’s Janssen COVID-19 vaccine overview and safety, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html (accessed 8 April 2021).
- 60. Ghosh T. Some U.S. areas stop using J&J COVID-19 vaccine after “adverse reaction” reports. Global News, 8 April 2021, https://globalnews.ca/news/7746651/johnson-and-johnson-adverse-reaction-covid-19/amp/ (accessed 8 April 2021).
- 61. Marks P. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine. U.S. Food & Drug Administration, 2021, https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine (accessed 13 April 2021).
- 62. World Health Organization. Interim recommendations for use of the AZD1222 (ChAdOx1-S (recombinant)) vaccine against COVID-19 developed by Oxford University and AstraZeneca, 2021, https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1 (accessed 13 April 2021).
- 63. Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384: 2092–2101, https://www.nejm.org/doi/full/10.1056/NEJMoa2104840 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384: 2124–2130, https://www.nejm.org/doi/full/10.1056/NEJMoa2104882 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021; 397: 671–681, https://pubmed.ncbi.nlm.nih.gov/33545094/ (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ikegame S, Siddiquey MNA, Hung CT, et al. Qualitatively distinct modes of Sputnik V vaccine-neutralization escape by SARS-CoV-2 spike variants. medRxiv 2021, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8020991/ (accessed 13 April 2021).
- 67. Ampel NM. The Sputnik V COVID-19 vaccine: promising interim results published. NEJM Journal Watch, 5 February 2021, https://www.jwatch.org/na53184/2021/02/05/sputnik-v-covid-19-vaccine-promising-interim-results (accessed 13 April 2021).
- 68. Jones I, Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021; 397: 642–643, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7906719/ (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Costamed Medical Group. Convidicea (Ad5-nCoV) from CanSino Biologics Inc. COVID-19 vaccines, 2021, https://www.costamed.com.mx/en/blog/post/convidicea-ad5-ncov-from-cansino-biologics-inc-covid-19-vaccines (accessed 13 April 2021).
- 70. Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J 2021; 14: 100517, https://www.sciencedirect.com/science/article/pii/S1939455121000119 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. World Health Organization. Coronavirus disease (COVID-19): vaccines, 2021, https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines (accessed 15 April 2021). [PubMed]
- 72. Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med 2021; 384: 643–649, https://www.nejm.org/doi/full/10.1056/nejmra2035343 (accessed 11 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Soucheray S. New hot spots emerge as states urge COVID-19 vaccination. Center for Infectious Disease Research and Policy, 2021, https://www.cidrap.umn.edu/news-perspective/2021/04/new-hot-spots-emerge-states-urge-covid-19-vaccination (accessed 3 April 2021).
- 74. Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer–BioNTech and Moderna vaccines against COVID–19 among hospitalized adults aged >65 years – United States, January-March 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 674–679, https://www.cdc.gov/mmwr/volumes/70/wr/mm7018e1.htm (accessed 3 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA 2021; 325: 2201–2202, https://jamanetwork.com/journals/jama/fullarticle/2778441 (accessed 14 April 2021). [DOI] [PubMed] [Google Scholar]
- 76. Merchant H. Increase in acute phase protein may have caused thrombocytopenia even in COVID-19. BMJ 2021; 373, https://www.bmj.com/content/373/bmj.n883/rapid-responses (accessed 14 April 2021). [Google Scholar]
- 77. Dyer O. COVID-19: EMA defends AstraZeneca vaccine as Germany and Canada halt rollouts. BMJ 2021; 373: n883, https://www.bmj.com/content/373/bmj.n883 (accessed 14 April 2021). [DOI] [PubMed] [Google Scholar]
- 78. Fisher J, Linder A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J Intern Med 2017; 281: 562–574, https://pubmed.ncbi.nlm.nih.gov/28370601/ (accessed 14 April 2021). [DOI] [PubMed] [Google Scholar]
- 79. Taquet M, Husain M, Geddes JR, et al. Cerebral venous thrombosis: a retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,971 people receiving a COVID-19 mRNA vaccine. OSF, 2021, https://osf.io/a9jdq/ (accessed 17 April 2021).
- 80. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020; 396: 1595–1606, https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(20)32137-1.pdf (accessed 14 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Harris RJ, Hall JA, Zaidi A, et al. Impact of vaccination on household transmission of SARS-CoV-2 in England. Public Health England, 2021, https://static.poder360.com.br/2021/04/public-health-1stdose-abr2021.pdf (accessed 3 April 2021). [DOI] [PMC free article] [PubMed]
- 82. Corum J, Zimmer C. Coronavirus variants and mutations. The New York Times, 5 March 2021, https://www.nytimes.com/interactive/2021/health/coronavirus-variant-tracker.html (accessed 15 April 2021).
- 83. NTK Institute. T cells recognise recent SARS-CoV-2 variants, 2021, https://ntk-institute.org/article/t-cells-recognise-recent-sars-cov-2-variants (accessed 14 April 2021).
- 84. Centers for Disease Control and Prevention. Benefits of getting a COVID-19 vaccine, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html (accessed 16 April 2021).
- 85. Cavanaugh AM, Spicer KB, Thoroughman D, et al. Reduced risk of reinfection with SARS–CoV–2 after COVID–19 vaccination – Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1081–1083, https://www.cdc.gov/mmwr/volumes/70/wr/mm7032e1.htm (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. World Health Organization. COVID-19 advice for the public: getting vaccinated, 2021, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed 15 April 2021).
- 87. Government of Canada. Vaccines and treatments for COVID-19: progress, 2021, https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/prevention-risks/covid-19-vaccine-treatment.html (accessed 15 April 2021).
- 88. Centers for Disease Control and Prevention. COVID-19: vaccines for COVID-19, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html (accessed 16 April 2021). [PubMed]
- 89. Pujari R, Thommana MV, Mercedes BR, et al. Therapeutic options for COVID-19: a review. Cureus 2020; 12: e10480, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7496561/ (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rastogi M, Pandey N, Shukla A, et al. SARS coronavirus 2: from genome to infectome. Respir Res 2020; 21: 318, https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-020-01581-z (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yousefi B, Valizadeh S, Ghaffari H, et al. A global treatments for coronaviruses including COVID-19. J Cell Physiol 2020; 235: 9133–9142, https://onlinelibrary.wiley.com/doi/10.1002/jcp.29785 (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dittmar M, Lee JS, Whig K, et al. Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-CoV-2. Cell Rep 2021; 35: 108959, https://www.cell.com/cell-reports/fulltext/S2211-1247(21)00273-4?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124721002734%3Fshowall%3Dtrue (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open 2021; 4: e210369, https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2776305 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chourasia M, Koppula PR, Battu A, et al. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules 2021; 26: 1200, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7956763/ (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chilamakuri R, Agarwal S. COVID-19: characteristics and therapeutics. Cells 2021; 10: 206, https://www.mdpi.com/2073-4409/10/2/206 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mishra P, Rizwana P, Agarwal NB. Role of vitamin D in risk reduction of COVID-19: a narrative review. Ann Natl Acad Med Sci 2021; 57: 36–40, https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0041-1724460 (accessed 13 April 2021). [Google Scholar]
- 97. Leaf DE, Ginde AA. Vitamin D3 to treat COVID-19: different disease, same answer. JAMA 2021; 325: 1047–1048, https://jamanetwork.com/journals/jama/fullarticle/2776736 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang X, Zhang Y, Fang F. Role of vitamin D in COVID-19 infections and deaths. J Evid Based Med 2021; 14: 5–6, https://onlinelibrary.wiley.com/doi/10.1111/jebm.12421 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ahmed U, Ashfaq UA, Khaliq S, et al. Current trends and possible therapeutic options against COVID-19. J Microbiol Infect Dis 2020; 10: 110–120, https://dergipark.org.tr/en/pub/jmid/issue/56321/790198 (accessed 13 April 2021). [Google Scholar]
- 100. Clark SA, Clark LE, Pan J, et al. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell 2021; 184: P2605–P2617.E18, https://www.cell.com/cell/fulltext/S0092-8674(21)00355-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS009286742100355X%3Fshowall%3Dtrue (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Maideen NMP. Recent updates in the pharmacological management of COVID-19. Lett Appl Nanobiosci 2021; 10: 1969–1980, https://nanobioletters.com/wp-content/uploads/2020/10/22846808101.19691980.pdf (accessed 13 April 2021). [Google Scholar]
- 102. U.S. Food & Drug Administration. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems, 2020, https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or (accessed 22 March 2022).
- 103. U.S. Food & Drug Administration. COVID-19 update: FDA broadens emergency use authorization for Veklury (remdesivir) to include all hospitalized patients for treatment of COVID-19, 2020, https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized (accessed 13 April 2021).
- 104. Kuritzkes D. Clinical outcomes and therapeutics of COVID-19. Virology: COVID-19 Online Educational Program, 2021, https://academicmedicaleducation.com/meeting/covid-19-online-educational-program-2020/video/clinical-outcomes-and-therapeutics-covid-19 (accessed 13 April 2021).
- 105. Islam S. COVID-19 – Present treatments and other options. Lancet. Epub ahead of print November 2020, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3713294 (accessed 13 April 2021).
- 106. Kandimalla R, John A, Abburi C, et al. Current status of multiple drug molecules, and vaccines: an update in SARS-CoV-2 therapeutics. Mol Neurobiol 2020; 57: 4106–4116, https://link.springer.com/article/10.1007%2Fs12035-020-02022-0 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Elliott W, Chan J. Nirmatrelvir and ritonavir tablets (Paxlovid). Int Med Alert 2022; 44: 1, https://www.proquest.com/openview/4ddefdf54fb1b4835865a2ad70dab891/1?pq-origsite=gscholar&cbl=136155 (accessed 7 January 2022). [Google Scholar]
- 108. Bernal AJ, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386: 509–520, https://www.nejm.org/doi/full/10.1056/NEJMoa2116044 (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. U.S. Food & Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19, 2021, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 (accessed 7 January 2022).
- 110. U.S. Food & Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19, 2021, https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19 (accessed 13 April 2021).
- 111. Kory R, Meduri GU, Varon J, et al. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther 2021; 28: E299–E318, https://journals.lww.com/americantherapeutics/fulltext/2021/06000/review_of_the_emerging_evidence_demonstrating_the.4.aspx (accessed 7 January 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 2021; 325: 1426–1435, https://jamanetwork.com/journals/jama/fullarticle/2777389 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178: 104787, https://www.sciencedirect.com/science/article/pii/S0166354220302011?via%3Dihub (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Babalola OE, Bode CO, Ajayi AA, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomised controlled double-blind, dose-response study in Lagos. QJM 2021; 114: 780–788, https://academic.oup.com/qjmed/article/114/11/780/6143037 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 2021; 9: 498–510, https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30566-X/fulltext (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Siddique R, Bai Q, Shereen MA, et al. Evidence and speculations: vaccines and therapeutic options for COVID-19 pandemic. Hum Vaccines Immunother 2020; 17: 1113–1121, https://www.tandfonline.com/doi/full/10.1080/21645515.2020.1824497 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384: 1491–1502, https://www.nejm.org/doi/full/10.1056/NEJMoa2100433 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med 2021; 384: 1503–1516, https://www.nejm.org/doi/full/10.1056/NEJMoa2028700 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med 2021; 384: 238–251, https://www.nejm.org/doi/10.1056/NEJMoa2035002?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ramakrishnan S, Nicolau DV, Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 illness: a randomised controlled trial. medRxiv 2021, https://www.medrxiv.org/content/10.1101/2021.02.04.21251134v1 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed]
- 121. Singh VK, Mishra A, Singh S, et al. Emerging prevention and treatment strategies to control COVID-19. Pathogens 2020; 9: 501, https://www.mdpi.com/2076-0817/9/6/501 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation. Res Sq. Epub ahead of print June 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7336704/ (accessed 13 April 2021).
- 123. Rabbani AB, Parikh RV, Rafique AM. Colchicine for the treatment of myocardial injury in patients with coronavirus disease 2019 (COVID-19)—an old drug with new life? JAMA Netw Open 2020; 3: e2013556, https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2767588 (accessed 13 April 2021). [DOI] [PubMed] [Google Scholar]
- 124. Nasiripour S, Zamani F, Farasatinasab M. Can colchicine as an old anti-inflammatory agent be effective in COVID-19? J Clin Pharmacol 2020; 60: 828–829, https://accp1.onlinelibrary.wiley.com/doi/full/10.1002/jcph.1645 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Parra-Medina R, Sarmiento-Monroy JC, Rojas-Villarraga A, et al. Colchicine as a possible therapeutic option in COVID-19 infection. Clin Rheumatol 2020; 39: 2485–2486, https://link.springer.com/article/10.1007/s10067-020-05247-5 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Piantoni S, Patroni A, Toniati P, et al. Why not use colchicine in COVID-19? An old anti-inflammatory drug for a novel auto-inflammatory disease. Rheumatology 2020; 59: 1769–1770, https://academic.oup.com/rheumatology/article/59/7/1769/5848955 (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lotfi M, Rezaei N. CRISPR/Cas13: a potential therapeutic option of COVID-19. Biomed Pharmacother 2020; 131: 110738, https://pubmed.ncbi.nlm.nih.gov/33152914/ (accessed 13 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. U.S. Food & Drug Administration. Recommendations for investigational COVID-19 convalescent plasma, 2021, https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-applications-inds-cber-regulated-products/recommendations-investigational-covid-19-convalescent-plasma (accessed 13 April 2021).
- 129. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America, 2021, https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed 14 April 2021). [DOI] [PMC free article] [PubMed]
- 130. Zhou H, Dcosta BM, Samanovic MI, et al. B.1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. bioRXiv 2021, https://www.biorxiv.org/content/10.1101/2021.03.24.436620v1 (accessed 3 April 2021). [DOI] [PMC free article] [PubMed]
- 131. Burckhardt RM, Dennehy JJ, Poon LLM, et al. Are COVID-19 vaccine boosters needed? The science behind boosters. J Virol 2022; 96: e0197321, https://pubmed.ncbi.nlm.nih.gov/34817198/ (accessed 23 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sanyaolu A, Marinkovic A, Prakash S, et al. Reactogenicity to COVID-19 vaccination in the United States of America. Clin Exp Vaccine Res 2022; 11: 104–115, https://pubmed.ncbi.nlm.nih.gov/35223671/ (accessed 23 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kumar US, Afjei R, Ferrara K, et al. Gold-nanostar-chitosan-mediated delivery of SARS-CoV-2 DNA vaccine for respiratory mucosal immunization: development and proof-of-principle. ACS Nano 2021; 15: 17582–17601, https://pubs.acs.org/doi/10.1021/acsnano.1c05002 (accessed 22 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mao T, Israelow B, Suberi A, et al. Unadjuvanted intranasal spike vaccine booster elicits robust protective mucosal immunity against sarbecoviruses. bioRxiv 2022, https://www.biorxiv.org/content/10.1101/2022.01.24.477597v1 (accessed 22 March 2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tav-10.1177_25151355221097559 for Current advancements and future prospects of COVID-19 vaccines and therapeutics: a narrative review by Adekunle Sanyaolu, Chuku Okorie, Aleksandra Marinkovic, Stephanie Prakash, Martina Williams, Nafees Haider, Jasmine Mangat, Zaheeda Hosein, Vyshnavy Balendra, Abu Fahad Abbasi, Priyank Desai, Isha Jain, Stephen Utulor and Amos Abioye in Therapeutic Advances in Vaccines and Immunotherapy


