Abstract
Objectives
The aim of this study was to determine the frequency and severity of adverse events (AEs) reported from use of an adjuvanted whole-cell autologous cancer vaccine in cats with solid tumors under field conditions.
Methods
The case accession database at Torigen Pharmaceuticals was searched to identify client-owned cats that underwent biopsy or surgical resection of their primary tumor, had histologic confirmation of neoplasia and received at least one subcutaneous dose of an adjuvanted whole-cell autologous cancer vaccine. Records were reviewed for any reported AEs.
Results
In total, 117 cats met the inclusion criteria and received 422 doses of autologous cancer vaccine. Six (5.1%) cats had seven reported AEs, with the majority of these (85.7%) being characterized as grade 1 or 2 (mild) and resolving without medical intervention.
Conclusions and relevance
AEs were infrequent in cats treated with an adjuvanted whole-cell autologous cancer vaccine under typical field use conditions. This form of active cancer immunotherapy appears to be well tolerated by cats and may represent a treatment option for owners who are concerned about AEs associated with chemotherapy or radiotherapy. Additional studies are warranted to determine the efficacy of this form of individualized immunotherapy in cats with solid tumors.
Keywords: Autologous cancer vaccine, cancer, cancer immunotherapy, safety
Introduction
Since the 1960s there has been a keen interest in how to direct a patient’s immune system to fight cancer. Autologous vaccines represent one of the earliest forms of immunotherapy studied, beginning with a rabbit model of viral-induced carcinoma. 1 Autologous cancer vaccines (ACVs) represent a form of active immunotherapy in which antigenic material is derived from the patient’s own tumor. 2 After ex vivo processing, tumor cells are returned to the patient with the goal of stimulating an immune response against multiple tumor antigens unique to the individual. A successful immune response involves cytotoxic T cells capable of recognizing tumor-associated antigens (TAAs) and tumor-specific antigens that may be abundantly expressed or unique to the patient. 3 Various methods of tumor processing have been used to create ACVs, including whole-cell vaccines, tumor-cell lysates, isolation of specific intracellular components (eg, heat shock proteins [HSPs]) and transfection of cells to induce novel antigen presentation. 4 Before being administered to the patient, the cancer cells are inactivated using chemical treatment, cell lysis or irradiation.5–7 Subcutaneous (SC), intramuscular (IM), intraperitoneal and intradermal routes of administration of ACVs have been described in dogs.5,6,8,9 To date, ACVs have not been evaluated in cats.
As early as 1964 it was recognized that ACVs were capable of inducing a humoral immune response in human patients with terminal cancer. 10 The method of tumor processing was also discovered to affect the TAAs available for presentation to the immune system, and various strategies were studied to chemically or enzymatically dissociate tumor cells to try and preserve the most TAAs. 11 Early development of ACVs was slow given the rudimentary understanding of the immune system, as well as the paucity of available reagents and instrumentation. Through the late twentieth century, studies of ACVs were conducted in people with melanoma, colorectal carcinoma, head and neck squamous cell carcinoma (SCC), non-small-cell lung cancer and renal cell carcinoma, among others.12–18 The interest in ACVs has continued over the past 50 years in human oncology, with multiple ongoing human clinical trials describing some form of ACV listed on the ClinicalTrials.gov website. 19
The development of ACVs in veterinary oncology has progressed more slowly than in human medicine. This is owing, in part, to the lack of validated reagents for studying the immune system in companion animals. As a result, there are only a handful of canine studies describing ACVs in the veterinary literature, including vaccines created from irradiated cancer cells transfected with human granulocyte–macrophage colony-stimulating factor, cancer cells transfected with the gene encoding Emm55, a Streptococcus pyogenes serotyping antigen, a whole-cell vaccine prepared using enzymatic cell dissociation and an autologous vaccine consisting of HSPs isolated from the patient’s tumor with a hydroxylapatite ceramic powder adjuvant.9,20–22
Similarly, there are limited published safety data regarding ACVs in veterinary medicine. There were no significant adverse events (AEs) described in the studies discussed above.9,20–22 Additional evidence that this form of immunotherapy is safe for dogs comes from two recently published studies. The first report described 150 IM injections of an adjuvanted autologous tumor lysate preparation to a group of 28 cancer-bearing dogs. 6 No significant AEs were reported. The second report described the AEs in 93 cancer-bearing dogs treated with an adjuvanted whole-cell ACV, which is the same ACV used in the present feline study. 5 Approximately 10% of the treated dogs developed mild AEs after vaccine administration. All of the described AEs were characterized as grade 1 (mild) on the Veterinary Comparative Oncology Group – Common Terminology Criteria for Adverse Events (VCOG-CTCAE) scale, 23 and included redness or discomfort at the injection site, mild lethargy, transient decrease in appetite or low-grade fever. None of the described AEs required medical intervention and all resolved spontaneously.
To date, there have been no published reports of the use of active cancer immunotherapy with an ACV in cats. The purpose of this study was to determine the frequency and severity of AEs in cats treated with an adjuvanted whole-cell ACV that has been previously evaluated in dogs. 5
Materials and methods
Autologous cancer vaccine protocol
After surgical excision, unfixed tumor tissue was placed into an empty, sterile container and shipped overnight on cold packs to the commercial laboratory (Torigen Pharmaceuticals, Farmington, CT, USA) for preparation of the ACV. The vaccine preparation has been described elsewhere. 5 Briefly, the tumor tissue was mechanically dissociated into a uniform cell suspension, cells were inactivated with 2.5% glutaraldehyde and eventually combined with a protein matrix immuno-modulator adjuvant (MIM-SIS; Cook Biotech). The final vaccine product was placed into a sterile vial and shipped overnight on cold packs to the submitting veterinarian for administration to the cat. Veterinarians were instructed to give 1 ml of the vaccine SC once every 7 days for three total doses. The attending veterinarian was further instructed to monitor the cat for acute AEs for 30 mins after each of the three injections. At hospital discharge, cat owners were also informed of possible vaccine reactions and instructed to report any observed abnormalities immediately upon their occurrence. Written owner informed consent was obtained before the vaccine was produced and administered as required by the United States Department of Agriculture for unlicensed biologics.
Case selection
The case accession database at Torigen Pharmaceuticals was queried to identify cats treated with the adjuvanted whole-cell ACV between November 2015 and November 2020. Cats were eligible for inclusion in this study if they had a histopathologic or cytologic diagnosis of cancer and received at least one dose of the vaccine. Cats were excluded from study if they had a histologic or cytologic diagnosis of a non-cancerous process, did not receive at least one dose of the adjuvanted whole-cell ACV, received adjuvant chemotherapy or radiation therapy concurrent with ACV treatment or had incomplete case information. Histopathologic and cytologic diagnoses were reported by board-certified veterinary pathologists via commercial laboratory services. Patient data collected included signalment, body weight, histology or cytology results and reported AEs following ACV administration. Details on AEs were collected from telephone or email contact with the cat owners and submitting veterinarians, or through a survey tool sent at regular intervals to each submitting veterinarian. Follow-up information for each cat with a reported AE was obtained through direct communication with the submitting veterinarian and medical records were requested for affected cats. An AE was considered to be any observation in treated cats that was unfavorable, unintended and occurred after the use of the investigational veterinary product, whether or not it was considered to be product-related. 24 AEs were classified based on the VCOG-CTCAE. 23
Summary statistics were generated using commercial software (XLSTAT Life Science 2020; Addinsoft). Results are reported as mean ± SD unless otherwise noted.
Informed consent
Informed written consent was obtained from the owner or legal custodian of all animals described in this work for the procedures undertaken regarding the clinical use of the investigational, commercially available, autologous cancer vaccine as required by the United States Department of Agriculture (USDA) for unlicensed veterinary biologics.
Results
Study population
There were 193 feline cases identified in the database. Of these cats, 61 (31.6%) did not receive any vaccine dose as a result of the owners or attending veterinarians opting for other treatment options, a non-cancerous diagnosis or the death of the patient before the vaccine could be administered. One of these patients that was coded as a Bengal cat was actually a Bengal tiger and therefore excluded from study. An additional 15 (7.8%) cats that received at least one dose of the vaccine were excluded from the study owing to concurrent use of chemotherapy (n = 10), no pathology report provided by the attending veterinarian (n = 4) or non-cancerous process (n = 1).
During the 5-year study period, 438 doses of the adjuvanted whole-cell ACV were administered to 117 cats that met the inclusion criteria. The mean number of vaccine doses administered per cat was 3.7 ± 3.29 (median 3.0; range 1–36). Eighteen (15.4%) cats were treated with >1 course (ie, more than 3 doses) of the adjuvanted whole-cell ACV during the study period, owing to cancer recurrence or the development of a new malignancy. Sixteen cats received six doses of the adjuvanted whole-cell ACV, one cat received 12 doses and one cat received 36 doses over the course of 4 years during the study period. The male:female ratio was approximately 1:1, and the majority (71.8%) of cats were described as domestic shorthairs. Summary data are presented in Table 1.
Table 1.
Summary data from 117 cancer-bearing cats treated with surgery and an adjuvanted whole-cell autologous cancer vaccine (ACV)
| Variable | |
|---|---|
| Age (years) | 11.1 ± 3.33 |
| Range | 3.0–19.9 |
| Weight (kg) | 5.1 ± 1.70 |
| Range | 1.8–13.4 |
| Sex | |
| Male | 58 (49.6) |
| Female | 59 (50.4) |
| Reproductive status | |
| Neutered | 114 (97.4) |
| Intact | 3 (2.6) |
| Breed | |
| Domestic shorthair | 84 (71.8) |
| Domestic longhair | 13 (11.1) |
| Maine Coon | 4 (3.4) |
| Other breeds | 16 (13.7) |
| Cancer origin | |
| Epithelial | 48 (41.0) |
| Mesenchymal | 59 (50.4) |
| Round cell | 10 (8.5) |
| Doses of Torigen ACV administered | 438 |
| Doses per cat | 3.7 ± 3.29 |
| Range | 1–36 |
| AEs | 7 |
| Affected cats | 6 (5.1) |
| Doses associated with AEs | 6 (1.4) |
| Doses associated with serious AEs | 1 (0.2) |
Data are n (%) or mean ± SD
AEs = adverse events
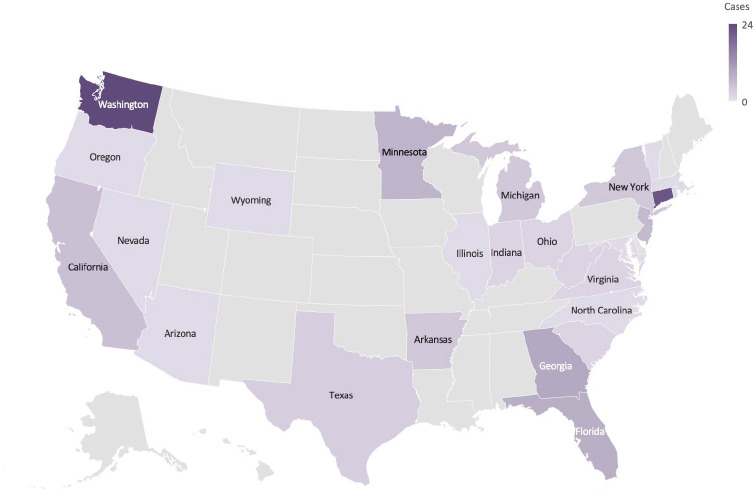
Tumor specimens were submitted from cats in 26 different states, with the largest proportion of cases coming from Connecticut and Washington (Figure 1). General practitioners submitted 76 (65.0%) cases, and 41 (35.0%) cases were submitted by specialists (surgeons and medical oncologists). There were 48 (41.0%) epithelial tumors, 59 (50.4%) mesenchymal tumors and 10 (8.5%) discrete round-cell tumors. The most commonly submitted epithelial tumors were mammary gland carcinomas (n = 19 [39.6%]) and SCCs (n = 7 [14.6%]). Soft tissue sarcomas (n = 53 [86.9%]), of which 18 were described as injection-site sarcomas, represented the most commonly submitted mesenchymal tumor. Lymphoma was the most frequent round-cell tumor submission (n = 5 [50%]).
Figure 1.
Geographic distribution of 117 cats treated with an adjuvanted whole-cell autologous cancer between November 2015 and November 2020
AEs
There were seven AEs reported in six (5.1%) cats, which were associated with six (1.4%) doses of the novel adjuvanted whole-cell ACV. The most common AE was lethargy, reported in four cats. Of the reported AEs, six (85.7%) were characterized as mild or moderate (grade 1 or 2), and there was only one severe (grade 5) AE reported. AEs were reported after the first adjuvanted whole-cell ACV dose in three (42.9%) cats, after the second dose in two (28.6%) cats and after the third dose in one (14.3%) cat. One cat with an oral SCC had two AEs (lethargy and anorexia) reported after the second dose of the vaccine; there were no reported AEs with the first or third doses, nor with three additional doses given 2 months after the initial series owing to local tumor progression. The remaining 15 cats treated with more than three doses of the adjuvanted whole-cell ACV had no reported AEs. Regarding the attribution of reported AEs to the adjuvanted whole-cell ACV, one (14.3%) was classified as unrelated, two (28.6%) were classified as unlikely, two (28.6%) were classified as possible and two (28.6%) were classified as probable.
The AE that was classified as unrelated, which was also the only severe AE, was reported in a cat with metastatic pulmonary carcinoma. The cat died at home 3 days after the first dose of the vaccine. Necropsy revealed pleural effusion, carcinomatosis and effacement of pulmonary tissue by neoplastic cell, making cancer the probable cause of death. For the two AEs considered unlikely to be related to the novel adjuvanted whole-cell ACV, the first was fever following the first dose of vaccine in a cat with laryngeal lymphoma. The cat owner was keeping the cat on an electric heating pad at the time the fever was reported, and the cat also suffered second-degree burns on the ventral body wall. The elevated body temperature was thought to be a result of exposure to the heating pad. The second AE unlikely to be related to the vaccine was lethargy, which was reported after the third dose in a cat with mammary carcinoma. An infection was concurrently diagnosed at the mastectomy incision site, which was likely the cause of the observed lethargy.
The two AEs that were classified as possibly related to the vaccine were reported in the cat with oral SCC described above. The two AEs that were classified as probable were reported in a cat with osteosarcoma shortly after the second vaccine dose, and in a cat with an injection-site swelling several hours after the first dose.
Discussion
For consistency with previously published safety and efficacy data in dogs treated with this ACV,5,25 cats described herein were also given a series of three SC injections spaced 1 week apart. The most effective interval for ACV administration remains unknown. Rodent data on the effect of dosing interval on efficacy are limited; studies that are available often do not detail which intervals are best to produce an immune response. A recent study in mice revealed that three doses of an autologous vaccine generated a superior immune response and better tumor control, compared with mice treated with either one or five doses. 26 Given the heterogeneity of ACV products, and a lack of conclusive mouse model data, it is difficult to extrapolate mouse model results to an effective dosing scheme in feline patients. In a study of an ACV given IM to dogs, no significant difference in AE rates was found between dogs receiving four weekly administrations and a group treated with four doses given at 4-week intervals. 6 Presently, there is no consensus on the optimal dosing interval for ACVs in humans, in part, owing to a comparative lack of data on clinically successful autologous vaccines relative to infectious disease vaccines. 27
The population of cats described herein was geographically diverse, and the observed middle age of the affected cats was similar to previous reports.28,29 The predominance of mesenchymal tumors within this population is different than the majority of epithelial tumors described in a review of the Swiss Feline Cancer Registry. 29 This may reflect the smaller population size herein, or be a result of the different genetic make-up of cats in the USA vs Switzerland. This could also represent case selection bias by the submitting veterinarians, if cat owners opt out of postoperative radiation therapy for managing soft tissue sarcomas; round-cell tumors and carcinomas have a wider array of available adjuvant treatment options. Likewise, a higher risk of female cats developing cancer was observed in the Swiss Feline Cancer Registry; 28 that finding was not replicated here. Unfortunately, there are no contemporary feline cancer registries in the USA to allow for comparison of breed distribution.The cancer-bearing cats treated with immunotherapy in this study all tolerated the novel adjuvanted whole-cell ACV well, with a few, mild AEs described. The AE rate in the group of cats described herein was even lower than previously reported in 93 dogs (11.8%), 5 as well as a group of 28 horses (14.3% [MD Lucroy, unpublished data]) treated with the same ACV.Given the retrospective nature of this study, and the potential for veterinarians or cat owners to not report observed AEs, it is possible that the observed AE rate could be lower than the actual rate. However, ACVs detailed in the human literature have a consistently reported low rate of AEs. In a human study of an ACV modified genetically with tag7/PGRP-S, none of the patients demonstrated any clinical significant signs of toxicity. 30 Additional studies of ACVs in humans with solid tumors have reported a similar lack of AEs or clinically significant events, with the worst AEs being infrequent and limited to grade 1/2 AEs.31,32 Moreover, the adjuvant (MIM-SIS) used in the ACV studied here is a non-irritating adjuvant that has a very low incidence of AEs, while also being capable of producing a robust immune response against co-administered antigens.33,34 Based on the reported safety of autologous cancer vaccines among various species, and the properties of MIM-SIS, the low AE rate reported in this population of cats is likely representative of what could be expected with more widespread use.
Only one of the 18 cats that received more than three doses of the novel adjuvanted whole-cell ACV during the study period was reported to have an AE. This was an episode of mild lethargy following the second vaccine dose. No AEs were reported following the other five doses. Sixteen cats received six doses of the novel adjuvanted whole-cell ACV, one cat received 12 doses and one cat received 36 doses during the 5-year study period, demonstrating that repeated exposure over time does not appear to increase the risk of an AE.
Although chemotherapy is a commonly used adjuvant treatment after cancer surgery, many cat owners are concerned about AEs associated with chemotherapy. A study of pet owners revealed that 58% would not pursue chemotherapy citing concerns about the risk for AEs. 35 This is not an unfounded fear. Neutropenia is most commonly the dose-limiting AE, and vomiting and diarrhea are commonly reported, with cats being particularly sensitive to diminished appetite and weight loss. 36 In a population of 70 cats treated with doxorubicin, alone or as part of a combination chemotherapy protocol, 34% had at least one episode of neutropenia, 44% were anemic and 29% developed acute kidney injury. 37 Appetite decrease, vomiting and diarrhea were also reported in 13%, 16% and 7% of instances, respectively, where recorded. Similarly, a retrospective study of cats with mast cell cancers treated with oral toceranib phosphate (Palladia; Zoetis) had a reported AE rate of 60%, including gastrointestinal and hematologic events, which necessitated a break in treatment. 38 Therefore, the adjuvanted whole-cell ACV used in the present study may represent an appealing option for cat owners concerned about potential risks associated with chemotherapy. Until efficacy data regarding this ACV are published, it will be impossible for veterinarians and cat owners to assess the risk:benefit ratio when making treatment decisions.
The strengths of the study include the large number of cats described herein that received >400 doses of the ACV, and that the reported observations represent the experience from typical use in the field. These results are valuable for veterinarians when discussing cancer treatments with cat owners. Including owner-reported AEs provides additional information not readily available from a medical record review. In human medicine, patient reports of AEs have been shown to be a credible source of information for care-related AEs. 39 A limitation of this study is the retrospective nature of case reviews.
Given the low rate of AEs described in this population of 117 cats under field conditions, and the previous report describing a low rate of AEs in a population of 93 similarly treated dogs at a single surgery practice, 5 this adjuvanted whole-cell ACV appears to be a comparatively safe cancer treatment. Further studies are warranted to determine the efficacy of this form of active immunotherapy in cancer-bearing cats.
Conclusions
AEs were infrequent in the population of cats treated with an adjuvanted whole-cell ACV. This form of active cancer immunotherapy appears to be well tolerated by cats and may represent an alternative treatment for owners who are concerned about AEs associated with chemotherapy or radiotherapy. Additional studies are warranted to determine the efficacy of this form of individualized immunotherapy in cats with solid tumors.
Footnotes
Accepted: 17 June 2021
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals) and data from prospective or retrospective studies. Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not required for publication in JFMS.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals), for the procedure(s) undertaken (prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Michael D Lucroy  https://orcid.org/0000-0002-1166-4991
https://orcid.org/0000-0002-1166-4991
References
- 1. Evans CA, Gorman LR, Ito Y, et al. Antitumor immunity in the Shope papilloma-carcinoma complex of rabbits. I. Papilloma regression induced by homologous and autologous tissue vaccines. J Natl Cancer Inst 1962; 29: 277–285. [PubMed] [Google Scholar]
- 2. Emens LA. A new twist on autologous cancer vaccines. Cancer Biol Ther 2003; 2: 161–163. [DOI] [PubMed] [Google Scholar]
- 3. Morse MA, Gwin WR, 3rd, Mitchell DA. Vaccine therapies for cancer: then and now. Target Oncol 2021; 16: 121–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas S, Prendergast GC. Cancer vaccines: a brief overview. Methods Mol Biol 2016; 1403: 755–761. [DOI] [PubMed] [Google Scholar]
- 5. Crossley RA, Matz A, Dew T, et al. Safety evaluation of autologous tissue vaccine cancer immunotherapy in a canine model. Anticancer Res 2019; 39: 1699–1703. [DOI] [PubMed] [Google Scholar]
- 6. Weir C, Oksa A, Millar J, et al. The safety of an adjuvanted autologous cancer vaccine platform in canine cancer patients. Vet Sci 2018; 5: 87. DOI: 10.3390/vetsci5040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curry WT, Jr, Gorrepati R, Piesche M, et al. Vaccination with irradiated autologous tumor cells mixed with irradiated GM-K562 cells stimulates antitumor immunity and T lymphocyte activation in patients with recurrent malignant glioma. Clin Cancer Res 2016; 22: 2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konduri V, Halpert MM, Baig YC, et al. Dendritic cell vaccination plus low-dose doxorubicin for the treatment of spontaneous canine hemangiosarcoma. Cancer Gene Ther 2019; 26: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yannelli JR, Wouda R, Masterson TJ, et al. Development of an autologous canine cancer vaccine system for resectable malignant tumors in dogs. Vet Immunol Immunopathol 2016; 182: 95–100. [DOI] [PubMed] [Google Scholar]
- 10. Aswaq M, Richards V, McFadden S. Immunologic response to autologous cancer vaccine. Arch Surg 1964; 89: 485–487. [DOI] [PubMed] [Google Scholar]
- 11. Peters LC, Brandhorst JS, Hanna MG., Jr. Preparation of immunotherapeutic autologous tumor cell vaccines from solid tumors. Cancer Res 1979; 39: 1353–1360. [PubMed] [Google Scholar]
- 12. Berd D, Maguire HC, Jr, McCue P, et al. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol 1990; 8: 1858–1867. [DOI] [PubMed] [Google Scholar]
- 13. Berd D, Murphy G, Maguire HC, Jr, et al. Immunization with haptenized, autologous tumor cells induces inflammation of human melanoma metastases. Cancer Res 1991; 51: 2731–2734. [PubMed] [Google Scholar]
- 14. de Weger VA, Turksma AW, Voorham QJ, et al. Clinical effects of adjuvant active specific immunotherapy differ between patients with microsatellite-stable and microsatellite-instable colon cancer. Clin Cancer Res 2012; 18: 882–889. [DOI] [PubMed] [Google Scholar]
- 15. Karcher J, Dyckhoff G, Beckhove P, et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res 2004; 64: 8057–8061. [DOI] [PubMed] [Google Scholar]
- 16. Perroud MW, Jr, Honma HN, Barbeiro AS, et al. Mature autologous dendritic cell vaccines in advanced non-small cell lung cancer: a phase I pilot study. J Exp Clin Cancer Res 2011; 30: 65. DOI: 10.1186/1756-9966-30-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirschowitz EA, Foody T, Kryscio R, et al. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol 2004; 22: 2808–2815. [DOI] [PubMed] [Google Scholar]
- 18. Tani K, Azuma M, Nakazaki Y, et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol Ther 2004; 10: 799–816. [DOI] [PubMed] [Google Scholar]
- 19. National Institutes of Health. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/results?cond=autologous+cancer+vaccine&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&recrs=m&age_v=&gndr=&type=&rslt= (2020, accessed Apr 7, 2020). [Google Scholar]
- 20. Hogge GS, Burkholder JK, Culp J, et al. Development of human granulocyte-macrophage colony-stimulating factor-transfected tumor cell vaccines for the treatment of spontaneous canine cancer. Hum Gene Ther 1998; 9: 1851–1861. [DOI] [PubMed] [Google Scholar]
- 21. Lawman M, Eidizadeh S, Selmon C, et al. Anti-tumor response induced by autologous cancer vaccine in canine lymphoma. Cancer Ther 2008; 6: 827–840. [Google Scholar]
- 22. Marconato L, Frayssinet P, Rouquet N, et al. Randomized, placebo-controlled, double-blinded chemoimmunotherapy clinical trial in a pet dog model of diffuse large B-cell lymphoma. Clin Cancer Res 2014; 20: 668–677. [DOI] [PubMed] [Google Scholar]
- 23. Veterinary Cooperative Oncology Group. Veterinary Cooperative Oncology Group – common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016; 14: 417–446. [DOI] [PubMed] [Google Scholar]
- 24. VICH. International co-operation on harmonisation of technical requirements for registration of veterinary medicinal products, good clinical practice. https://vichsec.org/en/guidelines/pharmaceuticals/pharma-efficacy/good-clinical-practice.html (accessed June 28, 2021). [Google Scholar]
- 25. Lucroy MD, Clauson RM, Suckow MA, et al. Evaluation of an autologous cancer vaccine for the treatment of metastatic canine hemangiosarcoma: a preliminary study. BMC Vet Res 2020; 16: 447. DOI: 10.1186/s12917-020-02675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu JT, Alson D, Lee TH, et al. Effect of multiple vaccinations with tumor cell-based vaccine with codon-modified GM-CSF on tumor growth in a mouse model. Cancers (Basel) 2019; 11: 368. DOI: 10.3390/cancers11030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maeng HM, Berzofsky JA. Strategies for developing and optimizing cancer vaccines. F1000Res 2019; 8: F1000 Faculty Rev-654. DOI: 10.12688/f1000research.18693.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graf R, Gruntzig K, Boo G, et al. Swiss Feline Cancer Registry 1965–2008: the influence of sex, breed and age on tumour types and tumour locations. J Comp Pathol 2016; 154: 195–210. [DOI] [PubMed] [Google Scholar]
- 29. Graf R, Gruntzig K, Hassig M, et al. Swiss Feline Cancer Registry: a retrospective study of the occurrence of tumours in cats in Switzerland from 1965 to 2008. J Comp Pathol 2015; 153: 266–277. [DOI] [PubMed] [Google Scholar]
- 30. Moiseyenko VM, Danilov AO, Baldueva IA, et al. Phase I/II trial of gene therapy with autologous tumor cells modified with tag7/PGRP-S gene in patients with disseminated solid tumors: miscellaneous tumors. Ann Oncol 2005; 16: 162–168. [DOI] [PubMed] [Google Scholar]
- 31. Morita S, Oka Y, Tsuboi A, et al. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Jpn J Clin Oncol 2006; 36: 231–236. [DOI] [PubMed] [Google Scholar]
- 32. Neelapu SS, Baskar S, Gause BL, et al. Human autologous tumor-specific T-cell responses induced by liposomal delivery of a lymphoma antigen. Clin Cancer Res 2004; 10: 8309–8317. [DOI] [PubMed] [Google Scholar]
- 33. Aachoui Y, Ghosh SK. Extracellular matrix from porcine small intestinal submucosa (SIS) as immune adjuvants. PLoS One 2011; 6: e27083. DOI: 10.1371/journal.pone.0027083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aachoui Y, Ghosh SK. Immune enhancement by novel vaccine adjuvants in autoimmune-prone NZB/W F1 mice: relative efficacy and safety. BMC Immunol 2011; 12: 61. DOI: 10.1186/1471-2172-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams J, Phillips C, Byrd MB. Factors which influence owners when deciding to use chemotherapy in terminally ill pets. Animals 2017; 7: 18. DOI: 10.3390/ani7030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kent MS. Cats and chemotherapy: treat as ’small dogs‘ at your peril. J Feline Med Surg 2013; 15: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kopecny L, Palm CA, Skorupski KA, et al. Risk factors associated with progressive increases in serum creatinine concentrations in cats with cancer receiving doxorubicin. J Vet Intern Med 2020; 34: 2048–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berger EP, Johannes CM, Post GS, et al. Retrospective evaluation of toceranib phosphate (Palladia) use in cats with mast cell neoplasia. J Feline Med Surg 2018; 20: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu J, Stuver SO, Epstein AL, et al. Can we rely on patients’ reports of adverse events. Med Care 2011; 49: 948–955. [DOI] [PubMed] [Google Scholar]