Abstract
Background
Expiratory muscle weakness and impaired airway clearance are early signs of respiratory dysfunction in Duchenne muscular dystrophy (DMD), a degenerative muscle disorder in which muscle cells are damaged and replaced by fibrofatty tissue. Little is known about expiratory muscle pathology and its relationship to cough and airway clearance capacity; however, the level of muscle replacement by fat can be estimated using MRI and expressed as a fat fraction (FF).
Research Question
How does abdominal expiratory muscle fatty infiltration change over time in DMD and relate to clinical expiratory function?
Study Design and Methods
Individuals with DMD underwent longitudinal MRI of the abdomen to determine FF in the internal oblique, external oblique, and rectus abdominis expiratory muscles. FF data were used to estimate a model of expiratory muscle degeneration by using nonlinear mixed effects and a cumulative distribution function. FVC, maximal inspiratory and expiratory pressures, and peak cough flow were collected as clinical correlates to MRI.
Results
Forty individuals with DMD (aged 6-18 years at baseline) participated in up to five visits over 36 months. Modeling estimated the internal oblique progresses most quickly and reached 50% replacement by fat at a mean patient age of 13.0 years (external oblique, 14.0 years; rectus abdominis, 16.2 years). Corticosteroid-untreated individuals (n = 4) reached 50% muscle replacement by fat 3 to 4 years prior to treated individuals. Individuals with mild clinical dystrophic phenotypes (n = 3) reached 50% muscle replacement by fat 4 to 5 years later than corticosteroid-treated individuals. Internal and external oblique FFs near 50% were associated with maximal expiratory pressures < 60 cm H2O and peak cough flows < 270 L/min.
Interpretation
These data improve understanding of the early phase of respiratory compromise in DMD, which typically presents as airway clearance dysfunction prior to the onset of hypoventilation, and links expiratory muscle fatty infiltration to pulmonary function measures.
Key Words: airway clearance, cough, Duchenne muscular dystrophy, expiratory muscles, MRI
Abbreviations: DMD, Duchenne muscular dystrophy; FF, fat fraction; MEP, maximal expiratory pressure; PCF, peak cough flow; PFT, pulmonary function test
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 601
Duchenne muscular dystrophy (DMD) is a pediatric-onset neuromuscular disorder in which absence of dystrophin protein at muscle cell membranes leads to muscle degeneration1 and resultant weakness of the respiratory muscles, including the diaphragm, intercostals, and abdominal muscles used in forced expiration.2 The first sign of respiratory impairment in DMD is often reduced expiratory muscle strength and function, leading to impaired coughing and airway clearance.2 Poor airway clearance can cause mucus retention, atelectasis, and pneumonia leading to hospitalization and slower recovery from respiratory infection.3 Maximal expiratory pressure (MEP) is a surrogate for expiratory muscle strength, and MEP values are one of the first pulmonary function measures to fall below normative values in young individuals with DMD.4 Peak cough flow (PCF) is another measure of expiratory ability; however, few normative pediatric reference data are available to help interpret PCF results in children with DMD.5,6 Interventions can improve airway clearance in the presence of expiratory muscle weakness, but recommendations for initiating interventions are based largely on expert opinions.7
The internal oblique, external oblique, and rectus abdominis are abdominal muscles activated during the expiratory phase of cough maneuvers.8,9 However, there are few data regarding disease progression in these critical expiratory muscles or how muscle pathology relates to clinical measures of expiratory function. We reported cross-sectional data describing the fatty infiltration of expiratory muscles in DMD using MRI with the goal of assessing the pathology of muscles of forced expiration.10 We found that fat fraction (FF), a noninvasive biomarker of muscle health in DMD, was increased the most in the internal oblique, followed by the external oblique and the rectus abdominis. Expiratory muscle FF was found to be correlated to a clinical measure, percent predicted MEP.
The current study is a 3-year longitudinal MRI follow-up of expiratory muscle health in a cohort of boys and young men with DMD. The three aims of the study were to: (1) characterize and model the evolution of expiratory muscle degeneration over time in DMD; (2) quantify the effect of corticosteroid treatment and mild clinical phenotypes on expiratory muscle FF; and (3) determine how expiratory muscle disease progression is related to pulmonary function. We hypothesized that given the early clinical signs of expiratory muscle weakness, there would be increases in expiratory muscle pathology over time that are directly related to expiratory function.
Study Design and Methods
Individuals with DMD and unaffected control subjects were recruited to participate in this observational study conducted at the University of Florida and approved by the Institutional Review Board (IRB201602123). Participants provided either informed consent (for those aged ≥ 18 years) or informed assent with consent of a parent (for those aged < 18 years). All participants underwent a baseline MRI examination and pulmonary function tests (PFTs), and individuals with DMD returned annually for up to 3 years for follow-up MRI and PFT assessment. Medical history information, including corticosteroid use, ambulatory status, and respiratory interventions, was collected at each visit. Unaffected control data were previously published and are not included here.10
The study began in March 2016, and rolling recruitment was conducted over the course of several years, ending in November 2020. Inclusion criteria were a clinical or genetic diagnosis of DMD, age 5 to 18 years at baseline, lack of respiratory conditions apart from those associated with DMD, and ability to tolerate an MRI examination. Individuals who were treated with investigational microdystrophin gene therapy were excluded.
MRI Acquisition and Analysis
After screening for contraindications to MRI examination, participants underwent chemical shift-encoded (Dixon) imaging of the lower abdomen on a Philips 3T Achieva MRI by using a 32-channel cardiac coil (Invivo). Dixon imaging was chosen over T2 mapping due to the ability to achieve shorter scan times, reduced respiratory motion, and higher resolution. Single-slice axial images were acquired at the base of the L4 vertebra, and if susceptibility artifacts from intestinal gas were present, a second slice was obtained approximately 2 cm lower. Follow-up scans were acquired at the same landmark and used scans from prior years to ensure slice location consistency. The scan was a three-point gradient echo mDIXON sequence (Philips R5.3) with the following: repetition time repetition time = 10 ms; echo time = 5.4, 6.4, and 7.4 ms; voxel size = 0.6 × 0.6 × 6 mm3; flip angle = 10°. Six signal averages were used to reduce the impact of respiratory motion and increase signal to noise. A short repetition time with low flip angle minimized the impact of T1 weighting, and the echo times were as short as possible to reduce T2∗ effects to avoid the need to correct for relaxation.11,12 Scan time per slice was approximately 3.5 min.
Water and fat images were reconstructed by using a precalibrated seven-peak lipid model (Philips 5.3.0 software) and exported as DICOM files. Using OsiriX MD (version 10.0.0; Pixmeo) and the reconstructed images, muscle FF was calculated for the abdominal expiratory respiratory muscles, including the rectus abdominis, the external oblique, and the internal oblique. Regions of interest were drawn just inside the borders of each abdominal muscle to ensure that non-muscle tissue was not included. FF was calculated within each region of interest by dividing fat image signal intensity by the fat and water image signal intensities. Due to relatively symmetric disease progression, muscles on the left were analyzed unless an artifact required analysis of the right side.13,14 For visualization of fat and water components, colorized fat-water fusion images were created in OSIRIX.
Disease Trajectory Modeling
Nonlinear mixed effects were used to estimate trajectories of expiratory muscle FF over time based on a Gaussian cumulative distribution function, which has been shown to accurately model lower extremity muscle fatty infiltration.15,16 The following mathematical equation was used to define the cumulative distribution function:
MRS FF at age t is estimated by FF(t) for individual i. A is the maximal value of the MRS FF (0.95; fixed effect), and C is the FF initial value (0.05; fixed effect).16 Parameters from model estimation include mu (μi) and sigma (σi). Mu represents the median of the Gaussian function and is equivalent to the age at 50% maximum FF in the cumulative distribution function. Sigma represents the SD of the Gaussian function, which affects the slope of the cumulative distribution function. With A = 0.95 and C = 0.05, the mu parameter gives the age at which a muscle FF = 0.50. Therefore, smaller mu values indicate earlier age at 50% FF (disease trajectory curve shifted leftward). Smaller sigma values indicate faster disease progression (steeper curve slope).
Pulmonary Function Testing
At each visit, PFTs were performed to determine FVC, maximal inspiratory pressure, maximal expiratory pressure (MEP), and PCF. Each test was performed at least three times, up to six trials, to achieve consistent values within 10%. The highest value was used for analysis. For percent predicted values in nonambulatory individuals, height was measured by using segmental summation (the sum of head to femoral greater trochanter length plus greater trochanter to lateral femoral condyle length plus lateral condyle to heel length). For FVC, Global Lung Initiative equations were used to determine percent predicted values,17 and for maximal inspiratory pressure and MEP, the equations published by Wilson et al18 were used to allow for consistency when transitioning between pediatric and adult ages. PCF values were expressed in absolute terms as no comprehensive prediction equations and limited normative values exist to determine percent predicted PCF in pediatric populations.5,6
Data Analysis and Statistical Approach
FF disease progression modeling was performed in RStudio (version 1.1.456) using the NLME, plyr, and ggplot2 packages; data visualization and statistical analyses were performed in GraphPad Prism (version 9.0.1). Mu and sigma values from FF modeling are reported as means with SDs. Participants were divided into three subgroups for comparison: corticosteroid-negative, steroid-treated, and mild phenotype individuals. Corticosteroid-negative participants were defined as individuals who never took corticosteroids or took them < 6 months. Mild phenotype participants were defined as individuals who enrolled with mutations likely resulting in DMD but who exhibited mild clinical phenotypes atypical of the natural history of disease progression.
Results
Forty individuals with DMD enrolled in this longitudinal cohort study. Participants joined the study on a rolling basis, with 15 participating across 36 months, 12 participating across 24 months, six participating across 12 months, and seven participating only at a baseline visit due to enrollment in the final year of the study. Thirty-four individuals participated until the study end, two were lost to follow-up, and four withdrew (claustrophobia, difficulty traveling, and no longer wanted to participate). Three individuals had one additional visit approximately 6 months from the annual visit, and these data were also included. Thirty-six individuals identified as White, two as Asian, one as Black, and one as American Indian/Alaska Native. Four participants identified as Hispanic/Latino. Table 1 summarizes cohort characteristics. Sixteen participants received a conditionally approved drug while in the study. Three individuals were categorized as having atypically mild clinical phenotypes, with one splice site mutation and two nonsense mutations within in-frame rod domain exons, which could theoretically produce small amounts of dystrophin.19
Table 1.
Participant Characteristics
| Characteristic | Baseline (N = 40) | 12 Months (n = 33a) | 24 Months (n = 27) | 36 Months (n = 15) |
|---|---|---|---|---|
| Age, y | ||||
| Mean ± SD | 12.5 ± 3.1 | 13.6 ± 3.1 | 14.9 ± 3.2 | 15.6 ± 3.3 |
| Range | 6-18 | 8-19 | 9-20 | 10-21 |
| Height, mean ± SD, cm | 132 ± 10 | 134 ± 10 | 136 ± 10 | 135 ± 9 |
| Weight, mean ± SD, kg | 40.7 ± 13.4 | 44.0 ± 13.8 | 47.3 ± 14.6 | 46.9 ± 14.5 |
| Corticosteroid treatment | 36/40 | 30/33 | 26/27 | 15/15 |
| Ambulatory | 28/40 | 20/33 | 16/27 | 6/15 |
One participant missed his 12-month visit; therefore, data represent n = 32 individuals.
Participant PFT results are consistent with trends seen in other contemporary observational studies in DMD (e-Fig 1 [includes PFT data according to approved therapy use], Fig 1).4 In terms of expiratory function, both absolute and percent predicted MEP showed early declines, with the majority of the cohort falling below 80% predicted MEP by age 12 years. PCF generally improved with increasing age into the early to mid-teens, although the vast majority of PCFs remained < 300 L/min.
Figure 1.
A-G, Pulmonary function characteristics of the study cohort. A, FVC. B, Percent predicted FVC. C, MEP. D, Percent predicted MEP. E, MIP. F, Percent predicted MIP. G, Peak cough flow values for the study cohort. Dots indicate test values at a single visit, and lines connect data from a single participant. Filled dots denote ambulatory time points, and open dots denote nonambulatory time points. When participants lost ambulation during the study, this is noted with a blue line. Note that percent predicted FVC uses height in estimation equations, and height measurement methods changed once a participant became nonambulatory. MEP = maximal expiratory pressure; MIP = maximal inspiratory pressure.
Axial fat-water fusion MRIs of representative participants reveal progressive fatty infiltration of the expiratory muscles over time, particularly in the internal oblique (Fig 2). Examining longitudinal changes in expiratory muscle FF, the internal oblique had the fastest progression, and the rectus abdominis had the slowest progression (e-Fig 2 [includes data according to approved therapy use], e-Table 1, Figs 3A, 3C, 3E). Noting that longitudinal changes in expiratory muscle FF exhibited a sigmoidal progression trajectory similar to that seen in lower extremity muscles in DMD,16 we fit the individual data to estimate a model of disease progression (Figs 3B, 3D, 3F). The estimated patient age (±SD) at 50% muscle fatty infiltration, or mu, was 13.0 ± 2.5 years for the internal oblique, 14.0 ± 2.7 years for the external oblique, and 16.2 ± 3.0 years for the rectus abdominis; sigma, representing the steepness of the trajectory curve (smaller numbers associated with steeper curves), was 3.3 ± 0.7 years for the internal oblique, 3.3 ± 1.0 years for the external oblique, and 5.4 + 1.1 years for the rectus abdominis.
Figure 2.
Expiratory muscle fatty infiltration over time. Axial fat-water fusion images from two participants at four time points illustrate the progressive fatty infiltration of the internal oblique, external oblique, and rectus abdominis muscles over time. The individual detailed in the top row was 12.3 years old at baseline and nonambulatory throughout the study; the individual detailed in the bottom row was 12.7 years old at baseline and remained ambulatory throughout. Abd = abdominis.
Figure 3.
A-F, Raw data and modeled trajectories of expiratory muscle fatty infiltration. A, C, E, Trajectories of expiratory muscle FF from each participant. Dots indicate FF at a single visit, and lines connect data from a single participant. The internal oblique had the fastest degeneration and increase in FF of the three muscles. Although the majority of participants were corticosteroid treated (Steroid +, blue dots), there were four corticosteroid-negative (Steroid -, red dots) participants. There was also three participants with mild clinical phenotypes (Mild Phenotype, gray dots). B, D, F, Modeled trajectories (solid line) and 25%/75% quartiles (dashed lines) for expiratory muscle FF changes with age. FF = fat fraction.
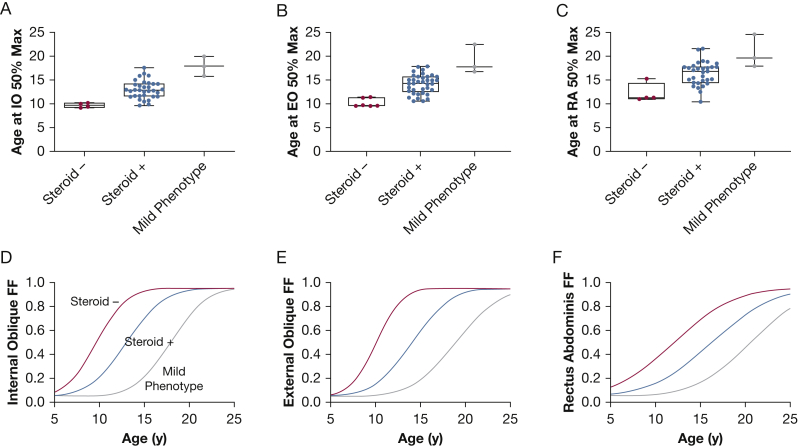
The four corticosteroid-negative individuals had higher expiratory muscle FFs compared with peers, whereas individuals with mild clinical phenotypes had lower expiratory muscle FFs compared with similarly aged peers (Figs 3A, 3C, 3E). When age at 50% FF values were compared between corticosteroid-negative and corticosteroid-treated individuals, there was a 3- to 4-year difference in age at 50% FF (Figs 4A-C, Table 2). In addition, the three individuals with clinically mild phenotypes had older modeled ages at 50% FF, linking slow clinical progression to slower accumulation of fat within the expiratory muscles. Figures 4D to 4F illustrate the estimated FF trajectories of each group.
Figure 4.
A-F, Estimated FF trajectories and parameters for subgroups. A-C, Mu values (age at muscle FF = 0.50) derived from modeled trajectories for each participant plotted as box and whisker plots with median and 25th and 75th percentile lines creating the box, and the minimum and maximum values creating the whiskers. The mu values of the corticosteroid-negative group (Steroid -, red dots), the corticosteroid-treated group (Steroid +, blue dots), and the mild clinical phenotype group (Mild Phenotype, gray dots) are plotted. D-F, Expiratory muscle trajectories were plotted by using the mean mu and sigma values from the participants in each group. For all three expiratory muscles, the corticosteroid-negative group (red line) was the most left shifted, denoting fatty infiltration reaching 50% at an earlier age, and the mild clinical phenotype group (gray line) was the most right shifted, reflecting an older age at 50% fatty infiltration (slower disease progression). FF = fat fraction.
Table 2.
Muscle Trajectory Modeling
| Variable | Corticosteroid Negative (n = 4) | Corticosteroid Treated (n = 33) | Mild Phenotype (n = 3) |
|---|---|---|---|
| Internal oblique age at 50% fat fraction, mu, y | 9.7 ±0.5 | 13.0 ± 1.9 | 17.9 ± 2.1 |
| Internal oblique curve steepness, sigma, y | 2.7 ± 0.3 | 3.3 ± 0.6 | 3.2 ± 1.2 |
| External oblique age at 50% fat fraction, mu, y | 10.1 ± 0.9 | 14.1 ± 2.0 | 18.9 ± 3.0 |
| External oblique curve steepness, sigma, y | 2.3 ± 1.0 | 3.4 ± 1.0 | 3.9 ± 1.7 |
| Rectus abdominis age at 50% fat fraction, mu, y | 12.1 ± 2.0 | 16.3 ± 2.5 | 20.6 ± 3.4 |
| Rectus abdominis curve steepness, sigma, y | 5.2 ± 0.4 | 5.4 ± 1.1 | 4.9 ± 0.4 |
Data are presented as mean ± SD. Larger sigma values indicate less steep trajectory curves, whereas smaller sigma values indicate steeper trajectory curves.
Expiratory muscle FF was also related to PFTs, particularly FVC and MEP. Combining all visits, data were divided into categories according to internal and external oblique FFs. With increasing FF, there was a tendency toward lower percent predicted FVC, although the decline was minimal (Figs 5A, 5B). In contrast, there was a precipitous decrease in percent predicted MEP with increasing FF, especially once internal or external oblique FF was ≥ 0.40 (Figs 5C, 5D). There were no clear trends between PCF and expiratory muscle FF (Figs 5E, 5F). In Figures 5G and 5H, we evaluated the relationship between muscle FF and MEP values above versus below 60 cm H2O; 60 cm H2O has been suggested as a threshold value below which airway clearance is recommended.7 Internal oblique FFs ≥ 0.60 and external oblique FFs ≥ 0.40 were associated with MEP values below this threshold.
Figure 5.
A-G, Comparison of expiratory muscle FF vs pulmonary function test results. A-F, Box and whisker plots illustrate pulmonary function test values for each of five internal oblique and external oblique FF groups. The median and 25th and 75th percentile lines create the box, and the minimum and maximum values create the whiskers. The + symbol indicates mean. For percent predicted FVC and MEP, the red shaded area indicates values ≤ 80%, where 80% is generally accepted as the lower limit of normal for these tests. For PCF, the line indicates 270 L/min; the red shaded area indicates values ≤ 160 L/min. G, Bars indicate the percentage of the participant visits with MEP values < 60 cm H2O (blue) and > 60 cm H2O (red) for each FF group. FF = fat fraction; MEP = maximal expiratory pressure.
Discussion
Airway clearance dysfunction is a common respiratory complication in neuromuscular disorders, leading to retained secretions and increased risk of atelectasis and pulmonary infections.20 Effective coughing comprises an inspiratory phase, compressive phase, and expiratory phase.21 In DMD, impaired expiratory function is one of the first signs of respiratory muscle involvement, and the expiratory phase of a cough is likely the most affected component initially. As inspiratory function becomes more affected later in the disease, it also contributes to impaired cough, whereas bulbar muscle weakness and poor glottic closure are less likely limiting factors for adequate cough.22,23 Surprisingly, little is known about expiratory muscle pathology in DMD and its contribution to effective coughing. In addition, evidence-based recommendations for airway clearance interventions are limited.24, 25, 26 The current study used high-resolution MRI to quantify abdominal expiratory muscle fatty infiltration longitudinally in a cohort of boys and young men with DMD. With these data, we generated mathematical models of expiratory muscle disease progression over time, including subanalyses for corticosteroid-treated, corticosteroid-negative, and mild phenotype individuals.
Using a modeling approach,16 the estimated patient age at muscle 50% fatty infiltration for the cohort was 13.0 years for the internal oblique, 14.0 years for the external oblique, and 16.2 years for the rectus abdominis, indicating that the internal oblique progresses most quickly, and all major abdominal expiratory muscles undergo significant degeneration by the mid-teens. The modeling approach allowed for estimation of the effects of not using corticosteroids on expiratory muscle disease progression. It was elegantly demonstrated in the Cooperative International Neuromuscular Research Group (CINRG) cohort that individuals who do not use corticosteroids have lower peak FVCs and reach their peak FVC about 5 years earlier, on average, than corticosteroid-treated individuals.4 Absolute MEP, percent predicted MEP, and PCF are also lower in corticosteroid-negative individuals, and FVC falls below 50% at a younger age.4,27,28 Although the current study was limited by a small sample of corticosteroid-untreated participants, the MRI and modeling findings mirror clinical observations, with corticosteroid-negative individuals reaching 50% fatty infiltration of the expiratory muscles 3 to 4 years earlier than treated individuals.
Three participants had dystrophin mutations that have been shown to produce small amounts of dystrophin,19,29 and these individuals had relatively mild clinical phenotypes. One participant had a splice site mutation and was ambulatory at the end of the study at age 21 years. The other two participants had nonsense mutations within in-frame exons, and at their last study visit, they also exhibited unexpectedly preserved ambulatory function. These individuals reached 50% fatty infiltration of the expiratory muscles an estimated 4 to 5 years after corticosteroid-treated participants with typical DMD. Although muscle biopsy results or dystrophin quantification data are unavailable, we speculate that the low levels of dystrophin production possible with these mutations could lead to appreciable slowing of expiratory muscle fatty infiltration and preservation of clinical respiratory abilities.30
When expiratory muscles reach a threshold level of degeneration and individuals feel they cannot adequately clear their airways, interventions are available to support productive coughing. Manually assisted cough techniques, with and without insufflation, and mechanical insufflation/exsufflation devices (cough assist) have proven superior over voluntary coughing alone.31,32 Currently, DMD care guidelines suggest a tiered approach to respiratory management beginning with initiation of lung volume recruitment when FVC drops below 60%, followed by manual or mechanically assisted cough once FVC < 50%, MEP < 60 cm H2O, or PCF < 270 L/min.7,33 However, these thresholds have limitations as they are primarily based on expert opinion,24 they are noted to apply only to older teenagers or adults with DMD,7 and MEP and PCF are not consistently evaluated at clinical visits.34
After critically evaluating the order of recommendations and thresholds within this study cohort, we found that thresholds for initiating cough assist were always met prior to criteria for initiating lung volume recruitment. Specifically, all individuals with FVC < 60% already had MEP < 60 cm H2O, and 79% of visits with MEP < 60 cm H2O were associated with FVC > 60%. This suggests that loss of expiratory pressure generation precedes significant declines in FVC, and order of intervention prescription may need reevaluation. Interpretation of PCF thresholds was challenging, as many individuals never achieved a PCF > 270 L/min and, conversely, the majority of individuals with PCF > 270 L/min had MEP < 60 cm H2O. Using available reference data, all but four participants had PCFs less than the fifth percentile.6 Therefore, care guideline threshold levels of function may provide conflicting information.
The results of the current study raise the possibility that expiratory muscle FF could be useful in defining a threshold level of muscle degeneration associated with the need for airway clearance interventions. When internal oblique FF approached 50% fatty infiltration (approximated here as FF = 0.40-0.59) (Fig 5), mean predicted FVC was 84%, MEP was 64 cm H2O, and predicted MEP was 49%. Similarly, an external oblique FF near 0.50 (ie, 0.40-0.59) was associated with a predicted FVC of 84%, an MEP of 55 cm H2O, and a predicted MEP of 46%. We thus hypothesize that as the internal and external obliques reach 50% fatty infiltration (estimated to occur at 13.0 and 14.0 years of age, respectively), FVC is relatively maintained while expiratory muscle strength (measured by using MEP) begins to decline dramatically. This could mark the beginning of airway clearance deficiencies, which continue to progress as the expiratory muscles further weaken and inspiratory muscle strength also declines. PCF primarily remained between 270 L/min and 160 L/min regardless of expiratory muscle degeneration, suggesting that these absolute values be interpreted judiciously in children.
Although this study provides valuable insight into disease progression of the abdominal expiratory muscles, a significant limitation is absence of data from other muscles involved in cough and airway clearance, including inspiratory muscles, upper airway muscles, and other expiratory muscles such as the internal intercostals and transverse abdominis, which are typically too small for quantitative MRI analysis. Inspiratory capacity, in particular, is an important determinant of cough and airway clearance efficacy that was not analyzed here.20 In addition, other factors beyond expiratory muscle composition/degeneration may affect cough efficacy, including lung and chest wall compliance, adiposity, and scoliosis. A second limitation of this study was the inability to evaluate relationships between airway clearance interventions and expiratory muscle FF. Mechanical insufflation/exsufflation was the primary intervention prescribed in the current cohort; however, devices were prescribed at widely disparate ages, and variable adherence to prescribed use further precluded analysis. Finally, to allow for free breathing MRI acquisition at high resolutions but short scan times, we only acquired a single axial slice. Multi-slice acquisition would provide more information about muscle health over the length of the expiratory muscles. There may be a small degree of T1 bias within the images; however, we minimized this effect by using a short repetition time and low flip angle.12
Interpretation
This study defined models of expiratory respiratory muscle degeneration in DMD by using noninvasive and nonvolitional MR biomarkers and expanded the analysis to evaluate the effects of corticosteroid treatment and mild phenotypes on expiratory muscle degeneration. These data improve understanding of the early phase of respiratory compromise in DMD, which typically presents as airway clearance dysfunction prior to the onset of nocturnal or daytime hypoventilation. This study also links expiratory muscle fatty infiltration to clinical pulmonary function measures with a critical assessment of current PFT thresholds recommended for initiating airway clearance interventions. Future studies may consider using MR quantification of FF to aid in evaluation of respiratory function in DMD or as a treatment response biomarker in clinical trials.
Take-home Points.
Study Question: How does abdominal expiratory muscle fatty infiltration change over time in DMD and relate to clinical expiratory function?
Results: Nonlinear mixed effects modeling estimates the internal oblique, external oblique, and rectus abdominis muscles reach 50% replacement by fat at 13.0, 14.0, and 16.2 years of age, respectively, in individuals with DMD, and internal/external oblique muscle fat fractions of approximately 0.50 are associated with decreased MEPs and peak cough flows.
Interpretation: When the internal and external oblique muscles reach about 50% replacement by fat in individuals with DMD, it can be expected that airway clearance can become impaired, and intervention may be required to produce adequate coughs.
Acknowledgments
Author contributions: G. A. W. takes responsibility for the content of the manuscript, including the data and analysis. A. M. B., D. J. L., W. T. T., R. J. W., S. C. F., W. D. R., B. K. S., K. V., and G. A. W. contributed to the design and conception of the study; A. M. B., D. J. L., A. B., and G. A. W. contributed to data collection; A. M. B., W. T. T., W. D. R., M. J. D., B. K. S., and G. A. W. contributed to data analysis and interpretation; A. M. B. drafted the manuscript; and D. J. L., A. B., W. T. T., R. J. W., S. C. F., W. D. R., M. J. D., B. K. S., K. V., and G. A. W. contributed to revising it critically for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: B. K. S. reports being a consultant for Sarepta Therapeutics and Amicus Therapeutics on content unrelated to the manuscript. K. V. reported funding from Italfarmaco, Sarepta Therapeutics, ML Bio Solutions, and Catabasis Pharmaceuticals via a research service agreement to the University of Florida (unrelated to the manuscript). G. A.,W. received grant support from the National Institutes of Health for the submitted study. None declared (A. M. B., D. J. L., A. B., W. T. T., R. J. W., S. C. F., W. D. R., M. J. D.).
Role of the sponsor: None of the funding sources had a role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors acknowledge Samuel Riehl, BS, for his assistance with data collection and analysis. They also acknowledge the MR technologists who assisted with MRI acquisition: Judy Steadman, RT, Christi Swiers, RT (R) (M) (MR), and Tammy Nicholson, BSRT (R) (MR). They appreciate the boys and young men who were participants in the study.
Additional information: The e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Dr Batra is currently at the Louisiana State University Health Sciences Center, New Orleans, LA.
FUNDING/SUPPORT: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health [P50 AR052646 which was formerly U54 AR052646 and R01 AR056973]. The first author was supported by T32 HL134621 and K12 HD055929 during the conduct of this work. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s Advanced Magnetic Resonance Imaging and Spectroscopy Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1644779 and DMR-1157490 and by the state of Florida.
DISCLAIMER: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Data
References
- 1.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Mayer O.H. Pulmonary function and clinical correlation in DMD. Paediatr Respir Rev. 2019;30:13–15. doi: 10.1016/j.prrv.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Dohna-Schwake C., Ragette R., Teschler H., et al. Predictors of severe chest infections in pediatric neuromuscular disorders. Neuromuscul Disord. 2006;16(5):325–328. doi: 10.1016/j.nmd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 4.McDonald C.M., Gordish-Dressman H., Henricson E.K., et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: long-term natural history with and without glucocorticoids. Neuromuscul Disord. 2018;28(11):897–909. doi: 10.1016/j.nmd.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Kotwal N., Shukla P.J., Perez G.F. Peak cough flow in children with neuromuscular disorders. Lung. 2020;198(2):371–375. doi: 10.1007/s00408-020-00340-7. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi C., Baiardi P. Cough peak flows: standard values for children and adolescents. Am J Phys Med Rehabil. 2008;87(6):461–467. doi: 10.1097/PHM.0b013e318174e4c7. [DOI] [PubMed] [Google Scholar]
- 7.Birnkrant D.J., Bushby K., Bann C.M., et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347–361. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques L., de Fregonezi G.A.F., Santos I.P., et al. Effects of positioning on cough peak flow and muscular electromyographic activation in Duchenne muscular dystrophy. Respir Care. 2020;65(11):1668–1677. doi: 10.4187/respcare.07426. [DOI] [PubMed] [Google Scholar]
- 9.LoMauro A., Aliverti A. Respiratory muscle activation and action during voluntary cough in healthy humans. J Electromyogr Kinesiol. 2019;49:102359. doi: 10.1016/j.jelekin.2019.102359. [DOI] [PubMed] [Google Scholar]
- 10.Barnard A.M., Lott D.J., Batra A., et al. Imaging respiratory muscle quality and function in Duchenne muscular dystrophy. J Neurol. 2019;266(11):2752–2763. doi: 10.1007/s00415-019-09481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C.-Y., McKenzie C.A., Yu H., et al. Fat quantification with IDEAL gradient echo imaging: correction of bias fromT1 and noise. Magn Reson Med. 2007;58(2):354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 12.Burakiewicz J., Sinclair C.D.J., Fischer D., et al. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol. 2017;264(10):2053–2067. doi: 10.1007/s00415-017-8547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischmann A., Hafner P., Gloor M., et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol. 2013;260(4):969–974. doi: 10.1007/s00415-012-6733-x. [DOI] [PubMed] [Google Scholar]
- 14.Stuberg W.A., Metcalf W.K. Reliability of quantitative muscle testing in healthy children and in children with Duchenne muscular dystrophy using a hand-held dynamometer. Phys Ther. 1988;68(6):977–982. doi: 10.1093/ptj/68.6.977. [DOI] [PubMed] [Google Scholar]
- 15.Naarding K.J., Reyngoudt H., van Zwet E.W., et al. MRI vastus lateralis fat fraction predicts loss of ambulation in Duchenne muscular dystrophy. Neurology. 2020;94(13):e1386–e1394. doi: 10.1212/WNL.0000000000008939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney W.D., Berlow Y.A., Triplett W.T., et al. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology. 2020;94(15):e1622–e1633. doi: 10.1212/WNL.0000000000009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quanjer P.H., Stanojevic S., Cole T.J., et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson S.H., Cooke N.T., Edwards R.H., Spiro S.G. Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax. 1984;39(7):535–538. doi: 10.1136/thx.39.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanigan K.M., Dunn D.M., Niederhausern A.von, et al. Nonsense mutation-associated Becker muscular dystrophy: interplay between exon definition and splicing regulatory elements within the DMD gene. Hum Mutat. 2011;32(3):299–308. doi: 10.1002/humu.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boitano L.J. Management of airway clearance in neuromuscular disease. Respir Care. 2006;51(8):913–922. discussion 922-924. [PubMed] [Google Scholar]
- 21.McCool F.D. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 22.Kang S.W., Bach J.R. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil. 2000;79(3):222–227. doi: 10.1097/00002060-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Suárez A.A., Pessolano F.A., Monteiro S.G., et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil. 2002;81(7):506–511. doi: 10.1097/00002060-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 24.McDonald C.M., Mercuri E. Evidence-based care in Duchenne muscular dystrophy. Lancet Neurol. 2018;17(5):389–391. doi: 10.1016/S1474-4422(18)30115-7. [DOI] [PubMed] [Google Scholar]
- 25.Morrow B., Argent A., Zampoli M., et al. Cough augmentation techniques for people with chronic neuromuscular disorders. Cochrane Database Syst Rev. 2021;4:CD013170. doi: 10.1002/14651858.CD013170.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatwin M., Toussaint M., Gonçalves M.R., et al. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respir Med. 2018;136:98–110. doi: 10.1016/j.rmed.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Daftary A.S., Crisanti M., Kalra M., et al. Effect of long-term steroids on cough efficiency and respiratory muscle strength in patients with Duchenne muscular dystrophy. Pediatrics. 2007;119(2):e320–e324. doi: 10.1542/peds.2006-1400. [DOI] [PubMed] [Google Scholar]
- 28.Trucco F., Domingos J.P., Tay C.G., et al. Cardiorespiratory progression over 5 years and role of corticosteroids in Duchenne muscular dystrophy: a single-site retrospective longitudinal study. Chest. 2020;158(4):1606–1616. doi: 10.1016/j.chest.2020.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Tuffery-Giraud S., Béroud C., Leturcq F., et al. Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum Mutat. 2009;30(6):934–945. doi: 10.1002/humu.20976. [DOI] [PubMed] [Google Scholar]
- 30.de Feraudy Y., Ben Yaou R., Wahbi K., et al. Very low residual dystrophin quantity is associated with milder dystrophinopathy. Ann Neurol. 2021;89(2):280–292. doi: 10.1002/ana.25951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S.-W., Kang Y.-S., Sohn H.-S., et al. Respiratory muscle strength and cough capacity in patients with Duchenne muscular dystrophy. Yonsei Med J. 2006;47(2):184–190. doi: 10.3349/ymj.2006.47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrow B., Zampoli M., van Aswegen H., Argent A. Mechanical insufflation-exsufflation for people with neuromuscular disorders. Cochrane Database Syst Rev. 2013;12:CD010044. doi: 10.1002/14651858.CD010044.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan D.W., Birnkrant D.J., Benditt J.O., et al. Respiratory management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142(suppl 2):S62–S71. doi: 10.1542/peds.2018-0333H. [DOI] [PubMed] [Google Scholar]
- 34.Andrews J.G., Conway K., Westfield C., et al. Implementation of Duchenne muscular dystrophy care considerations. Pediatrics. 2018;142(1) doi: 10.1542/peds.2017-4006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.