Key Points
Question
Are circulating levels of brodalumab, an interleukin 17 (IL-17) receptor α inhibitor, and antibrodalumab antibodies associated with response to therapy?
Findings
In this case series study including 20 consecutive patients with psoriasis with previous treatment failure with anti-IL-17A inhibitor therapy, subquantifiable brodalumab levels were associated with a lack of response at a statistically significant level. Antibrodalumab antibodies were not detected in any of the samples.
Meaning
Results of this case series study suggest that monitoring patient levels of circulating brodalumab may aid clinical decision-making toward the prevention of ineffective therapy.
Abstract
Importance
Given the possible treatment modalities in psoriasis management, little is known about whether drug monitoring is associated with response rate.
Objective
To determine whether drug monitoring is associated with response to brodalumab therapy.
Design
A multicenter case series study of patients with psoriasis treated with brodalumab whose treatment with previous IL-17A inhibitor therapy failed. Patients were recruited from the Departments of Dermatology at Gentofte and Aarhus University Hospitals, Denmark, between 2018 and 2020. Patient visits were conducted after 4 and 12 weeks of therapy. Patients not achieving Psoriasis Area and Severity Index 75% improvement from baseline (PASI 75) after 12 weeks were discontinued and considered nonresponders. Patients maintaining PASI 75 response were followed up for up to 52 weeks.
Exposure
Treatment with brodalumab, 210 mg, at weeks 0, 1, 2, then every 2 weeks.
Main Outcomes and Measures
Outcome measures were PASI reductions vs brodalumab levels and antibrodalumab antibodies.
Results
Twenty patients with psoriasis (13 [65%] were male; median age, 50 years [range, 19-66 years]) were included. After 12 weeks of therapy, patients with quantifiable levels of brodalumab (≥0.05 μg/mL) experienced significantly higher PASI reductions than those without (median, 93%; range, 61%-100% vs median, −3; range, −49% to 94%, respectively; P = .006). After 12 weeks of therapy, 4 of 5 patients (80%) not achieving PASI 75 had subquantifiable drug levels (<0.05 μg/mL), although this finding was seen for only 3 of 14 PASI 75 responders (21%). None of 7 patients (35%) with subquantifiable drug levels after 12 weeks of therapy maintained response. No antibrodalumab antibodies were detected in any of the tested samples.
Conclusions and Relevance
Results of this case series study suggest that circulating brodalumab level is a factor associated with clinical treatment response. Monitoring patient levels of circulating brodalumab may aid clinical decision-making and help prevent ineffective therapy.
This case series study assesses measured trough levels of circulating brodalumab in patients with psoriasis who experienced treatment failure with anti–interleukin 17A and whether brodalumab levels were associated with clinical response.
Introduction
Biological therapies targeting cytokines of inflammatory pathways, including tumor necrosis factor (TNF)-α, interleukin (IL)-12 or IL-12/23 inhibitors, or IL-17 inhibitors are indicated for moderate to severe psoriasis and have substantially improved treatment options.1 However, some patients do not respond well (primary nonresponders) or lose response over time (secondary nonresponders) to some or even all of these medications or have to cease therapy because of adverse events, which warrants further additions to the armamentarium of approved biological psoriasis therapies.
Brodalumab is a monoclonal fully human antibody of IgG2-isotype targeting the IL-17 receptor A (IL-17RA) subunit, thereby abrogating signaling by IL-17A, IL-17F, IL-17A/F, IL-17B, IL-17C, and IL-17E. Given this, brodalumab targets the broadest possible range of IL-17–mediated signaling.2,3,4 Among IL-17 and IL-23 pathway inhibitors, brodalumab has a comparable short time to onset of action,5 and rates of skin clearance in patients treated with brodalumab have been shown to be similar in patients who were naive to or previously treated with ustekinumab.6 These properties suggest that brodalumab may be a suitable candidate for rescue therapy in patients refractory to treatment in addition to being a candidate for first-line therapy. However, despite its long-term efficacy and safety shown in several clinical studies,7,8,9,10 brodalumab has not yet gained widespread use as a first-line antipsoriasis therapy. This finding is likely due to the US Food and Drug Administration boxed warning of suicidal ideation and behavior as a reported adverse effect.11
Given the many possible treatment modalities in psoriasis management, an emerging challenge is to estimate the ideal treatment choice for each individual patient. To date, no validated clinically useful biomarkers have been found to guide clinical decisions in this field, and treatment modalities are generally applied on a trial-and-error basis. Treatment strategies are often decided based on locally accepted guidelines, and monitoring of circulating drug levels and antidrug antibodies are not routinely implemented. In general, it is therefore not known whether patients who did not respond to a drug had a lack of response because of low bioavailability of the drug, formation of antidrug antibodies, or whether the biological target of the chosen therapy is simply not affecting the pathophysiology of these patients. In the case of low bioavailability of the drug, a change in dosing regimen might restore response, whereas the presence of neutralizing antidrug antibodies should prompt an immediate change of drug. Conversely, if drug levels are adequate, switching to a drug targeting a different pathway may be more appropriate.
In this study, we retrospectively measured trough levels of circulating brodalumab and antidrug antibodies in patients with prior failure to anti–IL-17A therapy to assess whether brodalumab levels are associated with clinical response, as measured by reductions in Psoriasis Area and Severity Index (PASI), and can be used as a marker of long term response.
Methods
As described previously,12 the study was conducted in accordance with the Declaration of Helsinki13 and approved by the local ethics committee, the data protection agency, and the Danish Medicines Agency. Written informed consent was obtained from all patients prior to inclusion. The study was monitored and conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice, and the protocol was registered in the EudraCT database prior to study start (2018-000097-30).
Patients
Consecutive patients were recruited from the Departments of Dermatology at Gentofte and Aarhus University Hospitals between May 2018 and June 2020. All patients had baseline PASI score of 6 or higher (score range, 0-72, with higher scores indicating severe disease), none received concomitant systemic treatment for psoriasis, and all patients experienced treatment failure receiving at least one IL-17A inhibitor prior to inclusion (eTable in the Supplement).
Four weeks prior to baseline, systemic psoriasis therapies were discontinued to avoid overlapping treatment effects. Following this discontinuation, all patients had their baseline PASI score assessed and initiated treatment with brodalumab, 210 mg, at weeks 0, 1, 2, then every 2 weeks. The PASI was assessed and blood was drawn immediately before the next dosing to measure trough levels of brodalumab and cytokines after 4, 12, 26, and 52 weeks of therapy. Although brodalumab was administered subcutaneously, circulating levels likely reflect its systemic bioavailability more correctly and were therefore used in this study. After 12 weeks of therapy and at all subsequent visits, patients who did not show PASI improvement of 75% from baseline (PASI 75) had their treatment discontinued and left the study. Patients discontinuing treatment for any reason were considered nonresponders at all subsequent visits. Missing data were analyzed according to nonresponder imputation.
Brodalumab Drug Levels and Antidrug Antibody Measurements by Inhibitor
Brodalumab levels were measured using in-house assays based on the Luminex platform. Human recombinant IL-17RA (Sino Biological Europe GmbH) was covalently coupled to Luminex MagPlex-beads (Luminex Corporation) as described by the manufacturer. For each run, a duplicate standard curve was prepared by serial dilutions of stock brodalumab injection-solution (LEO Pharma A/S).
The IL-17RA-coupled beads, as well as a vehicle only-coupled beadset, were preblocked in phosphate buffered saline (PBS) containing 0.05% Tween-20 (Merck Life Science A/S) and 10% fetal bovine serum (Merck Life Science A/S). Standard curve and patient samples were both diluted 1:5000 in PBS containing 0.05% Tween-20 and 1% fetal bovine serum (PBSTF) and incubated with the blocked beads. All wells were subsequently washed with PBS containing 0.05% Tween-20 (PBST) and incubated with a biotinylated mouse anti–human IgG-detection antibody (Merck Life Science A/S). After additional PBST washes, all wells were incubated with streptavidin-R-phycoerythrin (Agilent Technologies Denmark ApS) and analyzed on a Luminex 100IS-instrument (Luminex Corporation).
Blank-subtracted median fluorescence intensities from standard curve points were used to fit a sigmoidal equation using Bio-Plex Manager version 6.0 (Bio-Rad Denmark Aps) for quantifying brodalumab. Samples containing at least 2 μg/mL brodalumab were reanalyzed using a modified protocol for higher sensitivity as well as for indirect detection of antidrug antibodies against brodalumab. Brodalumab spiked and unspiked patient samples were incubated overnight. Standard curve, unspiked, and spiked samples were diluted 1:500 in PBSTF. Brodalumab recovery in spiked samples was compared with corresponding unspiked samples for quantifying antidrug antibodies (ADA). Samples were defined as ADA positive if spike recovery was less than or equal to 70% and ADA negative otherwise.
Antibrodalumab Antibody Measurements by Bridging Assay
Brodalumab-coupled beads, as well as vehicle-only coupled beads, were blocked in PBST with 50% fetal bovine serum. For each plate run, a duplicate standard curve was prepared by serial dilutions of rabbit polyclonal anti–human IgG (Jackson ImmunoResearch Inc). Standards and patient EDTA-samples were diluted 1:2 in PBSTF and incubated with blocked beads. Samples were subsequently washed with PBST and incubated with biotinylated brodalumab. After additional PBST washes, samples were incubated with streptavidin-R-phycoerythrin and analyzed on a Luminex 100IS instrument.
Cytokine Measurements
As described previously,12 plasma levels of IL-17A, IL-17F, IL-23, IL-6, interferon (IFN)-γ, and TNF-α were measured using a custom multiplex Luminex High-Performance Assay (Bio-techne Ltd), as instructed by the vendor. The stated sensitivities were 2.5 pg/mL for IL-17A, 9.2 pg/mL for IL-17F, 20.2 pg/mL for IL-23, 0.7 pg/mL for IL-6 1.9 pg/mL for IFN-γ, and 1.1 pg/mL for TNF-α.
Statistical Analysis
Assuming nonnormality, data are presented using medians and ranges. Categorical and count data are presented as counts with frequencies or count ranges. The Kruskal-Wallis test was used to compare differences in PASI reductions between samples with quantifiable and subquantifiable drug levels. The Wilcoxon signed rank test was used to compare paired samples from baseline and 12 weeks of brodalumab therapy. Fisher exact test was used to compare dichotomized variables. Statistical significance was determined at P ≤ .05. All analyses were 2-sided and were performed in RStudio version 1.4.1717 (RStudio Inc) using R version 4.1.0 (R Foundation for Statistical Computing).
Results
Patient Characteristics
Seven (35%) female and 13 (65%) male patients with plaque psoriasis, median age of 50 years (range, 19-66 years), median weight of 103 kg (range, 59-182 kg), median body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]) of 32 (range, 21-50), and median baseline PASI of 13 (range, 7-26) were included. All patients had treatment failure with at least one IL-17A inhibitor, ie, 14 (70%) patients had experienced treatment failure with secukinumab, 1 (5%) with ixekizumab, and 5 (25%) with both. Eighteen (90%) patients experienced treatment failure with at least one TNF-α or IL-12/-23 inhibitor (eTable in the Supplement).
Changes in PASI and Circulating Brodalumab Levels
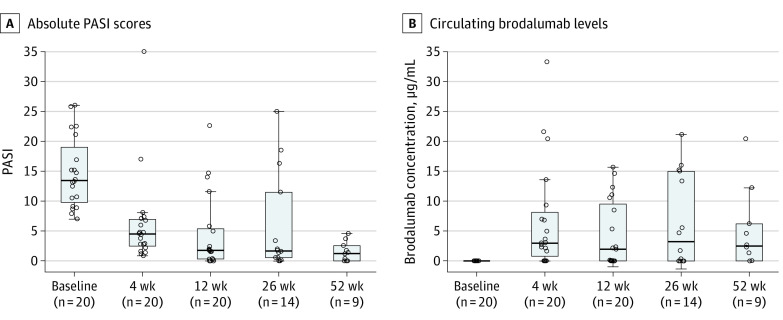
Compared with baseline, most patients showed notable reductions in PASI after 4 weeks of brodalumab therapy and even further reductions after 12 weeks of therapy. Median PASI at baseline was 13.5 vs 4.5 at 4 weeks (P < .001) and vs 1.8 at 12 weeks (P < .001) using the Wilcoxon signed test (Figure 1). As expected, trough levels of circulating brodalumab varied with concentrations ranging from nonquantifiable to 33.3 μg/mL (Figure 1). After 26 weeks of therapy, 6 patients discontinued treatment with brodalumab therapy because of insufficient response (5 of 6) or adverse events (1 of 6) comprising worsening of psoriasis. In addition, after 52 weeks of therapy, 5 patients discontinued treatment with brodalumab therapy because of insufficient response (3 of 5) or adverse events (2 of 5) comprising severe nausea (1 of 2) or back pain (1 of 2). Neither serious adverse events nor suicidal ideation or behavior in any of the participating patients was observed during the study period.
Figure 1. Psoriasis Area and Severity Index (PASI) Scores and Trough Plasma Brodalumab Levels by Weeks of Therapy.
A, The PASI scores (score range, 0-72, with higher scores indicating severe disease). B, Circulating brodalumab levels in μg/mL (lower limit of quantification is 0.05 μg/mL). The horizontal line inside the boxes indicates the median, and the lower and upper ends of the boxes are the first and third quartiles. The whiskers indicate the values within the IQR. Data outside the whiskers are outliers.
PASI Reductions vs Brodalumab Levels
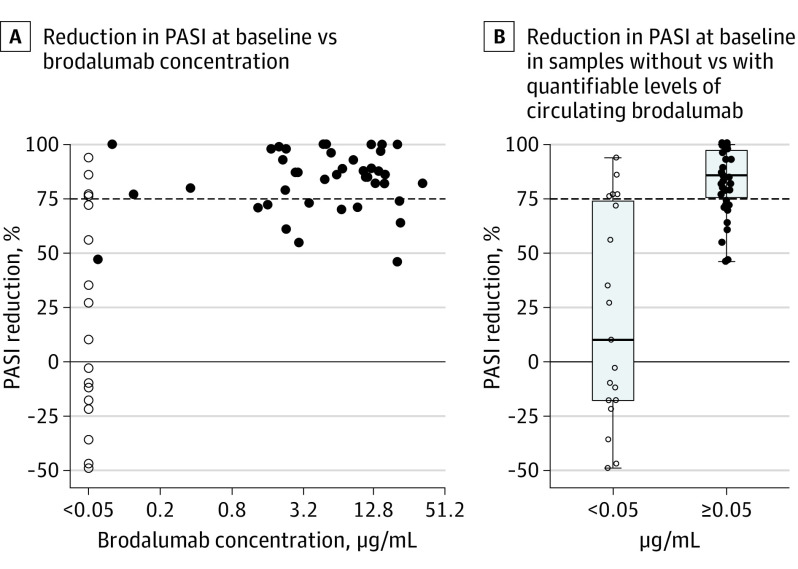
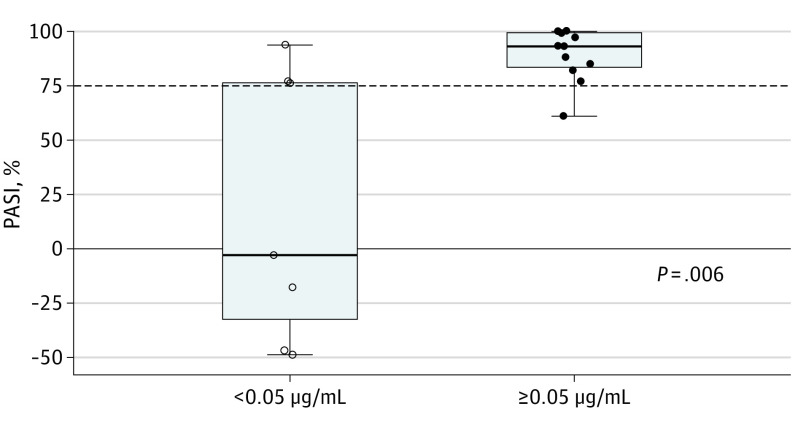
Although the absence of quantifiable drug levels was associated with a lack of response while quantifiable drug levels were associated with response, this finding was not numerically proportional (ie, higher drug levels did not mean better response) (Figure 2A). However, patients with quantifiable brodalumab levels (≥0.05 μg/mL) experienced higher PASI reductions compared with patients with subquantifiable brodalumab levels (<0.05 μg/mL) (Figure 2B). All samples with quantifiable brodalumab levels corresponded to PASI reductions, and none of the samples showing increases in PASI had quantifiable brodalumab levels. Thus, dichotomization by whether levels of brodalumab were quantifiable was performed, and after 12 weeks of therapy those with quantifiable drug levels had significantly higher responses (median: 93%; range, 61% to 100%) compared with those without (median,: −3%; range, −49% to 94%) (P = .006) (Figure 3).
Figure 2. Reduction in Psoriasis Area and Severity Index (PASI) Score vs Brodalumab Levels for All Times Combined.
PASI reduction compared with baseline values vs circulating brodalumab (for all visits). The dashed horizontal line marks 75% reduction of PASI compared with baseline. Open circles mark brodalumab concentrations below the lower limit of quantification (<0.05 μg/mL) and closed circles mark quantifiable brodalumab concentrations (≥0.05 μg/mL). The horizontal line inside the boxes indicates the median, and the lower and upper ends of the boxes are the first and third quartiles. The whiskers indicate the values within the IQR. Data outside the whiskers are outliers.
Figure 3. Reduction in Psoriasis Area and Severity Index (PASI) Score vs Quantifiable Brodalumab After 12 Weeks of Therapy.
PASI reductions compared with baseline values vs presence (≥0.05 μg/mL) or absence (<0.05 μg/mL) of quantifiable levels of circulating brodalumab, after 12 weeks of therapy. The dashed horizontal line marks 75% reduction of PASI compared with baseline. Open circles mark brodalumab concentrations below the lower limit of quantification and closed circles mark quantifiable brodalumab concentrations. The horizontal line inside the boxes indicates the median, and the lower and upper ends of the boxes are the first and third quartiles. The whiskers indicate the values within the IQR. Data outside the whiskers are outliers.
Dichotomization of PASI reductions after 12 weeks of therapy according to PASI 75 criteria showed that 4 of 5 (80%) nonresponders had subquantifiable drug levels, although this finding was the case in only 3 of 14 (21%) responders (Figure 3).
After 52 weeks of brodalumab therapy, 7 patients (35%), all of whom had quantifiable drug levels after 12 weeks of therapy, were still PASI 75 responders. In contrast, none of the 7 patients with subquantifiable drug levels after 12 weeks of therapy were PASI 75 responders after 52 weeks of therapy. Five patients with quantifiable drug levels after 12 weeks of therapy were not PASI 75 responders after 52 weeks of therapy. Among the latter, however, 1 had an 82% reduction in PASI after 26 weeks but left the study before 52 weeks because of adverse events, 1 patient reached a 71% reduction after 52 weeks, 1 reached a 61% reduction after 26 weeks and treatment was therefore discontinued at that point, while 2 were responders after 12 weeks of therapy but experienced relapse subsequently and did not have quantifiable levels of brodalumab at later visits.
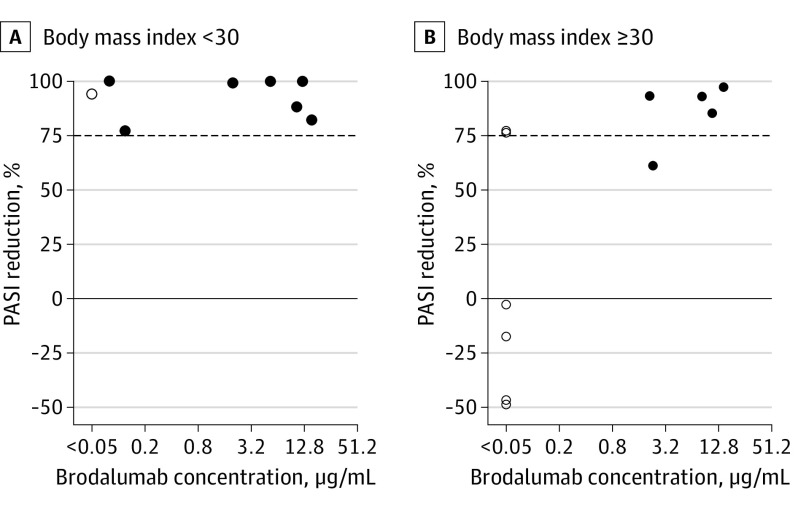
PASI Reductions and Brodalumab Levels vs Body Weight
After 12 weeks of therapy, all of the patients without obesity (BMI <30) (n = 8) had PASI reductions of at least 77% compared with baseline, and all but 1 (88%) had quantifiable levels of brodalumab (Figure 4A). For patients with obesity (BMI ≥30) (n = 12), subquantifiable brodalumab levels were seen in 6 of 12 patients (50%) (Figure 4B). Of the 6 patients with obesity with subquantifiable brodalumab levels, 4 had increased PASI after 12 weeks of therapy (Figure 4B). All patients with obesity with quantifiable brodalumab levels had a reduced PASI of at least 61% after 12 weeks of therapy (Figure 4B).
Figure 4. Reduction in Psoriasis Area and Severity Index (PASI) Score vs Brodalumab Levels After 12 Weeks of Therapy According to Body Mass Index.
PASI reductions vs circulating brodalumab levels (log-scale) after 12 weeks of therapy in patients without obesity (body mass index <30 [calculated as weight in kilograms divided by height in meters squared]) and with obesity (body mass index ≥30). The dashed horizontal line marks 75% reduction of PASI compared with baseline. Open circles mark brodalumab concentrations below the lower limit of quantification (<0.05 μg/mL) and closed circles mark quantifiable brodalumab concentrations.
IL-17A, IL-17F, IL-23, IL-6, TNF-α, and IFN-γ Levels vs Brodalumab Levels
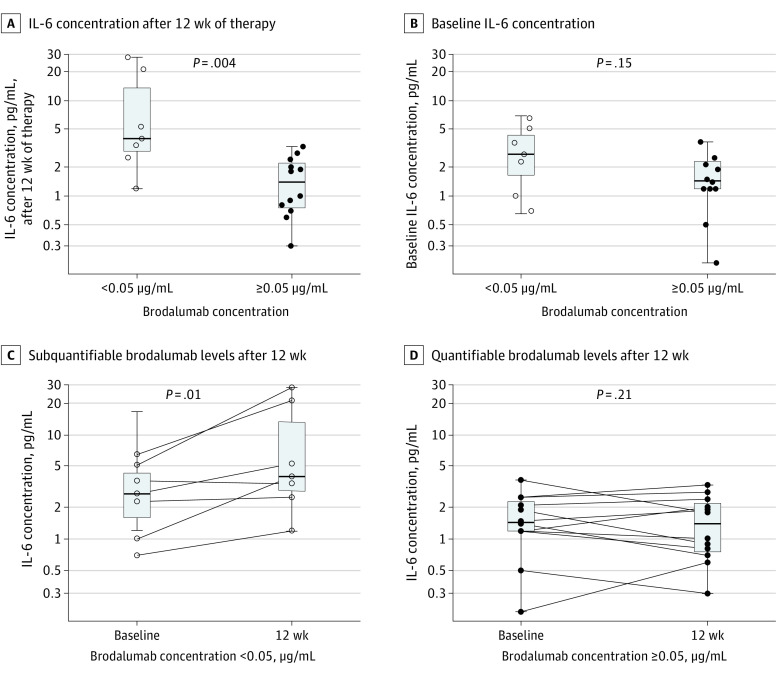
After 12 weeks of therapy, levels of circulating IL-6 were significantly higher in patients with subquantifiable brodalumab levels compared with patients with quantifiable brodalumab levels (median, 4.0 pg/mL [range, 1.2-28.2 pg/mL] in patients with subquantifiable brodalumab levels vs median, 1.4 pg/mL [range, 0.3-3.3 pg/mL] in patients with quantifiable brodalumab levels; P = .004) (Figure 5A). Although patients with subquantifiable brodalumab levels after 12 weeks of therapy also had slightly elevated levels of IL-6 at baseline, the finding was not statistically significant at baseline (Figure 5B). Further analyses showed that in patients with subquantifiable levels of brodalumab after 12 weeks of therapy, IL-6 levels were significantly increased compared with baseline (median, 4.0 pg/mL [range, 1.2-28.2 pg/mL] after 12 weeks of therapy vs median, 2.7 pg/mL [range, 0.7-6.5 pg/mL] at baseline; P = .01) (Figure 5C), whereas this was not the case for patients with quantifiable levels of brodalumab after 12 weeks of therapy (Figure 5D).
Figure 5. Differences in Levels of IL-6 Between Patients With Quantifiable vs Subquantifiable Levels of Brodalumab After 12 Weeks of Therapy.
Levels of circulating interleukin (IL)-6 in patients without or with quantifiable levels of brodalumab after 12 weeks of therapy. Open circles mark brodalumab concentrations below the lower limit of quantification (<0.05 μg/mL) and closed circles mark quantifiable brodalumab concentrations.
The levels of brodalumab were not associated with IL-17A, TNF-α, or IFN-γ. The IL-17F and IL-23 levels were below detection rate for nearly all patients and were therefore not investigated further.
Antibrodalumab Antibodies
All samples containing at least 2 μg/mL of brodalumab were tested for antidrug antibodies with 2 different in-house bead-based analyses. None of the tested samples contained detectable ADA levels, as assessed by either of the 2 assays.
Discussion
In this case series, we monitored the levels of brodalumab and antibrodalumab antibodies in 20 patients with psoriasis treated with brodalumab whose prior treatment failed with at least one IL-17A inhibitor and in most cases also failed with several other antibody-based therapies, including anti–IL-12/23, anti–IL23, and TNF-α inhibitors (eTable in the Supplement). As reported previously,12 the study did not include placebo controls for comparison but 14 of the 20 participants (70%) were PASI 75 responders after 12 weeks of therapy and 7 of the 20 participants (35%) were PASI 75 responders after 52 weeks of therapy, suggesting that brodalumab may have utility in these otherwise treatment-refractory patients.
All patients with quantifiable levels of brodalumab after 12 weeks of therapy experienced concomitant PASI reductions. However, 3 patients with subquantifiable brodalumab levels after 12 weeks of therapy also reached PASI 75 response. It can therefore be speculated that the therapeutic concentration range of brodalumab may extend to values lower than our in-house assay can quantify and that nontrough level samples may be useful in defining the optimal therapeutic window for brodalumab. The latter might be more reliable because brodalumab has been shown to undergo target-mediated drug disposition14 resulting in highly variable drug clearance rates at low drug concentrations. However, all 3 patients experienced relapse within the 52 weeks of the study, and trough levels of brodalumab within the range of this assay therefore seem necessary to maintain long-term response.
Antidrug antibodies against brodalumab appear to be a rare phenomenon.15,16 We measured all samples containing up to 2 μg/mL brodalumab using 2 in-house assays based on 2 different approaches; in 1 assay, samples were spiked with brodalumab and were subsequently measured with and without spike to assess brodalumab-neutralizing capability of the sample. In the other assay, a bridging format with brodalumab as both capture and detection antibody was used to measure antidrug antibodies. Antidrug antibodies toward brodalumab were not detected in any of the samples using either of these assays, so antidrug antibodies seem to explain neither the subquantifiable levels of brodalumab nor lack of response in this group of patients.
The risk of developing psoriasis has been shown to be significantly higher in patients with obesity compared with individuals without obesity.17 Similarly, a higher BMI has been shown to correlate with disease severity16,18 and BMI has also been shown to adversely affect the response to biological therapies.16,19,20 As for similar antibody-based therapeutics, the interindividual clearance variability for brodalumab is notable.14,21,22 In our study, levels of circulating brodalumab decreased from immediately after the induction period of 4 weeks to the assessment point after 12 weeks of therapy in most patients. Differences in body weight have been shown to be associated with some of this variation; thus, using simulated data, Timmermann and Hall14 predicted that a patient weighing 120 kg would have an at least 50% reduction in systemic exposure at steady state following the applied dosing scheme compared with a patient weighing 90 kg. It may also be speculated that the differences in circulating brodalumab levels observed among patients with obesity reflect differences in the degree of ongoing low-grade systemic inflammation as reflected by observed differences in the levels of IL-6 in the present study. Yamaguchi et al16 found a significant association between body weight and PASI and observed increased PASI responses with increased brodalumab levels. These observations are consistent with our findings of both increased PASI response and increased numbers of patients with quantifiably drug levels in patients without obesity compared with patients with obesity.
Our data also suggest that brodalumab bioavailability may be more important than BMI with respect to response because all patients with obesity with quantifiable brodalumab levels achieved a 61% or greater reduction in PASI after 12 weeks of therapy. The BMI, however, remains important because 42% of patients with obesity vs 88% of patients without obesity had quantifiable brodalumab levels. In a post hoc analysis of 2 phase 3 brodalumab-trials, Hsu et al23 concluded that the efficacy of brodalumab did not differ between patients with obesity and patients without obesity. However, in the same study, patients treated with ustekinumab who later received rescue therapy with brodalumab showed markedly lower responses in patients with obesity than in patients without obesity, suggesting the importance of body weight as a factor in patients with prior therapy failure.
Notably, none of the 7 patients (35%) with subquantifiable brodalumab levels after 12 weeks of therapy remained PASI 75 responders, suggesting that this subgroup could benefit from dosage increase or switch to a different treatment modality already at this time. However, these results should be validated in additional preferably larger cohorts with special attention to prior treatment failures and BMI.
Several authors of this study have previously reported significantly different baseline levels of IL-6 between responders and nonresponders in the current cohort.12 In the present study, we found significant differences in IL-6 levels between patients with and without quantifiable brodalumab levels after 12 weeks of therapy but, notably, these differences were not significant at baseline. Our data indicate that, on average, patients with quantifiable brodalumab levels maintain their IL-6 levels, while the average IL-6 levels seem to increase significantly from baseline to 12 weeks of therapy in patients without quantifiable brodalumab levels after 12 weeks of therapy. IL-6 plays an important role in psoriasis and is often considered a cytokine preceding the production of IL-17.24,25,26 However, IL-6 production may also be increased as a result of a feedback loop in which IL-6 production sustains and is, in turn, sustained itself by Th17-cells.27 When this loop is not effectively broken in patients with subquantifiable brodalumab levels, Th17-feedback stimulation of IL-6 production may increase IL-6 levels.
Because increased IL-6 expression seems consistent in the subset of nonresponsive patients, investigations of treatment with an IL-6 pathway inhibitor such as tocilizumab or sarilumab in these patients might be warranted. However, documentation of the outcomes associated with tocilizumab in psoriasis treatment are currently limited to case reports. Although several reports of favorable responses to tocilizumab treatment of drug-induced psoriasis and psoriatic lesions have been published, others have described the development of psoriasislike eruptions following its use, but these conditions seemed manageable using minor adjustments of tocilizumab dosing and/or adding topical corticosteroid treatment.28
Limitations
This study has limitations. The number of participants was relatively small. Inclusion of just patients with a history of treatment failure may limit the generalizability of the findings. In addition, the retrospective nature of the study may limit robust conclusions about potential dose adjustment outcomes in individual patients.
Conclusions
In this case series study, circulating trough levels of brodalumab were associated with concomitant response, and subquantifiable brodalumab levels after 12 weeks of therapy identified long-term nonresponders, at least in patients with previous response failure to other biological treatments.
eTable. Baseline Patient Characteristics
References
- 1.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945-1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 2.Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828-3836. doi: 10.4049/jimmunol.1301737 [DOI] [PubMed] [Google Scholar]
- 3.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556-567. doi: 10.1038/nri2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nies JF, Panzer U. IL-17C/IL-17RE: emergence of a unique axis in TH17 biology. Front Immunol. 2020;11:341. doi: 10.3389/fimmu.2020.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egeberg A, Andersen YMF, Halling-Overgaard AS, et al. Systematic review on rapidity of onset of action for interleukin-17 and interleukin-23 inhibitors for psoriasis. J Eur Acad Dermatol Venereol. 2020;34(1):39-46. doi: 10.1111/jdv.15920 [DOI] [PubMed] [Google Scholar]
- 6.Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179(2):320-328. doi: 10.1111/bjd.16464 [DOI] [PubMed] [Google Scholar]
- 7.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181-1189. doi: 10.1056/NEJMoa1109017 [DOI] [PubMed] [Google Scholar]
- 8.Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273-286. doi: 10.1111/bjd.14493 [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl M, Strober B, Menter A, et al. Phase 3 Studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318-1328. doi: 10.1056/NEJMoa1503824 [DOI] [PubMed] [Google Scholar]
- 10.Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183(6):1037-1048. doi: 10.1111/bjd.19132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebwohl M, Leonardi C, Armstrong A, et al. Three-year US pharmacovigilance report of brodalumab. Dermatol Ther. 2021;34(6):e15105. doi: 10.1111/dth.15105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loft N, Bregnhøj A, Fage S, et al. Effectiveness of brodalumab after previous treatment failure of interleukin-17A inhibitors in patients with psoriasis. Dermatol Ther. 2021;34(6):e15106. Published online August 27, 2021. doi: 10.1111/dth.15106 [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Timmermann S, Hall A. Population pharmacokinetics of brodalumab in patients with moderate to severe plaque psoriasis. Basic Clin Pharmacol Toxicol. 2019;125(1):16-25. doi: 10.1111/bcpt.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas LW, Lee EB, Wu JJ. Systematic review of anti-drug antibodies of IL-17 inhibitors for psoriasis. J Dermatolog Treat. 2019;30(2):110-116. doi: 10.1080/09546634.2018.1473552 [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi Y, Kanai Y, Kitabayashi H, Okada H, Nakagawa H. Relationship between serum trough levels and efficacy of brodalumab from a post hoc exploratory analysis of a Japanese study in patients with plaque psoriasis. J Dermatol. 2021;48(3):324-333. doi: 10.1111/1346-8138.15690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125(1):61-67. doi: 10.1111/j.0022-202X.2005.23681.x [DOI] [PubMed] [Google Scholar]
- 18.Fleming P, Kraft J, Gulliver WP, Lynde C. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg. 2015;19(5):450-456. doi: 10.1177/1203475415586332 [DOI] [PubMed] [Google Scholar]
- 19.Schwarz CW, Loft N, Rasmussen MK, et al. Predictors of response to biologics in patients with moderate-to-severe psoriasis: a Danish nationwide cohort study. Acta Derm Venereol. 2021;101(10):adv00579. doi: 10.2340/actadv.v101.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134(1):18-23. doi: 10.1038/jid.2013.326 [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548-558. doi: 10.1038/clpt.2008.170 [DOI] [PubMed] [Google Scholar]
- 22.Endres CJ, Salinger DH, Köck K, et al. Population pharmacokinetics of brodalumab in healthy adults and adults with psoriasis from single and multiple dose studies. J Clin Pharmacol. 2014;54(11):1230-1238. doi: 10.1002/jcph.334 [DOI] [PubMed] [Google Scholar]
- 23.Hsu S, Green LJ, Lebwohl MG, Wu JJ, Blauvelt A, Jacobson AA. Comparable efficacy and safety of brodalumab in obese and nonobese patients with psoriasis: analysis of two randomized controlled trials. Br J Dermatol. 2020;182(4):880-888. doi: 10.1111/bjd.18327 [DOI] [PubMed] [Google Scholar]
- 24.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183(5):3170-3176. doi: 10.4049/jimmunol.0803721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuner P, Urbanski A, Trautinger F, et al. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J Invest Dermatol. 1991;97(1):27-33. doi: 10.1111/1523-1747.ep12477880 [DOI] [PubMed] [Google Scholar]
- 26.Grossman RM, Krueger J, Yourish D, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989;86(16):6367-6371. doi: 10.1073/pnas.86.16.6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camporeale A, Poli V. IL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci (Landmark Ed). 2012;17(6):2306-2326. doi: 10.2741/4054 [DOI] [PubMed] [Google Scholar]
- 28.Choong DJ, Tan E. Does tocilizumab have a role in dermatology? A review of clinical applications, its adverse side effects and practical considerations. Dermatol Ther. 2021;34(4):e14990. doi: 10.1111/dth.14990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline Patient Characteristics