Abstract
It is well known that financial disadvantage is associated with alterations in brain development in regions critical to socioemotional well-being such as the hippocampus and the amygdala. Yet little is known about whether family income at different points in development is differentially associated with these structures. Furthermore, little is known about which environmental factors statistically mediate associations between income and subcortical structure. Using a longitudinal birth cohort and linear mixed-effects models, we identified associations between income-to-needs ratio (INR) at 6 timepoints throughout childhood and hippocampal and amygdala volumes at age 7–9 years (n = 41; 236 INR measurements; 41 brain measurements). Mediation analysis identified environmental sequelae of income that statistically accounted for INR–brain associations. Lower INR prior to age 4 was associated with smaller hippocampal volumes, whereas lower INR prior to age 2 was associated with smaller right amygdala volume. These associations were mediated by unmet basic needs (e.g., food, housing). These findings delineate the temporal specificity of associations between income and hippocampal and amygdala structures.
Keywords: amygdala, hippocampus, income, material hardship, sensitive period
1 |. INTRODUCTION
Approximately 13 million children in the United States (18%) live in poverty (United States Census Bureau, 2018c). An additional 16 million are low-income (income<200% of the federal poverty line; United States Census Bureau, 2018a). Financial disadvantage is associated with a wide range of poor mental health outcomes including depression, anxiety, and attention-deficit hyperactivity disorder (McLaughlin et al., 2011; Peverill et al., 2020; Sareen et al., 2011; Yoshikawa et al., 2012). One biological pathway by which income may be related to mental illness is through a reduction in hippocampal and amygdala volumes (Barch et al., 2020; Brito& Noble, 2014; Dufford et al., 2020), two subcortical brain regions critical to a wide range of cognitive, social, and emotional abilities (Adolphs, 2010; Eichenbaum, 2004; Hamilton et al., 2008; Videbech & Ravnkilde, 2004). Many studies that have found associations between income and these structures examined income at a single timepoint, whether prospectively measured (Ellwood-Lowe et al., 2018; Hair et al., 2015; McDermott et al., 2019), retrospectively reported (Staff et al., 2012), or measured concurrently with neuroimaging (Dufford et al., 2019; Hanson et al., 2011; Noble et al., 2012; Uban et al., 2020). Furthermore, the sign of income–amygdala associations has been inconsistent (Noble et al., 2012), perhaps due to the unique effects of income timing and chronicity (Hanson&Nacewicz, 2020; Merz et al., 2018). Prospective longitudinal approaches are needed to understand whether there exists a sensitive period (a developmental period of heightened sensitivity) for the association between income and the volumes of these subcortical structures in childhood. Longitudinal studies have shown that early childhood often constitutes a sensitive period for the neural correlates of early life adversity in general (Humphreys et al., 2019; Luby et al., 2016; Lupien et al., 2009). However, little is known about the effects of income at various developmental stages on subcortical volumes. Given the rapid rate of structural brain development during the first years of life (Knickmeyer et al., 2008), this may be a period of heightened vulnerability to the influence of financial disadvantage on the brain.
Also understudied are the environmental factors that mediate income–subcortex associations. In this context, mediators are environmental characteristics that are associated with income and are more temporally proximal to brain volumes. Importantly, statistical mediation does not prove causation, but identifying statistical mediators may inform future, experimental studies on the mechanisms linking income and poor outcomes. Possible mediators between income and subcortical volumes include environmental adversities that might vary with income such as material hardship (unmet basic needs; Mayer & Jencks, 1989), and parental distress (Goosby, 2007).
Using a prospective longitudinal birth cohort, we sought to delineate timing-specific effects of income across the first seven years of life on hippocampal and amygdala volumes at age 7–9 years. We hypothesized: (a) that INR in the first years of life would be more strongly associated with hippocampal and amygdala volumes in middle childhood than later poverty and (b) that INR-associated environmental adversities would mediate INR-brain associations. Identifying sensitive periods and mediators of INR-subcortex associations would have implications for the timing and types of interventions required to address neural and psychiatric disparities.
2 |. MATERIALS AND METHODS
2.1 |. Participants
Prenatal recruitment for the Sibling–Hermanos birth cohort (SHBC) began in 2008, and participants were a community sample of pregnant women from the South Bronx, Harlem, and Northern Manhattan who were already part of the Mothers and Newborns birth cohort (started in 1998; Cowell et al., 2017; Perera et al., 2006). Participants were excluded on the basis of prenatal maternal active smoking or if they gave birth before their scheduled third-trimester visit (third-trimester data are critical to the main aims of the SHBC).
Participants in the current sub-study included 53 children aged 7–9 years from the SHBC. Five participants opted against participating in the neuroimaging portion of our study. A trained research assistant visually inspected each T1-weighted image for head motion-related artifacts (e.g., ringing, blurring) and identified seven participants who did not provide artifact-free images. In total, 41 participants provided useable hippocampal and amygdala volumetric data. This study was approved by the institutional review boards at Columbia University and New York State Psychiatric Institute. All children and guardians provided written informed assent and consent, respectively. All research conforms to the recognized standards outlined in the Declaration of Helsinki and the U.S. Federal Policy for the Protection of Human Subjects.
2.2 |. Income-to-needs ratio
The income-to-needs ratio (INR) was calculated as mother-reported household income divided by the federal poverty threshold for each participant’s household size at child age 6 months and 1, 2, 3, 5, and 7 years (Supporting Information for additional details). INR was also measured at one timepoint during the third trimester. Our primary analyses focused on associations between postnatal INR and subcortical volumes because the putative mechanisms linking postnatal income (direct experience of adversity) to altered brain development are distinct from those of prenatal income (i.e., transplacental conferral of stress hormones, inflammation, and nutrition; Hantsoo et al., 2019; Lefmann & Combs-Orme, 2014; O’Donnell et al., 2009; Ramphal et al., 2020; Spann et al., 2020). Nevertheless, supplementary analyses included the prenatal INR timepoint.
2.3 |. Potential mediators linking INR and brain volumes
At each timepoint, mothers participated in a structured interview that included questions about environmental adversities. Potential mediators included material hardship (Mayer & Jencks, 1989) and two measures of maternal psychological distress: demoralization (Dohrenwend et al., 1980) and perceived stress (Cohen et al., 1994). A timeline of data collection (Table S1) is presented in the Supporting Information.
2.4 |. MRI acquisition and processing
Neuroimaging data were collected using a 3T GE 750 scanner with a 32-channel head coil at one timepoint between child age 7 and 9 years. Two structural T1 images were collected per participant using a 3D FSPGR sequence (11° flip angle, TE = 2.588 ms, TR = 6.412 ms, 180 slices, 1 mm isotropic resolution). Structural images were visually inspected, and subjects with apparent motion artifacts were excluded. T1 images were anatomically segmented using the recon-all command in FreeSurfer v6.0, segmentations were visually inspected by a trained research assistant for quality assurance, and amygdala and hippocampal volumes were extracted (Fischl et al., 2002, 2004).
2.5 |. Statistical analyses
Total (sum of left and right) hippocampal and total amygdala volumes were calculated and residualized for age, sex assigned at birth, and estimated total intracranial volume, given the effects of these variables on regional brain volumes (Koolschijn & Crone, 2013; Pintzka et al., 2015). These residualized volumes were used in all analyses to preserve degrees of freedom and because we did not have hemisphere-specific hypotheses. To test our first hypothesis, we used a linear mixed-effects model with a random effect for participant and tested the interaction between timepoint of INR measurement (age, ranging from 0.5 to 7 years) and hippocampus/amygdala volumes at age 7–9 years on INR. This method of analysis, which permits missing data, tests whether the slope relating brain volumes to INR varies linearly with the timepoint of INR measurement. INR was the dependent variable because it was the repeated measure, while timepoint of INR measurement, brain volumes, and their interaction were the independent variables. Bonferroni correction was applied for two brain regions (α = .025). Post hoc Johnson–Neyman analyses were performed to determine the developmental window during which INR-brain associations were significant. Although this approach of switching the independent and dependent variables is counterintuitive, it allows a parsimonious examination of timing-specific associations between INR and subcortical volumes and has been previously used (Chen et al., 2015).
We also used a complementary approach that preserves intuitive independent and dependent variable assignments and employs generalized estimating equations to simultaneously regress subcortical volumes on INR at multiple timepoints (Supporting Information). To ensure that our findings were due to financial dynamics rather than family compositional changes, we tested our model using raw income bins instead of derived INR values. To examine whether maternal education drove any observed effects, we implemented our main model again using subcortical volumes that were further residualized for years of maternal education.
Supplementary analyses examined how results varied when each nuisance covariate was residualized individually, the effects of prenatal INR on the estimation of INR’s sensitive periods, whether results varied by brain hemisphere, and whether the inclusion of timepoint2 and brain volume × timepoint2 term improved our models estimating INR’s sensitive periods.
To test our second hypothesis, we first identified which environmental adversities (measured at the timepoint after which INR first had an effect) were correlated with INR. Bonferroni correction was applied for four possible mediators (α = .0125). Next, we tested whether these mediated any associations between INR and subcortical volumes. The indirect effect of INR on brain volumes through a mediator was defined as the average causal mediation effect (ACME). The ACME equals the product, ab, of the regression coefficient, a, relating the independent variable (INR) to the mediator and the regression coefficient, b, relating the mediator to the dependent variable (brain volume) with the independent variable as a covariate. Whether the ACME was significantly different from zero was tested using a boot-strapping method implemented in the R mediation package with 10,000 resamples (Tingley et al., 2014). Bonferroni correction was applied for two brain regions (α = .025). All analyses were performed using R v4.0.0 (Core Team, 2015), employed pairwise deletion, statistical tests were two-sided, and model diagnostics are reported in the Supporting Information.
3 |. RESULTS
3.1 |. Participants
Sample characteristics are presented in Table 1, and correlations among all variables of interest in this study are presented in Table S2. Participants were predominantly low-income (INR < 2, i.e., having income less than twice the poverty line for their household size) with some participants being middle-income (2 < INR < 4; Figure S1). While those who were low- or middle- income at age 6 months predominantly remained in the same category at age 7 years (Figure S2), INR did fluctuate between timepoints for all participants.
TABLE 1.
Sample characteristics
| N = 41 | Mean (SD)/N (%) | Range |
|---|---|---|
| Sex assigned at birth (Female) | 24 (59%) | Female/Male |
| Age (Years) | 8.6 (0.7) | 7.0–9.8 |
| Income-to-needs ratio | ||
| Prenatal | 1.4 (1.0) | 0.2–4.3 |
| 6 months | 1.1 (0.7) | 0.2–3.0 |
| 1 year | 1.2 (0.8) | 0.2–3.4 |
| 2 years | 1.2 (0.8) | 0.2–1.6 |
| 3 years | 1.1 (0.8) | 0.2–4.1 |
| 5 years | 1.1 (0.7) | 0.2–4.0 |
| 7 years | 1.1 (0.7) | 0.2–3.5 |
| Across all timepoints | 1.2 (0.8) | 0.2–4.3 |
| Self-reported ethnicity | ||
| African-American | 19 (46%) | African-American/Hispanic |
| Maternal education (in third trimester) | ||
| No high school or equivalency | 12 (29%) | |
| High school or equivalency | 8 (20%) | |
| Some college | 11(27%) | |
| Associate’s degree | 6 (15%) | |
| Bachelor’s degree | 3 (7%) | |
| Graduate degree | 1 (2%) |
3.2 |. Income-to-needs ratio over the first seven years of life and hippocampal and amygdala volumes
The hippocampus × timepoint (β = −0.079, p = .020), and amygdala × timepoint (β = −0.108, p = .002) interactions were associated with INR (Figure 1, see Figure S3 for simple slopes), indicating that INR–brain associations varied based on the child age at which INR was measured. These results were corroborated with a complementary approach using generalized estimating equations (Table S3). Additionally, when a variable representing income bins (1–10) was used instead of INR, our findings persisted (Table S4). Finally, when subcortical volumes were additionally residualized for years of maternal education, our income findings remained significant (Table S5).
FIGURE 1.
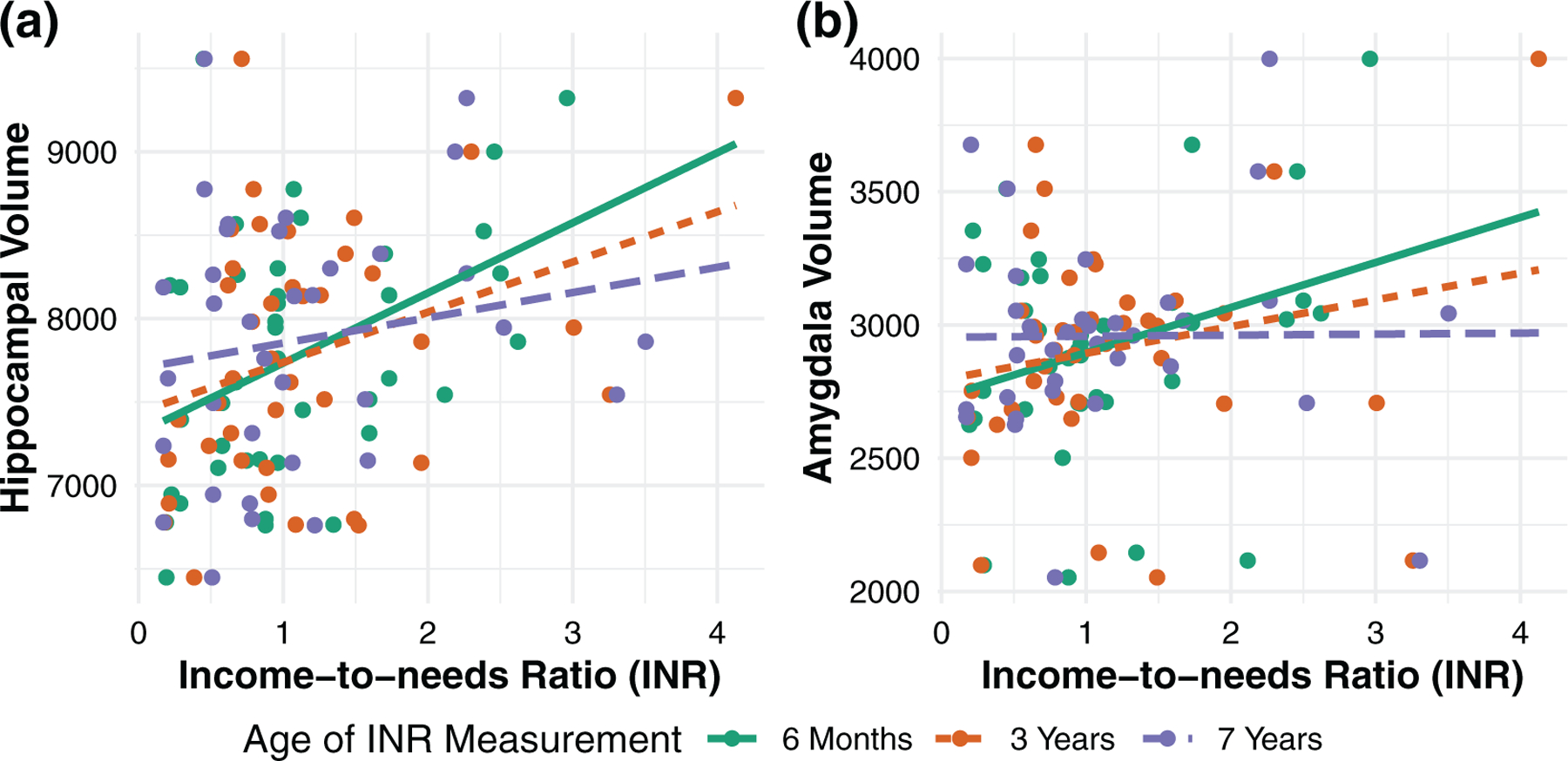
The association between income-to-needs ratio (INR) and residualized subcortical volumes (mm2) depends on when INR is measured. Earlier INR is more strongly associated with hippocampal and amygdala volumes than later INR
Johnson–Neyman analyses indicated that hippocampal volumes were positively associated with INR when it was measured before age 4.5 years, and amygdala volumes were positively associated with INR when it was measured before age 1.2 years (Figure S4).
In sensitivity analyses, effect sizes were similar to our main analyses when subcortical volumes were residualized for intracranial volume and reduced when not (Table S6). Including prenatal INR in our main model produced a similar pattern of results as the main analysis, though with attenuated effect sizes (Table S7). The bivariate association between prenatal INR and hippocampal volume was significant (r = .33, p = .04), whereas prenatal INR was not associated with amygdala volume (r = .19, p = .25). Multiple regression models with subcortical volumes as the dependent variable and both prenatal and 6-month INR as predictors reveal larger effect sizes for 6 month INR than prenatal INR (hippocampus: βprenatal = 0.16, β6-month = 0.37; amygdala: βprenatal = 0.01, β6-month = 0.35).
In supplementary analyses, there was no evidence for hemisphere-specific effects (volume × timepoint × hemisphere effect p-values > .5). Including additional terms for timepoint2 and timepoint2 × volume terms did not improve model fit (hippocampus: χ2(2) = 2.0, p = .73; amygdala: χ2(2) = 2.6, p = .62).
3.3 |. Material hardship mediates INR-subcortex associations
Income-to-needs ratio at age 6 months, the timepoint at which INR was most strongly associated with hippocampal and amygdala volumes at age 7–9 years, was used for subsequent analyses examining the mediating role of later stressors. INR at age 6 months was not correlated with maternal demoralization at age 1 (r = −.02, p = .90), or maternal perceived stress at age 1 (r = .02, p = .89); however, 6-month INR was negatively correlated with material hardship at child age 1 year (r = −.41, p = .009, Table S2). This time-staggered approach best described the INR-material hardship association (Table S8).
Material hardship at age 1 year was negatively correlated with both hippocampal (r = −.59, p < .001) and amygdala (r = −.5, p = .001) volumes. In multiple regression specificity analyses, material hardship at age 1-year was negatively associated with subcortical volumes, while material hardship in the third trimester and at age 5 years were not (Table S9).
The indirect effect of INR at 6 months on brain volumes through material hardship at age 1 year was significant for the hippocampus (average causal mediation effect (ACME) = 0.20, p = .009; proportion mediated = 0.49, p = .02), as well as the amygdala (ACME = 0.18, p = .008; proportion mediated = 0.60, p = 0.15).
4 |. DISCUSSION
4.1 |. Summary of results
In the current study, which is preliminary due to its small sample size, we demonstrate that positive associations between INR and hippocampal and amygdala volumes depend on when INR is measured. Specifically, we find that INR prior to age 4.5 years may be most critical for hippocampal volumes in middle childhood and prior to age 1.2 years may be most critical for amygdala volumes. We further show that material hardship mediates INR–brain associations.
4.2 |. Sensitive periods
The hippocampus and the amygdala are two brain regions commonly implicated as vulnerable to the effects of financial disadvantage, yet most previous studies on this topic measure income at a single timepoint (Dufford et al., 2019; Ellwood-Lowe et al., 2018; Hair et al., 2015; Hanson et al., 2011; McDermott et al., 2019; Noble et al., 2012; Staff et al., 2012; Uban et al., 2020). By leveraging a predominantly low-income longitudinal birth cohort with frequent income measurements, we demonstrate that earlier income is more associated with subcortical volumes than later income. Although very few participants moved from low- to middle-income or vice versa, our study was nevertheless able to detect differential effects between earlier and later INR. This suggests that the development of the hippocampus and the amygdala may be highly sensitive to small variation in income in this low-income sample. Indeed, small fluctuations in income can have substantive effects on a family’s ability to meet their needs in low-income contexts (Brownell et al., 2018; Rojas et al., 2020). Consistent with the idea that income variation can be impactful for low-income households, Noble and colleagues have previously shown that the association between income and cortical surface area is most pronounced in low-income children (Noble et al., 2015).
Our finding that early income is most related to brain volumes in middle childhood is likely due to the rapid rate of brain growth during the first few years of life (Knickmeyer et al., 2008). One study showed that prior to age five represents a sensitive period for associations between early life stressful events and hippocampal volumes (Humphreys et al., 2019). We extend this finding to income, to the amygdala, and provide further temporal specificity. Convergent with our findings, longitudinal studies have shown that INR prior to age five is more detrimental to physical (Kalil et al., 2016) and mental health (Mazza et al., 2017; McFarland, 2017) than later INR. Furthermore, it has been shown that adversity prior to age 3 years may constitute a sensitive period for DNA methylation (Dunn et al., 2019), a mechanism by which early experience may confer its effects on hippocampal volumes (Davis et al., 2017; Jia et al., 2019).
The sensitive period identified for the amygdala was more circumscribed than that of the hippocampus, potentially due to the earlier development of the amygdala’s stress response (Tottenham & Sheridan, 2010). Animal evidence suggests that by early postnatal life, the amygdala exhibits increased corticotropin-releasing hormone mRNA production following a stressor (Avishai-Eliner et al., 1996), while this phenomenon does not emerge in the hippocampus until substantially later (Fenoglio et al., 2004). In humans, the amygdala volume increases by approximately 105% in the first year of life, while the hippocampus grows by 84% (Gilmore et al., 2012). Thus, the relatively protracted functional and structural development of the hippocampus may explain its wider sensitive period.
Interestingly, although the fetal brain triples in size during the third trimester (Bouyssi-Kobar et al., 2016), the effects of income and material hardship in this time period were minimal. Because any influence of prenatal financial disadvantage on fetal development must be transmitted in the form of glucocorticoids, cytokines, toxicants, nutrition, or other transplacental molecules, any associations between prenatal income and brain outcomes are necessarily limited by the extent to which income is associated with these systemic effects (Gilman et al., 2017; Keenan-Devlin et al., 2017; Miller et al., 2017). Because our sample was predominantly low-income, it is possible that we did not have a sufficient range in prenatal income to detect effects mediated by these transplacental factors. Another possibility is that the contributions of prenatal INR to subcortical volumes in childhood are more complex, whether by exerting effects that are conditional on later disadvantage (O’Donnell & Meaney, 2017) or by exerting opposite effects (Qiu et al., 2013; Rifkin-Graboi et al., 2018). Future studies with larger samples and greater income ranges may be able to query the influences of prenatal income on brain outcomes via these mechanisms.
4.3 |. Material hardship
Material hardship mediated the effects of INR on hippocampal and amygdala volumes. The specific resources queried by our material hardship questionnaire included food, shelter, utilities, clothing, and healthcare. Indeed, when these needs are unmet, children experience more anxiety, depression, inattention, and hyperactivity, and parents face higher rates of mental illness (Knowles et al., 2016; Loukas & Prelow, 2004; Melchior et al., 2009, 2012; Slopen et al., 2010; Whitaker et al., 2006; Zilanawala & Pilkauskas, 2012). Few studies have examined the mediators through which income exerts its effects on hippocampal and amygdala volumes. One study found that harsh parenting mediated the association between income and reduced hippocampal but not amygdala volumes in a sample enriched for preschool depression (Luby et al., 2013). Though we did not examine parenting style, we considered two measures of maternal psychological distress: demoralization and perceived stress. Neither of these was significantly associated with concurrent or antecedent INR or material hardship. One possibility is that material hardship adversely affects parenting in a manner that is not captured by our distress variables. Another possibility is that unmet needs directly affect brain development, given the moderate correlations between material hardship and hippocampal and amygdala volumes. Indeed, animal models have shown that these brain regions are particularly vulnerable to poor nutrition (Coupé et al., 2009; Escobar & Salas, 1993; Hoeijmakers et al., 2015; Pravosudov et al., 2005) and unpredictable stress (Alfarez et al., 2003; Kosten et al., 2008).
4.4 |. Limitations
The current study should be interpreted as preliminary given our limited sample size. For example, prior studies suggest that associations between subcortical volumes and mental health (Videbech & Ravnkilde, 2004) and between subcortical volumes and stress (McLaughlin et al., 2019; Wang et al., 2019) are hemisphere- or subregion-specific. Although we tested volume × timepoint × hemisphere interactions, these analyses had limited power to detect potentially extant hemisphere-specific associations between INR and amygdala and hippocampal volumes. Future studies with larger sample sizes are needed to corroborate these findings.
Furthermore, additional factors across development likely contribute to and moderate the association between income-to-needs ratio and subcortical volumes, such as environmental toxicant burden or preterm birth which are known to vary based on socioeconomic factors (Cureton, 2011) or paternal distress, which was not collected in our study. Additionally, it is possible that INR-brain associations may be mediated by different environmental factors at different points in development. Future studies with longitudinal MRI designs are needed to investigate whether the associations demonstrated here persist into adolescence and adulthood.
4.5 |. Opportunities for intervention: timing and type
The current study, though limited due to its small sample size, highlights several potential timepoints and modalities for intervention against the neural consequences of financial disadvantage. By identifying sensitive periods for the INR–subcortical volume associations, we suggest that very early life may be a particularly critical time for beginning anti-poverty measures. That these effects are mediated by material hardship provides evidence that guaranteed access to food, housing, utilities, clothing, and healthcare may be beneficial to child brain development. This notion may perhaps seem unsurprising. However, 45% of households are rent-burdened (United States Census Bureau, 2019) and over one million children in the United States are homeless (National Center for Homeless Education, 2020). Four million children are medically uninsured with lower-income families facing lower rates of insurance (United States Census Bureau, 2018b, 2018d); Yet, quasi-experimental human studies have shown that income supplementation can be beneficial for child mental (Akee et al., 2015; Costello et al., 2003) and physical health (Hoynes et al., 2016).
Interventional studies are needed to more directly establish causality among the longitudinal associations we have demonstrated between income, material hardship, and brain structure (Luby et al., 2020). Nevertheless, in the absence of such studies, we have ample evidence that financial disadvantage is associated with child cognition, emotional wellbeing, and brain development (Farah, 2017; McLaughlin et al., 2011; Sareen et al., 2011; Yoshikawa et al., 2012). Furthermore, while individual-level intervention may be useful, it is equally important not to construe these disparities as individual-level phenomena. Thus, individual-level approaches must be coupled with policy interventions against the distal, clandestine, structural factors that permit and recapitulate neural and psychiatric disparities (Lende, 2012; Link & Phelan, 1995; Macvarish et al., 2014; Phelan et al., 2010). Resource inequality is rampant and expanding in the context of the COVID-19 pandemic (Coughlin et al., 2020; Dunn et al., 2020; Kinsey et al., 2020; Patrick et al., 2020). Yet, small experiments involving resource redistribution have produced powerful results for children (Akee et al., 2015; Costello et al., 2003). The adoption of alternative, equitable economic norms would necessarily be more far-reaching than ad-hoc individual-based intervention and perhaps more effective at alleviating health disparities more broadly (Link & Phelan, 1995; Phelan et al., 2010).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIEHS grants K23ES026239 and R01ES030950.
Funding information
NIEHS, Grant/Award Numbers: K23ES026239, R01ES030950
Footnotes
CONFLICTS OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- Adolphs R (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191(1), 42–61. 10.1111/j.1749-6632.2010.05445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akee R, Simeonova E, Costello EJ, & Copeland W (2015). How does household income affect child personality traits and behaviors? National Bureau of Economic Research Working Paper Series, No. 21562. 10.3386/w21562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarez DN, Joëls M,& Krugers HJ (2003). Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. European Journal of Neuroscience, 17(9), 1928–1934. 10.1046/j.1460-9568.2003.02622.x [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, & Baram TZ (1996). Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Brain Research Developmental Brain Research, 91(2), 159–163. 10.1016/0165-3806(95)00158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Shirtcliff EA, Elsayed NM, Whalen D, Gilbert K, Vogel AC, Tillman R, & Luby JL (2020). Testosterone and hippocampal trajectories mediate relationship of poverty to emotion dysregulation and depression. Proceedings of the National Academy of Sciences of the United States of America, 117(36), 22015. 10.1073/pnas.2004363117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyssi-Kobar M, du Plessis AJ, McCarter R, Brossard-Racine M, Murnick J, Tinkleman L, Robertson RL, & Limperopoulos C (2016). Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics, 138(5), e20161640. 10.1542/peds.2016-1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276. 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell M, Nickel NC, Chartier M, Enns JE, Chateau D, Sarkar J, Burland E, Jutte DP, Taylor C, & Katz A (2018). An Unconditional Prenatal Income Supplement Reduces Population Inequities In Birth Outcomes. Health Affairs, 37(3), 447–455. 10.1377/hlthaff.2017.1290 [DOI] [PubMed] [Google Scholar]
- United States Census Bureau (2018a). Age by ratio of income to poverty level in the past 12 months (Table S17024). In (2018: American Community Survey 1-Year Estimates Subject Tables ed.). [Google Scholar]
- United States Census Bureau (2018b). Health insurance coverage status and type by ratio of income to poverty level in the past 12 months (Table B27016). In (2018: American Community Survey 1-Year Estimates Subject Tables ed.). [Google Scholar]
- United States Census Bureau (2018c). Poverty status in the past 12 months (Table S1701). In (2018: American Community Survey 1-Year Estimates Subject Tables ed.). [Google Scholar]
- United States Census Bureau (2018d). Selected characteristics of health insurance coverage in the united states (Table S2701). In (2018: American Community Survey 1-Year Estimates Subject Tables ed.). [Google Scholar]
- United States Census Bureau (2019). Gross rent as a percentage of household income in the past 12 months (Table B25070). In (2019: American Community Survey 1-Year Estimates Subject Tables ed.). [Google Scholar]
- Chen Y-H, Ferguson KK, Meeker JD, McElrath TF, & Mukherjee B (2015). Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environmental Health, 14(1), 9. 10.1186/1476-069X-14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. Measuring stress: A guide for health and social scientists, 10, 1–2. [Google Scholar]
- R Core Team. (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: (2013). Supplementary Figure S, 2. [Google Scholar]
- Costello EJ, Compton SN, Keeler G, & Angold A (2003). Relationships between poverty and psychopathology: a natural experiment. Jama, 290(15), 2023–2029. 10.1001/jama.290.15.2023 [DOI] [PubMed] [Google Scholar]
- Coughlin CG, Sandel M, & Stewart AM (2020). Homelessness, Children, and COVID-19: A Looming Crisis. Pediatrics, 146(2), e20201408. 10.1542/peds.2020-1408 [DOI] [PubMed] [Google Scholar]
- Coupé B, Dutriez-Casteloot I, Breton C, Lefèvre F, Mairesse J, Dickes-Coopman A, Silhol M, Tapia-Arancibia L, Lesage J, & Vieau D (2009). Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. Journal of Neuroendocrinology, 21(1), 40–48. 10.1111/j.1365-2826.2008.01806.x [DOI] [PubMed] [Google Scholar]
- Cowell WJ, Stapleton HM, Holmes D, Calero L, Tobon C, Perzanowski M, & Herbstman JB (2017). Prevalence of historical and replacement brominated flame retardant chemicals in New York City homes. Emerging Contaminants, 3(1), 32–39. 10.1016/j.emcon.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton S (2011). Environmental victims: environmental injustice issues that threaten the health of children living in poverty. Reviews on Environmental Health, 26(3), 141–147. 10.1515/reveh.2011.021 [DOI] [PubMed] [Google Scholar]
- Davis EG, Humphreys KL, McEwen LM, Sacchet MD, Camacho MC, MacIsaac JL, Lin DTS, Kobor MS, & Gotlib IH (2017). Accelerated DNA methylation age in adolescent girls: associations with elevated diurnal cortisol and reduced hippocampal volume. Translational Psychiatry, 7(8), e1223–e1223. 10.1038/tp.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP, Shrout PE, Egri G, & Mendelsohn FS (1980). Non-specific psychological distress and other dimensions of psychopathology: Measures for use in the general population. Archives of General Psychiatry, 37(11), 1229–1236. 10.1001/archpsyc.1980.01780240027003 [DOI] [PubMed] [Google Scholar]
- Dufford AJ, Bianco H, & Kim P (2019). Socioeconomic disadvantage, brain morphometry, and attentional bias to threat in middle childhood. Cognitive, Affective & Behavioral Neuroscience, 19(2), 309–326. 10.3758/s13415-018-00670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford AJ, Kim P, & Evans GW (2020). Chapter Four - The impact of childhood poverty on brain health: Emerging evidence from neuroimaging across the lifespan. In Clow A & Smyth N (Eds.), International review of neurobiology (Vol. 150, pp. 77–105). Academic Press. [DOI] [PubMed] [Google Scholar]
- Dunn CG, Kenney E, Fleischhacker SE, & Bleich SN (2020). Feeding low-income children during the Covid-19 pandemic. New England Journal of Medicine, 382(18), e40. 10.1056/NEJMp2005638 [DOI] [PubMed] [Google Scholar]
- Dunn EC, Soare TW, Zhu Y, Simpkin AJ, Suderman MJ, Klengel T, Smith ADAC, Ressler KJ, & Relton CL (2019). Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective, longitudinal study. Biological Psychiatry, 85(10), 838–849. 10.1016/j.biopsych.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Homeless Education (2020). Federal Data Summary School Years 2015–16 to 2017–18 for the Education for Homeless Children and Youth Retrieved from https://nche.ed.gov/wp-content/uploads/2020/01/Federal-Data-Summary-SY-15.16-to-17.18-Published-1.30.2020.pdf
- Eichenbaum H (2004). Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 44(1), 109–120. 10.1016/j.neuron.2004.08.028 [DOI] [PubMed] [Google Scholar]
- Ellwood-Lowe ME, Humphreys KL, Ordaz SJ, Camacho MC, Sacchet MD, & Gotlib IH (2018). Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Developmental Cognitive Neuroscience, 30, 41–50. 10.1016/j.dcn.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, & Salas M (1993). Neonatal undernutrition and amygdaloid nuclear complex Development: An experimental study in the rat. Experimental Neurology, 122(2), 311–318. 10.1006/exnr.1993.1130 [DOI] [PubMed] [Google Scholar]
- Farah MJ (2017). The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron, 96(1), 56–71. 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, & Baram TZ (2004). Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology, 145(6), 2702–2706. 10.1210/en.2004-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A,Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, & Dale AM (2004). Sequence-independent segmentation of magnetic resonance images. Neuroimage, 23(1), S69–S84. 10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Gilman SE, Hornig M, Ghassabian A, Hahn J, Cherkerzian S, Albert PS, Buka SL, & Goldstein JM (2017). Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proceedings of the National Academy of Sciences of the United States of America, 114(26), 6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, & Shen D (2012). Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex, 22(11), 2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ (2007). Poverty duration, maternal psychological resources, and adolescent socioemotional outcomes. Journal of Family Issues, 28(8), 1113–1134. 10.1177/0192513X07300712 [DOI] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, & Pollak SD (2015). Association of child poverty, brain development, and academic achievement. JAMA Pediatrics, 169(9), 822–829. 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, & Gotlib IH (2008). Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry, 13(11), 993–1000. 10.1038/mp.2008.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between income and the hippocampus. PLoS One, 6(5), e18712–e18712. 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, & Nacewicz BM (2020). Amygdala allostasis and early life adversity: Considering excitotoxicity and inescapability in the sequelae of stress. PsyArXiv. 10.31234/osf.io/7gcuw [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantsoo L, Kornfield S, Anguera MC, & Epperson CN (2019). Inflammation: A proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biological Psychiatry, 85(2), 97–106. 10.1016/j.biopsych.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Lucassen PJ, & Korosi A (2015). The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Frontiers in Molecular Neuroscience, 7, 103. 10.3389/fnmol.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoynes H, Schanzenbach DW, & Almond D (2016). Long-Run Impacts of Childhood Access to the Safety Net. American Economic Review, 106(4), 903–934. 10.1257/aer.20130375 [DOI] [Google Scholar]
- Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, Ho TC, & Gotlib IH (2019). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Developmental Science, 22(3), e12775. 10.1111/desc.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T, Chu C, Liu Y, van Dongen J, Papastergios E, Armstrong NJ, Bastin ME, Carrillo-Roa T, den Braber A, Harris M, Jansen R, Liu J, Luciano M, Ori APS, Santiañez RR, Ruggeri B, Sarkisyan D, Shin J, Sungeun K, … Desrivières S (2019). Epigenome-wide meta-analysis of blood DNA methylation and its association with subcortical volumes: findings from the ENIGMA Epigenetics Working Group. Molecular Psychiatry. 10.1038/s41380-019-0605-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A, Duncan G, & Ziol-Guest KM (2016). Early childhood poverty: Short and long-run consequences over the lifespan. In: Mortimer JT & Shanahan MJ (eds.), Handbook of the life course, handbooks of sociology and social research (pp. 341–354). Springer Science. [Google Scholar]
- Keenan-Devlin LS, Ernst LM, Ross KM, Qadir S, Grobman WA, Holl JL, Crockett A, Miller GE, & Borders AEB (2017). Maternal income during pregnancy is associated with chronic placental inflammation at birth. American Journal of Perinatology, 34(10), 1003–1010. 10.1055/s-0037-1601353 [DOI] [PubMed] [Google Scholar]
- Kinsey EW, Kinsey D, & Rundle AG (2020). COVID-19 and Food Insecurity: An uneven patchwork of responses. Journal of Urban Health, 97(3), 332–335. 10.1007/s11524-020-00455-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, & Gilmore JH (2008). A structural MRI study of human brain development from birth to 2 years. The Journal of Neuroscience, 28(47), 12176. 10.1523/JNEUROSCI.3479-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M, Rabinowich J, Ettinger de Cuba S, Cutts DB, & Chilton M (2016). “Do you wanna breathe or eat?”: Parent perspectives on child health consequences of food insecurity, trade-offs, and toxic stress. Maternal and Child Health Journal, 20(1), 25–32. 10.1007/s10995-015-1797-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, & Crone EA (2013). Sex differences and structural brain maturation from childhood to early adulthood. Developmental Cognitive Neuroscience, 5, 106–118. 10.1016/j.dcn.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Galloway MP, Duman RS, Russell DS, & D’Sa C (2008). Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology, 33(7), 1545–1558. 10.1038/sj.npp.1301527 [DOI] [PubMed] [Google Scholar]
- Lefmann T, & Combs-Orme T (2014). Prenatal stress, poverty, and child outcomes. Child and Adolescent Social Work Journal, 31(6), 577–590. 10.1007/s10560-014-0340-x [DOI] [Google Scholar]
- Lende DH (2012). Poverty poisons the brain. Annals of Anthropological Practice, 36(1), 183–201. 10.1111/j.2153-9588.2012.01099.x [DOI] [Google Scholar]
- Link BG, & Phelan J (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 35, 80–94. 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- Loukas A, & Prelow HM (2004). Externalizing and internalizing problems in low-income latino early adolescents: Risk, resource, and protective factors. The Journal of Early Adolescence, 24(3), 250–273. 10.1177/0272431604265675 [DOI] [Google Scholar]
- Luby JL, Baram TZ, Rogers CE, & Barch DM (2020). Neurodevelopmental optimization after early-life adversity: Cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends in Neurosciences, 43, 744–751, 10.1016/j.tins.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, & Barch D (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167(12), 1135–1142. 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Harms MP, Tillman R, & Barch DM (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences of the United States of America, 113(20), 5742. 10.1073/pnas.1601443113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Macvarish J, Lee E, & Lowe P (2014). The ‘First Three Years’ movement and the infant brain: A review of critiques. Sociology Compass, 8(6), 792–804. 10.1111/soc4.12183 [DOI] [Google Scholar]
- Mayer SE, & Jencks C (1989). Poverty and the distribution of material hardship. The Journal of Human Resources, 24(1), 88–114. 10.2307/145934 [DOI] [Google Scholar]
- Mazza JRSE, Lambert J, Zunzunegui MV, Tremblay RE, Boivin M, & Côté SM (2017). Early adolescence behavior problems and timing of poverty during childhood: A comparison of lifecourse models. Social Science & Medicine, 177, 35–42. 10.1016/j.socscimed.2017.01.039 [DOI] [PubMed] [Google Scholar]
- McDermott CL, Seidlitz J, Nadig A, Liu S, Clasen LS, Blumenthal JD, Reardon PK, Lalonde F, Greenstein D, Patel R, Mallar Chakravarty M, Lerch JP, & Raznahan A (2019). Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. The Journal of Neuroscience, 39(8), 1365–1373. 10.1523/jneurosci.1808-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland MJ (2017). Poverty and problem behaviors across the early life course: The role of sensitive period exposure. Population Research and Policy Review, 36(5), 739–760. 10.1007/s11113-017-9442-4 [DOI] [Google Scholar]
- McLaughlin KA, Breslau J, Green JG, Lakoma MD, Sampson NA, Zaslavsky AM, & Kessler RC (2011). Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Social Science & Medicine, 73(7), 1088–1096. 10.1016/j.socscimed.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, & Bitrán D (2019). Childhood adversity and neural development: A systematic review. Annual Review of Developmental Psychology, 1(1), 277–312. 10.1146/annurev-devpsych-121318-084950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Caspi A, Howard LM, Ambler AP, Bolton H, Mountain N, & Moffitt TE (2009). Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics, 124(4), e564. 10.1542/peds.2009-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Chastang J-F, Falissard B, Galéra C, Tremblay RE, Côté SM, & Boivin M (2012). Food insecurity and children’s mental health: A prospective birth cohort study. PLoS One, 7(12), e52615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, & Noble KG (2018). Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 47(2), 312–323. 10.1080/15374416.2017.1326122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Borders AE, Crockett AH, Ross KM, Qadir S, Keenan-Devlin L, Leigha AK, Hama P, Mad J, Arevalod JMG, Ernst LM, & Cole SW (2017). Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain, Behavior, and Immunity, 64, 276–284. 10.1016/j.bbi.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, … Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–527. 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, & Meaney MJ (2017). Fetal origins of mental health: The developmental origins of health and disease hypothesis. American Journal of Psychiatry, 174(4), 319–328. 10.1176/appi.ajp.2016.16020138 [DOI] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, & Glover V (2009). Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Developmental Neuroscience, 31(4), 285–292. 10.1159/000216539 [DOI] [PubMed] [Google Scholar]
- Patrick SW, Henkhaus LE, Zickafoose JS, Lovell K, Halvorson A, Loch S, Letterie M, & Davis MM (2020). Well-being of parents and children during the COVID-19 pandemic: A national survey. Pediatrics, 146(4), e2020016824. 10.1542/peds.2020-016824 [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai W-Y, Tang D, Diaz D, Hoepner L, Barr D, Tu Y-H, Camann D, & Kinney P (2006). Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental Health Perspectives, 114(8), 1287–1292. 10.1289/ehp.9084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverill M, Dirks MA, Narvaja T, Herts KL, Comer JS, & McLaughlin KA (2020). Socioeconomic status and child psychopathology in the United States: A meta-analysis of population-based studies. Clinical Psychology Review,83, 101933. 10.1016/j.cpr.2020.101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JC, Link BG, & Tehranifar P (2010). Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. Journal of Health and Social Behavior, 51(1), S28–S40. 10.1177/0022146510383498 [DOI] [PubMed] [Google Scholar]
- Pintzka CWS, Hansen TI, Evensmoen HR, & Håberg AK (2015). Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Frontiers in Neuroscience, 9, 238–238. 10.3389/fnins.2015.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV, Lavenex P, & Omanska A (2005). Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behavioral Neuroscience, 119(5), 1368–1374. 10.1037/0735-7044.119.5.1368 [DOI] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD, Fortier MV, & Meaney MJ (2013). Maternal anxiety and infants’ hippocampal development: timing matters. Translational Psychiatry, 3(9), e306. 10.1038/tp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal B, Whalen DJ, Kenley JK, Yu Q, Smyser CD, Rogers CE, & Sylvester CM (2020). Brain connectivity and socioeconomic status at birth and externalizing symptoms at age 2 years. Developmental Cognitive Neuroscience, 45, 100811. 10.1016/j.dcn.2020.100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Quan J, Richmond J, Goh SKY, Sim LW, Chong YS, Bureau J-F, Chen H,&Qiu A (2018). Greater caregiving risk, better infant memory performance? Hippocampus, 28(7), 497–511. 10.1002/hipo.22949 [DOI] [PubMed] [Google Scholar]
- Rojas NM, Yoshikawa H, Gennetian L, Lemus Rangel M, Melvin S, Noble K, Duncan G, & Magunson K (2020). Exploring the experiences and dynamics of an unconditional cash transfer for low-income mothers: A mixed-methods study. Journal of Children and Poverty, 26(1), 64–84. 10.1080/10796126.2019.1704161 [DOI] [Google Scholar]
- Sareen J, Afifi TO, McMillan KA, & Asmundson GJG (2011). Relationship between household income and mental disorders: findings from a population-based longitudinal study. Archives of General Psychiatry, 68(4), 419–427. 10.1001/archgenpsychiatry.2011.15 [DOI] [PubMed] [Google Scholar]
- Slopen N, Fitzmaurice G, Williams DR, & Gilman SE (2010). Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 49(5), 444–452. 10.1097/00004583-201005000-00005 [DOI] [PubMed] [Google Scholar]
- Spann MN, Bansal R, Hao X, Rosen TS, & Peterson BS (2020). Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychology, 26(2), 170–188. 10.1080/09297049.2019.1648641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, & Whalley LJ (2012). Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology, 71(5), 653–660. 10.1002/ana.22631 [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K (2014). Mediation: R package for causal mediation analysis. Journal of Statistical Software, 59, 1–38.26917999 [Google Scholar]
- Tottenham N, & Sheridan MA (2010). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Kan E, Wozniak JR, Mattson SN, Coles CD, & Sowell ER (2020). The relationship between socioeconomic status and brain volume in children and adolescents with prenatal alcohol exposure. Frontiers in Human Neuroscience, 14, 85. 10.3389/fnhum.2020.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, & Ravnkilde B (2004). Hippocampal volume and depression: A meta-analysis of MRI studies. American Journal of Psychiatry, 161(11), 1957–1966. 10.1176/appi.ajp.161.11.1957 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang H, Wee C-Y, Lee A, Poh JS, Chong Y-S, Tan KH, Gluckman PD, Yap F, Fortier MV, Rifkin-Graboi A, & Qiu A (2019). Maternal sensitivity predicts anterior hippocampal functional networks in early childhood. Brain Structure and Function, 224(5), 1885–1895. 10.1007/s00429-019-01882-0 [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Phillips SM, & Orzol SM (2006). Food insecurity and the risks of depression and anxiety in mothers and behavior problems in their preschool-aged children. Pediatrics, 118(3), e859–e868. 10.1542/peds.2006-0239 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Aber JL, & Beardslee WR (2012). The effects of poverty on the mental, emotional, and behavioral health of children and youth: implications for prevention. American Psychologist, 67(4), 272–284. 10.1037/a0028015 [DOI] [PubMed] [Google Scholar]
- Zilanawala A, & Pilkauskas NV (2012). Material hardship and child socioemotional behaviors: Differences by types of hardship, timing, and duration. Children and Youth Services Review, 34(4), 814–825. 10.1016/j.childyouth.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


