Abstract
EFSA received a mandate from the European Commission to assess the risks related to a possible reduction of the waiting period after rabies antibody titration test to 30 days compared with 90 days of the current EU legislation, for dogs moving from certain non‐EU countries to the EU. This Scientific Report assessed the probability of introduction of rabies into the EU through commercial and non‐commercial movements of vaccinated dogs with a positive titration test (≥ 0.5 IU/mL) if the waiting period decreases from 90 to 30 days. Assuming that all the legal requirements are complied with, the risk of transmission of rabies through the movement of a vaccinated dog is related to the risk of introducing an animal incubating rabies that was infected before the day of vaccination or shortly after vaccination but before the development of immunity (21 days post‐vaccination). Using published data on the incubation period for experimental and field cases in dogs and considering the rabies incidence data in certain countries, the aggregated probability for the annual introduction of rabies through dogs was assessed. Considering the uncertainty related to the duration of the incubation period, the number of imported dogs, and the disease incidence in some countries it was concluded with a 95% certainty that the maximum number of rabies‐infected imported dogs complying with the regulations in a 20‐year period could increase from 5 to 20 when decreasing the waiting period from 90 to 30 days. Nevertheless, the potential impact of even a small increase in probability means the risk is increased for a region like the EU where rabies has long been a focus for eradication, to protect human and animal health.
Keywords: antibody titration test, dog, import, rabies, waiting period, vaccination
Summary
The European Food Safety Authority (EFSA) received a mandate by the European Commission for scientific and technical assistance on the risks related to a possible reduction of the waiting time after rabies antibody titration to 30 days compared with the current EU legislative regime of 90 days. This reduction is indeed being considered at international level as a proposed change to the Rabies Chapter of the World Organisation for Animal Health (OIE) Terrestrial Animal Health Code.
Vaccination against rabies is currently required for dogs, cats and ferrets before entry into the European Union according to Commission Delegated Regulation (EU) 2020/692. Animals must be at least 12 weeks of age at vaccination using approved vaccines according to Annex III to Regulation (EU) 576/2013, and dispatch of the animal must only occur at least 21 days following primary vaccination. Vaccination should be specified in an animal health certificate; also, a virus neutralising antibody titration test (VNT) must be carried out for commercial movements of animals originating in countries or regions specified in column 5 of Annex VIII to Commission Implementing Regulation (EU) 2021/404 or from countries or regions not listed in Annex II to Commission Regulation (EU) No 577/2013. This test must be carried out by a veterinarian authorised by the competent authority on a blood sample taken at least 30 days after the primary vaccination, and the test should demonstrate neutralising antibodies in a concentration ≥ 0.5 IU/mL.
Rabies vaccination is considered effective in animals capable of mounting neutralising antibodies, and the test is carried out to demonstrate this vaccination. However, rabies vaccination is not effective in preventing the development of fulminant rabies in animals already incubating the disease at primary vaccination. Therefore, a waiting period from a positive titration test until import from certain non‐EU countries is required for the commercial and non‐commercial movements of dogs to the EU from certain no‐EU countries where rabies is endemic. This period is currently 90 days (and thus in line with current OIE recommendations) but following a report by the OIE ad hoc Group on Rabies, OIE has proposed to be reduced to 30 days.
Therefore, the European Commission requested scientific advice for the assessment of the risks associated with the possible reduction of the waiting period following positive titration test before movement. Only the so‐called Type A risk (EFSA AHAW Panel, 2007), in which an animal is incubating the disease at vaccination, was considered relevant; Type B risk characterised by failure to induce protective immunity following vaccination was not included, because these animals are expected to test negative for neutralising antibodies. In the vaccinated dogs with Type A risk, this likelihood of becoming infected was considered to include the days before vaccination and 21 days after vaccination, whereas the waiting period following this would be expected to capture those dogs incubating disease as part of Type A risk.
An Extensive Literature Review (ELR) was carried out by an external contractor to characterise the incubation period in experimental trials and natural infections, stratified by vaccinated and unvaccinated dogs. Furthermore, data on dog movements for commercial purposes from non‐EU countries were collected from TRACES, the EU trade notification system. These data were used to characterise the import of dogs infected by Rabies Virus (RABV) and the total recorded imports of dogs for commercial purposes from non‐EU countries.
Incubation with RABV causes a variable onset of clinical signs in dogs. The results of the ELR revealed the following distribution of the incubation periods for non‐vaccinated dogs challenged intramuscularly: 90th percentile: 29 days, 95th percentile: 36 days, 99th percentile: 77 days, and maximum: 92 days. Recent field data indicate that incubation periods of > 30, > 60 and > 120 days may occur in 41, 16 and 4% of infected dogs, respectively.
Neutralising antibodies are generally observed 5–21 days following experimental intramuscular inoculation, and 3–21 days following vaccination.
An average of 23,000 dogs per year were recorded in TRACES 1 as imported from non‐EU countries into the EU for commercial purposes in the period 2019–2021 (Section 3.1.1.2 and Table C.1 of Appendix C). Of these, an average of 1,780 per year were from non‐EU countries, for which the antibody titration test and the waiting period afterwards is mandatory. However, in general, the numbers of non‐commercial movements of dogs into the EU are not registered.
Specifically, for the period 2001–2021, based on the reports found in the ELR, 20 dogs were imported into the EU from non‐EU countries and later confirmed to be RABV infected were identified (Table B.1 of Appendix B). Non‐compliance with existing rules were identified in most cases.
Table B.1.
Collected evidence regarding onset of clinical signs and time to death in unvaccinated dogs purposefully inoculated with rabies virus. This table includes data from experimental infection studies as well as unvaccinated (but challenged) control groups from vaccination studies
| Reference | Route | Dose |
Number of animals in groups |
Number of animals with clinical signs |
Clinical signs onset (days PI) |
Number of dead animals |
Death (days PI) |
|---|---|---|---|---|---|---|---|
| Schneider et al. (1965) | IM | – | 11 | 8 | 14–21 | 8 | 14–21 |
| Vaughn et al. (1965) | IM | 4.4 Log10 of MICLD50 | 3 | 3 | 10 to 16 | 3 | 11 to 17 |
| 5.0 Log10 of MICLD50 | 3 | 3 | 9 to 11 | 3 | 11 to 15 | ||
| 4.9 Log10 of MICLD50 | 8 | 7 | 13 to 20 | 7 | 14 to 24 | ||
| 6.4 Log10 of MICLD50 | 8 | 6 | 11 to 34 | 6 | 12 to 41 | ||
| 5.2 Log10 of MICLD50 | 7 | 7 | 13 to 24 | 7 | 15 to 27 | ||
| 5.2 Log10 of MICLD50 | 7 | 4 | 13 to 26 | 4 | 16 to 28 | ||
| 3.6 Log10 of MICLD50 | 9 | 3 | 15 to 17 | 3 | 19 to 21 | ||
| 4.7 Log10 of MICLD50 | 11 | 1 | 29 | 1 | 30 | ||
| 4.4 Log10 of MICLD50 | 12 | 1 | 42 | 1 | 45 | ||
| 6.4 Log10 of MICLD50 | 12 | 2 | 14 to 39 | 2 | 17 to 41 | ||
| 6.5 Log10 of MICLD50 | 13 | 2 | 26 to 34 | 2 | 33 to 36 | ||
| 6.0 Log10 of MICLD50 | 12 | 7 | 9 to 20 | 7 | 10 to 26 | ||
| 5.7 Log10 of MICLD50 | 12 | 8 | 9 to 27 | 8 | 10 to 29 | ||
| Fekadu and Baer (1980) | IC | 800,000 MICLD50 | 4 | 4 | 7 | 4 | 9 to 11 |
| IM | 800,000 MICLD50 | 3 | 3 | 7 | 2 | 8 to 9 | |
| Botros et al. (1979) | IM | 2 mL (6.6 Log10 MICLD50/mL) | 2 | – | – | 2 | 13 |
| 2 mL (8.5 Log10 MICLD50/mL) | 2 | – | – | 2 | 5 to 6 | ||
| IN | 2 mL (6.6 Log10 MICLD50/mL) | 1 | – | – | 1 | 33 | |
| Soulebot et al. (1982) (b) | IM | 4.8 Log10 of MICLD50 | 31 | 11.4 (average) | 29 | 13 (average) | |
| 3.8 Log10 of MICLD50 | 34 | 14.2 (average) | 30 | 15,9 (average) | |||
| 3.0 Log10 of MICLD50 | 9 | 19.7 (average) | 6 | 19,7 (average) | |||
| 6.0 Log10 of MICLD50 | 5 | 9.5 (average) | 3 | 13 (average) | |||
| 5.5 Log10 of MICLD50 | 5 | 19.3 (average) | 3 | 24 (average) | |||
| 4.8 Log10 of MICLD50 | 5 | 14 (average) | 4 | 15,5 (average) | |||
| 4.0 Log10 of MICLD50 | 5 | 11 (average) | 1 | 24 (average) | |||
| Fekadu et al. (1982a) | IC | 8.3 Log10 of MICLD50/mL | 4 | 4 | 7 | 4 | 8 to 10 |
| 6.3 Log10 of MICLD50/mL | 4 | 4 | 8 | 4 | 11 to 13 | ||
| 5.9 Log10 of MICLD50/mL | 4 | 4 | 7 to 9 | 4 | 9 to 13 | ||
| 4.0 Log10 of MICLD50/mL | 4 | 4 | 8 | 4 | 16 to 19 | ||
|
IM |
5.8 Log10 of MICLD50/mL | 5 | 4 | 9 to 10 | 4 | 9 to 13 | |
| 3.8 Log10 of MICLD50/mL | 5 | 3 | 16 to 28 | 3 | 17 to 30 | ||
| 2.8 Log10 of MICLD50/mL | 4 | 4 | 23 to 27 | 4 | 23 to 34 | ||
| 1.8 Log10 of MICLD50/mL | 5 | 2 | 30 to 42 | 2 | 30 to 48 | ||
| 4.7 Log10 of MICLD50/mL | 5 | 5 | 14 to 18 | 5 | 15 to 21 | ||
| 3.7 Log10 of MICLD50/mL | 5 | 5 | 19 to 29 | 5 | 21 to 34 | ||
| 2.7 Log10 of MICLD50/mL | 4 | 4 | 22 to 37 | 4 | 24 to 42 | ||
| Fekadu et al. (1982b) | IM | 5.8 Log10 of MICLD50/mL | 4 | 3 | 9 to 10 | 4 | 10 to 11 |
| 4.8 Log10 of MICLD50/mL | 4 | 4 | 11 to 21 | 4 | 12 to 25 | ||
| 3.8 Log10 of MICLD50/mL | 3 | 1 | 29 | 3 | 16 to 30 | ||
| 2.8 Log10 of MICLD50/mL | 4 | 3 | 16 to 27 | 4 | 23 to 29 | ||
| 1.8 Log10 of MICLD50/mL | 2 | 2 | 30 to 41 | 2 | 36 to 43 | ||
| 5.7 Log10 of MICLD50/mL | 5 | 5 | 12 to 15 | 5 | 14 to 17 | ||
| 4.7 Log10 of MICLD50/mL | 5 | 5 | 14 to 18 | 5 | 16 to 21 | ||
| 3.7 Log10 of MICLD50/mL | 5 | 5 | 19 to 29 | 5 | 21 to 34 | ||
| 2.7 Log10 of MICLD50/mL | 4 | 3 | 22 to 37 | 4 | 24 to 42 | ||
| 1.7 Log10 of MICLD50/mL | 3 | 3 | 36 to 69 | 3 | 40 to 69 | ||
| Hanlon et al. (2002) | IM | 0.5 mL (7 Log10 MICLD50/mL) | 5 | 5 | 11 to 13 | 11 to 13 euthanised | |
| McColl et al. (2007) | IM | 0.2 mL (5 Log10 TCID50) | 2 | 2 | 9 to 12 | 9 to 12 euthanised | |
| Gnanadurai et al. (2015) | IM | 0.3 mL (200 MICLD50) | 4 | 4 | 19 to 30 | 21 to 31 euthanised | |
| Wang et al. (2019) | IM | 6 × 104 MICLD50 | 4 | – | – | 4 | 11 |
| Cho and Lawson (1989) | IM | 106.3 MICLD50 | 13 | – | – | 12 | 12 to 17 |
| Haddad et al. (1994) | IM | 103.7 MICLD50/0.03 mL | 4 | 4 | – | 4 | 23 to 53 |
| Hammami et al. (1999) | IM | < 104 MICLD50 | 6 | 5 | 12 to 92 | 5 | 14 to 96 |
| Perrin et al. (1999) | IM | 40,000 MICLD50 | 2 | 2 | 12 to 15 | 2 | 19 to 20 |
| Kallel et al. (2006) | IM | 0.5 mL (104 DL50/mL) | 5 | 4 | 11 to 21 | 4 | – |
| Hu et al. (2006) | IM | 6 × 104 LD50 | 20 | 20 | 10 to 26 | 10 to 26 euthanised | |
| Manickam et al. (2008) | IM | 104.4 MICLD50/0.03 mL | 10 | 6 + (4 euthanised) | 32 to 58 (90 euthanised) | ||
| Liu et al. (2012) | IM | 6 × 104 LD50 | 10 | 10 | 8 to 24 | ||
| Webster and Casals (1942) | IM | – | 45 | – | – | 45 | 12 to 41 |
| 0.25 mL of a 1:400 dilution | 48 | – | – | 48 | 3 to 41 | ||
| Fields et al. (1976) | IM | 103.8 mouse LD50/0.03 mL | 10 | – | – | 8 | 13 to 23 |
| Gnanadurai et al. (2013) | IM | 100 mL viral suspension containing 200 MICLD50 | 38 | 8 | 11 to 21 | 6 | 13 to 22 |
| 5 | 5 | 13 | |||||
| 4 | 0 | ||||||
| Tierkel et al. (1949) | IM | 0.2 mL of 10% canine salivary gland suspension | 15 | 8 | 14 to 39, and 257(a) | ||
| Fekadu et al. (1992) | IM | 10^6.3 50% MICLD50 | 6 | – | – | 6 | – |
| Cliquet et al. (2007) | IM | 100 MICLD50 | 5 | 5 | 6–8 before death | 5 | 25–85 |
| (Cliquet et al., 2008) | IM | 3,150 MICLD50 | 6 | 6 | 12 to 18 | Euthanised | |
| Rupprecht et al. (2005) | IM | 107.4 MICLD50/mL, 0.5 mL | 12 | 12 | 11 to 12 | – | – |
| Zhugunissov et al. (2017) | IM | 105.0 MICLD50 | 2 | 2 | 6 | 2 | 11 and 13 |
| Blancou et al. (1989) | IM | 107.6 MICLD50 | 3 | 3 | 10, 11, 21 |
2 |
13, 14 |
IC: intracerebral; IM: intramuscular; IN: intranasal; MICLD50: mice intracerebral lethal doses 50; LD50 or DL50: median lethal dose; PI: post‐inoculation.
This unusually long time to death was specifically mentioned in the paper, and it is correctly registered here.
Data are provided as averages because this is the information in the publication.
The risk of introduction of rabies is dependent on the incidence in the country of origin. Recent estimates suggested an annual incidence of rabies in certain endemic countries between 100 and 500 infected dogs per 100,000 dogs, although this is likely to be an overestimate, so these along with an annual incidence of five infected dogs per 100,000 dogs were used. Using a deterministic approach, the probability of importing a dog incubating rabies but fully compliant with requirements (i.e. vaccination, positive titration test) and a waiting period of 30 days was estimated to 1.13 × 10–5, in regions with high incidence rate (500/100,000), using an incubation period estimated based on experimental data. For a waiting period of 90 days, this probability would be zero, because no dogs in the experimental data had a sufficiently long incubation period. For the incubation period derived from field data, the probability of importing a dog incubating rabies, but fully compliant with legislation from a high incidence region (with 500 rabies cases per 100,000 dogs), would be 14.8 × 10–5 for a 30‐day waiting period, while the probability with a 90‐day waiting period would be 2.96 × 10–5. These probabilities are lower for low prevalence regions. This implies that if 20,000 dogs are imported annually from high incidence regions and based on the incubation period as derived from the field data, then a dog incubating rabies will be imported every 0.34 years when applying a 30‐day waiting period, and every 1.4 year if applying a 90‐day waiting period. Because the estimates are very susceptible to the incubation period estimates, we also used data from experimental studies. These suggested that assuming the import of 20,000 dogs per year, one dog infected with rabies would be imported every 4.4 years if a waiting period of 30 days is applied; while for a waiting period of 90 days, import of a rabies‐infected dog would not occur. When the incubation period estimated based on field data was used, the average time it takes to import a RABV‐infected dog fully compliant with the regulation was reduced 4.2 times if the waiting period was reduced from 90 to 30 days irrespective of the incidence in the region of origin and the numbers of dogs introduced. This assessment was based on several assumptions subject to considerable uncertainty (number of dogs imported, incidence in the population of imported dogs, duration of the incubation period, etc.). Once the impact of all identified sources of uncertainty in the assessment was assessed collectively through expert judgement, it was concluded with a 95% certainty that the maximum number of RABV‐infected dogs imported in the EU in a 20‐year period from the countries considered in the assessment applying the current 90‐day waiting period would be five, while this number could be up to 20 using the proposed 30‐day waiting period.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Specific animal health requirements for entry into the Union of dogs, cats and ferrets are laid down in Commission Delegated Regulation (EU) 2020/692 2 . They mainly rely on preventing rabies from entering the EU territory from imported animals. To that end, the following conditions must be met:
Vaccination against rabies ‐ dogs, cats and ferrets must be vaccinated against rabies as follows:
the animals must be at least 12 weeks old at the time of vaccination;
the vaccine must comply with the requirements set out in Annex III to Regulation (EU) No 576/2013 3 ;
at the day of dispatch to the Union, at least 21 days must have elapsed since the completion of the primary vaccination against infection with rabies virus;
a certified copy of the vaccination details must be attached to the animal health certificate.
Rabies antibody test ‐ dogs, cats and ferrets coming from third countries or territories listed in Part I of Annex VIII to Commission Implementing Regulation (EU) 2021/404 4 , for which the specific condition “rabies antibody titration test” applies, must undergo a rabies antibody test, meeting certain criteria. That test:
must be carried out on a sample collected by a veterinarian authorised by the competent authority during the period commencing at least 30 days after the date of the primary vaccination, within a current valid vaccination series, and ending 3 months before the date of issue of the certificate;
must measure a titre of neutralising antibody to rabies virus equal to or greater than 0.5 IU/mL;
must be certified by an official report from the official laboratory as regards the result, and a copy of this report must be attached to the animal health certificate accompanying the animals to the Union;
does not have to be renewed on an animal which, following the antibody rabies titration test with satisfactory results, has been revaccinated against rabies within the period of validity of the primary vaccination and all subsequent valid vaccinations in the series.
These measures largely reflect the recommendations provided by EFSA in an opinion adopted on 11 December 2006 and published on 15 February 2007 regarding an “Assessment of the risk of rabies introduction into the UK, Ireland, Sweden, Malta, as a consequence of abandoning the serological test measuring protective antibodies to rabies” 5 . In this opinion, EFSA points out that the risk of transmission of rabies by pet movement is related to moving an animal incubating the disease and that the primary means of removing an individual from the population at risk is by vaccination, as inactivated rabies vaccines are highly efficient and induce rapid protective immunity that prevents infection and subsequent transmission. On the other hand, it also highlights that infection prior to vaccination protection cannot be controlled by immunisation. Therefore, further requirements should be based on whether rabies occurs in the pet population or not. If rabies occurs in the pet population where pets reside before primo‐vaccination, a waiting time following primo‐vaccination is recommended as the most efficient measure to reduce the risk of importing rabies‐infected pets. The higher is the actual prevalence, the longer should be the waiting time required in order to reach an acceptable level of risk. Finally, the opinion recognises that the implementation of serological testing or other risk‐reducing measures may be considered when the required waiting time exceeds 100 days.
As indicated above, the waiting time legally required in the EU legislation for movements from countries with a higher prevalence/unknown status is of at least 3 months after the blood sampling, which has to be undertaken at least 30 days after rabies vaccination. This requirement is also in line with the current recommendations included in the OIE Terrestrial Animal Health Code Chapter 8.14 on rabies (29th edition 2021) (OIE, 2021).
As shown in EFSA Opinion (EFSA AHAW Panel, 2007), the waiting time between vaccination and import is crucial, because vaccination does not prevent disease developing in already infected animals. Blancou et al. (1989) demonstrated that vaccination in an already infected animal does not significantly alter the clinical picture or development time of the disease. Therefore, it is possible that an animal infected prior to rabies vaccination would continue to incubate the disease despite developing a significant antibody titre. Another risk of rabies introduction is linked to pets which are not fully protected by the vaccination, either because they were recently vaccinated or they mounted an insufficient antibody response, before being infected.
From a general point of view, the risk that an animal is incubating disease at the time of vaccination is the same as the risk that an unvaccinated animal is incubating disease when it is imported, thus, the overall risk is very sensitive to the waiting time. It is also very sensitive to compliance with requirements (e.g. shorter than required wait, incorrect or no vaccination, falsified test result) (Wilsmore et al., 2006).
The OIE ad hoc Group on Rabies has started to work on modifying Article 8.14.7 of the OIE Terrestrial Animal Health Code and reducing the waiting time after a positive antibody titration test from 90 to 30 days. A concept paper 6 of the OIE ad hoc group describing the scientific evidence to support those changes was released with the February 2020 OIE Scientific Commission for Animal Diseases (“OIE Scientific Commission”) report 7 and was subsequently published in the scientific journal Vaccine 8 . The OIE Terrestrial Animal Health Standards Commission (“OIE Code Commission”) amended Article 8.14.7 and circulated for OIE Members countries’ (Members) comments after its September 2020 meeting. The OIE Scientific Commission agreed to consult subject‐matter experts to address Member’s concerns expressed after that round of consultation.
In December 2020, the European Union expressed concerns 9 that the presented data and drawn conclusions were not sufficient for a policy change and would request additional scientific evidence. To support its position, it submitted a scientific report prepared by experts of the European Union Reference Laboratory for Rabies (cf. p. 127–131 of the document under footnote 77).
In September 2021, after careful analysis of the Member’s concerns, the OIE Scientific Commission endorsed the expert opinion of the OIE Rabies Reference Laboratory network (RABLAB) which considered that the scientific basis for a 30‐day post‐titration waiting time was justified and that the conclusion of the 2019 OIE ad hoc Group on Rabies that reviewed dog importation standards should remain unchanged.
The OIE Scientific Commission opinion together with the experts’ rationale were forwarded to the OIE Code Commission for consideration. It is therefore likely that these changes will be proposed for adoption by OIE member countries, possibly as early as at the General Session of the OIE in May [2022].
1.1.1. Terms of Reference
In the context of Article 31 of Regulation (EC) No 178/2002, the Commission asks EFSA for scientific and technical assistance on the risks related to a possible reduction of the waiting time after rabies antibody titration to 30 days compared to the current EU legislative regime, taking into account:
-
–
the experience gained in the last years with the current waiting time laid down in the EU legislation;
-
–
the possible risks/limitations including those identified by the experts of the EU Reference Laboratory for Rabies in their February 2021 opinion;
-
–
newly available scientific information, and specifically the publication describing the scientific evidence to support the proposed changes released.
1.2. Interpretation of the Terms of Reference (if appropriate)
According to the background and terms of reference (ToRs) provided by the Commission, the request concerns the provisions for the dogs (Canis lupus) intended to be moved for commercial or non‐commercial purposes into the EU territory from non‐EU Countries to prevent the introduction of rabies in EU as described in Article 76 of the Commission Delegated Regulation (EU) 2020/692 in accordance with Article 8.14.7 of the OIE Terrestrial Code (last revised in 2019) (please refer to Appendix A).
More specifically, it concerns the waiting period from the neutralising antibody titration test and before dog shipment.
For this work, it is considered that all the requirements of EU legislation related to dog movements have been implemented. Specifically:
The dog is individually identified by means of an injectable transponder implanted that fulfils the technical requirements for means of identification (Article 74 Reg 2020/692) by a veterinarian, and the dog was individually identified before or at the time of primary vaccination (Annex III to the Regulation (EU) 576/2013) so the details correspond to those in the certificate or passport.
The dog has been vaccinated against rabies before shipment with a vaccine that complies with the validity requirements set out in Annex III to Regulation (EU) No 576/2013: (i) it is not a live modified vaccine and it is either an inactivated vaccine of at least one antigenic unit per dose (recommendation from the World Health Organisation); (ii) it has been granted an approval or a licence by the competent authority of the non‐EU country and (iii) it meets at least the requirements laid down in the relevant part of the chapter concerning rabies in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health.
The dog was at least 12 weeks old at the date on which the primary rabies vaccination was administered (Article 76 of Regulation 2020/692).
The period of validity of the vaccination starts from the establishment of protective immunity, which shall not be less than 21 days from the completion of the vaccination protocol required by the manufacturer for the primary vaccination, and continues until the end of the period of protective immunity, as prescribed in the technical specification of the marketing authorisation referred to in point 1(b) 10 or the approval or licence referred to in point 1(c) 11 for the anti‐rabies vaccine in the Member State or territory or non‐EU country where the vaccine is administered [point 2(e) Annex III Regulation (EU) No 576/2013].
As primary rabies vaccination is considered the first vaccination and any revaccination if it was not carried out within the period of validity of the previous vaccination (point 2e of Annex III to Regulation (EU) No 576/2013).
The vaccination has been conducted by an authorised veterinarian (Annex III Regulation (EU) 576/2013) and therefore good veterinary practice related to vaccination has been implemented. This also implies that the dog was healthy at the day of vaccination (based on the results of the clinical examination) and there was no suspicion of any disease including rabies (based on the medical history of the dog for the last days prior to vaccination).
The vaccinated dog must, at the time of import, remain within the protective immunity period of the vaccines according to the manufacturer’s instructions.
A certified copy of the vaccination details must be attached to the animal health certificate; the date of administration of the vaccine and the period of validity of the vaccination is indicated by an authorised veterinarian or an official veterinarian in the appropriate section of the identification document (article 76 Reg 2020/692, point 2(e) Annex III Regulation (EU) No 576/2013).
A rabies antibody titration test using a virus neutralisation test (VNT) to detect neutralising antibodies must be carried out on a blood sample collected not less than 3 months and not more than 12 months prior to the date of issue of the certificate for the shipment. In case of primary vaccination, the samples should be collected at least 30 days after the date of primary vaccination course, within a current valid vaccination series. The sample should be collected by a veterinarian authorised by the competent authority.
The VNT must comply with the validity requirements set out in Annex XXI to Regulation (EU) 2016/429.
The VNT before entry should be performed in a laboratory authorised 12 by the ANSES‐Nancy laboratory which is the European Union Reference Laboratory (EURL) 13 for rabies.
A neutralising antibody level ≥ 0.5 IU/mL is characterised as positive. Nevertheless, the test does not differentiate between infected and vaccinated animals and there are no laboratory tests able to differentiate between neutralising antibodies resulting from natural infection from those developed after vaccination.
The antibodies resulting from natural infection are only detectable when the animal is in the late stages and showing clinical signs, only dogs without clinical signs should travel, however only commercial consignments of dogs will be subject to a veterinary inspection before to travel.
Provided the VNT results are positive, the dogs are not allowed to travel immediately. A waiting period of at least 90 days (current regulation) and not more than 12 months, after the day of sampling for the antibody titration test, has been introduced to allow the clinical signs to manifest if animals were infected before vaccination or just after vaccination.
Once the dog is ready to travel, it should be clinically examined within a period of 48 h before to the time of loading for dispatch [article 13(3) of Reg 2020/692] and in the absence of clinical signs the shipment is allowed, and the certificate is provided. However, this is only applicable to commercial movements; for non‐commercial movements, there is no such requirement.
Dogs from countries not listed in Annex II to 577/2013 and all commercial consignments from outside the EU will have to enter through a Traveller's Point of Entry (TPE) or a Border Control Post (BCP), respectively, where veterinary checks can be undertaken.
Taking into consideration that all the above‐mentioned requirements are implemented, the risk of transmission of rabies through the movement of a vaccinated dog is related to the risk of moving a vaccinated animal incubating the disease.
The question to be addressed by this Scientific Report is how much the risk of introduction of rabies into EU increases through the movement of vaccinated dogs with a positive titration test (≥ 0.5 IU/mL) if the waiting period from sampling to movement decreases from 90 to 30 days.
All other parameters are considered identical across both options in the assessment besides the difference in the length of the waiting period (Figure 1).
Figure 1.

Schematic representation of the two options of the lengths of the waiting period (current of 90 days and proposed of 30 days) after the sampling and before travelling, given that the titration test is positive (≥ 0.5 IU/mL). In addition, some other time intervals are included for both options to support the comparison; pv: post‐vaccination
The length of the incubation period is considered the main epidemiological parameter for the purposes of this assessment.
To address the ToRs of the mandate, EFSA proposed and agreed with the European Commission that the assessment will be based on the results of an Extensive Literature Review (ELR), which would be conducted by an external contractor. The protocol of the literature review was shared and agreed with European Commission (Annex A; available under Supporting Information).
2. Data and methodologies
2.1. Data
Data on the incubation period were collected via an ELR conducted by an external contractor (OC/EFSA/ALPHA/2020/01) according to the ELR protocol in Annex A (available under Supporting Information), and following the overall methodology protocol agreed at the start of the process that is presented in Appendix D. This literature review included publications on experimental trials and natural infection in the field in unvaccinated and vaccinated dogs (please refer to Appendix B). Data were collected separately for vaccinated and unvaccinated dogs.
Experimental studies included purely experimental infection trials and vaccine trials in which the experimental infection preceded or followed the vaccination or was implemented to the control groups without vaccine administration.
Data on natural infection were obtained from publications, reports, and notification systems (e.g. ADIS, WAHIS) on dogs found infected after travelling from a non‐EU country to the EU territory.
Data on dog movements for commercial purposes (imports) from non‐EU countries into the EU territory were collected from TRACES online application1 (Table C.1 of Appendix C). There are no consistent data collected on non‐commercial dog movements in this database or in any other database at EU level and therefore official data are not available for this type of dog movements.
2.2. Methodologies
The main epidemiological parameter used for the assessment of the risk of rabies introduction to EU countries is the incubation period. The estimation of the length of the incubation period is based on the results of the literature review for which two different time intervals were considered: (i) the time from virus inoculation to the onset of clinical signs and/or death, for experimental infections and (ii) the time from entering the EU country of destination to the onset of clinical signs or death for natural infections in dogs moved from non‐EU countries.
For the estimation of the incubation period in unvaccinated animals, data from control groups in vaccine studies were used together with those data from experimental infection studies in which dogs have not been subjected to any treatment for rabies. Data from non‐EU countries of naturally infected dogs were also used to estimate the incubation period.
The ELR was carried out by an external contractor and the protocol is described in detail in Annex A (available under Supporting Information).
In a previous EFSA Opinion (EFSA AHAW Panel, 2007), two types of risk have been recognised for rabies transmission through dog movements: (i) Type A risk, related to the risk that an animal is already incubating rabies at the time of vaccination and (ii) Type B risk, related to the failure of inducing protective immunity following vaccination, and failure to correctly identify this condition.
Type B risk as described in the EFSA Opinion (EFSA AHAW Panel, 2007) is not as such relevant to this Scientific Report given that a positive titration test (≥ 0.5 IU/mL) at least 30 days post‐vaccination is a prerequisite for movement from countries where rabies is endemic or not controlled. The virus neutralising test is a managerial tool to minimise the risk of introducing rabies through dog movements.
A healthy dog with titration test undetectable or below the cut‐off levels (i.e. < 0.5 IU/mL) after vaccination cannot be considered as not having developed protective immunity as cellular immunity is mediated by vaccination as well. Nevertheless, this dog will not be allowed to travel. Conversely, a dog with immune system deficiencies that either fails to react to vaccination making the dog susceptible to infection, or when there was vaccine failure during the administration, will not reach a positive neutralising antibody titre ≥ 0.5 IU/mL within 30 days post‐vaccination and will not be allowed to travel. Therefore, Type B risk, the risk of infection upon vaccination failure to develop protective immunity, used in the above‐mentioned EFSA Opinion (EFSA AHAW Panel, 2007), is not considered relevant, because in the situation assessed here only dogs with a titre ≥ 0.5 IU/mL upon vaccination will be allowed to move into the EU.
In this Scientific Report, and for the purposes of the assessment, the following assumptions were applied:
Vaccinated dogs without clinical signs, which show neutralising antibody titres ≥ 0.5 IU/mL at least 30 days post‐vaccination, are fully protected against infection and this protection has been effective from day 21 post‐vaccination (21dpv) onwards.
The risk of infection before 21dpv is similar to Type A risk from a previous EFSA Opinion (EFSA AHAW Panel, 2007), and depends again on the incubation period, as the process of vaccination by an approved veterinarian constitutes a health check. These dogs can be infected either before vaccination or during the first 21 days (before the development of immunity) of the 30‐day interval from vaccination to sampling for the titration test.
The effectiveness of vaccination is 100% and a dog is fully protected from 21dpv. Given that the waiting period (30 or 90 days) starts from the blood sampling and that the blood is taken at least 30 days post‐vaccination, the dogs are effectively protected 10 days before the start of the waiting period (from the 21dpv). For a 30‐day waiting period, the dog is assumed protected and cannot be infected at least 40 days before movement (10 + 30), and accordingly 100 days (10 + 90) for a waiting period of 90 days (Figure 2).
100% of the dogs that show clinical signs of rabies before movement into EU are detected at border control or even before (sensitivity of clinical examination: SeClinical examination = 1) and the serological test (VNT) showing a titre ≥ 0.5 IU/mL is 100% specific (SpVNT = 1) (no false‐positive results). The clinical inspection is systematically performed 48 h before leaving the country of origin in dogs moving for commercial purposes while for non‐commercial movements clinical inspection is not always performed and the controls may be limited to document and identity checks.
On each day before the 21dpv, it was assumed that a dog could be infected at the same incidence rate (IR; the incidence rate expressed per day in the region), as the IR in a region was assumed to be constant.
Figure 2.

Timeline showing the window of susceptibility for RABV infection and the period when dogs are considered protected from RABV infection due to the development of the immunity in relation to the moment of vaccination (V) and the waiting period of 30 (WP30) and 90 days (WP90). Dogs are assumed to be protected against infection from day 21 post‐vaccination (21dpv)
The methodology used to apply the question was based on a deterministic approach.
For each day before 21dpv, the probability of the incubation period exceeding the end of the waiting period, is derived from the distribution of the incubation period for the experimental data (extracted by the contractor via an ELR) and from a lognormal distribution fitted to field data according to Crozet et al. (2022) (please refer to Section 3.2). The earlier the infection occurs, the lower the probability that the incubation period will exceed the waiting period.
The probability of a single imported dog being infected with RABV having an incubation period exceeding the length of the waiting period (90 or 30 days) (while in compliance with the requirements of EU regulation) equals to:
where IR is the annual incidence rate in the region of origin and p is the sum of the probabilities of having an incubation period that will exceed the waiting period on each day before 21dpv. Specifically, p = p0 + p1 + … + pd + … + p20, where pd is the probability that a dog infected at day ‘d’ post‐vaccination but before day 21 has an incubation period exceeding or greater than ‘WP – d’, where WP is the waiting period.
The overall annual probability of introducing at least one RABV‐infected dog incubating the RABV after the end of a certain waiting period (30 or 90 day) out of the total number of dogs (n) moved from a non‐EU country to EU, can therefore be calculated as:
2.3. Uncertainty
All sources of uncertainty identified during the assessment were recorded, and their impact on the scientific assessment was assessed collectively (the simplest option for this type of assessment; section 4.1 of EFSA Scientific Committee (2018)) after transforming the objective of the assessment into well‐defined quantities of interest (QoIs). In particular, considering that the mandate requested scientific and technical assistance on the risks related to a possible reduction of the waiting time after rabies antibody titration to 30 days, compared with the current practice of 90 days, two QoIs were defined:
QoI1: the number of RABV‐infected dogs that will be moved from countries or regions either not listed in Annex II to Regulation (EU) 577/2013 for pet dogs, or listed in Regulation (EU) 2020/404 (Annex VIII column 5) for commercial and non‐commercial movements compliant with the regulations (vaccinated as requested and passing a VNT test 30 days post‐vaccination) in a 20‐year period under the current waiting period of 90 days.
QoI2: the number of RABV‐infected dogs that will be moved from the same countries and conditions in the 20‐year period assuming a 30‐day waiting period is put in place.
The evidence included in this report and the sources of uncertainty identified during the assessment were summarised in an evidence dossier that was provided to the experts. A lower (0 dogs) and upper (50 dogs) bound delimiting the range of plausible values for both QoIs were then agreed within the Working Group during a meeting, and the Working Group experts were asked to provide their individual judgements on the most likely values for each QoI using the roulette method (EFSA, 2014). Individual judgements were then discussed and used to agree on the 95% percentile of the distribution for each QoI (i.e. the value below which experts were 95% certain 14 that the QoI would be), which were used to quantify the increase in risk related to the reduction of the waiting period considering all uncertainties.
3. Assessment
Rabies is a viral zoonotic disease of mammals, including humans, which causes encephalomyelitis and if left untreated is invariably fatal. Rabies disease is induced by neurotropic viruses of the Lyssavirus genus, Rhabdoviridae family, rabies virus (RABV), serotype 1 of multiple strains. Each strain is identified by its reservoir host species. Other related Lyssaviruses can cause identical neurological disease, and include the European bat lyssaviruses in Phylogroup I, but this assessment is only concerned with classical RABV.
Classical rabies is present worldwide, with the exception of some islands and countries with strict wildlife and import controls. There is no official rabies free status recognition by the OIE, but an OIE member country can declare itself to be free of rabies based on the requirements of the OIE Code if there have been no autochthonous acquired cases in humans or animals during the previous 2 years, in the presence of adequate surveillance and import regulations.
The EU has over the last 20 years invested heavily and successfully in the pathway to freedom from rabies in wildlife, companion animals and livestock. This has been based not only on widespread rabies vaccination programmes in EU countries, but also on import controls to verify the implementation of a series of measures for dogs intended to travel from a non‐EU country to EU territory: e.g. individual identification, mandatory vaccination and clinical examination before movement and in addition to (from some non‐EU countries) positive titration test followed by a waiting period of 90 days post‐titration and before movement.
The EU has for many years required import controls for dogs, cats and ferrets that includes vaccination as well as identification, health certificates and blood tests for some countries. However, many EU MS have been on a pathway to elimination of rabies in wildlife through annual oral vaccination programmes of the reservoir wildlife host (mostly the red fox) and monitoring/surveillance. As a result, most EU MS have not reported any rabies cases in wildlife and only a few MS have reported occasional cases in cats or dogs that have been illegally imported, having not been prepared properly for travel. Wildlife strains of rabies still occur as spill‐over cases into domestic animals in some EU MS where wildlife vaccination programmes are in place.
Specific animal health requirements for entry into the European Union of dogs are laid down in European legislation. They mainly rely on preventing rabies from entering the EU territory from imported animals. Each one of the above‐mentioned requirements verifies that some conditions are in place that are minimising the risk of rabies transmission through dog movement and there are several decisions to be made for each one (see Figure 3).
Figure 3.
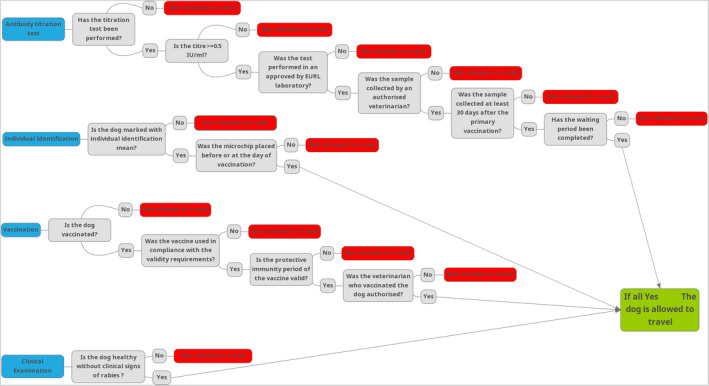
The process to be followed and the decisions to be taken in each step, for a dog to be allowed to enter the EU territory from non‐EU countries for which the specific condition for rabies antibody titration test applies as per Part I of Annex VIII to Commission Implementing Regulation (EU) 2021/404. The box representing clinical examination is not applicable for non‐commercial movements of dogs. MindMup app has been used to generate this figure
Dogs and also cats and ferrets that are moving for commercial purposes from certain non‐EU countries or territories listed in Part I of Annex VIII of the Commission Implementing Regulation (EU) 2021/404, must undergo rabies antibody titration test before travel into the EU (map in Figure 4), whereas other non‐EU countries may only apply a vaccination and the 21 days wait before entry into the EU. The criteria according to which the Countries are categorised into lists with different rules in terms of dog movements into EU territory, are not well described and are not based on epidemiological parameters. Those countries are approved for commercial movements of dogs with a certificate only are also approved for non‐commercial movement of dogs under 576/2013, in which a derogation is applied for the blood test and the waiting period. For all other countries, non‐commercial movements of dogs are allowed provided they apply the requirements (no derogations) in Regulations 576/2013 and 577/2013.
Figure 4.
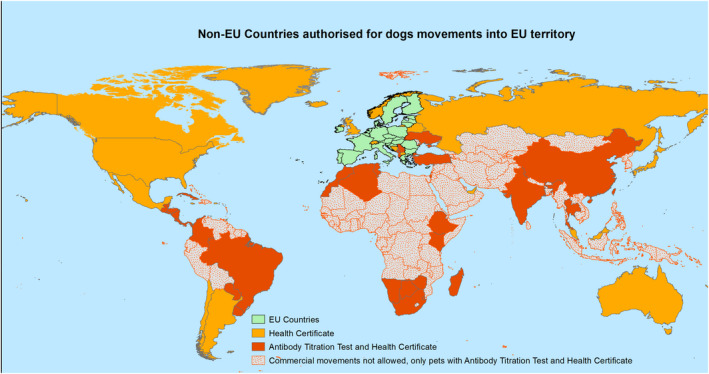
Map of non‐EU Countries from which dogs are allowed to travel to EU territory either with health certificate (orange) or with health certificate and rabies antibody titration test (red) according to Part I of Annex VIII to Commission Implementing Regulation (EU) 2021/404. ArcMap was used to create this map
3.1. ELR results
Abstracts of 4,215 publications were reviewed by two reviewers according to the protocol of the literature review shown in Annex A (available under Supporting Information). As described in the exclusion criteria of this protocol, only those publications rejected by both reviewers were finally excluded. In total, 527 publications were subjected to full‐text screening, and data were extracted from 124 publications. Data were collected to the highest level of detail available in the publication, and the results are summarised in the tables presented in Appendix B of this Scientific Report.
3.1.1. Incubation period
The data on the incubation period collected from experimental infections in unvaccinated (Table B.1 of Appendix B) and in vaccinated dogs (Table B.2 of Appendix B) and from naturally infected dogs travelling as non‐commercial consignments (Table B.4 of Appendix B) to the EU territory are presented below (Table 1 and Figure 5) and in the tables in Appendix B.
Table B.2.
Collected evidence regarding onset of clinical signs and time to death in vaccinated dogs purposefully inoculated with rabies virus. The route of virus inoculation was intramuscular in all studies
| Reference | Vaccine | Regimen | Inoculation | No. of animals in group | Clinical signs | Deaths |
Study end (days PI/PV) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Days PV | Dose | No. of animals | Onset (days PI) | No. of animals |
Onset (days PI) |
|||||
| Tierkel et al. (1949) | nonCOM | 1‐dose IM | 60 | 105.20 MICLD50/0.2 mL | 9 | – | – | 0 | – | – |
| 106.5 MICLD50/0.2 mL | 8 | – | – | 1 | 19 | – | ||||
| 106.25 MICLD50/0.2 mL | 8 | – | – | 0 | – | – | ||||
| 8.790 MICLD50/0.2 mL | 7 | – | – | 1 | 173 | – | ||||
| Fields et al. (1976) | Trimune | 1‐dose IM | 1,080 | 103.8 MLD50/0.03 mL | 24 | 0 | 0 | 0 | 0 | 30/2,110 |
| Kallel et al. (2006) | Rabisin | 1‐dose SC | 162 | 0.5 mL (104 DL50/mL) | 5 | 1 |
150/312 |
|||
| nonCOM | 1‐dose SC | 5 | 0 | |||||||
| (Hu et al., 2006) | Nobivac | 1‐dose SC | 175 | 60,000 LD50 | 20 | 0 | 0 | 0 | 0 | 180/355 |
| nonCOM | 1‐dose SC | 20 | 0 | 0 | 0 | 0 | ||||
| nonCOM | 2‐dose SC | 13 | 0 | 0 | 0 | 0 | ||||
| Bahloul et al. (2006) | Rabisin |
2‐dose IM Days 0, 21 |
1,400 | 104 LD50/mL | 4 | – | – | 0 | 0 | 120/1,520 |
| 1‐dose SC | 3 | – | – | 0 | 0 | |||||
| 1‐dose SC | 2 | – | – | 0 | 0 | |||||
| Lodmell et al. (2006) | RabVac | 1‐dose IM | 382 | 106.5 MICLD50/0.03 mL | 5 | 0 | 0 | – | – | 90/472 |
| Liu et al. (2012) | nonCOM | 1‐dose IM | 180 | 60,000 LD50 | 10 | 0 | 0 | – | – | 270 |
| Nobivac | 10 | 0 | 0 | – | – | |||||
| Gnanadurai et al., (2013) | nonCOM | 1‐dose | 28 | 200 MICLD50 | 4 | 0 | – | 4 euthanised | 30 | 30/58 |
| Darkaoui et al. (2016) | Rabivac |
2‐dose SC Days 0, 30 |
121 | 1 mL (105.6 MICLD50) | 8 | 0 | – | 1 (not rabies) | 58 | 70/191 |
IM: intramuscular; SC: subcutaneous; nonCOM: non‐commercial vaccine; PI: post‐inoculation; PV: post‐vaccination; –: information not given in the publication; MICLD50 : Mice Intracerebral Lethal Doses 50; LD50 or DL50 or MLD50: median lethal dose.
Notes: The route of virus inoculation was intramuscular in all studies. When it was explicitly reported that no animals were clinical or dead, the number of animals reported is zero.
Table B.4.
Reports of imported dogs later confirmed infected with rabies are summarised
| Country | Entry year | Dog age* |
Vaccination certificate |
Vaccination time to entry |
Days post entry to | Non‐compliance reported | No of people submitted to PET | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Destination (last) | Origin | Clinical Signs | Confirmation | |||||||
| Belgium | Morocco | 2007 | 3.5 m | No | No | 105 d | 111 d |
– Not titre tested – Not vaccinated |
40 |
Van Gucht and Le Roux (2008) |
| France |
Morocco |
2001 | 3 m | No | – | 49 d | 51 d |
– No vaccine certificate – Travel through Spain |
5 | WHO (2001b), Crozet et al. (2020a,b) |
| 2002 | 2.5 m |
No |
– | 13 d | – | Health certificate | 7 | Crozet et al. (2020a) | ||
| 2004 | 4 y | – | – | – | 5 d |
– No passport – Travel through Spain |
27 | Crozet et al. (2020a) | ||
| 2004 | 4 m | No | – | 37 d | 46 d | Illegal travel through Spain | 187 PET | Crozet et al. (2020a), Ribadeau‐Dumas et al. (2016), Servas et al. (2005) | ||
| 2004 | 6 m | No | – | On the way to France | – | Illegal travel through Spain | 11 PET | Crozet et al. (2020a), Ribadeau‐Dumas et al. (2016) | ||
|
2007 |
adult |
– | – |
15 d to France (22 d to Spain) |
Not tested |
– Not titre tested Illegally introduced trough Spain and Portugal |
152 vaccinated and several HRIG |
Collective French multidisciplinary investigation team (2008), Crozet et al. (2020a) | ||
| Gambia | 2008 | 6 m | Confirmed | 6 d |
3 d France (9 d Belgium) |
– |
– Vaccine date falsified – Not titre tested – Travel through Belgium |
32 PET |
WHO (2008), Crozet et al. (2020a) |
|
| Morocco | 2008 | 3 m | Not vaccinated | – | 18 d | Travel through Spain | 25 | Ribadeau‐Dumas et al. (2016) | ||
| Morocco | 2011 | 3m | Not vaccinated | – | 4 d | 11 d |
– Not vaccinated – No travel certificate – Illegal movement |
5 vaccinated and 8 HRIG | Crozet et al. (2020a), Mailles et al. (2011) | |
| Algeria | 2015 | 6–7 m | – | – | 7 d | 11 |
– Illegal travel |
24 | Crozet et al. (2020a); ADIS | |
| Morocco | 2020 | 3–5 m | Not vaccinated | – | 54 |
97 |
– Not vaccinated – Illegally moved |
7 |
Crozet et al. (2020a); ADIS |
|
| Germany | Azerbaijan | 2002 | 2 m | Yes vaccinated | 41 d | 2 d | 19 d | – | WHO (2001a) | |
| Morocco | 2004 | 8 m | No | – | 27 d | – |
– No vaccination – No passport – No Health Certificate |
Ribadeau‐Dumas et al. (2016) | ||
| Croatia (1) | 2008 | 6 w | – | – | 179 d | 181 d | – No vaccine certificate | WHO (2009) | ||
| Bosnia‐Herzegovina | 2010 | 2 m | Not vaccinated | 22 d | Ribadeau‐Dumas et al. (2016); | |||||
| Turkey | 2021 | 8 w | – | no | 6 d (died) | 13 d | – Entered the country illegally via Bulgaria | PROMED‐mail 2021‐09‐21; ADIS | ||
| Netherlands | Morocco | 2012 | 2 m | Health Certificate | no |
(11 d Spain) 3 d Netherlands |
(15 d Spain) 4 d Netherlands |
– Not vaccinated – No testing – Travelled via Spain |
21 vaccinated, 21 vaccinated and HRIG |
van Rijckevorse et al. (2012) |
| Spain | Morocco | 2013 | 4 y | Vaccinated for the 1st time 11 days before entering Morocco from France | 4,5 m | 50 d | 54 d |
– No titre testing – Entered illegally – Vaccine waiting time not respected – the requirement for reintroduction not compliant |
64 vaccinated, 118 vaccinated and HRIG |
Perez de Diego et al. (2015); ADIS |
| UK (2) | Sri Lanka | 2008 | 2.5 m | – | 2 d | 6 d | 8 d | In compliance, the dog was detected at the quarantine place | 11 vaccination and HRIG | Catchpole et al. (2008) |
HRIG: human rabies immune globulin; PET: post‐exposure treatment.
Croatia was not an EU MS in 2008.
UK was an EU MS in 2008 and was applying 6 months quarantine.
dog age: m: months, w: weeks, y: years.
Table 1.
Summary of the results from literature review in relation to the incubation period from experimental studies and from cases imported into the EU. For experimental studies, the distribution for the onset of clinical signs and death is provided at group level in the trials (earliest and latest days of the ranges of the groups) and for the individual dogs as exact days
| CS/D | No. of studies | No. of groups | No. of animals | Earliest and latest day of the ranges | Distribution (days PI) | No. of limits of the ranges (earliest, latest) or no. of individual values exceeded | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Median (25th, 75th percentiles) | > 30 days | > 40 days | > 90 days | > 100 days | ||||||||
| Ranges of onset of CS and D of the groups of dogs in experiments | Experimental studies | CS | 19 | 46 (a) | 214 (a) | Latest | 6 | 92 | 24 (17.25, 29.75) | 11 | 6 | 1 | 0 |
| Earliest | 6 | 42 | 13 (10.25, 15.785) | 2 | 1 | 0 | 0 | ||||||
| D | 19 | 48 (a) | 288 (a) | Latest | 6 | 257 | 28.5 (19.25, 41) | 19 | 15 | 2 | 1 | ||
| Earliest | 3 | 45 | 14.5 (12, 23) | 5 | 1 | 0 | 0 | ||||||
| Individuals (dogs) | Experimental studies | CS | 19 | 46 (a) | 159 (a) | 6 | 92 | 15 (12, 21.5) | 14 | 8 | 1 | 0 | |
| D | 19 (a) | 48 (a) | 152 (a) | 3 | 257 | 17 (14, 25) | 26 | 16 | 1 | 1 | |||
| Imported cases | CS | 18 (b) | 2 | 179 | 16.5 (6, 46) | 6 | 5 | 2 | 2 | ||||
PI = post‐inoculation.
Data on the onset of clinical signs (CS) and death (D) at individual level was not available for all groups of dogs in all studies. In some other groups information was provided only for the onset of CS or death or vice versa but not for both.
The total number of imported infected dogs were 20, but for one there was no information on the onset of the CS and for another one the CS started during travelling before arriving at the EU country of destination.
Figure 5.
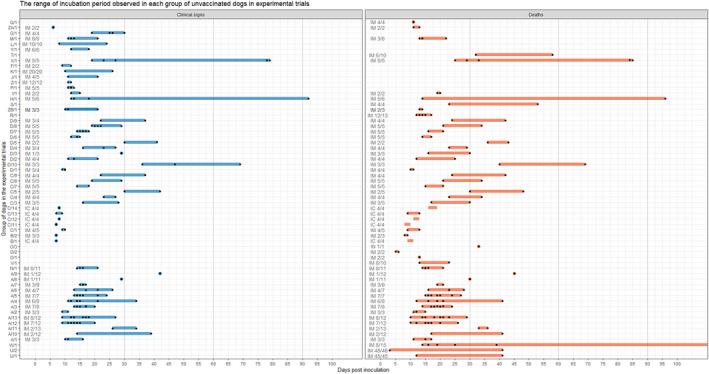
Range from the time of inoculation to the time of the onset of clinical signs (blue) and death (red), respectively, in each group of dogs of the experimental trial in studies retrieved from the literature review
- Each group of dogs has a unique identification code and was inoculated with different doses of RABV through intramuscular (IM), intracerebral (IC) and intranasal (IN) route. Black dots indicate the time to the onset of clinical signs and death individually for each dog of the group. For the group with code number W/1, the last dog died 257 days post‐inoculation but to maintain better visualisation of the whole graph the x‐axis is not extended up to the value 257.
3.1.1.1. Experimental trials
The experimental studies that were identified by the literature review were not harmonised in terms of the objectives and the level of details they included, their methodology and/or the presentation of the results. The dose of inoculation, the virus strains, and the route of inoculation (intramuscular, intracerebral, intranasal) varied in the different groups of animals.
Unvaccinated animals
In 27 experimental studies, 62 groups of dogs were identified as having been experimentally infected with various doses of different strains of RABV and through different routes of inoculation without being vaccinated or treated: 56 groups via intramuscular inoculation, 5 groups via intracerebral inoculation and 1 group via intranasal inoculation (Table B.1 of Appendix B).
Information on the onset of clinical signs and death at individual level was not available for all groups of dogs in all studies. Instead, the average or the range of the earliest and latest day of the onset of clinical signs or/and death in each group of dogs in the trial was provided. In some other groups, information was provided only for the onset of clinical signs or death but not for both.
The graph in Figure 5 presents the ranges from the time of inoculation to the time of the onset of the clinical signs and death, respectively, according to either the group of dogs in the studies or the days of the onset of the clinical signs in each dog when individual information was available.
One study provided the means for each group of dogs instead of ranges or individual information on the time from intramuscular virus inoculation to the onset of clinical signs or death (Soulebot et al., 1982). The means of the time between virus inoculation and the onset of clinical signs and death in the groups range from 9.5 to 19.7 days and from 13 to 24 days accordingly (Soulebot et al., 1982).
To estimate the incubation period, 48 groups of dogs from 25 studies that have been inoculated intramuscularly were used as the intramuscular inoculation is more similar to the natural route of infection compared with intranasal and intracerebral inoculation.
Vaccinated animals
Nine studies on experimental inoculation after vaccination were found through the literature review (Table B.2 of Appendix B). Clinical signs after immunisation were observed in three dogs in two studies, when non‐commercial vaccines (two animals) and Rabisin (one animal) respectively were used. Dogs were challenged 60 days post‐vaccination when non‐commercial vaccines were used, with doses 106.5 MICLD50/0.2 mL and 8,790 MICLD50/0.2 mL. One of the vaccinated dogs died 19 days after the challenge inoculation, or 79 days after vaccination. The second dog died 173 days after the challenge inoculation, or 233 days after vaccination (Tierkel et al., 1949). It should be considered that this is a study with live virus vaccines and phenolised vaccines produced in the 1940s that probably do not meet today’s international standards for market authorisation.
When Rabisin vaccine was used, the animal died after a 0.5 mL (104 DL50/mL) challenge dose, inoculation was performed 162 days after vaccination (Kallel et al., 2006). In the study conducted by Darkaoui et al. (2016), one dog out of eight died due to a mesenteric torsion accident after 58 days post‐inoculation with 1 mL (105.6 MICLD50) challenge virus. The infection was carried out 121 days post‐vaccination (Rabivac, two doses SC, days 0.30). No VNT titres were available for these studies, therefore it was not possible to extrapolate whether these animals would have been able to travel.
3.1.1.2. Cases imported into EU territory from infected non‐EU Countries
Information about dogs imported from non‐EU Countries and confirmed with rabies in the EU country of destination is summarised in Table B.4 of Appendix B.
For imported cases, no official positive serological testing of infected dogs before travel was reported. The infected dogs were either not tested or had no testing information reported. In one ProMED report, the animal was reported as ‘RFFIT positive’, but titres were not given. There have been no cases of imported dogs that have been prepared for travel to the EU according to the EU legislation, having been vaccinated and tested positive for neutralising antibodies (≥ 0.5 IU/mL) that were then found to be infected with rabies once they have arrived in the EU.
Based on the literature review, in total 20 cases of RABV‐infected dogs were imported into the EU territory, from 2001 to 2021 from Algeria, Azerbaijan, Bosnia and Herzegovina, Croatia (before becoming an EU Member State), Gambia, Morocco, Sri Lanka and Turkey. More than half of the records concerned France as the country of destination, with involved dogs mainly from Morocco (9/11 French imported cases). Germany is the country reporting the second highest number of imported rabid dogs with five cases since 2001.
The average importation frequency of rabid dogs into the EU amounts to one case every year. None of these cases complied with the regulations in force. Regarding the age of the dogs, more than half (65%) were under 6 months old and 5% were adult dogs over 1 year old.
Based on the available data from the literature review related to the imported cases (Table B.4 of Appendix B), the estimated period between the entry into the EU country of destination and the onset of first clinical signs ranged from 2 to 179 days, with an average of 33 days and a median of 16.5 days. In one case, the clinical signs started on the way from the country of origin (Morocco) to the country of destination (France) and was consequently excluded from this estimation. As the exact date of infection is not known, the incubation period cannot be defined accurately. Considering the rabies‐free status of the country of destination and/or the sequencing studies of the rabies virus strains, it is assumed that the infection occurred at the country of origin before entering the EU territory. However, the period between the entry into the EU country of destination and the onset of first clinical signs is a good indicator and reflects how long an incubation could last following a natural infection even if systematically underestimated as the infection occurred before the arrival in the country of destination. Although underestimated, these estimations remain however longer than the estimations of incubation periods issued from experimental studies. It should be also noted that longer durations than the maximum of 179 days observed in the EU have been observed outside the EU, as documented for example in an imported dog having travelled from Cameroon to the USA with 288 asymptomatic days (more than 9 months) and found infected with a west African dog rabies strain (CDC, 1987) and for a 3‐month‐old vaccinated imported dog (dog vaccinated in the country of departure with a non‐licensed rabies vaccine nor in Canada nor in the EU), in which the period between the entry into the country and onset of first clinical signs exceeded 7 months (Ministry of Agriculture Food and Rural Affairs of Canada, 2022).
Considering the impact on public health as a result of these illegal imports, for the 15 cases for which the information was available, 770 people that were in close contact, bitten or scratched by the infected animals were submitted for post‐exposure treatment (PET) with vaccination or vaccination and human rabies immune globulin (HRIG).
3.1.1.3. Estimation from field data
Based on field data from previous published studies (Ribadeau‐Dumas et al., 2016; Tojinbara et al., 2016) and the report from Great Britain by the Ministry of Agriculture Fisheries and Food (1971), Crozet et al. (2022) constructed a probability density (Figure 6) of the duration of the incubation period in which incubation > 30, > 60 and > 120 days was estimated to have a respective probability of 0.41, 0.16 and 0.04.
Figure 6.
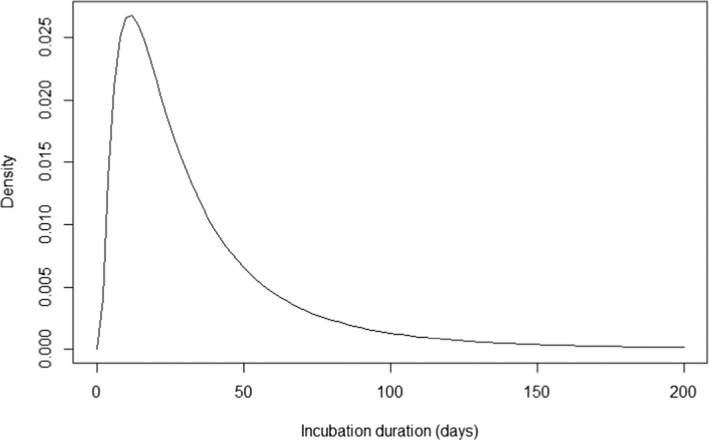
Probability density of the duration of the incubation period (Crozet et al., 2022)
3.1.2. Neutralising antibodies development
In human patients, neutralising antibody responses are usually only detectable at a late stage in the course of infection, by which point the infected individual has developed clinical signs and is unable to clear the virus (Gold et al., 2020). Some serological surveys in humans, dogs or wildlife do report the presence of anti‐rabies antibodies in otherwise healthy people or animals, suggesting the existence of subclinical infections or recovery from a clinical infection (Gold et al., 2020). There is, however, still controversy to what extent these results represent true or false positives.
Serological studies in unvaccinated dogs that are experimentally challenged with RABV yield a variable picture from no seroconversion to very high neutralising antibody titres upon challenge (Table B.3, B.5–B.7 of Appendix B). The antibody response is most likely to depend on the size of the virus inoculum and the route of inoculation, but most available studies demonstrate some degree of seroconversion with the build‐up of neutralising antibodies between 5 days and 3 weeks upon experimental inoculation.
Table B.3.
Available information on the detection of virus neutralising antibodies in unvaccinated animals after challenge with rabies virus. All serological tests presented targeted virus neutralising antibodies and the type of test is given in the table
| Reference |
Tissue type |
Test type |
Serology testing day PI |
No. of samples | Titre or concentration mean/range |
Serology end (days PI) |
Study end (days PI) |
|
|---|---|---|---|---|---|---|---|---|
| Tested | Positive | |||||||
| Fekadu and Baer (1980) | Serum | RFFIT |
6–12 30 50 |
7 1 1 |
7 1 1 |
7–250 > 1,000 1,600 |
Single time point for dying dogs 50 days for surviving dogs |
50 |
| Fekadu and Shaddock (1984) | ? | RFFIT | 5 | 7 | 7 | 0.3–11 IU/mL | Single time point | |
| Hanlon et al. (2002) | Serum | RFFIT |
0 3 7 11–13 |
5 5 5 5 |
5 5 5 5 |
< 5 < 5 < 5 to 7 13 to > 56 |
13 | 13 |
| McColl et al. (2007) | Serum | RFFIT |
7 12 |
2 2 |
2 2 |
1–3 IU/mL 6.3–30 IU/mL |
12 | 42 |
| Gnanadurai et al. (2015) | Serum | RFFIT |
7 21 |
4 3 |
? ? |
Mean = 0.12 IU/mL Mean = 0.32 IU/mL |
21 | 90 |
| Fekadu et al. (1992) | Serum | RFFIT |
–49 –42 0 7 14 |
6 6 6 6 6 |
0.1 IU/mL 0.1 IU/mL 0.1 IU/mL 0.1 IU/mL 0.3 IU/mL |
14 | 28 | |
| Hammami et al. (1999) | Serum | FAVN |
–33 –26 –19 –12 0 7 160 |
6 6 6 6 6 6 1 |
0.04 to 0.14 IU/mL 0.03 to 0,18 IU/mL 0.02 to 0.06 IU/mL 0.02 to 0.05 IU/mL 0.02 to 0.14 IU/mL 0.02 to 0.08 IU/mL 0.04 IU/mL |
160 | 160 | |
| Perrin et al. (1999) | Serum | RFFIT |
–231 0 |
2 2 |
< 0.5 UI/mL < 0.5 UI/mL |
0 | 20 | |
| Kallel et al. (2006) | Serum | RFFIT |
–162 –147 –132 –102 0 150 |
5 5 5 5 5 1 |
0.26 IU/mL (GM) 0.24 IU/mL (GM) 0.28 IU/mL (GM) 0.25 IU/mL (GM) 0.27 IU/mL (GM) 11.27 IU/mL (GM) |
150 | 150 | |
| Hu et al. (2006) | Serum | FAVN | –161 | 20 | 0 IU/mL | –161 | 180 | |
RFFIT: rapid fluorescent focus inhibition test; FAVN: fluorescent antibody virus neutralisation assay.
Timeline is given as days post‐inoculation (PI) (negative when before inoculation). All serological tests presented targeted virus neutralising antibodies and the type of test is given in the table.
Table B.5.
Available information on the detection of virus neutralising antibodies in unvaccinated animals after challenge with rabies virus All serological tests presented targeted virus neutralising antibodies
| Reference | Tissue type | Test type | Serology testing day PI | Number of samples tested |
Number of positive samples |
Titre |
Serology end (days PI) |
Study end (days PI) |
|---|---|---|---|---|---|---|---|---|
| Fekadu and Baer (1980) | Serum | RFFIT |
6–12 30 50 |
7 1 1 |
7 1 1 |
7–250 > 1,000 1,600 |
Single time point for dying dogs 50 days for surviving dog |
50 |
| CSF | RFFIT |
6–11 30 50 |
5 1 1 |
4 1 1 |
< 2–95 500 1,100 |
50 | ||
| Brain tissue | RFFIT | 8–11 | 6 | 6 | < 10–40 | 50 | ||
| Fekadu and Shaddock (1984) | ? | RFFIT | 5 | 7 | 7 | 0.3–11 IU/mL | Single time point | |
| Hanlon et al. (2002) | Serum | RFFIT |
0 3 7 11–13 |
5 5 5 5 |
5 5 5 5 |
< 5 < 5 < 5–7 13–> 56 |
13 | 13 |
| McColl et al. (2007) | Serum | RFFIT |
7 12 |
2 2 |
2 2 |
1–3 IU/mL 6.3–30 IU/mL |
12 | 42 |
| Gnanadurai et al. (2015) | Serum | RFFIT |
7 (RFFIT) 21 (RFFIT) |
4 3 |
? ? |
Mean = 0.12 IU/mL Mean = 0.32 IU/mL |
21 | 90 |
| CSF | RFFIT |
7 21 |
4 4 |
0 0 |
0 IU/mL 0 IU/mL |
21 | 90 |
CSF: cerebrospinal fluid; RFFIT: rapid fluorescent focus inhibition test.
Timeline is given as days post‐inoculation (PI).
Table B.7.
Virus neutralising antibodies titration in vaccination trials where animals were vaccinated, but not challenged with the rabies virus (sample specimen always serum)
| Reference | Vaccine type | Vaccine regimen | Age group | Serology testing day (PV) | Test type | Number of animals | Titre or concentration mean | Titre or concentration range | Serology end (days PV) | Study end (days PV) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive | ||||||||||
| Chomel et al. (1988) | Rabisin | 1‐dose SC | > 3 months | 90 | RFFIT | 137 | 135 | 11.13 IU/mL (GM) | 365 | 365 | |
| Rabisin | 1‐dose SC | > 3 months | 180 | RFFIT | 151 | 147 | 7.96 IU/mL (GM) | 365 | 365 | ||
| Rabisin | 1‐dose SC | > 3 months | 273 | RFFIT | 130 | 123 | 5.02 IU/mL (GM) | 365 | 365 | ||
| Rabisin | 1‐dose SC | > 3 months | 365 | RFFIT | 198 | 192 | 4.33 IU/mL (GM) | 365 | 365 | ||
| Tepsumethanon et al. (1991) | Rabdomun | 1‐dose IM | 3–6 months |
0 14 30 60 180 360 |
RFFIT |
32 32 31 29 27 18 |
0.04 IU/mL (GM) 2.03 IU/mL (GM) 1.69 IU/mL (GM) 0.44 IU/mL (GM) 0.12 IU/mL (GM) 0.04 IU/mL (GM) |
< 0.1 to 0.55 IU/mL < 0.1 to 17.66 IU/mL 0.08 to 16.18 IU/mL < 0.1 to 5.04 IU/mL < 0.1 to 2.31 IU/mL < 0.1 to 0.3 IU/mL |
360 | 360 | |
| Rabdomun | 1‐dose IM | 6–12 months |
0 14 30 60 180 360 |
RFFIT |
14 14 14 14 11 9 |
0.04 IU/mL (GM) 1.67 IU/mL (GM) 2.78 IU/mL (GM) 0.39 IU/mL (GM) 0.05 IU/mL (GM) 0.03 IU/mL (GM) |
< 0.1 to 0.27 IU/mL 0.24 to 49.77 IU/mL 0.6 to 19.23 IU/mL < 0.1 to 11.45 IU/mL < 0.1 to 1.64 IU/mL < 0.1 to 0.43 IU/mL |
360 | 360 | ||
| Rabdomun | 1‐dose IM | > 12 months |
0 14 30 60 180 360 |
RFFIT |
7 7 7 7 5 4 |
0.03 IU/mL (GM) 4.3 IU/mL (GM) 3.6 IU/mL (GM) 1.99 IU/mL (GM) 0.32 IU/mL (GM) 0.03 IU/mL (GM) |
< 0,1 to 0,25 IU/mL 1,02 to 19,24 IU/mL 0,39 to 17,64 IU/mL 0,18 to 9,64 IU/mL 0,15 to 0,63 IU/mL < 0,1 to 0,21 IU/mL |
360 | 360 | ||
| Sage et al. (1993) | Rabdomun | 1‐dose IM | > 3 months |
0 14 30 60 180 360 |
RFFIT |
24 25 21 26 23 16 |
0 25 21 19 17 11 |
< 0,1 IU/mL 0.75 to 10.06 IU/mL 1.06 to 9.64 IU/mL 0.29 to 4.38 IU/mL 0.25 to 6.39 IU/mL 0.26 to 7.17 IU/mL |
360 | 360 | |
| Rabdomun | 2‐dose IM days 0, 180 | > 3 months |
0 3 7 |
RFFIT |
2 2 2 |
1 1 2 |
0.24 to 1.26 IU/mL 0.26 to 1.02 IU/mL 7.12 to 36.76 IU/mL |
7 | 187 | ||
| Rabdomun |
2‐dose IM Days 0, 360 |
> 3 months |
0 3 7 |
RFFIT |
5 5 4 |
3 2 4 |
0.36 to 1.32 IU/mL 0.29 to 3.57 IU/mL 25.83 to 45.61 IU/mL |
7 | 367 | ||
| Rabguard‐TC | 1‐dose IM |
Adult 15 animals measured at different times |
3 months 5 months 7 months 8 months 9 months 11 months 12 months |
RFFIT |
1 2 2 1 2 3 4 |
1 1 1 0 2 2 2 |
0.66 IU/mL 0.2 to 0.53 IU/mL 0.31 to 0.72 IU/mL 0.37 IU/mL 0.69 to 0.82 IU/mL 0.31 to 1.5 IU/mL 0.34 to 1.26 IU/mL |
||||
| Rabguard‐TC | 2 to 4‐dose IM |
Adult 17 animals measured at different times |
2 years 3 years 4 years 5 years 6 years 7 years 8 years 9 years 10 years 11 years 12 years |
RFFIT |
2 2 1 2 1 2 2 1 2 1 1 |
2 2 1 2 0 2 2 1 2 1 1 |
2.31 to 2.6 IU/mL 0.85 to 1.38 IU/mL 1.32 IU/mL 2.69 to 5.99 IU/mL 0.34 IU/mL 0.97 to 1.6 IU/mL 3 to 5.26 IU/mL 2.07 IU/mL 2.58 to 4.15 IU/mL 1.64 IU/mL 1.9 IU/mL |
||||
| Sihvonen et al. (1995) | Madivak | 1‐dose SC |
0 to 40 350 to 370 |
RFFIT |
47 47 38 |
0 46 34 |
0.03 IU/mL (GM) 18.05 IU/mL (GM) 1.28 IU/mL (GM) |
0.17 IU/mL 1.5 to 81 IU/mL 0.5 t 81 IU/mL |
360 | 370 | |
| Rabisin | 1‐dose SC |
0 to 40 350 to 370 |
RFFIT |
78 83 68 |
4 80 54 |
0.02 IU/mL 17.03 IU/mL 0.91 IU/mL |
0.5 IU/mL 0.5 to 81 IU/mL |
360 | 370 | ||
| Beníšek et al. (1998) | Lyscelin | 1‐dose IM |
14 28 60 90 |
Virus neutralisation in mice |
6 6 6 6 |
153.3 ED50 153.3 ED50 98.2 ED50 92 ED50 |
90 | 90 | |||
| Rabisin | 1‐dose IM |
14 28 60 90 |
6 6 6 6 |
162.1 ED50 162.1 ED50 90.9 ED50 105.1 ED50 |
90 | 90 | |||||
| Reddy and Srinivasan (1999) | Raksharab | 1‐dose SC | 6–12 months |
0 180 365 540 730 900 1095 |
RFFIT |
30 24 24 19 15 12 12 |
< 0.12 IU/mL (GM) 7.2 IU/mL (GM) 5.4 IU/mL (GM) 3.8 IU/mL (GM) 2.9 IU/mL (GM) 1.8 IU/mL (GM) 1.2 IU/mL (GM) |
1095 | 1095 | ||
| Seghaier et al. (1999) | Rabirata |
2‐dose SC Days 0, 365 |
< 3 months |
0 30 182 365 395 |
RFFIT |
39 44 27 28 21 |
10 28 6 9 20 |
395 | 395 | ||
| Rabirata |
2‐dose SC Days 0, 365 |
3 months‐1 year |
0 30 182 365 395 |
RFFIT |
81 68 49 54 43 |
15 44 10 24 43 |
395 | 395 | |||
| Rabirata |
2‐dose SC Days 0, 365 |
1–3 years |
0 30 182 365 395 |
RFFIT |
93 86 81 58 53 |
27 65 32 17 44 |
395 | 395 | |||
| Rabirata |
2‐dose SC Days 0, 365 |
> 3 years |
0 30 182 365 395 |
RFFIT |
74 69 60 43 48 |
41 58 37 16 45 |
395 | 395 | |||
| HogenEsch et al. (2002) | Imrab |
5‐dose SC Days 112, 364, 728, 1092, 1456 |
8 weeks |
112 364 378 728 742 1092 1104 1456 1470 |
RFFIT |
5 5 5 5 5 5 5 5 5 |
400 0 3000 1000 3000 300 1000 500 2800 |
1470 | 1470 | ||
| Reddy et al. (2003) | Megavac |
2‐dose IM Days 0, 30 |
9–16 weeks |
0 90 180 360 540 720 |
RFFIT |
10 10 8 8 8 8 |
< 0.12 IU/mL 6.6 IU/mL 5.1 IU/mL 4 IU/mL 3.6 IU/mL 2.9 IU/mL |
720 | 720 | ||
| Shimazaki et al. (2003) | Non‐commercial |
2‐dose SC Days 0, 396 |
6–12 months |
30 91 182 275 395 427 455 |
RFFIT |
2 2 2 2 2 2 2 |
2 IU/mL (GM) 1.5 IU/mL (GM) 1.6 IU/mL (GM) 1.5 IU/mL (GM) 1 IU/mL (GM) 47 IU/mL (GM) 40 IU/mL (GM) |
455 | 455 | ||
| Non‐commercial |
2‐dose SC Days 0, 396 |
6–12 months |
30 91 182 275 395 427 455 |
RFFIT |
2 2 2 2 2 2 2 |
1.5 IU/mL (GM) 1 IU/mL (GM) 0.8 IU/mL (GM) 0.7 IU/mL (GM) 0.6 IU/mL (GM) 12 IU/mL (GM) 30 IU/mL (GM) |
455 | 455 | |||
| Non commercial |
2‐dose SC Days 0, 396 |
6–12 months |
30 91 182 275 395 427 455 |
RFFIT |
2 2 2 2 2 2 2 |
1 IU/mL (GM) 0.6 IU/mL (GM) 0.7 IU/mL (GM) 0.5 IU/mL (GM) 0.4 IU/mL (GM) 45 IU/mL (GM) 35 IU/mL (GM) |
455 | 455 | |||
| Blancou et al. (1989) | Rabisin | 1‐dose SC | 10 months |
35 90 180 365 540 730 1400 |
RFFIT |
4 4 4 4 4 4 2 |
8 IU/mL 0.6 IU/mL 0.9 IU/mL 1 IU/mL 0.5 IU/mL 0.5 IU/mL 0.1 IU/mL |
1400 | 1400 | ||
| Ramanna et al. (2007) | Rabivac | 1‐dose IM | 3–48 months |
0 30 180 360 540 720 |
RFFIT |
60 60 45 30 15 15 |
< 0.5 to 1.75 0.75 to 1.75 0.75 to 1.75 0.75 to 1.75 1.25 to 2 1.25 to 1.75 |
720 | 720 | ||
| Yuan et al. (2008) | Nobivak | 1‐dose IM | 3 months |
0 14 28 42 70 98 126 154 182 |
FAVN |
6 6 6 6 6 6 6 6 6 |
0 IU/mL (GM) 0.83 IU/mL (GM) 4.52 IU/mL (GM) 6.59 IU/mL (GM) 9.61 IU/mL (GM) 8.75 IU/mL (GM) 8.28 IU/mL (GM) 7.06 IU/mL (GM) 6.97 IU/mL (GM) |
182 | 182 | ||
| Bender et al. (2009) | Denfensor 3 | 1‐dose IM | > 5 months |
0 13 27 61 82 |
RFFIT |
16 16 16 16 16 |
0 IU/mL 0.65 IU/mL 0.65 IU/mL 0.61 IU/mL 0.6 IU/mL |
82 | 82 | ||
| Denfensor 3 | 1‐dose IM | > 5 months |
0 13 27 61 82 |
RFFIT |
16 16 16 16 16 |
0.15 IU/mL 0.46 IU/mL 0.64 IU/mL 0.6 IU/mL 0.6 IU/mL |
82 | 82 | |||
| Minke et al. (2009) | Rabisin | 1‐dose SC | 13–18 weeks |
0 14 28 56 84 112 120 |
FAVN |
15 15 15 15 15 15 15 |
0.06 IU/mL (GM) 2.53 IU/mL (GM) 2.03 IU/mL (GM) 1.07 IU/mL (GM) 0.61 IU/mL (GM) 0.51 IU/mL (GM) 0.4 IU/mL (GM) |
0.06 to 0.66 IU/mL 0.17 to 1377 IU/mL 0.29 to 7.92 IU/mL 0.22 to 3.46 IU/mL 0.1 to 2.62 IU/mL 0.1 to 2.62 IU/mL 0.07 to 1.51 IU/mL |
120 | 120 | |
| Nobivac | 1‐dose SC | 13–18 weeks |
0 14 28 56 84 112 120 |
FAVN |
15 15 15 15 15 15 15 |
0.06 IU/mL (GM) 1.26 IU/mL (GM) 0.74 IU/mL (GM) 0.11 IU/mL (GM) 0.11 IU/mL (GM) 0.16 IU/mL (GM) 0.11 IU/mL (GM) |
0.06 to 0.13 IU/mL 0.5 to 4.56 IU/mL 0.66 to 1.99 IU/mL 0.06 to 0.22 IU/mL 0.06 to 0.29 IU/mL 0.06 to 0.5 IU/mL 0.06 to 0.5 IU/mL |
120 | 120 | ||
| David et al. (2010) | RabVac |
2‐dose? Days 0, 255 |
1 year |
254 263 |
RFFIT |
1 1 |
0 1 |
0.7 IU/mL 49.91 IU/mL |
|||
| Judit et al. (2010) | Mevak | 1‐dose IM | 3–6 months |
0 14 30 90 450 |
RFFIT |
10 10 10 10 10 |
0.05 IU/mL 0.16 IU/mL 1.27 IU/mL 1.22 IU/mL 0.38 IU/mL |
SD = 0.03 SD = 0.05 SD = 1.14 SD = 1.14 SD = 0.2 |
450 | ||
| Mevak | 1‐dose IM | 3–6 months |
0 14 30 90 450 |
RFFIT |
10 10 10 10 10 |
0.04 IU/mL 0.33 IU/mL 3.29 IU/mL 2.88 IU/mL 0.45 IU/mL |
SD = 0.03 SD = 0.22 SD = 2.27 SD = 1.96 SD = 0.18 |
450 | |||
| Mevak | 1‐dose IM | 3–6 months |
0 14 30 90 450 |
RFFIT |
10 10 10 10 10 |
0.04 IU/mL 0.35 IU/mL 4.63 IU/mL 4.17 IU/mL 1.02 IU/mL |
SD = 0.13 SD = 0.18 SD = 3.01 SD = 2.21 SD = 0.4 |
450 | |||
| Rad et al. (2010) | Rabdomun | 1‐dose IM | 3–4 months |
180 540 |
RFFIT |
6 6 |
9.2 IU/mL 0.6 IU/mL |
540 | 540 | ||
| Durrani et al. (2012) | Rabisin |
2‐dose SC Days 0, 21 |
1–2 years |
0 21 30 60 90 120 150 180 210 240 270 300 |
RFFIT |
4 4 4 4 4 4 4 4 4 4 4 4 |
0 IU/mL 0.4 IU/mL 1.8 IU/mL 1.7 IU/mL 2.7 IU/mL 2.3 IU/mL 3.2 IU/mL 6.3 IU/mL 8.1 IU/mL 8.2 IU/mL 11 IU/mL 11 IU/mL |
300 | |||
| Hexadog DHP‐LR |
2‐dose SC Days 0, 21 |
1–2 years |
0 21 30 60 90 120 150 180 210 240 270 300 |
RFFIT |
4 4 4 4 4 4 4 4 4 4 4 4 |
0 IU/mL 0.1 IU/mL 0.8 IU/mL 1.2 IU/mL 2.9 IU/mL 4.5 IU/mL 5.5 IU/mL 5.8 IU/mL 7.4 IU/mL 7.4 IU/mL 6.8 IU/mL 6.9 IU/mL |
300 | ||||
| Rabisyva VP13 |
2‐dose SC Days 0, 21 |
1–2 years |
0 21 30 60 90 120 150 180 210 240 270 300 |
RFFIT |
4 4 4 4 4 4 4 4 4 4 4 4 |
0 IU/mL 0.4 IU/mL 1.9 IU/mL 2.4 IU/mL 8.7 IU/mL 14 IU/mL 14 IU/mL 15 IU/mL 16 IU/mL 16 IU/mL 16 IU/mL 16 IU/mL |
300 | ||||
| Hurisa et al. (2013) | Ethiorab | 1‐dose SC | 4–5 months |
0 7 15 21 30 60 90 |
FAVN |
6 6 6 6 6 6 6 |
0 5 6 6 6 6 6 |
1.55 IU/mL (GM) 1.7 IU/mL (GM) 3.57 IU/mL (GM) 3.154 IU/mL (GM) |
1.55 to 2.05 IU/mL 1.55 to 2.4 IU/mL 1.55 to 2.95 IU/mL 2.9 to 4.2 IU/mL 2.6 to 4.05 IU/mL 2.05 to 4.3 IU/mL |
90 | 120 |
| Asokkumar et al. (2014) | Raksharab | 1‐dose IM | 3–5 months |
0 14 28 |
RFFIT |
15 15 15 |
0 12 15 |
0.059 IU/mL 1.059 IU/mL 2.034 IU/mL |
0.03 to 0.07 IU/mL 0.41 to 1.55 IU/mL 1.6 to 2.44 IU/mL |
28 | 28 |
| Liu et al. (2014) | Merial G52 | 1‐dose SC | Adult |
0 41 21 60 120 180 240 270 |
(not given by authors) |
8 8 8 8 8 8 8 8 |
0 IU/mL (GM) 3 IU/mL (GM) 4.5 IU/mL (GM) 5 IU/mL (GM) 4 IU/mL (GM) 3.5 IU/mL (GM) 2.6 IU/mL (GM) 2 IU/mL (GM) |
270 | 270 | ||
| Shiraishi et al. (2014) | Commercial not specified | 3‐dose IM | 13–24 months |
0 14 21 28 40 47 54 61 90 120 151 181 213 243 274 305 335 365 395 426 549 671 760 |
FAVN |
10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 |
0 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 |
0 to 0 IU/mL 1.3 to 10 IU/mL 1.25 to 8 IU/mL 1.2 to 12 IU/mL 40 to 700 IU/mL 53 to 1094 IU/mL 45 to 750 IU/mL 60 to 500 IU/mL 40 to 1100 IU/mL 0.7 to 1300 IU/mL 0.6 to 1300 IU/mL 0.9 to 10 IU/mL 0.9 to 20 IU/mL 0.7 to 30 IU/mL 0.7 to 50 IU/mL 0.7 to 50 IU/mL 1.05 to 30 IU/mL 0.9 to 50 IU/mL 1.05 to 50 IU/mL 20 to 800 IU/mL 8 to 300 IU/mL 10 to 400 IU/mL 5 to 200 IU/mL |
760 | 760 | |
| Morters et al. (2015) | Rabisin | 1‐dose SC | 1–3 months |
0 30 |
FAVN |
2 19 |
0.06 IU/mL 2.0 to 90.5 IU/mL |
30 | 30 | ||
| Nobivac | 1‐dose SC | 2–3 months |
0 30 |
FAVN |
8 8 |
0.1 to 0.29 IU/mL > 5.9 IU/mL |
30 | 30 | |||
|
Asokkumar et al. (2016) |
Raksharab | 1‐dose IM |
0 14 28 |
RFFIT |
8 8 8 |
0.51 IU/mL 11.14 IU/mL 43.23 IU/mL |
SD = 0.17 SD = 2.15 SD = 8.42 |
28 | 28 | ||
| Raksharab | 1‐dose SC |
0 14 28 |
RFFIT |
8 8 8 |
0.17 IU/mL 16.71 IU/mL 62.35 IU/mL |
SD = 0.04 SD = 0.00 SD = 2.18 |
28 | 28 | |||
| Raksharab | 1‐dose ID |
0 14 28 |
RFFIT |
8 8 8 |
0.91 IU/mL 14.75 IU/mL 60.23 IU/mL |
SD = 0.11 SD = 0.87 SD = 2.47 |
28 | 28 | |||
| Raksharab | 1‐dose IM |
0 14 28 |
RFFIT |
8 8 8 |
0.07 IU/mL 0.72 IU/mL 1.44 IU/mL |
SD = 0.00 SD = 0.06 SD = 0.12 |
28 | 28 | |||
| Raksharab | 1‐dose SC |
0 14 28 |
RFFIT |
8 8 8 |
0.07 IU/mL 1.05 IU/mL 2.00 IU/mL |
SD = 0.00 SD = 0.08 SD = 0.08 |
28 | 28 | |||
| Raksharab | 1‐dose ID |
0 14 28 |
RFFIT |
8 8 8 |
0.07 IU/mL 0.57 IU/mL 1.84 IU/mL |
SD = 0.00 SD = 0.06 SD = 0.08 |
28 | 28 | |||
| Rabivac | 1‐dose SC | > 3 months |
0 30 |
FAVN |
919 919 |
220 845 |
0.03 to 6.01 IU/mL 0.03 to 21826 IU/mL |
30 | 30 | ||
| Darkaoui et al. (2016) | Rabivac | 1‐dose SC | > 3 months |
0 30 |
FAVN |
919 919 |
220 845 |
0,03 to 6,01 IU/mL 0,03 to 218,26 IU/mL |
30 | 30 | |
| Lankester et al. (2016) | Nobivac | 1‐dose SC |
0 28 |
FAVN |
50 50 |
0 |
< 0.5 IU/mL 1.8 IU/mL (GM) |
28 | |||
| Niu et al. (2016) | Commercial not specified |
2‐dose SC Days 0, 14 |
6 months |
14 28 |
FAVN |
10 10 |
4 IU/mL (GM) 8 IU/mL (GM) |
28 | 28 | ||
| Zhang et al. (2016) | ? | 1‐dose? | > 16 weeks |
< 3 4–7 8–14 15–30 31–90 91–180 180–270 > 270 |
FAVN |
5483 5483 5483 5483 5483 5483 5483 5483 |
0.02 IU/mL (GM) 1.65 IU/mL (GM) 2.66 IU/mL (GM) 2.7 IU/mL (GM) 1.86 IU/mL (GM) 0.86 IU/mL (GM) 0.75 IU/mL (GM) |
0.01 to 0.06 IU/mL 1.01 to 2.69 IU/mL 2.31 to 3.06 IU/mL 2.57 to 2.85 IU/mL 1.75 to 1.99 IU/mL 0.75 to 0.98 IU/mL 0.59 to 0.97 IU/mL |
|||
| Giel‐Moloney et al. (2017) | Commercial not specified | 1‐dose SC | 4 months |
−14 7 14 28 49 70 99 127 163 253 |
RFFIT |
2 2 2 2 2 2 2 2 2 2 |
0 IU/mL (GM) 4.5 IU/mL (GM) 10 IU/mL (GM) 2.7 IU/mL (GM) 1.8 IU/mL (GM) 1.5 IU/mL (GM) 1 IU/mL (GM) 0.9 IU/mL (GM) 0.8 IU/mL (GM) 1 IU/mL (GM) |
253 | 284 | ||
| Pimburage et al. (2017) | Nobivac | 1‐dose IM | 6 weeks–3 months |
0 30 180 360 |
RFFIT | 40 |
0 39 33 3 |
0.1 IU/mL (GM) 10.66 IU/mL (GM) 4.63 IU/mL (GM) 0.23 IU/mL (GM) |
0.02 to 0.44 IU/mL 0.4 to 49.09 IU/mL 0.004 to 32.5 IU/mL 0.004 to 18 IU/mL |
360 | 360 |
| Nobivac | 1‐dose IM | 3 months–1 year |
0 30 180 360 |
RFFIT | 47 |
37 45 44 37 |
15.99 IU/mL (GM) 34.77 IU/mL (GM) 27.09 IU/mL (GM) 21.59 IU/mL (GM) |
0.03 to 177.5 IU/mL 0.03 to 269.79 IU/mL 0.06 to 269.83 IU/mL 0.08 to 229 IU/mL |
360 | 360 | |
| Nobivac | 1‐dose IM | > 1 year |
0 30 180 360 |
RFFIT | 47 |
33 47 47 39 |
6.66 IU/mL (GM) 51.85 IU/mL (GM) 22.89 IU/mL (GM) 7.177 IU/mL (GM) |
0.02 to 49.09 IU/mL 1.96 to 269.76 IU/mL 0.4 to 100.4 IU/mL 0.19 to 49.09 IU/mL |
360 | 360 | |
| Nobivac | 1‐dose IM | 1–6 years |
0 30 180 360 |
RFFIT | 51 |
39 49 45 40 |
13.62 IU/mL (GM) 29.81 IU/mL (GM) 39.47 IU/mL (GM) 24.23 IU/mL (GM) |
0.03 to 214.63 IU/mL 0.03 to 269.79 IU/mL 0.06 to 269.83 IU/mL 0.36 to 229 IU/mL |
360 | 360 | |
| Wallace et al. (2017) | ? | 1‐dose? | < 12 week |
< 3 4–7 8–14 15–30 31–90 91–180 180–270 > 270 |
FAVN |
290 290 290 290 290 290 290 290 |
1.42 IU/mL (GM) 4.14 IU/mL (GM) 2.51 IU/mL (GM) 1.21 IU/mL (GM) 0.55 IU/mL (GM) 2.05 IU/mL (GM) 1.46 IU/mL (GM) |
0.27 to 7,39 IU/mL 2.89 to 5,93 IU/mL 1.99 to 3,16 IU/mL 0.87 to 1,68 IU/mL 0.28 to 1,12 IU/mL 1.58 to 2,66 IU/mL 0.67 to 3,19 IU/mL |
|||
| ? | 1‐dose? | 12–16 weeks |
< 3 4–7 8–14 15–30 31–90 91–180 180–270 > 270 |
FAVN |
2238 2238 2238 2238 2238 2238 2238 2238 |
0.01 IU/mL (GM) 0.23 IU/mL (GM) 1.68 IU/mL (GM) 2.13 IU/mL (GM) 1.56 IU/mL (GM) 0.74 IU/mL (GM) 0.51 IU/mL (GM) 0.03 IU/mL (GM) |
0 to 0.15 IU/mL 0 to 35.52 IU/mL 1 to 2.81 IU/mL 1.92 to 2.37 IU/mL 1.4 to 1.73 IU/mL 0.6 to 0.9 IU/mL 0.36 to 0.73 IU/mL 0 to 2.44 IU/mL |
||||
| ? | 1‐dose? | > 16 weeks |
< 3 4–7 8–14 15–30 31–90 91–180 180–270 > 270 |
FAVN |
5483 5483 5483 5483 5483 5483 5483 5483 |
0.02 IU/mL (GM) 1.65 IU/mL (GM) 2.66 IU/mL (GM) 2.7 IU/mL (GM) 1.86 IU/mL (GM) 0.86 IU/mL (GM) 0.75 IU/mL (GM) |
0.01 to 0.06 IU/mL 1.01 to 2.69 IU/mL 2.31 to 3.06 IU/mL 2.57 to 2.85 IU/mL 1.75 to 1.99 IU/mL 0.75 to 0.98 IU/mL 0.59 to 0.97 IU/mL |
||||
| Bouvet et al. (2018) | Rabisin | 1‐dose SC | 7–9 week |
0 14 27 42 70 119 182 272 363 |
FAVN |
0 IU/mL (GM) 8 IU/mL (GM) 16 IU/mL (GM) 11 IU/mL (GM) 3.5 IU/mL (GM) 8 IU/mL (GM) 9 IU/mL (GM) 10 IU/mL (GM) 3.5 IU/mL (GM) |
363 | 363 | |||
| Rabisin | 1‐dose SC | 7–9 week |
0 14 27 42 70 119 182 272 363 |
FAVN |
0 IU/mL (GM) 12 IU/mL (GM) 23 IU/mL (GM) 12 IU/mL (GM) 6 IU/mL (GM) 10 IU/mL (GM) 11 IU/mL (GM) 11,5 IU/mL (GM) 4 IU/mL (GM) |
363 | 363 | ||||
| Devi et al. (2018) | Rabipur |
2‐dose? Days 0, 21 |
3–6 months |
0 7 14 21 28 |
RFFIT |
6 6 6 6 6 |
2 log 2 titer 12 log 2 titer 12 log 2 titer 12 log 2 titer 12 log 2 titer |
||||
| Zhang et al. (2016) | Commercial not specified |
2‐dose IM Days 0, 730 |
> 2 years |
730 737 744 751 758 |
FAVN |
5 5 5 5 5 |
1.2 IU/mL (GM) 1.5 IU/mL (GM) 2.3 IU/mL (GM) 2.3 IU/mL (GM) 2.5 IU/mL (GM) |
758 | 758 | ||
| Commercial not specified | 1‐dose IM | 3 months |
14 28 |
FAVN |
5 5 |
0.7 IU/mL (GM) 1.2 IU/mL (GM) |
28 | 28 | |||
| Arega et al. (2020) | Defensor 3 |
2‐dose SC Days 0, 91 |
6 weeks |
42 63 112 |
RFFIT |
173 117 49 |
0.0064 IU/mL (GM) 1.47 IU/mL (GM) 2.73 IU/mL (GM) |
112 | 112 | ||
| Bommier et al. (2020) | Rabisin | 1‐dose SC | 4.5–5 months |
7 28 |
RFFIT |
6 6 |
6 6 |
0.16 IU/mL (Med) 7.92 IU/mL (Med) |
0.06 to 0.66 IU/mL 3.46 to 10.45 IU/mL |
28 | 84 |
| Rabisin | 1‐dose SC | 4.5–5 months |
7 28 |
RFFIT |
6 6 |
6 6 |
0.52 IU/mL (Med) 7.5 IU/mL (Med) |
0.29 to 3.46 IU/mL 4.56 to 72.27 IU/mL |
28 | 84 | |
| Paris et al. (2020) | Rabisin | 1‐dose SC | 3.5–5.5 months | 28 | RFFIT | 7 | 7 IU/mL (Med) | 2 to 13.8 IU/mL | 28 | 28 | |
| Non‐commercial | 1‐dose SC | 3.5–5.5 months | 28 | RFFIT | 6 | 1,6 IU/mL (Med) | 0.2 to 13.8 IU/mL | 28 | 28 | ||
| Non‐commercial | 1‐dose SC | 3.5–5.5 months | 28 | RFFIT | 7 | 2 IU/mL (Med) | 1.2 to 4.6 IU/mL | 28 | 28 | ||
| Non‐commercial | 1‐dose SC | 3.5–5.5 months | 28 | RFFIT | 6 | 1 IU/mL (Med) | 0.3 to 13.8 IU/mL | 28 | 28 | ||
| Non‐commercial | 1‐dose SC | 3.5–5.5 months | 28 | RFFIT | 7 | 10.5 IU/mL (Med) | 1.2 to 18.2 IU/mL | 28 | 28 | ||
| Lugelo et al. (2021) | Nobivac | 1‐dose SC | 3–60 months | 28 | FAVN | 163 | 139 | 1.8 IU/mL (GM) | 28 | 28 | |
| Nobivac | 1‐dose SC | 3–60 months | 28 | FAVN | 163 | 140 | 2.0 IU/mL (GM) | 28 | 28 | ||
| Molini et al. (2021) | Rabisin | 1‐dose? | ? | 28 | RFFIT | 2 | 2 | 28 | 28 | ||
FAVN: fluorescent antibody virus neutralisation assay; GM: geometric mean; RFFIT: rapid fluorescent focus inhibition test; PV: post‐vaccination.
Timelines are given in relation to the first vaccination day. All serological tests presented targeted virus neutralising antibodies, and the type of test is given in the table.
In experimental infections and vaccine trials, there are few records of an individual dog in which a value of 0.5 IU/mL was measured before onset of clinical signs. Fekadu et al. (1982a) reported average values beyond 0.5 IU/mL (at 1.5 IU/mL) 7 days post‐inoculation but 4 days prior the first onset of clinical signs in a vaccinated group in which five out of seven dogs showed clinical signs. However, there was no information on how these results were distributed in individual dogs.
Upon vaccination, most dogs seroconvert between 3 and 21 days. Antibody levels tend to reach a peak at 28–30 days post‐vaccination with titres well above 0.5 IU/mL. Van Gucht and Le Roux (2008) analysed serology results of 28,412 canine blood samples submitted between 2000 and 2005 under the EU Pet Travel Scheme to check the neutralising antibody titre upon vaccination. The vast majority of dogs tested positive. Only 6.35% of canine blood samples tested negative 1–12 months after vaccination (< 0.5 IU/mL). Up to 14% of dogs between the age of 3 and 6 months tested negative upon vaccination. Approximately 8% of dogs between the age of 6 and 12 months tested negative. Above the age of 1 year, the percentage of negative test results varied at ~ 3%. The seemingly lower percentage of negative tests in dogs older than 1 year is most likely because young dogs have been vaccinated only once, whereas older animals have often received one or more additional vaccinations earlier in life. The probability of a positive test result was highest when the sample was taken 1–2 months after vaccination (4.42% negatives). Intervals of 3 months or more are associated with a significantly higher probability of a negative test result (8.81% negatives). Zanoni et al. (2010) found similar results with ~ 5% of dogs testing negative at 1–2 months after primary vaccination. Antibody titres were highest at 1 month post‐vaccination and decreasing afterwards. At 7–12 months after the primary vaccination 30% of dogs tested negative. A double primary vaccination or repeat vaccination significantly increases the probability to reach a titre of ≥ 0.5 IU/mL.
3.1.3. Dogs imported into the EU territory from non‐EU countries
The consignments of dogs imported into the EU territory for commercial purposes are registered into the TRACES system, therefore information on number of dogs according to the country of origin and destination are available. The total number of imported dogs from all non‐EU countries and the number of dogs imported from the non‐EU countries where the positive titration test has been a requirement for the last 3 years (2019–2021) are presented in Table C.1 of Appendix C.
The number of dogs imported from non‐EU countries for non‐commercial reasons (as pets accompanied by their owner or an authorised person) is not known.
There are no officially available data for the number of dog movements into the EU each year. According to the study conducted by (Norman et al., 2020) and based on the data collected using an online survey, the number of pets moving for non‐commercial purposes was 10 times the number of commercial dogs, and the majority were from non‐Eu countries for which no titration test is required or EU Member States, that is 300,000 vs. 31,000 (Norman et al., 2020).
3.2. Assessment
3.2.1. Probability of importing a RABV‐infected dog from a country for which a titration test is mandatory
3.2.1.1. Based on the length of incubation period as derived from experimental studies
According to the results of the ELR, the inoculation dose used in experimental trials varied widely in the different groups of dogs (Table B.1 of Appendix B). For this risk assessment, 46 groups of dogs inoculated by intramuscular route were selected from 19 experimental studies resulting in 214 infected dogs with clinical signs. The time from inoculation to the onset of clinical signs was considered as the incubation period. The incubation period was available at individual level for 35 groups of dogs, whereas for the other 11 groups only the range of the incubation period of each group of dogs was provided in the publication. The upper limit of each of these 11 ranges was below 40 days and the incubation period of each one of the individual dogs was therefore less than 40 days. Therefore, the incubation periods of 55 dogs were imputed in the dataset assuming a uniform distribution of the individual incubation periods over the range of the respective groups.
In total, for 159 dogs, the incubation period was available, whereas for the remaining 55 dogs the incubation periods were randomly imputed into the data set assuming a uniform distribution of cases over their respective incubation period range.
The overall distribution of the 214 dogs had a minimum incubation period of 6 days and a maximum incubation period of 92 days; in eight dogs the incubation period exceeded 40 days (Figure 7).
Figure 7.

(Cumulative) Distribution of the number of days of the incubation period upon intramuscular challenge with rabies virus in unvaccinated dogs
In this data set, none of the incubation periods are long enough to reach WP90, as they would need to be longer than 100 days. Applying the methodology described in Section 2.2 to the available data the overall rate for a dog to be RABV infected and not present clinical signs before the end of WP30 equals 0.823 × IR, whereas this rate is 0.000 × IR for WP90.
According to Crozet et al. (2020b), the annual incidence of rabies in dogs in non‐EU countries, for which a blood test is required for dog movements either for commercial or non‐commercial purposes, varies from 100 to 500 cases per 100,000 dogs.
This implies that from the high incidence regions (500 cases per 100,000 dogs per year) an imported dog that is fully compliant with legislation and had a waiting period of 30 days has a probability of 1.13 × 10–5 to be RABV infected, whereas this probability is 2.25 × 10–6 if imported from a low incidence region (100 cases per 100,000 dogs per year). For a region with a very low incidence (five cases per 100,000 dogs per year), the probability would be 1.13 × 10–7.
3.2.1.2. Based on the length of incubation period as derived from field data
Crozet et al. (2022) fitted a lognormal distribution [dlnorm(x, meanlog = 3.2132377, sdlog = 0.8908552)] to incubation periods from Ribadeau‐Dumas et al. (2016), Tojinbara et al. (2016), and Appleton (1972). Figure 8 shows the comparison between this lognormal distribution and a lognormal distribution fitted to the data from Figure 7. Figure 8 shows that the field data contain longer incubation periods than those observed in experimental studies. According to this distribution, 29.6% of the incubation periods would exceed 40 days (WP30) and 5.9% would even exceed 100 days (WP90).
Figure 8.

Distribution of the duration of the incubation period (days) of rabies derived from experimental data from the literature review (red) and field data from Crozet et al. (2022) (green line). A log normal distribution was fitted to the datasets
Using this distribution of the incubation periods in the method described above, the overall rate for a dog to be RABV infected and not develop clinical signs before the end of WP30 equals 10.8 × IR, whereas this is 2.58 × IR for WP90 (Table 2 and Figure 9).
Table 2.
Probability of an individual dog being infected incubating rabies virus (RABV) and being moved to EU from a non‐EU country, even if the movement is fully in compliance with the EU legislation, and having an incubation period exceeding the waiting period (WP) of 30 days and 90 days, respectively, as a function of the incidence rate of rabies in the regions of dog origin
| Annual incidence rate (IR) | Probability of an imported dog incubating RABV based on the incubation period | |||
|---|---|---|---|---|
| Experimental data | Field data | |||
|
WP 30 days |
WP 90 days |
WP 30 days |
WP 90 days |
|
| High (500/100,000 dogs) | 1.13 × 10–5 | 0 | 14.8 × 10–5 | 3.53 × 10–5 |
| Medium (250/100,000 dogs) | 5.64 × 10–6 | 0 | 7.4 × 10–5 | 1.77 × 10–5 |
| Low (100/100,000 dogs) | 2.25 × 10–6 | 0 | 2.96 × 10–5 | 0.707 × 10–5 |
| Very Low (5/100,000 dogs) | 1.13 × 10–7 | 0 | 14.8 × 10–7 | 3.53 × 10–7 |
Figure 9.
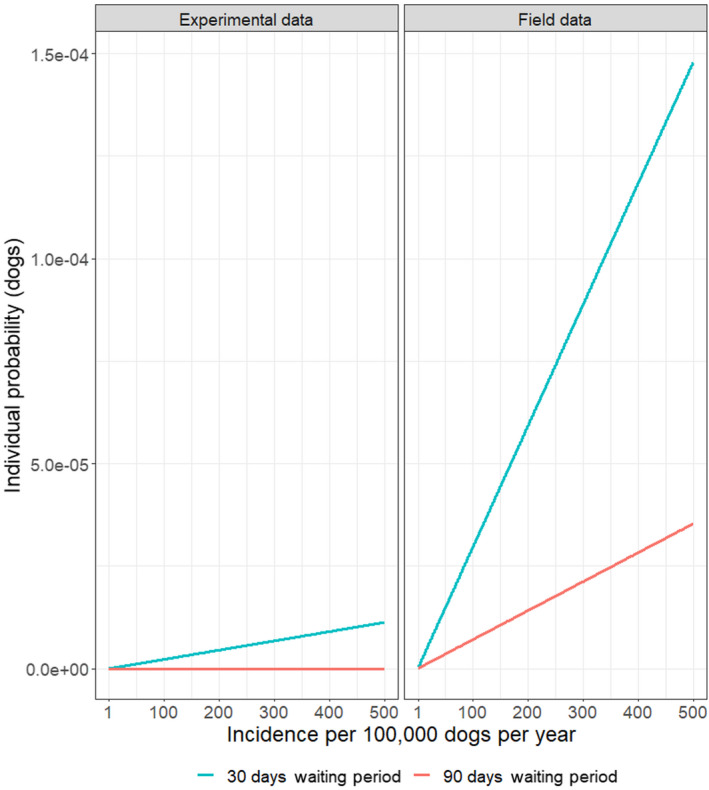
Probability of an individual dog imported being infected and having an incubation period exceeding the waiting period of 30 days and 90 days accordingly, as a function of the incidence rate of rabies in the regions of dog origin
- The Incidence Rate (IR) is calculated as the number of infected dogs per 100,000 dogs per year. When the incubation period is derived from the experimental data and the waiting period is 90 days, the individual probability is zero regardless the value of the IR.
From high‐incidence regions, the probability that an imported dog that is fully in compliance with legislation and had a waiting period of 30 days incubating RABV equals 14.8 × 10–5, whereas this probability is 2.96 × 10–5 if imported from a low‐incidence region and 14.8 × 10–7 if imported from a region with a very low incidence (Table 2 and Figure 9). For WP90, these probabilities are 3.53 × 10–5, 0.707 × 10–5 and 3.53 × 10–7, respectively (Table 2 and Figure 9).
Table 2 and Figure 9 show that the individual probability of an imported dog being infected and having an incubation period exceeding the waiting period, is higher when the dog has originated from regions with higher IR in both lengths of waiting periods (30 and 90 days). The linear relationship observed between the individual probability and the IR originates from the formula described in the Methodologies section.
3.2.2. Overall probability of RABV introduction from countries for which a titration test is mandatory
The estimation of the overall probability of RABV introduction from non‐EU countries for which a titration test is mandatory, based on the probability of importing one RABV‐infected dog and the number of dogs imported from such regions.
The total number of dogs moving into the EU from non‐EU countries for which the titration test is mandatory is not known and cannot be calculated. Information on the number of dogs and the country of origin is available (in TRACES) only for commercial movements. For non‐commercial movements of dogs this information is not available.
Based on the data from TRACES (Table C.1 of Appendix C) on commercial movements of dogs from non‐EU countries for which a titration test is mandatory, in the period between 2019 and 2021, the average number of dogs imported per year equals 1,780.
For the non‐commercial movements and based on the results of the study by Norman et al. (2020), the number of non‐commercial dogs imported is assumed to be 10 times as high as the number of commercial movements; approximately 20,000.
Consequently, for the purposes of this assessment in the following examples, two indicative values were used for the number of animals moved from non‐EU countries into EU per year; 1,780 and 20,000 (Table 3).
Table 3.
Examples of the estimated overall probability of introducing at least one rabies‐infected dog, and average number of years before this occurs, under different combinations of data source on incubation period, rabies incidence in non‐EU countries of origin and number of dogs imported into the EU per year
| Annual incidence rate in the non‐EU country (IR)(a) | Incubation period | No. of imported dogs (b) | Waiting period | |||
|---|---|---|---|---|---|---|
| 30 days | 90 days | |||||
| p | Years | p | Years | |||
|
High (500/100,000) |
Field data | 1,780 | 0.23 | 3.8 | 0.061 | 16 |
| 20,000 | 0.95 | 0.34 | 0.51 | 1.4 | ||
| Experimental data | 1,780 | 0.02 | 50 | 0.000 | N/A | |
| 20,000 | 0.20 | 4.4 | 0.000 | N/A | ||
|
Medium (250/100,000) |
Field data | 1,780 | 0.12 | 7.6 | 0.031 | 32 |
| 20,000 | 0.77 | 0.68 | 0.30 | 2.8 | ||
| Experimental data | 1,780 | 0.010 | 100 | 0.000 | N/A | |
| 20,000 | 0.11 | 8.9 | 0.000 | N/A | ||
|
Low (100/100,000) |
Field data | 1,780 | 0.051 | 19 | 0.013 | 79 |
| 20,000 | 0.45 | 1.7 | 0.13 | 7.0 | ||
| Experimental data | 1,780 | 0.004 | 250 | 0.000 | N/A | |
| 20,000 | 0.044 | 22 | 0.000 | N/A | ||
|
Very low (5/100,000) |
Field data | 1,780 | 0.003 | 380 | 0.001 | 1,590 |
| 20,000 | 0.029 | 34 | 0.007 | 141 | ||
| Experimental data | 1,780 | 0.000 | 4,983 | 0.000 | N/A | |
| 20,000 | 0.002 | 443 | 0.000 | N/A | ||
p = probability of introducing at least one RABV‐infected dog.
Years = average number of years to import one RABV‐infected dog.
The values for the Incident Rate (IR) used here are based on the publication of Crozet et al. (2020b) the low IR = 100/100,000 dogs, medium IR = 250/100,000 dogs, high IR = 500/100,000 dogs and for very low it was added to cover regions with lower IR.
The total number of animals used here are based on the average number of dogs per year imported from non‐EU countries for commercial purposes based on TRACES data for 2019–2021 (1,780); and on the assumption that the number of dogs imported into EU for non‐commercial purposes would be 10 times as high as the number for the commercial purposes (20,000).
In addition, the dogs come from areas that vary in risk; from high risk (500 cases/100,000 dogs per year) to very low risk (5 cases/100,000 dogs per year) (Tables 2 and 3).
The probability of introduction of at least one RABV‐infected dog per year and the average time (in years) it takes to introduce one RABV‐infected dog have been calculated for both waiting periods (30 and 90 days) and are presented in Table 3. These probabilities have been estimated separately for dogs coming from areas of high, medium, low and very low risk using the length of incubation period as derived from experimental and field data.
3.2.2.1. Based on the length of incubation period as derived from experimental studies
90 days waiting period
In the 90‐day waiting period scenario, the risk is zero according to the assessment based on experimentally derived incubation periods for both numbers of introduced dogs per year (1.780 and 20,000), because no incubation periods longer than 100 days were present in the data from experimental infections.
30 days waiting period
If the total number of imported dogs is 1,780 per year and assuming the dogs originate from a medium risk area, the likelihood for an individual dog to be RABV infected would be 5.64 × 10–6 (Table 2). This implies that the annual probability of importing at least one RABV‐infected dog into EU after a 30‐day waiting period, equals 1.0% (1 – (1 – 5.64 × 10–6)^1,780)) (Table 3). As a result, for a waiting period of 30 days, on average, once every 100 years a RABV‐infected dog would be introduced into the EU.
If the total number of dogs is 20,000 per year and assuming the dogs originate from a medium risk area the annual probability of importing at least one RABV‐infected dog after a 30‐day waiting period would be 11% and, on average, one RABV‐infected dog would be introduced into the EU every 9 years (Table 3).
3.2.2.2. Based on the length of incubation period as derived from field data
90 days waiting period
Assuming that the imported dogs originate from a medium risk area, the probability for an individual dog to be RABV infected after a 90‐day waiting period would be 1.77 × 10–5 (Table 2). If the total number of imported dogs is 1,780 per year, this implies that the annual probability that one or more RABV‐infected dogs are introduced to EU equals 3.1% (1 – (1 – 1.77 × 10–5)^1,780) (Table 3). As a result, for a waiting period of 90 days, on average, once every 32 years one RABV‐infected dog would be introduced into the EU.
If the total number of dogs is 20,000 per year, and assuming the dogs originate from a medium risk area, the probability of importing at least one RABV‐infected dog is 29.8%, and on average, one RABV‐infected dog would be imported every 2.8 years (Table 3).
30 days waiting period
If the total number of imported dogs is 1,780 per year and assuming the dogs originate from a medium risk area, the probability for an individual dog to be RABV infected after a 30‐day waiting period would be 7.4 × 10–5 (Table 2).
This implies that the annual probability that one or more RABV‐infected dogs are introduced to EU equals 12.3% (1 – (1 – 7.4 × 10–5)^1,780) in the 30‐day waiting period scenario (Table 2).
Phrased differently, the number of RABV‐infected dogs introduced into the EU would increase from one dog every 32 years to one dog every 7.6 years when the waiting period is reduced from 90 to 30 days, respectively, (Table 3) even if the movement is in compliance with the EU regulation.
If the total number of dogs is 20,000 per year and assuming the dogs originate from a medium risk area, the probability of importing at least one RABV‐infected dog is 77.2% for a waiting period of 30 days and on average one RABV‐infected dog would be imported per 250 days (or 1.5 rabies‐infected dogs per year) (Table 3).
Table 3 and Figure 10 show that the annual probability of importing at least one RABV‐infected dog into the EU and the number of years needed to import at least one infected dog increases as the number of dogs moving from the non‐EU countries to the EU is increasing.
Figure 10.
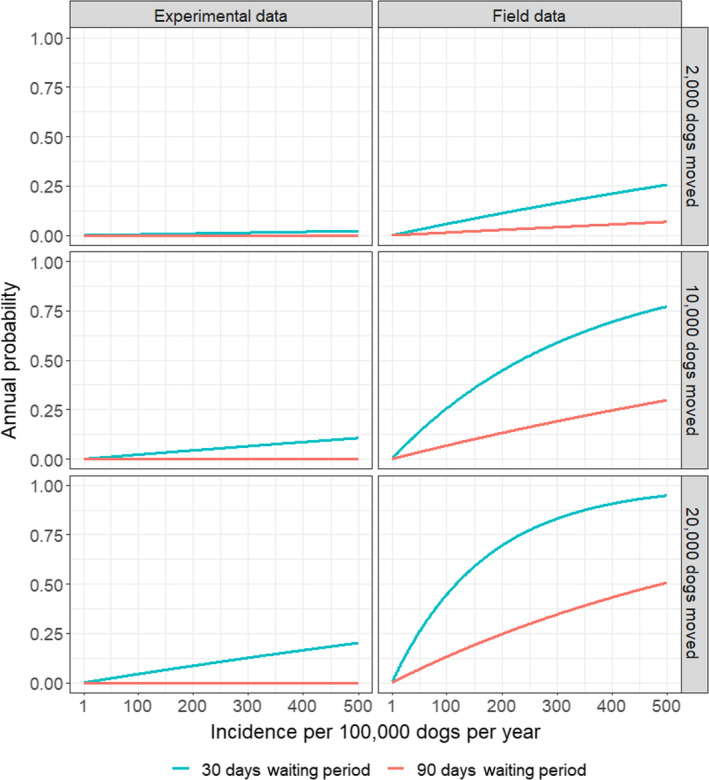
The overall annual probability of at least one RABV‐infected dog with an incubation period exceeding the waiting period of 30 days and 90 days by the incidence rate of rabies in the regions of dog origin
- Incidence Rate (IR) is calculated as the number of infected dogs per 100,000 dogs per year. When the incubation period is derived from the experimental data for a waiting period of 90 days, the individual probability is zero regardless the value of the IR.
Table 3 and Figure 10 show that using the incubation period as derived from the field data (Crozet et al., 2022), the average time it takes to import a RABV‐infected dog incubating RABV, while fully compliant with the regulation, is reduced 4.2 times if the waiting period is reduced from 90 to 30 days, irrespective of the incidence in the region of origin and the numbers of dogs introduced.
Although this relative difference is stable, the absolute outcomes (probability of introducing at least one RABV‐infected dog per year and the average number of years it takes to introduce one RABV‐infected dog) are very sensitive to the incidence in the region of origin and the numbers of dogs introduced from such regions. The median prevalence applies to certain regions in Africa and Asia mostly (Crozet et al., 2020b). For imports from countries where rabies is not endemic in the dog population, the very low incidence is more applicable. According to the assessment, the risk of importing a RABV‐infected dog from a very low‐risk region is much lower than from the median risk region as presented above (Table 2). Using the field‐derived incubation periods, the average time it takes to import one dog fully compliant with the regulations from a region with very low risk is reduced from 141 years in WP90 to 34 years in WP30. Although the relative increase of the risk of changing from 90 to 30 days waiting period is stable for differences in IR and numbers of dogs imported, it strongly depends on the distribution of the incubation period, in particular those longer than 40 days and 100 days, respectively. Table 2 shows that while assuming the experimentally derived incubation period distribution, the risk is reduced considerably in comparison with the incubation periods derived from the field data.
3.3. Uncertainty analysis
The analysis presented above is based on several assumptions as described in the Methodology in Section 2.2 that lead to considerable uncertainty regarding its results. The approach is as follows:
Uncertainty regarding the methodology used: the approach was based on a simple deterministic model and as such stochastic variability was not considered.
Uncertainty on the number of dogs entering the EU from the countries considered in the assessment: although information on the number of dogs imported for commercial purposes is available, no data on the number of non‐commercial movements exist, although it was estimated to be up to 10 times higher for some European countries. The assessment presented in Table 2 considered two scenarios (1,780 and 20,000), both of which can underestimate the true number of dogs imported from these countries every year. Conversely, only dogs subjected to their first vaccination or in which immunity has expired and therefore must be revaccinated could be incubating the disease while being imported and would thus be of interest for the assessment, but there is no information on what the proportion of those dogs may be among all imported from the countries considered in the assessment.
Uncertainty on the incidence of rabies in the population of dogs travelling from these countries into the EU while complying with the regulations: although some estimates on the incidence of rabies in the countries of origin of imported dogs exist, no data on the actual incidence in the dogs that are actually imported are available. Available incidence estimates for countries of origin do not take into consideration differences in dog subpopulations (e.g. indoor, free‐roaming and stray dog populations). Dogs travelling to the EU is more likely to be indoor well‐cared pets, though an unknown proportion may also be formed by dogs found in the street, abandoned or coming from a rescue centre, that would be therefore subjected to a much higher risk of exposure than indoor animals.
Uncertainty on the incubation period of naturally infected dogs: scientific articles retrieved through the literature review providing data on duration of the incubation period were mostly based on experimental infection, were typically very heterogeneous in terms of their objectives and methodology used (e.g. infection strain, dose and routes for experimental challenge studies, tests used, follow‐up periods, etc.), and often did not provide sufficient information to assess the responses recorded in each individual animal considered in each study (and provided ranges of days from infection to clinical signs, death, etc.). Furthermore, incubation periods derived from experimental studies may not accurately reflect the situation in which natural exposure exists and may lead to artificially shorter periods. Differences in the incubation periods coming from experimental and field studies may be due to several reasons, e.g. the way of inoculation, the virus dose and the daily observation. Moreover, the duration of some of the experiments may not have been long enough to detect very long incubation periods. Conversely, in the field situation the actual moment of infection may be uncertain.
Uncertainty on the degree of protection conferred by rabies vaccines and time between vaccination and development of a protective response: although in the report it was assumed that a dog with a titre > 0.5 IU/mL became fully protected (100% efficacy) 21 days post‐vaccination (as by that time most of the vaccinated dogs will have fully seroconverted), the protective immunity induced by the vaccines is most likely to increase in a more gradual way after vaccination. Therefore, before 21 days post‐vaccination some protection could be expected (i.e. not all vaccinated dogs will be fully susceptible), and therefore, the assumption used in the report is likely to be an overestimation of the susceptibility of vaccinated dogs within the first weeks post‐vaccination.
Uncertainty on the sensitivity of clinical inspection at the EU borders: dogs entering the EU must have a health certificate verifying they are in good health, and if a dog is symptomatic, it is assumed that it would be detected at the border control (100% sensitivity), and therefore only fully asymptomatic infected dogs at import were considered at risk of remaining undetected. However, there are no estimates of the sensitivity of the inspection at border control regarding the detection of symptomatic animals; if it is not 100% it would be possible for an infected dog to enter the EU even if the incubation period was (slightly) shorter than the waiting period.
Because of these uncertainties in Table 2 different scenarios are presented, IR varying from high to low, number of dogs varying from the ones documented in TRACES to 20,000 dogs and a narrow range incubation period (experimental) to a wide range (field derived data).
After considering the overall impact of these sources of uncertainty in the assessment and discussing the individual judgements of the experts, it was concluded with a 95% certainty that the number of RABV‐infected dogs that will be imported from countries or regions either not listed in Annex II to Regulation (EU) 577/2013 for pet dogs, or listed in Regulation (EU) 2020/404 (Annex VIII column 5) for commercial and non‐commercial movements that are compliant with the regulations (vaccinated as requested and passing a VNT test 30 days post‐vaccination) in a 20‐year period under the current waiting period of 90 days (QoI1) would be equal or lower than 5. When the alternative scenario (waiting period of 30 days) was considered, it was concluded with a 95% certainty that this number would be equal or lower than 20. The difference between the QoIs was due to the much larger uncertainty on the possible effect of the factors listed above on the number of rabies‐infected imported dogs that would remain undetected with a 30‐day waiting period, with the effect leading to an increase of the risk in most cases. Therefore, even though the expected number of RABV‐infected dogs that would enter the EU in a 20‐year period in this alternative scenario was still low (≤ 10) according to the judgements from most experts, it was not possible to rule out scenarios in which this number could be even twice as high.
4. Conclusions
Based on a review of published studies, in the experimental infections, the onset of clinical signs and deaths in dogs after intramuscular inoculation ranged from 6 to 92 and 3 to 257 days post‐inoculation, respectively. Similarly, in infected dogs imported from non‐EU countries not meeting EU requirements, the onset of clinical signs varied from 2 to 179 days after the arrival at the country of destination. Even if experimental infections might not reflect natural infection in the field, data from imported rabies cases in dogs suggest that the incubation period can be longer than 40 days and can exceed the 100 days, although rarely.
There is no official system at EU level requiring the registration of the non‐commercial movements of dogs from non‐EU countries to EU territory like TRACES for the commercial movements. Therefore, there are no available data on the number of dogs, and the countries of origin and destination to support the needs of the risk assessment.
No cases of imported RABV‐infected dogs have been associated with non‐commercial or commercial movements of dogs that have been correctly prepared for travel under EU rules. Cases of imported dogs that have been vaccinated, tested positive for neutralising antibodies (≥ 0.5 IU/mL) and found to be infected with rabies virus have not been found by the literature review.
Most, but not all vaccinated dogs would develop neutralising antibodies by 2 weeks after primary vaccination reaching peak levels at ~ 4–8 weeks. Failure to mount an antibody response of ≥ 0.5 IU/mL occurs in 5–30% of dogs after primary vaccination, but less than 5% after repeat vaccination. Neutralising antibodies titres below 0.5 IU/mL following vaccination does not necessarily mean absence of protection as lower titres might be protective and cellular immunity might also play a role.
Based on the limited data from experimental infections of unvaccinated dogs, seroconversion was usually observed close to or after the onset of clinical signs. There is no way to distinguish the serological response induced by infection or vaccination, but it is reasonable to assume that dogs that test positive for neutralising antibodies (≥ 0.5 IU/mL) upon infection will develop clinical signs and die as a result of the infection within 1 or 2 weeks. The assumption that most dogs are protected against rabies virus infection by week 3 after primary vaccination seems reasonable and relatively conservative.
Reducing the waiting period from 90 days to 30 days would increase the probability of one or more RABV‐infected dogs entering the EU, albeit to a small degree. Using an incubation period based on field data, the average time it would take to import a RABV‐infected dog fully compliant with the regulations would decrease by a factor of 4.2 when reducing the waiting period from 90 to 30 days, regardless of the number of dogs imported and the incidence in the country of origin. The increase in absolute risk of rabies introduction will depend on the incidence rate in the country of origin and the number of dogs imported.
Reporting rabies cases to the OIE on a regular basis is not always done in a harmonised manner in all countries. Therefore, it is difficult to assess the incidence in individual countries.
The introduction of RABV‐infected dogs has an impact on the public health sector. As an example, over 770 people have received PET in the EU following contact with 15 illegally imported infected dogs during the last 20 years. In addition, as rabies become less frequent in the EU, the awareness in the public, veterinary and medical sector decreases, and this might result in misdiagnoses and omission of preventive treatments in humans.
When all the sources of uncertainty were considered, it was concluded with a 95% certainty that under the current 90 days waiting period the number of rabies‐infected dogs that will be imported from the countries or regions considered in this assessment in a 20‐year period would be equal to or lower than 5. If the 30‐day waiting period was implemented instead, it was concluded with a 95% certainty that this number would be equal or lower than 20. This difference was due to the much larger uncertainty on the number of RABV‐infected imported dogs that would remain undetected under a 30‐day waiting period.
5. Recommendations
A further assessment of the number of dogs that enter the EU under the commercial and non‐commercial rules will reduce the uncertainty. In particular to focus on those dogs that do not have the same continual history of ownership or residency, have been mixing with animals of a different health status, including wildlife and therefore may be more likely to be exposed to rabies virus as a Type A risk.
The assessment of the countries or the regions regarding the risk of spread of RABV should be carried out based on epidemiological criteria to support the future risk management measures.
Further research with experimental and natural infection studies on the incubation period, the survival of infected animals and the development of antibodies will improve the evidence base and reduce the uncertainty of the analysis.
Awareness campaigns should be continued and focused on the risk of rabies introduction through dog movements from non‐EU countries and the importance of the correct implementation of the requirements of EU legislation including vaccination and titration test.
The compliance with vaccine quality, OIE laboratory test validation, case reporting, clinical inspections of animals before travel and official controls at the borders should be maintained at high level.
Abbreviations
- CSF
cerebrospinal fluid
- Dpv
days post‐vaccination
- GM
geometrical Mean
- FAVN
fluorescent antibody virus neutralisation assay
- HRIG
human rabies immune globulin
- IC
Intracerebral
- IM
Intramuscular
- IN
Intranasal
- IU
International Units
- MS
Member State(s)
- PET
post‐exposure treatment
- PI
post‐inoculation
- PV
post‐vaccination
- RABV
rabies virus
- RFFIT
rapid fluorescent focus inhibition test
- SC
subcutaneous
- ToR
Term of Reference
- VNT
virus neutralisation test
- WP
waiting period
Glossary
- Authorised veterinarian
Any veterinarian who has been authorised by the competent authority to carry out specific tasks in accordance with this Regulation or with acts adopted pursuant to this (Definitions in Regulation 576/2013)
- Official veterinarian
Any veterinarian appointed by the competent authority (Definitions in Regulation 576/2013) A veterinarian authorised by the competent authority and appropriately qualified to perform official activities in accordance with this Regulation; (definition Animal Health Law)
- Primary vaccination
The first vaccination and any revaccination if it was not carried out within the period of validity of the previous vaccination (point 2e of Annex III to Regulation (EU) No 576/2013).
Appendix A – Legislation
A. OIE Terrestrial Animal Health Code (29th edition – 2021) Chapter 8.14 on Infection with rabies virus
Article 8.14.7.: Recommendations for importation of dogs, cats and ferrets from countries or zones infected with rabies virus
Veterinary Authorities should require the presentation of an international veterinary certificate complying with the model of Chapter 5.11. attesting that the animals:
showed no clinical sign of rabies the day prior to or on the day of shipment;
were permanently identified and their identification number stated in the certificate;
-
and either:
were vaccinated or revaccinated in accordance with the recommendations of the manufacturer, with a vaccine that was produced in accordance with the Terrestrial Manual and were subjected not less than 3 months and not more than 12 months prior to shipment to an antibody titration test as prescribed in the Terrestrial Manual with a positive result of at least 0.5 IU/mL; or
were kept in a quarantine station for six months prior to shipment.
B. Commission Delegated Regulation (EU) 2020/692 of 30 January 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin.
Article 76: The dogs, cats and ferrets
-
Consignments of dogs, cats and ferrets shall only be permitted to enter the Union if the animals of the consignment comply with the following requirements
-
they have received a vaccination against infection with rabies virus that complies with the following conditions:
the animals must be at least 12 weeks old at the time of vaccination
the vaccine must comply with the requirements set out in Annex III to Regulation (EU) No 576/2013 of the European Parliament and of the Council;
at the day of dispatch to the Union, at least 21 days must have elapsed since the completion of the primary vaccination against infection with rabies virus;
a certified copy of the vaccination details must be attached to the animal health certificate referred to in Article 3(1)(c)(i);
they must have undergone a valid rabies antibody titration test, in accordance with point 1 of Annex XXI.
-
By way of derogation of paragraph 1(b), dogs, cats and ferrets originating in third countries or territories or zones thereof included in the list set out in Commission Implementing Regulation (EU) No 577/2013 shall be permitted to enter the Union without being subjected to the rabies titration test
Consignments of dogs shall be permitted to enter into a Member State with disease‐free status for Echinococcus multilocularis or an approved eradication programme for infestation with that disease, if the animals of the consignment have been treated against this infestation in accordance with Part 2 of Annex XXI
Annex XXI to Regulation (EU) 2020/692:
Specific Requirements as Regards Dogs, Cats and Ferrets Intended For Entry Into The Union
1) Antibody Rabies Titration Test Requirements
must be carried out on a sample collected by a veterinarian authorised by the competent authority during the period commencing at least 30 days after the date of the primary vaccination, within a current valid vaccination series, and ending 3 months before the date of issue of the certificate;
must measure a titre of neutralising antibody to rabies virus equal to or greater than 0.5 IU/mL;
must be certified by an official report from the official laboratory as regards the result, and a copy of this report must be attached to the animal health certificate accompanying the animals to the Union;
does not have to be renewed on an animal which, following the antibody rabies titration test with satisfactory results, has been revaccinated against rabies within the period of validity of the primary vaccination referred to in point (a) and all subsequent valid vaccinations in the series.
C. Regulation (EU) No 576/2013 of the European Parliament and of the Council of 12 June 2013 on the non‐commercial movement of pet animals:
Article 10 : Conditions applicable to the non‐commercial movement of pet animals of the species listed in Part A of Annex I
-
Pet animals of the species listed in Part A of Annex I shall not be moved into a Member State from a territory or a third country unless they fulfil the following conditions:
they are marked in accordance with Article 17(1);
they have received an anti‐rabies vaccination that complies with the validity requirements set out in Annex III;
they have undergone a rabies antibody titration test that complies with the validity requirements set out in Annex IV;
they comply with any preventive health measures for diseases or infections other than rabies adopted pursuant to Article 19(1);
they are accompanied by an identification document duly completed and issued in accordance with Article 26.
Annex III to Regulation (EU) No 576/2013:
Validity requirements for anti‐rabies vaccinations
-
The anti‐rabies vaccine must
-
a
be a vaccine other than a live modified vaccine and fall within one of the following categories:
an inactivated vaccine of at least one antigenic unit per dose (recommendation from the World Health Organisation); or
a recombinant vaccine expressing the immunising glycoprotein of the rabies virus in a live virus vector;
-
b
where it is administered in a Member State, it must have been granted a marketing authorisation in accordance with:
Article 5 of Directive 2001/82/EC; or
Article 3 of Regulation (EC) No 726/2004;
-
c
where it is administered in a territory or a third country, have been granted an approval or a licence by the competent authority and meet at least the requirements laid down in the relevant part of the Chapter concerning rabies in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health.
-
a
-
An anti‐rabies vaccination must fulfil the following conditions
the vaccine was administered by an authorised veterinarian;
the pet animal was at least 12 weeks old at the date on which the vaccine was administered;
the date of administration of the vaccine is indicated by an authorised veterinarian or an official veterinarian in the appropriate section of the identification document;
the date of administration referred to in point (c) does not precede the date of application of the transponder or tattoo or the date of reading of the transponder or the tattoo indicated in the appropriate section of the identification document;
the period of validity of the vaccination starts from the establishment of protective immunity, which shall not be less than 21 days from the completion of the vaccination protocol required by the manufacturer for the primary vaccination, and continues until the end of the period of protective immunity, as prescribed in the technical specification of the marketing authorisation referred to in point 1(b) or the approval or licence referred to in point 1(c) for the anti‐rabies vaccine in the Member State or territory or third country where the vaccine is administered. The period of validity of the vaccination is indicated by an authorised veterinarian or an official veterinarian in the appropriate section of the identification document;
a revaccination must be considered a primary vaccination if it was not carried out within the period of validity referred to in point (e) of the previous vaccination.
Annex IV to Regulation (EU) No 576/2013:
Validity requirements for the rabies antibody titration test
The collection of the sample of blood necessary to carry out the rabies antibody titration test must be carried out and documented by an authorised veterinarian in the appropriate section of the identification document;
The rabies antibody titration test
must be carried out on a sample collected at least 30 days after the date of vaccination and:
-
not less than three months before the date of:
-
–
the non‐commercial movement from a territory or a third country other than those listed in the implementing acts adopted pursuant to Article 13(1) or (2), or
-
–
the transit through such a territory or third country, where the conditions laid down in point (c) of Article 12 are not fulfilled, or
-
ii
before the pet animal left the Union for movement to or transit through a territory or a third country other than those listed pursuant to Article 13(1) or (2); the identification document in the format provided for in Article 21(1) must confirm that a rabies antibody titration test was carried out with a favourable result before the date of movement;
-
b
must measure a level of neutralising antibody to rabies virus in serum equal to or greater than 0.5 IU/mL and using a method prescribed in the relevant part of the Chapter concerning rabies in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health;
-
c
must be performed in a laboratory approved in accordance with Article 3 of Decision 2000/258/EC;
-
d
does not have to be renewed following a satisfactory result described in point (b), provided that the pet animal is revaccinated within the period of validity referred to in point 2(e) of Annex III to the previous vaccination.
-
–
Appendix B – Results from Literature Review
Incubation Period
Antibodies production in unvaccinated animals after challenge
Table B.6 Available information on the detection of virus neutralising antibodies in unvaccinated animals belonging to the control group of vaccine trial studies, after challenge with the rabies virus. All serological tests presented targeted virus neutralising antibodies, and the type of test is given in the table
| Reference | Tissue type | Test type |
Serology testing day PI |
Number of samples tested |
Number Positive |
Titre or concentration mean | Titre or concentration range |
Serology end (days PI) |
Study end (days PI) |
|---|---|---|---|---|---|---|---|---|---|
| Cho and Lawson (1989) | Serum | RFFIT |
–49 –42 0 7 14 |
6 6 6 6 6 |
0.1 IU/mL 0.1 IU/mL 0.1 IU/mL 0.1 IU/mL 0.3 IU/mL |
14 | 28 | ||
| Haddad et al. (1994) | Serum | RFFIT |
–120 0 |
4 4 |
0? 0.01? |
0 | 53 | ||
| Hammami et al. (1999) | Serum | FAVN |
–33 –26 –19 –12 0 7 160 |
6 6 6 6 6 6 1 |
0.04–0.14 0.03–0.18 0.02–0.06 0.02–0.05 0.02–0.14 0.02–0.08 0.04 |
160 | 160 | ||
| Perrin et al. (1999) | Serum | RFFIT |
–231 0 |
2 2 |
< 0.5 UI/mL < 0.5 UI/mL |
0 | 20 | ||
|
Kallel et al. (2006) |
Serum | RFFIT |
–162 –147 –132 –102 0 150 |
5 5 5 5 5 1 |
0.26 IU/mL (GM) 0.24 IU/mL (GM) 0.28 IU/mL (GM) 0.25 IU/mL (GM) 0.27 IU/mL (GM) 11.27 IU/mL (GM) |
150 | 150 | ||
| Hu et al. (2006) | Serum | FAVN | –161 | 20 | 0 IU/mL | –161 | 180 | ||
| Lodmell et al. (2006) | Serum | RFFIT |
–22 7 90 |
10 10 10 |
0.1 IU/mL (GM) 25 IU/mL (GM) 300 IU/mL (GM) |
90 | 90 | ||
| Manickam et al. (2008) | Serum | RFFIT |
7 14 28 90 |
10 10 10 4 |
0 1 2 3 |
0 1 1 1 to 2 |
90 | 90 | |
| Gnanadurai et al. (2013) | Serum | RFFIT |
–28 2 |
8 8 |
0.05–9.85 UI/mL | 90 |
FAVN: fluorescent antibody virus neutralisation assay; GM: geometric mean; RFFIT: rapid fluorescent focus inhibition test.
Timeline is given as days post‐inoculation (PI) (negative when before inoculation). All serological tests presented targeted virus neutralising antibodies, and the type of test is given in the table.
Antibodies production in vaccinated animals after challenge
Vaccinated, not challenged
Vaccinated and challenged
Table B.8 Virus neutralising antibodies titration in vaccination trials where animals were vaccinated, and later challenged with the rabies virus (sample specimen always serum)
| Reference | Vaccine type | Vaccine regimen | Age group |
Time to inoculation (days PV) |
Serology testing day (PV) | Test type | Number of animals | Titre or concentration mean | Titre or concentration range |
Serology end (days PV) |
Study end (days PV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive | |||||||||||
| Tierkel et al. (1949) | Non‐commercial | 1‐dose IM | 6–51 months | 60 |
0 22 43 60 |
Virus neutralisation test through injection in mice |
3 2 3 2 |
< 1:2 to 1:6 1:25 to 1:105 1:20 to 1:70 1:15 to 1:50 |
60 | 60 | ||
| Non‐commercial | 1‐dose IM | 6–51 months | 60 |
0 22 43 60 |
4 4 4 4 |
< 1:2 1:160 to 1:315 1:55 to 1:330 1:65 to 1:255 |
60 | 60 | ||||
| Non‐commercial | 1‐dose IM | 6–51 months | 60 |
0 22 43 60 |
3 3 3 3 |
1:2 to 1:10 1:30 to > 1:512 1:20 to 1:130 1:15 to 1:95 |
60 | 60 | ||||
| Non‐commercial | 1‐dose IM | 6–51 months | 60 |
0 22 43 60 |
4 3 3 4 |
< 1:2 to 1:3 1:20 to > 1:512 1:3 to > 1:512 1:4 to > 1:512 |
60 | 60 | ||||
| Fields et al. (1976) | Trimune | 1‐dose IM | > 12 month | 1,080 |
0 30 330 720 1,080 1,110 |
Standard serum‐dilution method |
24 24 22 19 19 19 |
< 2 titre (median) 512 titre (median) 146 titre (median) 19 titre (median) 6 titre (median) 217 titre (median) |
< 2 titre 165 to 789 titer 45 to 512 titre 3 to 194 titre < 2 to 458 titre 32 to 1,024 titre |
|||
| Cho and Lawson (1989) | Non‐commercial | 1‐dose IM | 1–3 years | 56 |
7 14 35 56 63 70 84 |
RFFIT |
7 7 7 7 7 |
0.1 IU/mL 0.1 IU/mL 0.1 IU/mL 0.1 IU/mL 0.3 IU/mL 1.5 IU/mL 1.5 IU/mL |
130 | |||
| Non‐commercial | 1‐dose IM | 1–3 years | 56 |
7 14 35 56 63 70 84 |
RFFIT |
5 5 5 5 5 5 5 |
0.3 IU/mL 1.5 IU/mL 1.5 IU/mL 1.5 IU/mL 37 IU/mL 37 IU/mL 37 IU/mL |
130 | ||||
| Non‐commercial | 1‐dose IM | 1–3 years | 56 |
7 14 35 56 63 70 84 |
RFFIT |
5 5 5 5 5 5 5 |
0.1 IU/mL 1.5 IU/mL 1.5 IU/mL 1.5 IU/mL 37 IU/mL 37 IU/mL 37 IU/mL |
130 | ||||
| Kallel et al. (2006) | Rabisin | 1‐dose SC | 5–6 months | 162 |
0 15 30 60 162 312 |
RFFIT |
5 5 5 5 5 4 |
0.29 IU/mL (GM) 6.1 IU/mL (GM) 5 IU/mL (GM) 2.8 IU/mL (GM) 1.06 IU/mL (GM) 3.62 IU/mL (GM) |
312 | 312 | ||
| Hu et al. (2006) | Nobivac | 1‐dose SC | 75–100 days | 175 |
14 28 112 |
FAVN |
20 20 20 |
10.36 IU/mL 26.3 IU/mL 14.2 IU/mL |
112 | 355 | ||
| Bahloul et al. (2006) | Rabisin |
2‐dose IM Days 0, 21 |
10 months | 1,400 |
35 90 180 365 540 730 1,400 |
RFFIT |
4 4 4 4 4 4 2 |
38 IU/mL 1.6 IU/mL 1.4 IU/mL 1.3 IU/mL 1.2 IU/mL 1.1 IU/mL 0.3 IU/mL |
1,400 | 1,520 | ||
| Rabisin | 1‐dose SC | 2 months | 1,400 |
0 27 90 1,400 |
RFFIT |
3 3 3 3 |
0.5 IU/mL 7.1 IU/mL 3.6 IU/mL 0.2 IU/mL |
1,400 | 1,520 | |||
| Rabitun | 1‐dose SC | 2 months | 1,400 |
0 27 90 1,400 |
RFFIT |
3 3 3 2 |
0.5 IU/mL 0.9 IU/mL 0.4 IU/mL 0.25 IU/mL |
1,400 | 1,520 | |||
|
Lodmell et al. (2006) |
RabVac | 1‐dose IM | 12–14 months | 382 |
60 120 180 240 300 360 389 472 |
RFFIT |
5 5 5 5 5 5 5 5 |
240 IU/mL (GM) 150 IU/mL (GM) 120 IU/mL (GM) 90 IU/mL (GM) 110 IU/mL (GM) 90 IU/mL (GM) > 1,000 IU/mL (GM) 900 IU/mL (GM) |
472 | 472 | ||
| Manickam et al. (2008) | Nobivak |
5‐dose IM Days 0, 3, 7, 14, 28 |
8–12 months | 0 |
3 7 14 21 28 90 |
RFFIT | 10 |
< 0.5 IU/mL 2.2 IU/mL 1.8 IU/mL 6.3 IU/mL 11.2 IU/mL 2.3 IU/mL |
SD = 2,3 SD = 2,2 SD = 4,8 SD = 5,3 SD = 1,3 |
90 | 90 | |
| Rabisin |
5‐dose IM Days 0, 3, 7, 14, 28 |
8–12 months | 0 |
3 7 14 21 28 90 |
RFFIT | 10 |
< 0.5 IU/mL 1.3 IU/mL 6.3 IU/mL 9.6 IU/mL 12.4 IU/mL 3.4 IU/mL |
SD = 1.1 SD = 5.7 SD = 5.7 SD = 8.1 SD = 1.9 |
90 | 90 | ||
| Nobivak |
3‐dose IM Days 0, 5, 28 |
8–12 months | 0 |
7 14 21 28 90 |
RFFIT | 10 |
1.5 IU/mL 6.2 IU/mL 14.6 IU/mL 20.0 IU/mL 2.5 IU/mL |
SD = 1.4 SD = 5.5 SD = 10.8 SD = 11.3 SD = 2.1 |
90 | 90 | ||
| Liu et al. (2012) | Non‐commercial | 1‐dose IM | 3–4 months | 180 |
0 30 60 90 120 150 180 |
FAVN |
10 10 10 10 10 10 10 |
0 IU/mL (GM) 11.5 IU/mL (GM) 9.5 IU/mL (GM) 8 IU/mL (GM) 7.8 IU/mL (GM) 7.6 IU/mL (GM) 7.5 IU/mL (GM) |
180 | 270 | ||
| Nobivac | 1‐dose IM | 3–4 months | 180 |
0 30 60 90 120 150 180 |
FAVN |
10 10 10 10 10 10 10 |
0 IU/mL (GM) 12 IU/mL (GM) 11 IU/mL (GM) 10,5 IU/mL (GM) IU/mL (GM)10 9 IU/mL (GM) 8 IU/mL (GM) |
180 | 270 | |||
| Gnanadurai et al. (2013) | Non‐commercial | 1‐dose IM | 5 months | 28 |
0 30 |
RFFIT |
4 4 |
4 4 |
9.85 to 29.6 IU/mL 0.2 to 2.9 IU/mL |
30 | ||
| Non‐commercial | 1‐dose IM | 5 months | 28 |
0 30 |
4 4 |
0 |
0 IU/mL |
30 | ||||
| Darkaoui et al. (2016) | Rabivac |
2‐dose SC Days 0, 30 |
3–6 months | 121 |
1 7 14 21 28 35 49 56 64 70 77 84 91 98 105 112 119 |
FAVN |
8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 8 |
0 7 8 8 7 8 8 8 8 7 7 6 6 7 6 7 6 |
0.04 IU/mL (GM) 1.41 IU/mL (GM) 3.34 IU/mL (GM) 5.51 IU/mL (GM) 4.36 IU/mL (GM) 7.16 IU/mL (GM) 18.3 IU/mL (GM) 5.42 IU/mL (GM) 3.54 IU/mL (GM) 3.29 IU/mL (GM) 1.29 IU/mL (GM) 1.07 IU/mL (GM) 0.71 IU/mL (GM) 0.57 IU/mL (GM) 0.39 IU/mL (GM) 0.77 IU/mL (GM) 0.71 IU/mL (GM) |
0.04 to 0.06 IU/mL 0.17 to 4.56 IU/mL 0.5 to 10.45 IU/mL 1.99 to 18.15 IU/mL 0.04 to 13.77 IU/mL 1.51 to 23.93 IU/mL 1.15 to 54.82 IU/mL 0.29 to 18.15 IU/mL 0.29 to 10.45 IU/mL 0.17 to 10.45 IU/mL 0.22 to 4.56 IU/mL 0.13 to 3.46 IU/mL 0.04 to 1.99 IU/mL 0.07 to 1.99 IU/mL 0.07 to 1.15 IU/mL 0.06 to 2.62 IU/mL 0.04 to 1.99 IU/mL |
119 | 120 |
| Zhang et al. (2016) | PCEV |
4‐dose IM Days 0, 7, 14, 28 |
0 |
0 7 14 21 28 |
FAVN |
15 15 15 15 15 |
0 IU/mL 0.5 IU/mL 3 IU/mL 3.5 IU/mL 4 IU/mL |
28 | 45 | |||
| PIKA‐RV |
4‐dose IM Days 0, 7, 14, 28 |
0 |
0 7 14 21 28 |
FAVN |
15 15 15 15 15 |
0 IU/mL 0.5 IU/mL 6 IU/mL 11.5 IU/mL 16 IU/mL |
28 | 45 | ||||
| PCEV |
4‐dose IM Days 0, 7, 14, 28 |
0 |
0 7 14 21 28 |
FAVN |
15 15 15 15 15 |
0 IU/mL 0.5 IU/mL 4 IU/mL 6 IU/mL 10 IU/mL |
28 | 45 | ||||
| PIKA‐RV |
4‐dose IM Days 0, 7, 14, 28 |
0 |
0 7 14 21 28 |
FAVN |
15 15 15 15 15 |
0 IU/mL 0.5 IU/mL 7 IU/mL 12 IU/mL 16 IU/mL |
28 | 45 | ||||
FAVN: fluorescent antibody virus neutralisation assay; GM: geometric mean; PV: post‐vaccination; RFFIT: rapid fluorescent focus inhibition test; Vaccine regimen; IM: intramuscular, SC: subcutaneous.
Timelines are given in relation to the first vaccination day. All serological tests presented targeted virus neutralising antibodies, and the type of test is given in the table.
Appendix C – Dogs imported into EU countries from non‐EU countries and from listed non‐EU countries for commercial purposes
Table C.1.
Number of dogs imported from non‐EU countries in 2019–2021 as registered in TRACES
| EU country of destination | Number of dogs imported from non‐EU countries for commercial purposes per year | |||||||
|---|---|---|---|---|---|---|---|---|
| All non‐EU countries from which dogs imports are allowed | Non‐EU countries for which the antibody titration test and the waiting period afterwards is mandatory | |||||||
| 2019 | 2020 | 2021 | Mean | 2019 | 2020 | 2021 | Mean | |
| Austria | 288 | 261 | 528 | 359 | 124 | 72 | 130 | 109 |
| Belgium | 241 | 448 | 2,059 | 916 | 34 | 30 | 86 | 50 |
| Bulgaria | 50 | 52 | 273 | 125 | 10 | 22 | 9 | 14 |
| Croatia | 39 | 25 | 143 | 69 | 1 | 12 | 105 | 39 |
| Cyprus | 326 | 103 | 179 | 203 | 7 | 15 | 19 | 14 |
| Czechia | 39 | 79 | 653 | 257 | 7 | 10 | 13 | 10 |
| Denmark | 218 | 767 | 950 | 645 | 31 | 14 | 34 | 26 |
| Estonia | 32 | 631 | 1,849 | 837 | 10 | 8 | 3 | 7 |
| Finland | 1,127 | 756 | 1,315 | 1,066 | 69 | 64 | 38 | 57 |
| France | 2,375 | 2,276 | 4,575 | 3,075 | 91 | 89 | 248 | 143 |
| Germany | 3,701 | 5,968 | 10,599 | 6,756 | 309 | 344 | 410 | 354 |
| Greece | 31 | 45 | 80 | 52 | 2 | 8 | 3 | 4 |
| Hungary | 253 | 249 | 506 | 336 | 1 | 24 | 71 | 32 |
| Ireland | 477 | 501 | 795 | 591 | 172 | 116 | 168 | 152 |
| Italy | 839 | 1,097 | 1,945 | 1,294 | 75 | 78 | 100 | 84 |
| Latvia | 9 | 523 | 913 | 482 | 0 | 3 | 0 | 1 |
| Lithuania | 9 | 100 | 1,183 | 431 | 0 | 0 | 0 | 0 |
| Luxembourg | 21 | 24 | 39 | 28 | 4 | 5 | 20 | 10 |
| Malta | 32 | 29 | 118 | 60 | 18 | 5 | 25 | 16 |
| Netherlands | 648 | 1,281 | 2,370 | 1,433 | 105 | 207 | 297 | 203 |
| Poland | 61 | 196 | 3,814 | 1357 | 22 | 24 | 25 | 24 |
| Portugal | 347 | 301 | 543 | 397 | 277 | 202 | 296 | 258 |
| Romania | 15 | 457 | 1,825 | 766 | 3 | 4 | 15 | 7 |
| Slovakia | 7 | 162 | 22 | 64 | 2 | 3 | 3 | 3 |
| Slovenia | 7 | 8 | 64 | 26 | 4 | 0 | 1 | 2 |
| Spain | 472 | 526 | 1,318 | 772 | 107 | 68 | 155 | 110 |
| Sweden | 610 | 921 | 1,758 | 1,096 | 33 | 41 | 79 | 51 |
| Total | 12,274 | 17,786 | 40,416 | 23,492 | 1,518 | 1,468 | 2,353 | 1,780 |
Appendix D – Protocol for the assessment
Assessment of the risk of importing to EU infected with rabies dogs related to a possible reduction of the waiting time after rabies antibody titration from 90 days to 30 days.
Background as provided by the requestor
Specific animal health requirements for entry into the Union of dogs, cats and ferrets are laid down in Commission Delegated Regulation (EU) 2020/6922. They mainly rely on preventing rabies from entering the EU territory from imported animals. To that end, the following conditions must be met:
Vaccination against rabies ‐ dogs, cats and ferrets must be vaccinated against rabies as follows:
the animals must be at least 12 weeks old at the time of vaccination;
the vaccine must comply with the requirements set out in Annex III to Regulation (EU) No 576/2013 15 ;
at the day of dispatch to the Union, at least 21 days must have elapsed since the completion of the primary vaccination against infection with rabies virus;
a certified copy of the vaccination details must be attached to the animal health certificate.
Rabies antibody test ‐ dogs, cats and ferrets coming from third countries or territories listed in part I of Annex VIII to Commission Implementing Regulation (EU) 2021/404 16 for which the specific condition ‘rabies antibody titration test’ applies must undergo a rabies antibody test, meeting certain criteria. That test:
must be carried out on a sample collected by a veterinarian authorised by the competent authority during the period commencing at least 30 days after the date of the primary vaccination, within a current valid vaccination series, and ending 3 months before the date of issue of the certificate;
must measure a titre of neutralising antibody to rabies virus equal to or greater than 0.5 IU/mL;
must be certified by an official report from the official laboratory as regards the result, and a copy of this report must be attached to the animal health certificate accompanying the animals to the Union;
does not have to be renewed on an animal which, following the antibody rabies titration test with satisfactory results, has been revaccinated against rabies within the period of validity of the primary vaccination and all subsequent valid vaccinations in the series.
These measures largely reflect the recommendations provided by EFSA in an opinion adopted on 11 December 2006 and published on 15 February 2007 regarding an ‘Assessment of the risk of rabies introduction into the UK, Ireland, Sweden, Malta, as a consequence of abandoning the serological test measuring protective antibodies to rabies’.9 In this opinion, EFSA points out that the risk of transmission of rabies by pet movement is related to moving an animal incubating the disease and that the primary means of removing an individual from the population at risk is by vaccination, as inactivated rabies vaccines are highly efficient and induce rapid protective immunity that prevents infection and subsequent transmission. On the other hand, it also highlights that infection prior to vaccination protection cannot be controlled by immunisation. Therefore, further requirements should be based on whether rabies occurs in the pet population or not. If rabies occurs in the pet population where pets reside before primo‐vaccination, a waiting time following primo‐vaccination is recommended as the most efficient measure to reduce the risk of importing rabies‐infected pets. The higher is the actual prevalence, the longer should be the waiting time required in order to reach an acceptable level of risk. Finally, the opinion recognises that the implementation of serological testing or other risk‐reducing measures may be considered when the required waiting time exceeds 100 days.
As indicated above, the waiting time legally required in the EU legislation for movements from countries with a higher prevalence/unknown status is of at least 3 months after the blood sampling, which has to be undertaken at least 30 days after rabies vaccination. This requirement is also in line with the current recommendations included in the OIE Terrestrial Animal Health Code Chapter 8.14 on rabies (29th edition 2021).
As shown in EFSA 2007 opinion, the waiting time between vaccination and import is crucial, because vaccination does not prevent disease developing in already infected animals. Blancou et al. (1989) demonstrated that vaccination in an already infected animal does not significantly alter the clinical picture or development time of the disease. Therefore, it is possible that an animal infected prior to rabies vaccination would continue to incubate the disease despite developing a significant antibody titre. Another risk of rabies introduction is linked to pets which are not fully protected by the vaccination, either because they were recently vaccinated or they mounted an insufficient antibody response, before being infected.
From a general point of view, the risk that an animal is incubating disease at the time of vaccination is the same as the risk that an unvaccinated animal is incubating disease when it is imported, thus, the overall risk is very sensitive to the waiting time. It is also very sensitive to compliance with requirements (e.g. shorter than required wait, incorrect or no vaccination, falsified test result) (Wilsmore et al., 2006).
The OIE ad hoc Group on Rabies has started to work on modifying Article 8.14.7 of the OIE Terrestrial Animal Health Code and reducing the waiting time after a positive antibody titration test from 90 to 30 days. A concept paper of the OIE ad hoc group describing the scientific evidence to support those changes was released with the February 2020 OIE Scientific Commission for Animal Diseases (‘OIE Scientific Commission’) report7 and was subsequently published in the scientific journal Vaccine 17 . The OIE Terrestrial Animal Health Standards Commission (‘OIE Code Commission’) amended Article 8.14.7 and circulated for OIE Members Countries’ (Members) comments after its September 2020 meeting. The OIE Scientific Commission agreed to consult subject‐matter experts to address Member’s concerns expressed after that round of consultation.
In December 2020, the European Union expressed concerns that the presented data and drawn conclusions were not sufficient for a policy change and would request additional scientific evidence. To support its position, it submitted a scientific report prepared by experts of the European Union Reference Laboratory for Rabies (cf. p. 127‐131 of the document under footnote 77).
In September 2021, after careful analysis of the Member’s concerns, the OIE Scientific Commission endorsed the expert opinion of the OIE Rabies Reference Laboratory network (RABLAB) which considered that the scientific basis for a 30‐day post‐titration waiting time was justified and that the conclusion of the 2019 OIE ad hoc Group on Rabies that reviewed dog importation standards should remain unchanged.
The OIE Scientific Commission opinion together with the experts’ rationale were forwarded to the OIE Code Commission for consideration. It is therefore likely that these changes will be proposed for adoption by OIE member countries, possibly as early as at the General Session of the OIE in May 2022.
ToRs as provided by the requestor
In the context of Article 31 of Regulation (EC) No. 178/2002, the Commission asks EFSA for scientific and technical assistance on the risks related to a possible reduction of the waiting time after rabies antibody titration to 30 days compared to the current EU legislative regime, taking into account:
-
‐
the experience gained in the last years with the current waiting time laid down in the EU legislation;
-
‐
the possible risks/limitations including those identified by the experts of the EU Reference Laboratory for Rabies in their February 2021 opinion;
-
‐
newly available scientific information, and specifically the publication describing the scientific evidence to support the proposed changes released.
Problem formulation
Here, a summary of the initial considerations taken to deliver on the mandate are described. With this mandate the EC requests EFSA’s support in assessing the excess risk associated with a potential delay in the waiting period prior to the importation of dogs from non‐EU countries. The request concerns the provisions for the dogs (Canis lupus) intended to be moved as non‐commercial pets or imported as commercial dogs into the EU territory from non‐EU countries to prevent the introduction of rabies in EU as described in Article 76 of the Commission Delegated Regulation (EU) 2020/692 in accordance with the article 8.14.7 of the OIE Terrestrial Code (last revised in 2019) (please refer to Appendix A).
For this work it is considered that all the requirements of EU legislation related to dog movements have been implemented. Specifically:
the dog is individually identified by means of an injectable transponder implanted which fulfils the technical requirements for means of identification (Article 74 Reg 2020/692) by a veterinarian, and the dog was individually identified before or at the time of primary vaccination (Annex III to the Regulation (EU) 576/2013) so the details correspond to those in the certificate or passport.
the dog has been vaccinated against rabies before shipment with a vaccine that complies with the validity requirements set out in Annex III to Regulation (EU) No 576/2013: (i) it is not a live modified vaccine and it is either an inactivated vaccine of at least one antigenic unit per dose (recommendation from the World Health Organisation); (ii) it has been granted an approval or a licence by the competent authority of the third country; and (iii) it meets at least the requirements laid down in the relevant part of the chapter concerning rabies in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health.
the dog was at least 12 weeks old at the date on which the primary rabies vaccination was administered (Article 76 Reg 2020/692).
the period of validity of the vaccination starts from the establishment of protective immunity, which shall not be less than 21 days from the completion of the vaccination protocol required by the manufacturer for the primary vaccination, and continues until the end of the period of protective immunity, as prescribed in the technical specification of the marketing authorisation referred to in point 1(b)10 or the approval or licence referred to in point 1(c) for the anti‐rabies vaccine in the Member State or territory or third country where the vaccine is administered (point 2(e) Annex III Regulation (EU) No 576/2013).
as primary rabies vaccination is considered the first vaccination and the any revaccination if it was not carried out within the period of validity of the previous vaccination (point 2e of Annex III to Regulation (EU) No 576/2013.
the vaccination has been conducted by an authorised veterinarian (Annex III Regulation (EU) 576/2013) and therefore good veterinary practice related to vaccination has been implemented. This also implies that the dog was healthy at the day of vaccination (based on the results of the clinical examination) and there was no suspicion of any disease including rabies (based on the medical history of the dog for the last days prior to vaccination).
the vaccinated dog must, at the time of import, remain within the protective immunity period of the vaccines according to the manufacturer’s instructions
a certified copy of the vaccination details must be attached to the animal health certificate; the date of administration of the vaccine and the period of validity of the vaccination is indicated by an authorised veterinarian or an official veterinarian in the appropriate section of the identification document (article 76 Reg 2020/692, point 2(e) Annex III Regulation (EU) No 576/2013).
a rabies antibody titration test using a virus neutralisation test (VNT) to detect neutralising antibodies must be carried out on a blood sample collected not less than 3 months and not more than 12 months prior to the date of issue of the certificate for the shipment. In case of primary vaccination, the samples should be collected at least 30 days after the date of primary vaccination course, within a current valid vaccination series. The sample should be collected by a veterinarian authorised by the competent authority.
the VNT must comply with the validity requirements set out in Annex XXI to Regulation (EU) 2016/429.
the VNT before entry should be performed in a laboratory authorised12 by the ANSES‐Nancy laboratory which is the European Union Reference Laboratory (EURL)13 for rabies.
a neutralising antibody level ≥ 0.5 IU/mL is characterised as positive. Nevertheless, the test does not differentiate between infected and vaccinated animals and there are no laboratory tests able to differentiate between neutralising antibodies resulting from natural infection from those developed after vaccination.
as antibodies resulting from natural infection are only detectable when the animal is in the late stages and showing clinical signs, only healthy animals should travel, however only commercial consignments of dogs will be subject to a veterinary inspection prior to travel.
provided the results are positive, the dogs are not allowed to travel immediately. A waiting period of at least 90 days (current regulation) and not more than 12 months, after the day of sampling for the antibody titration test, has been introduced to allow the clinical signs to manifest if animals were infected before vaccination or just after vaccination.
once the dog is ready to travel, it should be clinically examined within a period of 24–48 h prior to the time of loading for dispatch (article 13(3) of Reg 2020/692) and in the absence of clinical signs the shipment is allowed, and the certificate is provided. However, this is only applicable to commercial movements; for non‐commercial movements, there is no such requirement.
dogs from countries not listed in Annex II to 577/2013 and all commercial consignments from outside the EU will have to enter through a Traveller's Point of Entry (TPE) or a Border Control Point (BCP), respectively, where veterinary checks can be undertaken.
Taking into consideration that all the above‐mentioned requirements are implemented, the risk of transmission of rabies through the movement of a vaccinated dog is related to the risk of moving a vaccinated animal incubating the disease.
Clarifications of the scope of the request: framework, population and geographical area of concern, definitions
Scope: Recommendations for importation of dogs from countries or zones infected with rabies virus
Geographical area:
-
–
Countries of dog origin: countries or zones infected with rabies virus (OIE) or coming from third countries or territories listed in part I of Annex VIII to Commission Implementing Regulation (EU) 2021/404 for which the specific condition ‘rabies antibody titration test’ applies.
-
–
Countries of destination: EU countries
Population: Dogs over 12 weeks of age, intended to be exported from countries or zones infected with rabies virus and have been subjected (at least 30 days post‐vaccination and not more than 12 months prior to shipment, to antibody titration test with a positive result of at least 0.5 IU/mL.
Translation of ToRs into assessment questions and subquestions
The main question to be addressed by this Scientific Report is: ‘How much does the risk of introduction of rabies into EU increase through the movement of vaccinated dogs with a positive titration test (≥ 0.5 IU/mL) if the waiting period from sampling to movement decreases from 90 to 30 days?’.
The ToRs have been translated into three subquestions, and these subquestions have been further broken into more specific questions:
Subquestion 1: How can we identify vaccinated dogs, with positive results (≥ 0.5 IU/mL) to virus neutralisation test that are incubating rabies before their movement?
Within this subgroup, questions to be discussed and addressed are:
When do clinical signs of rabies (including death) appear and can be identified through clinal examination? This should be considered after natural and experimental infection, and before and after vaccination.
When does the production of the neutralising antibodies start following vaccination and natural and experimental infection? How does the titre change over time?
Are there any reports of dogs with a titre of neutralisation antibodies ≥ 0.5 IU/mL appearing with clinical signs of rabies?
What is the effect of different vaccination schemes (in terms of the types of the vaccines, the times of vaccination shots and the age of the dogs (e.g. vaccination in puppies < 16 weeks old) on the development of neutralising antibodies in dogs following vaccination?
Method/data: The subquestions can be addressed through literature review including plausible grey literature, and data retrieved from endemic countries that could help with the questions related to natural infection. Expert knowledge from WG members will also be used for answering the questions.
Subquestion 2: Are the results presented in the February 2020 OIE Scientific Commission report, and published in the scientific journal Vaccine, valid?
Within this subgroup, questions to be discussed and addressed are:
Is the methodology used for the review appropriate (e.g., terms of search, criteria of inclusion and exclusion)?
Were all relevant publications included in the assessment?
What are the limitations and strengths of the evidence provided?
Method/data: By a critical appraisal of evidence provided by OIE in the February 2020 OIE Scientific Commission report and published in the scientific journal Vaccine. By including experts contributing to this publication in the WG (as Hearing Experts), EFSA can retrieve further information on the methodology followed in the OIE report.
Subquestion group 3: Were there any cases of infected dogs imported into the EU during the period while the measures described in the EU legislation were in place?
Sub questions:
Were there any cases of infected dogs imported into the EU after the implementation of these measures?
Are there any records of the number of dogs imported to EC?
How many cases of vaccinated and tested for antibodies imported dogs were found to be infected with rabies?
Are there any cases in the literature of vaccinated and tested positive for neutralising antibodies imported dogs found to be infected with rabies?
Method/data: by analysis of documented/reported cases of rabies in dogs imported into EU in the period between 2019 and 2021. Aside, a literature review of publications on cases of imported infected dogs will be carried out.
Assessing and synthesising evidence (including uncertainty analysis)
Details of the methodology used for the analysis of the data retrieved by the literature review as well as data from other sources will be provided in the methodology section of the opinion.
All sources of uncertainty identified during the assessment will be recorded, and their impact on the scientific assessment will be assessed collectively (the simplest option for this type of assessment (section 4.1 of EFSA Scientific Committee (2018)) after transforming the objective of the assessment into one or several well‐defined quantities of interest (QoI). Evidence dossiers will be provided to the experts for their assessment. A lower and upper bound delimiting the range of plausible values for the QoIs will be agreed within the Working Group during a meeting, and the Working Group experts will be asked to provide their individual judgements on the most likely values for each QoI using the roulette method (EFSA, 2014). Individual judgements will be then discussed and used to agree on the 95% percentile of the distribution for each QoI.
Annex I – Legal Requirements
A. OIE Terrestrial Animal Health Code (29th edition 2021) Chapter 8.14 on rabies (version adoption in 2019as they described in article 8.14.7 of Terrestrial Code.
Article 8.14.7.: Recommendations for importation of dogs, cats and ferrets from countries or zones infected with rabies virus.
Veterinary Authorities should require the presentation of an international veterinary certificate complying with the model of Chapter 5.11. attesting that the animals:
showed no clinical sign of rabies the day prior to or on the day of shipment;
were permanently identified and their identification number stated in the certificate;
-
and either:
were vaccinated or revaccinated in accordance with the recommendations of the manufacturer, with a vaccine that was produced in accordance with the Terrestrial Manual and were subjected not less than 3 months and not more than 12 months prior to shipment to an antibody titration test as prescribed in the Terrestrial Manual with a positive result of at least 0.5 IU/mL; or
were kept in a quarantine station for six months prior to shipment.
B. Commission Delegated Regulation (EU) 2020/692 of 30 January 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin.
Article 76: The dogs and the cats
-
Consignments of dogs, cats and ferrets shall only be permitted to enter the Union if the animals of the consignment comply with the following requirements:
-
a
they have received a vaccination against infection with rabies virus that complies with the following conditions:
the animals must be at least 12 weeks old at the time of vaccination
the vaccine must comply with the requirements set out in Annex III to Regulation (EU) No 576/2013 of the European Parliament and of the Council (21);
at the day of dispatch to the Union, at least 21 days must have elapsed since the completion of the primary vaccination against infection with rabies virus;
-
a certified copy of the vaccination details must be attached to the animal health certificate referred to in Article 3(1)(c)(i);
-
b
they must have undergone a valid rabies antibody titration test, in accordance with point 1 of Annex XXI.
-
b
-
a
By way of derogation of paragraph 1(b), dogs, cats and ferrets originating in third countries or territories or zones thereof included in the list set out in Commission Implementing Regulation (EU) No 577/2013 (22) shall be permitted to enter the Union without being subjected to the rabies titration test.
Consignments of dogs shall be permitted to enter into a Member State with disease‐free status for Echinococcus multilocularis or an approved eradication programme for infestation with that disease, if the animals of the consignment have been treated against this infestation in accordance with Part 2 of Annex XXI.
Annex XXI:
Specific Requirements As Regards Dogs, Cats And Ferrets Intended For Entry Into The Union
-
Antibody Rabies Titration Test Requirements:
must be carried out on a sample collected by a veterinarian authorised by the competent authority during the period commencing at least 30 days after the date of the primary vaccination, within a current valid vaccination series, and ending 3 months before the date of issue of the certificate;
must measure a titre of neutralising antibody to rabies virus equal to or greater than 0,5 IU/mL;
must be certified by an official report from the official laboratory as regards the result, and a copy of this report must be attached to the animal health certificate accompanying the animals to the Union;
does not have to be renewed on an animal which, following the antibody rabies titration test with satisfactory results, has been revaccinated against rabies within the period of validity of the primary vaccination referred to in point (a) and all subsequent valid vaccinations in the series.
C. Regulation (EU) 576/2013 of the European Parliament and of the Council of 12 June 2013 on the non‐commercial movement of pet animals:
Article 10: Conditions applicable to the non‐commercial movement of pet animals of the species listed in Part A of Annex I
-
Pet animals of the species listed in Part A of Annex I shall not be moved into a Member State from a territory or a third country unless they fulfil the following conditions:
they are marked in accordance with Article 17(1);
they have received an anti‐rabies vaccination that complies with the validity requirements set out in Annex III;
they have undergone a rabies antibody titration test that complies with the validity requirements set out in Annex IV;
they comply with any preventive health measures for diseases or infections other than rabies adopted pursuant to Article 19(1);
they are accompanied by an identification document duly completed and issued in accordance with Article 26.
1. Annex III:
2. Validity requirements for anti‐rabies vaccinations
-
The anti‐rabies vaccine must:
-
be a vaccine other than a live modified vaccine and fall within one of the following categories:
an inactivated vaccine of at least one antigenic unit per dose (recommendation from the World Health Organisation); or
a recombinant vaccine expressing the immunising glycoprotein of the rabies virus in a live virus vector;
-
where it is administered in a Member State, it must have been granted a marketing authorisation in accordance with:
Article 5 of Directive 2001/82/EC; or
Article 3 of Regulation (EC) No 726/2004;
where it is administered in a territory or a third country, have been granted an approval or a licence by the competent authority and meet at least the requirements laid down in the relevant part of the Chapter concerning rabies in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health.
-
-
An anti‐rabies vaccination must fulfil the following conditions:
the vaccine was administered by an authorised veterinarian;
the pet animal was at least 12 weeks old at the date on which the vaccine was administered;
the date of administration of the vaccine is indicated by an authorised veterinarian or an official veterinarian in the appropriate section of the identification document;
the date of administration referred to in point (c) does not precede the date of application of the transponder or tattoo or the date of reading of the transponder or the tattoo indicated in the appropriate section of the identification document;
-
the period of validity of the vaccination starts from the establishment of protective immunity, which shall not be less than 21 days from the completion of the vaccination protocol required by the manufacturer for the primary vaccination, and continues until the end of the period of protective immunity, as prescribed in the technical specification of the marketing authorisation referred to in point 1(b) or the approval or licence referred to in point 1(c) for the anti‐rabies vaccine in the Member State or territory or third country where the vaccine is administered.
The period of validity of the vaccination is indicated by an authorised veterinarian or an official veterinarian in the appropriate section of the identification document;
a revaccination must be considered a primary vaccination if it was not carried out within the period of validity referred to in point (e) of the previous vaccination.
Annex IV: Validity requirements for the rabies antibody titration test
The collection of the sample of blood necessary to carry out the rabies antibody titration test must be carried out and documented by an authorised veterinarian in the appropriate section of the identification document;
-
The rabies antibody titration test:
-
a
must be carried out on a sample collected at least 30 days after the date of vaccination and:
-
a
-
i
not less than three months before the date of:
-
–
the non‐commercial movement from a territory or a third country other than those listed in the implementing acts adopted pursuant to Article 13(1) or (2), or
-
–
the transit through such a territory or third country, where the conditions laid down in point (c) of Article 12 are not fulfilled, or
-
–
-
ii
before the pet animal left the Union for movement to or transit through a territory or a third country other than those listed pursuant to Article 13(1) or (2); the identification document in the format provided for in Article 21(1) must confirm that a rabies antibody titration test was carried out with a favourable result before the date of movement;
-
b
must measure a level of neutralising antibody to rabies virus in serum equal to or greater than 0,5 IU/mL and using a method prescribed in the relevant part of the Chapter concerning rabies in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health;
-
c
must be performed in a laboratory approved in accordance with Article 3 of Decision 2000/258/EC;
-
d
does not have to be renewed following a satisfactory result described in point (b), provided that the pet animal is revaccinated within the period of validity referred to in point 2(e) of Annex III to the previous vaccination.
-
b
Annex II: Documents
OIE Terrestrial Animal Health Code (29th edition 2021) Chapter 8.14 on rabies (version adoption in 2019) (https://www.oie.int/en/what‐we‐do/standards/codes‐and‐manuals/terrestrial‐code‐online‐access/)
OIE Terrestrial Manual Chapter, Chapter 3.1.17. on Rabies (version adoption in May 2018) https://www.oie.int/en/what‐we‐do/standards/codes‐and‐manuals/terrestrial‐manual‐online‐access/
Commission Delegated Regulation (EU) 2020/6921: Rules for import into the EU (https://eur‐lex.europa.eu/eli/reg_del/2020/692/oj)
Commission Delegated Regulation (EU) 2020/689: Rules for surveillance, eradication programmes, and disease‐free status for certain listed and emerging diseases (https://eur‐lex.europa.eu/legal‐ontent/EN/TXT/?uri=CELEX%3A32020R0689&qid=1643032540018)
Regulation (EU) No 576/2013 of the European Parliament and of the Council of 12 June 2013 on the non‐commercial movement of pet animals (https://eur‐lex.europa.eu/legal‐content/EN/TXT/?uri=CELEX%3A32013R0576&qid=1643032787787)
Commission Delegated Regulation (EU) 2021/404: lists of third countries, territories or zones from which import of products and animals is permitted (https://eur‐lex.europa.eu/legal‐content/EN/TXT/?uri=CELEX%3A32021R0404&qid=1643224144905)
EFSA Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission regarding an assessment of the risk of rabies introduction into the UK, Ireland, Sweden, Malta, as a consequence of abandoning the serological test measuring protective antibodies to rabies. (https://www.efsa.europa.eu/en/efsajournal/pub/436)
Report of the meeting of the OIE Scientific Commission for Animal Diseases (the Commission) 3–7 February 2020 https://www.oie.int/en/what‐we‐do/standards/standards‐setting‐process/scientific‐commission/#ui‐id‐2
Report of the meeting of the OIE Terrestrial Animal Health Standards Commission (SCAD) 1‐10 September 2020 (https://ec.europa.eu/food/system/files/2020‐12/ia_standards_oie_eu_comments_tahsc‐report_202012.pdf) https://www.oie.int/en/what‐we‐do/standards/standards‐setting‐process/scientific‐commission/#ui‐id‐2
Report of the meeting of the OIE Scientific Commission for Animal Diseases (the Commission) 13–14 September 2021 https://www.oie.int/en/what‐we‐do/standards/standards‐setting‐process/scientific‐commission/#ui‐id‐2
Annex A – Literature Review protocol as provided by the contractors
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7350
Supporting information
Literature Review protocol as provided by the contractors
Suggested citation: EFSA (European Food Safety Authority) , Alvarez J, Nielsen SS, Robardet E, Stegeman A, Van Gucht S, Vuta V, Antoniou S‐E, Aznar I, Papanikolaou A and Roberts HC, 2022. Scientific Report on the risks related to a possible reduction of the waiting period for dogs after rabies antibody titration to 30 days compared with 90 days of the current EU legislative regime. EFSA Journal 2022;20(6):7350, 78 pp. 10.2903/j.efsa.2022.7350
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00159
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank the following hearing experts for the support provided to this scientific output: Thomas Müller and Florence Cliquet. EFSA wishes to acknowledge Guillaume Crozet for the data provided for this scientific output, COVETLAB for the literature review and Verena Oswaldi for her support.
Approved: 17 May 2022
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 6: © Guillaume Crozet
This output was first published on the EFSA website on 31 May.
Notes
TRACES is the European Commission's online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Available online: https://ec.europa.eu/food/animals/traces_en
Commission Delegated Regulation (EU) 2020/692 of 30 January 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin, OJ L 174, 3.6.2020, p. 379.
Regulation (EU) No 576/2013 of the European Parliament and of the Council of 12 June 2013 on the non‐commercial movement of pet animals and repealing Regulation (EC) No 998/2003, OJ L 178, 28.6.2013, p. 1.
Commission Implementing Regulation (EU) 2021/404 of 24 March 2021 laying down the lists of third countries, territories or zones thereof from which the entry into the Union of animals, germinal products and products of animal origin is permitted in accordance with Regulation (EU) 2016/429 of the European Parliament and the Council, OJ L 114, 31.3.2021, p. 1.
Commentary in Vaccine, 39, 2496–2499. https://doi.org/10.1016/j.vaccine.2021.03.064
https://www.oie.int/en/what‐we‐do/standards/standards‐setting‐process/scientific‐commission/#ui‐id‐2
Smith TG, Fooks AR, Moore SM, Freuling CM, Müller T, Torres G and Wallace RM, 2021. Negligible risk of rabies importation in dogs thirty days after demonstration of adequate serum antibody titer. Vaccine. https://doi.org/10.1016/j.vaccine.2021.03.064. (https://www.sciencedirect.com/science/article/pii/S0264410X21003686?via%3Dihub).
https://ec.europa.eu/food/system/files/2020‐12/ia_standards_oie_eu_comments_tahsc‐report_202012.pdf
Is referred to Point 1(b) of Annex III Regulation (EU) No 576/2013.
Is referred to Point 1(c) of Annex III Regulation (EU) No 576/2013.
Approved rabies serology laboratories in EU and non‐EU Countries: https://ec.europa.eu/food/animals/movement‐pets/approved‐rabies‐serology‐laboratories_en
The link of the EURL: https://eurl‐rabies.anses.fr/en/minisite/rabies/european‐union‐reference‐laboratory‐eurl‐rabies
% certainty is used in this Opinion to express the Panel’s % probability for the more probable outcome, as recommended by EFSA’s guidance on communication of uncertainty (EFSA, 2019).
2 Regulation (EU) No 576/2013 of the European Parliament and of the Council of 12 June 2013 on the non‐commercial movement of pet animals and repealing Regulation (EC) No 998/2003, OJ L 178, 28.6.2013, p. 1.
3 Commission Implementing Regulation (EU) 2021/404 of 24 March 2021 laying down the lists of third countries, territories or zones thereof from which the entry into the Union of animals, germinal products and products of animal origin is permitted in accordance with Regulation (EU) 2016/429 of the European Parliament and the Council, OJ L 114, 31.3.2021, p. 1.
Smith TG, Fooks AR, Moore SM, Freuling CM, Müller T, Torres G and Wallace RM, 2021. Negligible risk of rabies importation in dogs thirty days after demonstration of adequate serum antibody titer. Vaccine. https://doi.org/10.1016/j.vaccine.2021.03.064. (https://www.sciencedirect.com/science/article/pii/S0264410X21003686?via%3Dihub)
References
- Appleton D, 1972. Report of a committee of enquiry on rabies (June 1971). Laboratory Animals, 6, 241–242. 10.1258/002367772781006202 [DOI] [PubMed] [Google Scholar]
- Arega S, Conan A, Sabeta CT, Crafford JE, Wentzel J, Reininghaus B, Biggs L, Leisewitz AL, Quan M, Toka F and Knobel DL, 2020. Rabies vaccination of 6‐week‐old puppies born to immunized mothers: a randomized controlled trial in a high‐mortality population of owned, free‐roaming dogs. Trop Med Infect Dis, 5. 10.3390/tropicalmed5010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokkumar M, Ramesh A, Gunaseelan L, Tirumurugaan KG and Anuradha P, 2014. Comparison of two different methods to quantify the neutralizing rabies antibodies in vaccinated dogs. Indian Veterinary Journal, 91, 23–25. [Google Scholar]
- Asokkumar M, Ganesan PI, Sekar M, Anuradha P and Balakrishnan S, 2016. Vaccination studies against rabies in farm and pet animals using different immunization routes. Indian Veterinary Journal, 93, 33–36. [Google Scholar]
- Bahloul C, Taieb D, Diouani MF, Ahmed SBH, Chtourou Y, B'chir BI, Kharmachi H and Dellagi K, 2006. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine, 24, 1063–1072. 10.1016/j.vaccine.2005.09.016 [DOI] [PubMed] [Google Scholar]
- Bender SC, Bergman DL, Wenning KM, Miller LA, Slate D, Jackson FR and Rupprecht CE, 2009. No adverse effects of simultaneous vaccination with the immunocontraceptive GonaCon (TM) and a commercial rabies vaccine on rabies virus neutralizing antibody production in dogs. Vaccine, 27, 7210–7213. 10.1016/j.vaccine.2009.09.026 [DOI] [PubMed] [Google Scholar]
- Beníšek Z, Süliová J, Švrcek Š, Závadová J, Ondrejka R and Durove A, 1998. The effectiveness of inactivated, purified and concentrated experimental rabies vaccine for veterinary use: antigenic activity. Experiments on Domestic Dogs. Veterinarni Medicina, 43, 45–49. [PubMed] [Google Scholar]
- Blancou J, Artois M, Brochier B, Thomas I, Pastoret PP, Desmettre P, Languet B and Kieny MP, 1989. Safety and efficacy of an antirabies vaccine consisting of recombinant vaccinia‐rabies virus administered orally to the fox, dog and cat. Annales De Recherches Veterinaires, 20, 195–204. [PubMed] [Google Scholar]
- Bommier E, Chapat L, Guiot AL, Hilaire F, Cariou C, Poulet H, Pialot D and De Luca K, 2020. Multivariate analysis of the immune response to different rabies vaccines. Veterinary Immunology and Immunopathology, 220, 109986. 10.1016/j.vetimm.2019.109986 [DOI] [PubMed] [Google Scholar]
- Botros BAM, Lewis JC and Kerkor M, 1979. A study to evaluate non‐fatal rabies in animals. Journal of Tropical Medicine and Hygiene, 82, 137–141. [PubMed] [Google Scholar]
- Bouvet J, Cariou C, Poulard A, Oberli F, Cupillard L and Guigal PM, 2018. Compatibility between a rabies vaccine and a combined vaccine against canine distemper, adenovirosis, parvovirosis, parainfluenza virus and leptospirosis. Veterinary Immunology and Immunopathology, 205, 93–96. 10.1016/j.vetimm.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Catchpole M, Thomas L, Morgan D, Brown K, Turbitt D and Kirkbride H, 2008. Imported rabies in a quarantine centre in the United Kingdom. Eurosurveillance Weekly, 13. 10.2807/ese.13.19.18868-en [DOI] [PubMed] [Google Scholar]
- CDC , 1987. Morbidity and mortality weekly report (MMWR): an imported case of rabies in an immunized dog. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00000874.htm [PubMed]
- Cho HC and Lawson KF, 1989. Protection of dogs against death from experimental rabies by postexposure administration of rabies vaccine and hyperimmune globulin (human). Canadian Journal of Veterinary Research, 53, 434–437. [PMC free article] [PubMed] [Google Scholar]
- Chomel B, Chappuis G, Bullon F, Cardenas E, de Beublain TD, Lombard M and Giambruno E, 1988. Mass vaccination campaign against rabies: are dogs correctly protected? The Peruvian experience. Reviews of Infectious Diseases, 10(Suppl 4), S697–S702. 10.1093/clinids/10.supplement_4.s697 [DOI] [PubMed] [Google Scholar]
- Cliquet F, Gurbuxani JP, Pradhan HK, Pattnaik B, Patil SS, Regnault A, Begouen H, Guiot AL, Sood R, Mahl P, Singh R, Meslin FX, Picard E, Aubert MF and Barrat J, 2007. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine, 25, 3409–3418. 10.1016/j.vaccine.2006.12.054 [DOI] [PubMed] [Google Scholar]
- Cliquet F, Barrat J, Guiot AL, Cael N, Boutrand S, Maki J and Schumacher CL, 2008. Efficacy and bait acceptance of vaccinia vectored rabies glycoprotein vaccine in captive foxes (Vulpes vulpes), raccoon dogs (Nyctereutes procyonoides) and dogs (Canis familiaris). Vaccine, 26, 4627–4638. 10.1016/j.vaccine.2008.06.089 [DOI] [PubMed] [Google Scholar]
- Collective French multidisciplinary investigation team , 2008. Identification of a rabid dog in France illegally introduced from Morocco. Eurosurveillance, 13. [DOI] [PubMed]
- Crozet G, Julie R, Dufour B, Robardet E and Cliquet F, 2020a. BILAN DES CAS DE RAGE IMPORTÉS EN France: COMMENT Y FAIRE FACE ? ‐ Le Point Vétérinaire n° 410 du 01/10/2020.
- Crozet G, Riviere J, Canini L, Cliquet F, Robardet E and Dufour B, 2020b. Evaluation of the worldwide occurrence of rabies in dogs and cats using a simple and homogenous framework for quantitative risk assessments of rabies reintroduction in disease‐free areas through pet movements. Veterinary Sciences, 7. 10.3390/vetsci7040207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozet G, Rivière J, Rapenne E, Cliquet F, Robardet E and Dufour B, 2022. Quantitative risk assessment of rabies being introduced into mainland France through worldwide non‐commercial dog and cat movements (Manuscript submitted for publication). [DOI] [PubMed] [Google Scholar]
- Darkaoui S, Fassi Fihri O, Schereffer JL, Aboulfidaa N, Wasniewski M, Zouine K, Bouslikhane M, Yahia KI and Cliquet F, 2016. Immunogenicity and efficacy of Rabivac vaccine for animal rabies control in Morocco. Clinical and Experimental Vaccine Research, 5, 60–69. 10.7774/cevr.2016.5.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D, Bellaiche M and Yakobson BA, 2010. Rabies in two vaccinated dogs in Israel. Veterinary Record, 167, 907–908. 10.1136/vr.c3614 [DOI] [PubMed] [Google Scholar]
- Devi S, Sharma MC, Singh RP, Dimri U, Patel AC, Kumar P and Singh RD, 2018. Effect of mineral supplementation on humoral immunity against rabies vaccine in dog pups. Indian Journal of Animal Research, 52, 615–618. [Google Scholar]
- Durrani UF, Khan MS, Khan MA, Hanlon C and Moore S, 2012. Comparison of immunogenic efficacy of mono‐ and polyvalent rabies vaccines in dogs. Pakistan Journal of Zoology, 44, 291–296. [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA AHAW Panel , 2007. Opinion of the Scientific Panel on Animal Health and Welfare AHAW on a request from the Commission regarding an ‘Assessment of the risk of rabies introduction into the UK, Ireland, Sweden, Malta, as a consequence of abandoning the serological test measuring protective antibodies to rabies’. EFSA Journal 2007;5(2):436, 45 pp. 10.2903/j.efsa.2007.436 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2018. Guidance on Uncertainty Analysis in Scientific Assessments. 1831–4732 (Electronic) 1831‐4732 (Linking). e05123 pp. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32625671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority , Hart A, Maxim L, Siegrist M, Von Goetz N, da Cruz C, Merten C, Mosbach‐Schulz O, Lahaniatis M, Smith A and Hardy A, 2019. Guidance on Communication of Uncertainty in Scientific Assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekadu M and Baer GM, 1980. Recovery from clinical rabies of 2 dogs inoculated with a rabies virus strain from Ethiopia. American Journal of Veterinary Research, 41, 1632–1634. [PubMed] [Google Scholar]
- Fekadu M, Chandler FW and Harrison AK, 1982a. Pathogenesis of rabies in dogs inoculated with an Ethiopian rabies virus strain. Immunofluorescence, histologic and ultrastructural studies of the central nervous system. Archives of Virology, 71, 109–126. 10.1007/BF01314881 [DOI] [PubMed] [Google Scholar]
- Fekadu M, Shaddock JH and Baer GM, 1982b. Excretion of rabies virus in the saliva of dogs. Journal of Infectious Diseases, 145, 715–719. [DOI] [PubMed] [Google Scholar]
- Fekadu M and Shaddock JH, 1984. Peripheral distribution of virus in dogs inoculated with two strains of rabies virus. American Journal of Veterinary Research, 45, 724–729. [PubMed] [Google Scholar]
- Fekadu M, Sumner JW, Shaddock JH, Sanderlin DW and Baer GM, 1992. Sickness and recovery of dogs challenged with a street rabies virus after vaccination with a vaccinia virus recombinant expressing rabies virus N protein. Journal of Virology, 66, 2601–2604. 10.1128/JVI.66.5.2601-2604.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields M, Ament RD, Lamb D and Blades J, 1976. Suckling‐mouse‐brain rabies vaccine (SMBV): duration of immunity in dogs. Veterinary Medicine, Small Animal Clinician, 71, 37–40. [PubMed] [Google Scholar]
- Giel‐Moloney M, Rumyantsev AA, David F, Figueiredo M, Feilmeier B, Mebatsion T, Parrington M, Kleanthous H and Pugachev KV, 2017. A novel approach to a rabies vaccine based on a recombinant single‐cycle flavivirus vector. Vaccine, 35, 6898–6904. 10.1016/j.vaccine.2017.08.055 [DOI] [PubMed] [Google Scholar]
- Gnanadurai CW, Zhou M, He W, Leyson CM, Huang CT, Salyards G, Harvey SB, Chen Z, He B, Yang Y, Hooper DC, Dietzchold B and Fu ZF, 2013. Presence of virus neutralizing antibodies in cerebral spinal fluid correlates with non‐lethal rabies in dogs. PLoS Neglected Tropical Diseases, 7, e2375. 10.1371/journal.pntd.0002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanadurai CW, Yang Y, Huang Y, Li Z, Leyson CM, Cooper TL, Platt SR, Harvey SB, Hooper DC, Faber M and Fu ZF, 2015. Differential host immune responses after infection with wild‐type or lab‐attenuated rabies viruses in dogs. PLoS Neglected Tropical Diseases, 9, e0004023. 10.1371/journal.pntd.0004023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Donnelly CA, Nouvellet P and Woodroffe R, 2020. Rabies virus‐neutralising antibodies in healthy, unvaccinated individuals: what do they mean for rabies epidemiology? PLoS Neglected Tropical Diseases, 14, e0007933. 10.1371/journal.pntd.0007933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad N, Khelifa RB, Matter H, Kharmachi H, Aubert MFA, Wandeler A and Blancou J, 1994. Assay of oral vaccination of dogs against rabies in Tunisia with the vaccinal strain SADBern. Vaccine, 12, 307–309. [DOI] [PubMed] [Google Scholar]
- Hammami S, Schumacher C, Cliquet F, Tlatli A, Aubert A and Aubert M, 1999. Vaccination of Tunisian dogs with the lyophilised SAG2 oral rabies vaccine incorporated into the DBL2 dog bait. Veterinary Research, 30, 607–613. [PubMed] [Google Scholar]
- Hanlon CA, Niezgoda M and Rupprecht CE, 2002. Postexposure prophylaxis for prevention of rabies in dogs. American Journal of Veterinary Research, 63, 1096–1100. 10.2460/ajvr.2002.63.1096 [DOI] [PubMed] [Google Scholar]
- HogenEsch H, Dunham AD, Scott‐Moncrieff C, Glickman LT and DeBoer DJ, 2002. Effect of vaccination on serum concentrations of total and antigen‐specific immunoglobulin E in dogs. American Journal of Veterinary Research, 63, 611–616. 10.2460/ajvr.2002.63.611 [DOI] [PubMed] [Google Scholar]
- Hu R, Zhang S, Fooks AR, Yuan H, Liu Y, Li H, Tu C, Xia X and Xiao Y, 2006. Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type‐2 encoding the rabies virus glycoprotein. Microbes and Infection, 8, 1090–1097. 10.1016/j.micinf.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Hurisa B, Tegbaru B, Nolkes D, Mengesha A, Kebede G, Kerga S, Adhanom A, Godana A, Nigussie D, Newayesilasie B, Gebrewold G, Bankovskiy D, Metlin A and Urga K, 2013. Safety and immunogenicity of ETHIORAB rabies vaccine. Journal of Vaccines and Vaccination, 4. [Google Scholar]
- Judit S, Beníšek Z, Ondrejková ANA, Ondrejka R and Prokeš M, 2010. A new rabies vaccine with an experimental adjuvant for domestic animals. Acta Veterinaria, 60, 597–603. [Google Scholar]
- Kallel H, Diouani MF, Loukil H, Trabelsi K, Snoussi MA, Majoul S, Rourou S and Dellagi K, 2006. Immunogenicity and efficacy of an in‐house developed cell‐culture derived veterinarian rabies vaccine. Vaccine, 24, 4856–4862. 10.1016/j.vaccine.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Lankester FJ, Wouters P, Czupryna A, Palmer GH, Mzimbiri I, Cleaveland S, Francis MJ, Sutton DJ and Sonnemans DGP, 2016. Thermotolerance of an inactivated rabies vaccine for dogs. Vaccine, 34, 5504–5511. 10.1016/j.vaccine.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang S, Zhang F and Hu R, 2012. Adaptation of a Chinese ferret badger strain of rabies virus to high‐titered growth in BHK‐21 cells for canine vaccine development. Archives of Virology, 157, 2397–2403. 10.1007/s00705-012-1436-2 [DOI] [PubMed] [Google Scholar]
- Liu X, Yang Y, Sun Z, Chen J, Ai J, Dun C, Fu ZF, Niu X and Guo X, 2014. A recombinant rabies virus encoding two copies of the glycoprotein gene confers protection in dogs against a virulent challenge. PLoS One, 9, e87105. 10.1371/journal.pone.0087105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell DL, Ewalt LC, Parnell MJ, Rupprecht CE and Hanlon CA, 2006. One‐time intradermal DNA vaccination in ear pinnae one year prior to infection protects dogs against rabies virus. Vaccine, 24, 412–416. 10.1016/j.vaccine.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Lugelo A, Hampson K, Czupryna A, Bigambo M, McElhinney LM, Marston DA, Kazwala R and Lankester F, 2021. Investigating the efficacy of a canine rabies vaccine following storage outside of the cold‐chain in a passive cooling device. Frontiers in Veterinary Science, 8, 728271. 10.3389/fvets.2021.728271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailles A, Boisseleau D, Dacheux L, Michalewiscz C, Gloaguen C, Poncon N, Bourhy H, Callon H, Vaillant V, Dabosville I and Morineau‐Le Houssine P, 2011. Rabid dog illegally imported to France from Morocco, August 2011. Euro Surveill, 16. [PubMed]
- Manickam R, Basheer MD and Jayakumar R, 2008. Post‐exposure prophylaxis (PEP) of rabies‐infected Indian street dogs. Vaccine, 26, 6564–6568. [DOI] [PubMed] [Google Scholar]
- McColl KA, Chamberlain T, Lunt RA, Newberry KM and Westbury HA, 2007. Susceptibility of domestic dogs and cats to Australian bat lyssavirus (ABLV). Veterinary Microbiology, 123, 15–25. 10.1016/j.vetmic.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture Fisheries and Food (Ministry of Agriculture Fisheries and Food) , 1971. Report of the Committee of Inquiry on Rabies: Final report, HMSO, Great Britain. [Google Scholar]
- Ministry of Agriculture Food and Rural Affairs of Canada , online. Update on wildlife rabies and recent cases of imported canine rabies in Ontario. Available online: https://www.omafra.gov.on.ca/english/food/inspection/ahw/rabies‐update.htm
- Minke JM, Bouvet J, Cliquet F, Wasniewski M, Guiot AL, Lemaitre L, Cariou C, Cozette V, Vergne L and Guigal PM, 2009. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Veterinary Microbiology, 133, 283–286. 10.1016/j.vetmic.2008.06.024 [DOI] [PubMed] [Google Scholar]
- Molini U, Hassel R, Ortmann S, Vos A, Loschke M, Shilongo A, Freuling CM and Muller T, 2021. Immunogenicity of the oral rabies vaccine strain SPBN GASGAS in dogs under field settings in Namibia. Frontiers in Veterinary Science, 8, 737250. 10.3389/fvets.2021.737250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morters MK, McNabb S, Horton DL, Fooks AR, Schoeman JP, Whay HR, Wood JL and Cleaveland S, 2015. Effective vaccination against rabies in puppies in rabies endemic regions. Veterinary Record, 177, 150. 10.1136/vr.102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Liu Y, Yang L, Qu H, Zhao J, Hu R, Li J and Liu W, 2016. Immunogenicity of multi‐epitope‐based vaccine candidates administered with the adjuvant Gp96 against rabies. Virologica Sinica, 31, 168–175. 10.1007/s12250-016-3734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C, Stavisky J and Westgarth C, 2020. Importing rescue dogs into the UK: reasons, methods and welfare considerations. Veterinary Record, 186, 248. 10.1136/vr.105380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (World Animal Health Organisation) , 2021. Infection with Rabies virus (Terestrial Code). Available online: https://www.oie.int/en/what‐we‐do/standards/codes‐and‐manuals/terrestrial‐code‐online‐access/?id=169&L=1&htmfile=chapitre_rabies.htm
- Paris S, Chapat L, Martin‐Cagnon N, Durand PY, Piney L, Cariou C, Bergamo P, Bonnet JM, Poulet H, Freyburger L and De Luca K, 2020. ß‐Glucan as trained immunity‐based adjuvants for rabies vaccines in dogs. Frontiers in Immunology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Diego AC, Vigo M, Monsalve J and Escudero A, 2015. The One Health approach for the management of an imported case of rabies in mainland Spain in 2013. Eurosurveillance Weekly, 20. 10.2807/1560-7917.es2015.20.6.21033 [DOI] [PubMed] [Google Scholar]
- Perrin P, Jacob Y, Aguilar‐Setien A, Loza‐Rubio E, Jallet C, Desmezieres E, Aubert M, Cliquet F and Tordo N, 1999. Immunization of dogs with a DNA vaccine induces protection against rabies virus. Vaccine, 18, 479–486. 10.1016/s0264-410x(99)00247-9 [DOI] [PubMed] [Google Scholar]
- Pimburage RMS, Gunatilake M, Wimalaratne O, Balasuriya A and Perera K, 2017. Sero‐prevalence of virus neutralizing antibodies for rabies in different groups of dogs following vaccination. BMC Veterinary Research, 13, 133. 10.1186/s12917-017-1038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad MA, Jamshidi S, Shoaei S, Bahonar AR, Simani S, Fayaz A, Zavarei AR, Janani AR, Taghikhani M, Aldavood SJ, Aliyari A, Howaizi N, Eslami N, Biglari P and Rad I, 2010. Comparative study of antibody titers produced against two BHK rabies vaccines in field trial experimental condition in dogs by RFFIT. Iranian Journal of Veterinary Research, 11, 279–282. [Google Scholar]
- Ramanna BC, Swain A and Wakankar CC, 2007. A study on the seroconversion in dogs vaccinated with cell culture rabies vaccine. Journal of Communicable Diseases, 39, 165–170. [PubMed] [Google Scholar]
- Reddy GS, Sarma MSR and Srinivasan VA, 2003. Duration of immunity conferred by combined vaccine containing Canine distemper, Canine hepatitis, Canine parvo, Rabies and Leptospira antigens. Indian Veterinary Journal, 80, 604–607. [Google Scholar]
- Reddy GS and Srinivasan VA, 1999. Kinetics of serum neutralizing (SN) antibodies in dogs vaccinated with tissue culture rabies vaccine. Indian Veterinary Journal, 76, 1108–1110. [Google Scholar]
- Ribadeau‐Dumas F, Cliquet F, Gautret P, Robardet E, Le Pen C and Bourhy H, 2016. Travel‐associated rabies in pets and residual rabies risk, Western Europe. Emerging Infectious Diseases, 22, 1268–1271. 10.3201/eid2207.151733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijckevorse GG, Swaan CM, van den Bergh JP, Goorhuis A, Baayen D, Isken L, Timen A and van den Hoek A, 2012. Rabid puppy‐dog imported into the Netherlands from Morocco via Spain, February 2012. Eurosurveillance, 17. 10.2807/ese.17.10.20112-en [DOI] [PubMed] [Google Scholar]
- Rupprecht CE, Hanlon CA, Blanton J, Manangan J, Morrill P, Murphy S, Niezgoda M, Orciari LA, Schumacher CL and Dietzschold B, 2005. Oral vaccination of dogs with recombinant rabies virus vaccines. Virus Research, 111, 101–105. 10.1016/j.virusres.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Sage G, Khawplod P, Wilde H, Lobaugh C, Hemachudha T, Tepsumethanon W and Lumlertdaecha B, 1993. Immune response to rabies vaccine in Alaskan dogs: failure to achieve a consistently protective antibody response. Transactions of the Royal Society of Tropical Medicine and Hygiene, 87, 593–595. 10.1016/0035-9203(93)90099-c [DOI] [PubMed] [Google Scholar]
- Schneider MD, Furusho Y and Iiyama S, 1965. Paper electrophoresis study of sera of dogs immune to wild rabies virus. Canadian Journal of Comparative Medicine and Veterinary Science, 29, 97–101. [PMC free article] [PubMed] [Google Scholar]
- Seghaier C, Cliquet F, Hammami S, Aouina T, Tlatli A and Aubert M, 1999. Rabies mass vaccination campaigns in Tunisia: are vaccinated dogs correctly immunized? American Journal of Tropical Medicine and Hygiene, 61, 879–884. 10.4269/ajtmh.1999.61.879 [DOI] [PubMed] [Google Scholar]
- Servas V, Mailles A, Neau D, Castor C, Manetti A, Fouquet E, Ragnaud JM, Bourhy H, Paty MC, Melik N, Astoul J, Cliquet F, Moiton MP, François C, Coustillas M, Minet JC, Parriaud P, Capek I and Filleul L, 2005. An imported case of canine rabies in Aquitaine: investigation and management of the contacts at risk, August 2004‐March 2005. Euro surveillance : bulletin européen sur les maladies transmissibles = European communicable disease bulletin, 10, 222–225. [PubMed]
- Shimazaki Y, Inoue S, Takahashi C, Gamoh K, Etoh M, Kamiyama T and Makie H, 2003. Immune response to Japanese rabies vaccine in domestic dogs. Journal of Veterinary Medicine Series B, 50, 95–98. 10.1046/j.1439-0450.2003.00627.x [DOI] [PubMed] [Google Scholar]
- Shiraishi R, Nishimura M, Nakashima R, Enta C and Hirayama N, 2014. Neutralizing antibody response in dogs and cats inoculated with commercial inactivated rabies vaccines. Journal of Veterinary Medical Science, 76, 605–609. 10.1292/jvms.13-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen L, Kulonen K, Neuvonen E and Pekkanen K, 1995. Rabies antibodies in vaccinated dogs. Acta Veterinaria Scandinavica, 36, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulebot JP, Brun A, Chappuis G, Guillemin F and Tixier G, 1982. Rabies virus pathogenicity and challenge influence of the method of preparation, the route of inoculation, and the species. Comarison of the characteristics of the modified, fixed and wild strains. Comparative Immunology, Microbiology and Infectious Diseases, 5, 71–78. [DOI] [PubMed] [Google Scholar]
- Tepsumethanon W, Polsuwan C, Lumlertdaecha B, Khawplod P, Hemachudha T, Chutivongse S, Wilde H, Chiewbamrungkiat M and Phanuphak P, 1991. Immune response to rabies vaccine in Thai dogs: a preliminary report. Vaccine, 9, 627–630. 10.1016/0264-410x(91)90186-a [DOI] [PubMed] [Google Scholar]
- Tierkel ES, Koprowski H, Black J and Gorrie RH, 1949. Preliminary observations in the comparative prophylactic vaccination of dogs against rabies with living virus vaccines and phenolized vaccine. Veterinary Research, 361–367. [Google Scholar]
- Tojinbara K, Sugiura K, Yamada A, Kakitani I, Kwan NCL and Sugiura K, 2016. Estimating the probability distribution of the incubation period for rabies using data from the 1948–1954 rabies epidemic in Tokyo. Preventive Veterinary Medicine, 123, 102–105. 10.1016/j.prevetmed.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Van Gucht S and Le Roux I, 2008. Rabies control in Belgium: From eradication in foxes to import of a contaminated dog. Vlaams Diergeneeskundig Tijdschrift, 77, 376–384. [Google Scholar]
- Vaughn JB Jr, Gerhardt P and Newell KW, 1965. Excretion of street rabies virus in the saliva of dogs. JAMA: the Journal of the American Medical Association, 193, 363–368. [DOI] [PubMed] [Google Scholar]
- Wallace RM, Pees A, Blanton JB and Moore SM, 2017. Risk factors for inadequate antibody response to primary rabies vaccination in dogs under one year of age. PLoS Neglected Tropical Diseases, 11, e0005761. 10.1371/journal.pntd.0005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang Z, Xiong J, Yang K, Chi Y, Tang X, Ma L, Zhang R, Deng F, Lan K and Zhou D, 2019. A chimpanzee adenoviral vector‐based rabies vaccine protects beagle dogs from lethal rabies virus challenge. Virology, 536, 32–38. [DOI] [PubMed] [Google Scholar]
- Webster LT and Casals J, 1942. Non‐virulent, single‐dose rabies vaccine for prophylactic immunization of dogs. Journal of Experimental Medicine, 76, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) , 2001a. Rabies in a vaccinated dog imported from Azerbaijan to Germany. Available online: https://www.who‐rabies‐bulletin.org/sites/default/files/resource/Journal/Archive/Bulletin_2001_4.pdf
- WHO (World Health Organization) , 2001b. A puppy illegally imported from Morocco brings rabies to France. Available online: https://www.who‐rabies‐bulletin.org/sites/default/files/resource/Journal/Archive/Bulletin_2001_3.pdf
- WHO (World Health Organization) , 2008. Two cases of imported canine rabies in the Brussels area within six months time. Available online: https://www.who‐rabies‐bulletin.org/sites/default/files/resource/Journal/Archive/Bulletin_2008_1.pdf
- WHO (World Health Organization) , 2009. Rabies exposure due to an illegally imported dog in Germany. Available online: https://www.who‐rabies‐bulletin.org/sites/default/files/resource/Journal/Archive/Bulletin_2009_2.pdf
- Wilsmore T, Hamblin C, Taylor N, Taylor W and Watson B, 2006. Qualitative veterinary risk assessment of the introduction of rabies into the United Kingdom. A report prepared for Defra (Department for Environment, Food and Rural Affairs). [Google Scholar]
- Yuan Z, Zhang S, Liu Y, Zhang F, Fooks AR, Li Q and Hu R, 2008. A recombinant pseudorabies virus expressing rabies virus glycoprotein: safety and immunogenicity in dogs. Vaccine, 26, 1314–1321. 10.1016/j.vaccine.2007.12.050 [DOI] [PubMed] [Google Scholar]
- Zanoni RG, Bugnon P, Deranleau E, Nguyen TM and Brugger D 2010. Walking the dog and moving the cat: rabies serology in the context of international pet travel schemes. Schweizer Archiv Fuer Tierheilkunde, 152, 561–568. 10.1024/0036-7281/a000125 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang S, Li L, Hu R, Lin H, Liu H, Liu F, Shao H and Liu Y, 2016. Ineffectiveness of rabies vaccination alone for post‐exposure protection against rabies infection in animal models. Antiviral Research, 135, 56–61. 10.1016/j.antiviral.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Zhugunissov K, Bulatov Y, Taranov D, Yershebulov Z, Koshemetov Z, Abduraimov Y, Kondibayeva Z, Samoltyrova A, Amanova Z, Khairullin B and Sansyzbay A, 2017. Protective immune response of oral rabies vaccine in stray dogs, corsacs and steppe wolves after a single immunization. Archives of Virology, 162, 3363–3370. 10.1007/s00705-017-3499-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature Review protocol as provided by the contractors


