Abstract
Background
Albumin has multiple functions and is used in the clinical assessment of liver function, kidney function and nutritional status. However, few epidemiological studies have evaluated the association between sleep duration and albumin. Therefore, we carried out a cross-sectional study to address this issue. The aim of the study was to investigate the association between sleep duration and albumin in American adults based on the NHANES (National Health and Nutrition Examination Survey).
Methods
A total of 9,973 participants aged 20 years were included in this study from NHANES 2015–2018. Weighted data were calculated according to analytical guidelines. Linear regression models and smooth curve fitting were used to assess and describe the relationship between sleep duration and albumin. The inflection point was determined by a two-step recursive method. Moreover, univariate and stratified analyses were performed.
Results
There was an inverted U-shaped association between sleep duration and albumin levels. Albumin levels were highest when the sleep duration was 7.5 h. Compared to 7–8 h of sleep, short sleep duration was linked to lower albumin levels [sleep duration 5 h: β -1.00, 95% CI (-1.26, -0.74), P < 0.0001]. Compared to 7–8 h of sleep, long sleep duration was related to lower albumin levels [sleep duration 9 h: β -0.48, 95% CI (-0.68, -0.27), P < 0.0001].
Conclusions
Sleep duration had an inverted U-shaped relationship with albumin, with short or long sleep duration associated with significantly lower albumin levels.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-022-13524-y.
Keywords: Albumin, Sleep duration, Sleep timing, NHANES
Background
Human serum albumin is an important carrier protein and drug binding protein for various substances in plasma that possesses multiple functions, including endogenous and exogenous molecule binding and, transport, antioxidant activity, inflammatory responses, and immune modulation [1, 2]. Additionally, albumin is essential in plasma for maintaining the osmotic pressure of the blood [3]. Albumin levels are clinically useful in assessing liver function, kidney function, and nutritional status [4]. A retrospective study showed that hypoalbuminemia in patients with coronavirus disease 2019 was associated with illness progression to more severe stages and increased mortality [5]. Additionally, a recent study found that among patients with sepsis, the sleep deprivation group had significantly lower albumin than the good sleep group [6].
Sleep is critical to human health and well-being, and it is a complex and highly regulated process [7]. Serum albumin is a major antioxidant agent [8]. A study found that serum albumin levels were significantly lower in patients with sleep disorders than in those without sleep disorders [9]. The biological mechanism by which sleep duration is related to albumin levels is unclear. Among adults with objectively short sleep durations, physiological indicators of hyperarousal were increased. Increased cortisol and norepinephrine levels, result in changes in systemic metabolic rate, increased high frequency cortical dynamics, and brain glucose metabolism, and decreased gamma-aminobutyric acid levels. Regarding the effects of sleep loss on transcriptome dynamics, there is evidence that partial sleep loss during the night induces the upregulation of a gene set that includes the major circadian regulator, several immediate early genes that mark cell signaling, and multiple inflammatory response genes [10]. Albumin is the main protein synthesized in the liver and can be negatively affected by inflammation [11]. However, there is currently a limited amount of data available on the association between serum albumin levels and sleep duration. Previous studies have shown that sleep durations that are insufficient or extremely long are associated with markers of inflammation, such as C-reactive protein (CRP), CRP/ALB (CRP to albumin ratio), and the gamma gap (calculated by subtracting albumin from total protein) [12–14]. In addition, decreased albumin lipid concentrations stimulate lipoprotein production in the liver, increase blood concentrations, lead to increased peripheral vascular resistance and increased cardiovascular risk, and are associated with an increase in multiple chronic diseases, which may affect sleep.
The National Sleep Foundation (NSF) recommended 7–9 h of sleep for young adults and 7–8 h for older adults [15]. However, only 45% of individuals in the US had sleep durations that mapped onto the National Sleep Foundation recommendations on weekdays [16]. Regarding the different patterns of sleep on weekdays and weekends, a study on NHANES provided a series of benchmark estimates of sleep duration and sleep timing for the US population [17]. Shorter or longer sleep duration was linked to an increased risk of hypertension, diabetes, obesity, renal dysfunction, cancer, and mortality [18–20]. However, few epidemiological studies have assessed the relationship between sleep duration and albumin in the average adult.
Thus, we conducted this cross-sectional study to investigate the association between sleep duration and albumin levels in American adults according to NHANES.
Methods
Study design
NHANES is a United States national stratified multistage probability sample surveyed by the NCHS (National Center for Health Statistics) to assess nutritional status and its relationship to health promotion and disease prevention. The survey is characterized by a combination of interviews and physical examinations. Highly trained medical personnel administer the examinations and laboratory tests. Detailed descriptions of NHANES’ participant recruitment, survey design, and data collection procedures are available online at http://www.cdc.gov/nchs/nhanes/aboutnhanes.htm.
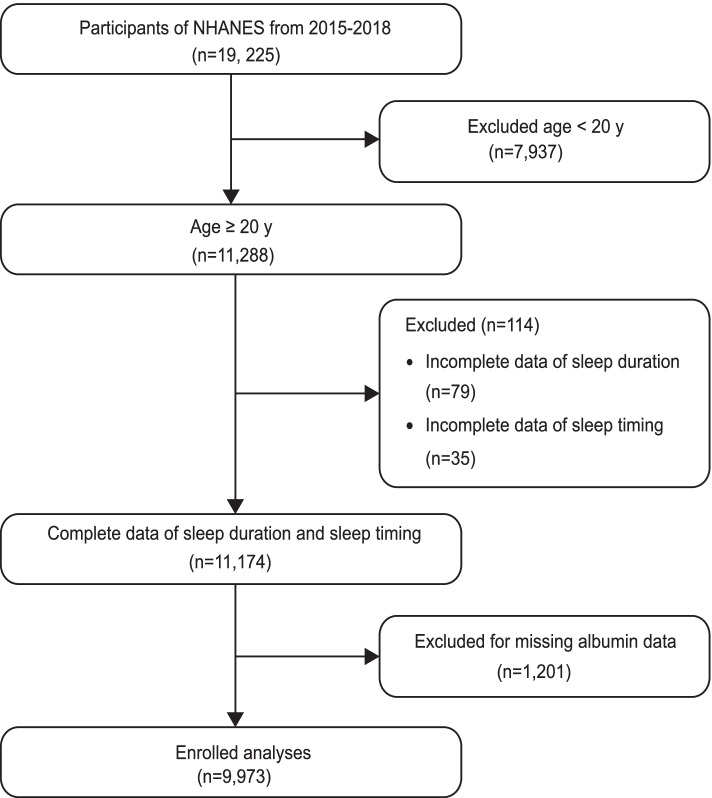
This study, which used data from NHANES 2015–2018, was approved by the NCHS Research Ethics Review Board (ERB) and obtained written consent from the Centers for Disease Control and Prevention (CDC) for all surveyed individuals. Through the rigorous screening, of 19,225 participants, a total of 9,973 participants aged 20 years with a complete set of sleep-related data and albumin data were included in this study. The detailed screening process of participants is shown in Fig. 1.
Fig. 1.
Flowchart of the participants included
Measurements
Sleep duration and sleep timing
The definition was based on the “sleep disorders” data in the “NHANES Questionnaire”. Question SLQ012 asked “Number of hours usually sleep on weekdays or workdays.” The participants answered with their sleep duration, and hours were rounded to the nearest half-hour. Sleep duration was analysed as both a continuous and categorical variable. The sleep duration was classified and presented in 6 groups: sleep duration 5 h, 5 h sleep duration 6 h, 6 h sleep duration 7 h, 7 h sleep duration 8 h, 8 h sleep duration 9 h, sleep duration 9 h. They are described as follows: 5 h, 5–6 h, 6–7 h, 7–8 h, 8-9 h, 9 h [21]. (“short” sleep duration: 5 h; “long” sleep duration: 9 h) [22, 23]. Question SLQ300 asked “ What time {do you/does SP} usually fall asleep on weekdays or workdays?” The participants answered sleep timing.
Sleep timing was recorded in 24-h notation, where HH (from 00 to 23) was the number of full hours elapsed since midnight and MM (from 00 to 59) was the number of full minutes elapsed since the last full hour. Sleep timing was divided into 4 groups: 18:00–21:59, 22:00–22:59, 23:00–23:59, and later than 00:00 [24, 25].
Albumin
Albumin is abbreviated as ALB. The method for measuring albumin concentration used the dye bromocresol purple. The NHANES Quality Control and Quality Assurance Protocol (QA/QC) complies with the requirements of the Clinical Laboratory Improvement Act of 1988.
Covariates
The covariates in this study included: demographic characteristics (age, sex, race, marital status), chronic disease (hypertension, high cholesterol, cancer or malignancy), lifestyle behavior (moderate work activity), examination data (BMI), and laboratory data (TP, ALT, AST, Cr, HS-CRP, GLU, UACR) [26].
The categorical variables among the demographic characteristics included: sex (male, female); race (Mexican American, non-Hispanic white, non-Hispanic black, other race); and marital status (married or living with a partner, living alone [never married, separated, divorced, or widowed]). Chronic diseases were assessed by self-reported medical history, and the participants were asked if they were told by a doctor or other health professional to have a specific health condition, including hypertension (yes, no, unknown), high cholesterol (yes, no, unknown), cancer or malignancy (yes, no, unknown). Moderate work activity (yes or no) was obtained from the adult section of the Physical Activity Questionnaire and categorized by answering the question, does your “work involve moderate-intensity activity that causes small increases in breathing or heart rate?” The body measurement data were collected, in the Mobile Examination Center, by trained health technicians, and BMI was calculated as weight in kilograms divided by height in meters squared. Biochemical profiles included: TP, ALT, AST, Cr, HS-CRP, GLU, and UACR. Furthermore, the estimated glomerular filtration rate (eGFR) was determined using the MDRD formula [27]: eGFR (ml/min/1.73m2) 175 SCr (mg/dl)−1.154 Age−0.203 0.742 (if female).
Statistical analysis
Weighted data were calculated according to analytical guidelines (NHANES:2015–2016 and 2017–2018). Various sample weights, such as interview weight (wtint2yr), MEC (Mobile Examination Center) exam weight (wtmec2yr), and several subsample weights, are available in the NHANES data release file. The selection of the correct sample weights for the analyses depends on the variables used. All the interview and MEC exam weights covered in this study are available in the demographic files. Since the MEC examined sample persons are a subset of those interviewed in the survey, we used the combined MEC exam weight for analysis. NHANES2015-2018 involved a combination of two survey cycles (four years), and the data were weighted according to the information that the NCHS provided analysts on how to combine multiple cycles and construct appropriate weights. MEC4YR = 1/2 WTMEC2YR [28]. Linear regression models were used to describe the relationship between sleep duration and albumin levels, and three models were constructed: Model 1, was not adjusted for any variables; Model 2, was adjusted for sex, age, race, marital status, and moderate work activity; Model 3, was adjusted for all the covariates (sex, age, race, marital status, moderate work activity, TP, ALT, AST, Cr, UACR, HS-CRP, GLU, BMI, hypertension, high cholesterol, cancer or malignancy). Smooth curve fitting was used to resolve the relationship between sleep duration and albumin. The log-likelihood ratio test was used to assess whether a threshold existed, and the inflection point was determined using a two step recursive method. We performed univariate and stratified analyses to identify independent effect between sleep duration and albumin levels. In addition, an interaction test of these factors was also performed. Continuous variables are represented by means SEs (standard errors), while percentages are used to represent classified variables. The effect value was expressed as β and corresponding 95% CIs (confidence intervals). P values 0.05 were considered statistically significant. All data in the study were analysed using the statistical package R (http://www.r-project.org; the Version: 3.4.3, 2018–02-18) and Empower Stats (http://www.empowerstats.com; X&Y Solutions Inc).
Results
Table 1 shows the description of the characteristics of the participants grouped by sleep duration. Among the 9973 participants, 2926 slept 7–8 h, accounting for 29.3% of the total, 694 slept 5 h, 6.96% of the total, and 1164 slept 9 h, accounting for 11.67% of the total. The total average sleep duration was 7.65 1.578 h. The average ALB level was the highest in the 7–8 h group, and its highest value was 42.50 3.54 g/L. Based on the sleep timing distribution, 72.83% of the participants with a sleep duration 5 h fell asleep after 0:00.
Table 1.
Characteristics of participants by sleep duration groups
| sleep duration (h) | |||||||
|---|---|---|---|---|---|---|---|
| 5 | 5–6 | 6–7 | 7–8 | 8–9 | 9 | P value | |
| N | 694 | 1051 | 2248 | 2926 | 1890 | 1164 | |
| sleep duration (ungrouped, h) | 4.46 ± 0.67 | 5.89 ± 0.21 | 6.85 ± 0.23 | 7.80 ± 0.24 | 8.78 ± 0.25 | 10.28 ± 0.86 | < 0.0001 |
| sleep timing (%) | < 0.0001 | ||||||
| 18:00–21:59 | 2.35 | 7.14 | 11.15 | 17.54 | 28.37 | 38.11 | |
| 22:00–22:59 | 9.58 | 16.60 | 29.03 | 36.15 | 35.51 | 27.66 | |
| 23:00–23:59 | 15.25 | 33.20 | 32.32 | 28.50 | 19.45 | 17.22 | |
| later than 00:00 | 72.83 | 43.06 | 27.50 | 17.82 | 16.67 | 17.01 | |
| age (years) | 49.01 ± 16.10 | 47.33 ± 15.13 | 47.36 ± 15.73 | 47.93 ± 16.65 | 49.10 ± 18.54 | 49.82 ± 20.32 | 0.0002 |
| sex (%) | < 0.0001 | ||||||
| male | 54.43 | 55.38 | 55.74 | 46.80 | 38.49 | 39.59 | |
| female | 45.57 | 44.62 | 44.26 | 53.20 | 61.51 | 60.41 | |
| race | < 0.0001 | ||||||
| Mexican American | 17.56 | 18.54 | 16.56 | 13.58 | 14.84 | 17.12 | |
| non-Hispanic white | 48.41 | 56.31 | 62.47 | 68.50 | 67.58 | 59.90 | |
| non-Hispanic black | 23.87 | 15.92 | 10.56 | 7.89 | 8.20 | 13.83 | |
| other race | 10.16 | 9.23 | 10.41 | 10.03 | 9.38 | 9.15 | |
| marital status (%) | < 0.0001 | ||||||
| married or living with partner | 52.72 | 63.89 | 67.28 | 67.04 | 63.62 | 52.60 | |
| living alone | 47.28 | 36.11 | 32.72 | 32.96 | 36.38 | 47.40 | |
| moderate work activity (%) | < 0.0001 | ||||||
| yes | 51.69 | 54.68 | 47.91 | 47.82 | 45.29 | 37.73 | |
| no | 48.31 | 45.32 | 52.09 | 52.18 | 54.71 | 62.27 | |
| TP (g/L) | 71.68 ± 4.57 | 70.94 ± 4.39 | 71.25 ± 4.29 | 70.97 ± 4.19 | 70.75 ± 4.46 | 71.12 ± 4.61 | < 0.0001 |
| ALT (IU/L) | 26.43 ± 21.66 | 25.58 ± 16.57 | 24.92 ± 16.58 | 24.01 ± 16.41 | 23.43 ± 16.29 | 22.40 ± 15.94 | < 0.0001 |
| AST (IU/L) | 25.63 ± 18.35 | 24.23 ± 12.30 | 24.06 ± 13.01 | 23.62 ± 10.20 | 23.68 ± 11.96 | 23.57 ± 13.98 | 0.0135 |
| Crlog2 (μmol/L) | 6.25 ± 0.40 | 6.23 ± 0.38 | 6.23 ± 0.36 | 6.20 ± 0.37 | 6.18 ± 0.38 | 6.23 ± 0.46 | < 0.0001 |
| UACRlog2 (mg/g) | 3.26 ± 1.52 | 3.07 ± 1.45 | 3.07 ± 1.56 | 3.08 ± 1.47 | 3.14 ± 1.41 | 3.51 ± 1.78 | < 0.0001 |
| HS-CRP (mg/L) | 3.65 ± 4.55 | 4.38 ± 8.20 | 3.51 ± 5.98 | 3.86 ± 7.47 | 3.81 ± 6.31 | 4.84 ± 10.44 | < 0.0001 |
| GLU (mmol/L) | 5.71 ± 2.09 | 5.63 ± 1.87 | 5.55 ± 1.75 | 5.52 ± 1.90 | 5.52 ± 1.71 | 5.73 ± 2.02 | 0.0079 |
| BMI (kg/m2) | 30.42 ± 7.62 | 30.52 ± 7.52 | 29.84 ± 7.14 | 29.27 ± 6.67 | 29.58 ± 7.20 | 29.22 ± 7.02 | < 0.0001 |
| hypertension | < 0.0001 | ||||||
| yes | 40.07 | 34.95 | 30.46 | 30.79 | 31.12 | 38.41 | |
| no | 59.78 | 65.05 | 69.45 | 69.13 | 68.73 | 61.43 | |
| unknown | 0.15 | 0.09 | 0.08 | 0.15 | 0.16 | ||
| high cholesterol (%) | < 0.0001 | ||||||
| yes | 34.32 | 33.75 | 31.49 | 33.10 | 34.84 | 36.24 | |
| no | 64.71 | 65.69 | 68.20 | 66.80 | 64.78 | 62.62 | |
| unknown | 0.97 | 0.56 | 0.31 | 0.11 | 0.38 | 1.14 | |
| cancer or malignancy (%) | < 0.0001 | ||||||
| yes | 9.26 | 8.44 | 8.85 | 10.38 | 13.88 | 13.19 | |
| no | 90.74 | 91.28 | 91.13 | 89.62 | 85.96 | 86.81 | |
| unknown | 0.27 | 0.03 | 0.16 | ||||
| ALB (g/L) | 41.41 ± 3.48 | 41.81 ± 3.44 | 42.49 ± 3.68 | 42.50 ± 3.54 | 42.04 ± 3.67 | 41.66 ± 3.98 | < 0.0001 |
N referred to sample size
Mean ± SEs for continuous variables, P values were calculated by weighted linear regression model
Percentage (%) for categorical variables, P values were calculated by weighted chi-square test
Abbreviations: ALB Albumin, TP Total Protein, ALT Alanine aminotransferase, AST Aspartate aminotransferase, Cr Creatinine, HS-CRP High Sensitivity C-reactive Protein, GLU Glucose, BMI Body Mass Index, UACR Urinary Albumin-creatinine Ratio
UACR and Cr were estimated after logarithmic transformation
Sleep duration and albumin (ALB) data were grouped by sleep duration, and the relationship between covariates and ALB was analysed through univariate analyses, as shown in Supplemental Table S1. Table 2 shows that in multivariable regression models, ALB levels in those with short-sleep or long-sleep durations was lower than that in those with sleep durations of 7–8 h. In Model 3, which was adjusted for all covariates, when the sleep duration of the participants was 5 h, it can be concluded that the effect size was β = -1.00, the 95% confidence interval was (-1.26, -0.74), and the p value was less than 0.0001. Similarly, when the sleep duration of participants was 9 h, conclusions can be drawn that the effect size was β -0.48, the 95% confidence interval was (-0.68, -0.27), and the p value was less than 0.0001. Table 2 also shows that compared with the ALB levels of the participants who fell asleep between 22:00 and 22:59, ALB levels showed a more significant decrease after 00:00. In Model 3, compared with the ALB levels of the participants who fell asleep between 22:00–22:59, the ALB levels in the participants sleeping after 00:00 decreased (β, -0.43; 95% CI, -0.59, -0.28; P < 0.0001). An inverted U-shaped association between sleep duration and albumin was found in the GAM (generalized additive model), as presented in Fig. 2. where the solid line represents a smooth curve fit between the variables, and the blue bars represent the 95% confidence interval for the fit. We adjusted for all covariates (sex, age, race, marital status, moderate work activity, TP, ALT, AST, Cr, UACR, HS-CRP, GLU, BMI, hypertension, high cholesterol, and cancer or malignancy). Furthermore, we performed a log-likelihood ratio test and then compared the one-line linear regression model with the two-segment regression model. The inflection point K 7.5 h was obtained through a two-step recursive method. The results showed that ALB levels were highest when the sleep duration was 7.5 h. The results are shown in Table 3.
Table 2.
Relationship between sleep duration/ sleep onset timing and albumin in different models
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β(95%CI) | P value | β(95%CI) | P value | β(95%CI) | P value | |
| sleep duration (h) | ||||||
| 7–8 | Ref | Ref | Ref | |||
| ≤ 5 | -1.09 (-1.43, -0.76) | < 0.0001 | -0.93 (-1.24, -0.61) | < 0.0001 | -1.00 (-1.26, -0.74) | < 0.0001 |
| 5–6 | -0.70 (-0.96, -0.44) | < 0.0001 | -0.72 (-0.96, -0.47) | < 0.0001 | -0.45 (-0.65, -0.25) | < 0.0001 |
| 6–7 | -0.02 (-0.21, 0.18) | 0.8581 | -0.14 (-0.32, 0.05) | 0.1420 | -0.13 (-0.28, 0.02) | 0.0919 |
| 8–9 | -0.47 (-0.67, -0.26) | < 0.0001 | -0.26 (-0.45, -0.07) | 0.0079 | -0.22 (-0.38, -0.06) | 0.0063 |
| > 9 | -0.84 (-1.11, -0.58) | < 0.0001 | -0.54 (-0.79, -0.30) | < 0.0001 | -0.48 (-0.68, -0.27) | < 0.0001 |
| sleep timing (HH:MM) | ||||||
| 22:00–22:59 | Ref | Ref | Ref | |||
| 18:00–21:59 | -0.35 (-0.56, -0.13) | 0.0014 | -0.27 (-0.46, -0.07) | 0.0091 | -0.16 (-0.33, 0.01) | 0.0580 |
| 23:00–23:59 | -0.16 (-0.36, 0.03) | 0.0901 | -0.23 (-0.41, -0.05) | 0.0128 | -0.18 (-0.33, -0.03) | 0.0166 |
| later than 00:00 | -0.35 (-0.55, -0.16) | 0.0003 | -0.61 (-0.80, -0.43) | < 0.0001 | -0.43 (-0.59, -0.28) | < 0.0001 |
β was the effect size (g/L) of the change in albumin, and the 95% CI indicated the confidence interval
Model 1 adjust for: none
Model 2 adjust for: sex, age, race, marital status, moderate work activity
Model 3 adjust for: sex, age, race, marital status, moderate work activity, TP, ALT, AST, Cr, UACR, HS-CRP, GLU, BMI, hypertension, high cholesterol, cancer or malignancy
Fig. 2.
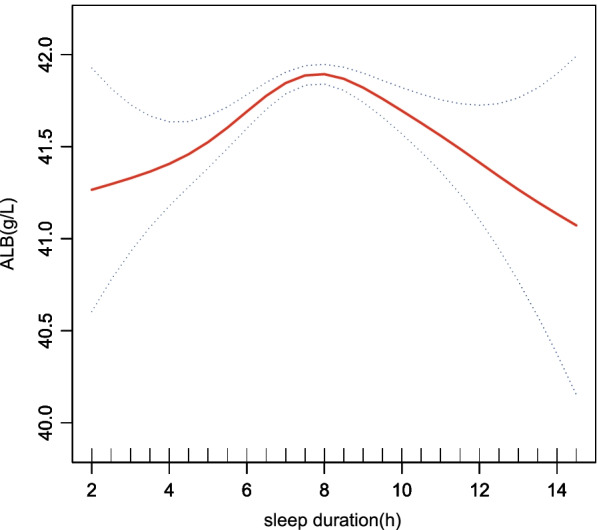
A threshold, nonlinear association between sleep duration and albumin levels was found in a generalized additive model (GAM). The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval around the fit. The model was adjusted for sex, age, race, marital status, moderate work activity, TP, ALT, AST, Cr, UACR, HS-CRP, GLU, BMI, hypertension, high cholesterol, cancer or malignancy
Table 3.
Threshold effect analysis of sleep duration and albumin using piece-wise linear regression
| Models | β (95%CI) | P value |
|---|---|---|
| Model I | ||
| One line effect | 0.04 (0.00, 0.08) | 0.0366 |
| Model II | ||
| Inflection point (K) | 7.5 | |
| sleep duration 7.5 | 0.30 (0.23, 0.38) | 0.0001 |
| sleep duration 7.5 | -0.18 (-0.25, -0.12) | 0.0001 |
| P for log-likelihood ratio test | 0.001 | |
Model I, one-line linear regression; Model II, two-segment regression
β was the effect size and the 95% CI indicated the confidence interval
adjust for: sex, age, race, marital status, moderate work activity, TP, ALT, AST, Cr, UACR, HS-CRP, GLU, BMI, hypertension, high cholesterol, cancer or malignancy
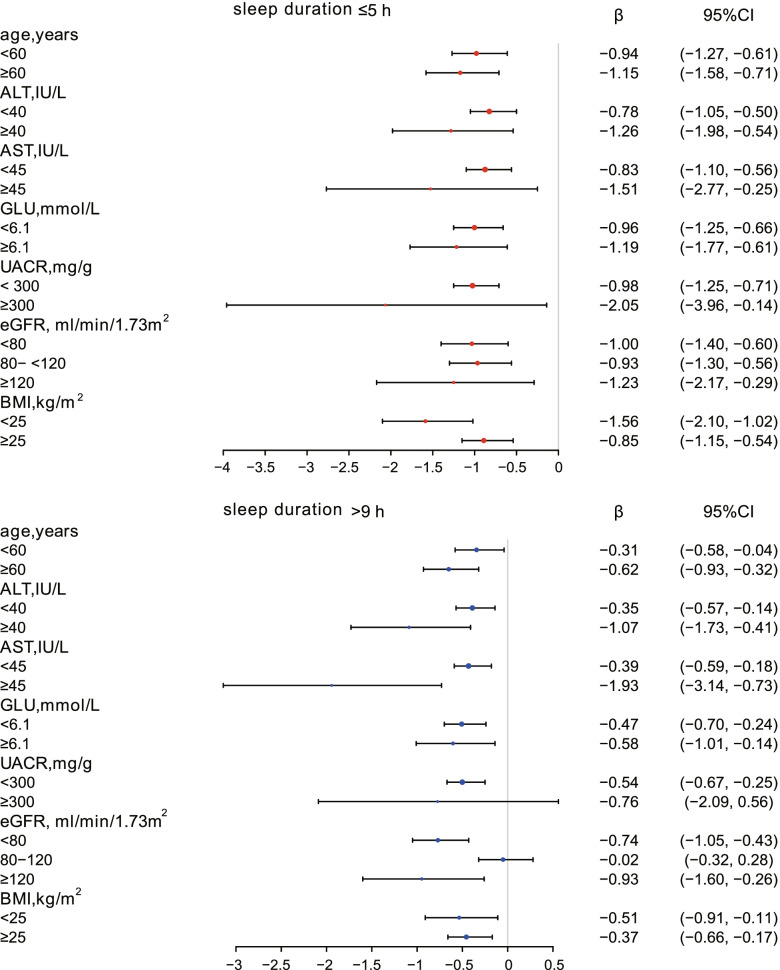
We evaluated the effect size of sleep duration on albumin in prespecified and exploratory subgroups. Stratified analyses and interaction tests were performed, as shown in Supplemental Table S2. Sex, race, marital status, and high cholesterol categorical variables as well as AST, and eGFR (after classification by clinical significance) had significant interactions. (P for interaction 0.05), as shown in Table 4. In terms of sex, the decreases in albumin levels were more pronounced in females than in males when sleep duration was short or long. When stratified by race, decreases in albumin levels were most marked in non-Hispanic whites with short or long sleep duration compared to albumin levels in those with 7–8 h sleep duration. When the participants slept less than 5 h, ALB decreased by 1. 67 g/L, 95% CI (-2.16, -1.17); when the participants slept more than 9 h, ALB decreased by 0.65 g/L, 95% CI (-1.00, -0.30). When stratified by marital status, the albumin of married individuals or those living with a partner fell appreciably when sleep duration was 5 h (β, -1.67; 95% CI, -2.16, -1.17) or 9 h (β, -0.65; 95% CI, -1.00, -0.30). The participants with high cholesterol and short or long sleep durations had lower levels of ALB than those without hypercholesterolaemia. When sleep duration was 5 h, ALB levels of hypercholesterolaemic participants decreased by 1. 23 g/L, 95% CI (-1.65, -0.81); when sleep duration was 9 h, their albumin levels decreased by 0. 65 g/L, 95% CI (-1.00, -0.30). The data for continuous variables (age, ALT, AST, eGFR, UACR, GLU, BMI) were stratified and analysed according to their clinical significance. The effect size (β) and 95% confidence interval (95% CI) of changes in ALB when sleep duration ≤ 5 h or > 9 h are shown in forest plots (Fig. 3).
Table 4.
Effect size of sleep duration on albumin in prespecified and exploratory subgroups
| sleep duration (h) | P for interaction | ||||||
|---|---|---|---|---|---|---|---|
| 7–8 | 5 | 5–6 | 6–7 | 8–9 | 9 | ||
| sex | 0.0418 | ||||||
| male | Ref | -0.94 (-1.29, -0.60) | -0.45 (-0.71, -0.18) | 0.09 (-0.12, 0.29) | -0.18 (-0.42, 0.06) | -0.28 (-0.59, 0.03) | |
| P value | < 0.0001 | 0.0011 | 0.4020 | 0.1493 | 0.0739 | ||
| female | Ref | -0.95 (-1.34, -0.56) | -0.47 (-0.77, -0.17) | -0.38 (-0.60, -0.15) | -0.26 (-0.47, -0.05) | -0.58 (-0.86, -0.31) | |
| P value | < 0.0001 | 0.0024 | 0.0009 | 0.0159 | < 0.0001 | ||
| race | 0.0010 | ||||||
| Mexican American | Ref | -0.86 (-1.34, -0.39) | -0.40 (-0.76, -0.03) | -0.19 (-0.48, 0.10) | 0.00 (-0.31, 0.32) | -0.05 (-0.43, 0.33) | |
| P value | 0.0004 | 0.0324 | 0.2015 | 0.9940 | 0.7933 | ||
| non-Hispanic white | Ref | -1.67 (-2.16, -1.17) | -0.53 (-0.89, -0.18) | -0.15 (-0.41, 0.10) | -0.32 (-0.58, -0.05) | -0.65 (-1.00, -0.30) | |
| P value | < 0.0001 | 0.0033 | 0.2339 | 0.0182 | 0.0003 | ||
| non-Hispanic black | Ref | 0.05 (-0.40, 0.50) | -0.13 (-0.55, 0.29) | 0.02 (-0.35, 0.39) | -0.02 (-0.43, 0.40) | -0.06 (-0.50, 0.38) | |
| P value | 0.8126 | 0.5405 | 0.9224 | 0.9433 | 0.7906 | ||
| other race | Ref | -0.02 (-0.67, 0.62) | -0.15 (-0.67, 0.37) | 0.10 (-0.28, 0.47) | -0.18 (-0.59, 0.23) | -0.43 (-0.96, 0.10) | |
| P value | 0.9466 | 0.5760 | 0.6143 | 0.3842 | 0.1095 | ||
| marital status | 0.0023 | ||||||
| married or living with partner | Ref | -1.38 (-1.75, -1.01) | -0.67 (-0.93, -0.41) | -0.23 (-0.42, -0.04) | -0.26 (-0.47, -0.06) | -0.69 (-0.97, -0.40) | |
| P value | < 0.0001 | < 0.0001 | 0.0190 | 0.0116 | < 0.0001 | ||
| living alone | Ref | -0.54 (-0.91, -0.17) | -0.06 (-0.38, 0.27) | 0.08 (-0.17, 0.33) | -0.12 (-0.38, 0.13) | -0.17 (-0.46, 0.13) | |
| P value | 0.0047 | 0.7374 | 0.5184 | 0.3454 | 0.2722 | ||
| high cholesterol | 0.0177 | ||||||
| yes | Ref | -1.23 (-1.65, -0.81) | -0.61 (-0.93, -0.28) | -0.51 (-0.76, -0.26) | -0.41 (-0.66, -0.15) | -0.83 (-1.15, -0.50) | |
| P value | < 0.0001 | 0.0002 | < 0.0001 | 0.0018 | < 0.0001 | ||
| no | Ref | -0.80 (-1.14, -0.46) | -0.40 (-0.66, -0.14) | 0.05 (-0.14, 0.25) | -0.14 (-0.34, 0.07) | -0.28 (-0.55, -0.02) | |
| P value | < 0.0001 | 0.0023 | 0.5709 | 0.1879 | 0.0367 | ||
| AST, IU/L | 0.0004 | ||||||
| < 45 | Ref | -0.83 (-1.10, -0.56) | -0.50 (-0.71, -0.30) | -0.12 (-0.27, 0.03) | -0.21 (-0.37, -0.05) | -0.39 (-0.59, -0.18) | |
| P value | < 0.0001 | < 0.0001 | 0.1197 | 0.0104 | 0.0003 | ||
| ≥ 45 | Ref | -1.51 (-2.77, -0.25) | 0.82 (-0.33, 1.97) | -0.18 (-1.11, 0.74) | -0.20 (-1.18, 0.79) | -1.93 (-3.14, -0.73) | |
| 0.0190 | 0.1629 | 0.7016 | 0.6945 | 0.0018 | |||
| eGFR, ml/min/1.73m2 | 0.0019 | ||||||
| < 80 | Ref | -1.00 (-1.40, -0.60) | -0.66 (-0.99, -0.33) | -0.31 (-0.55, -0.06) | -0.40 (-0.66, -0.15) | -0.74 (-1.05, -0.43) | |
| P value | < 0.0001 | < 0.0001 | 0.0134 | 0.0018 | < 0.0001 | ||
| 80–120 | Ref | -0.93 (-1.30, -0.56) | -0.48 (-0.75, -0.21) | -0.10 (-0.30, 0.10) | -0.10 (-0.32, 0.12) | -0.02 (-0.32, 0.28) | |
| P value | < 0.0001 | 0.0005 | 0.3282 | 0.3589 | 0.8870 | ||
| ≥ 120 | Ref | -1.23 (-2.17, -0.29) | 0.26 (-0.44, 0.96) | 0.21 (-0.33, 0.76) | 0.02 (-0.55, 0.59) | -0.93 (-1.60, -0.26) | |
| P value | 0.0106 | 0.4718 | 0.4431 | 0.9469 | 0.0064 | ||
Each stratification adjusted for all factors (sex, age, race, marital status, moderate work activity, TP, ALT, AST, Cr, UACR, HS-CRP, GLU, BMI, hypertension, high cholesterol, cancer or malignancy) except the stratification factor itself
Fig. 3.
In subgroups analysed by age, ALT, AST, eGFR, UACR, GLU, and BMI, the effect size (β) and 95% confidence interval (95% CI) of changes in ALB when sleep duration ≤ 5 h and sleep duration 9 h are shown in forest plots
Discussion
The main aim of this study was to explore the associations of sleep duration with ALB levels in a nationally representative sample of American adults. The results of this study showed that sleep duration and ALB levels showed an inverted U-shaped relationship. It was demonstrated that ALB levels were highest when the sleep duration was 7.5 h, which was in the 7–8 h group (7 h sleep duration 8 h). When sleep duration was 5 h (“short” sleep duration) or sleep duration was 9 h (“long” sleep duration), the albumin levels significantly decreased, so we performed a focused comparative analysis of 3 sleep duration categories (sleep duration 5 h, 7 h sleep duration 8 h, sleep duration 9 h).
Short sleep duration or long sleep duration was significantly associated with lower ALB levels than 7–8 h sleep duration even after adjusting for confounding factors. An animal experimental study found that sleep deprivation resulted in significantly lower serum albumin levels [29]. Albumin is an important indicator for evaluating nutritional status, and sleep duration 6 h or 9 h was indicated to increase the malnutrition risk of older individuals [21].
We evaluated the effect size of different sleep duration groups on albumin levels in prespecified and exploratory subgroups and the results showed differences. There was a significant correlation between sleep duration and ALB levels in patients with renal insufficiency, and the reason was considered that urinary albumin causes albumin levels to decrease. A cross-sectional study from the KNHANES confirmed the U-shaped relationship between sleep duration and urinary albumin in Korean adults [30]. Stratified and interaction analyses showed that sleep duration had a greater impact on albumin levels in patients with abnormal liver function. Albumin is synthesized by the liver, and impairments in liver function can lead to the blockage of albumin synthesis. ALB plays a crucial role in immunomodulation during chronic liver disease processes, and albumin is negatively correlated with infection in patients with chronic liver diseases [31]. The participants with high cholesterol or high blood sugar had more significant changes in their ALB levels with short or long sleep duration. A NHANES study showed a significant U-shaped relationship between sleep duration and metabolic syndrome severity score [32]. Circadian misalignment leads to an increased risk of metabolic disease in individuals with late sleep time [33]. Sleeping late can disrupt the rhythm of the body’s biological clock. We also found that ALB levels in those with late bedtimes were lower than in those falling asleep at 22:00–22:59, especially when the sleep timing was later than 0:00. A recent study showed that delayed sleep is the cause of short sleep duration [17]. Additionally, a study in the American population found that sleep duration had a U-shaped association with leading chronic diseases [34]. Chronic diseases often lead to a decline in the body’s immunity and nutritional status, which in turn results in a decline in albumin levels.
This study has some significance. First, our findings can provide supportive evidence to clinical work. The results of this study are consistent with NSF recommendations regarding healthy sleep durations. For adults with low albumin levels or hypoalbuminemia, especially those with chronic diseases, the sleep timing should be adjusted to ensure the optimal sleep duration. Second, to our knowledge, this is the first study to study the association between sleep duration and album levels in US adults. In addition, our findings revealed potential public health concern. We found racial differences in sleep duration, racial/ethnic minorities, particularly non-Hispanic blacks, reported shorter sleep durations than non-Hispanic whites. This is consistent with the findings of a recent study showing significant differences in sleep duration by race and ethnicity, with the incidence of unrecommended sleep durations consistently higher in black individuals [35]. These persistent disparities may lead to other persistent racial and ethnic disparities in health.
The study had certain limitations. First, due to the cross-sectional study design, causal associations cannot be established between sleep information and ALB levels. Future longitudinal studies could improve the reliability of the findings. Second, sleep duration and sleep timing were obtained from self-reported information. Studies have shown that self-reported or perceived sleep time is different from objective assessments of sleep duration accomplished by actigraphy [36, 37]. In addition, recall bias and seasonal variations may lead to information bias in the reporting of sleep duration [38]. However, in large population-based studies, self-reported data allow us to obtain more representative population estimates. Although validated sleep instruments are considered to provide objective indicators for the measurement of sleep architecture, self-reported inventories are often used to measure sleeping health owing to the advantages of being affordable, quick, and feasible to administer in large samples [39]. Finally, there might be other mixed factors that were not adequately taken into account. Despite these limitations, this is a population-based study looking at the relationship between sleep duration and ALB levels in US adults.
Conclusions
This study shows that sleep duration has an inverted U-shaped relationship with albumin levels, in a nationally representative sample of American adults. ALB levels were highest when the sleep duration was 7.5 h. Short or long sleep durations were significantly associated with lower ALB levels compared with sleep durations of 7–8 h.
Supplementary Information
Additional file 1: Table S1. Univariate analysis for albumin(g/L).
Additional file 2: Table S2. Effect size of sleep duration on albumin in prespecified and exploratory subgroups.
Acknowledgements
Not applicable.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- NSF
National Sleep Foundation
- NCHS
National Center for Health Statistics
- ERB
Ethics Review Board
- CDC
Centers for Disease Control and Prevention
- QA/QC
Quality Control and Quality Assurance Protocol
- MEC
Mobile Examination Center
- 95% CI
95% Confidence interval
- ALB
Albumin
- TP
Total protein
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- Cr
Creatinine
- HS-CRP
High sensitivity C-reactive protein
- GLU
Glucose
- BMI
Body mass index
- UACR
Urinary albumin-creatinine ratio
- eGFR
Estimated glomerular filtration rate
Authors’ contributions
JX.L proposed the theme of this study, conducted data collection and statistical analysis, and completed the writing of the first draft. LZ.G supervised and checked the manuscript writing. The manuscript was finally completed through repeated revisions. The final version of the manuscript was approved by all authors.
Author information
Jingxian Li is the first author.
Funding
Not applicable.
Availability of data and materials
The datasets generated and analysed for the current study are available in the NHANES repository. These data can be accessed using the following link: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Declarations
Ethics approval and consent to participate
The study analysed data downloaded from the National Health and Nutrition Examination Survey public database. The National Center for Health Statistics Ethics Review Committee granted ethics approval. The methods involved in this study were conducted in accordance with relevant guidelines and regulations (Declaration of Helsinki). All individuals provided written informed consent before participating in the study. Details are available at https://www.cdc.gov/nchs/nhanes/irba98.htm. The current study was deemed exempt from further review because the data used are deidentified and publicly accessible.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Duran-Güell M, Flores-Costa R, Casulleras M, López-Vicario C, Titos E, Díaz A, Alcaraz-Quiles J, Horrillo R, Costa M, Fernández J, et al. Albumin protects the liver from tumor necrosis factor α-induced immunopathology. FASEB J. 2021;35(2):e21365. doi: 10.1096/fj.202001615RRR. [DOI] [PubMed] [Google Scholar]
- 2.Oda E. Decreased serum albumin predicts hypertension in a Japanese health screening population. Intern Med. 2014;53(7):655–660. doi: 10.2169/internalmedicine.53.1894. [DOI] [PubMed] [Google Scholar]
- 3.Rabbani G, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: a natural cargo. Int J Biol Macromol. 2019;123:979–990. doi: 10.1016/j.ijbiomac.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Liew A, Bavanandan S, Prasad N, Wong MG, Chang JM, Eiam-Ong S, Hao CM, Lim CY, Lim SK, Oh KH, et al. Asian pacific society of nephrology clinical practice guideline on diabetic kidney disease - an executive summary. Nephrology (Carlton) 2020;25(11):809–817. doi: 10.1111/nep.13804. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang XA, Wang DW. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63(11):1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Wu Y, Xu D, Xiao P, Xie B, Huang H, Shang Y, Yuan S, Zhang J. Very-short-term sleep deprivation slows early recovery of lymphocytes in septic patients. Front Med (Lausanne) 2021;8:656615. doi: 10.3389/fmed.2021.656615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjot T, Ray DW, Williams FR, Tomlinson JW, Armstrong MJ. Sleep and liver disease: a bidirectional relationship. Lancet Gastroenterol Hepatol. 2021;6(10):850–863. doi: 10.1016/S2468-1253(21)00169-2. [DOI] [PubMed] [Google Scholar]
- 8.Faure P, Tamisier R, Baguet JP, Favier A, Halimi S, Lévy P, Pépin JL. Impairment of serum albumin antioxidant properties in obstructive sleep apnoea syndrome. Eur Respir J. 2008;31(5):1046–1053. doi: 10.1183/09031936.00062707. [DOI] [PubMed] [Google Scholar]
- 9.Miyaaki H, Hiraoka A, Haraguchi M, Uojima H, Kawaratani H, Hiramatsu A, Hanai T, Hiasa Y, Yoshiji H, Okita K, et al. Proposal for new sleep disorder criteria in patients with chronic liver disease: Influence of liver-related complications. Hepatol Res. 2022;52(4):364–370. doi: 10.1111/hepr.13731. [DOI] [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 11.Ishida S, Hashimoto I, Seike T, Abe Y, Nakaya Y, Nakanishi H. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: a retrospective study. J Med Invest. 2014;61(3–4):361–368. doi: 10.2152/jmi.61.361. [DOI] [PubMed] [Google Scholar]
- 12.Bakour C, Schwartz S, O'Rourke K, Wang W, Sappenfield W, Couluris M, Chen H. Sleep duration trajectories and systemic inflammation in young adults: results from the national longitudinal study of adolescent to adult health (add health) Sleep. 2017;40(11):zsx156. doi: 10.1093/sleep/zsx156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olgun Yazar H, Yazar T, Özdemir S, Kasko Arici Y. Serum C-reactive protein/albumin ratio and restless legs syndrome. Sleep Med. 2019;58:61–65. doi: 10.1016/j.sleep.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Izci-Balserak B, Zhu B, Wang H, Bronas UG, Gooneratne NS. Independent associations between sleep duration, gamma gap, and cognitive function among older adults: results from the NHANES 2013–2014. Geriatr Nurs. 2022;44:1–7. doi: 10.1016/j.gerinurse.2021.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Adams Hillard PJ, Katz ES, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Mireku MO, Rodriguez A. Sleep duration and waking activities in relation to the national sleep foundation's recommendations: an analysis of US population sleep patterns from 2015 to 2017. Int J Environ Res Public Health. 2021;18(11):6154. doi: 10.3390/ijerph18116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao C, Yang L. Sex and racial/ethnic disparities in sleep duration and timing on weekdays and weekends across lifespan in the US population: sex and racial/ethnic disparities in sleep. Sleep Epidemiology. 2022;2:100026. doi: 10.1016/j.sleepe.2022.100026. [DOI] [Google Scholar]
- 18.Li Q. The association between sleep duration and excess body weight of the American adult population: a cross-sectional study of the national health and nutrition examination survey 2015–2016. BMC Public Health. 2021;21(1):335. doi: 10.1186/s12889-021-10369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMullan CJ, Curhan GC, Forman JP. Association of short sleep duration and rapid decline in renal function. Kidney Int. 2016;89(6):1324–1330. doi: 10.1016/j.kint.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Cai S, Ling Y, Mi S, Fan C, Zhong Y, Shen Q. Association between total sleep time and all cancer mortality: non-linear dose-response meta-analysis of cohort studies. Sleep Med. 2019;60:211–218. doi: 10.1016/j.sleep.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Zhao WY, Zhang Y, Jia SL, Ge ML, Hou LS, Xia X, Liu XL, Yue JR, Dong BR. The association of sleep quality and sleep duration with nutritional status in older adults: Findings from the WCHAT study. Maturitas. 2021;145:1–5. doi: 10.1016/j.maturitas.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Jansen EC, Prather A, Leung CW. Associations between sleep duration and dietary quality: results from a nationally-representative survey of US adults. Appetite. 2020;153:104748. doi: 10.1016/j.appet.2020.104748. [DOI] [PubMed] [Google Scholar]
- 23.Luyster FS, Shi X, Baniak LM, Morris JL, Chasens ER. Associations of sleep duration with patient-reported outcomes and health care use in US adults with asthma. Ann Allergy Asthma Immunol. 2020;125(3):319–324. doi: 10.1016/j.anai.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikbakhtian S, Reed AB, Obika BD, Morelli D, Cunningham AC, Aral M, et al. Accelerometer-derived sleep onset timing and cardiovascular disease incidence: a UK Biobank cohort study. European Heart Journal - Digital Health. 2021;2(4):658-666. [DOI] [PMC free article] [PubMed]
- 25.Hu C, Zhang Y, Wang S, Lin L, Peng K, Du R, Qi H, Zhang J, Wang T, Zhao Z, et al. Association of bedtime with the risk of non-alcoholic fatty liver disease among middle-aged and elderly Chinese adults with pre-diabetes and diabetes. Diabetes Metab Res Rev. 2020;36(6):e3322. doi: 10.1002/dmrr.3322. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Guo J, Hu L, Veronese N, Smith L, Yang L, Cao C. Association between intake of energy and macronutrients and memory impairment severity in US older adults, national health and nutrition examination survey 2011–2014. Nutrients. 2020;12(11):3559. doi: 10.3390/nu12113559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb EJ, Tomson CR, Roderick PJ. Estimating kidney function in adults using formulae. Ann Clin Biochem. 2005;42(Pt 5):321–345. doi: 10.1258/0004563054889936. [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Jia G, Tang S, Zheng P, Hu L. Association of low-level blood lead with serum uric acid in U.S. adolescents: a cross-sectional study. Environ Health. 2019;18(1):86. doi: 10.1186/s12940-019-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakada T, Kato T, Numabe Y. Effects of fatigue from sleep deprivation on experimental periodontitis in rats. J Periodontal Res. 2015;50(1):131–137. doi: 10.1111/jre.12189. [DOI] [PubMed] [Google Scholar]
- 30.Yu JH, Han K, Kim NH, Yoo HJ, Seo JA, Kim SG, Choi KM, Baik SH, Kim NH. U-shaped association between sleep duration and urinary albumin excretion in Korean adults: 2011–2014 Korea National Health and Nutrition Examination Survey. PLoS ONE. 2018;13(2):e0192980. doi: 10.1371/journal.pone.0192980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, Fan Y, Wieser A, Zhang J, Regel I, Nieß H, Mayerle J, Gerbes AL, Steib CJ. Albumin might attenuate bacteria-induced damage on kupffer cells for patients with chronic liver disease. Cells. 2021;10(9):2298. doi: 10.3390/cells10092298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smiley A, King D, Bidulescu A. The association between sleep duration and metabolic syndrome: the NHANES 2013/2014. Nutrients. 2019;11(11):2582. doi: 10.3390/nu11112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron KG, Reid KJ, Wolfe LF, Attarian H, Zee PC. Phase relationship between DLMO and sleep onset and the risk of metabolic disease among normal weight and overweight/obese adults. J Biol Rhythms. 2018;33(1):76–83. doi: 10.1177/0748730417745914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Wheaton AG, Chapman DP, Croft JB. Sleep duration and chronic diseases among US adults age 45 years and older: evidence from the 2010 behavioral risk factor surveillance system. Sleep. 2013;36(10):1421–1427. doi: 10.5665/sleep.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caraballo C, Mahajan S, Valero-Elizondo J, Massey D, Lu Y, Roy B, Riley C, Annapureddy AR, Murugiah K, Elumn J, et al. Evaluation of temporal trends in racial and ethnic disparities in sleep duration among us adults, 2004–2018. JAMA Netw Open. 2022;5(4):e226385. doi: 10.1001/jamanetworkopen.2022.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, Hall MH, Loredo JS, Mossavar-Rahmani Y, Ramos AR, et al. Comparison of self-reported sleep duration with actigraphy: results from the hispanic community health study/study of latinos sueño ancillary study. Am J Epidemiol. 2016;183(6):561–573. doi: 10.1093/aje/kwv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17(3):295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 38.Allebrandt KV, Teder-Laving M, Kantermann T, Peters A, Campbell H, Rudan I, Wilson JF, Metspalu A, Roenneberg T. Chronotype and sleep duration: the influence of season of assessment. Chronobiol Int. 2014;31(5):731–740. doi: 10.3109/07420528.2014.901347. [DOI] [PubMed] [Google Scholar]
- 39.Dunietz GL, Jansen EC, Hershner S, O'Brien LM, Peterson KE, Baylin A. Parallel assessment challenges in nutritional and sleep epidemiology. Am J Epidemiol. 2021;190(6):954–961. doi: 10.1093/aje/kwaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate analysis for albumin(g/L).
Additional file 2: Table S2. Effect size of sleep duration on albumin in prespecified and exploratory subgroups.
Data Availability Statement
The datasets generated and analysed for the current study are available in the NHANES repository. These data can be accessed using the following link: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.