SUMMARY
We developed a new phage-typing method and evaluated its application in combination with XbaI macrorestriction analysis by pulsed-field gel electrophoresis (PFGE) as a useful tool for the long-term epidemiology of Salmonella enterica serovar Infantis. In this study, we investigated 1008 S. Infantis isolates recovered from humans, various animal species and food products from 1973 to 2009. The typing scheme is based on 17 typing phages, defining 61 different patterns within the strain collection. The experiments showed that phage typing is a reliable method for differentiation of outbreaks and sporadic clinical cases as well as for elucidation of chains of transmission. The combined analysis of phage typing and PFGE revealed the existence of epidemic clones with a high stability over time like PT29/XB27 which was identified in nosocomial salmonellosis, community outbreaks as well as in broiler chickens from 2002 to 2009.
Key words: ESBL, macrorestriction analysis, outbreak investigations, phage typing, S. Infantis
INTRODUCTION
Salmonella enterica serovar Infantis (S. Infantis) primarily causes gastroenteritis in adults, but in newborns and small children serious infections with lethal outcome are also reported [1, 2]. Of special concern is its ability to persist in hospitals for long periods posing significant public-health problems [3–5]. Current trends in the epidemiology of S. Infantis show that it is increasingly involved in human infections in several countries, e.g. Hungary, Israel and Japan [6–8]. In Germany, S. Infantis is the third most common serovar after S. Enteritidis and S. Typhimurium in human infections in recent years [9].
The reservoirs for salmonellosis in humans are primarily seen in animals and asymptomatic human carriers associated with food preparation [10]. The European Union (EU) baseline survey on the prevalence of Salmonella in broiler flocks for 2005–2006 revealed that S. Enteritidis (37%) and S. Infantis (20%) were the most frequently isolated serovars [11]. Further studies of the European Food Safety Authority (EFSA) from 2008 to 2010 showed that S. Infantis was the most frequently reported serovar in broiler meat in the EU [12]. In Germany S. Infantis was mostly detected in broilers and in slaughter-age pigs [11–13]. According to regulation EU No. 517/2011 S. Infantis belongs to the zoonotic agents, which are targeted for reduction in breeding flocks of Gallus gallus in the European Union in the near future [14]. These facts stress the risk that S. Infantis of animal origin represents for human health and underlines the importance of achieving a better understanding of the epidemiology of S. Infantis infections.
Epidemiological typing methods are essential for identifying the source of infection and transmission pathways as well as for routine surveillance of epidemic S. Infantis strains. Common typing methods used include traditional serotyping and antimicrobial susceptibility testing as well as a range of molecular methods developed more recently [15]. Of these methods macrorestriction analysis by pulsed-field gel electrophoresis (PFGE) is accepted as the ‘gold standard’ for Salmonella subtyping due to its excellent discriminatory power for surveillance and outbreak investigations. In addition, phage typing is an established method for Salmonella monitoring used throughout the world. The successful application of phage typing for the detection of outbreaks as well as for source attribution or transmission of epidemic strains has been demonstrated in different studies [16, 17].
The aim of our study was the development of novel phage-typing scheme for S. Infantis and assessment of its application in combination with PFGE analysis as a tool for routine typing.
MATERIALS AND METHODS
Bacterial strains
From 1973 to 2009 medical microbiology and veterinary laboratories throughout Germany sent 2746 S. Infantis strains to the National Reference Centre for Salmonella (NRC). Identification of S. Infantis was confirmed at the NRC by serotyping according to the White–Kauffmann–Le Minor scheme [18] by slide agglutination with O and H antigen-specific sera (Sifin Diagnostics, Germany) [19]. For this study we selected randomly 1008 isolates for further characterization and typing. The collection included isolates from foodborne outbreaks (187), sporadic cases (327), broiler chicken carcasses (199), pigs (71), cattle (39), layer hens (16), turkeys (2), ducks (4), geese (1), wild animals (16), dogs (6), cake (2), raw sausage (5), chicken meat (3), pork (26), beef (2), food ([16], without further information), feed (21), environmental samples (59) and autogenous vaccines for poultry (6). Isolates of German broiler chicken carcasses (n = 169, 85%) were sampled from slaughterhouses throughout Germany from 1996 to 2009. Isolates of Hungarian broiler chicken carcasses (n = 30, 15%; transported from Hungary to Germany) were originally sampled from three slaughterhouses in the German Federal States of North Rhine-Westphalia, Lower Saxony and Brandenburg during 2003–2009.
Antimicrobial susceptibility testing
All S. Infantis isolates were subjected to antimicrobial susceptibility testing against 14 antimicrobial substances (Sigma-Aldrich, Germany) as follows: ampicillin (AMP), mezlocillin (MEZ), cefotaxime (CTX), ceftazidime (CAZ), cefoxitin (FOX), gentamicin (GEN), amikacin (AMK), streptomycin (STR), nalidixic acid (NAL), chloramphenicol (CHL), oxytetracycline (OTE), ciprofloxacin (CIP), sulfameracin (SMZ) and sulfamethoxazole-trimethoprim (SXT) by broth microdilution according the DIN 58940 method [20]. Multidrug resistance was defined as resistance to three or more antimicrobial groups (quinolones, tetracyclines, β-lactams, aminoglycosides, sulfonamide, chloramphenicol). In strains resistant to third-generation cephalosporins (cefotaxime and/or ceftazidime) the production of extended-spectrum β-lactamases (ESBL) was confirmed by Etest® ESBL strips (bioMérieux, Germany) according to the manufacturer's instructions.
Isolation of typing phages and development of a phage-typing scheme
Fourteen typing phages were directly isolated from 14/123 lysogenic strains of S. Infantis by mitomycin (Sigma-Aldrich, Germany) induction [21]. Strains were cultivated at 37°C for 5 h with agitation in 10 ml double-strength nutrient broth (Becton Dickinson GmbH, Germany) containing 2·5 μg/ml mitomycin. After centrifugation (10 000 g for 10 min) the supernatants were carefully removed and sterilized by passage through a Millipore filter (0·2 μm pore size). In order to extend the typing scheme, three additional typing phages were obtained by adaptation of three phages (nos. 12, 16, 17) on three different strains of S. Infantis [22, 23]. Briefly, several dilutions of the phage were spotted on different S. Infantis strains plated on nutrient agar (Difco, USA). From each culture a single plaque was removed and inoculated into 3 ml nutrient broth, followed by incubation for 5 h at 37 °C. The propagated phages, which represent new lines of phages adapted by restriction/modification or phage module exchange, were obtained from the culture supernatant and titrated on the respective sensitive strains. Routine test dilutions of each typing phage were applied to nutrient Difco agar plates with a lawn of the respective reference strains using a multipoint inoculator. They were incubated for 17 h at 37°C until phage-induced lysis could be observed.
PFGE
Out of the 1008 selected S. Infantis strains 325 isolates (1999–2009) belonging to 31 frequent phage types were selected for further subtyping. These isolates from different origins [humans (206), broiler chicken carcasses (45), layer hens (10), pigs (8), cattle (5), pork (8), chicken meat (3), beef (1), raw sausage (4), cake (2), food ([11], without further information), wild animals (3), feed (6) and environmental samples (13)] were typed according to the CDC PulseNet protocol as described by Hunter et al. [24] using the restriction enzyme XbaI (Roche, Germany) and a CHEF DRIII instrument (Bio-Rad, Germany). Gel images were evaluated using BioNumerics, version 5.1 (Applied Maths, Belgium), and compared by similarity clustering using the unweighted pair-group matching algorithm (UPGMA) and the Dice correlation coefficient with a tolerance of 1·0% and an optimization of 1·5%. The PFGE patterns were designated arbitrarily by numbering according to Barrett et al. [25]; letters (a, b, c) in addition to the numbers were used to designate closely ‘related patterns’ differing only by one or two fragments from the primary pattern.
Designation of clones
Clones were designated by a number consisting of the phage type (PT01, PT02, etc.) and PFGE patterns type (XB01, XB02, etc.). For example, clone PT01/XB34 has the phage type designated PT01 and the PFGE pattern XB34.
Statistical analysis
Typability, i.e. the percentage of strains that could be assigned to a distinct phage type (e.g. PT01, PT02, etc.) was calculated as described by Struelens [26]. To evaluate the discriminatory power of phage typing and PFGE, Simpson's diversity index (DI), 95% confidence intervals (CI) and Wallace's index were calculated using Ridom EpiCompare software version 1.0 (Ridom GmbH, Germany).
RESULTS
Phage typing
The final typing scheme was established using 17 typing phages (Supplementary Table S1). In this study, we achieved a typability of 98% by the novel phage-typing scheme when typing more than 1000 isolates. Twenty-three (2%) isolates were untypable. Of the 985 isolates, 61 different phage types (DI 86·4, 95% CI 84·2–87·1) were identified. Table 1 shows the distribution of the detected phage types in various sources. The most prevalent phage types: PT29 (28%), PT01 (20%), PT11 (7%), PT09 (7%), PT24 (6%), PT04 (4%) and PT08 (3%) were found in humans and food as well as in animals. Interestingly, these frequent phage types have been detected in the majority of human sporadic cases (n = 228, 23%) as well as outbreaks (n = 168, 17%). Different phage types (e.g. PT01, PT09, PT11, PT29) have emerged in humans between 1973 and 2009, but only two phage types (PT01, PT29) account for the majority of clinical cases and most outbreaks of salmonellosis. During the 36-year study period a change in dominant phage types, e.g. PT01, PT09 and PT29 could be not observed.
Table 1.
Distribution of phage types of Salmonella Infantis strains isolated from various sources
| Source | Phage type |
|---|---|
| Humans (n = 514) | 1, 4, 8, 9, 11, 19, 21, 24, 29, 30, 33, 38, 51, 53, 57, 59, 60, nt |
| Broilers* (n = 199) | 1, 4, 6, 8, 9, 10, 11, 14, 15, 17, 24, 25, 26, 29, 32, 33, 35, 38, 41, 46, 49, 58, 61 |
| Chicken meat (n = 3) | 9, 11, 24 |
| Layer hens (n = 16) | 8, 10, 29, 30, 45, nt |
| Pigs (n = 71) | 1, 2, 3, 5, 8, 9, 11, 21, 24, 25, 29, 30, 33, 39, 55, 61, nt |
| Pork (n = 26) | 1, 2, 4, 8, 11, 21, 24, 29 |
| Cattle (n = 39) | 1, 9, 11, 18, 24, 29 |
| Beef (n = 2) | 29, 39 |
| Turkeys (n = 2) | 8, 29 |
| Ducks (n = 4) | 61 |
| Geese (n = 1) | 29 |
| Wild animal (n = 16) | 1, 4, 5, 8, 9, 15, 17, 40, nt |
| Dogs (n = 6) | 8, 9, 10, 11, 29, 41 |
| Feed (n = 21) | 1, 9, 10, 11, 13, 24, 29, 41, 42, 53 |
| Environment (n = 59) | 1, 4, 5, 8, 9, 10, 11, 15, 23, 24, 26, 28, 29, 30, 33, 43, nt |
| Cake (n = 2) | 53 |
| Raw sausage (n = 5) | 1, 29, 33 |
| Food† (n = 16) | 1, 8, 9, 11, 19, 26, 29, 53 |
| Vaccine‡ (n = 6) | 11, 26, 47, 54, 56 |
nt, Non-typable by the present phage-typing scheme.
Bold values indicate the occurrence of some phage type in diverse sources.
Isolates from broiler chicken carcasses.
Isolates without further information.
Isolates from autogenous vaccines for poultry.
Combination of PFGE and phage typing
The analysis of the selected 325 isolates revealed 58 different PFGE types (DI 95·0, 95% CI 85·2–97·5) and 31 defined phage types (DI 82·4, 95% CI 72·1–89·0). Supplementary Table S2 shows the distribution of the 325 isolates for each PFGE type and phage type. Cluster analysis of the 58 PFGE profiles revealed a similarity coefficient of 0·45. The resulting concordance showed that all outbreak isolates (n = 89, 27%) and the majority of sporadic isolates (n = 150, 46%) could be assigned to concordant phage types and PFGE types (Fig. 1). The probability of a pair of isolates with the same phage type also sharing the corresponding PFGE type was 71% (Wallace's index 0·71). Isolates of the three predominant PFGE types (XB05, XB27, XB34) showing an association with a certain phage type, e.g. isolates of XB34 (n = 25) were assigned to the same phage type (PT01) (Supplementary Table S2). On the other hand, the results confirmed the ability of phage typing to further discriminate several frequent PFGE types, e.g. XB04, XB06 and XB16 (Supplementary Table S2). One example is isolates of PT53 (n = 11) and PT29 (n = 8) which were involved in two outbreaks but share a single PFGE type (XB06). Since 27 infrequent PFGE types (e.g. XB01, XB12) were represented by only single isolates the potential ability of phage typing to further discriminate within these PFGE types could not be evaluated.
Fig. 1.
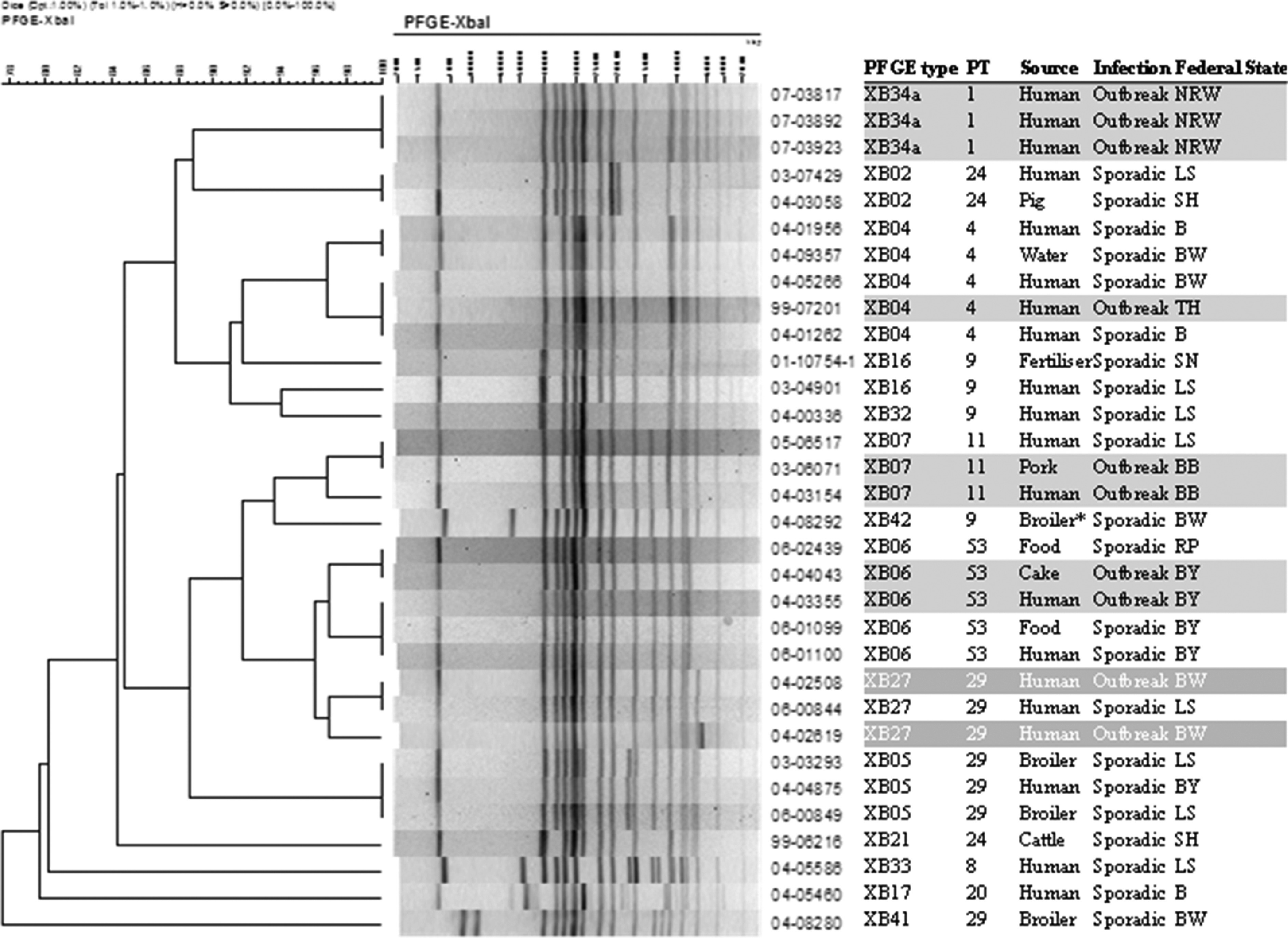
Macrorestriction patterns of 32 Salmonella Infantis isolates obtained with the restriction enzyme XbaI. The dendrogram shows the relationship of different XbaI patterns. Similarity analysis was performed using the Dice coefficient and the clustering was generated by UPGMA. S. Infantis isolates associated with outbreaks or sporadic cases could be assigned to a single combination of phage – and PFGE type (highlighted in grey). * Isolates from broiler chicken carcasses.
Isolates belonging to clones PT29/XB27 (13%), PT29/XB05 (10%), PT01/XB34 (8%), PT53/XB06 (6%), PT24/XB02 (6%), PT29/XB27a (5%), PT04/XB04 (5%), and PT11/XB07 (5%) were epidemiologically predominant and found in humans, food, broiler chickens (Germany, Hungary) as well as in isolates from various animal species (Supplementary Table S3). Within the German broiler chicken isolates 11 clones, e.g. PT29/XB05 (n = 15), PT29/XB27 (n = 4), PT29/XB27a (n = 3) and PT11/XB07 (n = 3) were found whereas in Hungarian broiler chicken isolates only four clones, e.g. PT29/XB05 (n = 8), PT29/XB14a (n = 1), PT29/XB05a (n = 1) and PT46/XB07a (n = 1) could be detected.
Outbreak investigations
We typed 51 foodborne outbreaks with PT29/XB27 as the most prominent clone (Table 2, Supplementary Table S3). Clone PT29/XB27 was identified in hospital outbreaks, community outbreaks and in broiler chickens during 2002–2009. This clone was repeatedly detected in a rehabilitation clinic in Baden-Württemberg from 2002 to 2009 (Fig. 1). According to information from the clinic several episodes of S. Infantis gastroenteritis had occurred in this clinic but only in summertime, and this persisted for about 3–4 weeks every year. The sources of the latest infection in 2009 were a cross-contamination in the kitchen and carriers among the clinic personnel. These findings demonstrate the stability of the phage and PFGE types over a long period of time thereby supporting the applicability of the approach to outbreak investigations.
Table 2.
Fifty-one foodborne outbreaks in Germany, 1974–2009
| Federal state | Year | Type of outbreak | Source | Phage type and/or PFGE type | ||
|---|---|---|---|---|---|---|
| Family | Community | Nosocomial | ||||
| Baden-Württemberg | 2002 | x | —* | PT24 | ||
| 2002 | x | — | PT29/XB27 | |||
| 2003 | x | — | PT29/XB27 | |||
| 2004 | x | — | PT29/XB27 | |||
| 2007 | x | — | PT29/XB27 | |||
| 2008 | x | — | PT29/XB27 | |||
| 2009 | x | Food or carrier | PT29/XB27 | |||
| Bavaria | 1997 | x | Raw sausage | PT09 | ||
| 2004 | x | Cake or carrier | PT53/XB06 | |||
| 2004 | x | Cake or carrier | PT53/XB06 | |||
| Brandenburg | 2004 | x | Pork | PT11/XB07 | ||
| Berlin | 2002 | x | — | PT01 | ||
| Hesse | 1975 | x | — | PT23 | ||
| 1997 | x | — | PT33 | |||
| Mecklenburg-West Pomerania | 1998 | x | — | PT29 | ||
| 1999 | x | — | PT01 | |||
| 1999 | x | — | PT01 | |||
| 2002 | x | — | PT01 | |||
| Lower Saxony | 1974 | x | Rice pudding | PT01 | ||
| 1998 | x | Raw eggs | PT08/XB43a | |||
| 2002 | x | — | PT29 | |||
| 2006 | x | — | PT29 | |||
| 2007 | x | — | PT29/XB06 | |||
| 2007 | x | — | PT29/XB27a | |||
| North Rhine-Westphalia | 1996 | x | — | PT29 | ||
| 1999 | x | — | PT01 | |||
| 2000 | x | — | PT24/XB02 | |||
| 2007 | x | Carrier or roast pork | PT01/XB34a | |||
| 2008 | x | — | PT21/XB01a | |||
| 2008 | x | Broiler chicken | PT01/XB34 | |||
| Saxony-Anhalt | 1996 | x | Carrier | PT29 | ||
| 1997 | x | — | PT30 | |||
| 2001 | x | — | PT01 | |||
| 2001 | x | — | PT01 | |||
| 2002 | x | — | PT01 | |||
| 2003 | x | — | PT11 | |||
| 2004 | x | Carrier | PT29 | |||
| 2005 | x | — | PT24/XB02 | |||
| 2005 | x | Carrier | PT30 | |||
| 2007 | x | — | PT29/XB06 | |||
| Schleswig-Holstein | 2003 | x | — | PT01 | ||
| Thuringia | 1999 | x | — | PT04/XB04 | ||
| 2000 | x | — | PT19 | |||
| 2000 | x | — | PT09 | |||
| 2002 | x | — | PT11 | |||
| 2003 | x | — | PT24 | |||
| 2006 | x | — | PT29/XB27 | |||
| 2007 | x | — | PT29/XB27a | |||
| 2007 | x | Carrier | PT29/XB27 | |||
| 2007 | x | — | PT29/XB27b | |||
| 2008 | x | — | PT29/XB27a | |||
Unknown source.
Two large outbreaks with 188 reported cases in two clinics (distance apart 130 km) were observed in Bavaria in 2004 and traced back to bakery products (Fig. 1). All isolates obtained from humans and food could be identified as an identical clone PT53/XB06 (Table 2, Supplementary Table S3). Two other outbreaks in North Rhine-Westphalia in 2007 and 2008 caused by contaminated roast pork and chicken kebabs were assigned to the related clones (PT01/XB34a, PT01/XB34) (Fig. 1). Clone PT01/XB34 was also detected in isolates of broiler chickens (n = 1), pigs (n = 2), cattle (n = 1), pork (n = 2), and raw sausage (n = 1). Moreover, 11 S. Infantis infections in three German Federal States: Thuringia, North Rhine-Westphalia and Lower Saxony were caused by clone PT29/XB27a; this clone was also found in three samples of broiler chicken (Supplementary Table S3). Other outbreak clones (PT11/XB07, PT21/XB01a, PT24/XB02) were also indentified in pork.
Antimicrobial susceptibility testing
The majority (809/1008, 80%) of the investigated isolates were susceptible (n = 317), resistant to one (n = 393) or resistant at least to two (n = 99) of the 14 tested antimicrobial substances. The most frequent epidemic clones PT29/XB27 and PT01/XB34 were resistant only to one or two antimicrobial groups. Moreover, 199 (20%) isolates were multidrug-resistant. Notably, multidrug-resistant clones PT11/XB07 and PT29/XB27a were resistant to three antimicrobial groups (AMP-MEZ-OTE-SMZ). In this study we observed an increase of resistance to β-lactams, sulfonamides, tetracyclines, aminoglycosides and quinolones in 93 isolates of broiler chickens since 2002. The predominant multidrug-resistant clone (31 isolates; NAL-STR-SMZ-OTE) was PT29/XB05. Only four isolates of the present study (two from humans and two from broiler chickens) were resistant to cefotaxime or ceftazidime. In these isolates ESBL production was confirmed by Etest® ESBL (bioMérieux).
DISCUSSION
The present study demonstrates that the combination of a newly developed phage-typing scheme and XbaI macrorestriction analysis is a reliable epidemiological tool for the routine typing of S. Infantis isolates at the NRC for Salmonella. We were able to type 985/1008 isolates with the established phage scheme, resulting in 61 different phage types. Previously only two phage-typing schemes existed for S. Infantis and these were developed more than 20 years ago by Laszlo et al. [27] and by Kasatiya et al. [28] in Hungary and Canada, respectively. However, in both typing systems several virulent phages or/and temperate phages were selected that cross-reacted with strains of different enterobacterial species as well as several Salmonella serovars (e.g. S. Thomson, S. Newport, S. Cerro). In contrast, phages of S. Infantis that we isolated for the new phage scheme are highly specific; they enable real-time monitoring of S. Infantis infections, and the results could be confirmed by PFGE analysis as well as by the study of Hauser et al. [17].
Phage typing demonstrated a high level of diversity in random isolates (61 phage types) and was also confirmed by results based on PFGE analysis with a low similarity coefficient of 0·45. In contrast, Hauser et al. [17] reported that the lowest observed similarity coefficient between strains was 0·72. Such heterogeneity between studies might be caused by differences in selection criteria of investigated isolates, e.g. variety of sources, number of isolates, and period of time. The combined typing showed the emergence of epidemic clones in humans (PT29/XB27, PT01/XB34) and the occurrence of the specific clone PT29/XB05 which is mainly associated with broiler chicken (Supplementary Table S3). These epidemic clones could be detected over many years in Germany indicating a highly genetic stability of these clones. Hauser et al. [17] reported that the S. Infantis clone PT29/XINF10 was predominantly found in Germany in humans, pigs, broiler chickens, and broiler meat. This clone, PT29/XINF10, is equivalent to clone PT29/XB27, according to the present study. However, at the moment there is no clear answer why this most dominant broiler chicken clone PT29/XB05 causes salmonellosis relative rarely (only four human cases). In contrast, other outbreak clones (e.g. PT01/XB34, PT29/XB27a, PT11/XB07) were also found in livestock animals or meat products (Supplementary Table S3). These findings indicate that broiler chickens or other contaminated foods are probable sources of S. Infantis infections in humans. A direct confirmation of the source of infection was possible for five outbreaks within the present study (Table 2).
Recent studies of the EFSA have shown that the serovar S. Infantis is very common in poultry flocks in the EU [11]. Furthermore, several studies suggest the occurrence of country-specific clones disseminated in humans and broilers in Hungary, Israel, Germany and Japan [6, 7, 17, 29]. Interestingly, clone PT29/XB05 was most prevalent in broiler chicken isolates from Germany and Hungary. Due to a lack of complete information on the broiler chicken strains that were sent to the NRC it is not entirely clear if this clone is in fact disseminated in German broiler flocks or if the strains transmitted in Germany are through the import of Hungarian broilers since 2003.
Two of the most prevalent clones, PT29/XB05 and PT29/XB27, are indistinguishable by phage typing but clone PT29/XB05 shows two additional bands of 70 kbp and 170 kbp in macrorestriction pattern. This is probably due to the presence of plasmids harbouring resistance genes resulting in additional resistance of clone PT29/XB05 to NAL-STR-SMZ-OTE. Corresponding to these results Nógrády et al. [6] reported recently that S. Infantis clones PT213 and PT217 were commonly found in Hungary in humans as well as in broilers. In the phage-typing scheme of Laszlo et al. [27], PT213 is equivalent to PT29 according to the phage-typing scheme presented in this work. Furthermore, results of other studies indicated the dissemination of an epidemic multidrug-resistant clone of S. Infantis from broilers in Germany, Hungary, Austria, and Poland [30]. However, it can only be hypothesized that the multidrug-resistant clone PT29/XB05 in the present study corresponds to the multidrug-resistant clone described by Nógrády et al. [30]. The presence of a plasmid conferring multidrug resistance could have facilitated the rapid spread of this multidrug-resistant clone in humans and livestock. Therefore, a continuous surveillance, especially of the multidrug-resistant clone PT29/XB05, is recommended.
Notably, four S. Infantis isolates from humans and broiler chickens belonging to epidemic clones PT01/XB34 and PT29/XB27 were found to be resistant to ceftazidime and cefotaxime due to ESBL production, although the use of cephalosporin in broilers has not been approved in Germany [31]. A possible reason for this might be the transfer of resistance plasmids from E. coli to S. Infantis since recently a high prevalence of ESBL has been described for E. coli from poultry in The Netherlands and Germany [32, 33]. ESBL-producing S. Infantis isolates have been increasingly reported as a source of nosocomial outbreaks posing a serious threat especially in paediatric and neonatology wards [5]. Although outbreaks with ESBL-producing S. Infantis are not known in Germany, continuous surveillance is required for early detection of these multidrug-resistant isolates.
The successful application of novel phage typing combined with PFGE analysis is demonstrated in the detailed analysis of outbreaks in a rehabilitation clinic in Baden-Württemberg diring 2002–2009. The cause of these outbreaks was the epidemic clone PT29/XB27, which was repeatedly identified over a period of 8 years. Retrospective investigations revealed that S. Infantis infections had been observed in this clinic since 1992. However, since, no patient isolates were preserved before 2002 it can only be hypothesized that clone PT29/XB27 has been endemic in the clinic for more than 8 years. Previous studies have also shown the ability of S. Infantis to persist in hospitals but the time spans reported were shorter than in the outbreaks described here [3, 4]. Possible explanations for the repeated isolation of clone PT29/XB27 might be readmission of carrier patients or repeated re-introduction by clinic personnel or contaminated food. A study of Murakami et al. [10] implied that asymptomatic carriers of S. Infantis associated with food preparation are a possible reservoir for infections. However, the potential duration of carriage is currently uncertain.
In conclusion, it has been shown that a novel phage-typing scheme is suitable for identification of outbreaks of S. Infantis as well as for long-term surveillance in Germany. The combined use of phage typing and PFGE analysis has proven to be an advantageous method for S. Infantis strains. The combined typing facilitates the detailed investigation of the dissemination of epidemic S. Infantis clones in humans, food, and livestock nationwide and internationally.
Supplementary Material
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Acknowledgements
We especially thank Professor Gerald-Friedrich Gerlach for valuable discussion and comments on the final draft of the manuscript. We thank Heidemarie Gattermann, Susanne Kulbe, Gerlinde Bartel, Erika Kleindienst, and Dorothea Eitze for excellent assistance. Furthermore, we thank Burkhard Malorny and Andreas Schroeter for providing the bacterial strains. This work was funded by the Ministry of Health, Germany. Tatjana Miller was supported by a scholarship from German Konrad Adenauer-Stiftung.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S095026881300037X.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hasenson L, et al. Epidemiological and microbiological studies on salmonellosis in Russia. Zentralblatt für Hygiene Umweltmedizin 1995; 198: 97–116. [PubMed] [Google Scholar]
- 2.Moraes BA, et al. Epidemiological analysis of bacterial strains involved in hospital infection in a university hospital from Brazil. Revista do Instituto de Medicina Tropical de São Paulo 2000; 42: 201–207. [DOI] [PubMed] [Google Scholar]
- 3.Pessoa-Silva CL, et al. Infection due to extended-spectrum beta-lactamase-producing Salmonella enterica subsp. enterica serotype infantis in a neonatal unit. Journal of Pediatrics 2002; 141: 381–387. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca EL, et al. Clonality and antimicrobial resistance gene profiles of multidrug-resistant Salmonella enterica serovar infantis isolates from four public hospitals in Rio de Janeiro, Brazil. Journal of Clinical Microbiology 2006; 44: 2767–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naas T, et al. Outbreak of Salmonella enterica serotype Infantis producing ArmA 16S RNA methylase and CTX-M-15 extended-spectrum β-lactamase in a neonatology ward in Constantine, Algeria. International Journal of Antimicrobial Agents 2011; 38: 135–139. [DOI] [PubMed] [Google Scholar]
- 6.Nógrády N, et al. Emergence of multidrug-resistant clones of Salmonella Infantis in broiler chickens and humans in Hungary. Journal of Antimicrobial Chemotherapy 2007; 60: 645–648. [DOI] [PubMed] [Google Scholar]
- 7.Bassal R, et al. Recent trends in the epidemiology of non-typhoidal Salmonella in Israel, 1999–2009. Epidemiology and Infection 2012; 140: 1446–1453. [DOI] [PubMed] [Google Scholar]
- 8.Iwabuchi E, et al. Prevalence of Salmonella isolates and antimicrobial resistance patterns in chicken meat throughout Japan. Journal of Food Protection 2011; 2: 270–273. [DOI] [PubMed] [Google Scholar]
- 9.Miller T, et al. Epidemiological relationship between S. Infantis isolates of human and broiler origin. Lohmann Information 2010; 2: 27–31. [Google Scholar]
- 10.Murakami K, et al. Features of Salmonella serovars among food handlers in Kyushu, Japan. New Microbiologica 2007; 30: 155–159. [PubMed] [Google Scholar]
- 11.European Food Safety Authority. Report of the task force on zoonoses data collection on the analysis of the baseline survey on the prevalence of Salmonella in broiler flocks of Gallus gallus. EFSA Journal 2007; 98: 1–85. [Google Scholar]
- 12.European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA Journal 2012; 10: 440–442. [Google Scholar]
- 13.European Food Safety Authority. Report of the task force on zoonoses data collection on the analysis of the baseline survey on the prevalence of Salmonella in slaughter pigs. EFSA Journal 2008; 135: 1–111. [Google Scholar]
- 14.European Union Council Regulation. Regulation No. 517/2011 of 25 May 2011 implementing council regulation No. 2160/2003 of the European Parliament and the council with regard to a target of the European Union to reduce the prevalence of certain Salmonella serotypes in laying hens of Gallus gallus and amending regulation No. 2160/2003 and regulation (EU) No. 200/2010 of implementing council. Official Journal of the European Union 2011; 138: 45–51. [Google Scholar]
- 15.Ross IL, Heuzenroeder MW. A comparison of three molecular typing methods for the discrimination of Salmonella enterica serovar Infantis. FEMS Immunology and Medical Microbiology 2008; 53: 375–384. [DOI] [PubMed] [Google Scholar]
- 16.Rabsch W, et al. Molecular epidemiology of Salmonella enterica serovar Agona: characterisation of a diffuse outbreak caused by aniseed-fennel-caraway infusion. Epidemiology and Infection 2005; 133: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser E, et al. Clonal dissemination of Salmonella enterica serovar Infantis in Germany. Foodborne Pathogens and Disease 2012; 9: 352–360. [DOI] [PubMed] [Google Scholar]
- 18.Grimont PAD, Weill FX. Antigenic formulae of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella. Institute Pasteur, 2007; 9: 166. [Google Scholar]
- 19.Shipp CR, Rowe B. A mechanised microtechnique for Salmonella serotyping. Journal of Clinical Pathology 1980; 33: 595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Standards. DIN 58940. Medical microbiology susceptibility testing of pathogens to antimicrobial agents. Part 8. Microdilution. General method specific requirements. In: DIN-Taschenbuch 222: Medizinische Mikrobiologie und Immunologie-Diagnostische Verfahren. Beuth-Verlag: 2004; 36: S342–353. [Google Scholar]
- 21.Miller T, Development of a phage typing system for Salmonella Infantis for epidemiological purposes (dissertation). Leipzig, Germany: University of Leipzig, 2009, 38 pp. [Google Scholar]
- 22.Ward LR, de Sa JD, Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiology and Infection 1987; 99: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schicklmaier P, et al. A comparative study on the frequency of prophages among natural isolates of Salmonella and Escherichia coli with emphasis on generalised transducers. Antonie Van Leeuwenhoek 1998; 73: 49–54. [DOI] [PubMed] [Google Scholar]
- 24.Hunter SB, et al. Establishment of a universal size standard strain for use with the PulseNet standardised pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. Journal of Clinical Microbiology 2005; 43: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett TJ, Gerner-Smidt P, Swaminathan B. Interpretation of pulsed-field gel electrophoresis patterns in foodborne disease investigations and surveillance. Foodborne Pathogens and Disease 2006; 3: 20–31. [DOI] [PubMed] [Google Scholar]
- 26.Struelens MJ. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clinical Microbiology and Infection 1996; 2: 2–11. [DOI] [PubMed] [Google Scholar]
- 27.Laszlo VG, Csak K, Csorian ES. A phage typing system for Salmonella infantis. Acta Microbiologica Hungarica 1988; 35: 55–69. [PubMed] [Google Scholar]
- 28.Kasatiya S, Caprioli T, Champoux S. Bacteriophage typing scheme for Salmonella infantis. Journal of Clinical Microbiology 1979; 10: 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda T, et al. Chicken meat is an infection source of Salmonella serovar Infantis for humans in Japan. Foodborne Pathogens and Disease 2010; 7: 727–735. [DOI] [PubMed] [Google Scholar]
- 30.Nógrády N, et al. Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. International Journal of Food Microbiology 2012; 157: 108–112. [DOI] [PubMed] [Google Scholar]
- 31.European Union Council Regulation. Regulation No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Official Journal of the European Union L15 2010; 23: 53–55. [Google Scholar]
- 32.Overdevest I, et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerging infectious diseases 2011; 17: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kola A, et al. High prevalence of extended-spectrum-ß-lactamase-producing Enterobacteriaceae in organic and conventional retail chicken meat, Germany. Journal of Antimicrobial Chemotherapy. Published online: 6 August 2012. doi: 10.1093/jac/dks295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S095026881300037X.
click here to view supplementary material


