Abstract
Objective:
Although gabapentin has demonstrated efficacy in mitigating alcohol withdrawal symptoms and preventing relapse drinking in individuals with Alcohol Use Disorder (AUD), the neurobiological mechanisms of action underlying these therapeutic effects remain unknown. The present study evaluated dorsal anterior cingulate cortex (dACC) GABA and glutamate changes as candidate mechanisms of action within a 16-week randomized clinical trial of gabapentin for AUD.
Methods:
Sixty-eight adults with AUD, including a history of Alcohol Withdrawal Syndrome, received gabapentin 1200mg/d (n=37) or placebo (n=31) and 9 medical-management visits following ≥72-hours of abstinence. Proton Magnetic Resonance Spectroscopy (1H-MRS) estimates of dACC GABA (n=67) and glutamate (n=64) levels were acquired at pretreatment and approximately 14-days post-randomization. Percent days abstinent (PDA) were reported via Timeline-Followback interview.
Results:
Effects of gabapentin on GABA and glutamate levels depended on participants’ PDA during early treatment (GABA: β=−0.48, p=0.002; glutamate: β=1.68, p=0.005). Specifically, gabapentin was associated with, 1) greater increases in glutamate and greater decreases in GABA levels in participants who remained mostly/entirely abstinent, yet 2) the opposite in participants who drank >1/2 of the days preceding the second scan. Furthermore, gabapentin-treated participants with greater increases in glutamate levels during early treatment had more PDA across the remainder of the study, relative to placebo-treated participants (β=0.30, p< 0.006).
Conclusions:
In addition to providing insight into the mechanisms through which gabapentin may promote abstinence in individuals with AUD, the present study also provides evidence for a biomarker of efficacious treatment that may be used to evaluate other glutamatergic and/or GABAergic medications for AUD and related conditions.
INTRODUCTION
Gabapentin, a safe and well-tolerated medication that is FDA-approved to treat post-herpetic neuralgia, partial seizures, and restless-leg syndrome, has garnered considerable attention for its efficacy in mitigating alcohol withdrawal symptoms (1) and preventing relapse drinking in individuals with Alcohol Use Disorder (AUD) (1–3). Though much research has focused on the neurobiological mechanisms of gabapentin’s (“on-label”) anticonvulsant, anxiolytic, and analgesic effects, few studies have focused on the mechanisms of gabapentin’s (“off-label”) therapeutic effects in AUD, which likely diverge due to the interacting effects of drinking and withdrawal on the brain (4). The present study represents the first controlled investigation of the neurobiological mechanisms of gabapentin in people with AUD.
Although gabapentin was designed as a structural analog to γ-Aminobutyric Acid (GABA), it is inert at GABA receptors and synapses (5). Instead, gabapentin binds with high affinity to the α2δ−1 protein and exerts its primary therapeutic effects through selective blockade of presynaptic voltage-gated calcium channels containing the α2δ−1 subunit (5). Recent research has revealed that gabapentin’s therapeutic effects may also involve, 1) α2δ−1 interaction with non-calcium-channel proteins including N-methyl-D-aspartate (NMDA) receptors, neurexin-1α, and thrombospondin (6), as well as 2) α2δ−1-independent mechanisms, including activation of KCNQ3/5 voltage-gated potassium channels (7) and expression of δ-subunit-containing GABAA receptors (8). Each of these molecular-mechanisms converge to reduce excitatory/glutamatergic and/or enhance inhibitory/GABAergic neurotransmission.
Proton magnetic resonance spectroscopy (1H-MRS) provides the opportunity to use MRI to better understand these issues in humans via measurement of brain GABA and glutamate levels in vivo. 1H-MRS studies in healthy volunteers (9, 10) and adults with epilepsy (11, 12) have confirmed that gabapentin increases brain GABA levels by 25–50%, with lower baseline GABA levels and relatively-higher doses of gabapentin associated with larger increases in GABA (9, 11).
Comparatively few studies have investigated the mechanisms of gabapentin’s effects on alcohol drinking and withdrawal in AUD. Because the neurobiological effects of drinking (i.e., inhibition of neuronal signaling via binding to GABA receptors and inhibiting glutamatergic synapses (13, 14)) involve the same neurochemical mechanisms as gabapentin, the existing literature on gabapentin’s mechanisms cannot be easily applied to AUD. For example, a seminal preclinical investigation of gabapentin demonstrated decreased anxiogenic effects of withdrawal and dependence-induced self-administration of alcohol, mediated by decreased GABAergic transmission in the central amygdala (15). Subsequent studies have found that gabapentin, 1) prevents excessive excitatory synaptogenesis, by antagonizing the interaction of α2δ−1 with thrombospondins, upregulated following intermittent ethanol exposure (16), and 2) normalizes alcohol-induced deficits in delta (1–4Hz) power during slow wave sleep (17). Conversely, a recent 1H-MRS study found no association between parieto-occipital cortex GABA levels and ≥1-week of gabapentin treatment in a cross-sectional convenience sample of AUD individuals in early recovery (18).
It is against this background that we report results of the first controlled investigation of the neurobiological mechanisms of gabapentin treatment for AUD. Specifically, in this MRI sub-study of a previously-reported clinical trial (2), individuals with AUD, including a history of alcohol withdrawal syndrome (AWS), and ≥72-hours of abstinence were randomized to gabapentin (1200mg/day) or placebo treatment for 16-weeks. Eligible participants additionally completed two 1H-MRS/MRI scans, one immediately preceding the first dose of study drug and the other following approximately 2-weeks of treatment. Here, we report the prospective effects of 1) early gabapentin treatment on dorsal anterior cingulate cortex (dACC) GABA and glutamate levels, in the context of variable relapse drinking among participants, and 2) GABAergic and glutamatergic medication effects on further drinking across the remainder of the trial.
METHODS AND MATERIALS
Participants and Procedure.
1H-MRS data for the present report were collected as part of a larger (n=96), 16-week, randomized, double-blind, placebo-controlled trial of gabapentin (titrated over 5-days to 1200mg/day) for individuals with AUD and a history of AWS. A history of posttraumatic stress disorder with stable symptoms was allowed given its comorbidity with AUD and potential response to gabapentin (19). The study protocol was approved by the Medical University of South Carolina Institutional Review Board (IRB). Briefly, after providing written, IRB-approved informed consent and subsequent assessment, participants who met DSM-V (20) criteria for AUD and a history of AWS (with no current major psychiatric illnesses, other substance dependence, or clinically-significant AWS at baseline [defined as, Clinical Institute Withdrawal Assessment-Revised score ≥10] (21)) were randomized to gabapentin or placebo for 16-weeks, with drinking assessed during this period using a daily calendar method (22). The primary report found that significantly more gabapentin-treated individuals had no heavy-drinking days and total abstinence compared with placebo (2). Consented and evaluable participants of the 1H-MRS sub-study (n=68; CONSORT diagram in Figure 1) completed their first scan immediately prior to treatment randomization (following ≥3-days of abstinence from alcohol, M=4.24, SD=2.03) and their second scan approximately 14-days post-randomization (Median=14, Range=[11,21]). The initial SIEMENS Trio (n=47) MRI scanner was upgraded to a Prisma-Fit (n=21) (both 3T; Siemens, Germany) partway through the study, with the same 32-channel phased-array head coil used across scanners. Time-from-randomization and scanner were examined as covariates across statistical models, but were eliminated, as they were universally non-significant (ps>0.10).
Figure 1.
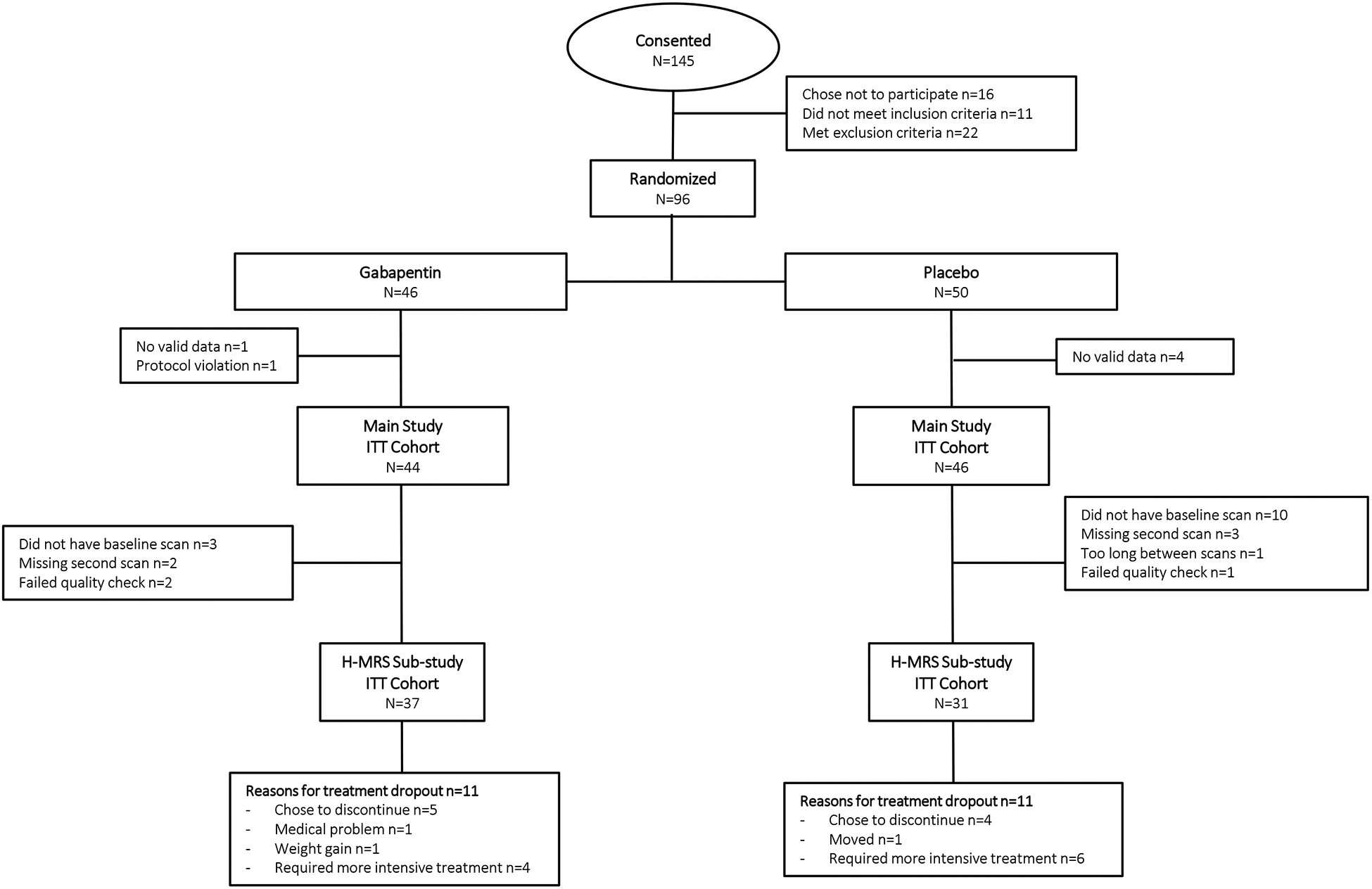
Consort Diagram.
1H-MRS Acquisition and Processing.
A structural scan was taken for 1H-MRS voxel placement and tissue segmentation (256 sagittal slices; 1mm thick/50% gap). A 2.5×2.5×3cm3 dACC voxel was placed on midsagittal T1-weighted images, posterior to the genu of the corpus callosum with the ventral edge aligned to the dorsal edge of the callosum (23). dACC was chosen given its demonstrated role in brain response to alcohol cues, relapse to heavy alcohol drinking, and core neurobehavioral deficits associated with AUD, and because most prior 1H-MRS AUD studies have measured this region (24–27). See Figure 2 for visualization of the voxel and sample spectrum. Following placement of saturation bands 1-cm away from voxel faces and shimming via FASTESTMAP (28), single-voxel water-suppressed 1H-MRS spectra were acquired using a MEGA-PRESS sequence (TR=2000ms; TE=68ms; number of averages=256) with symmetric editing-pulse frequencies for macromolecule suppression (1.9ppm,1.5ppm) (29) and a PRESS sequence sensitive to glutamate (TR=2000ms; TE=40ms; number of averages=128) (30). Each sequence was followed by a matched water-unsuppressed acquisition for phase and eddy-current correction and concentration referencing. Common sequence parameters included TR = 2000ms, 16-step phase cycling, spectral width=2.5 kHz, and 2048 complex data points. MEGA-PRESS data were processed using the Gannet MATLAB toolbox (version-3.1), with frequency/phase correction applied prior to fitting (31). PRESS data were processed using LCModel 6.3 with a vendor-supplied, GAMMA-simulated basis set and an analysis window of 0.2–4.0ppm (32). Within-voxel tissue fractions of gray (GM) and white (WM) matter and cerebrospinal fluid (CSF) were calculated based on automated segmentation in Statistical Parametric Mapping 12 (SPM12, Wellcome Department of Cognitive Neurology), using a volume mask generated in Gannet (33). Metabolite concentrations were normalized to unsuppressed-water and corrected for within-voxel CSF fraction.
Figure 2.
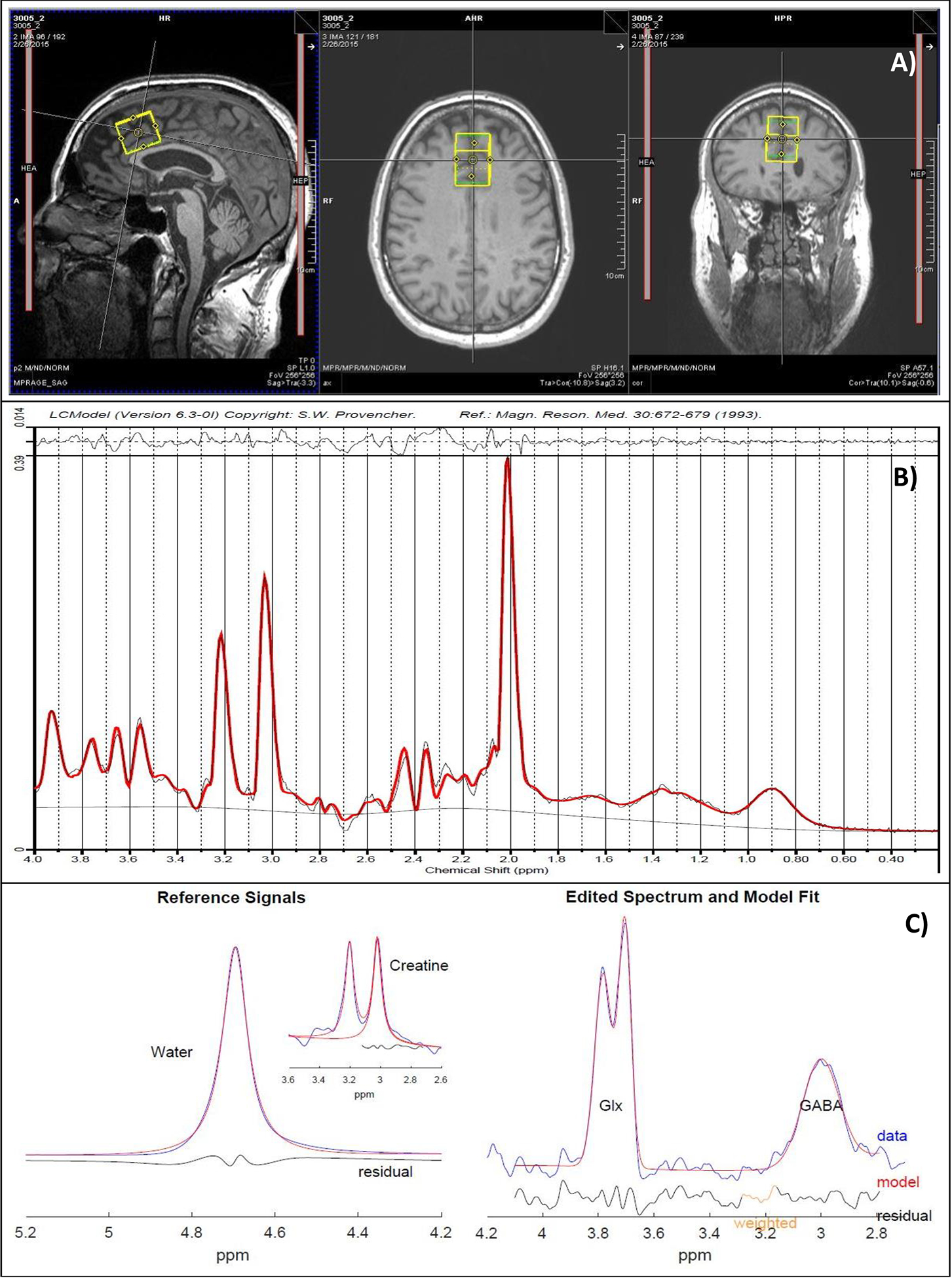
a) Representative dorsal anterior cingulate cortex (dACC) voxel placement, b) PRESS spectrum fitted in LCModel, and c) MEGA-PRESS GABA difference spectrum (right), along with reference water and creatine signals (left), fitted in Gannet.
Data Analytic Plan.
Frist, to evaluate the generalizability of our previously-reported findings to the present subsample, the number of evaluable individuals with no drinking days (abstinent) over the entire 16-week trial was analyzed using a 2-sample z test to produce a χ2 P value; individuals with missing drinking data were assumed to have been drinking (2). 1H-MRS quality control was conducted based on visual inspection of spectra (34), conducted blind to condition by JJP and TRB, along with evaluation of spectral fit errors relative to their observed distribution. Specifically, spectra with fit errors >3*interquartile range above the 3rd quartile or below the 1st quartile were excluded from analysis. Change variables were created for both glutamate and GABA by subtracting initial scan values from those at their second scan. Grey matter to brain matter ratio (GM:BM) was calculated as the amount of within-voxel grey matter over the sum of within-voxel grey and white matter. The relationship between change in metabolite values (the dependent variable) and medication group, drinking levels between scans (percent days abstinent, PDA), and their interaction was estimated with linear models. Each model included covariates measuring baseline PDA, the metabolite value at the first scan, and GM:BM. To further examine the interaction between medication group and between-scan drinking, PROCESS Model 1 was used to calculate Johnson-Neyman significance regions (35). For the second part of the analysis, linear mixed effect models were estimated using REML in the R package nlme (36). These models predicted weekly PDA over the remainder of the study (seven, two-week periods) following each subject’s second scan and used all available longitudinal data from each participant, providing unbiased parameter estimates and standard errors when missing values are at least missing at random (37). Medication group, change in metabolite values, and their interaction were included as predictors. Baseline PDA was incorporated as a covariate. These models were re-estimated with baseline metabolite values entered in place of change in metabolite values, to evaluate whether baseline metabolite levels could serve a similar treatment-response prediction function as did change in metabolite levels.
RESULTS
There were no significant differences between gabapentin- and placebo-treated participants on any demographic or clinical characteristic (see Table 1). Sixty participants (88%) from the 16-week parent study (i.e., gabapentin-treated n=33, placebo-treated n=27) provided full longitudinal drinking data (see Supplementary Table 3 for PDA by treatment group by week). Participants demonstrated good medication compliance, determined via pill counts, that did not differ by treatment group (gabapentin: M=92.7%, SD=8.8%; placebo: M=92.7%, SD=12.1%, p=0.988). The previously reported effect of gabapentin on total abstinence (2) was upheld in the present subsample, with significantly more gabapentin-treated individuals abstinent (8 of 37 [22%]) compared with placebo (1 of 31 [3%]), a difference of 19% (p=0.026).
Table 1.
Study Population Demographic and Drinking Characteristics
| No. (%) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total (n=68) | Gabapentin (n=37) | Placebo (n=31) | p valuea | |||
| Age, mean (SD), y | 49.3 | 10.6 | 49.6 | 10.4 | 48.9 | 10.9 | 0.78 |
| PTSD (current or past) | 18 | 26.5% | 9 | 24.3% | 9 | 29.0% | 0.66 |
| Nicotine use | 31 | 45.6% | 17 | 45.9% | 14 | 45.2% | 0.95 |
| Antidepressant use | 18 | 26.5% | 9 | 24.3% | 9 | 29.0% | 0.66 |
| Sex (male) | 50 | 73.5% | 28 | 75.7% | 22 | 71.0% | 0.66 |
| Married/cohabitating | 29 | 42.6% | 19 | 51.4% | 10 | 32.3% | 0.11 |
| Education (≤ 12 y) | 8 | 11.8% | 5 | 13.5% | 3 | 9.7% | 0.62 |
| Employed | 49 | 72.1% | 26 | 70.3% | 23 | 74.2% | 0.72 |
| Race (white) | 65 | 95.6% | 35 | 94.6% | 30 | 96.8% | 0.66 |
| Alcohol use and severity indicators, mean (SD) | |||||||
| Drinks per dayb | 11.0 | 4.6 | 10.8 | 4.3 | 11.2 | 5.0 | 0.77 |
| Drinks per drinking dayb | 13.0 | 4.8 | 13.2 | 4.9 | 12.8 | 4.8 | 0.72 |
| Days abstinent, %b | 14.1% | 21.3% | 14.9% | 22.7% | 13.3% | 19.8% | 0.76 |
| Heavy drinking days, %b | 83.0% | 22.3% | 82.8% | 23.6% | 83.2% | 21.0% | 0.94 |
| Days abstinent prior to randomization | 4.2 | 2.0 | 4.2 | 2.1 | 4.2 | 1.9 | 0.97 |
| Alcohol Dependence Scalec | 18.6 | 7.5 | 19.7 | 7.8 | 17.4 | 7.1 | 0.22 |
| OCDSd | 27.4 | 9.4 | 27.9 | 9.1 | 26.7 | 9.9 | 0.62 |
| Alcohol Withdrawal Symptom Checkliste | 10.5 | 7.0 | 10.9 | 7.4 | 9.9 | 6.4 | 0.53 |
| DSM-5 alcohol withdrawal items positive | 4.5 | 1.3 | 4.7 | 1.1 | 4.4 | 1.5 | 0.36 |
| CIWA | 2.8 | 1.8 | 2.8 | 2.0 | 2.8 | 1.6 | 0.95 |
| Past alcohol: | |||||||
| Treatments | 19 | 27.9% | 13 | 35.1% | 6 | 19.4% | 0.15 |
| Detoxifications | 9 | 13.2% | 7 | 18.9% | 2 | 6.5% | 0.13 |
| Alcohol blood tests (biomarkers) | |||||||
| %dCDT≥1.7 | 50 | 74.6% | 26 | 72.2% | 24 | 77.4% | 0.63 |
| GGT > 36 U/L | 52 | 76.5% | 29 | 78.4% | 23 | 74.2% | 0.69 |
Abbreviations: PTSD, Posttraumatic Stress Disorder; DSM-5, Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition); GGT, y-glutamyltransferase; OCDS, Obsessive Compulsive Drinking Scale; %dCDT, percentage of disialo carbohydrate-deficient transferrin.
The χ2 statistic was used for all the categorical variables, and the ANOVA statistic was used for all the continuous variables.
Calculated using the 90 days prior to screening.
Range, 0–47.
Range, 0–56.
Effects of gabapentin on early-treatment changes in glutamate and GABA levels.
Four participants (3 gabapentin, 1 placebo) were excluded from glutamate, and one (gabapentin) from GABA, analyses because their 1H-MRS scans failed quality control. Within-voxel GM:BM tissue fraction did not significantly differ between gabapentin- (M=0.65, SD=0.043) and placebo-treated (M=0.67, SD=0.039, p=0.081) participants. Please see Supplementary Table 1 for 1H-MRS quality control metrics and Supplementary Table 2 for summary statistics of metabolite levels organized by scan and by group.
Controlling for pretreatment glutamate level (β=−0.57, p< 0.001) and PDA over the 90-days preceding screening (β=−0.14, p=0.745), as well as PDA between pretreatment and posttreatment scans (β=−0.81, p=0.069) and GM:BM tissue fraction (β=0.92, p=0.703), gabapentin-treated participants had greater decreases (and/or smaller increases) in glutamate between scans (β=−1.26, p=0.008). However, this main effect of treatment was qualified by a significant interaction with PDA between scans (β=1.68, p=0.005). Specifically, Johnson-Neyman analyses demonstrated that gabapentin-treated participants with <50% of days abstinent between scans had greater decreases (and/or smaller increases) in glutamate relative to placebo-treated participants (ps< 0.05), whereas gabapentin-treated participants with >95–100% of days abstinent between scans had greater increases (and/or smaller decreases) in glutamate relative to placebo-treated participants (p=0.08) (see Figure 3, left panel). Please see Supplementary Figure 1 for spaghetti plots of glutamate levels by between scan drinking by treatment group.
Figure 3.
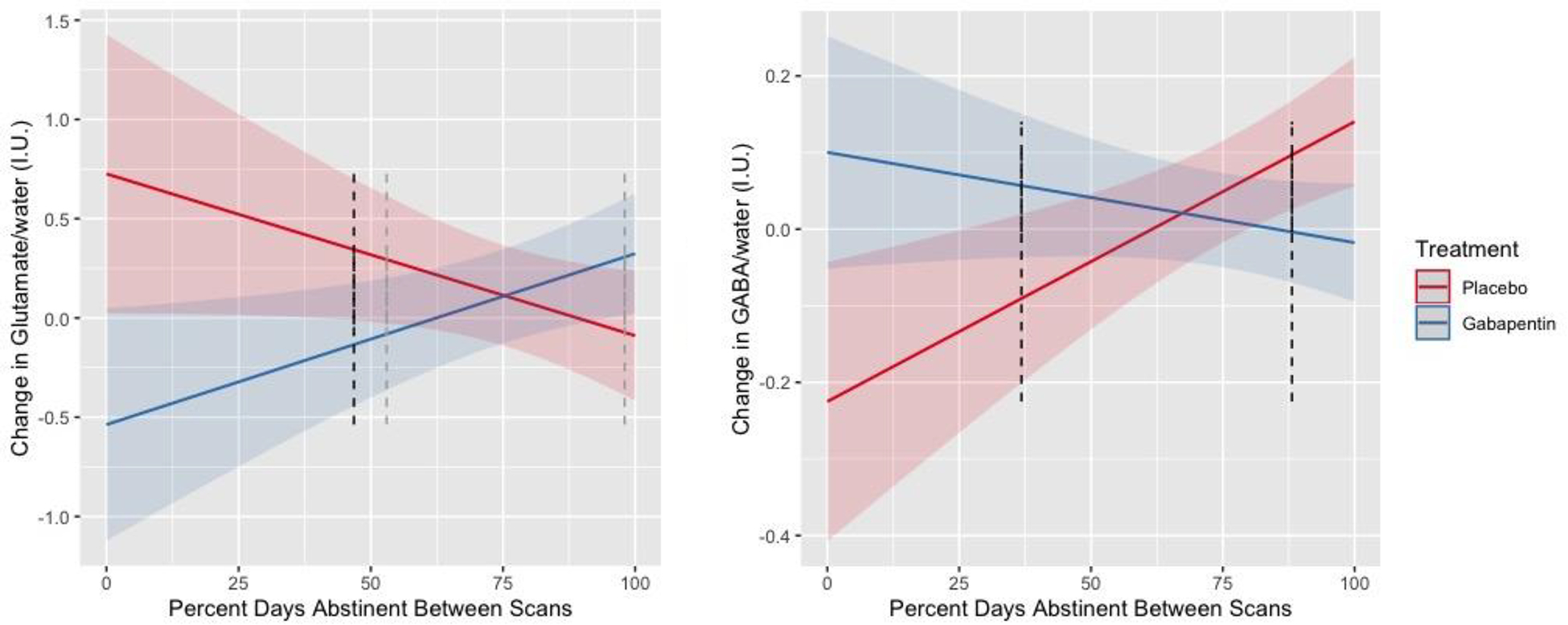
Change in dorsal anterior cingulate cortex (dACC) glutamate (n=64, left) and GABA (n=67, right) (y-axes) levels, normalized to water and expressed in Institutional Units (I.U.), by percent days abstinent between scans (x-axis) by treatment group (red line = placebo, blue line = gabapentin). Error bands represent 95% confidence intervals. Dark and light dotted lines represent the p < 0.05 and p < 0.10 thresholds, respectively, of Johnson-Neyman significance regions.
Conversely, controlling for pretreatment GABA level (β=−0.77, p<0.001) and pretreatment PDA (β=0.09, p=0.445), as well as PDA between scans (β=0.37, p=0.002) and GM:BM fraction (β=−1.23, p=0.038), gabapentin-treated participants had greater increases (and/or smaller decreases) in GABA between scans (β=0.33, p=0.009). However, similar to glutamate, this main effect of treatment was qualified by a significant interaction with PDA between scans (β=−0.48, p=0.002). Specifically, Johnson-Neyman analyses demonstrated that gabapentin-treated participants with <40% of days abstinent between scans had greater increases (and/or smaller decreases) in GABA relative to placebo-treated participants (ps< 0.05), whereas gabapentin-treated participants with >85% of days abstinent between scans had greater decreases (and/or smaller increases) in GABA relative to placebo-treated participants (ps< 0.05) (see Figure 3, right panel). Please see Supplementary Figure 2 for spaghetti plots of GABA levels by between scan drinking by treatment group.
Effects of gabapentin-induced changes in glutamate and GABA levels on subsequent abstinence.
Controlling for pretreatment PDA (β=0.57, p=0.003) and time (averaged over 2-week blocks) in study (β=−0.01, p=0.268), there was a significant interaction between medication group (main effect, β<−0.01, p=0.909) and change in between-scan glutamate levels (main effect, β=−0.14, p=0.051; interaction, β=0.28, p=0.007) on PDA during the remaining study-treatment period. Specifically, Johnson-Neyman analyses demonstrated that gabapentin-treated participants with decreased glutamate levels between scans (i.e., < −0.88 IUs; Z< −1.24 or 7.8% of the sample) had fewer PDA for the remainder of the study relative to placebo-treated participants (ps<0.05), whereas gabapentin-treated participants with increased glutamate levels between scans (i.e., >0.79 IUs; Z>0.86 or 18.8% of the sample) had more PDA relative to placebo-treated participants (ps< 0.05) (see Figure 4, left panel). Substituting changes-in for baseline glutamate levels, we found a significant interaction between medication group (main effect, β=3.24, p=0.040) and baseline glutamate levels on PDA during the remaining study-treatment period (main effect, β=0.22, p=0.028; interaction, β=−0.29, p= 0.040) (see Supplementary Figure 2).
Figure 4.
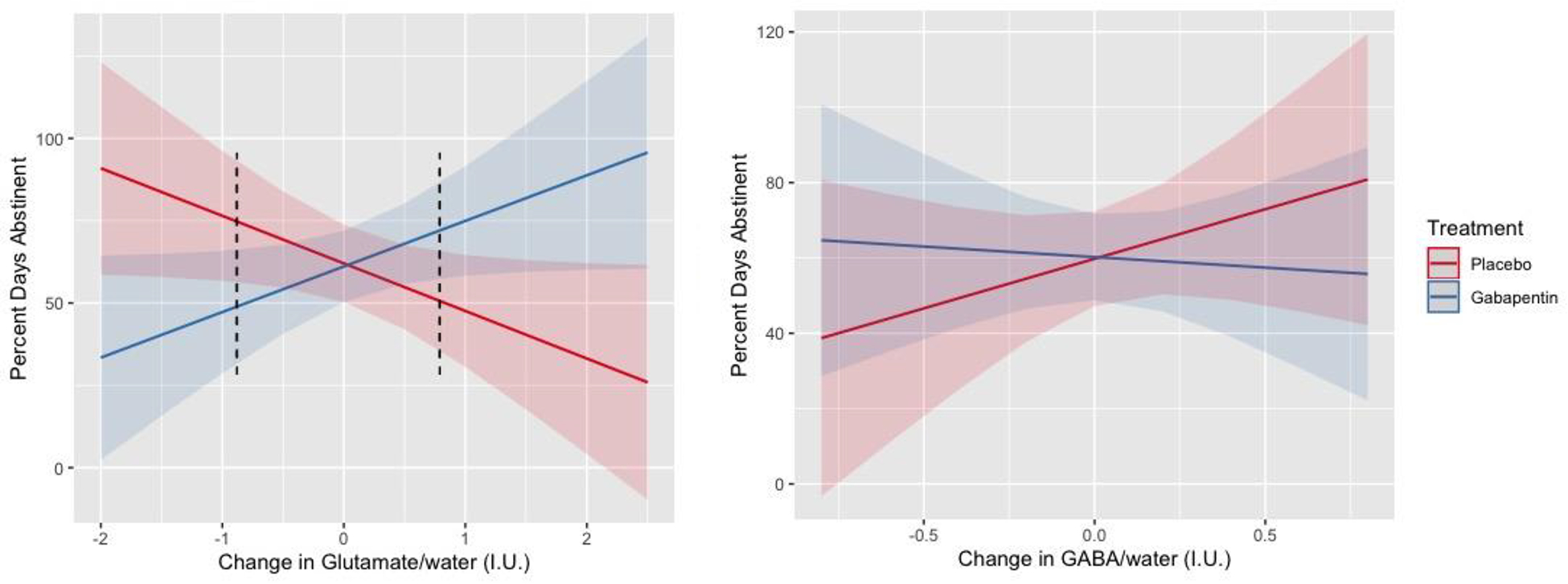
Percent days abstinent following scan 2 (i.e., early treatment; y-axis) by change in dorsal anterior cingulate cortex (dACC) glutamate (n=64, left) and GABA (n=67, right) levels (x-axes), normalized to water and expressed in Institutional Units (I.U.), by treatment group (red line = placebo, blue line = gabapentin). Error bands represent 95% confidence intervals. Dark dotted lines represent the p < 0.05 threshold of Johnson-Neyman significance regions.
In contrast, controlling for pretreatment PDA (β=0.54, p=0.017) and time in study (β=−0.01, p=0.299), there were no significant main effects of medication group (β=0.004, p=0.959) or change in between-scan GABA levels (β=0.26, p=0.287), nor their interaction (β=−0.32, p=0.327), on PDA during the remaining study-treatment period (see Figure 4, right panel). Substituting changes-in for baseline GABA levels provided similarly null findings (i.e., ps>0.50)
DISCUSSION
Following recent demonstration of the anti-drinking efficacy of gabapentin in individuals with AUD and a history of AWS (2), the present report provides insight into the neurobiological mechanisms through which gabapentin may have worked to promote abstinence in that study. This pre-planned investigation was based on the knowledge that adaptations in brain GABA and glutamate systems underlie AUD in general (4), and AWS in particular (23), and that potential gabapentin effects on those systems might explain its treatment efficacy (15). Consistent with the preclinical and 1H-MRS gabapentin literature, gabapentin treatment was associated with significantly increased GABA levels and significantly decreased glutamate levels in dACC, a fronto-cortical brain region. However, these associations were significantly moderated by (i.e., depended on) participants’ percentage of abstinent days during the first few weeks of treatment, not surprising given the large and dynamic impact that drinking itself has on brain glutamate and GABA levels (14). Specifically, gabapentin (relative to placebo) was associated with, 1) greater increases in glutamate and greater decreases in GABA levels in participants who remained mostly, or entirely, abstinent, but 2) greater decreases in glutamate and greater increases in GABA levels in participants who drank on more than approximately half of the days preceding the second (within-treatment-group) scan. Furthermore, gabapentin-treated participants with greater increases in glutamate (but not GABA) levels during the early weeks of treatment had significantly more percent days abstinent across the remaining study-treatment period, relative to placebo-treated participants. Finally, lower baseline metabolite levels were associated with greater-magnitude metabolite-level changes across the first few weeks of treatment, and gabapentin-treated participants with lower baseline glutamate levels had significantly more percent days abstinent across the study-treatment period.
Consistent with the inhibitory effects of alcohol drinking on glutamatergic synapses, AUD has been consistently associated with reduced fronto-cortical glutamate levels in 1H-MRS studies (38–42), with the notable exception of during acute, clinically-significant alcohol withdrawal, which has been associated with transient spikes in glutamate levels (23). Though the present study focused on AUD individuals with a history of AWS, those experiencing acute, clinically-significant alcohol withdrawal were excluded from participation to ensure their safety (i.e., if receiving placebo). As a result, gabapentin-induced increases in dACC glutamate levels in the present study likely represented neurochemical shifts in the direction of abstinence-induced “normalization” rather than acute alcohol-withdrawal effects, and this normalization depended on maintaining abstinence during the early treatment period. In contrast, a 1H-MRS investigation of acamprosate in AUD individuals undergoing inpatient detoxification treatment for clinically-significant alcohol withdrawal symptoms (with >60% of participants receiving benzodiazepines) reported that acamprosate significantly decreased ACC glutamate levels relative to placebo, which was interpreted as a therapeutic “normalization” from the transient spike in glutamate levels that accompanies clinically-significant alcohol withdrawal (43). Unlike that study, however, we additionally demonstrated that medication-induced increases in glutamate prospectively predicted future abstinence.
Consistent with the facilitatory effects of alcohol drinking on GABAergic synapses, 1H-MRS-measured GABA levels were initially reported to be increased in AUD relative to healthy volunteers (44), though subsequent studies failed to replicate this finding (40, 45). GABA results from the present study could be viewed as consistent with results from that initial study, in that decreases in GABA levels were associated with increased percent days abstinent in gabapentin-treated participants, albeit only during the early treatment period. GABA results from the present study are also consistent with the seminal preclinical investigation of gabapentin for AUD, where gabapentin reduced excessive alcohol drinking by decreasing GABAergic transmission, thereby normalizing the alcohol-induced effect, in the central amygdala (15).
Although the present study affirms 1H-MRS as a potentially-valuable tool for exploring neurochemical drug effects, interpretability of 1H-MRS findings is fundamentally limited by the methodology’s relatively-low spatiotemporal resolution (46, 47). Future studies using 1H-MRS as a translational bridge (i.e., including both rodents and people) could overcome these limitations (23), and provide more-detailed molecular explanations for the gabapentin effects observed in people with AUD. Given the dynamic nature of neurochemical adaptation to alcohol drinking and withdrawal (14, 48), more frequent 1H-MRS scanning during the initial days/weeks of gabapentin treatment could help disentangle the temporal interaction of changes in glutamate and GABA levels and percent days abstinent during the early phase of treatment. For example, consistent with findings from placebo-treated participants in the present study, we recently demonstrated that treatment-naïve individuals with AUD had depleted dACC GABA levels which normalized within 72-hours of monitored abstinence, and which remained normal across a subsequent 5-day period of abstinence (25). Though we found that both baseline, and change in, dACC glutamate levels were associated with gabapentin’s promotion of abstinence in the present study, findings involving glutamate-level changes were nearly 6-times more statistically-reliable than were findings involving baseline glutamate levels.
A notable strength of the present study was that, unlike most 1H-MRS GABA studies (49), we used a specialized MEGA-PRESS acquisition sequence that eliminated co-edited “macromolecules” which comprise 50% of the “GABA” signal (therefore denoted “GABA+”) acquired via traditional MEGA-PRESS acquisition (29)). Other strengths included a relatively-large sample (e.g., >2x that of (43)), and relatively long duration of gabapentin treatment (i.e., 16-weeks, to address the often-protracted nature of alcohol withdrawal symptoms (50)), delivered in the context of a randomized, double-blind, placebo-controlled, trial.
Limitations of the present study include, 1) acquisition of 1H-MRS data from a single brain region, precluding statements concerning the regional specificity of findings, 2) an MRI scanner upgrade that occurred partway through the study, though analyses demonstrated that results did not differ by scanner, 3) exclusion of AUD individuals experiencing acute, clinically-significant alcohol withdrawal at the time of scanning, 4) primary reliance on self-report alcohol consumption data, and 5) inability to predict, at the individual level, who might respond best to gabapentin.
In sum, results from the present study suggest that gabapentin-treatment promotes early abstinence partly by increasing dACC glutamate levels that are subsequently associated with gabapentin’s efficacy in reducing drinking over an extended period in individuals with AUD and a history of AWS. These novel findings contribute significantly to our understanding of how gabapentin may work to prevent relapse drinking in certain AUD individuals who attempt abstinence and are consistent with our previous report of gabapentin efficacy (2). They also provide evidence for a biomarker of efficacious treatment (i.e., increased dACC glutamate levels) that may be used to evaluate other glutamatergic and/or GABAergic medications for individuals with AUD, and potentially other conditions marked by dACC glutamate and/or GABA deficiency (e.g., cannabis use disorder (51), co-occurring bipolar disorder and substance use disorder (52)).
Supplementary Material
ACKNOWLEDGMENTS
The present study was funded by an R01 grant from NIH/NIAAA to Dr. Anton (R01AA022364). The study has been listed on clinicaltrials.gov (NCT02349477).
DISCLOSURES
In the past 3 years, Dr. Anton has been a consultant for Alkermes, Allergan, Dicerna, Indivior, Insys, Labortorio Farmaceutico C.T., Life Epigenetics (Foxo Bioscience), Xenoport (Arbor). He also received grant funding from Labortorio Farmaceutico C.T. He is a chair and participant in the Alcohol Clinical Trials Initiative (ACTIVE) sponsored by the American Society of Clinical Psychopharmacology (ASCP) but has been supported (in the past or currently) from Abbvie, Alkermes, Amygdala, Arbor, Dicerna, Ethypharm, Glaxo Smith Kline, Indivior, Janssen, Eli Lilly, Lundbeck, Mitsubishi, Otsuka, Pfizer, and Schering. In the past 3 years, Dr. Prisciandaro has been a consultant for, and received grant funding from, Laboratorio Farmaceutico CT.
REFERENCES
- 1.Kranzler HR, Feinn R, Morris P, Hartwell EE. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction (Abingdon, England). 2019;114(9):1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton RF, Latham P, Voronin K, Book S, Hoffman M, Prisciandaro J, et al. Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients With Alcohol Withdrawal Symptoms: A Randomized Clinical Trial. JAMA internal medicine. 2020;180(5):728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA internal medicine. 2014;174(1):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry. 2016;3(8):760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current opinion in pharmacology. 2006;6(1):108–13. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CP, Harris EW. Analgesia with Gabapentin and Pregabalin May Involve NMDA Receptors, Neurexins and Thrombospondins. Journal of Pharmacology and Experimental Therapeutics. 2020:jpet.120.266056. [DOI] [PubMed] [Google Scholar]
- 7.Manville RW, Abbott GW. Gabapentin Is a Potent Activator of KCNQ3 and KCNQ5 Potassium Channels. Mol Pharmacol 2018;94(4):1155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Wang DS, Bonin RP, Penna A, Alavian-Ghavanini A, Zurek AA, et al. Gabapentin increases expression of δ subunit-containing GABA(A) receptors. EBioMedicine. 2019;42:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T (1)H-MRS study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(13):2764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58(3):368–72. [DOI] [PubMed] [Google Scholar]
- 11.Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41(6):675–80. [DOI] [PubMed] [Google Scholar]
- 12.Petroff OA, Rothman DL, Behar KL, Lamoureux D, Mattson RH. The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Annals of neurology. 1996;39(1):95–9. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhoff DJ. Chapter 19 - Brain proton magnetic resonance spectroscopy of alcohol use disorders. In: Sullivan EV, Pfefferbaum A, editors. Handbook of Clinical Neurology. 125: Elsevier; 2014. p. 313–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberto M, Varodayan FP. Synaptic targets: Chronic alcohol actions. Neuropharmacology. 2017;122:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartzwelder HS, Park MH, Acheson S. Adolescent Ethanol Exposure Enhances NMDA Receptor-Mediated Currents in Hippocampal Neurons: Reversal by Gabapentin. Scientific reports. 2017;7(1):13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlers CL, Sanchez-Alavez M, Wills D. Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology (Berl). 2018;235(6):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyerhoff DJ, Murray DE, Durazzo TC, Pennington DL. Brain GABA and Glutamate Concentrations Following Chronic Gabapentin Administration: A Convenience Sample Studied During Early Abstinence From Alcohol. Frontiers in psychiatry. 2018;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S, Bachu R, Kotapati P, Adnan M, Ahmed R, Farooq U, et al. Use of Gabapentin in the Treatment of Substance Use and Psychiatric Disorders: A Systematic Review. Frontiers in psychiatry. 2019;10:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: 2013. [Google Scholar]
- 21.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British journal of addiction. 1989;84(11):1353–7. [DOI] [PubMed] [Google Scholar]
- 22.Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 23.Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry. 2012;71(11):1015–21. [DOI] [PubMed] [Google Scholar]
- 24.Luijten M, Machielsen MWJ, Veltman DJ, Hester R, de Haan L, Franken IHA. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci 2014;39(3):149–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment-naive individuals with alcohol use disorder. Addiction Biology. 2020;25(6):e12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 2013;18(1):121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin 2016;13:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magnetic resonance in medicine. 2000;43(2):319–23. [DOI] [PubMed] [Google Scholar]
- 29.Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, et al. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: A MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magnetic resonance in medicine. 2013;69(2):317–20. [DOI] [PubMed] [Google Scholar]
- 30.Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magnetic resonance in medicine. 2008;60(4):964–9. [DOI] [PubMed] [Google Scholar]
- 31.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of magnetic resonance imaging : JMRI. 2014;40(6):1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine. 1993;30(6):672–9. [DOI] [PubMed] [Google Scholar]
- 33.Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of magnetic resonance imaging : JMRI. 2015;42(5):1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreis R Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR in biomedicine. 2004;17(6):361–81. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 2nd ed. Little TD, editor: Guilford Press; 2017. [Google Scholar]
- 36.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. Linear and Nonlinear Mixed Effects Models. https://CRAN.R-project.org/package=nlme; 2017. [Google Scholar]
- 37.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken: Wiley; 2002. [Google Scholar]
- 38.Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, et al. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(8):1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, et al. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcoholism, clinical and experimental research. 2013;37(10):1643–9. [DOI] [PubMed] [Google Scholar]
- 40.Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug and alcohol dependence. 2012;125(1–2):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF. Associations Between Recent Heavy Drinking and Dorsal Anterior Cingulate N-Acetylaspartate and Glutamate Concentrations in Non-Treatment-Seeking Individuals with Alcohol Dependence. Alcoholism, clinical and experimental research. 2016;40(3):491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, et al. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(7):1359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Archives of general psychiatry. 2010;67(10):1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. The American journal of psychiatry. 1999;156(6):952–4. [DOI] [PubMed] [Google Scholar]
- 45.Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, et al. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biological psychiatry. 2006;59(1):85–93. [DOI] [PubMed] [Google Scholar]
- 46.Egerton A The potential of 1H-MRS in CNS drug development. Psychopharmacology (Berl). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waschkies CF, Bruns A, Müller S, Kapps M, Borroni E, von Kienlin M, et al. Neuropharmacological and neurobiological relevance of in vivo 1H-MRS of GABA and glutamate for preclinical drug discovery in mental disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(10):2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Current topics in behavioral neurosciences. 2013;13:511–40. [DOI] [PubMed] [Google Scholar]
- 49.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caputo F, Cibin M, Loche A, De Giorgio R, Zoli G. The recognition and management of protracted alcohol withdrawal may improve and modulate the pharmacological treatment of alcohol use disorder. Journal of Psychopharmacology. 2020;34(11):1171–5. [DOI] [PubMed] [Google Scholar]
- 51.Prescot AP, Renshaw PF, Yurgelun-Todd DA. γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug and alcohol dependence. 2013;129(3):232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prisciandaro JJ, Tolliver BK, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Translational Psychiatry. 2017;7(7):e1163–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


