Abstract
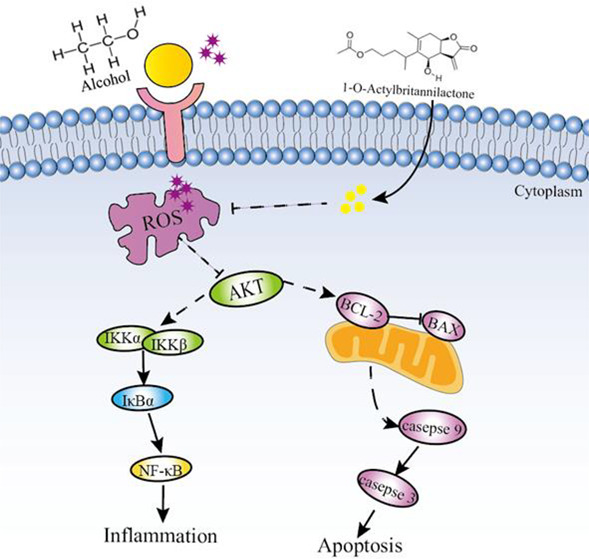
1-O-Acetylbritannilactone (ABL) is a marker component of Inula britannica L. and is reported to exhibit multiple pharmacological activities, including antiaging, anti-inflammatory, and antidiabetic properties. Although the protective effect of Inula britannica L. on animal models of liver injury has been widely reported, the effect of ABL on alcohol-induced liver damage has not been confirmed. The present study was designed to investigate the protective effect of ABL against alcohol-induced LO2 human normal liver cell injury and to further clarify the underlying mechanism. Our results revealed that ABL at concentrations of 0.5, 1, and 2 μM could remarkably suppress the decreased viability of LO2 cells stimulated by alcohol. In addition, ABL pretreatment improved alcohol-induced oxidative damage by decreasing the level of reactive oxygen species (ROS) and the excessive consumption of glutathione peroxidase (GSH-Px), while increasing the level of catalase (CAT) in LO2 cells. Moreover, Western blotting analysis showed that ABL pretreatment activated protein kinase B (Akt) phosphorylation, increased downstream antiapoptotic protein Bcl-2 expression, and decreased the phosphorylation level of the caspase family including caspase 9 and caspase 3 proteins, thereby attenuating LO2 cell apoptosis. Importantly, we also found that ABL significantly inhibits the activation of the nuclear factor-kappa B (NF-κB) signaling pathway by reducing the secretion of proinflammatory factors including tumor necrosis factor-α (TNF-α) and interleukin (IL-1β). In conclusion, the current research clearly suggests that the protective effect of ABL on alcohol-induced hepatotoxicity may be achieved in part through regulation of the ROS/Akt/NF-κB signaling pathway to inhibit inflammation and apoptosis in LO2 cells. (The article path map has not been seen.)
1. Introduction
Alcoholic liver disease (ALD) is the main cause of chronic liver disease worldwide. Its clinical manifestations are liver enlargement, abnormal liver function, and liver failure, and it can even lead to hepatocyte necrosis and apoptosis.1 It is estimated that 75 million people worldwide have severe alcoholic fatty liver disease. With the rapid increase in the global consumption of alcohol, the incidence of ALD has shown a continuous increase, and it has gradually become one of the world’s health problems.2 The mechanism of alcohol-induced hepatotoxicity is multifactorial. However, accumulated studies have shown that oxidative stress, apoptosis, and inflammation are considered to be the important elements of the pathogenesis of ALD.3 Previous studies have found that alcohol produces a large amount of reactive oxygen species (ROS) through various pathways in the body’s metabolism, which leads to an imbalance of the body’s antioxidant defense system. Under normal circumstances, antioxidant enzymes are involved in ROS regulation. When too much ROS are produced, the antioxidant factors in the body cannot remove it, which may cause ROS-mediated oxidative stress, mitochondrial dysfunction, and ultimately liver cell damage and abnormal liver function.4 Meanwhile, ROS overproduction promotes the translocation of apoptotic protein Bax-induced cell apoptosis via inhibiting the activation of the PI3K/Akt signaling pathway. Furthermore, the production of numerous inflammatory mediators and cytokines plays a key role in the formation of acute alcoholic liver injury. It has been reported that alcohol activates nuclear transcription factor-κB (NF-κB) to a certain extent by inhibiting the activation of Akt and stimulates the release of proinflammatory factors TNF-α and IL-1β. The production of multiple inflammatory cytokines further intensifies the alcohol-induced inflammatory response and hepatocyte apoptosis.5
Inula britannica L. is a relatively important medicinal plant resource in China, mainly distributed in its northern, northeastern, and eastern provinces. Studies have confirmed that Inula britannica L. contains a variety of chemical components such as sesquiterpenoids, flavonoids, and polysaccharides that play a key role in anti-inflammatory,6 anticancer,7 and liver damage processes.8 Importantly, 1-O-actylbritannilactone (ABL) is the most abundant and most active monomer compound in I. britannica L. Modern pharmacological studies have found that ABL has a variety of biological activities such as antitumor,9 antidiabetic,10 and antioxidation.11 It was previously reported that the water extract of Inula britannica L. could effectively reduce liver injury induced by LPS/PA in mice via regulating the imbalance of the Th1/Th2 ratio of T cells.12 It is worth noting that the steroidal compound taraxacum sterol acetate extracted from Inula britannica L. relieves CCl4-induced ALD by inhibiting the expression of inflammatory factors and the activation of NF-κB.13
Although Inula britannica L. has been confirmed by a number of studies to make an important contribution to liver protection, the protective effects of ABL against alcohol-induced hepatotoxicity have not been studied so far. Thus, the aim of this study was to investigate the mechanism of action of ABL on alcohol-induced liver injury by establishing an in vitro LO2 cell injury model.
2. Materials and Methods
2.1. Sample and Reagents
1-O-Actylbritannilactone (ABL) is manufactured in our laboratory by the following process.
Using ultrasound-assisted technology, a powder of Inula britannica L. was extracted three times with 70% ethanol. The filtrate was combined, filtered, and evaporated under reduced pressure. Hydrochloric acid was added to the filtrate to keep the pH in the range 1–2. The sample was stirred well and left overnight, and then, the resulting precipitate was dissolved in distilled water. AB-8 macroporous resin was used for the crude separation, and the 70% ethanol fraction was purified by silica gel column chromatography and a polyacrylamide gel column to obtain ABL. The purity was determined to be 99.4% by HPLC analysis, as shown in Figure 1A. RPMI 1640 medium, antibiotics (100 units/mL penicillin and 100 mg/mL streptomycin), and fetal bovine serum (FBS) were purchased from Gibco BRL. GSH-Px and CAT assay kits, a BCA protein assay kit (cat. A045-4-2), and an annexin V-FITC/PI double staining apoptosis detection kit (cat. G003-1-2) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). TNF-α and IL-1β ELISA kits were purchased from the R&D company (Minneapolis, MN). A Hoechst 33258 staining kit and reactive oxygen species (ROS) detection kits were provided from Beyotime Biotechnology Co., Ltd. (Shanghai, China). The primary monoclonal antibodies used included β-actin, Akt, p-Akt, NF-κB, p-NF-κB, IκBα, p-IκBα, IKKα/β, p-IKKα/β, Bax, Bcl-2, caspase 3/9, cl-caspase 3/9, and secondary antibodies, which were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals such as alcohol were of analytical grade and provided by Beijing Chemical Factory (Beijing, China).
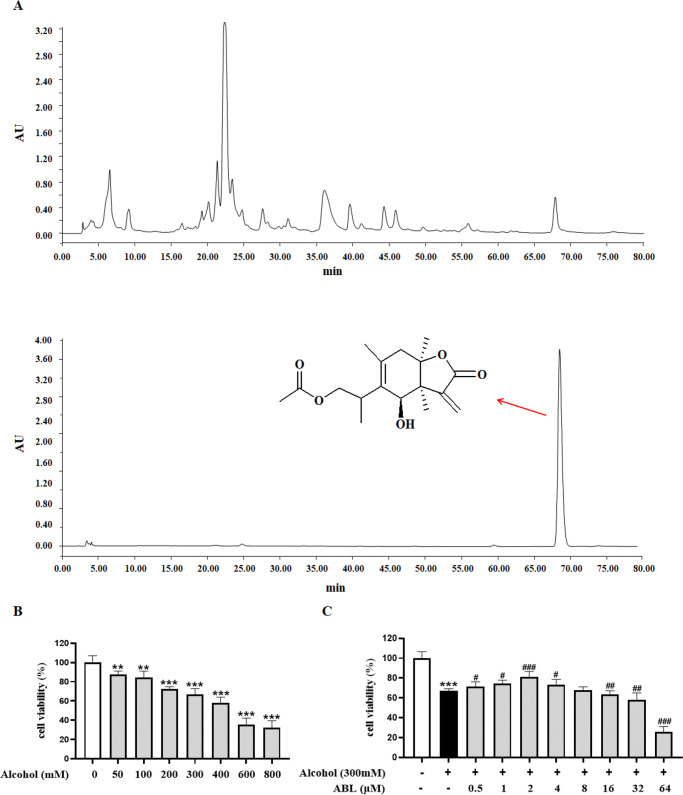
Figure 1.
Effect of ABL on viability in alcohol-induced LO2 cells. (A) Chemical structural formula of ABL. (B) Effects of different concentrations of alcohol (0, 50, 100, 200, 300, 400, 600, and 800 mM) on LO2 cell viability. (C) Protective effect of ABL (0–64 μM) against alcohol-induced LO2 cell injury. Data are mean ± SD (n = 6). ** p < 0.01, *** p < 0.001 vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs alcohol-treated group.
2.2. Cell Culture
Human normal LO2 liver cells were obtained from ATCC (Gaithersburg, MD). The cells were cultured in RPMI 1640 medium supplemented with 10% FBS and antibiotics (100 units/mL penicillin and 100 mg/mL streptomycin) in a 5% CO2 incubator at 37 °C.
2.3. Cell Viability Assay
The cells were seeded in a 96-well plate at a density of 6 × 104 cells per well and were incubated in a 37 °C, 5% CO2 incubator. When the density reached 70–80% coverage, the cells were treated with alcohol at different concentrations (50, 100, 200, 300, 400, 600, and 800 mM) for 24 h. Cell viability was measured by MTT assay according to the previous operation in the laboratory.14 Briefly, 20 μL of MTT solution (5 mg/mL) was added to the wells and incubated at 37 °C for 4 h. Then, the culture supernatant was discarded, and the residual cell layer was dissolved with 150 μL of DMSO. Finally, cell viability was evaluated by the determination of absorbance at 490 nm by a microplate reader (SPECTROstar Nano), and the optimal administration doses of alcohol were determined.
We next examined the protective effect of ABL on alcohol-induced cell damage. First, cells were supplemented with ABL with different concentrations of 0.5, 1, 2, 4, 8, 16, 32, and 64 μM for 24 h and then exposed to alcohol (300 mM) or not for 24 h. After treatment, the cell viability was determined as described above.
2.4. Determination of Antioxidant Enzymes
LO2 cells in the logarithmic growth phase were seeded into a 6-well plate at a density of 5 × 105 cells/mL per well. After pretreatment with ABL and alcohol, the culture supernatant was discarded, and cells were washed with phosphate-buffered saline (PBS) two times. The scraped cells were collected and resuspended in PBS. After that, the cell membrane was disrupted by a homogenizer, and the cell lysates were centrifuged at 3500g and 4 °C for 10 min. The levels of GSH-Px and CAT in cell homogenates of each group were determined using an assay kit according to the manufacturer’s protocol.
2.5. Proinflammatory Cytokine Measurements
Cell processing was performed according to the above method. The contents of TNF-α and IL-1β in the supernatant of cells were determined using ELISA assay kits according to the manufacture’s protocols (R&D, Minneapolis, MN). The absorbance was measured at 450 nm by an ELISA reader (Bio-Rad, Hercules, CA).
2.6. ROS Staining
The DCFH-DA (2,7-dichlorofluorescein diacetate) probe itself is a nonfluorescent substance. It can penetrate through the cell membrane and enter the cell to be hydrolyzed into DCFH by esterase. In addition, DCFH is oxidized by intracellular ROS to form fluorescent DCF. Therefore, the fluorescence expression of intracellular ROS was measured by DCFH-DA.
LO2 cells were inoculated in 6-well microplates, incubated with different concentrations of ABL for 24 h, and then treated with 300 mM alcohol for 24 h. The medium was discarded, and ROS staining was performed as previously described.15 Briefly, 5 μL of DCFH-DA fluorescent dye was added to each well and incubated at 37 °C for 30 min in the dark. Then, the medium was removed, and cells were washed with PBS two times. Alcohol (300 mM) was used for a positive control, and the relative ROS fluorescence intensity of treated cells was expressed as a percentage of the alcohol-induced group (Leica DM750, Solms, Germany).
2.7. Hoechst 33258 Staining
After the cells were treated with the above method, nuclear morphological changes of LO2 cells were analyzed by Hoechst 33258 staining.16 Simply put, the cells were fixed with 4% paraformaldehyde for 10 min and washed with PBS. Next, 1 mL of 0.2% Triton solution was added to each well for 10 min to increase the permeability of the cell membrane. Then, cells were washed two times by PBS. Hoechst 33258 dye solution at a concentration of 10 μg/mL was added for 5 min of incubation. Stained nuclei with blue fluorescence were detected by fluorescence microscopy (Leica TCS SP8). The result was quantified by Image-Pro plus 6.0 software (Media Cybernetics, Rockville, MD).
2.8. Flow Cytometry with Annexin V/PI Staining
Flow cytometry with annexin V/PI staining was performed according to the protocol provided by the annexin V-FITC/PI double staining apoptosis kit. Briefly, the treated cell culture was discarded, and a appropriate amount of trypsin digested cells were collected into a centrifuge tube and centrifuged at 1200g for 5 min. Buffer was added to the cell precipitate, followed by FITC annexin V staining solution (green light). The sample was thoroughly mixed and stained for 5 min; then, PI staining solution (red light) was added, and the mixture was incubated for 5 min.
After staining, an appropriate amount of PBS (100–500 μL) was added according to the number of cells. Apoptotic cell rates were analyzed using flow cytometry (FACSCalibur).
2.9. Western Blot Analysis
Western blot analysis was carried out as described previously.17 The total proteins from cells were extracted with RIPA lysis buffer containing 1% PMSF, and the protein concentration was determined using the BCA protein assay kit following the provided protocol. The protein lysate was denatured by boiling it at 100 °C for 7 min. Protein samples from LO2 cells were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes by electrophoretic transfer. Membranes were blocked at room temperature for 2 h with 5% skimmed milk. The membranes were incubated with the primary antibodies including Akt (1:1000), p-Akt (1:1000), caspase 3/9 (1:1000), cl-caspase 3/9 (1:1000), Bcl-2 (1:1000), Bax (1:1000), NF-κB (1:1000), p-NF-κB (1:1000), IKKα/β (1:1000), p-IKKα/β (1:1000), IκBα (1:1000), p-IκBα (1:1000), and β-actin (1:5000) overnight at 4 °C. Following that, samples were washed three times with TBST containing 0.1% Tween-20 and were then incubated with second antibody at room temperature for 1 h. After the end of the incubation, the blotting membrane was fully washed. Protein bands were detected by chemiluminescence using the ECL detection kit (Pierce Chemical Co., Rockford, IL). A density analysis was performed using Quantity One software.
2.10. Statistical Analysis
All data were presented with mean ± standard deviation (mean ± S.D.). The difference between groups was analyzed by a two-tailed Student’s t test or one-way analysis of variance (ANOVA). Results as data plots were created using GraphPad Prism 8.2 software (GraphPad Software, Inc., San Diego, CA). For statistical tests, p < 0.05, p < 0.01, and p < 0.001 were set to measure the level of significance.
3. Results
3.1. Effect of ABL on Viability in Alcohol-Induced LO2 Cells
To evaluate the cytotoxicity induced by alcohol, the viability of LO2 cells stimulated with different concentrations (50–800 mM) of ethanol for 24 h was determined using an MTT assay. As shown in Figure 1B, the cell viability of LO2 decreased in a manner dependent on the increase of alcohol concentration. With an alcohol concentration of 300 mM, LO2 cells had a moderate degree of toxicity. At this time, the cell survival rate was reduced to 66.87 ± 2.25%; therefore, an alcohol concentration of 300 mM was selected for subsequent administration.
In addition, we investigated whether ABL has a protective effect on alcohol-induced damage to LO2 cells. As shown in Figure 1C, the results showed that the concentration of ABL was in the range 0.5–2 μM; the survival rate of LO2 cells was significantly improved, and the cytotoxicity was effectively reduced (p < 0.05, p < 0.01). Based on the above results, the ABL doses of 0.5, 1, and 2 μM were selected for further mechanistic experimental studies.
3.2. Effect of ABL on Oxidative Stress of LO2 Cells Induced by Alcohol
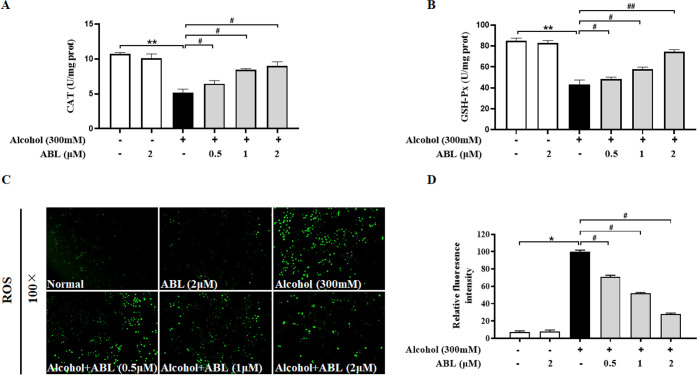
As shown in Figure 2A,B, in comparison with the control group, the activities of GSH-Px and CAT decreased abnormally under exposure to alcohol; this decreasing trend was significantly reversed by ABL pretreatment in a dose-dependent manner (p < 0.05, p < 0.01). Furthermore, the DCFH-DA probe was used to detect the fluorescence expression of ROS in LO2 cells in this study. As shown in Figure 2C,D, the positive expression of ROS after alcohol stimulation was significantly increased as compared to the control (p < 0.05); however, compared with alcohol treatment alone, the green fluorescence intensity of ABL was significantly reduced after 24 h of pretreatment (p < 0.05). The above results fully confirm that LO2 cells were protected from alcohol-induced oxidative stress by ABL.
Figure 2.
Effects of different concentrations of ABL against alcohol-induced oxidative stress. Levels of (A) catalase (CAT) and (B) glutathione peroxidase (GSH-Px) in alcohol-induced LO2 cell injury. (C) Improvement effects of ABL with different concentrations (0.5, 1, and 2 μM) on reactive oxygen species generation in alcohol-induced LO2 cells. (D) The relative levels of fluorescence intensity were quantified at a magnification of ×100. * p < 0.05, ** p < 0.01 vs control group; #p < 0.05, ##p < 0.01 vs alcohol-treated group.
3.3. Effect of ABL on Alcohol-Induced Apoptosis in LO2 Cells
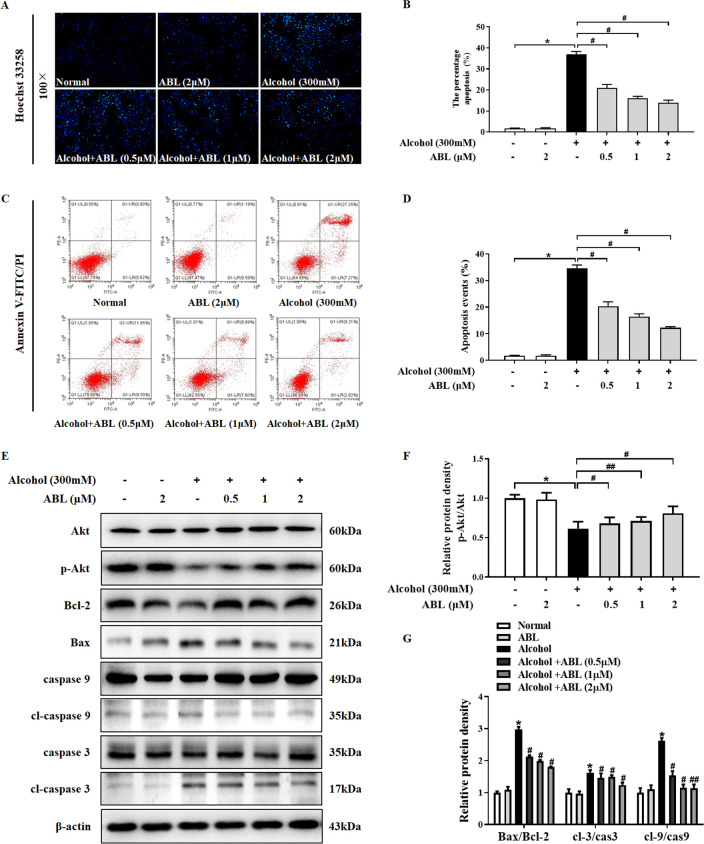
To examine the protective effects of ABL on alcohol-induced cell apoptosis, Hoechst 33258 staining was used to observe morphological changes of cell apoptosis. As shown in Figure 3A,B, no apoptotic cell nuclei were observed in the control group, and there is a complete morphology and clear outline. Simultaneously, the nuclei of the group with alcohol treatment alone showed dense staining and granular bright blue fluorescence, indicating that LO2 cells underwent apoptosis after alcohol stimulation. However, the preadministration of ABL effectively reversed the abnormal phenomenon of LO2 cell nuclei in a concentration-dependent manner (p < 0.05). Moreover, we analyzed the apoptosis rate by annexin V-FITC/PI double staining. As indicated in Figure 3C,D, the average apoptotic rate of LO2 cells after 24 h of incubation in 300 mM alcohol was 35.42%, which was significantly higher than 2.21% of the control group (p < 0.05). Compared with the alcohol treatment group, ABL was pretreated at 0.5, 1, and 2 μM concentrations for 24 h; the percentage of apoptotic cells was reduced to 21.4%, 17.5%, and 13.31% (p < 0.05), respectively. The above results confirmed that ABL pretreatment prevented alcohol-induced LO2 cell apoptosis.
Figure 3.
Effect of ABL against alcohol-induced apoptosis in LO2 cells. (A) LO2 cells were stained with Hoechst 33258 (100×). (B) Quantitative analysis of alcohol-induced apoptosis in each group. (C) The effect of ABL on early apoptosis of LO2 cells was tested by flow cytometry. (D) Quantitative analysis of early cell apoptosis rate of LO2 cells. * p < 0.05 vs control group; #p < 0.05 vs alcohol-treated group. (E) Western blot analysis of the protein expressions of Akt, p-Akt, Bax, Bcl-2, caspase 3/9, and cl-caspase 3/9. (F) The ratio of p-Akt/Akt was quantitatively analyzed. (G) Quantitative analysis of the ratios of Bax/Bcl-2, cl-caspase 3/caspase 3, and cl-caspase 9/caspase 9. * p < 0.05 vs control group; #p < 0.05, ##p < 0.01 vs alcohol-treated group.
3.4. ABL Improves Alcohol-Induced Cell Apoptosis by Regulating Akt and Caspase Signaling Pathways
To further clarify the regulatory mechanism of ABL pretreatment on alcohol-induced hepatotoxicity. We analyzed the expression of Akt and its downstream apoptosis-related proteins by Western blot. As shown in Figure 3E–G, when stimulated by alcohol alone, the phosphorylation of Akt was reduced in LO2 cells; meanwhile, the expression levels of pro-apoptotic protein Bax, cl-caspase 3, and cl-caspase 9 were significantly increased (p < 0.05), and the expression of antiapoptotic protein Bcl-2 was significantly decreased (p < 0.05). However, as the concentration of ABL increased, Akt was activated; the ratio of Bcl-2/Bax increased, and the phosphorylation levels of caspase 3 and caspase 9 were significantly inhibited (p < 0.05, p < 0.01). These results suggest that the protective effect of ABL on alcohol-induced hepatotoxicity may be achieved by activating Akt to inhibit hepatocyte apoptosis.
3.5. ABL Inhibits the Inflammatory Response Mediated by the NF-κB Signaling Pathway
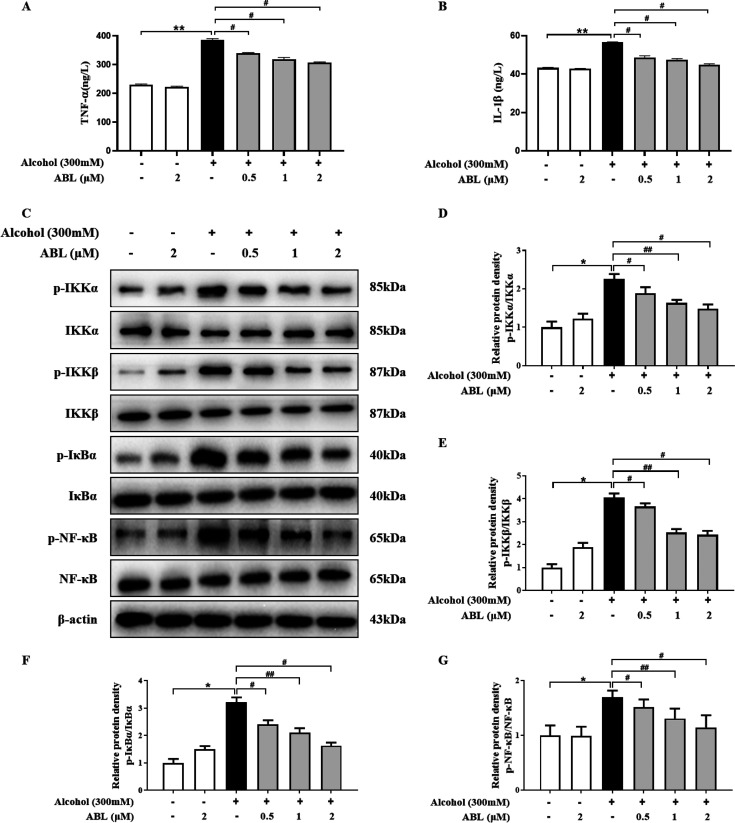
To investigate whether alcohol-induced hepatotoxicity was related to inflammation, we measured the content of inflammatory cytokines in LO2 cells by using an ELISA kit, including TNF-α and IL-1β. As shown in Figure 4A,B, the levels of TNF-α and IL-1β increased significantly after alcohol treatment alone (p < 0.01). However, the different doses of ABL significantly inhibited the secretion of proinflammatory factors (p < 0.05), suggesting that ABL has a potential anti-inflammatory effect on ALD.
Figure 4.
Effects of ABL against alcohol-induced cytotoxicity by regulating the inflammation response. (A) Determination of TNF-α using ELISA assay kits. (B) Determination of IL-1β using ELISA assay kits. (C) The expression levels of IKKα/β, p-IKKα/β, IκBα, p-IκBα, NF-κB, and p-NF-κB were measured by Western blotting. The bar graph shows the relative expression of the protein for (D) p-IKKα/IKKα, (E) p-IKKβ/IKKβ, (F) p-IκBα/IκBα, and (G) p-NF-κB/NF-κB. The results are expressed as mean ± SD (n = 3). * p < 0.05 vs control group; #p < 0.05, ##p < 0.01 vs alcohol-treated group.
To further explore the anti-inflammatory effect of ABL, the phosphorylation and total protein expression levels of NF-κB signaling pathway-related proteins were tested by Western blotting analysis. The results showed that the phosphorylation levels of NF-κB p65 and its upstream regulatory factors IKKα/β and IκBα were significantly decreased by ABL treatment (p < 0.05). In addition, we also found that the ratios of p-IKKα/IKKα, p-IKKβ/IKKβ, p-IκBα/IκBα, and p-NF-κB/NF-κB were significantly reduced in a dose-dependent manner (p < 0.05, p < 0.01), which demonstrated that ABL can effectively improve hepatocyte inflammation evoked by alcohol (Figure 4C–G).
4. Discussion
With the continuous expansion of the drinking population, the damage of alcohol to the body and its influence on social lifestyles have gradually become prominent, which has become a medical problem of global concern. Due to the key role of the liver in the process of alcohol intake and metabolism, the effect of alcohol on liver tissue and liver function, especially the damage effect on liver cells, has attracted the attention of many researchers.18 ALD refers to a series of progressive liver diseases caused by the body drinking a large amount of alcohol, including alcoholic fatty liver, alcoholic hepatitis, and alcoholic liver fibrosis leading to severe cirrhosis and liver cancer.19 In recent years, with an in-depth understanding of alcoholic liver injury by domestic and foreign researchers, it has been found that oxidative stress-related signaling pathways, inflammation, and apoptosis play an important regulatory role in alcohol-evoked hepatotoxicity.20 In addition, some prospective studies have shown that Chinese herb extracts have obvious advantages in the prevention and treatment of ALD and have a great promotion effect on the treatment of liver diseases.21−23
In this study, we evaluated the protective effects of ABL against alcohol-induced hepatotoxicity using an LO2 cell injury model. The results showed that ABL alleviated oxidative stress by inhibiting ROS and increasing the activity of antioxidant enzymes. Furthermore, we found that ABL pretreatment attenuated the expression of caspase-family apoptosis-related proteins and inhibited NF-κB pathway-mediated inflammatory responses through activation of Akt proteins. These results preliminarily reveal that the hepatoprotective effect of ABL may be partially achieved through regulation of the ROS/Akt/NF-κB signaling pathway.
Multiple studies have shown that oxidative stress plays a vital role in alcoholic liver disease.24 The cytochrome P450 2E1 enzyme (CYP2E1) is involved in alcohol metabolism when the alcohol concentration in the body is elevated. Under the action of CYP2E1, a large amount of ROS is produced. Due to the accumulation of ROS, the endogenous antioxidant capacity of the body is weakened, and it is difficult to remove excessive free radicals, resulting in the lipid peroxidation reaction of the liver cell membrane and ultimately liver cell damage.25 Previous studies have confirmed that alcohol intake induces an increase in the level of lipid peroxidation parameter MDA in the liver of mice, thereby causing liver tissue damage and the destruction of the antioxidant defense system.26 However, SOD, CAT, and GSH-Px are important components of the antioxidant enzyme defense system. They play a protective role in oxidative stress by catalyzing the breakdown of hydrogen peroxide in cells and blocking the damage caused by oxygen free radicals.27 Our data showed that, compared with the control group, the activity of the two antioxidant enzymes CAT and GSH-Px in the group with alcohol treatment alone was significantly reduced. However, the activity of these enzymes was preserved through ABL pretreatment, which prevented alcohol-induced oxidative damage to LO2 cells. Moreover, our research has found that the excessive accumulation of ROS after alcohol stimulation is effectively inhibited by treatment with different concentrations of ABL, which is consistent with previous reports.28
In the pathogenesis of alcohol-induced liver toxicity, apoptosis is also considered to be a major feature.29 Previous studies have found that alcohol accumulation in mitochondria causes a large amount of ROS production and mitochondrial dysfunction, resulting in increased mitochondrial permeability, and induces apoptosis.30 Our research found that, after alcohol stimulation, LO2 cells showed typical chromatin condensation and nuclear lysis, confirming that liver cells undergo apoptosis. Interestingly, the increase in the apoptosis rate of LO2 cells detected by annexin V-FITC/PI also supports this result. ABL treatment gradually restored abnormal nuclear morphology and inhibited hepatocyte apoptosis. Importantly, Akt, as a key protein in the downstream signaling pathway of PI3K, participates in the regulation of cell proliferation, metabolism, apoptosis, and migration.31 Much research has suggested that excessive accumulation of ROS promotes the translocation of apoptotic proteins to mitochondria by inhibiting the activation of the PI3K/Akt signaling pathway.32 After Akt is activated, its downstream target proteins (such as Bcl-2 and Bax) are regulated, while inhibiting the activity of the proteolytic enzyme caspase 9, preventing the initiation of the apoptotic cascade and inhibiting cell apoptosis.33 In this study, we found that Akt activation was inhibited after alcohol treatment, while Bax and caspase 3/9 phosphorylation levels increased, and Bcl-2 expression levels decreased. However, ABL significantly reversed the expression of these proteins. Our results further demonstrated that ABL effectively inhibits alcohol-induced apoptosis of LO2 cells and promoted signaling for cell survival.
NF-κB, as a downstream pathway of the PI3K/Akt pathway, is also one of the critical pathological mechanisms of ALD.34 The NF-κB signaling pathway not only regulates transcription factors, including chemokines, cytokines, and adhesion molecules, but also regulates the activity of the gene itself. Excessive production of inflammatory cytokines including IL-1β and TNF-α leads to the accumulation of inflammatory cells, thereby causing damage to liver tissue. Inhibition of the release of inflammatory factors can restore abnormal liver function. It has been reported that, after alcohol stimulation, the inhibitory subunit IκB is released from the complex; the p65 subunit is translocated from the cytoplasm to the nucleus, and the transcription of specific target genes is initiated in the nucleus, which in turn leads to liver damage.35 Previous studies have confirmed and proved that ABL can reduce the inflammatory response induced by LPS by blocking the nuclear translocation of NF-κB p65 and inhibiting the phosphorylation and degradation of IκBα.36 Some data also suggest that alcohol exposure promotes the release of cytokines IL-1β and TNF-α.37 Therefore, it reduces alcohol-induced liver toxicity by preventing the production of IL-1β and TNF-α. In our study, ABL significantly inhibited the increase of IL-1β and TNF-α caused by alcohol. In addition, we detected the related proteins of the NF-κB signaling pathway by Western blotting and further found that ABL effectively inhibits the activation of NF-κB in alcohol liver toxicity by reducing the expression levels of p-IKKα/β, p-IκBα, and p-NF-κB. These results support the potential role of ABL in improving the inflammatory response of ALD.
In summary, our study reveals for the first time the protective effect of ABL on alcohol-induced liver injury in vitro by reducing ROS-induced oxidative stress, inhibiting cell apoptosis and NF-κB-mediated inflammation by regulating the Akt. The unique pharmacological action of ABL in the treatment of liver diseases was further expounded. Meanwhile, our study lays a sufficient material foundation and theoretical reference for the in-depth development of Inula britannica L. chemical composition liver protection drugs.
Acknowledgments
This work was supported by the grant of Jilin Science & Technology Development Plan (20200301037 RQ and 20200404007YY). L.-y.X., Z.Y., S.J., and W.L. received funding from 20200301037 RQ. Y.W., J.-n.H., Y.-w.L., and H.Z. received funding from 20200404007YY.
Glossary
Abbreviations
- ABL
1-O-acetylbritannilactone
- ALD
alcoholic liver disease
- LO2
human normal liver cell
- ROS
reactive oxygen species
- GSH-Px
glutathione peroxidase
- CAT
catalase
- Akt
protein kinase B
- p-Akt
phosphorylated protein kinase B
- Bcl-2
B-cell-lymphoma-2
- Bax
Bcl2-associated X protein
- cl-caspase 3
cleaved-caspase 3
- cl-caspase 9
cleaved-caspase 9
- TNF-α
tumor necrosis factor-α
- IL-1β
interleukin
- NF-κB
nuclear factor-kappa B
- IKB
I kappa B kinases
- IKK
inhibitor of kappa B kinase complex
Author Contributions
§ L.-y.X. and Z.Y. contributed equally to this work and are considered as cofirst authors. W.L. and S.J. conceived and designed the experiments. Z.Y. contributed to the reagents/materials/experimental instruments. L.-y.X. drafted and revised the manuscript. J.-n.H. revised the manuscript. L.-y.X. and Y.W. completed the experiment together. Y.-w.L. and H.Z. jointly analyzed the recorded data. All authors reviewed and approved the contents of the manuscript.
The authors declare no competing financial interest.
References
- Li B.; Mao Q.; Zhou D.; Luo M.; Gan R.; Li H.; Huang S.; Saimaiti A.; Shang A.; Li H. Effects of Tea against Alcoholic Fatty Liver Disease by Modulating Gut Microbiota in Chronic Alcohol-Exposed Mice. Foods 2021, 10 (6), 1232. 10.3390/foods10061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi F. A.; Sajja K. C.; Latt N. L. Current Management of Alcohol-Associated Liver Disease. Gastroenterol Hepatol (N Y) 2020, 16 (11), 561–570. [PMC free article] [PubMed] [Google Scholar]
- Pan J. H.; Lee K. Y.; Kim J. H.; Shin H.; Lee J. H.; Kim Y. J. Prunus mume Sieb. et Zucc. fruit ameliorates alcoholic liver injury in mice by inhibiting apoptosis and inflammation through oxidative stress. J. Funct Foods 2016, 25, 135–148. 10.1016/j.jff.2016.04.024. [DOI] [Google Scholar]
- Hsu M. F.; Koike S.; Mello A.; Nagy L. E.; Haj F. G. Hepatic protein-tyrosine phosphatase 1B disruption and pharmacological inhibition attenuate ethanol-induced oxidative stress and ameliorate alcoholic liver disease in mice. Redox Biol. 2020, 36, 101658. 10.1016/j.redox.2020.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L.; Kang K.; Sun Y.; Li Y.; Chang B. FOXO4 ameliorates alcohol-induced chronic liver injury via inhibiting NF-kappaB and modulating gut microbiota in C57BL/6J. mice. Int. Immunopharmacol 2021, 96, 107572. 10.1016/j.intimp.2021.107572. [DOI] [PubMed] [Google Scholar]
- Wang X.; Tang S. A.; Wang R.; Qiu Y.; Jin M.; Kong D. Inhibitory Effects of JEUD-38, a New Sesquiterpene Lactone from Inula japonica Thunb, on LPS-Induced iNOS Expression in RAW264.7 Cells. Inflammation 2015, 38 (3), 941–948. 10.1007/s10753-014-0056-2. [DOI] [PubMed] [Google Scholar]
- Lee J.; Hwangbo C.; Lee J. J.; Seo J.; Lee J. H. The sesquiterpene lactone eupatolide sensitizes breast cancer cells to TRAIL through down-regulation of c-FLIP expression. Oncol. Rep. 2010, 23 (1), 229–237. [PubMed] [Google Scholar]
- Dong M.; Hong T.; Liu S.; Zhao J.; Meng Y.; Mu J. Hepatoprotective effect of the flavonoid fraction isolated from the flower of Inula britannica against D-Galactosamine-induced hepatic injury. Mol. Med. Rep 2013, 7 (6), 1919–23. 10.3892/mmr.2013.1443. [DOI] [PubMed] [Google Scholar]
- Bosio C.; Tomasoni G.; Martinez R.; Olea A. F.; Carrasco H.; Villena J. Cytotoxic and apoptotic effects of leptocarpin, a plant-derived sesquiterpene lactone, on human cancer cell lines. Chem-Biol. Interact 2015, 242, 415–421. 10.1016/j.cbi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Kobayashi T.; Song Q. H.; Hong T.; Kitamura H.; Cyong J. C. Preventative effects of the flowers of Inula britannica on autoimmune diabetes in C57BL/KsJ mice induced by multiple low doses of streptozotocin. Phytother Res. 2002, 16 (4), 377–382. 10.1002/ptr.868. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Diao Y.; Wang C.; Qu W.; Zhao X.; Ma H.; Shan J.; Sun G. Structural characters and protecting beta-cells of a polysaccharide from flowers of Inula japonica. Int. J. Biol. Macromol. 2017, 101, 16–23. 10.1016/j.ijbiomac.2017.03.044. [DOI] [PubMed] [Google Scholar]
- Song Q. H.; Kobayashi T.; Iijima K.; Hong T.; Cyong J. C. Hepatoprotective effects of Inula britannica on hepatic injury in mice. Phytother Res. 2000, 14 (3), 180–186. . [DOI] [PubMed] [Google Scholar]
- Iijima K.; Kiyohara H.; Tanaka M.; Matsumoto T.; Cyong J. C.; Yamada H. Preventive effect of taraxasteryl acetate from Inula britannica subsp. japonica on experimental hepatitis in vivo. Planta Med. 1995, 61 (1), 50–53. 10.1055/s-2006-957998. [DOI] [PubMed] [Google Scholar]
- Xing J. J.; Hou J. G.; Ma Z. N.; Wang Z.; Ren S.; Wang Y. P.; Liu W. C.; Chen C.; Li W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Proliferat 2019, 52 (4), 11137–11149. 10.1111/cpr.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Yan X. T.; Zhao L. C.; Ren S.; He Y. F.; Liu W. C.; Wang Z.; Li X. D.; Jiang S.; Li W. alpha-Mangostin, a Dietary Xanthone, Exerts Protective Effects on Cisplatin-Induced Renal Injury via PI3K/Akt and JNK Signaling Pathways in HEK293 Cells. Acs Omega 2020, 5 (32), 19960–19967. 10.1021/acsomega.0c01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. N.; Xu X. Y.; Jiang S.; Liu Y.; Liu Z.; Wang Y. P.; Gong X. J.; Li K. K.; Ren S.; Li W. Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells. Kaohsiung J. Med. Sci. 2020, 36 (9), 732–740. 10.1002/kjm2.12220. [DOI] [PubMed] [Google Scholar]
- Xing J. J.; Hou J. G.; Liu Y.; Zhang R. B.; Jiang S.; Ren S.; Wang Y. P.; Shen Q.; Li W.; Li X. D.; Wang Z. Supplementation of Saponins from Leaves of Panax quinquefolius Mitigates Cisplatin-Evoked Cardiotoxicity via Inhibiting Oxidative Stress-Associated Inflammation and Apoptosis in Mice. Antioxidants 2019, 8 (9), 347. 10.3390/antiox8090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J.; Wang F.; Wong N. K.; He J.; Zhang R.; Sun R.; Xu Y.; Liu Y.; Li W.; Koike K.; He W.; You H.; Miao Y.; Liu X.; Meng M.; Gao B.; Wang H.; Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol 2019, 71 (1), 212–221. 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Bertola A.; Mathews S.; Ki S. H.; Wang H.; Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc 2013, 8 (3), 627–637. 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W.; Chen C.; Feng H.; Li G.; Peng W.; Liu X.; Yang J.; Hu X. Protection of Ficus pandurata Hance against acute alcohol-induced liver damage in mice via suppressing oxidative stress, inflammation, and apoptosis. J. Ethnopharmacol 2021, 275, 114140. 10.1016/j.jep.2021.114140. [DOI] [PubMed] [Google Scholar]
- Rong S.; Zhao Y.; Bao W.; Xiao X.; Wang D.; Nussler A. K.; Yan H.; Yao P.; Liu L. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine 2012, 19 (6), 545–550. 10.1016/j.phymed.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Liu X.; Wang Y.; Wu D.; Li S.; Wang C.; Han Z.; Wang J.; Wang K.; Yang Z.; Wei Z. Magnolol Prevents Acute Alcoholic Liver Damage by Activating PI3K/Nrf2/PPARgamma and Inhibiting NLRP3 Signaling Pathway. Front Pharmacol 2019, 10, 1459–1469. 10.3389/fphar.2019.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; He X.; Zhao J.; Huang W. Hepatoprotection by Ginsenoside Rg1 in alcoholic liver disease. Int. Immunopharmacol 2021, 92, 107327. 10.1016/j.intimp.2020.107327. [DOI] [PubMed] [Google Scholar]
- Li L.; Wu Y.; Yin F.; Feng Q.; Dong X.; Zhang R.; Yin Z.; Luo L. Fructose 1, 6-diphosphate prevents alcohol-induced liver injury through inhibiting oxidative stress and promoting alcohol metabolism in mice. Eur. J. Pharmacol. 2017, 815, 274–281. 10.1016/j.ejphar.2017.09.034. [DOI] [PubMed] [Google Scholar]
- Wang C.; Nie G.; Yang F.; Chen J.; Zhuang Y.; Dai X.; Liao Z.; Yang Z.; Cao H.; Xing C.; Hu G.; Zhang C. J. J. o. h. m. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells 2020, 383, 121157. 10.1016/j.jhazmat.2019.121157. [DOI] [PubMed] [Google Scholar]
- El-Newary S. A.; Shaffie N. M.; Omer E. A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac J. Trop Med. 2017, 10 (4), 361–371. 10.1016/j.apjtm.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Weydert C. J.; Cullen J. J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc 2010, 5 (1), 51–66. 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S.; Tang J. J.; Zhang C. C.; Tian J. M.; Guo J. T.; Zhang Q.; Li H.; Gao J. M. Semisynthesis and in vitro cytotoxic evaluation of new analogues of 1-O-acetylbritannilactone, a sesquiterpene from Inula britannica. Eur. J. Med. Chem. 2014, 80, 71–82. 10.1016/j.ejmech.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Pan J. H.; Kim H.; Tang J.; Beane K. E.; Park J. W.; Kong S.; Kong B. C.; Kim Y. J.; Shin E. C.; Kim J. H.; Zhao J.; Lee J. H.; Kim J. K. Acute alcohol consumption-induced let-7a inhibition exacerbates hepatic apoptosis by regulating Rb1 in mice. Alcohol 2020, 85, 13–20. 10.1016/j.alcohol.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Ma X. Y.; Zhang M.; Fang G.; Cheng C. J.; Wang M. K.; Han Y. M.; Hou X. T.; Hao E. W.; Hou Y. Y.; Bai G. Ursolic acid reduces hepatocellular apoptosis and alleviates alcohol-induced liver injury via irreversible inhibition of CASP3 in vivo. Acta Pharmacol Sin 2021, 42 (7), 1101–1110. 10.1038/s41401-020-00534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Li D.; Wei M.; Du R.; Yan Z. The ester derivatives obtained by C-ring modification of podophyllotoxin induce apoptosis and inhibited proliferation in PC-3M cells via down-regulation of PI3K/Akt signaling pathway. Bioorg. Med. Chem. Lett. 2021, 46, 128174. 10.1016/j.bmcl.2021.128174. [DOI] [PubMed] [Google Scholar]
- Li X. L.; Chen M. C.; Yao Z. C.; Du H. Z.; Zhang T. F.; Wang H. Y.; Xie Y.; Li Z. N. Jujuboside B induces mitochondrial-dependent apoptosis in colorectal cancer through ROS-mediated PI3K/Akt pathway in vitro and in vivo. J. Funct Foods 2021, 87, 104796. 10.1016/j.jff.2021.104796. [DOI] [Google Scholar]
- Zhang J. J.; Wang J. Q.; Xu X. Y.; Yang J. Y.; Wang Z.; Jiang S.; Wang Y. P.; Zhang J.; Zhanga R.; Li W. Red ginseng protects against cisplatin-induced intestinal toxicity by inhibiting apoptosis and autophagy via the PI3K/AKT and MAPK signaling pathways. Food & Function 2020, 11 (5), 4236–4248. 10.1039/D0FO00469C. [DOI] [PubMed] [Google Scholar]
- Wen B.; Zhang C.; Zhou J.; Zhang Z.; Che Q.; Cao H.; Bai Y.; Guo J.; Su Z. Targeted treatment of alcoholic liver disease based on inflammatory signalling pathways. Pharmacol Ther 2021, 222, 107752. 10.1016/j.pharmthera.2020.107752. [DOI] [PubMed] [Google Scholar]
- Liu X. Y.; Chen G. N.; Du G. M.; Pan Y.; Song W. Q.; Jiang T. W.; Liu H. L. Berbamine ameliorates ethanol-induced liver injury by inhibition of hepatic inflammation in mice. Chin J. Nat. Med. 2020, 18 (3), 186–195. 10.1016/S1875-5364(20)30020-0. [DOI] [PubMed] [Google Scholar]
- Liu Y. P.; Wen J. K.; Wu Y. B.; Zhang J.; Zheng B.; Zhang D. Q.; Han M. 1,6-O,O-diacetylbritannilactones inhibits IkappaB kinase beta-dependent NF-kappaB activation. Phytomedicine 2009, 16 (2–3), 156–160. 10.1016/j.phymed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lai J. R.; Hsu Y. W.; Pan T. M.; Lee C. L. Monascin and Ankaflavin of Monascus purpureus Prevent Alcoholic Liver Disease through Regulating AMPK-Mediated Lipid Metabolism and Enhancing Both Anti-Inflammatory and Anti-Oxidative Systems. Molecules 2021, 26 (20), 320–334. 10.3390/molecules26206301. [DOI] [PMC free article] [PubMed] [Google Scholar]