Abstract
An ecofriendly resorcinol derivative, dimethyl-4,6-dihydroxyisophthalate (DDIP) is examined as an anticorrosion agent for low carbon steel (CS) in a 0.5 mol L–1 HCl solution. Electrochemical and chemical methods are used to determine the effectiveness of the inhibitor. The DDIP compound decreased the rate of CS corrosion. The mitigation efficiency rose from 61.8 to 79.9% as the DDIP dose increased from 50 to 300 ppm in the corrosive medium. At 300 ppm, however, the efficiency decreased from 79.9 to 70.05% as the temperature increased from 25 to 55 °C. Physical quantities and thermodynamic parameters are discussed. The compound’s adsorption follows Langmuir’s concept. Adsorption of the DDIP compound is a mix of physisorption and chemisorption. The difference in Ecorr values is less than 85 mV, indicating that the examined compound is a mixed-type inhibitor. Scanning electron microscopy and atomic force microscopy revealed the development of a coherent film at CS in the presence of the DDIP inhibitor. The results obtained using various techniques were closely related, indicating validity and accuracy. The interaction between the DDIP molecules and the CS was explained by the density functional theory and Monte Carlo simulation. The quantum characteristics confirmed that the DDIP compound is a promising inhibitor.
1. Introduction
Carbon steel (CS) is widely used for drilling and transportation pipelines in the oil and gas industry. The bulk of acidic industrial applications relies on carbon steel, such as pickling, industrial cleaning, and crude oil refining, descaling, and petrochemical processes. CS alloys are also inexpensive and have properties superior to those of other metal alloys. HCl is commonly used in the industrial sector for cleaning and descaling procedures. The fundamental problem with this procedure is that when carbon steel is exposed to an acidic environment, it rusts.1−5 In addition, the acid is also produced during the cracking of petroleum as a result of salt hydrolysis.6
Corrosion occurs when clean Fe metal reverts to its original state, Fe2O3, due to electrochemical reactions with nearby media such as H2S, CO2, and H2O. Corrosion impacts all metallic infrastructure in the oil field such as pipes, tanks, and separators at all stages of production. Pipes, tanks, and plumbing systems develop cracks or pits.7,8 In terms of corrosion, hydrochloric acid is by far the most commonly used acid.9
The use of adsorption inhibitors in acidic conditions is a standout among many other ideals and material ways of managing steel corrosion.10 Because of its importance in applications, the corrosion behavior of CS in acid environments is of great importance. The most efficient procedure for protecting metals from corrosion is using chemical compounds as corrosion inhibitors. These compounds reduce the corrosion rate.11−18 The substance that is considered to be eco-friendly and safe for humans when prepared is the best choice for use as a corrosion inhibitor. Many investigations about the utilization of drugs as potential candidates for metal corrosion mitigation have recently been conducted. Their structures permit the formation of complexes with metallic ions on the metal surfaces.19−23
Resorcinol, a safe compound, is used as an antiseptic and disinfectant in pharmaceutical drugs. Furthermore, it is inexpensive, widely available, nontoxic, and environmentally benign. Because of these characteristics, resorcinol derivatives were selected for corrosion studies.
The primary goal of this research is to examine the inhibition performance of the DDIP compound for CS corrosion in a 0.5 mol L–1 HCl solution. Potentiodynamic polarization (PP), electrochemical impedance spectroscopy (EIS), and weight loss (WL) methods are used in this study. Multiple adsorption isotherms are used to obtain further details concerning the manner of adsorption of the DDIP on the surface of CS. Some thermodynamic and activation parameters were also estimated. Furthermore, the interaction between DDIP molecules and CS was described using DFT and MC simulation.
2. Experimental Details
2.1. Materials and Solutions
The working electrode was a low CS with a composition (wt %) as follows: C 0.20, Mn 0.60, P 0.04, Si 0.003, and Fe the remainder. The chemicals utilized are resorcinol, sodium carbonate, hydrochloric acid (annular acid 37%), sulfuric acid, acetone, ethanol, and methanol, purchased from Sigma-Aldrich. Pt and saturated calomel electrodes (SCE) were utilized as counter and reference electrodes, respectively.
2.2. Synthesis of Resorcinol Derivative, DDIP
The synthesis procedure of the resorcinol derivative was as reported in ref (24). Resorcinol was obtained from Sigma-Aldrich Company. 4,6-Dihydroxyisophthalic acid was used to obtain the DDIP compound by dissolving 10 g in 100 mL of absolute MeOH and adding 5 mL of concentrated H2SO4 dropwise to the solution. The mix was refluxed in a water bath for 6 h, and then it was allowed cool to room temperature. The mixture was poured into Na2CO3 solution and filtered, and the precipitate was crystallized from ethanol with a yield of 78%. Scheme 1 shows the derivative’s schematic preparation.
Scheme 1. Synthesis of Resorcinol Derivative (DDIP).
2.3. WL Procedure
For WL measurements, steel coupons with dimensions of 2 × 2 × 0.2 cm were used. The exposed surface area was mechanically polished with varying grades of emery paper prior to all measurements. The samples were thoroughly washed with bidistilled water, degreased, and dried. The volume of solution was 100 mL, and the immersion duration for the WL was 60, 120, 180, 240, 300, and 360 min at 25, 35, 45, and 55 °C, respectively. Simultaneous triplicate experiments were conducted, and the mean WL value of the three CS sheets was determined.
2.4. Electrochemical Investigations
The electrochemical experiments were done in a three-electrode cell, with Pt gauze serving as the counter electrode, SCE as the reference electrode, and CS as the working electrode. Prior to the test, a 1 cm2 area of the working electrode was treated as in WL experiments and submerged in a testing solution for 30 min to achieve a steady-state potential (OCP). PP measurements were performed by automatically varying the electrode potential of CS from −900 to −100 mV vs SCE. The scan rate was 0.2 mV s–1. For each dose of the DDIP compound and inhibitor-free solutions, the Stern–Geary method25 was used to calculate corrosion current. This was done by extrapolating anodic and cathodic Tafel lines to the point that gave log Icorr and the related corrosion potential (Ecorr). All tests were repeated three times to ensure reliability, and all measurements were performed at 25 °C.
AC pulses at OCP with an amplitude of 5 mV peak to peak were utilized to measure impedance (EIS) in the frequency region of 100 kHz to 50 MHz. The Nyquist and Bode representations of EIS diagrams are provided. A BioLogic SP-150 potentiostat with EC-LAB software was used in all electrochemical studies for potentiodynamic and electrochemical impedance measurements. The data were collected using a personal computer. Origin 2018 and Microsoft Office 2016 were used for plotting, graphing, and fitting data.
2.5. Surface Analysis Study
The CS surface was handled with various abrasive sheets (grades 250 to 1200). The coupons were then rinsed with deionized water before being immersed in 0.5 mol L–1 HCl for 48 h in the absence and presence of the examined organic compound (300 ppm). The coupons were then treated with deionized water, dried, and inserted into a spectrometer with no additional processing. A JEOL JSM-6510 LV scanning electron microscope (SEM) and atomic force microscopy (AFM) (Key sight 5600LS large stage, made in the USA) were used to obtain the images.
2.6. Calculations Involving Quantum Chemistry
The quantum chemical parameters were determined using DFT/6-31+G(d,p) and MC simulations.
3. Results and Discussion
3.1. WL Study
The WL (ΔW) is determined from eq 1.
| 1 |
where ΔW is the WL of the carbon steel specimen and W1 and W2 are the metal weights prior to and after exposure to the corrosive solution, respectively. The corrosion rate, CR, is calculated from eq 2.
| 2 |
where ΔW denotes the WL value, A denotes the total area per cm2, and t signifies the time spent in minutes. Table 1 demonstrates the results of CS tests in 0.5 mol L–1 HCl acid with and without distinct amounts of the DDIP compound. The inhibition efficacy of the compound increases as its amount in the corrosive solution increases.
Table 1. Data of WL Measurements for CS in 0.5 mol L–1 HCl Solution in the Absence and Presence of Different Concentrations of Investigated Compound at 25 °C (Immersion Time = 240 min).
| DDIP | |||
|---|---|---|---|
| concn (ppm) | CR (kg·m–2·s–1) × 10–9 | θ | %IE |
| blank | 78.5 ± 0.3 | ||
| 50 | 30.0 ± 0.2 | 0.618 | 61.8 ± 0.2 |
| 100 | 27.5 ± 0.1 | 0.649 | 65.1 ± 0.1 |
| 150 | 24.2 ± 0.5 | 0.692 | 69.2 ± 0.3 |
| 200 | 20.4 ± 0.4 | 0.740 | 74.0 ± 0.2 |
| 250 | 18.8 ± 0.2 | 0.760 | 76.0 ± 0.4 |
| 300 | 15.8 ± 0.6 | 0.799 | 79.9 ± 0.3 |
3.2. Impact of Temperature on Inhibition Efficacy
The influence of solution temperature on the inhibition efficacy is determined at 25, 35, 45, and 55 °C, and the findings are shown in Tables 2 and 3. The effectiveness of inhibition decreases as the temperature increases. The decrease in inhibition efficacy is ascribed to the detachment of the compound molecules from the steel surface as the temperatures increases.
Table 2. Carbon Steel Corrosion Rate after Immersion in 0.5 mol L–1 HCl with and without Different Concentrations of the DDIP Compound at Different Temperatures.
| DDIP | ||||
|---|---|---|---|---|
| CR (kg·m–2·s–1 × 10–9) at 240 min |
||||
| concn (ppm) | 25 °C | 35 °C | 45 °C | 55 °C |
| blank | 78.00 ± 0.2 | 80.00 ± 0.3 | 83.00 ± 0.5 | 86.00 ± 0.2 |
| 50 | 30.07 ± 0.3 | 33.75 ± 0.2 | 37.08 ± 0.3 | 43.75 ± 0.4 |
| 100 | 27.5 ± 0.5 | 30.42 ± 0.4 | 35.42 ± 0.1 | 39.58 ± 0.1 |
| 150 | 24.25 ± 0.2 | 27.08 ± 0.5 | 32.08 ± 0.4 | 36.67 ± 0.3 |
| 200 | 20.43 ± 0.1 | 25.42 ± 0.4 | 29.17 ± 0.3 | 33.75 ± 0.2 |
| 250 | 18.82 ± 0.3 | 20.83 ± 0.1 | 27.08 ± 0.1 | 32.5 ± 0.5 |
| 300 | 15.86 ± 0.4 | 20.00 ± 0.2 | 21.67 ± 0.2 | 25.83 ± 0.6 |
Table 3. Data of Weight Loss Measurements at 240 min for CS in 0.5 mol L–1 HCl in the Absence and Presence of Different Concentrations of DDIP Compound at Different Temperatures.
| DDIP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 °C |
35 °C |
45 °C |
55 °C |
||||||||
| concn (ppm) | θ | %IE | concn (ppm) | θ | %IE | concn (ppm) | θ | %IE | concn (ppm) | θ | %IE |
| 0 | 0 | 0 | 0 | ||||||||
| 50 | 0.618 | 61.8 ± 0.2 | 50 | 0.574 | 57.37 ± 0.3 | 50 | 0.544 | 54.36 ± 0.3 | 50 | 0.493 | 49.28 ± 0.3 |
| 100 | 0.649 | 65.1 ± 0.1 | 100 | 0.616 | 61.58 ± 0.2 | 100 | 0.564 | 56.41 ± 0.1 | 100 | 0.541 | 54.11 ± 0.2 |
| 150 | 0.692 | 69.2 ± 0.3 | 150 | 0.658 | 65.79 ± 0..3 | 150 | 0.605 | 60.51 ± 0.3 | 150 | 0.575 | 57.49 ± 0.4 |
| 200 | 0.740 | 74.0 ± 0.5 | 200 | 0.679 | 67.89 ± 0.4 | 200 | 0.641 | 64.1 ± 0.4 | 200 | 0.609 | 60.87 ± 0.1 |
| 250 | 0.760 | 76.0 ± 0.4 | 250 | 0.737 | 73.68 ± 0.2 | 250 | 0.667 | 66.67 ± 0.2 | 250 | 0.623 | 62.32 ± 0.5 |
| 300 | 0.799 | 79.9 ± 0.3 | 300 | 0.747 | 74.74 ± 0.4 | 300 | 0.733 | 73.33 ± 0.2 | 300 | 0.700 | 70.05 ± 0.2 |
3.3. Thermodynamic Activation Parameters of the Corrosion Reaction
Thermodynamic parameters are a crucial and significant tool to understand inhibitor adsorption behavior. The activation energy (Ea*), enthalpy change (ΔHa*), and entropy change (ΔSa*) of activation for the dissolution of CS in 0.5 mol L–1 HCl solution were estimated. Arrhenius and transition-state equations were used to calculate the parameters in the absence and presence of the DDIP chemical compound.
| 3 |
| 4 |
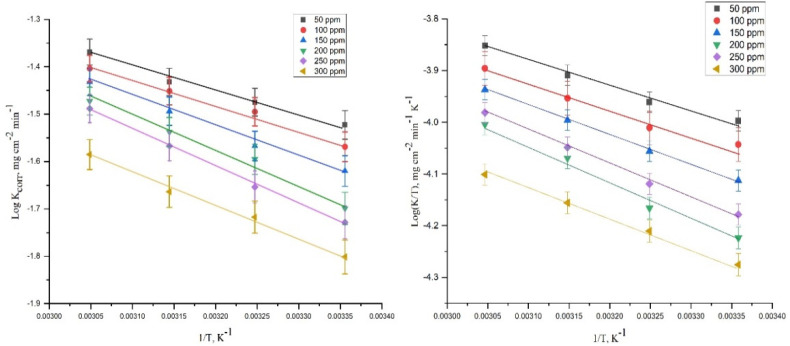
where k is the dissolution rate, R is the gas constant, kB is the Boltzmann constant, T is the Kelvin temperature, and h indicates Planck’s constant. The Arrhenius plot (log k vs 1/T) and transition-state plots (log k/T vs 1/T) of the DDIP compound are shown in Figure 1.
Figure 1.
Arrhenius plots (log k vs 1/T) and transition-state plots (log k/T vs 1/T) for corrosion of carbon steel in 0.5 mol L–1 HCl without and with different concentrations of the compound DDIP.
The Arrhenius plot shows a straight line with a slope of −Ea*/2.303R, through which the Ea value of the impeded dissolution process of CS is evaluated and listed in Table 4. The Ea* has a value of 3.5 kJ mol–1 for the blank solution. Because the DDIP compound inhibits the corrosion process, the activation energy significantly increases with varying concentrations of the organic compound. This variation might be ascribed either to the precipitation of the DDIP compound at the CS surface or a variation in the potential difference of the metal solution boundary caused by adsorption. Transition-state plots exhibit straight lines with slopes of −(ΔHa*)/(2.303R) and intercept log(kB/h) + ΔSa*/R), by which ΔSa* and ΔHa* quantities were evaluated (Table 4). The positivity of ΔHa* reveals that the formation of the activated complex is an endothermic process. The negativity of ΔSa* suggests that the molecules of DDIP were adsorbed in an organized way over the CS surface.26
Table 4. Activation Parameters for the Dissolution of CS in the Absence and Presence of Different Doses of the Compound DDIP in 0.5 mol L–1 HCl.
| concn (ppm) | Ea* (kJ mol–1) | R2 | ΔH* (kJ mol–1) | –ΔS* (J mol–1 K–1) |
|---|---|---|---|---|
| blank | 3.5 | 0.94 | 0.9 | 263 |
| 50 | 5.1 | 0.92 | 2.5 | 268 |
| 100 | 6.5 | 0.95 | 4.2 | 263 |
| 150 | 8.0 | 0.95 | 5.4 | 61 |
| 200 | 8.4 | 0.94 | 5.8 | 60 |
| 250 | 9.6 | 0.94 | 7.1 | 57 |
| 300 | 10.1 | 0.95 | 7.5 | 56 |
3.4. Adsorption Considerations
The adsorption isotherm is required to understand the adsorption mechanism. The experimental data were examined using many adsorption isotherms such as Langmuir, Freundlich, Temkin, and Flory–Huggins to learn more about the DDIP’s adsorption at CS. For the Langmuir model, the best fit is recognized with a regression coefficient of R2 = 0.9994, confirming the feasibility of this approach.27 The Langmuir adsorption isotherm presumes that all adsorption centers are equivalent and possess similar energy characteristics.28 The preceding formula is used to compute the Langmuir isotherm.
| 5 |
Kads denotes the equilibrium constant for adsorption. The Langmuir isotherm of the compound DDIP at the CS surface is shown in Figure 2. Table 5 comprises data gathered from this isotherm. The departure of the slopes from unity is ascribed to interactions of the adsorbed organic species, a component that is not considered throughout the development of the Langmuir equation. The following equation is used to compute the standard free energy change of the adsorption (ΔG°ads).29
| 6 |
where 55.5 is the molar quantity of H2O. The fact that ΔG°ads values are negative confirms the durability of the adsorbed film at the CS surface.30 It has been reported that ΔG°ads values up to −20 kJ mol–1 are preserved for electrostatic attraction between charged molecules and charged metals. However, those greater than −40 kJ mol–1 implicate electron transfer from the inhibitor to the metal surface to form a coordinating bond.31 The ΔG°ads reported in this study ranged between −25.8 and −28.4 kJ mol–1. This verifies that the DDIP inhibitor reduces the rate of corrosion of CS via physisorption and chemisorption processes. A good agreement with the Langmuir isotherm, according to Latour, does not imply that the model is acceptable for the adsorption process. Perhaps the value obtained for Keq could be regarded as a semiquantitative descriptor of the isotherm shape.32
Figure 2.
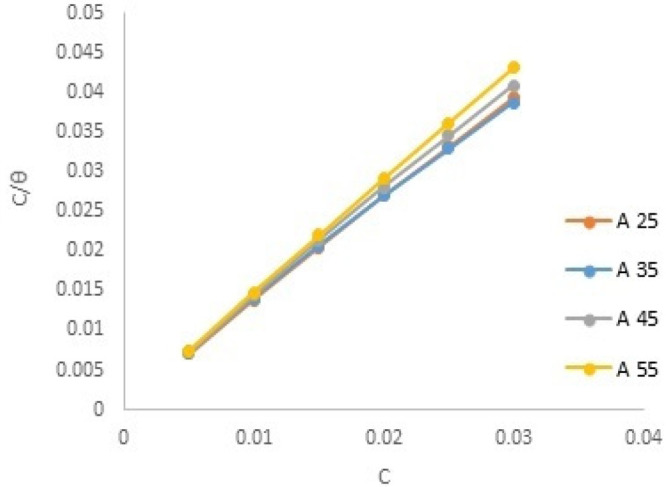
Langmuir adsorption isotherms for CS in 0.5 mol L–1 HCl in the presence of the DDIP compound at different temperatures.
Table 5. Equilibrium Constant Kads and Standard Free Energy ΔG°ads of Adsorption of the DDIP Compound on CS in 0.5 mol L–1 HCl at Different Temperatures.
| DDIP | |||||
|---|---|---|---|---|---|
| temp (°C) | slope | intercept × 10–5 | Kads × 10–3 (mol–1) | –ΔG° (kJ mol–1) | R2 |
| 25 | 1.29 | 91.5 | 1092.8 | 27.3 | 0.9995 |
| 35 | 1.26 | 137.6 | 726.6 | 25.8 | 0.9995 |
| 45 | 1.34 | 96.8 | 1033.5 | 26.7 | 0.9995 |
| 55 | 1.43 | 48.9 | 2084.1 | 28.4 | 0.9999 |
3.5. PP Measurements
Figure 3 depicts the Tafel plots of CS in 0.5 mol L–1 HCl in the presence and absence of varying dosages of the DDIP organic compound at 25 °C. Table 6 shows the derived kinetic factors such as corrosion current (Icorr), Tafel slopes (βc and βa), corrosion potential (Ecorr), and inhibition efficacy (IE). In the presence of the DDIP compound, Icorr decreases; however, %IE increases. Also, βa and βc were unaffected. The Tafel lines ran parallel, indicating that the inhibitory action of the DDIP compound is caused by simply blocking the accessible surface area by adsorption; that is, the examined compound reduces metal disintegration and hydrogen evolution while not affecting the reaction mechanism.33 The difference in Ecorr values does not attain 85 mV, indicating that the examined compound is a mixed-type inhibitor.
Figure 3.
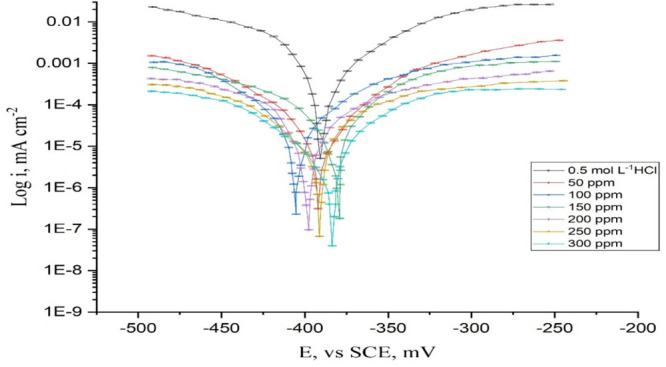
Plots of potentiodynamic polarization measurements for dissolution of CS without and with different concentrations of compound DDIP at 25 °C.
Table 6. Potentiodynamic Polarization Parameters of CS in 0.5 mol L–1 HCl Containing Different Concentrations of the Compound DDIP at 25 °C.
| DDIP | ||||||
|---|---|---|---|---|---|---|
| concn (ppm) | –Ecorr (V vs SCE) × 10–3 | βa (V dec–1) × 10–3 | βc (−V dec–1) × 10–3 | Icorr (A/cm2) × 10–3 | θ | %IE |
| blank | 391 | 242 | 322 | 1.52 | ||
| 50 | 393 | 228 | 288 | 0.60 | 0.605 | 60.5 ± 0.2 |
| 100 | 406 | 207 | 250 | 0.53 | 0.651 | 65.1 ± 0.1 |
| 150 | 380 | 190 | 248 | 0.49 | 0.677 | 67.7 ± 0.3 |
| 200 | 398 | 189 | 220 | 0.41 | 0.730 | 73.0 ± 0.4 |
| 250 | 390 | 182 | 190 | 0.35 | 0.769 | 76.9 ± 0.5 |
| 300 | 384 | 118 | 150 | 0.27 | 0.822 | 82.2 ± 0.1 |
3.6. EIS Measurements
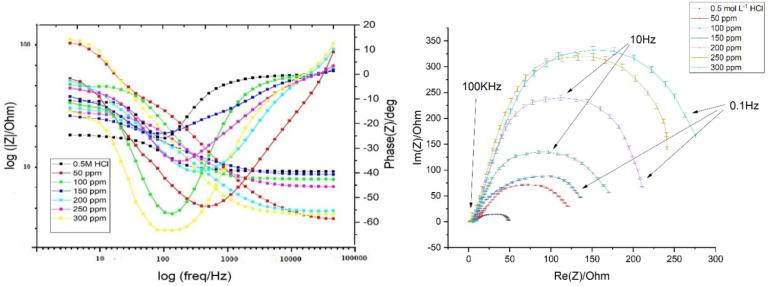
Table 7 summarizes the data derived from EIS measurements for the dissolution of CS in the presence of the DDIP compound and 0.5 mol L–1 HCl at room temperature. Figure 4 depicts the Nyquist (right) and Bode (left) plots of CS in 0.5 mol L–1 HCl, in the absence and presence of varying dosages of the DDIP compound. The impedance spectra have only one semicircle. This suggests that the dissolution of CS in 0.5 mol L–1 HCl acid is associated with the charge transfer and double-layer nature of the corrosion process.34 The diameter of the capacitive loop increases with DDIP dosage. This implies that, as the inhibitor dose increases, so does the impedance of the inhibited substrate. The frequency dispersion effect may be seen because these impedance spectra are not perfectly symmetrical semicircles. This abnormal behavior is commonly attributed to the imperfections and nonuniformity of the CS surface.35 As seen in Figure 4 (left), the impedance spectra of the Bode plots show only one semicircle correlating to one time constant. It is reasonable to conclude that the charge transfer resistance Rct increases when inhibitor dose increases. On the other hand, the double-layer capacitance (Cdl) values decrease. This is mainly due to a reduction in the local dielectric constant and/or a growth in the thickness of the double layer.36 The equivalent circuit shown in Figure 5 is being used to analyze the Nyquist curves, which comprises Rs and CPE (constant phase element) parallel to the Rct.
Table 7. EIS Data of CS in 0.5 mol L–1 HCl in the Absence and Presence of Different Concentrations of the DDIP Compound at 25 °C.
| DDIP | ||||
|---|---|---|---|---|
| concn (ppm) | Rct (Ω cm2) | Cdl (F cm–2) × 10–6 | θ | %IE |
| blank | 48.6 | 108 | ||
| 50 | 120.3 | 45 | 0.596 | 59.6 ± 0.3 |
| 100 | 135.7 | 38 | 0.642 | 64.2 ± 0.1 |
| 150 | 170.4 | 26 | 0.715 | 71.5 ± 0.4 |
| 200 | 210.6 | 22 | 0.769 | 76.9 ± 0.2 |
| 250 | 241.0 | 17 | 0.798 | 79.8 ± 0.1 |
| 300 | 275.7 | 15 | 0.824 | 82.4 ± 0.4 |
Figure 4.
Nyquist (right) and Bode (left) plots for the corrosion of CS in 0.5 mol L–1 HCl without and with different concentrations of the DDIP compound at 25 °C.
Figure 5.
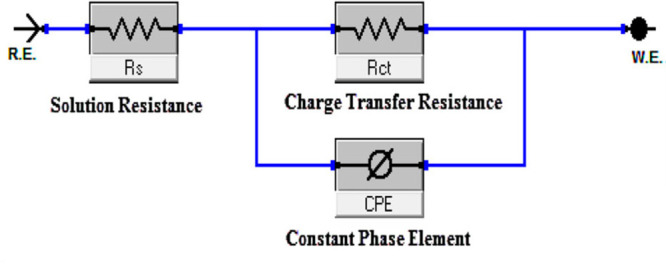
Equivalent circuit model used to fit experimental EIS data.
It can be observed that the magnitudes of mitigating efficiency estimated by PP and EIS methodologies differ just a little. The discrepancy between the values was attributed to variations in the surface state of the electrode material.37
3.7. Surface Studies
3.7.1. SEM Investigation
Figure 6 shows SEM image of the polished surface of CS before immersion in the test solution (0.5 mol L–1 HCl). Figures 7 and 8 show the morphology of CS specimens in the absence and presence of 300 ppm of the organic compound after 48 h of exposure in 0.5 mol L–1 HCl solution at room temperature. The CS surface of the blank specimen suffers greatly from pitting corrosion (Figure 7). However, in the presence of the DDIP compound, the surface is smooth and compact (Figure 8). The surface became isolated from the corrosive medium because of the adsorption of the DDIP compound on the CS surface and the formation of a protective film.
Figure 6.
Pure CS after polishing.
Figure 7.
CS after being immersed in 0.5 mol L–1 HCl.
Figure 8.
CS after being immersed in 0.5 mol L–1 HCl + 300 ppm DDIP.
3.7.2. AFM Characterization
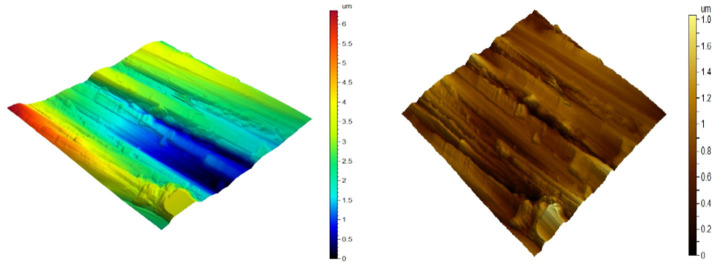
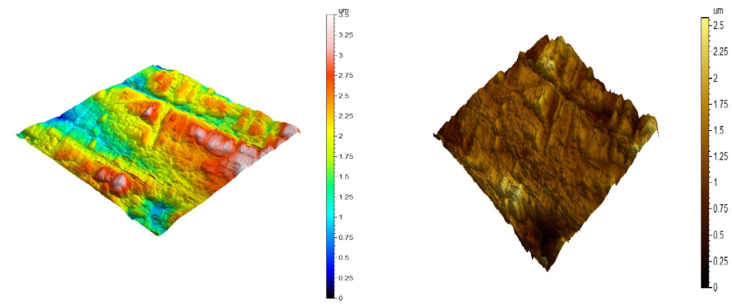
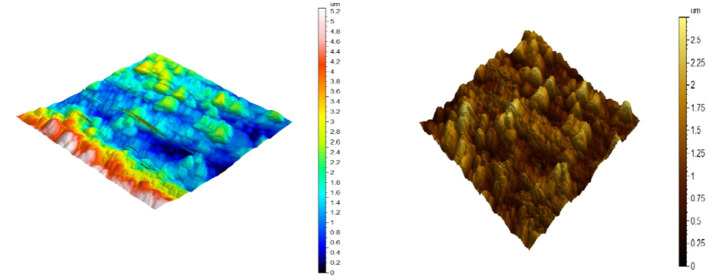
AFM is utilized to test the surface appearance at the pico- to microscales. It is a stunning and innovative tool for examining the effect of the inhibitor on the corrosion process at the CS solution boundary. Figures 9–11) illustrate the three-dimensional (3D) AFM morphologies of CS in 0.5 mol L–1 HCl in the presence and absence of the optimum concentration (300 ppm) of the DDIP compound. The surface of the CS in 0.5 mol L–1 HCl is significantly more damaged than the surface of the CS in the presence of the organic compound. In addition, the average roughness of CS in the blank solution is 0.254 μm. In the presence of the DDIP compound, the mean roughness is reduced to 0.155 μm. This implies that the organic compound is adsorbed on the CS surface and constitutes a protective film that isolates the CS surface from the aggressive HCl solution. All height parameters are given in Table 8, with the arithmetic mean height (Sa), the root-mean-square height (Sq), the maximum peak height (Sp), the maximum pit height (Sv), and the maximum height (Sz) computed in micrometer units according to ISO 25178.38
Figure 9.
AFM three-dimensional picture of pure CS.
Figure 11.
AFM three-dimensional picture of CS after being immersed for 48 h in 0.5 mol L–1 HCl + 300 ppm DDIP.
Table 8. AFM Parameters of CS.
| substance | Sa (μm) | Sq (μm) | Sp (μm) | Sv (μm) | Sz (μm) |
|---|---|---|---|---|---|
| control | 0.045 | 0.062 | 0.234 | 0.436 | 0.670 |
| 0.5 mol L–1 HCl | 0.254 | 0.351 | 6.040 | 3.560 | 9.600 |
| DDIP | 0.155 | 0.208 | 0.855 | 1.510 | 2.370 |
Figure 10.
AFM three-dimensional picture of CS surface after being immersed for 48 h in 0.5 mol L–1 HCl.
3.8. DFT Results
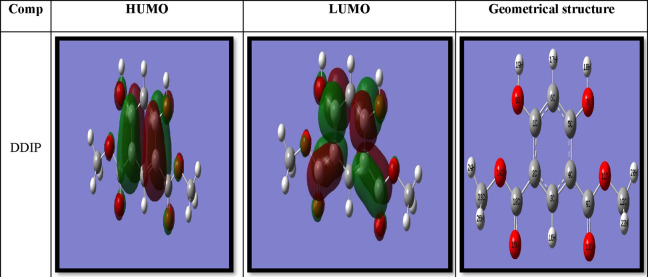
DFT simulations were utilized to investigate the interaction type between the DDIP compound’s adsorption centers and the CS surface. The highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) for the optimized structure are presented in Figure 12. The energy gap (ΔE) between EHOMO and ELUMO is computed. Other variables that influence the nature of the interaction between the DDIP molecule and the CS, such as ionization potential (I), electron affinity (A), electronegativity (χ), chemical potential (μ), hardness (η), softness (σ), electrophilicity (ω), total negative charge (TNC), and total energy (Et) were estimated by the subsequent relationships39 and are listed in Table 9.
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
Figure 12.
Geometrical structure and charge density distribution of HOMO and LUMO levels of the inhibitor compound DDIP.
Table 9. Calculated Quantum Chemical Parameters Obtained from DFT Theory.
| HOMO (au) | LUMO (au) | ΔE (au) | D (Debye) | η (au) | σ (au) | μ (au) | χ (au) | ω (au) | TNC | total energy (Et) | volume (cm3/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| –0.250 | –0.053 | 0.197 | 7.178 | 0.099 | 10.101 | –0.152 | 0.152 | 0.117 | –5.667 | –838.45 | 134.66 |
Quantum mechanics computations were used to assess the effect of structural parameters on inhibitor efficacy and to investigate the mechanism of adsorption at the CS surface. The DDIP’s molecular and electronic characteristics were determined by optimizing the lengths and angles of its bonds and the distortion angles. Figure 12 depicts the estimated configurations with the lowest energy gained from these calculations. As a result of the electron sharing between the oxygen in the DDIP and the CS, the studied inhibitor may adsorb at the CS surface.40 The computations revealed that the geometrical structures of the examined organic compound are virtually planar, as illustrated in Figure 12. The chemical reactivity is determined by the interaction of HOMO and LUMO levels.41
EHOMO denotes the molecule’s ability to give electrons to a convenient acceptor with vacant molecular orbitals, whereas ELUMO denotes its ability to gain electrons. The smaller the value of ELUMO, the greater the molecule’s capability to receive electrons.42 The larger the inhibitor’s EHOMO value, the easier it is to supply electrons to the vacant d-orbital of the CS and the better the inhibiting efficacy. The DFT results revealed that the organic inhibitor had a high EHOMO of −0.250 au, as shown in Table 9. This could explain why the DDIP inhibitor has a great potential to adsorb on the CS surface.
The structure was discussed and rationalized using the dipole moment, D.43D is the energy’s first derivative. Table 9 shows a strong correlation between D and inhibition efficiency. Another quantum parameter derived from the computations is the molecule volume. The progressive increase in inhibitor molecular volume indicates that the metal surface area covered/protected by the inhibitor molecules is gradually enhanced by extending the length of the hydrophobic chain. This observation is consistent with the findings of the polarization and impedance experiments. The inhibitor DDIP has a large molecular volume of 134.66 cm3 mol–1. This improves the inhibitor’s inhibition efficacy by expanding the surface contact between the inhibitor’s molecules and the metal surface. This is consistent with the experimental findings. The η and σ are essential qualities that assess a molecule’s stability and reactivity, and they agree with experimental evidence. Furthermore, the calculations revealed that the DDIP inhibitor has a low χ and ω and a high TNC (−5.667e) (Table 9), which increases its donating capability to the CS surface and, as a result, its inhibitory efficacy.
The preceding section concludes that the quantum characteristics confirm that the DDIP compound has high inhibition efficiency, according to the experimental findings. In addition, the HOMO level of the inhibitor is mainly localized at the lone pairs of oxygen atoms in the OCH3 moiety and as the π-bonding character of the C–C bond of the phenyl moiety. This indicates that these moieties are the favorite centers for the electrophilic attack on the CS surface, as shown in Figure 12. This also implies that the phenyl moiety, which has a high coefficient of HOMO density, was orientated toward the CS surface, and that adsorption happens most likely via its π-electrons.
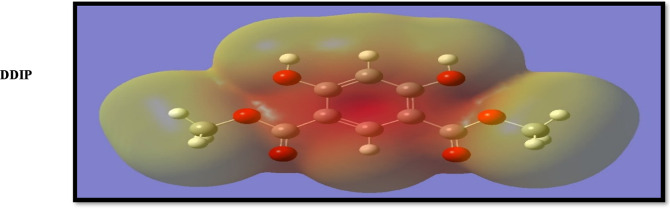
Moreover, the calculations revealed that the charge density of the LUMO level is localized as the antibonding character of the C–C phenyl moiety and C=O groups for the examined compound, implying that these moieties might be reacted as an electrophile, as shown in Figure 13. Molecular electrostatic potentials are immensely beneficial in that negative regions could be viewed as nucleophile sites, while positive electrostatic potential regions can be considered potential electrophile sites. In addition, the electrostatic potential reveals the polarization of the electron density. The results revealed that the oxygen atom has a negative electrostatic potential, implying that these centers are energetically favorable for attachment to the metal surface (Figure 13). According to the results obtained, the quantum chemical calculations demonstrate a strong link between the examined inhibitor’s quantum chemical characteristics and its experimental inhibition efficacy for CS corrosion.
Figure 13.
Electron density plots of molecular electrostatic potentials of the DDIP inhibitor.
3.9. Monte Carlo Simulation
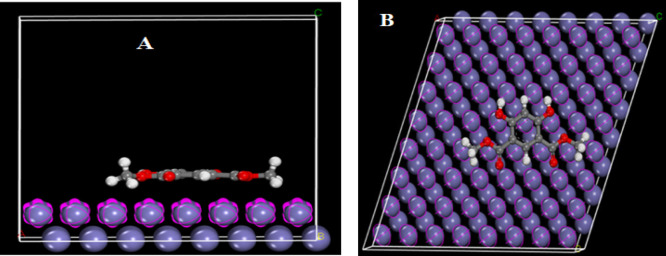
Monte Carlo simulation is a molecular mechanics-based simulation in which simulated annealing is used as an optimization method.44 The advantage of MC simulation over quantum mechanical simulation is that it is much less time-consuming and hence causes cost reduction. The type of adsorption is determined by the inhibitor’s molecular structure. The MC simulations were used to investigate the steel surface’s adsorption behavior and the mechanism of interactions between the inhibitor molecule and the metal surface. The side and top views of the adsorption modeling of the studied inhibitor on the surface of CS are shown in Figure 14. The following equation was used to calculate the interaction energy (binding energy) between the examined molecule and the CS surface:
| 14 |
where Einh and EFe are the total energy of the inhibitor and the total energy of the Fe surface, respectively. The data obtained from MC simulation (Table 10) show that the DDIP inhibitor’s binding energy and Fe surface is −528.38 kJ mol–1. These findings corroborate the findings of the experiments.
Figure 14.
Side view (A) and top view (B) for the adsorption of the DDIP inhibitor on CS.
Table 10. Descriptors Calculated by the Monte Carlo Simulation for Adsorption of the Inhibitor on the CS Surface.
| molecule | total energy (kJ mol–1) | adsorption energy (kJ mol–1) | deformation energy (kJ mol–1) | rigid adsorption energy (kJ mol–1) |
|---|---|---|---|---|
| DDIP | –43.93 | –528.38 | –33.35 | –495.15 |
4. Conclusions
A resorcinol derivative, DDIP, is examined as a corrosion inhibitor for low CS in 0.5 mol L–1 HCl acid. The mitigation efficacy increases from 61.8 to 79.9% as the DDIP dose increases from 50 to 300 ppm. However, inhibition efficiency decreases to 70.05% as the temperature reaches 55 °C. The compound’s inhibition is due to creating a protective film at the CS. Polarization findings showed that the compound functions as a mixed-type inhibitor. The DDIP compound’s adsorption obeys the Langmuir model. The results gathered through multiple methodologies were closely connected, indicating validity and correctness. The lower energy gap between the HUMO and LUMO leads to a more substantial contact between the DDIP molecule and the CS via electron donation and acceptance. The molecular dynamic simulation confirmed that the nature of the adsorption depends on the molecular structure of the inhibitor. The binding energy for the DDIP inhibitor and Fe surface is −528.38 kJ mol–1. The quantum characteristics confirmed that the inhibitor has high inhibition efficiency, which is consistent with the findings of the experimental results. SEM and AFM revealed the development of a uniform layer at CS in the presence of the DDIP.
Acknowledgments
We do acknowledge for General Petroleum Company for providing lab facilities for this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00153.
The 1H NMR and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Abbasov V. M.; Abd El-Lateef H. M.; Aliyeva L. I.; Qasimov E. E.; Ismayilov I. T.; Khalaf M. M. A study of the corrosion inhibition of mild steel C1018 in CO2-saturated brine using some novel surfactants based on corn oil. Egypt. J. Pet. 2013, 22, 451–470. 10.1016/j.ejpe.2013.11.002. [DOI] [Google Scholar]
- Olasunkanmi L. O.; Sebona M. F.; Ebenso E. E. Influence of 6-phenyl-3 (2H)-pyridazinone and 3-chloro-6-phenylpyrazine on mild steel corrosion in 0.5 M HCl medium: experimental and theoretical studies. J. Mol. Struct. 2017, 1149, 549–559. 10.1016/j.molstruc.2017.08.018. [DOI] [Google Scholar]
- Migahed M.A.; EL-Rabiei M. M.; Nady H.; Elgendy A.; Zaki E.G.; Abdou M.I.; Noamy E. S. Novel Ionic Liquid Compound Act as Sweet Corrosion Inhibitors for X-65 Carbon Tubing Steel: Experimental and Theoretical Studies. J. Bio. Tribo- Corros. 2017, 3, 31. 10.1007/s40735-017-0092-1. [DOI] [Google Scholar]
- Seter M.; Thomson M. J.; Stoimenovski J.; MacFarlane D. R.; Forsyth M. Dual active ionic liquids and organic salts for inhibition of microbially influenced corrosion. Chem. Commun. 2012, 48, 5983. 10.1039/c2cc32375c. [DOI] [PubMed] [Google Scholar]
- Pandey A.; Verma C.; Singh B.; Ebenso E. E. Synthesis, characterization and corrosion inhibition properties of benzamide–2- chloro-4-nitrobenzoic acid and anthranilic acid–2-chloro-4-nitrobenzoic acid for mild steel corrosion in acidic medium. J. Mol. Struct. 2018, 1155, 110–122. 10.1016/j.molstruc.2017.10.114. [DOI] [Google Scholar]
- Popoola L. T.; Grema A. S.; Latinwo G. K.; Gutti B.; Balogun A. S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 35. 10.1186/2228-5547-4-35. [DOI] [Google Scholar]
- Subramania A.; Kalyana Sundaram N. T.; Sathiya Priya R.; Saminathan K.; Muralidharan V. S.; Vasudevan T. Aldimines, Aldimines – effective corrosion inhibitors for mild steel in hydrochloric acid solution. J. Appl. Electrochem. 2004, 34, 693–696. 10.1023/B:JACH.0000031033.73171.f4. [DOI] [Google Scholar]
- Migahed M. A.; EL-Rabiei M. M.; Nady H.; Elgendy A.; Zaki E. G.; Abdou M. I.; Noamy E. S. Novel Ionic Liquid Compound Act as Sweet Corrosion Inhibitors for X-65 Carbon Tubing Steel: Experimental and Theoretical Studies. J. Bio. Tribo-Corros. 2017, 3, 31. 10.1007/s40735-017-0092-1. [DOI] [Google Scholar]
- Hasan B. O.; Sadek S. A. The effect of temperature and hydrodynamics on carbon steel corrosion and its inhibition in oxygenated acid–salt solution. J. Ind. Eng. Chem. 2014, 20, 297–307. 10.1016/j.jiec.2013.03.034. [DOI] [Google Scholar]
- Migahed M. A.; Al-Sabagh A. M.; Zaki E. G.; Mostafa H. A.; Fouda A. S. Synthesis of Some Novel Cationic Surfactants and Evaluation of Their Performance as Corrosion Inhibitors for X-65 type Carbon Steel under H2S Environment. Int. J. Electrochem. Sci. 2014, 9, 7693–7711. [Google Scholar]
- Ahamad I.; Prasad R.; Quraishi M.A. Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion inhibitor. J. Solid State Electrochem 2010, 52, 933–942. 10.1016/j.corsci.2009.11.016. [DOI] [Google Scholar]
- Yurt A.; Aykin O. Diphenolic Schiff bases as corrosion inhibitors for aluminium in 0.1 M HCl: Potentiodynamic polarisation and EQCM investigations. Corros. Sci. 2011, 53, 3725–3732. 10.1016/j.corsci.2011.07.018. [DOI] [Google Scholar]
- Chetouani A.; Hammouti B.; Benhadda T.; Daoudi M. Inhibitive action of bipyrazolic type organic compounds towards corrosion of pure iron in acidic media. Appl. Surf. Sci. 2005, 249, 375–385. 10.1016/j.apsusc.2004.12.034. [DOI] [Google Scholar]
- Quraishi M. A.; Sardar R. Aromatic Triazoles as Corrosion Inhibitors for Mild Steel in Acidic Environments. Corrosion 2002, 58, 748–755. 10.5006/1.3277657. [DOI] [Google Scholar]
- Juttner K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–5108. 10.1016/0013-4686(90)80004-8. [DOI] [Google Scholar]
- Nathiya R. S.; Perumal S.; Moorthy M.; Murugesan V.; Rangappan R.; Raj V. Synthesis, Characterization and Inhibition Performance of Schif Bases for Aluminium Corrosion in 1 M H2SO4 Solution. J. Bio Tribo Corros. 2020, 6, 5. 10.1007/s40735-019-0291-z. [DOI] [Google Scholar]
- Chen S.; Zhu B.; Liang X. Corrosion Inhibition Performance of Coconut Leaf Extract as a Green Corrosion Inhibitor for X65 Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2020, 15, 1–15. 10.20964/2020.01.39. [DOI] [Google Scholar]
- Fouda A. S.; Rashwan S. M.; Kamel M. M.; Haleem E. A. Chemical, electrochemical and surface morphology investigation of Cichorium intybus extract (CIE) as beneficial inhibitor for Al in 2 M HCl acid. Lett. Appl. NanoBioScience 2021, 9 (2), 1064–1073. [Google Scholar]
- Fouda A. S.; Rashwan S. M.; Kamel M. M.; Haleem E. A. Juglans Regia Extract (JRE) as Eco-Friendly Inhibitor for Aluminum Metal in Hydrochloric Acid Medium. Biointerface Res. Appl. Chem. 2020, 10 (5), 6398–6416. 10.33263/BRIAC105.63986416. [DOI] [Google Scholar]
- Kamel M.; Hegazy M.; Rashwan S.; El Kotb M. Innovative surfactant of Gemini-type for dissolution mitigation of steel in pickling HCl medium. Chin. J. Chem. Eng. 2021, 34, 125–133. 10.1016/j.cjche.2020.09.051. [DOI] [Google Scholar]
- Nafie M. S.; Arafa K.; Sedky N. K.; Alakhdar A. A.; Arafa R. K. Triaryl Dicationic DNA Minor-Groove Binders with Antioxidant Activity Display Cytotoxicity and Induce Apoptosis in Breast Cancer. Chemico-Biological Interactions 2020, 324, 109087. 10.1016/j.cbi.2020.109087. [DOI] [PubMed] [Google Scholar]
- Nafie M. S.; Amer A. M.; Mohamed A. K.; Tantawy E. S. Discovery of Novel Pyrazolo[3,4-b]Pyridine Scaffold-Based Derivatives as Potential PIM-1 Kinase Inhibitors in Breast Cancer MCF-7 Cells. Bioorg. Med. Chem. 2020, 28 (24), 115828. 10.1016/j.bmc.2020.115828. [DOI] [PubMed] [Google Scholar]
- Kamel M. M.; Mohsen Q.; Anwar Z. M.; Sherif M. A. An expired ceftazidime antibiotic as an inhibitor for disintegration of copper metal in pickling HCl media. J. Mater. Res. Technol. 2021, 11, 875–886. 10.1016/j.jmrt.2021.01.055. [DOI] [Google Scholar]
- Zeng H.; Miller R. S.; Flowers R. A.; Gong B. A Highly Stable, Six-Hydrogen-Bonded Molecular Duplex. J. Am. Chem. Soc. 2000, 122, 2635–2644. 10.1021/ja9942742. [DOI] [Google Scholar]
- Stern M.; Geaby A. L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104 (1), 56–63. 10.1149/1.2428496. [DOI] [Google Scholar]
- Perez N. Electrochemistry and corrosion science 2016, 1–23. 10.1007/978-3-319-24847-9_1. [DOI] [Google Scholar]
- Elachouri M.; Hajji M. S.; Salem M.; Kertit S.; Aride J.; Coudert R.; Essassi E. Some Nonionic Surfactants as Inhibitors of the Corrosion of Iron in Acid Chloride Solutions. Corrosion 1996, 52, 103. 10.5006/1.3292100. [DOI] [Google Scholar]
- Bentiss F.; Lebrini M.; Lagrenée M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/ 2,5-bis(n-thienyl)-1,3,4-thiadiazoles/ hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. 10.1016/j.corsci.2005.05.034. [DOI] [Google Scholar]
- Langmuir I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. 10.1021/ja02268a002. [DOI] [Google Scholar]
- Schorr M.; Yahalom J. The significance of the energy of activation for the dissolution reaction of metal in acids. Corros. Sci. 1972, 12 (11), 867–868. 10.1016/S0010-938X(72)80015-5. [DOI] [Google Scholar]
- Ekanem U. F.; Umoren S. A. Inhibition of mild steel corrosion in h2so4 using exudate gum from pachylobus edulis and synergistic potassium halide additives. Chem. Eng. Commun. 2010, 197, 1339–1356. 10.1080/00986441003626086. [DOI] [Google Scholar]
- Latour R. A. The langmuir isotherm: A commonly applied but misleading approach for the analysis of protein adsorption behavior. J. Biomed. Mater. Res., Part A 2015, 103 (3), 949–958. 10.1002/jbm.a.35235. [DOI] [PubMed] [Google Scholar]
- Gopi D.; Govindaraju K. M.; Kavitha L. Investigation of triazole derived Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid medium. J. Appl. Electrochem. 2010, 40, 1349–1356. 10.1007/s10800-010-0092-z. [DOI] [Google Scholar]
- Essaghouani A. L.; Elmsellem H.; Boulhaoua M.; Ellouz M.; El Hafi M.; Sebbar N. K.; Essassi E. M.; Bouabdellaoui M.; Aouniti A.; Hammouti B. Adsorption proprieties and inhibition of mild steel corrosion in HCl solution by 1-Benzyl-4-phenyl-2,3-dihydro-1H-1,5-benzodiazepin-2-one. Der Pharma Chemica. 2016, 8 (2), 347–355. [Google Scholar]
- Singh A. K.; Quraishi M. A. Investigation of the Effect of Disulfiram on Corrosion of Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2011, 53, 1288–1297. 10.1016/j.corsci.2011.01.002. [DOI] [Google Scholar]
- Issaadi T.; Douadi A.; Zouaoui S.; Chafaa M.; Khan A.; Bouet G. Novel thiophene symmetrical Schiff base compounds as corrosion inhibitor for mild steel in acidic media. Corros. Sci. 2011, 53, 1484–1488. 10.1016/j.corsci.2011.01.022. [DOI] [Google Scholar]
- Kamel M. M.; Fouda A. S.; Rashwan S. M.; Abdelkader O. Paprika extract: a green inhibitor for mitigating carbon steel disintegration in 1 M HCl pickling solution. Green Chem. Lett. Rev. 2021, 14 (4), 600–611. 10.1080/17518253.2021.1985173. [DOI] [Google Scholar]
- Introduction to Surface Roughness Measurement, International Organization for Standardization ISO 25178; https://sernia.ru/upload/pdf_files/Introduction%20to%20surface%20roughness%20measurement.pdf (accessed 2022-04-19).
- Hegazy M. A.; Rashwan S. M.; Meleek S.; Kamel M. M. Synthesis, characterization and mitigation action of innovative Schiff base on steel disintegration in sulfuric acid solution. Mater. Chem. Phys. 2021, 267 (5), 124697. 10.1016/j.matchemphys.2021.124697. [DOI] [Google Scholar]
- Lalitha A.; Ramesh S.; Rajeswari S. Surface protection of copper in acid medium by azoles and surfactants. Electrochim. Acta 2005, 51, 47. 10.1016/j.electacta.2005.04.003. [DOI] [Google Scholar]
- Zhang D. Q.; Gao L. W.; Zhou G. D. Inhibition of copper corrosion in aerated hydrochloric acid solution by heterocyclic compounds containing a mercapto group. Corros. Sci. 2004, 46, 3031. 10.1016/j.corsci.2004.04.012. [DOI] [Google Scholar]
- Negm N. A.; Migahed M. A.; Farag R. K.; Fadda A. A.; Awad M. K.; Shaban M. M. High performance corrosion inhibition of novel tricationic surfactants on carbon steel in formation water: Electrochemical and computational evaluations. J. Mol. Liq. 2018, 262, 363. 10.1016/j.molliq.2018.04.092. [DOI] [Google Scholar]
- Martınez S. Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 2003, 77, 97–102. 10.1016/S0254-0584(01)00569-7. [DOI] [Google Scholar]
- Kirkpatrick S.; Gelatt C. D.; Vecchi M. P. Optimization by Simulated Annealing. Science 1983, 220, 671–680. 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.