Abstract
Purpose of review:
This review provides a risk stratified and evidence-based management for subsets of systemic sclerosis (SSc) patients in the first five years from disease onset.
Recent findings:
Cardio-pulmonary disease remains the primary cause of mortality in SSc patients. Morbidity and mortality in SSc-associated pulmonary arterial hypertension has improved with combination treatment, in either an upfront or sequential treatment pattern. Traditional therapies for interstitial lung disease (SSc-ILD) have targeted those with clinically significant and progressive ILD with immunosuppression. New data suggest a possible paradigm shift, introducing immunosuppressive therapy to patients before they develop clinically significant or progressive ILD. 2019 saw the approval of the first FDA-approved therapy for SSc-associated interstitial lung disease, using an anti-fibrotic agent previously approved for idiopathic pulmonary fibrosis. To date only autologous hematopoietic stem cell transplant has demonstrated a mortality benefit for SSc-ILD, albeit in a narrow spectrum of SSc-ILD patients.
Summary:
SSc is a highly heterogeneous autoimmune disease typified by varying clinical trajectories. Its management may be stratified within the first five years by sub-classifying patients based on factors that have important prognostic significance: skin distribution and auto-antibody status.
Keywords: Systemic Sclerosis, Management, Treatment
I. INTRODUCTION
Systemic Sclerosis
Systemic sclerosis (SSc) is a chronic, heterogeneous autoimmune disease characterized by a triad of immune dysregulation, vasculopathy, and overproduction of collagen leading to skin and internal organ fibrosis1. This clinical heterogeneity may be codified into disease subsets, a critical insight allowing the provider to anticipate internal organ involvement and disease progression. Classification based upon the distribution of affected skin areas and autoantibody status informs the management of disease-related complications.
This article focuses on disease stratification and management in the first five years from onset of SSc. We support algorithmic approaches to management of disease subsets using recently published data.
II. EARLY SYSTEMIC SCLEROSIS
Early Disease
The majority of internal organ involvement in SSc will occur within the first two to five years from the disease onset (typically defined as the appearance of the first non-Raynaud’s phenomenon symptom). Classifying SSc patients into an early disease subset allows for tailored screening and management strategies, with an aim to institute therapeutic intervention to prevent irreversible organ damage.
Classification
Patients with SSc may be classified based on the extent of skin involvement: limited cutaneous (affected skin is distal to the elbows and knees, and may include the face), diffuse cutaneous (affected skin is both distal and proximal to the elbows and knees, and may include the face, chest, trunk, and thighs), or absent (SSc sine scleroderma). The 2013 ACR/EULAR classification criteria improved upon the performance of the 1980 classification criteria in terms of recognition of the disease, especially in limited disease and the early stages when skin fibrosis is less advanced: the sensitivity improved (91%, from 75%), as well as the specificity (90%, from 72%)2.
Patients may also be classified based on autoantibody status: antibodies are detected in more than 95% of patients with SSc, rarely found in healthy populations, and are mutually exclusive (the presence of one generally precludes the presence of another). These serological markers precede the onset of symptoms and are useful in making an early diagnosis3. Table 1 provides an overview of the likelihood of clinical feature development of SSc stratified by auto-antibody status. Anti-centromere antibody has a high specificity for limited cutaneous SSc, (95%) 4,5. Anti-SCL-70 (anti- topoisomerase I antibody) is typically associated with diffuse cutaneous SSc, however up to one third of patients with anti- topoisomerase I antibodies may have limited cutaneous SSc6. Commercially available ELISA based assays for this antibody have been associated with high false positivity7. Anti-RNA polymerase III antibodies are associated with diffuse cutaneous SSc (90%)8.
Table 1:
Organ Involvement Within the First Five Years, Stratified by Auto-Antibody Status
| Anti-Centromere | Anti-SCL-70 | Anti-RNA Polymerase III | ANA Positive, ENA Negative | |
|---|---|---|---|---|
|
| ||||
| Skin | ||||
|
| ||||
| Limited Cutaneous | ++ | + | + | Unclear |
| Diffuse Cutaneous | − | +++ | +++ | Unclear |
| Cardiopulmonary | ||||
|
| ||||
| Pulmonary Arterial Hypertension | +* | +/− | + | + |
| Clinically Significant Interstitial Lung Disease | +/− | +++ | ++ | ++ |
| Cardiomyopathy | +/− | + | +/− | + |
| Renal | ||||
|
| ||||
| Scleroderma Renal Crisis | +/− | + | +++ | ++ |
| Malignancy | ||||
|
| ||||
| Presence | − | + | +++ | Unclear |
− Very Rare
+/− Rare
+* Rare within the first 5 years
+ Less Common
++ Common
+++ More Common
Prognostication
Factors present in the first five years of disease are predictive of development of major outcomes in SSc (e.g., development of interstitial lung disease, pulmonary hypertension, scleroderma renal crisis, death)9–15.
Patients with limited cutaneous SSc typically have a burden of non-lethal signs and symptoms, notably a longstanding course of Raynaud’s phenomenon, digital ulcerations, gastrointestinal involvement, and later-stage development of pulmonary arterial hypertension. Compared to patients with diffuse cutaneous SSc, they have a lower mortality rate and incidence of developing severe interstitial lung disease16,17. Those with diffuse cutaneous SSc, particularly in the early stage, will have rapid progression of skin thickening, musculoskeletal involvement, higher frequency of clinically-significant interstitial lung disease, renal disease, and mortality.
Autoantibody status has better predictive value, compared to the extent of skin distribution, in predicting scleroderma organ involvement6,18. Patients with anti-centromere antibody positivity have a favorable prognosis compared to those with anti-SCL-70 antibody; they are more likely to develop ulcerations, gangrene, and tuft resorption of the digits, calcinosis, and are lower risk for arthritis or myositis. This antibody is associated with a higher risk for pulmonary arterial hypertension19,20. Patients with anti-SCL-70 antibody have a higher prevalence of arthritis, tendon friction rubs, severe pulmonary fibrosis, severe cardiac disease, and scleroderma renal crisis. The risk of interstitial lung disease in anti-SCL-70 positive patients is similar independent of the extent of skin involvement21. RNA polymerase III antibody positive patients have a high prevalence of scleroderma renal crisis (25%)22.
III. MANAGEMENT
Table 2 provides a screening strategy for internal organ involvement by skin and auto-antibody status, noting areas of high priority.
Table 2:
Screening Stratified by Skin Involvement and Auto-Antibody Status
| Limited SSc | Diffuse SSc | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Anti-Centromere | Anti-SCL-70 | Anti-SCL-70 | Anti-RNA Polymerase III | ANA Positive, ENA Negative | |
|
| |||||
| Screening | |||||
|
| |||||
| Cardiopulmonary Involvement | |||||
| Electrocardiogram | ++ | ++ | ++ | ++ | ++ |
| Transthoracic Echocardiogram | ++ | ++ | ++ | ++ | ++ |
| Pulmonary Function Testing | ++ | ++ | ++ | ++ | ++ |
| High Resolution Chest CT | + | ++ | ++ | ++ | ++ |
| Blood Pressure Monitoring for | |||||
| Scleroderma Renal Crisis | + | + | + | ++ | + |
| Age-appropriate Cancer Screening | + | + | + | ++ | + |
+ Routine Clinical Care
++ High Priority
Interstitial Lung Disease
All patients should be screened with HRCT and routine use of pulmonary function testing for monitoring purposes. The majority (55–65%) of scleroderma patients will have HRCT positive interstitial lung disease; that number increases to 96% of those with abnormal pulmonary function testing23,24. Routine pulmonary testing (spirometry and DLco), especially in the first 5 years, is to identify those patients developing progressive interstitial lung disease25,26. Patients with only minor impairment in the forced vital capacity (FVC) after more than 5 years of disease duration are much less likely to develop severe fibrotic lung disease later in their disease course. Reduced FVC within 4 years of the onset of symptoms is an important predictor of the eventual development of severe lung disease (FVC ≤ 50%)4. The greatest risk of progression for SSc ILD appears to be early in the disease course, particularly in those with diffuse SSc, male gender, African-American race, and positive anti-SCL-70 antibodies27.
Traditional management focuses on treating those with significant baseline impairment in FVC, extensive involvement on HRCT, or evidence of progressive disease. Proposed definitions identifying those with clinically-significant disease include an FVC less than 70%, and extensive ILD on baseline HRCT of greater than 20%, and a decline of FVC by ≥ 5–10 percent and/or DLco of >10–15% within a 12 month period28,29. The goal of treatment is disease attenuation and retardation of progression with the use of cyclophosphamide or mycophenolate mofetil, as demonstrated in the Scleroderma Lung Study I and II trials30,31. Importantly, SLS-II demonstrated that mycophenolate mofetil with a target dose of 3g/day was comparable in efficacy to 1 year of oral cyclophosphamide, was better tolerated with fewer adverse hematological events. In patients with early diffuse SSc, a recent open-label single-institution study showed promising evidence of lung and skin benefit with rituximab therapy32.
The landscape of treatment is showing signs of changing in terms of targeted populations and mechanisms of action. Within the last year, clinical trials in SSc-ILD have shown data to suggest benefit of tocilizumab in reducing the rate of FVC decline compared to placebo in those with mild impairment on pulmonary function testing in early diffuse SSc patients, with elevated inflammatory markers and positive SCL 70 antibody33,34. A landmark phase III, randomized, double-blind, placebo-controlled trial showed an anti-fibrotic medication, nintedanib, to slow the rate of decline in FVC decline in SSc-ILD35. This medication has demonstrated efficacy in those with progressive fibrotic lung disease despite being on immune suppression and those with a UIP pattern deriving significant benefit from anti-fibrotic therapy36.
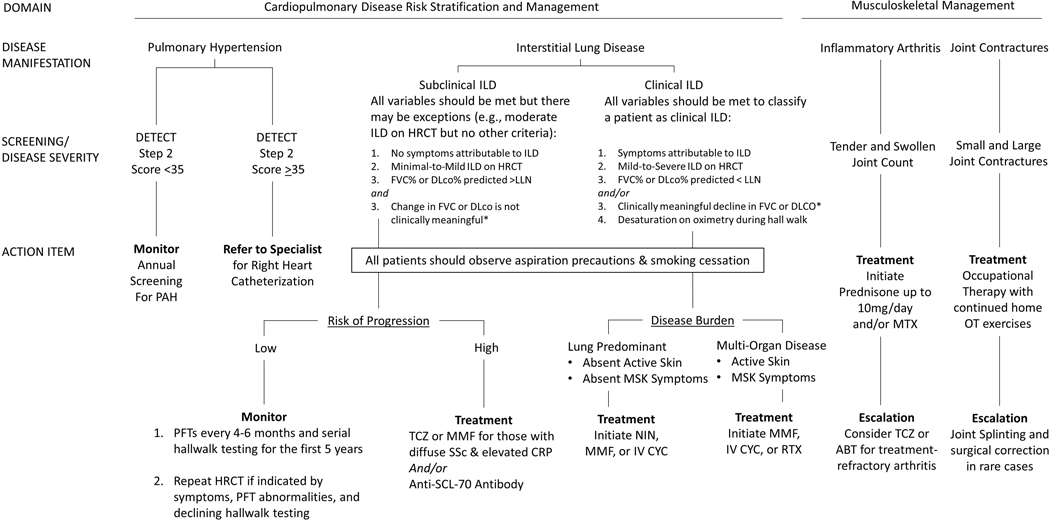
There are no universally agreed-upon treatment algorithms at this time, but several have been proposed34,37,38. A recent European consensus statement, achieved through a modified Delphi process, yielded a clinical management algorithm for SSc-ILD. Nintedanib may be appropriate for treatment initiation or escalation, and used as monotherapy or in combination with mycophenolate mofetil 3g/day39,31,35. We recommend stratifying based on disease severity (subclinical vs. clinical ILD) and tailoring therapy based on risk of progression and the burden of disease (e.g., if lung predominant or multi-organ involvement). Figure 1a outlines a recommended treatment strategy based on this approach.
Figure 1a:
General management of early systemic sclerosis
Clinically Meaningful Change:
*if >1 PFT available, a clinically meaningful decline is defined as FVC levels of >10% from baseline or decline in FVC >5% to <10% and >15% relative decline in DLCO.
Medication/Treatment Acronyms
ABT: Abatacept
CYC: Cyclophosphamide
MMF: Mycophenolate Mofetil
MTX: Methotrexate
NIN: Nintedanib
OT: Occupational Therapy
RTX: Rituximab
TCZ: Tocilizumab
Testing Acronyms
Anti-SCL-70: Anti-Topoisomerase I Antibody
CRP: C-Reactive Protein
DLco: Diffusion Capacity of Carbon Monoxide
FVC: Forced Vital Capacity
HRCT: High Resolution Chest CT
LLN: Lower Limit of Normal
PFT: Pulmonary Function Testing
Disease Acronyms
ILD: Interstitial Lung Disease
MSK: Musculoskeletal
SSc: Systemic Sclerosis
The use of autologous hematopoietic stem cell transplantation should be reserved for those with early diffuse scleroderma, less than 65 years of age, with severe visceral organ involvement (e.g., SSc-ILD) but without cardiac disease40. The experience of the treating medical team is considered to be of high importance when considering this modality41. Lung transplant should be considered in patients with progressive ILD despite aggressive medical therapy.
Pulmonary Arterial Hypertension
All patients with SSc are risk for developing of pulmonary arterial hypertension (PAH), however there is increased risk in those with longer disease duration, male gender, the number of telangiectasias, reduced capillary nail fold density, and anti-centromere antibody positivity. It is important to differentiate between pre-capillary pulmonary hypertension (due to PAH vs. PH-ILD) and post-capillary PH. PAH accounts for 17–30% of deaths among SSc patients42,43. Early detection and prompt initiation of therapy for PAH is essential; those with early diagnosis have more pronounced benefit with therapy44,45. In 2018 a revised definition of PH was proposed, lowering the threshold of right heart catheterization-derived mean pulmonary arterial pressure from ≥25mmHg to >20mmHg46. This shift was in accord with data showing those with an elevated mPAP have an increased risk for morbidity and mortality compared to normal mPAP47,48. Its implementation did not significantly impact the diagnosis of PH of those in two different screening cohorts 49.
Patients with longer duration of disease and limited cutaneous involvement are more likely to develop this complication50,51, however patients within their first five years52 and those with diffuse cutaneous involvement may also be affected, largely due to PH-related ILD. A recent single-center review of SSc showed a high rate of co-existing interstitial lung disease (>20% extent of lung involvement) and WHO Group III PH53. As a result, all patients should receive EKG, pulmonary function testing, echocardiography, and NT-proBNP screening for this complication at the time of diagnosis. A screening algorithm, as proposed by recent 6TH World Symposium on Pulmonary Hypertension, should be performed annually54. Any new symptoms or signs should prompt consideration for referral for right heart catheterization.
Treatment for patients with PAH includes use of PDE5 inhibitors (e.g., sildenafil, tadalafil), endothelin receptor antagonists (e.g., bosentan, macitentan, ambrisentan), and prostacyclins (iloprost, epoprostenil, and treprostinil), with a goal to achieve NYHA functional class II or higher (mild shortness of breath) and slight limitation during ordinary activity55. Recent data from 3 large clinical trials (AMBITION, SERAPHIN, GRIPHON) suggest benefit of targeting multiple pathways in treatment of PAH56–58. The AMBITION trial showed Ambrisentan and Tadalafil combination therapy was superior to monotherapy for either medication59. The SERAPHIN trial showed the addition of macitentan (compared to placebo) and patients in the GRIPHON study receiving the addition of selexipag to combination therapy reduced the risk of morbidity/mortality 56,57,60,61. Treatment of PH-ILD includes management of underlying ILD and 02 therapy, although many patients may have an overlap for PAH and PH-ILD53.
Scleroderma Heart Involvement
The majority of cardiac involvement in early SSc is subclinical62–64. Cardiac involvement may be separated into fibrotic disease that can affect any component of the heart (pericardium, myocardium, conduction system, and less commonly the valves) and secondary involvement due to other sites of SSc involvement (e.g., PAH, SSc-ILD, renal disease)65,66. Myocardial involvement may present in early disease; it presents more commonly with diastolic (rather than systolic) dysfunction as heart failure with preserved ejection fraction67–69.
Cardiac assessment should include considerations of myocardial fibrosis, coronary artery disease, co-occurring pulmonary hypertension, arrhythmias, and myocarditis. Hung et al., provide a diagnostic algorithm that includes an initial work-up of cardiac involvement including electrocardiogram, chest x-ray, transthoracic echocardiogram, troponin, CK-MB, and NT-proBNP measurements66. If abnormal or symptomatic, an appropriate work up should include a Holter monitor and appropriate referral to Cardiology should be made. Speckle tracking echocardiography is a technique recently shown to detect LV and RV dysfunction not detected by conventional 2D echo70. Cardiac MRI is a noninvasive, radiation-free, operator independent technique for identifying myocardial fibrosis and perfusion defects even in early disease. Those patients with modifiable risk factors for coronary artery disease (e.g., hypertension, dyslipidemia, diabetes, smoking) should be counseled.
Scleroderma Renal Crisis
Scleroderma renal crisis is the new onset of accelerated arterial hypertension and/or rapidly progressive oliguric renal failure during the course of scleroderma71; this is significantly more likely in diffuse SSc (12%) compared to limited SSc (2%)72. Features predictive of scleroderma renal crisis include disease symptoms less than 4 years, diffuse cutaneous skin involvement, rapid progression of skin thickening, the presence of anti-RNA polymerase III antibody, new anemia, new pericardial effusion or congestive heart failure, and antecedent high-dose corticosteroids.
Providers should become concerned for renal crisis if the SSc patient has an elevated BP of >150/85mmHg or if there is an increase of ≥20mmHg from baseline systolic blood pressure on two occasions in a 24-hour period73. These patients should be directed to the emergency department immediately. A decline in renal function (increase of 50% from baseline creatinine or an absolute increase of 0.3mg/dL, even if within normal range) and/or presence of proteinuria (>2+) and/or hematuria 1+ should prompt initiation of an ACE inhibitor74. A small proportion of patients may develop normotensive renal crisis, especially in those with background ACE inhibitor. Supportive features of this diagnosis include a microangiopathic hemolytic anemia, retinopathy typical of an acute hypertensive crisis, new onset of urinary red blood cells, flash pulmonary edema, and oliguria/anuria71,73. Clinical features include dyspnea, headache, blurred vision, encephalopathy, and seizures.
Management includes education for those at high risk regarding the importance of routine blood pressure monitoring, and close communication of new symptom development (headache, dyspnea, dizziness, syncope). Patients with scleroderma renal crisis should be hospitalized and prompt initiation of angiotensin converting enzyme inhibitor with close monitoring to avoid hypotensive nephropathy75. Other antihypertensive agents may be used if the blood pressure remains unacceptably high, with the exception of beta blockers. The use of ACE inhibitors in a prophylactic role has been found to be detrimental; and one study, exposure to ACE inhibitors prior to the onset of scleroderma renal crisis was associated with a greater than twofold increased risk of mortality76.
Gastrointestinal Disease
GI involvement is the most common site of internal organ involvement, and may affect anywhere in the tract: gastroesophageal reflux disease, dysphagia due to altered contractility of the esophagus, delayed gastric emptying, delayed motility with resulting postprandial bloating and small intestinal bacterial overgrowth, chronic constipation, and vascular complications like gastric antral vascular ectasia77.
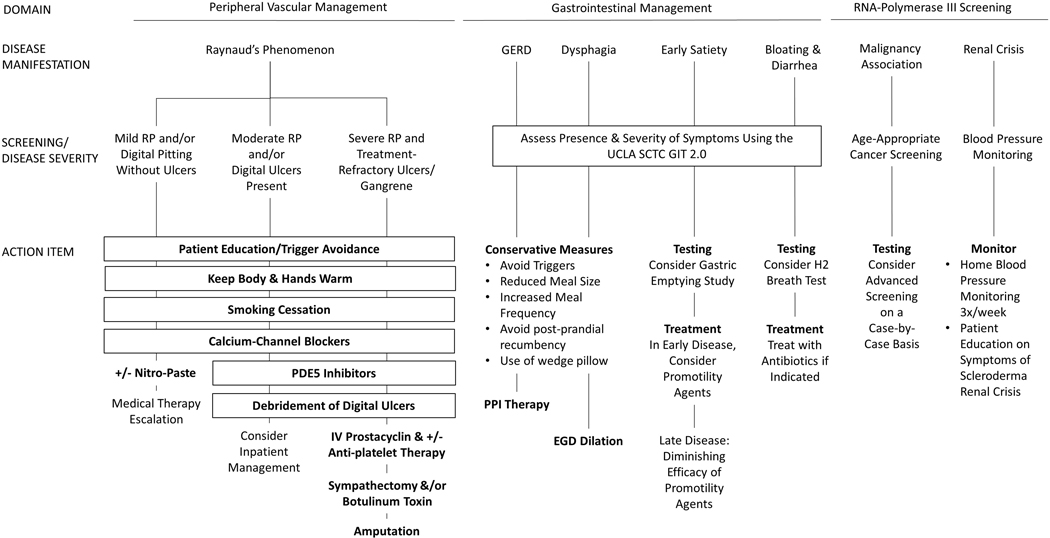
Management is based on symptom development. Immunosuppression and stem cell transportation has not demonstrated correction of the underlying gastrointestinal dysmotility associated with SSc. Education about silent aspiration and precautions to avoid choking should be instituted early on. We recommend conservative measures like remaining upright during meals, using liquids between swallowing solid foods, and avoiding recumbency for at least 4 hours following a meal to allow gravity to facilitate bolus transit.
Treatments include proton pump inhibitor for esophageal reflux disease, serial esophageal dilatation for persisting dysphasia, nutritional supplementation for those with a restricted diet and/or malabsorption, antibiotics for bacterial overgrowth, and photocoagulation for those patients with GAVE. We recommend co-management with a gastroenterologist when considering use of promotility agents or Botox injections into the esophagus. There are data to suggest sustained benefit from intravenous immunoglobulin therapy for gastrointestinal involvement78. Use of phosphodiesterase inhibitors and calcium channel blockers can impair the lower esophageal sphincter from functioning, and make esophageal reflux worse. Care should be taken to avoid pill esophagitis with common culprits (e.g., bisphosphonates, doxycycline), and consider common infections like candida as a source of esophageal discomfort.
Musculoskeletal/Cutaneous Involvement
SSc may affect several structures of the musculoskeletal system. Inflammatory arthritis (occurring in 16% (1,191 of 7,286) of a large European registry) and tendon friction rubs (occurring in 11% of patients (802 of 7,7286)) are commonly found in dcSSc, affecting the hands, wrists, elbows, knees, and ankles79. In addition to skin thickening, cutaneous disease involves the presence of calcinosis, occurring in 20–40% of SSc patients and seen more frequently in those with limited SSC with positive anti-centromere antibody positivity. Pruritus results as a consequence of small fiber neuropathy.
Patients with inflammatory arthritis may be treated similarly to those with rheumatoid arthritis80. Use of nonsteroidal anti-inflammatory drugs should be conducted with caution, given the risk of gastroesophageal abnormalities, GAVE in a small subset of patients, and those with impaired renal function. Low dose corticosteroids (less than 10 milligrams/day) may have value for symptomatic treatment of inflammatory arthritis. Providers should be cautious not to give doses above 15 milligrams/day to those patients with early diffuse SSc and especially those with RNA polymerase III positivity for fear of induction of scleroderma renal crisis. RA approved therapies may be considered, including abatacept and tocilizumab for treatment-refractory arthritis, although this recommendation is based on expert opinion81.
Treatment options for skin involvement appear to have modest benefit; efficacy in treatment is confounded by a treatment-independent regression of skin thickening (typically by five years past the first non-Raynaud’s phenomenon onset). Treatments include methotrexate, mycophenolate mofetil, with recent trials of tocilizumab and abatacept failing to show significant differences in modified Rodnan skin score compared to placebo, but significant improvements in global assessment of disease with abatacept82. The role of intravenous immunoglobulin therapy on skin manifestations in SSc remain unclear, but promising83. Hematopoietic stem cell transplant may be an option for a narrow spectrum of patients with early, rapidly progressive diffuse SSc with poor prognosis but an absence of advanced organ involvement.
Hand therapy includes paraffin wax treatments, resistance training, home therapy exercises as directed by an occupational therapist, and splinting84. Hand surgery is reserved for those with severe fixed deformities with functional limitations, ulcerations, and calcinosis refractory to treatment. The focus of surgery is to reposition digits and fuse the joints, immobilizing them to reduce pain and further digital complications of severely flexed PIP joints.
The efficacy of treatment of calcinosis remains disappointing. To date, there are little data to support the use of calcium channel blockers, bisphosphonates, minocycline, warfarin, and elective surgical excision. Gabapentin may have therapeutic role in treating small-fiber neuropathic pruritus.
Screening for Malignancy
There are data to suggest that SSc may be a paraneoplastic syndrome85,86. Maria et al. provide a comprehensive review of the subject to date87. In one cohort of 2,383 patients with scleroderma, 205 or 8.6% had a diagnosis of cancer. Patients with RNA polymerase III antibody positivity had a standardized incidence ratio of 2.84 (95% confidence interval 1.89–4.10); those who did not have scleroderma specific auto antibody positivity had a standardized incidence ratio of 1.83 (95% confidence interval 1.1–2.86). Those who were anti-centromere antibody positive had a lower risk of cancer during follow-up, with a standardized incidence ratio of 0.59 (95% confidence interval 0.44–0.76)88.
IV. APPROACH TO CLINICAL CARE
Management of early SSc
For patients with early SSc, we begin by counseling and educating the patient on his/her disease, the expected distribution and severity of organ involvement based on their skin and auto-antibody profile, and reinforce the varied trajectories of clinical outcomes depending on development of disease progression. Figures 1a and 1b outline the general management of early SSc.
Figure 1b:
General management of early systemic sclerosis
Medication/Treatment Acronyms
EGD: Esophagogastroduodenoscopy
PDE5: Phosphodiesterase 5
PPI: Proton Pump Inhibitor
Testing Acronyms
H2: Hydrogen
UCLA SCTC GIT 2.0: UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract Questionnaire
Disease Acronyms
GERD: Gastroesophageal Reflux Disease
RP: Raynaud’s Phenomenon
All patients should be screened for cardiac disease, interstitial lung disease, and pulmonary arterial hypertension; we recommend baseline EKG, echocardiogram, pulmonary function testing, and HRCT for all patients. Pulmonary arterial hypertension is rare to develop within the first five years, but the onset of shortness of breath is insidious and a screening algorithm such as the DETECT algorithm89 is advocated; echocardiogram is insufficient as a screening tool for PAH. High resolution chest CT is the gold standard in diagnosing ILD. Those patients with clinically-significant ILD, high risk for progression, or evidence of progressive disease should be initiated on immunosuppressive or anti-fibrotic therapy34. It is unclear if mild or subclinical ILD with limited SSc and anti-centromere antibody should be offered therapy. For those with positive anti-SCL-70 antibody status or elevated CRP levels in the setting of mild ILD on HRCT and mild deficits on FVC % predicted, we recommend initiation of tocilizumab or mycophenolate mofetil33 as these patients are at an increased risk of progression. For those with symptomatic ILD, mild-to-severe ILD on HRCT, FVC% predicted or DLco% predicted less than the lower limit of normal and/or clinically meaningful decline in FVC or DLco (if >1 PFT is available) accompanied by desaturation on oximetry during hall walk, we recommend mycophenolate mofetil. For those with progressive disease or non-tolerability to MMF, we add/replace with nintedanib 31,90. Those with extensive skin, musculoskeletal, and lung disease receive mycophenolate mofetil, cyclophosphamide, or rituximab30,31,91.
Nearly all patients will have gastrointestinal symptoms at the time of initial contact with rheumatology; patients should institute reflux/aspiration precautions, increase the frequency and decrease food consumption size per meal, and initiate proton pump inhibitor for GERD symptoms. Symptoms of small intestinal bacterial overgrowth should be screened for at each visit; we administer UCLA SCTC GIT 2.0 to every patient to assess for symptoms and severity of GI involvement (https://umich.qualtrics.com/jfe/form/SV_3eBP4A4umBwnSvj). We refer patients to gastroenterology who continue to have symptoms despite pharmacologic therapy.
Inflammatory arthritis and advancing skin thickening may simultaneously be treated with escalating immune suppressive therapy33,82,92, but continues to lead to considerable morbidity and remains a focus in the unmet needs of this subset of patients93. Patients with dcSSc and anti-SCL-70 antibody positivity are more likely than others to develop digital ulcerations; vasodilation, pain management, and prevention of/treatment for osteomyelitis remain a top priority94,95. Patients should be evaluated for the severity and frequency of Raynaud’s phenomenon, with particular attention paid to the presence and monitoring of digital ulcerations; tobacco abstinence should be a top priority for several health benefits, in addition to its detrimental vasoconstriction effect96.
Those with RNA Polymerase III antibody positivity should be counseled as above for risk of renal crisis. Those patients, and those with triple-negative antibody screening (negative anti-centromere, SCL-70, and RNA Polymerase III) should achieve up-to-date age-appropriate cancer screening88.
Finally, enrollment in clinical treatment trials provides an option for investigational use of medications not yet approved by the FDA for SSc. Clinical research trials are advancing the goal of improving outcomes for SSc patients and stratifying therapies for SSc subsets97.
V. CONCLUSIONS
Systemic sclerosis is a highly heterogeneous autoimmune disease, with varying clinical trajectories. Identifying patients within the first five years and sub-classifying patients based on skin distribution and auto-antibody status allows practitioners the best opportunity to intervene before advanced fibrosis sets in and cannot be reversed. Patients should be educated on the challenges ahead, limitations to treatment, and empowered to optimize their participation in maintaining their health. We encourage all our patients to explore their disease and management options at www.selfmanagescleroderma.com and scleroderma.org. Depending on the patient’s SSc subset, risk stratification allows for timely follow-up and close monitoring for the development of and response to therapy.
Key Points.
Identifying patients within the first five years and sub-classifying patients based on skin distribution and auto-antibody status allows practitioners the best opportunity to intervene before advanced fibrosis sets in and cannot be reversed.
All patients should be screened with HRCT for SSc-ILD and routinely monitored for the development of dyspnea, cough, or exercise limitation alongside pulmonary function testing.
Early detection and prompt initiation of therapy for PAH is essential.
Those with RNA Polymerase III antibody positivity should be counseled for risk of renal crisis and remain up-to-date on age-appropriate cancer screening.
Enrollment in clinical treatment trials provides an option for investigational use of medications not yet approved by the FDA for SSc.
BIBLIOGRAPHY
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. [DOI] [PubMed] [Google Scholar]
- 2.Johnson SR. New ACR EULAR guidelines for systemic sclerosis classification. Curr Rheumatol Rep. 2015;17(5). [DOI] [PubMed] [Google Scholar]
- 3.Steen VD, Powell D, Medsger TA. Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31(2):196–203. [DOI] [PubMed] [Google Scholar]
- 4.Reveille JD, Solomon DH. Evidence-based guidelines for the use of immunologic tests: Anticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum. 2003;49(3):399–412. [DOI] [PubMed] [Google Scholar]
- 5.Chung L, Utz PJ. Antibodies in scleroderma: direct pathogenicity and phenotypic associations. Curr Rheumatol Rep. 2004;6(2):156–163. [DOI] [PubMed] [Google Scholar]
- 6.Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2007;66(6):754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homer KL, Warren J, Karayev D, et al. Performance of Anti–Topoisomerase I Antibody Testing by Multiple-Bead, Enzyme-Linked Immunosorbent Assay and Immunodiffusion in a University Setting. JCR J Clin Rheumatol. 2018;00(00):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker JC, Burlingame RW, Webb TT, Bunn CC. Anti-RNA polymerase III antibodies in patients with systemic sclerosis detected by indirect immunofluorescence and ELISA. Rheumatology. 2008;47(7):976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. [DOI] [PubMed] [Google Scholar]
- 10.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: A prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plastiras SC, Karadimitrakis SP, Ziakas PD, Vlachoyiannopoulos PG, Moutsopoulos HM, Tzelepis GE. Scleroderma lung: Initial forced vital capacity as predictor of pulmonary function decline. Arthritis Care Res. 2006;55(4):598–602. [DOI] [PubMed] [Google Scholar]
- 12.Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2007;66(6):754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66(6):1625–1635. [DOI] [PubMed] [Google Scholar]
- 14.Scussel-Lonzetti L, Joyal F, Raynauld J-P, et al. Predicting mortality in systemic sclerosis: analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore). 2002;81(2):154–167. http://www.ncbi.nlm.nih.gov/pubmed/11889414. [DOI] [PubMed] [Google Scholar]
- 15.Mihai C, Landewé R, Van Der Heijde D, et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann Rheum Dis. 2016;75(4):681–686. [DOI] [PubMed] [Google Scholar]
- 16.Bryan C, Howard Y, Brennan P, Black C, Silman A. Survival following the onset of scleroderma: Results from a retrospective inception cohort study of the UK population. Br J Rheumatol. 1996;35(11):1122–1126. [DOI] [PubMed] [Google Scholar]
- 17.Nikpour M, Baron M. Mortality in systemic sclerosis: Lessons learned from population-based and observational cohort studies. Curr Opin Rheumatol. 2014;26(2):131–137. [DOI] [PubMed] [Google Scholar]
- 18. Nihtyanova S, Sari A, Harvey JC, et al. Using Autoantibodies and Cutaneous Subset to Develop Outcome-Based Disease Classification in Systemic Sclerosis. Arthritis Rheum. 2019;0(0):1–12. **This article provides evidence for the importance of autoantibodies and extent of skin involvement in stratifying SSc patients.
- 19.Mitri GM, Lucas M, Fertig N, Steen VD, Medsger TA. A comparison between anti-TH/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum. 2003;48(1):203–209. [DOI] [PubMed] [Google Scholar]
- 20.Stochmal A, Czuwara J, Trojanowska M, Rudnicka L. Antinuclear Antibodies in Systemic Sclerosis: an Update. Clin Rev Allergy Immunol. 2019;(January 2019):40–51. [DOI] [PubMed] [Google Scholar]
- 21.Perera A, Fertig N, Lucas M, et al. Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis Rheum. 2007;56(8):2740–2746. [DOI] [PubMed] [Google Scholar]
- 22.Steen VD. Autoantibodies in Systemic Sclerosis. 2005. [Google Scholar]
- 23.Launay D, Remy-Jardin M, Michon-Pasturel U, et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J Rheumatol. 2006;33(2006):1789–1801. [PubMed] [Google Scholar]
- 24.De Santis M, Bosello S, La Torre G, et al. Functional, radiological and biological markers of alveolitis and infections of the lower respiratory tract in patients with systemic sclerosis. Respir Res. 2005;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steen VD, Conte C, Owens GR, Medsger Ta. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37(9):1283–1289. [DOI] [PubMed] [Google Scholar]
- 26.Khanna D, Tseng CH, Farmani N, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: Analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum. 2011;63(10):3078–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J. Aetiology, Risk Factors, and Biomarkers in Systemic Sclerosis with Interstitial Lung Disease. Am J Respir Crit Care Med. 2019:1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Au K, Khanna D, Clements PJ, Furst DE, Tashkin DP. Current concepts in disease-modifying therapy for systemic sclerosis-associated interstitial lung disease: Lessons from clinical trials. Curr Rheumatol Rep. 2009;11(2):111–119. [DOI] [PubMed] [Google Scholar]
- 29.Goh NSL, Desai SR, Veeraraghavan S, et al. Interstitial Lung Disease in Systemic Sclerosis. Am J Respir Crit Care Med. 2008;177(11):1248–1254. [DOI] [PubMed] [Google Scholar]
- 30.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus Placebo in Scleroderma Lung Disease. N Engl J Med. 2006;354(25):2655–2666. [DOI] [PubMed] [Google Scholar]
- 31.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford). 2018;57(12):2106–2113. [DOI] [PubMed] [Google Scholar]
- 33.Khanna D, Lin CJF, Kuwana M, et al. Efficacy and Safety of Tocilizumab for the Treatment of Systemic Sclerosis : Results from a Phase 3 Randomized Controlled Trial. Arthritis Rheumatol. 2018;70(Suppl 10):Suppl 10. [Google Scholar]
- 34.Roofeh D, Jaafar S, Vummidi D, Khanna D. Management of systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2019:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Distler O, Highland KB, Gahlemann M, et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N Engl J Med. 2019;380(26):2518–2528. ** This well-designed and executed trial showed the annual rate of decline in FVC was lower with Nintedanib compared to placebo, leading to the first FDA-approved medication for SSc-ILD.
- 36.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. [DOI] [PubMed] [Google Scholar]
- 37.Mackintosh J, Stainer A, Barnett J, Renzoni E. Systemic Sclerosis Associated Interstitial Lung Disease: A Comprehensive Overview. Semin Respir Crit Care Med. 2019;40:208–226. [DOI] [PubMed] [Google Scholar]
- 38.Roofeh D, Distler O, Allanore Y, Denton CP, Khanna D. Treatment of systemic sclerosis– associated interstitial lung disease: Lessons from clinical trials. J Scleroderma Relat Disord. 2020;In Press.:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis : evidence-based European consensus statements. Lancet Rheumatol. 2020;9913(19):1–13. [DOI] [PubMed] [Google Scholar]
- 40.Walker UA, Saketkoo LA, Distler O. Haematopoietic stem cell transplantation in systemic sclerosis. RMD Open. 2018;4(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–1339. [DOI] [PubMed] [Google Scholar]
- 42.Steen V, Medsger TA. Predictors of Isolated Pulmonary Hypertension in Patients With Systemic Sclerosis and Limited Cutaneous Involvement. 2003;48(2):516–522. [DOI] [PubMed] [Google Scholar]
- 43.Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, et al. Registry of the Spanish network for systemic sclerosis: Survival, prognostic factors, and causes of death. Med (United States). 2015;94(43):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin V V, Shillington A, Rich S. Survival in primary pulmonary hypertension: The impact of epoprostenol therapy. Circulation. 2002;106(12):1477–1482. [DOI] [PubMed] [Google Scholar]
- 45.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: Prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–788. [DOI] [PubMed] [Google Scholar]
- 46.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs G, Avian A, Tscherner M, et al. Characterization of Patients With Borderline Pulmonary Arterial Pressure. Chest. 2014;146(6):1486–1493. [DOI] [PubMed] [Google Scholar]
- 48.Assad TR, Maron BA, Robbins IM, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol. 2017;2(12):1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaafar S, Visovatti S, Young A, et al. Impact of the revised haemodynamic definition on the diagnosis of pulmonary hypertension in patients with systemic sclerosis. Eur Respir J. 2019;54(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avouac J, Airò P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol. 2010;37(11):2290–2298. [DOI] [PubMed] [Google Scholar]
- 51.Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J. 2005;35(1):28–33. [DOI] [PubMed] [Google Scholar]
- 52.Hachulla E, Launay D, Mouthon L, et al. Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest. 2009;136(5):1211–1219. [DOI] [PubMed] [Google Scholar]
- 53.Young A, Vummidi D, Visovatti S, et al. Prevalence, Treatment, and Outcomes of Coexistent Pulmonary Hypertension and Interstitial Lung Disease in Systemic Sclerosis. Arthritis Rheumatol. 2019;71(8):1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2018:1801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundaram SM, Chung L. An Update on Systemic Sclerosis-Associated Pulmonary Arterial Hypertension: a Review of the Current Literature. Curr Rheumatol Rep. 2018;20(2). [DOI] [PubMed] [Google Scholar]
- 56.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. [DOI] [PubMed] [Google Scholar]
- 57.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533. [DOI] [PubMed] [Google Scholar]
- 58.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. [DOI] [PubMed] [Google Scholar]
- 59.Coghlan JG, Galiè N, Barberà JA, et al. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): Subgroup analysis from the AMBITION trial. Ann Rheum Dis. 2017;76(7):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coghlan JG, Channick R, Chin K, et al. Targeting the Prostacyclin Pathway with Selexipag in Patients with Pulmonary Arterial Hypertension Receiving Double Combination Therapy: Insights from the Randomized Controlled GRIPHON Study. Am J Cardiovasc Drugs. 2018;18(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jansa P, Pulido T. Macitentan in Pulmonary Arterial Hypertension: A Focus on Combination Therapy in the SERAPHIN Trial. Am J Cardiovasc Drugs. 2018;18(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mavrogeni SI, Bratis K, Karabela G, et al. Cardiovascular magnetic resonance imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflamm Allergy - Drug Targets. 2015;14(1):29–36. [DOI] [PubMed] [Google Scholar]
- 63.Mavrogeni S, Sfikakis PP, Karabela G, et al. Cardiovascular magnetic resonance imaging in asymptomatic patients with connective tissue disease and recent onset left bundle branch block. Int J Cardiol. 2014;171(1):82–87. [DOI] [PubMed] [Google Scholar]
- 64.Boueiz A, Mathai SC, Hummers LK, Hassoun PM. Cardiac complications of systemic sclerosis: Recent progress in diagnosis. Curr Opin Rheumatol. 2010;22(6):696–703. [DOI] [PubMed] [Google Scholar]
- 65.Parks JL, Taylor MH, Parks LP, Silver RM. Systemic Sclerosis and the Heart. Rheum Dis Clin North Am. 2014;40(1):87–102. [DOI] [PubMed] [Google Scholar]
- 66.Hung G, Mercurio V, Hsu S, Mathai SC, Shah AA, Mukherjee M. Progress in Understanding, Diagnosing, and Managing Cardiac Complications of Systemic Sclerosis. Curr Rheumatol Rep. 2019;21(12):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30(SUPPL.71). [PMC free article] [PubMed] [Google Scholar]
- 68.Meune C, Khanna D, Aboulhosn J, et al. A right ventricular diastolic impairment is common in systemic sclerosis and is associated with other target-organ damage. Semin Arthritis Rheum. 2016;45(4):439–445. [DOI] [PubMed] [Google Scholar]
- 69.Allanore Y, Meune C, Vonk MC, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69(1):218–221. [DOI] [PubMed] [Google Scholar]
- 70.Mukherjee M, Chung SE, Ton VK, et al. Unique Abnormalities in Right Ventricular Longitudinal Strain in Systemic Sclerosis Patients. Circ Cardiovasc imaging. 2016;176(1):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Denton CP, Hudson M. Renal Crisis and Other Renal Manifestations of Scleroderma. In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, eds. Scleroderma: From Pathogenesis to Comprehensive Management. 2nd ed. Springer Healthcare; 2017:317–330. [Google Scholar]
- 72.Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: Patient characteristics and long-term outcomes. Qjm. 2007;100(8):485–494. [DOI] [PubMed] [Google Scholar]
- 73.Bruni C, Cuomo G, Rossi FW, Praino E, Bellando-Randone S. Kidney involvement in systemic sclerosis: From pathogenesis to treatment. J Scleroderma Relat Disord. 2018;3(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lynch BM, Stern EP, Ong V, Harber M, Burns A, Denton CP. UK Scleroderma Study Group (UKSSG) guidelines on the diagnosis and management of scleroderma renal crisis. Clin Exp Rheumatol. 2016;34:106–109. [PubMed] [Google Scholar]
- 75.Nagaraja V. Management of scleroderma renal crisis. Curr Opin Rheumatol. 2019;31(3):223–230. [DOI] [PubMed] [Google Scholar]
- 76.Hudson M, Baron M, Tatibouet S, et al. Exposure to ACE inhibitors prior to the onset of scleroderma renal crisis-results from the international scleroderma renal crisis survey. Semin Arthritis Rheum. 2014;43(5):666–672. [DOI] [PubMed] [Google Scholar]
- 77.Denton CP. Overview of Gastrointestinal Tract Involvement. In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, eds. Scleroderma: From Pathogenesis to Comprehensive Management. 2nd ed. Springer Healthcare; 2017:423–426. [Google Scholar]
- 78.Raja J, Nihtyanova SI, Murray CD, Denton CP, Ong VH. Sustained benefit from intravenous immunoglobulin therapy for gastrointestinal involvement in systemic sclerosis. Rheumatol (United Kingdom). 2016;55(1):115–119. [DOI] [PubMed] [Google Scholar]
- 79.Avouac J, Walker U, Tyndall A, et al. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: Results from the EULAR Scleroderma Trial and Research Group (EUSTAR) database. J Rheumatol. 2010;37(7):1488–1501. [DOI] [PubMed] [Google Scholar]
- 80.Pellar RE, Pope JE. Evidence-based management of systemic sclerosis: Navigating recommendations and guidelines. Semin Arthritis Rheum. 2017. [DOI] [PubMed] [Google Scholar]
- 81.Fernández-Codina A, Walker KM, Pope JE. Treatment Algorithms for Systemic Sclerosis According to Experts. Arthritis Rheumatol. 2018;70(11):1820–1828. [DOI] [PubMed] [Google Scholar]
- 82.Khanna D, Spino C, Johnson S, et al. Abatacept in Early Diffuse Cutaneous Systemic Sclerosis – Results of a Phase 2 Investigator-Initiated, Multicenter, Double-Blind Randomized Placebo-Controlled Trial. Arthritis Rheumatol. 2019;72(1):125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poelman CL, Hummers LK, Wigley FM, Anderson C, Boin F, Shah AA. Intravenous immunoglobulin may be an effective therapy for refractory, active diffuse cutaneous systemic sclerosis. J Rheumatol. 2015;42(2):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy SL, Barber MW, Homer K, Dodge C, Cutter GR, Khanna D. Occupational Therapy Treatment to Improve Upper Extremity Function in Individuals with Early Systemic Sclerosis: A Pilot Study. Arthritis Care Res. 2018;70(11):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bonifazi M, Tramacere I, Pomponio G, et al. Systemic sclerosis (scleroderma) and cancer risk: Systematic review and meta-analysis of observational studies. Rheumatol (United Kingdom). 2013;52(1):143–154. [DOI] [PubMed] [Google Scholar]
- 86.Olesen AB, Sværke C, Farkas DK, Sørensen HT. Systemic sclerosis and the risk of cancer: A nationwide population-based cohort study. Br J Dermatol. 2010;163(4):800–806. [DOI] [PubMed] [Google Scholar]
- 87.Maria ATJ, Partouche L, Goulabchand R, et al. Intriguing relationships between cancer and systemic sclerosis: Role of the immune system and other contributors. Front Immunol. 2019;10(JAN):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Igusa T, Hummers LK, Visvanathan K, et al. Autoantibodies and scleroderma phenotype define subgroups at high-risk and low-risk for cancer. Ann Rheum Dis. 2018;77(8):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coghlan JG, Denton CP, Grünig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann Rheum Dis. 2014;73(7):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–2528. [DOI] [PubMed] [Google Scholar]
- 91.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatol. 2018;57(12 PG-2106–2113):2106–2113. [DOI] [PubMed] [Google Scholar]
- 92.Pellar RE, Pope JE. Evidence-based management of systemic sclerosis: Navigating recommendations and guidelines. Semin Arthritis Rheum. 2017;46(6):767–774. [DOI] [PubMed] [Google Scholar]
- 93.Khanna D, Allanore Y, Denton CP, et al. Patient perception of disease burden in diffuse cutaneous systemic sclerosis. J Scleroderma Relat Disord. 2019;5(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morrisroe K, Stevens W, Sahhar J, et al. Digital ulcers in systemic sclerosis: Their epidemiology, clinical characteristics, and associated clinical and economic burden. Arthritis Res Ther. 2019;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giuggioli D, Manfredi A, Lumetti F, Colaci M, Ferri C. Scleroderma skin ulcers definition, classification and treatment strategies our experience and review of the literature. Autoimmun Rev. 2018;17(2):155–164. [DOI] [PubMed] [Google Scholar]
- 96.Pauling JD, Hughes M, Pope JE. Raynaud’s phenomenon—an update on diagnosis, classification and management. Clin Rheumatol. 2019;38(12):3317–3330. [DOI] [PubMed] [Google Scholar]
- 97.Quinlivan A, Ross L, Proudman S. Best Practice & Research Clinical Rheumatology Systemic sclerosis : Advances towards strati fi ed medicine. Best Pract Res Clin Rheumatol. 2019;(Article In Press):1–22. [DOI] [PubMed] [Google Scholar]