Abstract
A 63‐year‐old Japanese man with amyopathic dermatomyositis treated with immunosuppressants became aware of distortion of his left visual field, and a metastatic choroidal tumor was suspected. His chest computed tomography (CT) showed a pulmonary nodule in the right upper lobe and mediastinal lymphadenopathy, and he was diagnosed with advanced lung adenocarcinoma with choroidal metastasis. Malignancies associated with dermatomyositis (DM) are often rapidly progressive and, in choroidal metastasis associated with lung cancer, a choroidal lesion is often diagnosed prior to lung cancer; therefore, CT performed at the time of diagnosis of choroidal metastasis may show lung cancer lesions. When ocular symptoms are observed in DM patients, metastatic malignancies should be suspected, and systemic examinations, such as positron emission tomography (PET)‐CT, should also be performed.
Keywords: adenocarcinoma, choroidal metastasis, dermatomyositis
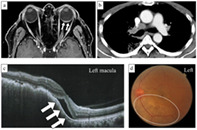
Here, we present the first case of rapidly progressive lung adenocarcinoma and choroidal metastasis in a patient with ADM complicated with ILD undergoing immunosuppressant treatment, in whom the rapidness of initiation and progression of lung cancer was observed incidentally. Chest CT showed mediastinal lymphadenopathies (a), and contrast‐enhanced magnetic resonance imaging of the head showed left retinal thickening (white arrow) (b). Optical coherence tomography (OCT) showed mild serous retinal detachment in the macula (white arrows) (c), and fundoscopy evaluation showed white spots in the lower part of the left optic nerve papilla (white circle) (d).
INTRODUCTION
Dermatomyositis (DM) is one of the most common autoimmune diseases that can be complicated by malignancies. 1 DM with malignancy is often rapidly progressive, affecting patient prognosis. 2 , 3 , 4 , 5
Metastatic choroidal tumors are the most frequent form of metastatic ocular tumors, 6 , 7 , 8 and the primary sites of metastatic choroidal tumors are breast (40%–53%) and lung cancer (20%–29%). 6 , 8 , 9 , 10 Compared to patients with breast cancer with metastatic choroidal tumors, ocular symptoms and metastatic choroidal tumors tend to be found and diagnosed before diagnosing in patients with lung cancer. 7 , 8 , 9 , 10
There have been no reports of lung cancer with choroidal metastasis in patients with DM so far. Herein, we present, to the best of our knowledge, the first case of rapidly progressive lung adenocarcinoma in a patient with amyopathic dermatomyositis (ADM) complicated with interstitial lung disease (ILD) presenting with choroidal metastasis.
CASE REPORT
A 63‐year‐old Japanese man noticed left visual field distortion and was evaluated in the ophthalmology department. He was diagnosed with ADM complicated with ILD when he was 62 years old and treated with prednisolone (5 mg/day) and tacrolimus (3 mg/day). One year later, computed tomography (CT) performed during a routine visit revealed bilateral peripheral pulmonary interstitial reticular opacities with no obvious shadows suspicious of malignancy (Figure 1a). At the ophthalmology department, fundoscopy showed a 6 × 6 mm choroidal tumor with petechial white spots in the lower part of the left optic nerve papilla, and optical coherence tomography showed mild serous retinal detachment in the left macula (Figure 2). His systemic CT revealed a nodule in the right upper lobe with mediastinal lymphadenopathy, and multiple bone metastases that had not been seen 2 months prior (Figure 1b). Upon admission to the Respiratory Medicine department, his laboratory findings showed increased serum levels of sialylated carbohydrate antigen Krebs von den Lungen‐6 (1678 IU/ml) and carcinoembryonic antigen (43.7 ng/ml); anti‐aminoacyl tRNA synthetase antibody was positive (Table 1). In addition, choroidal thickening on the left was observed on contrast‐enhanced magnetic resonance imaging of the head (Figure 1c). Bronchoscopic mediastinal lymph node biopsies yielded a diagnosis of lung adenocarcinoma without driver gene mutation (clinical stage IVB). After cessation of tacrolimus, first‐line chemotherapy with carboplatin and nab‐paclitaxel was started. This treatment was chosen because of its low risk of ILD exacerbation. 11 The lung and metastatic left choroidal tumors responded to this treatment, with a 50% reduction in size; furthermore, his serous retinal detachment and left visual field improved (Figure 3).
FIGURE 1.
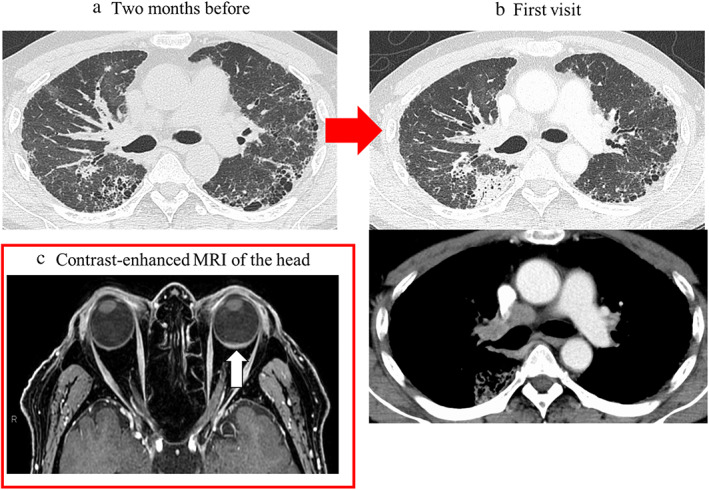
(a) Computed tomography (CT) scan image acquired 2 months prior to the first visit to our department and (b) at the time of the first visit to our department (b). (a) Reticular shadows and cystic clusters with a predominance of subpleural areas in bilateral lungs as ADM‐associated interstitial lung disease are seen. (b) New mass shadow in the right upper lobe approximately 3.0 cm in length and diameter and mediastinal lymphadenopathies are newly observed. (c) Contrast‐enhanced magnetic resonance imaging of the head, showing left retinal thickening (white arrow). Abbreviations: ADM: amyopathic dermatomyositis; CT: computed tomography; MR: magnetic resonance imaging
FIGURE 2.
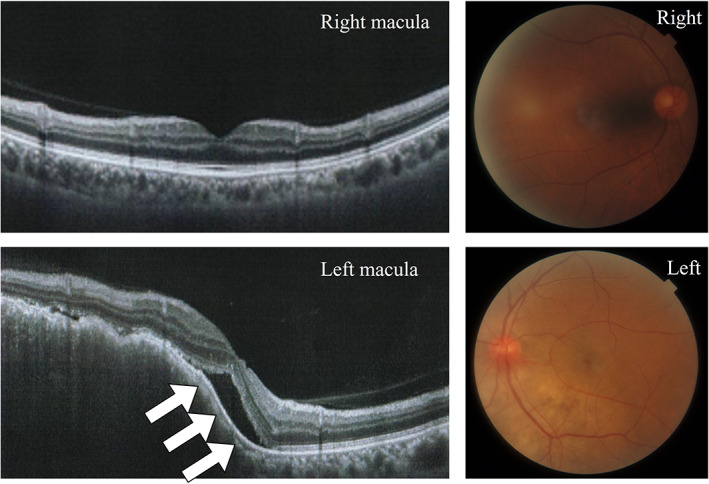
Optical coherence tomography, showing mild serous retinal detachment in the macula (white arrows). Moreover, the fundoscopy evaluation shows white spots in the lower part of the left optic nerve papilla (white circle). Abbreviation: OCT, optical coherence tomography
TABLE 1.
Laboratory findings on admission
| Blood cell counts | Blood chemistry | Serology | |||
|---|---|---|---|---|---|
| WBC | 12 200/μl | TP | 7.7 g/dl | CRP | 2.19 Mg/dl |
| Neutrophils | 74.9% | Alb | 4.0 g/dl | KL‐6 | 1678 U/ml |
| Lymphocytes | 16.4% | AST | 15 IU/l | SP‐D | 312 ng/ml |
| Eosinophils | 2.7% | ALT | 9 IU/l | CEA | 43.7 ng/ml |
| Monocytes | 6.0% | LDH | 253 IU/l | CYFRA | 3.3 ng/ml |
| Basophils | 0.0% | ALP | 153 IU/l | Rheumatoid factor | 8.3 U/ml |
| RBC | 468 × 104/μl | γ‐GTP | 18 IU/l | ANA | <40 titer |
| Hb | 13.7 g/dl | BUN | 14 mg/dl | Anti ARS antibody | 60.1 CI |
| Ht | 36.3% | Cre | 0.72 mg/dl | Anti MDA‐5 antibody | <1.0 U/ml |
| Platelets | 31.6 × 104/μl | CK | 37 IU/l | Anti TIF1‐γ antibody | <1.0 U/ml |
| Na | 141 mmol/l | Anti Mi‐2 antibody | <1.0 U/ml | ||
| K | 3.9 mmol/l | β‐D glucan | <6.0 pg/ml | ||
| Cl | 102 mmol/l | Aspergilous antigen | 0.1 CI | ||
Abbreviations: Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibody; ARS, aminoacyl tRNA synthetase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CEA, carcinoembryonic antigen; CK, creatine kinase; Cre, creatinine; CRP, C‐reactive protein; CYFRA, cytokeratin 19 fragment; Hb, hemoglobin; Ht, hematocrit; KL‐6, sialylated carbohydrate antigen Krebs von den Lungen‐6; LDH, lactate dehydrogenase; MDA5, melanoma differentiation‐associated gene 5; RBC, red blood cell; SP‐D, pulmonary surfactant protein‐D; TIF1‐γ, transcriptional intermediary factor 1‐γ; TP, total protein; WBC, white blood cell; γ‐GTP, gamma‐glutamyl transferase.
FIGURE 3.
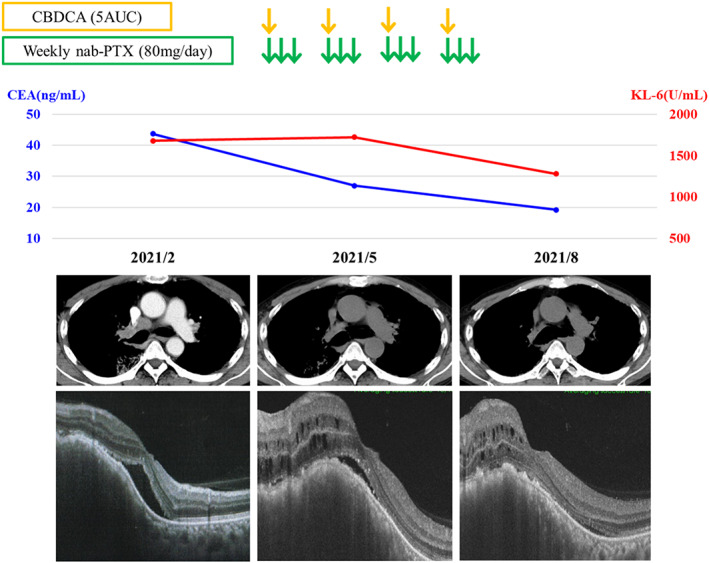
Clinical course of the patient. Abbreviations: CBDCA, carboplatin; AUC, area under the concentration‐time curve; nab‐PTX, nab‐paclitaxel; KL‐6, sialylated carbohydrate antigen Krebs von den Lungen‐6, CEA, carcinoembryonic antigen
DISCUSSION
Herein, we present the first case of rapidly progressive lung adenocarcinoma and choroidal metastasis in a patient with ADM complicated with ILD undergoing immunosuppressant treatment, in whom the rapidness of initiation and progression of lung cancer was observed incidentally.
DM is an autoimmune disease with muscular and skin manifestations, and 15%–30% of patients with DM are reported to develop malignancy. 1 In addition, malignancies diagnosed within 3 years after DM are often in the advanced stages at the time of diagnosis. 2 , 3 , 4 Even in our patient, the diagnosis of advanced‐stage lung adenocarcinoma was made only about a year after the diagnosis and treatment of DM with immunosuppressants. The prognosis of patients with DM‐related malignancies is reported to be poor. 1 , 3 , 4 , 5 , 12 However, further data are needed on the prognosis of lung cancer in patients with ADM initially treated with immunosuppressants after discontinuation of immunosuppressants.
Immunosuppressants were also considered as one of the precipitating factors for the rapidly progressive lung adenocarcinoma in this patient. Indeed, our patient used tacrolimus, one of the calcineurin inhibitors, which increases the expressions of several factors related to carcinogenesis, including transforming growth factor‐β (contributes to tumor cell invasion and metastasis), 13 , 14 vascular endothelial growth factor (promotes angiogenesis and vascular tumor growth), 15 and interleukin‐6 (induces activation of B cells that promote the development of lymphoproliferative diseases). 16 In addition, while the tumor doubling time (TDT) of the lung tumor was reported to be around 3–6 months, 17 faster TDT was reported in patients treated with immunosuppressants (as fast as 40–70 days). 18 Therefore, tacrolimus treatment might have influenced the rapid progression of lung adenocarcinoma in our patient.
Previous reports have shown that 4.7% of metastatic choroidal tumors were clinically diagnosed among 213 patients with malignancies, while 12.0% were diagnosed during autopsy in 230 autopsy cases with malignancies. 19 , 20 The common primary sites of metastatic choroidal tumors are the lung and breast. 6 , 8 , 9 , 10 Symptoms of metastatic choroidal tumors include distortion or loss of the visual field, and loss of vision. 7 , 8 However, patients with metastatic choroidal tumors are often asymptomatic as tumors located away from the macula might not lead to ocular symptoms. 7 , 8 , 10 Compared with metastatic choroidal tumors of primary tumors such as breast cancer, metastatic choroidal tumors of lung cancer are often preceded by ocular symptoms; thus, choroidal tumors can serve as an indication of future lung cancer diagnosis in some patients with lung cancer. 7 , 8 , 10
Our patient showed a good response to systemic chemotherapy for advanced lung adenocarcinoma, with shrinking of the lung and choroidal tumors with improved ocular symptoms due to the resolution of serous retinal detachment. Regarding tumor responsiveness to systemic chemotherapy, the response rate of lung cancer with metastatic choroidal metastasis has previously been reported to be about 68%. 7
Here, we present the first case of rapidly progressive lung adenocarcinoma and choroidal metastasis in a patient with ADM complicated with ILD undergoing immunosuppressant treatment. Malignancies associated with DM can progress rapidly clinically, have poor prognosis and optic insufficiency, and can be metastatic choroidal tumors from lung and other cancers. If a patient with DM presents with ocular symptoms, systemic evaluations, such as positron emission tomography‐CT, should be undertaken to ensure early diagnosis of malignancies.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Kawaguchi T, Yamasaki K, Shingu T, Manabe T, Koga S, Naruse S, et al. Advanced lung adenocarcinoma detected by choroidal metastasis in a patient with amyopathic dermatomyositis: A case report. Thorac Cancer. 2022;13(11):1739–1743. 10.1111/1759-7714.14440
REFERENCES
- 1. Li Y, Jia X, Sun X, Shi L, Lin F, Gan Y, et al. Risk factors for cancer‐associated myositis: a large‐scale multicenter cohort study. Int J Rheum Dis. 2021;24:268–73. [DOI] [PubMed] [Google Scholar]
- 2. Kang EH, Lee SJ, Ascherman DP, Lee YJ, Lee EY, Lee EB, et al. Temporal relationship between cancer and myositis identifies two distinctive subgroups of cancers: impact on cancer risk and survival in patients with myositis. Rheumatology (Oxford). 2016;55:1631–41. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Xu L, Wu H, Zhao N, Tang Y, Li X, et al. Characteristics and predictors of malignancy in dermatomyositis: analysis of 239 patients from northern China. Oncol Lett. 2018;16:5960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. András C, Bodoki L, Nagy‐Vincze M, Griger Z, Csiki E, Dankó K. Retrospective analysis of cancer‐associated myositis patients over the past 3 decades in a Hungarian myositis cohort. Pathol Oncol Res. 2020;26:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tiniakou E, Mammen AL. Idiopathic inflammatory myopathies and malignancy: a comprehensive review. Clin Rev Allergy Immunol. 2017;52:20–33. [DOI] [PubMed] [Google Scholar]
- 6. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–76. [DOI] [PubMed] [Google Scholar]
- 7. Shah SU, Mashayekhi A, Shields CL, Walia HS, Hubbard GB III, Zhang J, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121:352–7. [DOI] [PubMed] [Google Scholar]
- 8. Mathis T, Jardel P, Loria O, Delaunay B, Nguyen AM, Lanza F, et al. New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res. 2019;68:144–76. [DOI] [PubMed] [Google Scholar]
- 9. Konstantinidis L, Rospond‐Kubiak I, Zeolite I, Heimann H, Groenewald C, Coupland SE, et al. Management of patients with uveal metastases at the Liverpool ocular oncology Centre. Br J Ophthalmol. 2014;98:92–8. [DOI] [PubMed] [Google Scholar]
- 10. Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. a clinicopathologic study of 227 cases. Arch Ophthalmol. 1974;92:276–86. [DOI] [PubMed] [Google Scholar]
- 11. Asahina H, Oizumi S, Takamura K, Harada T, Harada M, Yokouchi H, et al. A prospective phase II study of carboplatin and nab‐paclitaxel in patients with advanced non‐small cell lung cancer and concomitant interstitial lung disease (HOT1302). Lung Cancer. 2019;138:65–71. [DOI] [PubMed] [Google Scholar]
- 12. Motomura K, Yamashita H, Yamada S, Takahashi Y, Kaneko H. Clinical characteristics and prognosis of polymyositis and dermatomyositis associated with malignancy: a 25‐year retrospective study. Rheumatol Int. 2019;39:1733–9. [DOI] [PubMed] [Google Scholar]
- 13. Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell‐autonomous mechanism. Nature. 1999;397:530–4. [DOI] [PubMed] [Google Scholar]
- 14. Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, et al. Tacrolimus enhances transforming growth factor‐beta1 expression and promotes tumor progression. Transplantation. 2003;76:597–602. [DOI] [PubMed] [Google Scholar]
- 15. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. [DOI] [PubMed] [Google Scholar]
- 16. Walz G, Zanker B, Melton LB, Suthanthiran M, Strom TB. Possible association of the immunosuppressive and B cell lymphoma‐promoting properties of cyclosporine. Transplantation. 1990;49:191–4. [DOI] [PubMed] [Google Scholar]
- 17. Usuda K, Saito Y, Sagawa M, Sato M, Kanma K, Takahashi S, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer. 1994;74:2239–44. [DOI] [PubMed] [Google Scholar]
- 18. Kawamura K, Ichikado K, Muranaka H, Ichikado K, Muranaka H, Gushima Y, et al. Solid malignancy in patients who received cyclosporine for fibrosing interstitial pneumonia. Nihon Kokyuki Gakkai Zasshi. 2010;48:261–6. [PubMed] [Google Scholar]
- 19. Albert DM, Rubenstein RA, Scheie HG. Tumor metastasis to the eye. I. Incidence in 213 adult patients with generalized malignancy. Am J Ophthalmol. 1967;63:723–6. [PubMed] [Google Scholar]
- 20. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol. 1971;85:673–5. [DOI] [PubMed] [Google Scholar]


