Abstract
Purpose
This study aimed to compare the value of a modified chest tube drainage strategy to a traditional drainage strategy in single‐port thoracoscopic pulmonary wedge resection.
Methods
From January 2019 to July 2021, we collected clinical data on 405 patients who underwent single‐port thoracoscopic pulmonary wedge resection in the No.1 Department of Thoracic Surgery at Fujian Medical University Union Hospital, with 121 (29.9%) cases in the modified drainage strategy group and 284 (70.1%) cases in the traditional drainage strategy group. The propensity score matching method (Match Ratio = 1:1) was used to reduce differences in clinical characteristics between the two groups.
Results
Following 1:1 propensity score matching, 120 matched pairs (240 patients) were included in the study. There was no significant difference in general clinical characteristics between the two groups. There was no statistical difference in intraoperative factors except for operative times (71.42 ± 22.98 min vs. 86.80 ± 36.75 min, p < 0.001). In terms of postoperative factors, there were significant differences in postoperative chest tube duration (0.00 ± 0.00 h vs. 32.68 ± 18.51 h, p < 0.001), total drainage volume (143.03 ± 118.33 ml vs. 187.73 ± 140.82 ml, p = 0.008), postoperative hospital stay (2.61 ± 0.70 days vs. 3.27 ± 1.88 days, p < 0.001), number of additional pain relief (0.14 ± 0.40 vs. 0.42 ± 0.74, p < 0.001), facial pain score (2.7 ± 1.8 vs. 3.6 ± 2.7, p = 0.005) and adverse events (p = 0.046). Furthermore, there was a statistical difference between the two groups regarding CTCAE grade‐1 complication, but no statistical difference in CTCAE grade‐2 complication.
Conclusions
A modified drainage strategy in single‐port thoracoscopic pulmonary wedge resection is safe and feasible, allowing for less postoperative rehabilitation time, pain relief, reduced postoperative pleural effusion, and reduced clinical workload.
Keywords: closed thoracic drainage, pulmonary wedge resection, rapid recovery, uni‐portal, video‐assisted thoracic surgery
A modified chest drainage strategy is shown. In this study, we evaluated the effect of a modified drainage strategy (MDS) in patients with single‐port thoracoscopic pulmonary wedge resection via propensity sore‐matched analysis. The results demonstrate that the application of MDS is safe and feasible, allowing for less postoperative rehabilitation time and pain relief, reduced postoperative pleural effusion, and reduced clinical workload. In addition, MDS has the potential to reduce patients' medical burden.
INTRODUCTION
Following thoracic surgery, the application of chest drain tubes is critical for removing air leaks and/or pleural effusions. All surgeons want to remove a chest tube as soon as possible because it can aggravate pain, delay recovery of lung function and the 6‐min walking distance, and lengthen hospitalization. 1 Chest tube management is still an important aspect following lung resection because it affects the recovery phase and the length of hospital stay. A strategy without using a chest tube has previously been reported in the literature. 2 , 3 , 4 In preliminary research, the tubeless strategy reduced pain and shortened hospital stays compared to standard VATs. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 This method, however, is not frequently encouraged due to security concerns.
In this study, the drainage strategy was modified by omitting the traditional silica gel drainage tube (modified drainage strategy, MDS) and compared to the traditional drainage strategy (TDS) to investigate the safety and reliability of the MDS used in single‐port video‐assisted thoracoscopic (VATs) pulmonary wedge resection (PWR) for the treatment of peripheral pulmonary nodules. Finally, a resource for clinical decision‐making is offered.
PATIENTS AND METHODS
This is a retrospective case–control study. From January 2019 to July 2021, 405 patients underwent single‐port thoracoscopic PWR at Fujian Medical University Union Hospital's No.1 Thoracic Department. Patients were divided into two groups based on whether or not a traditional silica gel drainage tube was used: the modified drainage strategy group (MDS group) and the traditional drainage strategy group (TDS group). PSM was carried out using a 1:1 matching algorithm to reduce the influence of confounders in the MDS and TDS groups (Figure 1). Finally, 240 patients were enrolled in the current study, with 120 patients (50%) in the MDS group and 120 patients (50%) in the TDS group. This study was reviewed and approved by the institutional review board of Fujian Medical University Union Hospital (No. 2021KY113), which waived the need for informed consent of the patients.
FIGURE 1.
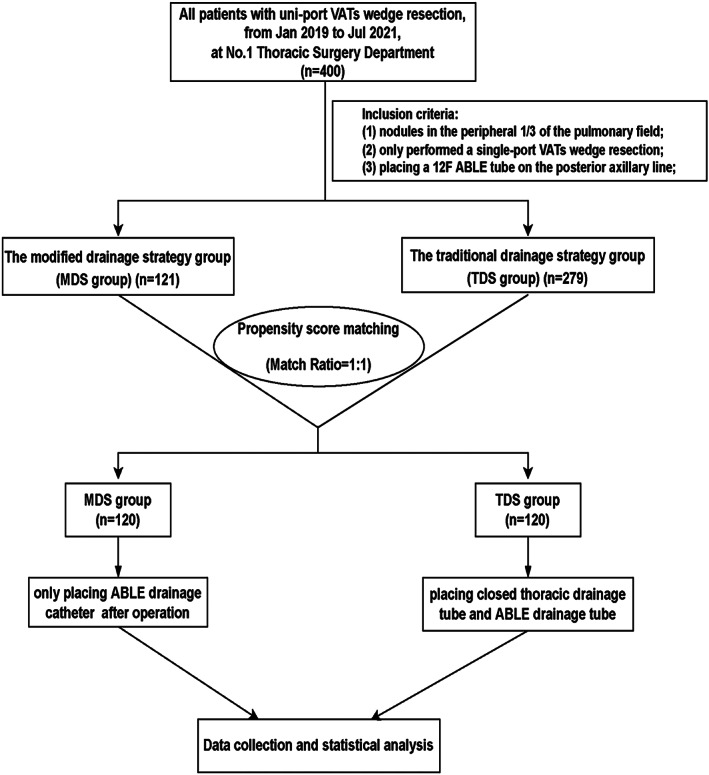
The flow diagram of the study. VATs, video‐assisted thoracic surgery
The following were the inclusion criteria: (1) a CT scan reveals one or more nodules in the peripheral third of the pulmonary field; (2) a single‐port thoracoscopic PWR was performed, with or without mediastinal lymph node sampling; (3) the linear cutting lines were well‐formed during the operation, or the wound was sutured continuously with 3–0/4–0 Prolen sutures when the cutting lines deformed; (4) a 12F drainage tube (ABLE, disposable abdomen drainage catheter set) was placed through the seventh or eighth intercostal space on the posterior axillary line; and (5) cardiopulmonary function allowed single‐port thoracoscopic PWR.
The following were the exclusion criteria: (1) severe emphysema; (2) severe pleural adhesions discovered during surgery; (3) ipsilateral thoracic operation, radiotherapy, or chemotherapy within the previous 3 months; and (4) undergoing other surgery during the same period, such as segmentectomy or lobectomy.
Surgical procedures
All of the patients had single‐port thoracoscopic surgery. The patients were positioned lateral decubitus. An incision was made on the midaxillary line at the fourth intercostal space for pulmonary wedge resection. The incision was approximately 3–4 cm long.
Under general anesthesia, single‐port VATs procedures were performed with double‐cavity endotracheal intubation and single‐lung ventilation. The nodule was located using the finger‐touch method based on the CT scan image. An endoscopic linear cutting stapler (Ethicon Endo‐Surgery, Inc. or REACH SURGICAL, Inc. or Medtronic, Inc.) was used to resect the pulmonary nodules.
Following PWR, we carefully examined the wound of the stapler line. When the cutting lines deformed, the wound was sutured continuously with 3–0/4–0 Prolen sutures. Generally, air leakage tests were not performed inside the thorax. After the procedure, we used 20 ml of 1% ropivacaine to perform a multilevel intercostal nerve block on the intercostal level of the incision and one level above and below. A 12F drainage tube (ABLE drainage catheter) was inserted into the posterior axillary line through the seventh or eighth intercostal space. The incision was closed with discontinuous suturing, and the knots were not tied immediately. Following surgery, two different chest tube drainage strategies were used.
MDS group
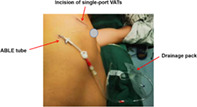
A 22# or 24# drainage tube for the MDS group was placed along the surgical incision, and the ABLE drainage tube was closed. The muscle layer suture was tightened to keep the pleural cavity closed. A silicone tube was temporarily inserted through the incision into the pleural cavity, while the tube's outer end was immersed in normal saline, as shown in Figure 2. Anesthesiologists performed lung inflation manually to achieve an airway pressure of 20 cmH2O. The lungs were inflated until all the air had been expelled with no air leakage. Finally, the silicone tube was removed and the incision was closed. Layer by layer, the incision was closed. The ABLE catheter was inserted into the posterior axillary line through the seventh or eighth intercostal space (Figure 3).
FIGURE 2.
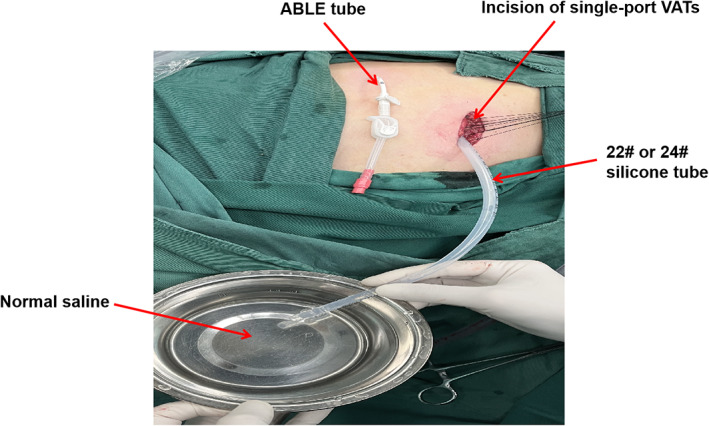
The air leakage test was performed in the MDS group. A 22# or 24# silicone tube was placed along the surgical incision, and the ABLE drainage tube was closed. The muscle layer suture was tightened to keep the pleural cavity closed. A silicone tube was temporarily inserted into the pleural cavity through the incision, and the tube's outer end was immersed in normal saline
FIGURE 3.
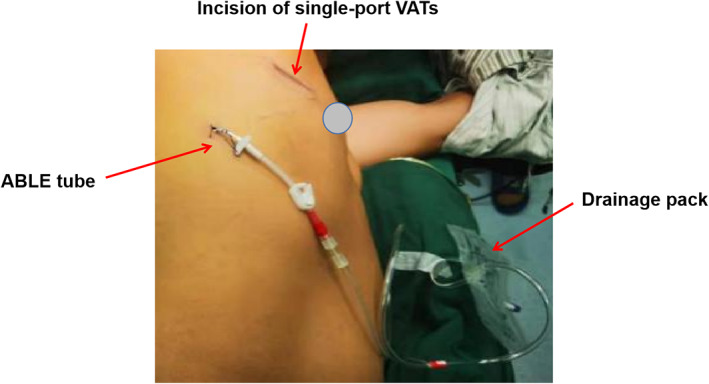
The MDS group's drainage. The ABLE catheter was inserted into the posterior axillary line through the seventh or eighth intercostal space
TDS group
A 22# or 24# silicone tube was inserted through the incision at the top of the pleural cavity. Layers of sutures were used to close the incision. The drainage tube was reserved and connected with a water‐sealed bottle (Figure 4). Bilateral lung ventilation was then re‐established, and the lungs were re‐expanded by a 20 cm H2O airway pressure. The incision was finally closed layer by layer.
FIGURE 4.
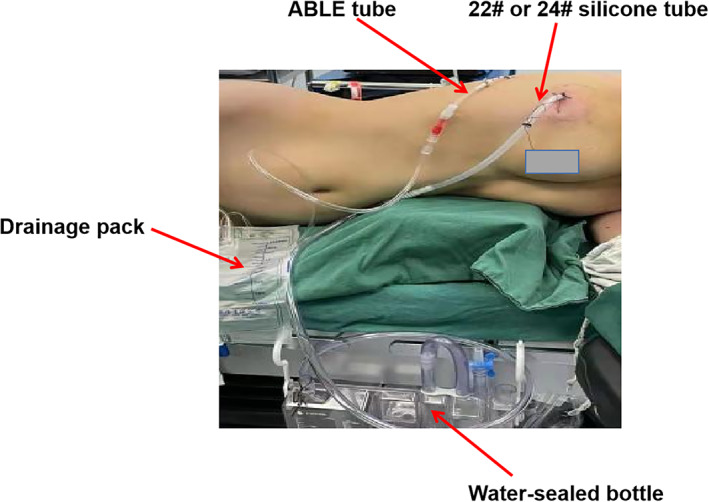
The drainage of the TDS group. A 22# or 24# silicone tube was inserted into the pleural cavity through the incision. The ABLE catheter was inserted into the posterior axillary line through the seventh or eighth intercostal space
Postoperative management
All patients' endotracheal tubes were removed in the anesthesia recovery room. Sufentanil (1 μg, qh) and flobiprofen axetil (50 mg, bid) were administered intravenously for postoperative analgesia. When patients could not tolerate the pain, bucinnazine hydrochloride injection (1 mg/kg) or morphine hydrochloride injection (10 mg) was used. A chest X‐ray was scheduled for the MDS group on the first or second postoperative day. If the postoperative day 1 X‐ray showed 30% lung compression, the X‐ray was repeated after 24 h. If the pneumothorax did not worsen, no treatment was given.
On the contrary, catheterization was performed in the second intercostal space. The standards for chest tube removal in the TDS group were no air leakage and satisfactory lung inflation. When fluid leakage was less than 100 ml per day, both groups' tubes (ABLE drainage catheter) were removed.
Statistical analysis
SPSS v22.0 software was used for statistical analysis (IBM Corp). Propensity score matching (PSM) was used to reduce the potential influence of confounding factors. Some confounding factors, such as age, gender, and smoking status, may skew results.
PSM was performed with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score and a 1:1 matching protocol without replacement (greedy‐matching algorithm). Continuous data were presented as mean ± standard deviation (SD), and categorical variables were presented as absolute frequencies and proportions (%). p < 0.05 was regarded as significant.
RESULTS
Clinicopathologic characteristics of all 405 patients prior to PSM and 240 patients following PSM
A total of 405 patients underwent uni‐portal VATs PWR in No1. Department of Thoracic Surgery in Fujian Medical University Union Hospital, including 121 in the MDS group and 284 in the TDS group. All patients' clinicopathological parameters are displayed in Table 1. Neither group showed significant differences regarding body mass index, emphysema, pulmonary function, lesion location, maximum lesion diameter, and pathological type (p > 0.05). On the contrary, for the aspect age, sex, and smoking, there was a significant difference (p < 0.05). To reduce the potential influence of confounding factors, we conducted PSM analysis, which resulted in 120 matched pairs, namely the MDS group (120/121, 99.17%) and the TDS group (120/284, 42.26%). None of the clinicopathological parameters were statistically different between the two groups following PSM (p > 0.05; Table 1).
TABLE 1.
Clinicopathological characteristics of the MDS and TDS groups (before and after propensity score matching)
| Characteristics | Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|---|
| MDS group (n = 121) | TDS group (n = 279) | T/χ 2 | p | MDS group (n = 120) | TDS group (n = 120) | T/χ 2 | p | |
| Age (years) | 50.86 ± 11.46 | 53.76 ± 10.95 | −2.400 | 0.017 | 51.15 ± 11.05 | 51.03 ± 11.60 | 0.08 | 0.936 |
| Sex (%) | 4.804 | 0.028 | 0.019 | 0.891 | ||||
| Male | 40 (33.1) | 125 (44.8) | 40 (33.3) | 41 (34.2) | ||||
| Female | 81 (66.9) | 154 (55.2) | 80 (66.7) | 79 (65.8) | ||||
| BMI a | 23.04 ± 3.16 | 23.12 ± 3.31 | −0.175 | 0.861 | 45.60 ± 247.53 | 23.02 ± 3.41 | 0.999 | 0.319 |
| Smoking (%) | 15 (12.4) | 63 (22.6) | 5.576 | 0.018 | 15 (12.5) | 16 (13.3) | 0.037 | 0.847 |
| Emphysema (%) | 1 (0.8) | 12 (4.3) | 3.241 | 0.072 | 1 (0.8) | 2 (1.7) | 0.338 | 0.561 |
| Pulmonary function | ||||||||
| FEV1 (L) | 2.65 ± 0.64 | 2.64 ± 0.67 | 0.177 | 0.859 | 2.64 ± 0.64 | 2.68 ± 0.70 | −0.453 | 0.651 |
| Actual FEV1/pre‐FEV1 (%) | 100.23 ± 16.54 | 99.33 ± 20.09 | 0.432 | 0.666 | 100.10 ± 16.55 | 101.34 ± 24.51 | −0.461 | 0.645 |
| MVV (L) | 90.83 ± 19.25 | 90.44 ± 20.32 | 0.177 | 0.859 | 90.71 ± 19.29 | 91.89 ± 20.99 | −0.453 | 0.651 |
| Actual MVV/pre‐MVV (%) | 89.17 ± 15.67 | 86.95 ± 12.30 | 1.523 | 0.128 | 88.44 ± 13.49 | 88.51 ± 11.64 | −0.04 | 0.968 |
| Lesion location (%) b | 10.028 | 0.348 | 0.338 | 0.845 | ||||
| Single lobe | 112 (92.6) | 256 (91.8) | 111 (92.5) | 110 (91.7) | ||||
| Two lobes | 1 (0.8) | 3 (1.1) | 1 (0.8) | 2 (1.7) | ||||
| Three lobes | 8 (6.6) | 20 (7.1) | 8 (6.7) | 8 (6.6) | ||||
| Maximum lesion diameter (cm) | 1.06 ± 0.63 | 1.10 ± 0.60 | −0.436 | 0.663 | 1.02 ± 0.56 | 1.02 ± 0.54 | −0.024 | 0.981 |
| Pathological types (%) | 6.8 | 0.147 | 7.012 | 0.135 | ||||
| Primary cancer | 57 (47.1) | 102 (36.6) | 55 (45.8) | 50 (41.7) | ||||
| Metastatic tumor | 3 (2.5) | 12 (4.3) | 45 (37.5) | 58 (48.3) | ||||
| Benign disease | 51 (42.2) | 138 (49.5) | 13 (10.8) | 7 (5.8) | ||||
| Mixture c | 6 (4.9) | 23 (8.2) | 2 (1.7) | 4 (3.3) | ||||
| Others | 4 (3.3) | 4 (1.4) | 5 (4.2) | 1 (0.9) | ||||
BMI refers to body mass index, which is the weight (kg) divided by height (m)^2.
Lesion location refers to a single lobe, or two or three lobes where the lesions are removed.
Mixture refers to patients who have multiple nodules at the same time. The pathological result is a mixture of lesions, for example primary lung cancer and benign lesions.
Intraoperative characteristics of 120 paired samples after PSM
Table 2 shows the intraoperative characteristics after matching, such as pleural adhesion, intraoperative bleeding volume, number of resected lesions, number of linear staples, and operation time. Except for operation time, there were no significant differences in pleural adhesion condition, intraoperative bleeding volume, the number of resected lesions, or the number of staples between the two groups (p > 0.05). The MDS group's operation time was less than that of the TDS group (p < 0.001).
TABLE 2.
Intraoperative characteristics of the MDS and TDS groups after PSM
| Characteristics | MDS group (n = 120) | TDS group (n = 120) | T/χ 2 | p |
|---|---|---|---|---|
| Pleural adhesion a (%) | 6 (5.0) | 10 (8.3) | 1.071 | 0.301 |
| Number of linear staples | 3.58 ± 1.48 | 3.85 ± 1.85 | −1.234 | 0.218 |
| Intraoperative bleeding volume (ml) | 17.52 ± 13.11 | 20.88 ± 18.08 | −1.643 | 0.102 |
| Operation time b (min) | 71.42 ± 22.98 | 86.80 ± 36.75 | −3.888 | 0.000 |
Only a few pleural adhesions were found in the included cases.
Operation time refers to the time between the incision of the skin and the end of the suture.
Postoperative rehabilitation of 240 patients after PSM
Table 3 shows the postoperative rehabilitation after PSM. Chest tube duration, total drainage volume, number of additional pain relievers, facial pain score, postoperative hospital stay, and incision adverse events were significantly lower in the MDS group than in the TDS group (p < 0.05). CTCAE grade‐1 complications (including mild subcutaneous emphysema and pneumothorax) were significantly higher in the MDS group than in the TDS group (78.3% vs. 58.3% p = 0.001). Furthermore, no statistically significant differences in CTCAE grade‐2 complications (only pulmonary infection occurred in the study) were found between the two groups (p > 0.05). Interestingly, the average hospitalization cost in the MDS group (30197.94 yuan) was lower than that in the TDS group (32619.73 yuan), but the statistics do not support the difference (p > 0.05).
TABLE 3.
Postoperative rehabilitation of the MDS and TDS groups after PSM
| Characteristics | MDS group (n = 120) | TDS group (n = 120) | T/χ 2 | p |
|---|---|---|---|---|
| Chest tube duration a (h) | 0.00 ± 0.00 | 32.68 ± 18.51 | −19.337 | 0.000 |
| Total drainage volume (ml) | 143.03 ± 118.33 | 187.73 ± 140.82 | −2.663 | 0.008 |
| Number of additional pain relief b | 0.14 ± 0.40 | 0.42 ± 0.74 | −3.591 | 0.000 |
| Facial pain score (postoperative day 1) | 2.7 ± 1.8 | 3.6 ± 2.7 | 2.829 | 0.005 |
| CTCAE 1 complications | 94 | 70 | 11.091 | 0.001 |
| Subcutaneous emphysema c | 61 (50.8) | 49 (40.8) | 2.417 | 0.120 |
| Pneumothorax | 33 (27.5) | 21 (17.5) | 3.441 | 0.064 |
| CTCAE 2 complications | ||||
| Pulmonary infection d | 3 (2.5) | 4 (3.3) | 0.000 | 1.000 |
| Postoperative hospital stay (days) | 2.61 ± 0.70 | 3.27 ± 1.88 | −3.598 | 0.000 |
| Incision adverse events e | 3 (2.5%) | 10 (8.3%) | 3.985 | 0.046 |
| Total in‐hospital costs | 30 197.94 ± 24 019.53 | 32 619.73 ± 5809.62 | −1.074 | 0.284 |
Chest tube duration refers to the duration of the thoracic closed drainage tube through the fourth intercostal incision.
The number of additional pain relief according to the times of using additional painkillers in the doctor's advice.
For the convenience of statistics, subcutaneous emphysema was confirmed by postoperative chest X‐ray.
Pulmonary infection refers to postoperative fever ≥38°C, leukocytosis, and lung inflammation revealed in chest X‐ray.
Incision adverse events refer to poor incision healing.
DISCUSSION
Adverse effects of placing a closed thoracic drainage tube
Closed thoracic drainage (CTD) is a standard procedure in traditional thoracic surgery that removes pleural effusion and air leakage after the operation. The placement of a closed chest drainage tube, on the other hand, has several adverse consequences. First, the tube's placement aggravates postoperative pain. Strong analgesic medications (such as opioid analgesics) failed to provide pain relief for some patients. Furthermore, the potential side effects of painkillers should not be overlooked. Second, CTD slowed the recovery of postoperative pulmonary function. 15 , 16 , 17 Third, previous studies reported that the tube was the primary cause of intercostal nerve injury, which was thought to resolve shortly after the chest tube was removed. 18 , 19 Fourth, stimulating the pleural causes an increase in the volume of pleural fluid. Fifth, the incidence of secondary pain increased significantly during extubation. Extubation also increased the risk of pneumothorax. Sixth, CTD may reduce patient compliance, resulting in delayed recovery and a longer postoperative hospital stay. Finally, CTD increases the workload of healthcare providers.
Clinicians have devised strategies to deal with the negative effects of thoracic tube placement, such as removing the chest tube as soon as possible 19 and improving pain‐relief regimens. The most effective of these is to avoid using closed thoracic drainage tubes. Nonetheless, a completely tubeless procedure is still not widely used due to safety concerns in clinical practice.
The feasibility and safety of a modified chest drainage strategy
Recently, some studies have reported the feasibility of completely tubeless surgery in a small number of cases. 1 , 20 , 21 , 22 However, due to different experiences in different medical centers, omitting all drainage tubes was limited by the surgeons' technical skills and management concepts. Furthermore, the strategy does not allow for the detection of air leakage, pleural effusion, or bleeding, making it unsuitable for widespread use. 2
Air leakage, as we know, can be effectively controlled by performing an air leakage test and repairing leaks during surgery; however, pleural effusion is difficult to control. As a result, the modified strategy for chest tube drainage has been summarized. Unlike the TDS group, the MDS group only received a small ABLE catheter. We believe that MDS could be used to reduce postoperative discomfort and speed up recovery in most patients following wedge resection.
This study investigated how MDS affected periprocedural management in patients undergoing wedge resection via PSM. The results showed that the MDS group had a significantly shorter operation time than the TDS group (p < 0.001), indicating that MDS simplifies the procedure and reduces operation time. In terms of postoperative pain, the number of additional pain relief in the MDS group was significantly lower than in the TDS group (p < 0.001), indicating that MDS aids in reducing postoperative pain, which is consistent with other studies. 8 , 23 Furthermore, we discovered that the total drainage volume (p = 0.008) and postoperative hospital stay (p = 0.001) in the MDS group were significantly lower than in the TDS group. The reasons could be that MDS reduces pleural stimulus and pleural exudate. 24 In terms of safety, while CTCAE grade‐1 complications (such as small pneumothorax and subcutaneous emphysema) were higher in the MDS group than in the TDS group, the complications could resolve independently. Furthermore, no differences in the occurrence of CTCAE grade‐2 complications were found between the two groups. These results demonstrate that MDS is as feasible and safe as TDS.
The advantages of the modified chest drainage strategy
First, it was only necessary to perform an air leak test once before closing the incision. The experimental results are accurate and reliable, and the operation procedure is straightforward, which simplifies drainage tube management and reduces postoperative nursing workload.
Second, the ABLE catheter could drain pleural effusion smoothly, allowing for real‐time evaluation of common complications without frequent X‐rays.
Third, patients in the MDS group reported a significant reduction in pain caused by a thoracic closed drainage tube and improved satisfaction with wound healing following surgery.
Fourth, patients in the MDS group avoided the negative effects of chest tube removal, such as fear psychology and postextubation complications.
Fifth, MDS streamlined the postoperative management workflow. Meanwhile, the postoperative complications were not significantly different between the two groups, implying that MDS is safe and feasible.
Sixth, MDS shortened the postoperative hospital stay and accelerated postoperative recovery, allowing the rapid rehabilitation concept to be implemented.
MDS complications and the corresponding measures
In this study, all included cases did not reveal any serious complications. Following the implementation of MDS, the following precautions are advised in the event of a CTCAE grade‐2 or more serious complication. First, for patients who are actively bleeding or have a chylothorax, an ABLE catheter can drain pleural effusion or hemothorax smoothly, which can help to evaluate the complication without taking X‐rays frequently, allowing treatment to be administered on time. Second, the ABLE catheter can be used to treat a severe pneumothorax (volume of compressed lung >30%) directly by drawing out air leakage through the ABLE catheter. Third, if a mild pneumothorax (volume of compressed lung 30%) occurs, X‐rays will be taken again in 24 h. If the pneumothorax is not aggravating, no further treatment is required.
MDS is safe and acceptable for patients with single‐port thoracoscopic pulmonary wedge resection. MDS is recommended for patients who meet the following conditions: (1) no emphysema; (2) no or mild pleural adhesion; (3) number of linear staplers ≤5; (4) the cutting lines form well; and (5) no air leakage.
Limitations in the present study
The research is single‐center, nonrandomized, and retrospective in nature. Although we used PSM to reduce differences in clinical baseline characteristics, this study is still susceptible to case selection bias. For example, there were lower in‐hospital costs in the MDS group than in the TDS group. However, there was no significant difference between the groups, which may be due to case selection bias. As a result, we need to conduct another prospective, randomized controlled study to confirm our findings.
CONCLUSION
Applying a modified drainage strategy in single‐port thoracoscopic pulmonary wedge resection is safe and feasible, allowing for less postoperative rehabilitation time and pain relief, reduced postoperative pleural effusion and clinical workload. In addition, MDS has the potential to reduce patients' medical burden.
DECLARATION OF FINANCIAL/OTHER RELATIONSHIPS
All authors have no relevant financial or other relationships to disclose.
ACKNOWLEDGMENTS
We thank Prof. Chunfu‐Zheng (https://cumming.ucalgary.ca/departments/microinfect/chunfu-zheng) for language and grammar editing.
Xu G, Du J, Zhang J, Chen H, Zheng B, Yang Z, et al. A propensity sore‐matched study: Applying a modified chest tube drainage strategy in rapid rehabilitation following uni‐portal thoracoscopic pulmonary wedge resection. Thorac Cancer. 2022;13(11):1657–1663. 10.1111/1759-7714.14438
Guobing Xu and Jianting Du contributed equally to the article and should be considered as co‐first authors.
Funding informationOur study was supported by Startup Fund for scientific research, Fujian Medical University (Grant number: 2020QH1072).
Contributor Information
Zhang Yang, Email: fjxh_zhang2021@163.com.
Chun Chen, Email: chenchun0209@fjmu.edu.cn.
REFERENCES
- 1. Deng B, Qian K, Zhou JH, Tan QY, Wang RW. Optimization of chest tube management to expedite rehabilitation of lung cancer patients after video‐assisted thoracic surgery: a meta‐analysis and systematic review. World J Surg. 2017;41:2039–45. [DOI] [PubMed] [Google Scholar]
- 2. Cui F, Liu J, Li S, Yin W, Xin X, Shao W, et al. Tubeless video‐assisted thoracoscopic surgery (VATS) under non‐intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis. 2016;8:2226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yao F, Wang J, Yao J, Hang F, Cao S, Qian J, et al. Early chest tube removal after thoracoscopic esophagectomy with high output. J Laparoendosc Adv Surg Tech A. 2016;26:17–22. [DOI] [PubMed] [Google Scholar]
- 4. Nakanishi R, Fujino Y, Kato M, Miura T, Yasuda M, Oda R, et al. Early chest tube removal after thoracoscopic lobectomy with the aid of an additional thin tube: a prospective multi‐institutional study. Gen Thorac Cardiovasc Surg. 2018;66:723–30. [DOI] [PubMed] [Google Scholar]
- 5. Nakashima S, Watanabe A, Mishina T, Obama T, Mawatari T, Higami T. Feasibility and safety of postoperative management without chest tube placement after thoracoscopic wedge resection of the lung. Surg Today. 2011;41:774–9. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez‐Rivas D, Yang Y, Guido W, Jiang G. Nonintubated (tubeless) uniportal video‐assisted thoracoscopic lobectomy. Ann Cardiothorac Surg. 2016;5:151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mineo TC, Tamburrini A, Perroni G, Ambrogi V. 1000 cases of tubeless video‐assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol. 2016;12:13–8. [DOI] [PubMed] [Google Scholar]
- 8. Yang SM, Wang ML, Hung MH, Hsu HH, Cheng YJ, Chen JS. Tubeless uniportal thoracoscopic wedge resection for peripheral lung nodules. Ann Thorac Surg. 2017;103:462–8. [DOI] [PubMed] [Google Scholar]
- 9. Li S, Jiang L, Ang KL, Chen H, Dong Q, Yang H, et al. New tubeless video‐assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg. 2017;51:689–93. [DOI] [PubMed] [Google Scholar]
- 10. Petersen RH, Holbek BL, Hansen HJ, Kehlet H. Video‐assisted thoracoscopic surgery‐taking a step into the future. Eur J Cardiothorac Surg. 2017;51:694–5. [DOI] [PubMed] [Google Scholar]
- 11. Zhao ZR, Lau RWH, Ng CSH. Anaesthesiology for uniportal VATS: double lumen, single lumen and tubeless. J Visc Surg. 2017;3:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia Z, Qiao K, He J. Recent advances in the management of pulmonary tuberculoma with focus on the use of tubeless video‐assisted thoracoscopic surgery. J Thorac Dis. 2017;9:3307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng G, Liu M, Luo Q, Chen H, Yin W, Wang W, et al. Spontaneous ventilation anesthesia combined with uniportal and tubeless thoracoscopic lung biopsy in selected patients with interstitial lung diseases. J Thorac Dis. 2017;9:4494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu CY, Hsu PK, Chien HC, Hsieh CC, Ting CK, Tsou MY. Tubeless single‐port thoracoscopic sublobar resection: indication and safety. J Thorac Dis. 2018;10:3729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mueller XM, Tinguely F, Tevaearai HT, Ravussin P, Stumpe F, von Segesser LK. Impact of duration of chest tube drainage on pain after cardiac surgery. Eur J Cardiothorac Surg. 2000;18:570–4. [DOI] [PubMed] [Google Scholar]
- 16. Refai M, Brunelli A, Salati M, Xiume F, Pompili C, Sabbatini A. The impact of chest tube removal on pain and pulmonary function after pulmonary resection. Eur J Cardiothorac Surg. 2012;41:820–2. [DOI] [PubMed] [Google Scholar]
- 17. Miyazaki T, Sakai T, Yamasaki N, Tsuchiya T, Matsumoto K, Tagawa T, et al. Chest tube insertion is one important factor leading to intercostal nerve impairment in thoracic surgery. Gen Thorac Cardiovasc Surg. 2014;62:58–63. [DOI] [PubMed] [Google Scholar]
- 18. Miyazaki T, Sakai T, Tsuchiya T, Yamasaki N, Tagawa T, Mine M, et al. Assessment and follow‐up of intercostal nerve damage after video‐assisted thoracic surgery. Eur J Cardiothorac Surg. 2011;39:1033–9. [DOI] [PubMed] [Google Scholar]
- 19. Xing T, Li X, Liu J, et al. Early removal of chest tubes leads to better short‐term outcome after video‐assisted thoracoscopic surgery lung resection.[J]. Ann Transl Med. 2020;4:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueda K, Haruki T, Murakami J, Tanaka T, Hayashi M, Hamano K. No drain after thoracoscopic major lung resection for cancer helps preserve the physical function. Ann Thorac Surg. 2019;108:399–404. [DOI] [PubMed] [Google Scholar]
- 21. Murakami J, Ueda K, Tanaka T, Kobayashi T, Kunihiro Y, Hamano K. The validation of a no‐drain policy after thoracoscopic major lung resection. Ann Thorac Surg. 2017;104:1005–11. [DOI] [PubMed] [Google Scholar]
- 22. Ueda K, Hayashi M, Tanaka T, Hamano K. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardiothorac Surg. 2013;44:225–9. [DOI] [PubMed] [Google Scholar]
- 23. Liao HC, Yang SM, Hung MH, Cheng YJ, Hsu HH, Chen JS. Thoracoscopic surgery without drainage tube placement for peripheral lung nodules. Ann Thorac Surg. 2020;109(3):887–93. [DOI] [PubMed] [Google Scholar]
- 24. Yu P, Wang G, Zhang C, Liu H, Wang Y, Yu Z, et al. Clinical application of enhanced recovery after surgery (ERAS) in pectus excavatum patients following Nuss procedure. J Thorac Dis. 2020;12(6):3035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


