Abstract
Background
Sleeve lobectomy is recognized as an alternative surgical operation to pneumonectomy because it preserves the most pulmonary function and has a considerable prognosis. In this study, we aimed to investigate the implications of residual status for patients after sleeve lobectomy.
Methods
In this retrospective cohort study, we summarized 58 242 patients who underwent surgeries from 2015 to 2018 in Shanghai Chest Hospital and found 456 eligible patients meeting the criteria. The status of R2 was excluded. The outcomes were overall survival (OS) and recurrence‐free survival (RFS). We performed a subgroup analysis to further our investigation.
Results
After the propensity score match, the baseline characteristic was balanced between two groups. The survival analysis showed no significant difference of overall survival and recurrence‐free survival between R0 and R1 groups (OS: p = 0.053; RFS: p = 0.14). In the multivariate Cox analysis, we found that the margin status was not a dependent risk factor to RFS (p = 0.119) and OS (p = 0.093). In the patients of R1, N stage and age were closely related to OS, but we did not find any significant risk variable in RFS for R1 status. In the subgroup analysis, R1 status may have a worse prognosis on patients with more lymph nodes examination. On further investigation, we demonstrated no differences among the four histological types of margin status.
Conclusion
In our study, we confirmed that the margin status after sleeve lobectomies was not the risk factor to prognosis. However, patients with more lymph nodes resection should pay attention to the margin status.
Keywords: margin, sleeve lobectomy, surgery
Sleeve lobectomy is recognized as an alternative surgical operation to pneumonectomy because it preserves the most pulmonary function and has a considerable prognosis. A positive margin is inevitable during the surgery sometimes. In our study, we confirmed that the margin status after sleeve lobectomies was not the risk factor to prognosis.
INTRODUCTION
According to Global Cancer Statistics in 2020, there were nearly 19.3 million new cases and 10 million cancer deaths in 2020. Lung cancer is still at the top of lethal cancers. 1 During the past few decades, pneumonectomy has remained an indispensable surgical operation for central localized non–small cell lung cancer (NSCLC) until the sleeve techniques were demonstrated to be superior. The sleeve lobectomy preserves the most pulmonary function and has a considerable prognosis. However, the decision to have a sleeve operation or the pneumonectomy depended on the surgeons individually under most circumstances and the relevant guidelines are not quite clear. A positive margin is inevitable during the surgery because of its central location. The International Association for the Study of Lung Cancer (IASLC) lung cancer staging project has extended the understanding of proposals for residual tumors for NSCLC in 2019. 2 Although the adjuvant chemoradiotherapy will be taken to minimize the hazard of the positive margin, the influence of a positive margin on prognosis after the sleeve operation is still unclear. Should an extensive sleeve lobectomy, pneumonectomy, or no‐operation be performed after the frozen section report of a positive margin? It remains mysterious and needs further investigation. In our study, we compared the overall survival (OS) and recurrence‐free survival (RFS) between two margin statuses: R0 (negative) and R1 (positive residual under a microscope), which may contribute to the decision during the sleeve lobectomies.
METHODS
Patients
In this study, we retrospectively collected 58 242 patients who underwent surgeries from 2015 to 2018 in Shanghai Chest Hospital. A total of 575 patients accepted sleeve operation. Only 456 cases met the criteria according to the inclusion principles. Among those people, 71 patients were lost to follow‐up, 20 were benign tumors, and 21 were metastatic lung tumors or small cell lung cancer. In addition, one case was excluded for postoperative cheek sarcoma, one for granulosa cell carcinoma of the trachea, one for malignant bronchial melanoma, one for squamous cell carcinoma with isolated small cell carcinoma, and two patients without pathology were also excluded (Figure 1).
FIGURE 1.
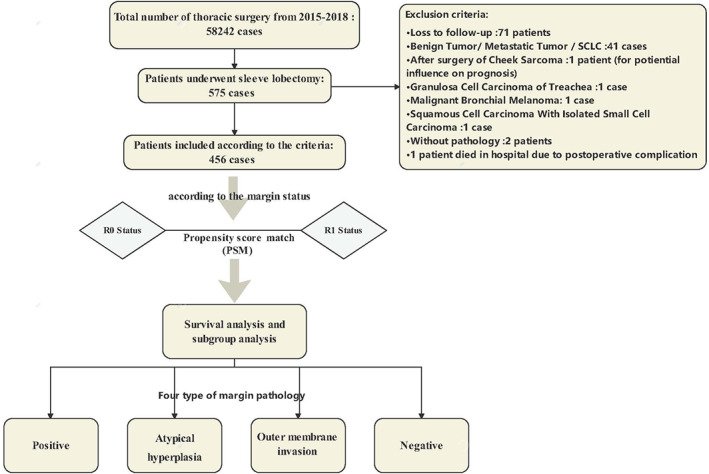
The design and participants of this study
Study design
The clinical information of patients was retrieved from the clinical medical system in the Shanghai Chest Hospital. This study was a retrospective cohort study in a single center. We aimed to investigate the implication of margin status on the prognosis after sleeve lobectomy. According to residual status, we compared groups of two margin statuses: R0 (negative) and R1 (positive residual under the microscope). The status of R2 (macroscopic residual tumor) was not taken into consideration. We adopted the American Joint Committee on Cancer (AJCC) eighth edition to classify the TMN stage. The outcomes were OS and RFS. A subgroup for OS was performed to identify the patients at risk. Considering the pathology results of margin, we also compared the patients according to pathological type: positive, atypical hyperplasia/tumor in situ, outer membrane invasion, and negative.
Statistical analysis
Continuous variables were expressed in the format of mean ± standard deviation (SD). Two independent sample t‐tests were applied to calculate the difference of the continuous variables and the categorical variables were analyzed by Fisher exact test or χ2 test. The baseline characteristic of the two groups was balanced by propensity scores match. The product‐limit method (Kaplan–Meier method) and the log‐rank test were used to evaluate and compare the OS and RFS. The univariate Cox regression analysis and multivariate Cox regression analysis where we used the method of “enter” were adopted to select the risk factors correlated to RFS/OS after sleeve lobectomies. A subgroup analysis of OS between R0 and R1 was carried out based on Cox analysis. We executed the pairwise comparisons using the log‐rank test for four types of histology and used the method of Benjamini‐Hochberg (BH) to adjust the p‐value. The p‐value of 0.05 in the study was deemed as a borderline of significant difference. The statistic procedure was assisted by software of SAS version 9.4 and R software version 4.0.3.
Preoperative preparation and surgical techniques
All the patients before surgery had multidisciplinary consultation and comprehensive examinations. The enhanced thorax computed tomography (CT) scan was a conventional examination to have the preliminary recognition of the lesion. An enhanced CT or magnetic resonance imaging (MRI) of the head plus an optional positron emission tomography (PET)‐CT was used to evaluate the distant progress and mediastinal lymph nodes metastatic. A biopsy through the bronchoscope was necessary to specify the extent of the tumor and the histology whether it was small cell lung cancer. The surgical tolerability was assessed by pulmonary function, echocardiography, electrocardiograph, plate movement, and arterial blood gas analysis.
In our hospital, we mainly have three surgical techniques: open, video‐assisted thoracic surgery (VATS), and robot‐assisted thoracic surgery (RATS) from 2015 to 2018. The thoracoscope operation was mainly manipulated by a single aperture or three apertures. The patients with great vessels invasion, such as superior vena cava invasion and pulmonary artery, would accept angioplasty after thorough evaluation. The frozen section pathology was routinely performed to confirm the status of the margin. When it was R1 (positive under the microscope), the corresponding surgical decision depended on surgeons individually whether to extend resection of sleeve operation, pneumonectomy, or if no extra operation should be taken. As for lymph nodes examination, we routinely resected groups 2R, 4R, 7, 8, 9, 10R, and 11R for right‐side surgery and 4L, 5, 6, 7, 8, 9, 10L, and 11L for left‐sided surgery.
RESULTS
Baseline characteristics of R0 and R1 groups
The baseline characteristics of the two groups were listed in Table 1. The major histology in the sleeve operation was squamous cell carcinoma that tended to be located in the center. There was only one patient who died in hospital because of postoperative complications, and we excluded this case from the list. In our study, 10 patients underwent superior vena cava replacement and 56 patients suffered pulmonary artery angioplasty. A majority of patients had the T1–T2 stage, and it seemed that the N stage varied averagely from N0 to N2. In addition, approximately 13.60% patients accepted neoadjuvant therapy and 71.18% patients accepted adjuvant therapy.
TABLE 1.
Baseline characteristics of patients suffering sleeve lobectomy before PSM
| Variable | Summarize | R0 group | R1 group | p value |
|---|---|---|---|---|
| Sex (n) | ||||
| Male | 416 | 333 (91.74%) | 83 (89.25%) | 0.4183 |
| Female | 40 | 30 (8.26%) | 10 (10.75%) | |
| Age (y) | 60.46 ± 8.93 | 60.99 ± 8.88 | 58.40 ± 8.88 | 0.0123 < 0.05 |
| BMI (kg/m2) | 23.23 ± 2.97 | 23.28 ± 2.91 | 23.06 ± 3.22 | 0.5378 |
| Hospital days (d) | 16.15 ± 8.13 | 17.77 ± 7.41 | 17.63 ± 10.36 | 0.1063 |
| Death in hospital (n) | 1 | 1 | 0 | |
| Laterality (n) | 0.0040 < 0.05 | |||
| Left | 285 | 215 (59.23%) | 70 (70.27%) | |
| Right | 171 | 148 (40.77%) | 23 (24.73%) | |
| Tumor size (cm) | 3.77 ± 1.53 | 3.78 ± 1.54 | 3.72 ± 1.49 | 0.7414 |
| Surgical technology (n) | 0.5196 | |||
| Open | 387 | 310 (85.40%) | 77 (82.80%) | |
| VATS + RATS | 69 | 53 (14.60%) | 16 (17.20%) | |
| T stage (n) | 0.1367 | |||
| T1 + T2 | 332 | 270 (74.38%) | 62 (66.67%) | |
| T3 | 56 | 45 (12.40%) | 11 (11.83%) | |
| T4 | 68 | 48 (13.22%) | 20 (21.51%) | |
| N stage (n) | 0.0037 < 0.05 | |||
| N0 | 167 | 146 (40.22%) | 21 (22.58%) | |
| N1 | 159 | 116 (31.96%) | 43 (46.24%) | |
| N2 | 130 | 101 (27.82%) | 29 (31.18%) | |
| Pathological stage (n) | 0.0143 < 0.05 | |||
| I | 107 | 94 (25.10%) | 13 (13.98%) | |
| II | 157 | 127 (34.99%) | 30 (32.26%) | |
| III | 192 | 142 (39.13%) | 50 (53.76%) | |
| Lymph nodes resection (n) | ||||
| Total | 16.29 ± 6.58 | 16.31 ± 6.80 | 16.20 ± 5.70 | 0.8771 |
| N1 | 6.67 ± 3.66 | 6.62 ± 3.66 | 6.85 ± 3.68 | 0.5943 |
| N2 | 9.60 ± 4.94 | 9.66 ± 5.11 | 9.37 ± 4.27 | 0.5648 |
| Histology (n) | 0.1671 | |||
| SCC | 340 | 274 (75.48%) | 66 (70.97%) | |
| Adenocarcinoma | 63 | 52 (14.33%) | 11 (11.83%) | |
| ACC and others | 53 | 37 (10.19%) | 16 (17.20%) | |
| Superior vena cava invasion | 0.1256 | |||
| No | 446 | 357 (98.35%) | 89 (95.70%) | |
| Yes | 10 | 6 (1.65%) | 4 (4.30%) | |
| Pulmonary artery angioplasty | 0.1555 | |||
| No | 400 | 314 (86.50%) | 86 (92.47%) | |
| Yes | 56 | 49 (13.50%) | 7 (7.53%) | |
| Neoadjuvant therapy (n) | 0.0621 | |||
| No | 394 | 308 (84.85%) | 86 (92.47%) | |
| Yes | 62 | 55 (15.15%) | 7 (7.53%) | |
| Adjuvant therapy (n) | 0.6026 | |||
| No | 125 | 102 (28.10%) | 23 (24.73%) | |
| Yes | 331 | 261 (71.90%) | 70 (75.27%) | |
| Comorbidity (n) | ||||
| Cardiovascular system | 181 | 141 | 40 | |
| Nervous system | 240 | 192 | 48 | |
| Hypertension | 87 | 69 | 18 | |
| Diabetes | 42 | 35 | 7 | |
| FEV1 | 2.88 ± 0.38 | 2.89 ± 0.39 | 2.84 ± 0.37 | 0.3183 |
| FEV1% | 79.63 ± 15.76 | 79.97 ± 15.90 | 78.04 ± 15.03 | 0.32 |
| DLCO% | 86.90 ± 19.59 | 87.37 ± 19.81 | 84.70 ± 18.46 | 0.2692 |
Propensity scores match to balance the baseline
The significant differences between two groups were observed in age, laterality, N stage and clinical stage before PSM. The ratio of PSM was 1:2 and the caliper was set at 0.02. The results of PSM were recorded in Table 3. Before the balance, the comparison of OS between two groups through log‐rank test showed that the p value was 0.091. After PSM, a difference that nearly reached statistical significance (p = 0.053) between R0 and R1 groups was observed. As for RFS, the p values were 0.043 (before PSM) and 0.14 (after PSM), which showed no obvious statistical differences (Figure 2).
TABLE 3.
Baseline characteristics of patients suffering sleeve lobectomy after PSM
| Variable | Summarize | R0 group | R1 group | p value |
|---|---|---|---|---|
| Sex (n) | 0.617 | |||
| Male | 164 | 110 ( 90.2%) | 54 (87.1%) | |
| Female | 20 | 12 (9.8%) | 8 (12.9%) | |
| Age (y) | 60.41 ± 8.254 | 60.93 ± 8.11 | 59.37 ± 8.51 | 0.226 |
| BMI (kg/m2) | 23.40 ± 3.02 | 23.60 ± 2.98 | 23.02 ± 3.08 | 0.219 |
| Hospital days (d) | 15.89 ± 5.86 | 15.66 ± 6.36 | 16.35 ± 4.729 | 0.446 |
| Laterality (n) | 0.855 | |||
| Left | 126 | 83 (68.0%) | 43 (69.4%) | |
| Right | 58 | 39 (320%) | 19 (30.6%) | |
| Tumor size (cm) | 3.75 ± 1.56 | 3.73 ± 1.56 | 3.80 ± 1.57 | 0.763 |
| Surgical technology (n) | 0.527 | |||
| Open | 154 | 104 (85.2%) | 50 (80.6%) | |
| VATS + RATS | 30 | 18 (14.8%) | 12 (19.4%) | |
| T stage (n) | 0.155 | |||
| T1 + T2 | 146 | 100 (82/0%) | 46 (74.2%) | |
| T3 | 17 | 12 (9.8%) | 5 (8.1%) | |
| T4 | 21 | 10 (8.2%) | 11 (17.7%) | |
| N stage (n) | 0.641 | |||
| N0 | 51 | 35 (28.7%) | 16 (25.8%) | |
| N1 | 76 | 52 (42.6%) | 24 (38.7%) | |
| N2 | 57 | 35 (28.7%) | 22 (35.5%) | |
| Pathological stage (n) | 0.468 | |||
| I | 39 | 28 (23.0%) | 11 (17.7%) | |
| II | 70 | 48 (39.3%) | 22 (35.5%) | |
| III | 75 | 46 (37.7%) | 29 (46.8%) | |
| Lymph nodes resection (n) | ||||
| Total | 16.20 ± 6.11 | 16.48 ± 6.28 | 15.65 ± 5.763 | 0.38 |
| N1 | 6.96 ± 3.51 | 7.27 ± 3.52 | 6.34 ± 3.45 | 0.089 |
| N2 | 9.24 ± 4.47 | 9.20 ± 4.48 | 9.31 ± 4.48 | 0.885 |
| Histology (n) | 0.954 | |||
| SCC | 137 | 91 (74.6%) | 46 (74.2%) | |
| Non‐SCC | 47 | 31 (25.4%) | 16 (25.8%) | |
| Superior vena cava invasion | 0.112 | |||
| No | 180 | 121 (99.2%) | 59 (95.2%) | |
| Yes | 4 | 1 (0.8%) | 3 (4.8%) | |
| Pulmonary artery angioplasty | 0.793 | |||
| No | 167 | 110 (90.2%) | 57 (91.9%) | |
| Yes | 17 | 12 (9.8%) | 5 (8.1%) | |
| Neoadjuvant therapy (n) | ||||
| No | 167 | 111 (91.0%) | 56 (90.3%) | |
| Yes | 17 | 11 (9.0%) | 6 (9.7%) | |
| Adjuvant therapy (n) | 0.725 | |||
| No | 48 | 33 (27.0%) | 15 (24.2%) | |
| Yes | 136 | 89 (73.0%) | 47 (75.8%) | |
FIGURE 2.
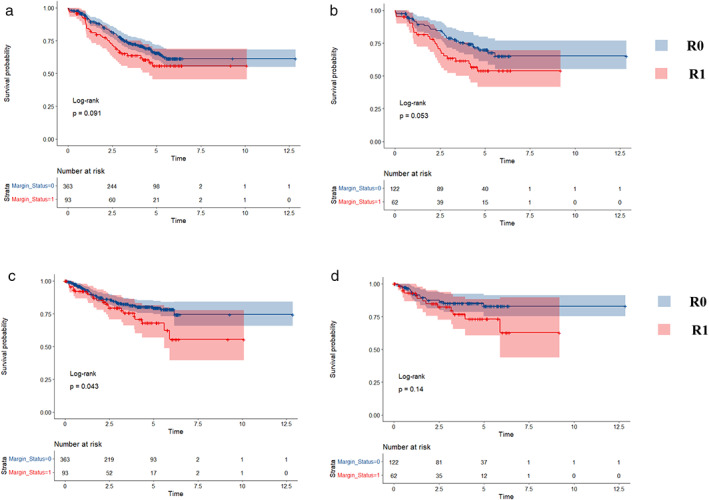
Survival analyses between R0 and R1 before PSM and after PSM. (a) Overall survival before PSM. (b) Overall survival after PSM. (c) Recurrence‐free survival before PSM. (d) Recurrence‐free survival after PSM
Postoperative complications of patients during hospitalization
The most frequent complications in the R0 group were hypokalemia (n = 54), atelectasis (n = 63), and hypoxemia (n = 45, three patients had ARDS). The second was bacterial infection or pneumonia, and one patient was infected by MASA. In the R1 group, hypoxemia (n = 21) and hypokalemia (n = 23) were also the common postoperative complications. In this group, one patient with empyema was infected by MASA and another one suffered acute syndrome derived from anastomotic stenosis. Seven patients had anastomotic fistula in the R0 group and three in the R1 group, respectively. Cases of BPF were found in four patients and one patient in two groups. Only one patient had a re‐operation because of an anastomotic fistula (Table 2).
TABLE 2.
Postoperative complications during hospitalization of patients after sleeve pneumonectomy
| Diseases | Total cases | R0 group (n = 363) | R1 group (n = 93) |
|---|---|---|---|
| Hypercapnia | 19 | 6 | 13 |
| Hypoxemia | 66 | 45 (3 for ARDS) | 21 |
| Acid‐base disturbance | 153 | 110 | 40 |
| Hypokalemia | 77 | 54 | 23 |
| Bacterial infection or pneumonia | 39 | 38 (1 for MASA) | 11 |
| Atelectasis or pneumothorax | 77 | 63 | 14 |
| Anastomotic fistula | 10 | 7 (1 for re‐operation) | 3 |
| Anastomotic‐stenosis | 4 | 2 | 2 (1 for acute syndrome) |
| Empyema | 10 | 7 | 3 (1 for MASA) |
| BPF | 5 | 4 | 1 |
| Respiratory failure | 3 | 2 | 1 |
| Pulmonary edema | 1 | 1 | 0 |
| Hemoptysis | 2 | 1 | 1 |
| Chylothorax | 2 | 0 | 2 |
| Embolization | 9 | 7 | 4 |
| ACS | 3 | 2 | 1 |
| Heart hernia | 2 | 1 | 1 |
| Arrhythmia | 6 | 4 | 2 |
Risk factors correlated to prognosis after sleeve lobectomy
The variables with p‐value <0.05 were selected into the multivariable Cox model and the method was “enter.” In Table 4, we found that age at diagnosis (95% CI, 1.008–1.067, p = 0.011) and N stage especially N2 stage (95% CI, 1.169–7.784, p = 0.022) would influence the post‐operative recurrence, but there was a borderline effect in which survival analysis demonstrated a somewhat higher hazard rate for tumor size (95% CI, 0.999–1.415, p = 0.051). In the analysis of overall survival, age at diagnosis (95% CI, 1.011–1.054, p = 0.003), neoadjuvant therapy (95% CI, 1.106–2.614, p = 0.016), superior vena cava invasion (95% CI, 1.144–6.215, p = 0.023), tumor size (95% CI, 1.077–1.401, p = 0.002), and N stage (95% CI, 5.265–12.05, p = 0.000) had significant differences. In addition, we also performed a survival analysis based on the Cox regression for the R1 population. Age at diagnosis (95% CI, 1.014–1.117, p = 0.012) and N stage (95% CI, 1.205–35.52, p = 0.030) was still confirmed to be significant to OS, but to our surprise, we did not find any risk factors to RFS. In addition, the margin status was not a relevant factor to RFS or OS.
TABLE 4.
Univariate and multivariate analyses of prognostic factors of OS and RFS
| Variable | RFS | OS | ||
|---|---|---|---|---|
| Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | |
| HR (95% CI) p value | HR (95% CI) p value | HR (95% CI) p value | HR (95% CI) p value | |
| Age at diagnosis | 1.032 (1.003–1.060) 0.027 | 1.037 (1.008–1.067) 0.011 | 1.032 (1.011–1.054) 0.003 | 1.033 (1.011–1.054) 0.003 |
| Sex | 1.143 (0.550–2.375) 0.720 | 0.700 (0.356–1.376) 0.301 | ||
| BMI | 0.976 (0.906–1.052) 0.523 | 0.970 (0.916–1.028) 0.304 | ||
| In‐hospital days | 1.021 (1.001–1.040) 0.034 | 1.013 (0.991–1.036) 0.260 | 1.022 (1.008–1.037) 0.002 | 1.013 (0.999–1.028) 0.075 |
| Laterality (left/right) | 0.748 (0.465–1.202) 0.230 | 0.758 (0.530–1.086) 0.131 | ||
| Neoadjuvant therapy | 1.366 (0.753–2.476) 0.304 | 1.780 (1.168–2.713) 0.007 | 1.704 (1.106–2.624) 0.016 | |
| Adjuvant therapy | 0.936 (0.568–1.542) 0.795 | 0.901 (0.620–1.310) 0.584 | ||
| Carina reconstruction | 2.109 (1.014–4.385) 0.046 | 1.844 (0.772–4.401) 0.168 | 1.266 (0.644–2.491) 0.494 | |
| Superior vena cava invasion | 1.021 (0.142–7.359) 0.984 | 4.440 (1.947–10.13) 0.000 | 2.667 (1.144–6.215) 0.023 | |
| Surgical technique | ||||
| Open | Control in dummy variable | |||
| VATS + RATS | 0.899 (0.508–1.592) 0.716 |
Reference 0.951 (0.579–1.561) 0.842 |
||
| Pulmonary artery angioplasty | 1.072 (0.515–2.229) 0.853 | 1.398 (0.841–2.325) 0.196 | ||
| Margin status (positive/negative) | 0.598 (0.368–0.972) 0.038 | 1.506 (0.900–2.519) 0.119 | 1.390 (0.947–2.041) 0.093 | |
| Tumor size | 1.186 (1.022–1.375) 0.024 | 1.189 (0.999–1.415) 0.051 | 1.267 (1.136–1.413) 0.000 | 1.228 (1.077–1.401) 0.002 |
| Histology | 0.668 | 0.132 | ||
| SCC | Control in dummy variable | Reference | ||
| Adenocarcinoma | 0.810 (0.402–1.629) 0.554 | 1.474 (0.950–2.288) 0.083 | ||
| ACC and others | 0.745 (0.341–1.629) 0.745 | 0.792 (0.435–1.441) 0.445 | ||
| T stage | 0.314 | 0.173 | ||
| T1 + T2 | Control in dummy variable | Reference | ||
| T3 | 1.091 (0.539–2.208) 0.808 | 1.558 (0.971–2.499) 0.066 | ||
| T4 | 1.577 (0.877–2.838) 0.128 | 1.180 (0.713–1.952) 0.519 | ||
| N stage | 0.015 | 0.072 | 0.000 | 0.000 |
| N0 | Control in dummy variable | Reference | Reference | Reference |
| N1 | 1.309 (0.750–2.284) 0.343 | 1.574 (0.759–3.264) 0.223 | 1.747 (1.069–2.856) 1.747 | 1.787 (0.946–3.376) 0.073 |
| N2 | 2.198 (1.270–3.805) 0.005 | 3.016 (1.169–7.784) 0.022 | 4.705 (2.996–7.389) 0.000 | 5.226 (2.265–12.05) 0.000 |
| No. of N1 resection | 0.952 (0.892–1.016) 0.141 | 1.020 (0.976–1.066) 0.382 | ||
| No. of N2 resection | 0.950 (0.904–0.999) 0.045 | 0.999 (0.913–1.093) 0.982 | 0.987 (0.953–1.022) 0.458 | |
| No. of total nodes | 0.956 (0.921–0.993) 0.020 | 0.953 (0.891–1.019) 0.158 | 1.001 (0.975–1.026) 0.969 | |
| Pathological stages | 0.028 | 0.659 | 0.000 | 0.815 |
| Stage I | Control in dummy variable | Reference | Reference | Reference |
| Stage II | 2.113 (0.881–5.068) 0.094 | 0.738 (0.317–1.718) 0.481 | 1.753 (0.977–3.145) 0.060 | 0.933 (0.438–1.986) 0.857 |
| Stage III | 3.412 (1.494–7.792) 0.003 | 0.603 (0.202–1.799) 0.365 | 4.176 (2.442–7.142) 0.000 | 0.758 (0.284–2.023) 0.581 |
Subgroup analysis and pairwise comparisons between histology types
Because the survival analysis using the log‐rank test for OS showed a borderline statistical significance (p = 0.053), we conducted a subgroup analysis for OS for further study (Figure 3). In the subgroup analysis for OS, we discovered that patients with open techniques, N1 stage, clinical stage II may harm prognosis. Besides, it seemed that more lymph nodes examination would cause poorer survival when the margin was positive.
FIGURE 3.
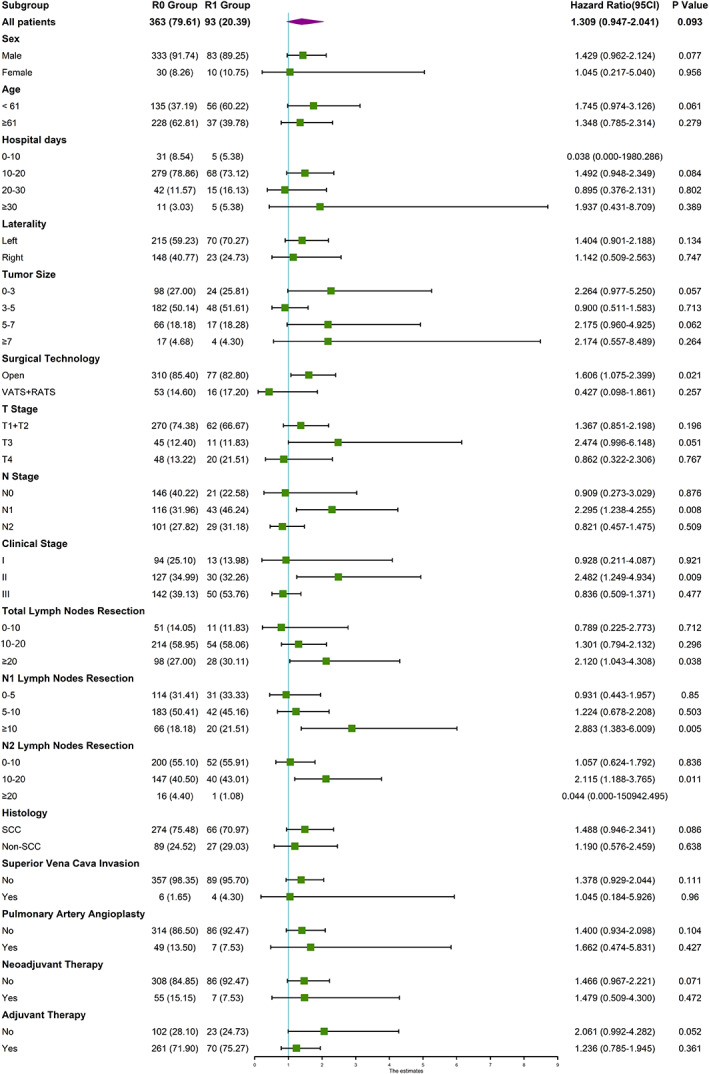
Subgroup analysis of margin status for overall survival
Furthermore, we aimed at the number of lymph nodes resection and had a cutoff analysis based on KM curve and the log‐rank test. The breakpoints of N number and N1 number were 12 and 8, respectively. As for the N2 number, although the Figure showed the breakpoint was 4, the log‐rank test and survival curve concluded a p‐value of no statistical implication (p = 0.1) (Figure 4).
FIGURE 4.
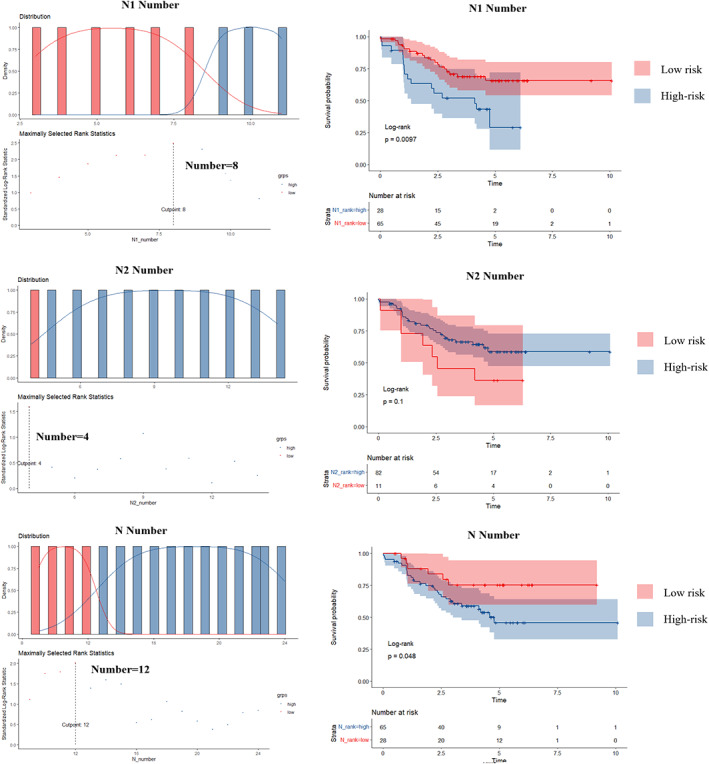
The cutoff of the lymph nodes resection (the cutoffs were 8, 4, and 12 for N1, N2, and N number)
There were no patients with the margin of atypical hyperplasia or tumor in situ who died after surgeries, and we compared the other three histology types, which indicated no significant differences. The pairwise comparisons using the log‐rank test for RFS revealed no differences (Figure 5).
FIGURE 5.
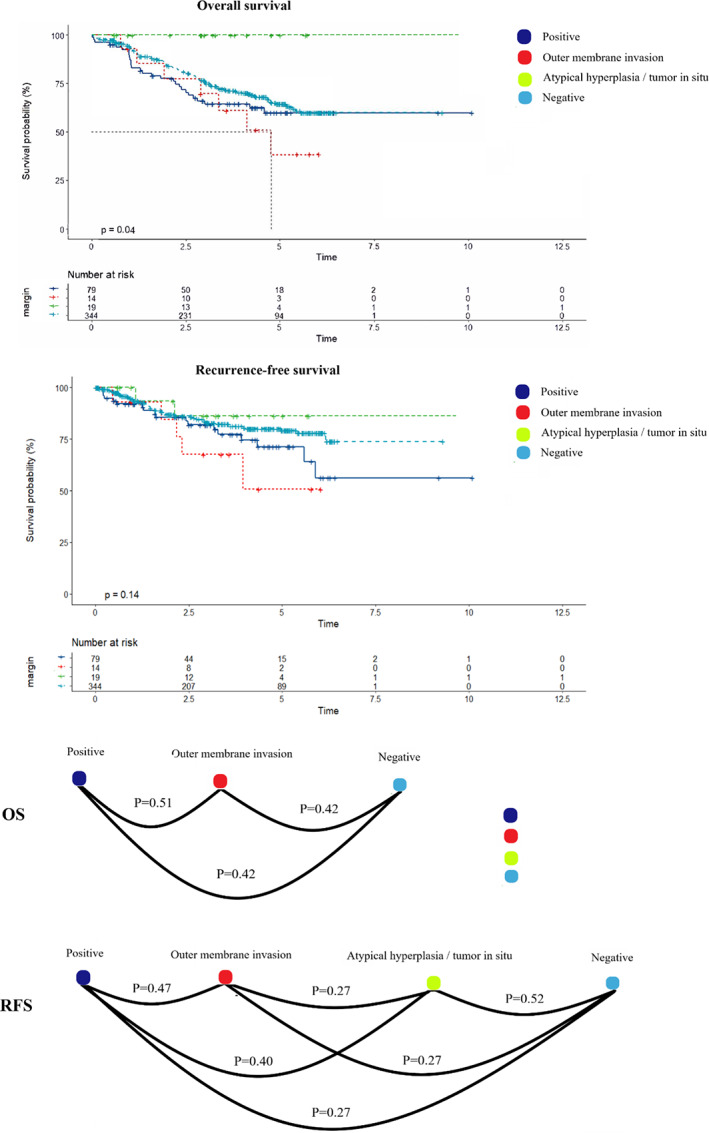
Pairwise comparisons of histological types of margin status
DISCUSSION
With the development of surgical techniques, sleeve operation has been widely recognized as an alternative to pneumonectomy when it is a centrally located lesion. Sleeve lobectomy not only preserves maximum lung function (PF), but also has a better prognosis than pneumonectomy. However, the sleeve operations are always companied with a disputable problem that the margin of resection will be inevitably positive sometimes. In response to this, Lee et al. 3 explored a novel evaluation for resectability of sleeve lobectomy with the aid of CT features. Some articles reported the frequency of R1 resection ranging from 1.2% to 17%. 4 , 5 The management of a positive margin is always controversial and usually depends on the decision of individual surgeons. There has not been any clear consensus to guide the surgeon on whether to have extensive sleeve lobectomy, a pneumonectomy, or no‐operation after reporting the frozen section results.
In our study, we found that the margin status was not related to the prognosis of both RFS and OS although the OS between R0 and R1 tended to be significant (p = 0.053). It indicated that a positive margin might not be that serious and an extensive sleeve or a pneumonectomy might not necessary. Similarly, Hong et al. 6 drew the homologous conclusion in 2020 that R1 after sleeve operations generally showed long‐term survival and are not significantly jeopardized in terms of oncologic outcomes. Among those participants, nearly 75.8% (47/62) patients accepted adjuvant therapy including radiology. The local control of disease may be in part because of radiotherapy that was proposed by Massard et al. 7 as early as 2000. As National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines) refers, it is widely recognized that radiotherapy plus selective chemotherapy is the standard treatment for positive margin. However, the radiotherapy sometimes increases the anastomotic complications to some extent, especially in advanced patients. The IASLC lung cancer staging project argued that the R status has some importance in prognosis evaluation and should be considered in the design of trials. 2 Because Wind et al. 4 interpreted that the negative effect of R1status has mainly been observed in patients with clinical stage III–IV and the patients of R0 with stage I–II had obvious better prognosis compared to R1 patients. However, in our subgroup and multivariable analysis, we did not find such above tendency and the clinical stage was even not an independent risk factor to both OS and RFS.
In the past relative study, the 5‐year OS of patients with R0 resection who accepted sleeve lobectomy varied from 30% to 60%. 8 , 9 In our study, the 5‐year OS and RFS of R0 resection had reached 64.9% and 78.3%, respectively, and reached 55% and 62.4% for R1 resection, which was closed to R0 resection. Considering the potential implication of margin histology, we further discussed its histologic subgroup. Nevertheless, there were no patients with atypical hyperplasia or tumor in situ who died and no statistical difference of OS was found between the other three types. Neither, the RFS between four types mentioned above had no significant difference. However, some articles mentioned that the margin of in situ did not have any negative influence on survival and had comparable progress to radical resection, and they also insisted that the invasive margin had a progressive tendency of recurrence. 6 , 7 Therefore, our study needs further investigation to analyze the histology subgroup.
Compared to margin status, the N stage was a more momentous risk factor. Because the lymph system was the potential metastatic pathway of tumor cells, and the positive mediastinal lymph nodes indicated to be prone to local progress. The 5‐year OS of N0, N1, and N2 were 78.5%, 66.0%, 37.4%, and the corresponding RFS were 80.9%, 76.2%, 65.3% in our study where significant differences were found among them. In the subgroup analysis, R1 status had a potentially negative effect on patients with more lymph nodes resection. Lymph node dissection helped remove potential local micro‐metastases, however, it disrupts the patient's innate immune system. How to maintain a balance between them needs to be determined. In the subgroup analysis, we also found that the margin status might affect the prognosis of patients with clinical II or N1 stage. We conjectured that, in patients of N2 stage or clinical stage III‐IV, the disease itself had a more important contribution to tumor progress compared to margin status. However, we cannot explain why margin status was not a risk factor to patients with early‐stage such as clinical stage I and N0 stage.
In addition to these factors, neoadjuvant chemotherapy is also an emerging technique to sleeve lobectomy. In the multivariate analysis, we concluded that neoadjuvant chemotherapy could not improve the outcome of RFS, but was an independent variable to OS. As early as 1997, Rendina et al. 10 had demonstrated the safety and efficacy of bronchus reconstruction after induction chemotherapy for NSCLC. A series of trials have been currently undertaken. It has been recognized that patients with neoadjuvant chemotherapy can acquire a better prognosis 11 , 12 and the neoadjuvant chemotherapy will not increase surgical morbidity, anastomotic complications as well as mortality. 13 , 14 However, neoadjuvant radiology is associated with increased anastomotic complications such as anastomotic fistula. 15
Our study has some limitations. First, it was a retrospective cohort study in a single center of Shanghai Chest Hospital rather than a randomized controlled trial although we performed the propensity scores to balance the baseline. Second, the sample size of our study was a little small, which took some bias in the survival analysis. Third, the follow‐up time of 5 years was insufficient and not all patients were followed up for 5 years.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Ren J, Zhu M, Xu Y, Liu R, Ren T, Guo Z, et al. The outcomes of margin status after sleeve lobectomy for patients of non–small cell lung cancer. Thorac Cancer. 2022;13(11):1664–1675. 10.1111/1759-7714.14441
Funding informationNational Natural Science Foundation of China Project No. 81871497.
Jianghao Ren, Mingyang Zhu, and Yuanyuan Xu are contributed equally to this work and are co‐first authors.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Edwards JG, Chansky K, Van Schil P, et al. The IASLC lung cancer staging project: analysis of resection margin status and proposals for residual tumor descriptors for non‐small cell lung cancer. J Thorac Oncol. 2020;15:344–59. [DOI] [PubMed] [Google Scholar]
- 3. Lee JH, Yoon SH, Kim YT, Kang CH, Park IK, Park S, et al. Sleeve lobectomy for non‐small cell lung cancers: predictive CT features for resectability and outcome analysis. AJR Am J Roentgenol. 2019;213:807–16. [DOI] [PubMed] [Google Scholar]
- 4. Wind J, Smit EJ, Senan S, Eerenberg JP. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg. 2007;32:29–34. [DOI] [PubMed] [Google Scholar]
- 5. Pezzetta E, Stupp R, Zouhair A, et al. Comparison of neoadjuvant cisplatin‐based chemotherapy versus radiochemotherapy followed by resection for stage III (N2) NSCLC. Eur J Cardiothorac Surg. 2005;27:1092–8. [DOI] [PubMed] [Google Scholar]
- 6. Hong TH, Kim J, Shin S, et al. Clinical outcomes of microscopic residual disease after bronchial sleeve resection for non‐small cell lung cancer. J Thorac Cardiovasc Surg. 2021;161:267–77. [DOI] [PubMed] [Google Scholar]
- 7. Massard G, Doddoli C, Gasser B, Ducrocq X, Kessler R, Schumacher C, et al. Prognostic implications of a positive bronchial resection margin. Eur J Cardiothorac Surg. 2000;17:557–65. [DOI] [PubMed] [Google Scholar]
- 8. Cerfolio RJ, Bryant AS: Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non‐small cell lung cancer. Ann Thorac Surg 83:1971–6; discussion 1976–7, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K, et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non‐small‐cell lung cancer with N2 disease. Lung Cancer. 2016;96:56–62. [DOI] [PubMed] [Google Scholar]
- 10. Rendina EA, Venuta F, De Giacomo T, et al. Safety and efficacy of bronchovascular reconstruction after induction chemotherapy for lung cancer. J Thorac Cardiovasc Surg. 1997;114:830–5; discussion 835‐7. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Zhang L, Yan B, Zeng Z, Hui Z, Zhang R, et al. Feasibility of sleeve lobectomy after neo‐adjuvant chemo‐immunotherapy in non‐small cell lung cancer. Transl Lung Cancer Res. 2020;9:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gómez‐Caro A, Boada M, Reguart N, et al. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg. 2012;41:1052–8. [DOI] [PubMed] [Google Scholar]
- 13. Comacchio GM, Schiavon M, Azzolina D, Mammana M, Marulli G, Zuin A, et al. Does induction therapy increase anastomotic complications in bronchial sleeve resections? World J Surg. 2019;43:1385–92. [DOI] [PubMed] [Google Scholar]
- 14. Milman S, Kim AW, Warren WH, et al: The incidence of perioperative anastomotic complications after sleeve lobectomy is not increased after neoadjuvant chemoradiotherapy. Ann Thorac Surg 88:945–50; discussion 950–1, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Koryllos A, Lopez‐Pastorini A, Zalepugas D, Ludwig C, Hammer‐Helmig M, Stoelben E. Bronchus anastomosis healing depending on type of neoadjuvant therapy. Ann Thorac Surg. 2020;109:879–86. [DOI] [PubMed] [Google Scholar]


