Abstract
Objectives
Whether curative‐intent radiotherapy could be safely applied to lung cancer patients with interstitial lung diseases (ILD) remains unclear. We aim to evaluate radiation induced lung toxicities (RILTs) and the efficacy of intensity‐modulated radiotherapy (IMRT) in these patients. ILD is characterized by inflammation or fibrosis in the interstitial tissue of the lung.
Materials and Methods
Stage III non–small cell lung cancer (NSCLC) and ILD patients treated with curative‐intent IMRT between 2010 and 2019 were retrospectively reviewed. Pre‐radiation computed tomography (CT) was scored according to a thin‐section CT scoring system for idiopathic pulmonary fibrosis.
Results
A total of 85 of 1261 stage III NSCLC patients were found with ILD. Seventeen (20%) of them developed G3+ (greater than or equal to grade 3) RILTs. The incidence abruptly dropped to 11.1%, 3.8%, and 0% for patients with honeycombing score ≤1, V20 <20%, or both, respectively. Multivariate analysis showed that honeycombing score >1 and V20 ≥20% were independently associated with higher risk of G3+ RILTs. The median overall survival (OS) and progression‐free survival (PFS) were 14.0 months and 7.4 months in the whole group, whereas 26.5 months and 10.6 months in the low‐risk group (patients with honeycombing score <1 and V20 <20%). In the univariate analysis for overall survival, G3+ RILTs were evaluated as risk factors (p = 0.026) and low‐risk group as the only protective factor (p = 0.063). In the multivariate analysis, G3+ RILTs were the only independent risk factor for OS.
Conclusion
Honeycombing score >1 and V20 ≥20% were associated with high incidence of RILTs. However, patients with low risk might benefit from IMRT with acceptable toxicities and durable OS.
Keywords: honeycombing, pulmonary fibrosis, pulmonary toxicities, radiotherapy, survival
Stage III NSCLC patients with ILD had poor survival in general. Honeycombing score >1 and V20 ≥20% were associated with high incidence of RILTs. Patients with low risk might benefit from IMRT with acceptable toxicities and durable OS
INTRODUCTION
Interstitial lung diseases (ILD) are characterized by inflammation or fibrosis in the interstitial tissue of the lung, eventually leading to respiratory failure. 1 It is now typically subclassified as fibrotic or nonfibrotic and patients with honeycombing change on chest computed tomography (CT) were considered as fibrotic ILD ones. 2 Fibrotic ILD (FILD) are predictive of more severe radiation pneumonitis (RP) after thoracic radiation therapy (TRT). Concurrent chemoradiotherapy (cCRT) with consolidation immunotherapy plays a crucial role in the definitive treatment of inoperable locally advanced non–small cell lung cancer (LA‐NSCLC) patients. 3 However, 2.4%–10.9% of newly diagnosed lung cancer patients have concurrent ILD, 4 whereas they may not be able to tolerate cCRT or sequential CRT. Some studies have shown that both radiotherapy and surgery can significantly increase pulmonary toxicities in patients with pre‐existing ILDs, leading to acute exacerbation (AE) of ILD or severe RP. 5 , 6 , 7 Clinicians are particularly careful of using radiation therapy, which increases the risk of pulmonary fibrosis. Several small‐sample studies have discussed the optimal therapeutic strategies for ILD patients, trying to balance between efficacy and safety. Chemotherapy alone for LA‐NSCLC patients with even mild ILD can yield very poor survival, with a median survival time of <16 months. 8 Kobayashi et al. 9 reported a durable median survival period of 34.6 months in patients treated with cCRT, despite increased lung toxicities (grade 3 ILD AE: 46%; grade 5 ILD AE: 2.7%). They also showed that patients without usual interstitial pneumonia (UIP) pattern, which is a favorable ILD subtype on CT, could predict lower risk of ILD AE and longer overall survival (OS) durations than the UIP pattern. Therefore, radiotherapy can improve survival and local control in unresectable lung cancer patients with ILD, but also aggravate lung toxicities. Two studies proved that patients with interstitial lung abnormities (ILA), which are considered subclinical ILD, could receive cCRT with controllable RP and long survival. 10 , 11 However, whether TRT can be applied to patients with fibrotic ILD needs further exploration. Intensity modulated radiotherapy (IMRT) is a modern radiotherapy (RT) technique implemented since the 1990s. It allows radiation beam intensities to target the tumor precisely and reduce the dose of organs at risk. A significant reduction of lung toxicity was observed in patients with LA‐NSCLC receiving IMRT. 12 The aim of this study was to determine the efficacy and safety of IMRT in stage III NSCLC patients with ILD. We also investigated risk factors of lung toxicities and survival to formulate a risk‐based radiotherapy in the modern era.
MATERIAL AND METHODS
Patients
We retrieved the medical records of patients with locally advanced lung cancer treated with radiotherapy at our institution between January 2010 and December 2019. Both concurrent/sequential chemoradiotherapy and TRT alone as the first‐line treatment were included in the analysis. Patients who were pathologically diagnosed with NSCLC and whose clinical stage was IIIA or IIIB as per the American Joint Committee of Cancer (AJCC) 7th edition Cancer Staging Manual 13 were included. The nodal stage (N) was diagnosed on the basis of CT findings. Eastern Cooperative Oncology Group‐Performance status (ECOG‐PS) system was used to evaluate patients' functional status. Patients receiving surgery for lung cancer or those diagnosed with multiple primary tumors were excluded. Furthermore, patients who underwent epidermal growth factor receptor–tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) were excluded from the analysis.
Radiotherapy and chemotherapy
All patients underwent curative‐intent IMRT. 12 X‐rays at 6 MV were used, with a median total dose of 60 Gy (40–70 Gy) in 30 (20–35) fractions. Irradiation dose no less than 50 Gy was considered as curative‐intent. Lung V20 and V5 indicated the percentage of total lung volume minus gross tumor volume (GTV) receiving 20 Gy and 5 Gy, respectively. Etoposide/cisplatin and paclitaxel/carboplatin regimen were primarily used in cCRT or sequential CRT. Platinum‐based doublet agents with several combinations such as etoposide, paclitaxel, gemcitabine, and pemetrexed were generally used.
Diagnoses of ILD and RILTs
The pre‐radiation thin‐section CT reports of 1261 stage III NSCLC patients who were described as having interstitial or fibrotic change were first sorted as candidates for inclusion. A total of 85 of them were picked then their diagnostic CTs were reviewed by two radiologists (Z.S.J. and W.L.F.) and one pulmonologist (H.H.), respectively. Their medical records were also reviewed and we combined the medical history and CT image to exclude lung changes caused by non‐ILD diseases, like infectious pneumonia or cardiopulmonary diseases. Next, they were scored according to a thin‐section CT scoring system for idiopathic pulmonary fibrosis (IPF). 14 We used this score system to evaluate honeycombing change on CT: 0, no discrete honeycombing, with interlobular septal thickening; 1, honeycombing involving 0%–5% of the lobe; 2, honeycombing 6%–24% of the lobe; 3, honeycombing involving 25%–49% of the lobe; 4, honeycombing involving 50%–74% of the lobe; and 5, honeycombing involving >75% of the lobe (Figure 1). Honeycombing change on CT was defined as clustered cystic airspaces, typically of comparable diameters of the order of 3–10 mm, which are usually subpleural and have well‐defined walls. 15 Patients with honeycombing score more than 0 points were considered as fibrotic ILD. 2 The gender, age, and pulmonary physiology (GAP) index used in our study was modified by eliminating the percent predicted carbon monoxide diffusing capacity (%DLCO), because this is not routinely performed in NSCLC‐ILD patients during clinical practice, which can also be used as a clinical prognostic factor for NSCLC‐IPF patients instead of the original GAP index. 16 The modified GAP (mGAP) index was used to obtain a total score ranging between 0 and 5: sex (female, 0; male, 1), age (years; ≤60, 0; 61–65, 1; >65, 2), and forced vital capacity (FVC) % (>75%, 0; 50%–75%, 1; <50%, 2). Based on a previous study on mGAP, 16 we chose point 3 as the cut‐off value for subsequent analysis. No commonly used laboratory or imaging tests can definitively identify RILTs, because it is a diagnosis of exclusion. 17 We excluded pneumonia caused by infectious or cardiopulmonary diseases and made the diagnosis based on chest CT, clinical symptoms, and physical examination. Patients with radiation lung toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
FIGURE 1.
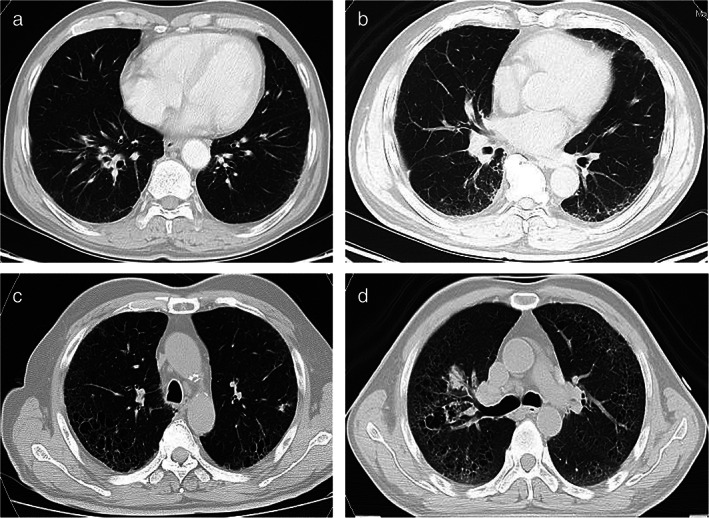
(a) Honeycombing score = 0 (no discrete honeycombing, with interlobular septal thickening); (b) honeycombing score = 1 (0%–5%); (c) honeycombing score = 2 (6%–24%); (d) honeycombing score = 3 (25%–49%)
The OS was defined as the time from the start of initial treatment to death. All patients were followed up 1 month after discharged from the hospital and then every 3 months for 2 years, and then every 6 months for 3 years, with chest CT required. Patients with symptoms like cough, dyspnea, or fever were followed up more frequently.
This study was approved by the institutional review board of the National Cancer Center, Chinese Academy of Medical Sciences and Peking Union Medical College (IRB No. NCC2612).
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics for Windows, version 25.0 (IBM) and R (version 4.0.0). Comparison of patient characteristics between patients with and without severe RILTs (grade ≥3) was performed using Mann–Whitney U, χ2, and Kruskal–Wallis tests, as appropriate. Univariate and multivariate analyses with logistic regression models and Cox proportional hazards approach were performed to identify the risk factor(s) of severe RP and OS, respectively. We estimated OS using the Kaplan–Meier method and compared groups using the log‐rank test. All p‐values are two‐sided, and p < 0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
Among 1261 stage III patients, 85 (6.74%) were found with pre‐existing ILD and included in the analysis. Pulmonary function test results were available in 73 patients. As shown in Table 1, the mGAP scores of most patients were from 2 to 4 points. A total of 51 (60%) patients, who had honeycombing change on pretreatment CT, were scored over 0 points on CT and were classified as fibrotic ILD. A total of 68 (80%) of the patients received sequential chemoradiotherapy (sCRT) or RT alone because 70% of them were evaluated as ECOG score 2 and most of these patients performed poorly in pulmonary function test with low median FVC% (68%, 44%–125%) because of underlying interstitial lung diseases. There were no patients with honeycombing score >3. In Table 2, 20% (17/85) of the whole cohort developed G3+ RILTs, and we compared the patients' characteristics between G3+ RILTs group and non‐G3+ group. Patients with severe RILTs showed significantly higher honeycombing score on pre‐treatment CT (p = 0.010) than those who developed asymptomatic or intermediate RP. The baseline of the other variables was quite balanced between these two groups.
TABLE 1.
Patient, tumor, and treatment characteristics (n = 85)
| Characteristic | No. or median (range) |
|---|---|
| Sex | |
| Male | 80 |
| Female | 5 |
| Age (y) | 67 (46–82) |
| Performance status | |
| 1 | 31 |
| 2 | 54 |
| Smoking status | |
| Never | 15 |
| Current or former smoker | 70 |
| Treatment pattern | |
| cCRT | 17 |
| sCRT | 26 |
| RT alone | 42 |
| Honeycombing score on CT | |
| 0 | 34 |
| 1 | 20 |
| 2 | 23 |
| 3 | 8 |
| Platinum‐based chemotherapy | |
| Paclitaxel | 21 |
| Pemetrexed | 6 |
| Etoposide | 10 |
| Gemcitabine | 6 |
| mGAP score (n = 73) | |
| 1 | 8 |
| 2 | 16 |
| 3 | 21 |
| 4 | 23 |
| 5 | 5 |
| Clinical stage | |
| IIIA | 33 |
| IIIB | 52 |
| Tumor location | |
| Lower lobe | 31 |
| Non‐lower lobe | 54 |
| Histology | |
| Squamous | 54 |
| Non‐squamous | 31 |
| %FVC (n = 73) | 68.6 (41.00–125.30) |
| MLD (Gy) (n = 83) | 13.71 (2.30–20.69) |
| V5% (n = 83) | 52.74 (16.61–86.54) |
| V20% (n = 83) | 23.00 (8.77–30.39) |
| Radiation dose (Gy) | 60.00 (40.00–70.00) |
Abbreviations: cCRT, concurrent chemoradiotherapy; CRT, chemoradiotherapy; RT, radiotherapy; mGAP, modified age gender, and pulmonary physiology index; FVC, forced vital capacity; MLD, mean lung dose; V5, the percentage of lung volume minus gross tumor volume receiving >5 Gy; V20, the percentage of lung volume minus gross tumor volume receiving >20 Gy.
TABLE 2.
The comparison of characteristics between G3+ RILTs group and non G3+ RILTs group (n = 85)
| Variable | RILTs (grade ≥3) (n = 17) | RILTs (grade <3) (n = 68) | p‐value | |
|---|---|---|---|---|
| Sex | Male/Female | 17/0 | 63/5 | 0.249 |
| Age (y) | Median (range) | 65 (54–78) | 68 (46–82) | 0.347 |
| Performance status | 1 vs. 2 | 8/9 | 23/45 | 0.311 |
| Smoking status | Never vs. current or former smoker | 2/15 | 13/55 | 0.477 |
| Clinical stage | IIIA vs. IIIB | 5/12 | 28/40 | 0.373 |
| Tumor location | Lower lobe vs. non‐lower lobe | 7/10 | 24/44 | 0.652 |
| Histology | Squamous vs. non‐squamous | 9/8 | 45/23 | 0.599 |
| Treatment | cCRT vs. sequential CRT or RT alone | 6/11 | 11/57 | 0.078 |
| Radiation dose | Median (range) | 60.00 (40.00–66.00) | 60.00 (40.00–70.00) | 0.771 |
| %FVC (n = 73) | Median (range) | 70.10 (59.80–107.30) | 68.60 (41.00–125.30) | 0.178 |
| MLD (Gy) (n = 83) | Median (range) | 13.71 (2.44–16.63) | 13.36 (2.31–20.69) | 0.179 |
| V5% (n = 83) | Median (range) | 59.65 (29.39–86.54) | 51.27 (16.61–84.76) | 0.148 |
| V20% (n = 83) | Median (range) | 24.43 (12.99–28.60) | 21.55 (8.77–30.39) | 0.107 |
| Honeycombing score on CT | 0–1 vs. 2–3 | 6/11 | 48/20 | 0.010 |
| mGAP score (n = 73) | >3 vs. ≤3 | 3/9 | 25/36 | 0.298 |
Note: The data are reported as the number or median (range). The p‐value was calculated by comparing patients with and without G3+ RILTs.
Abbreviations: RILTs, radiation‐induced lung toxicities; cCRT, concurrent chemoradiotherapy; CRT, chemoradiotherapy; RT, radiotherapy; mGAP, modified age gender, and pulmonary physiology index; FVC, forced vital capacity; MLD, mean lung dose; V5, the percentage of lung volume minus gross tumor volume receiving >5 Gy; V20, the percentage of lung volume minus gross tumor volume receiving >20 Gy.
Incidence and characterization of lung toxicities
As summarized in Table 2, of the 85 patients, 17 (20%) developed RILTs of grade 3 or higher, all of which happened within 1 year after the last irradiation. The median interval from the onset of radiation to the day of severe radiation pneumonitis was 2.6 months (range: 0.6–5.43 months). Of the 31 patients whose honeycombing on CT involved 6%–49% of the lobe, the rate of grade 3 or higher RILTs reached 35.5% specifically. No patients experienced grade 4 pneumonitis. A total of 10 patients died from pulmonary AEs, all occurring within 6 months from the onset of radiation. The median value of V5, V20, and mean lung dose (MLD) was significantly higher in the 10 patients (p = 0.017; p = 0.027; p = 0.044). The details of patients with fatal pneumonitis were listed in Table S1.
Predictors of severe RILTs
The univariate and multivariate analyses on the relationship between the incidence of severe RILTs and each factor in patients with stage III NSCLC and ILD treated with IMRT are shown in Table 3. Multivariate analysis showed that ILD with honeycombing score >1 was significantly associated with the rate of G3+ RILTs (odds ratio [OR], 4.53; 95% confidence interval [CI], 1.39–14.77; p = 0.012). The relationship between fibrosis score and G3+ RILTs was strong (area under curve [AUC] = 0.76). We described the distribution of lung AEs among different CT groups in Figure 2, which showed that the higher the honeycombing scores are, the severer the lung toxicities became. V20 ≥20% was also an independent risk factor for severe lung toxicities (OR, 9.40; 95% CI, 1.13–77.89; p = 0.038). MLD was not related to sever lung toxicities with median value as the cutoff point (p = 0.197). The incidence of G3+ RILTs abruptly dropped to 3.8% (1/26), 11.1% (6/54), and 0% (0/19) for patients with V20 <20%, honeycombing score ≤1 point, or both, respectively, as compared with 20% in the whole group. Therefore, patients with honeycombing score <1 and V20 <20% were defined as low‐risk group (n = 13). No RILTs ≥grade 2 were observed in low‐risk group. The therapy modality (cCRT vs. sCRT or RT alone) displayed a tendency in that cCRT could worsen lung toxicities, but this was not significant (p = 0.086). Primary tumor location (lower vs. non‐lower lobes), smoking history, FVC%, and mGAP score were not related to the risk of RILTs.
TABLE 3.
Univariate and multivariate analyses of severe RILTs (grade ≥3) in patients with stage III non‐small‐cell lung cancer treated with thoracic radiation (n = 85)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p‐value | Odds ratio | 95% CI | p‐value | |
| Age, y (≥65 vs. <65) | 0.65 | 0.22–1.91 | 0.438 | |||
| Performance status (2 vs. 1) | 0.56 | 0.20–1.69 | 0.314 | |||
| Smoking history (never vs. current or former smoker) | 1.73 | 0.36–8.73 | 0.482 | |||
| Honeycombing score on CT (0–1 vs. 2–3) | 4.40 | 1.43–13.53 | 0.010 | 4.53 | 1.39–14.77 | 0.012 |
| mGAP score (>3 vs. ≤3) (n = 73) | 0.48 | 0.12–1.95 | 0.305 | |||
| Clinical stage (IIIB vs. IIIA) | 1.68 | 0.53–5.30 | 0.376 | |||
| Histology (squamous vs. non‐squamous) | 0.75 | 0.25–2.22 | 0.599 | |||
| Tumor location (lower lobe vs. non‐lower lobe) | 1.28 | 0.43–3.80 | 0.653 | |||
| %FVC (<75% vs. ≥75%) (n = 73) | 0.96 | 0.27–3.37 | 0.951 | |||
| MLD (<13.71 Gy vs. ≥13.71 Gy) (n = 83) | 2.07 | 0.69–6.26 | 0.197 | |||
| V5 (≥55% vs. <55%) (n = 83) | 1.89 | 0.64–5.58 | 0.250 | |||
| V20 (≥20% vs. <20%) (n = 83) | 10.00 | 1.25–80.14 | 0.030 | 9.40 | 1.13–77.89 | 0.038 |
| Treatment (cCRT vs. sCRT or RT alone) | 2.83 | 0.86–9.25 | 0.086 | |||
| Paclitaxel or gemcitabine vs. other chemotherapy | 2.10 | 0.54–8.18 | 0.285 | |||
Abbreviations: RILTs, radiation induced lung toxicities; mGAP, modified age gender, and pulmonary physiology index; FVC, forced vital capacity; MLD, mean lung dose; V5, the percentage of lung volume minus gross tumor volume receiving >5 Gy; V20, the percentage of lung volume minus gross tumor volume receiving >20 Gy; cCRT, concurrent chemoradiotherapy; sCRT, sequential chemoradiotherapy; RT, radiotherapy.
FIGURE 2.
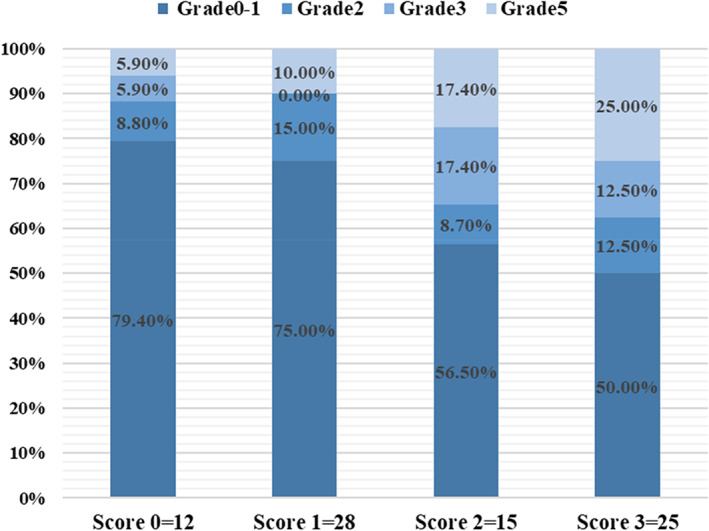
Grade 0–5 refers to the grade of radiation induced lung toxicities; score 0–3 refers to the honeycombing score on CT
Survival benefit of TRT in different risk groups
For all the patients, the mean follow‐up time was 46.77 months. The median OS was 14 months, with the 1‐year and 3‐year OS being 57.6% and 10.8%, respectively (Figure 3(a)). The median progression‐free survival (PFS) was 7.4 months (Figure 3(b)). The 90‐day mortality was 7%. For the low‐risk patients, the median OS and PFS were 26.5 months and 10.6 months, respectively, which were longer than those with at least one risk factor or the whole group, although no significance was noted (hazard ratio [HR], 0.52, 95% CI, 0.27–1.04, p = 0.063) (Figure 3(c)). The univariate and multivariate analyses of prognostic factors were presented in Table 4. Univariate or multivariate analysis of OS showed that G3+ RILTs (HR, 2.40, 95% CI, 1.32–4.36, p = 0·004) (Figure 3(d)) was a significant independent factor of poor OS.
FIGURE 3.
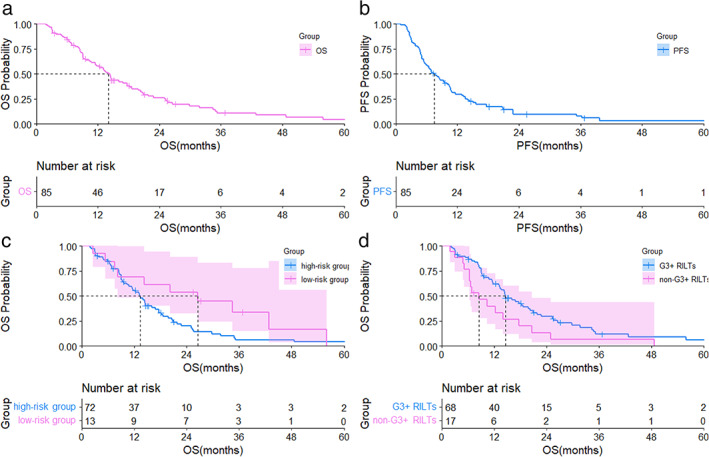
(a) Overall survival curve for patients with stage III NSCLC and ILD treated with TRT; (b) PFS curve for patients with stage III NSCLC and ILD treated with thoracic radiation therapy; (c) overall survival curve for 13 patients in the low‐risk group and 72 patients in the high‐risk group; (d) overall survival curve for 17 patients with G3+ RILTs and 68 patients without G3 RILTs. OS, overall survival; PFS, progression‐free survival; low‐risk group, the patients with V20 <20% and honeycombing score <1; NSCLC, non‐small‐cell lung cancer; ILD, interstitial lung disease; RILTs, radiation‐induced lung toxicities; CI, confidence interval; HR, hazard ratio
TABLE 4.
Univariate and multivariate analyses of overall survival in patients with stage III non‐small‐cell lung cancer and ILD treated with thoracic radiotherapy (n = 85)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p‐value | Hazard ratio | 95% CI | p‐value | |
| mGAP score (>3 vs. ≤3) (n = 67) | 1.08 | 0.63–1.84 | 0.782 | |||
| Honeycombing score on CT (0 vs. 1–3) | 1.19 | 0.73–1.92 | 0.486 | |||
| Clinical stage (IIIB vs. IIIA) | 1.52 | 0.94–2.48 | 0.088 | 1.52 | 0.92–2.49 | 0.097 |
| Histology (squamous vs. non‐squamous) | 0.73 | 0.45–1.19 | 0.210 | |||
| Tumor location (lower lobe vs. non‐lower lobe) | 1.37 | 0.84–2.22 | 0.204 | |||
| %FVC (<75% vs. ≥75%) (n = 67) | 0.91 | 0.54–1.53 | 0.726 | |||
| MLD (<13.71 Gy vs. ≥13.71 Gy) (n = 76) | 1.21 | 0.75–1.95 | 0.437 | |||
| V5 (≥55% vs. <55%) (n = 76) | 1.41 | 0.89–2.28 | 0.155 | |||
| V20 (≥20% vs. <20%) (n = 76) | 1.38 | 0.81–2.34 | 0.227 | |||
| Low risk group (V20 <20% and CT honeycombing score <1) | 0.52 | 0.27–1.04 | 0.063 | |||
| Treatment (cCRT vs. sCRT or RT alone) | 0.65 | 0.36–1.19 | 0.164 | 0.56 | 0.30–1.06 | 0.074 |
| RILTs (grade ≥3 vs. grade <3) | 1.90 | 1.08–3.33 | 0.026 | 2.40 | 1.32–4.36 | 0.004 |
Abbreviations: cCRT, concurrent chemoradiotherapy; FVC, forced vital capacity; ILD, interstitial lung disease; mGAP, modified age gender, and pulmonary physiology index; MLD, mean lung dose; RILTs, radiation‐induced lung toxicities; RT, radiotherapy.; sCRT, sequential chemoradiotherapy; V20, the percentage of total lung volume minus gross tumor volume receiving >20 Gy; V5, the percentage of total lung volume minus gross tumor volume receiving >5 Gy.
DISCUSSION
The majority (60%) of patients included in our study showed honeycombing on CT, which was considered as a typical manifestation for fibrotic ILD. To our knowledge, this study is the first to report pulmonary toxicities and OS of curative‐intent IMRT in stage III NSCLC patients with fibrotic ILD and also the first to define a low‐risk group with superior survival and durable lung toxicities. Our study showed that fibrosis score and radiation dosimetric parameters were significantly associated with the incidence of lung toxicities among this group of patients. We investigated the risk factors of lung toxicities and OS to formulate a risk‐based radiotherapy in the modern IMRT era. Relevant or similar studies are summarized in Table S2.
As shown in Table S3, NSCLC patients with fibrotic ILD treated with IMRT developed a higher incidence of severe RILTs than those 426 NSCLC patients without ILD treated with definitive RT at our institution from 2014 to 2016 (20% vs. 3.7%). Our results are consistent with previous reports, 9 , 18 which concluded that the presence of ILD was a risk factor for high‐grade RP, even fatal lung toxicities. 19 Some research demonstrated that ILD classification on CT was an important risk factor of ILD AE, and the diagnosis of a non‐UIP pattern could predict lower risk of AE of ILD and longer OS durations. 9 Furthermore, a retrospective study of 87 subclinical ILD with locally advanced NSCLC patients in China suggested that the volume of subclinical ILD ≥25% of the lung field could indicate an increased risk of grade 3 RILTs. 20 MLD is another key dosimetric risk factor for RILT shown in many studies. 21 , 22 On the basis of the abovementioned studies, we aimed to explore the connection between ILD fibrosis score with the lung toxicities and found that fibrosis score <1 point represented a lower risk of severe RILTs. We also intended to validate and determine how V20 and MLD make a difference in pulmonary adverse events. The current National Comprehensive Cancer Network (NCCN) guideline (NSCLC Version 5.2021) encourages more conservative limits for lung V20 with a diagnosis of UIP, but dose constraints for these patients are not well characterized. Our analyses showed that V20 ≥20% rather than 25% was a predictive factor for G3+ RILTs, which might be because of a stricter dosimetric request and a wide implantation of IMRT technology in our clinical setting, 12 with no significant correlation between MLD and severe RILTs. The rate of severe lung toxicities in our study were similar to what was reported by Li et al. 23 (G3+ RILTs: 20% vs. 20.9%). However, the incidence of fatal pneumonitis was obviously higher than previous studies, with 11.8% of the patients suffering from grade 5 RILTs. 2 Because CT honeycombing is identified as a typical manifestation for fibrotic ILD and associated with high mortality rate in ILD, 24 , 25 we believe the high proportion of fibrotic ILD (60%) might account for the high mortality, as well as the unsatisfactory survival. Moreover, it is shown that the dosimetric parameters including V5, V20, and MLD were significantly higher in 10 patients with fatal pneumonitis, which indicates that dose constraints need to be kept as low as possible comprehensively in patients with fibrotic ILD.
Notably, the OS was rather unsatisfactory compared with the reports from other studies. 5 , 9 , 10 As mentioned, G3+ RILTs was the only significant prognostic factor for OS, which was primarily observed in patients with V20 ≥20% and/or honeycombing scores >1 point. Therefore, we reviewed previous literatures 5 , 9 , 10 and speculate that one of the most disadvantageous factors is the high proportion of CT honeycombing (60%), which most likely results in the poor survival in the whole group. Apart from that, 10 patients died from lung‐related adverse events during a short interval from their last radiation, the median OS of which was only 6.4 months. Although the whole group analysis yielded a poor survival outcome, it is encouraging that patients with V20 ≤20% and honeycombing scores <1 point showed much better OS and less toxicities, whose median OS and PFS were 26.5 months and 10.6 months respectively, with no grade 2–5 RILTs observed. Compared to patients treated with chemotherapy alone, with a median OS no more than 16 months reported in a previous study, 8 our results suggest that IMRT in low‐risk patients might improve OS safely. Kim et al. 26 and Ono et al. 27 suggest that compared to X‐ray therapy, proton therapy may be more helpful to reduce acute and fatal complications. However, they did not include a direct comparison between IMRT and proton therapy, which deserves further research. Moreover, proton technique and related equipment are not available in most cancer centers yet, which indicates that the discussion on RILTs in IMRT compared to 3D‐CRT technique is realistic in clinical practice.
Our study has some limitations. First, this was a retrospective, non‐randomized study conducted at a single center with inherently introduced bias. Second, distinguishing RILTs and exacerbation of existing ILD can be challenging. Those with significant ILD may be more likely to have its exacerbation while labeled as RILTs. Moreover, cCRT with consolidation immunotherapy is the mainstream therapy in LA‐NSCLC, whereas none of the patients included in our study had ever received immune checkpoint inhibitors (ICIs) therapy, probably because of the concern of ICIs‐induced lung toxicities and poor OS. 28 , 29 Therefore, further studies are needed to explore the optimizing CRT in stage III NSCLC patients with fibrotic ILD and whether ICIs can be consolidated after chest irradiation.
In conclusion, receiving curative‐intent radiation therapy could trigger fatal lung toxicities in patients with ILD‐LC, especially those with fibrotic ILD. Honeycombing score >1 point and V20 ≥20% were significantly associated with the high incidence of severe RILTs. However, patients at low risk might benefit from IMRT with acceptable toxicities and considerable OS.
CONFLICT OF INTEREST
The authors declare no conflicts of interest
Supporting information
Table S1 Details of patients with fatal pneumonitis
Table S2 Selected studies reporting outcomes with TRT in patients with ILD
Table S3 Radiation induced lung toxicities grade
ACKNOWLEDGMENTS
The authors wished to thank all their families.
Wu L, Zhao S, Huang H, Wang W, Zhang T, Zhou Z, et al. Treatment outcomes of patients with stage III non–small cell lung cancer and interstitial lung diseases receiving intensity‐modulated radiation therapy: A single‐center experience of 85 cases. Thorac Cancer. 2022;13(11):1583–1591. 10.1111/1759-7714.14418
Funding informationThis work was supported by the National Natural Sciences Foundation Key Program (81872474); Capital's Funds for Health Improvement and Research (2020‐2‐4022); and Sanming Project of Medicine in Shenzhen (SZSM201612063)
Contributor Information
Nan Bi, binan_email@163.com.
Luhua Wang, Email: wlhwq@yahoo.com.
REFERENCES
- 1. Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. 2020;383(10):958–68. 10.1056/NEJMra2005230 [DOI] [PubMed] [Google Scholar]
- 2. Goodman CD, Nijman SFM, Senan S, Nossent EJ, Ryerson CJ, Dhaliwal I, et al. A primer on interstitial lung disease and thoracic radiation. J Thoracic Oncol. 2020;15(6):902–913. 10.1016/j.jtho.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 3. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377(20):1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 4. Goto T, Maeshima A, Oyamada Y, Kato R. Idiopathic pulmonary fibrosis as a prognostic factor in non‐small cell lung cancer. Int J Clin Oncol. 2014;19(2):266–73. 10.1007/s10147-013-0566-1 [DOI] [PubMed] [Google Scholar]
- 5. Tsujino K, Hashimoto T, Shimada T, Yoden E, Fujii O, Ota Y, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non‐small‐cell lung cancer. J Thoracic Oncol. 2014;9(7):983–90. 10.1097/jto.0000000000000187 [DOI] [PubMed] [Google Scholar]
- 6. Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147(5):1604–11. 10.1016/j.jtcvs.2013.09.050 [DOI] [PubMed] [Google Scholar]
- 7. Kenmotsu H, Naito T, Kimura M, Ono A, Shukuya T, Nakamura Y, et al. The risk of cytotoxic chemotherapy‐related exacerbation of interstitial lung disease with lung cancer. J Thoracic Oncol. 2011;6(7):1242–6. 10.1097/JTO.0b013e318216ee6b [DOI] [PubMed] [Google Scholar]
- 8. Ogawa K, Takahashi Y, Murase K, Hanada S, Uruga H, Takaya H, et al. Treatment outcome of patients with unresectable stage III non‐small cell lung cancer and interstitial pneumonia. Respir Investig. 2019;57(4):388–94. 10.1016/j.resinv.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi H, Naito T, Omae K, Omori S, Nakashima K, Wakuda K, et al. Impact of interstitial lung disease classification on the development of acute exacerbation of interstitial lung disease and prognosis in patients with stage III non‐small‐cell lung cancer and interstitial lung disease treated with Chemoradiotherapy. J Cancer. 2018;9(11):2054–60. 10.7150/jca.24936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higo H, Kubo T, Makimoto S, Makimoto G, Ihara H, Masaoka Y, et al. Chemoradiotherapy for locally advanced lung cancer patients with interstitial lung abnormalities. Jpn J Clin Oncol. 2019;49(5):458–64. 10.1093/jjco/hyz016 [DOI] [PubMed] [Google Scholar]
- 11. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. 10.1056/NEJMoa1007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J, Zhou Z, Liang J, Feng Q, Xiao Z, Hui Z, et al. Intensity‐modulated radiation therapy may improve local‐regional tumor control for locally advanced non‐small cell lung cancer compared with three‐dimensional conformal radiation therapy. Oncologist. 2016;21(12):1530–7. 10.1634/theoncologist.2016-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 14. Kazerooni EA, Martinez FJ, Flint A, Jamadar DA, Gross BH, Spizarny DL, et al. Thin‐section CT obtained at 10‐mm increments versus limited three‐level thin‐section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol. 1997;169(4):977–83. 10.2214/ajr.169.4.9308447 [DOI] [PubMed] [Google Scholar]
- 15. Johkoh T, Sakai F, Noma S, Akira M, Fujimoto K, Watadani T, et al. Honeycombing on CT; its definition, pathologic correlation, and future direction of its diagnosis. Eur J Radiol. 2014;83(1):27–31. 10.1016/j.ejrad.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi H, Omori S, Nakashima K, Wakuda K, Ono A, Kenmotsu H, et al. Modified GAP index for prediction of acute exacerbation of idiopathic pulmonary fibrosis in non‐small cell lung cancer. Respirol. 2017;22(7):1379–85. 10.1111/resp.13075 [DOI] [PubMed] [Google Scholar]
- 17. Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation‐induced lung injury: assessment and management. Chest. 2019;156(1):150–62. 10.1016/j.chest.2019.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ueki N, Matsuo Y, Togashi Y, Kubo T, Shibuya K, Iizuka Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thoracic Oncol. 2015;10(1):116–25. 10.1097/jto.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 19. Minami‐Shimmyo Y, Ohe Y, Yamamoto S, Sumi M, Nokihara H, Horinouchi H, et al. Risk factors for treatment‐related death associated with chemotherapy and thoracic radiotherapy for lung cancer. J Thoracic Oncol. 2012;7(1):177–82. 10.1097/JTO.0b013e31823c4c07 [DOI] [PubMed] [Google Scholar]
- 20. Li F, Liang S, Wu H, Xu Y, Chen M. Risk factors of radiation pneumonitis in lung cancer patients with subclinical interstitial pulmonary disease after thoracic radiotherapy: a prospective observational study. Int J Radiat Oncol Biol Phys. 2019;105(1):E489–E90. 10.1016/j.ijrobp.2019.06.1390 [DOI] [Google Scholar]
- 21. Jan N, Guy C, Reshko LB, Hugo GD, Weiss E. Lung and heart dose variability during radiation therapy of non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98(3):683–90. 10.1016/j.ijrobp.2017.02.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawkins PG, Boonstra PS, Hobson ST, Hearn JWD, Hayman JA, Ten Haken RK, et al. Radiation‐induced lung toxicity in non‐small‐cell lung cancer: understanding the interactions of clinical factors and cytokines with the dose‐toxicity relationship. Radiother Oncol. 2017;125(1):66–72. 10.1016/j.radonc.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li F, Liu H, Wu H, Liang S, Xu Y. Risk factors for radiation pneumonitis in lung cancer patients with subclinical interstitial lung disease after thoracic radiation therapy. Radiat Oncol. 2021;16(1):70. 10.1186/s13014-021-01798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adegunsoye A, Oldham JM, Bellam SK, Montner S, Churpek MM, Noth I, et al. Computed tomography honeycombing identifies a progressive fibrotic phenotype with increased mortality across diverse interstitial lung diseases. Ann Am Thorac Soc. 2019;16(5):580–8. 10.1513/AnnalsATS.201807-443OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence‐based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim H, Pyo H, Noh JM, Lee W, Park B, Park HY, et al. Preliminary result of definitive radiotherapy in patients with non‐small cell lung cancer who have underlying idiopathic pulmonary fibrosis: comparison between X‐ray and proton therapy. Radiat Oncol. 2019;14(1):19. 10.1186/s13014-019-1221-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ono T, Hareyama M, Nakamura T, Kimura K, Hayashi Y, Azami Y, et al. The clinical results of proton beam therapy in patients with idiopathic pulmonary fibrosis: a single center experience. Radiat Oncol. 2016;11:56–63. 10.1186/s13014-016-0637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shibaki R, Murakami S, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association of immune‐related pneumonitis with the presence of preexisting interstitial lung disease in patients with non‐small lung cancer receiving anti‐programmed cell death 1 antibody. Cancer Immunol Immunother. 2020;69(1):15–22. 10.1007/s00262-019-02431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre‐existing pulmonary fibrosis is a risk factor for anti‐PD‐1‐related pneumonitis in patients with non‐small cell lung cancer: a retrospective analysis. Vol 125. Amsterdam, Netherlands; Lung cancer; 2018. p. 212–7. 10.1016/j.lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Details of patients with fatal pneumonitis
Table S2 Selected studies reporting outcomes with TRT in patients with ILD
Table S3 Radiation induced lung toxicities grade


