Abstract
Background
Immune checkpoint inhibitors (ICIs) have become standard‐of‐care in patients with pretreated advanced esophageal squamous cell carcinoma (ESCC). However, reliable biomarkers for clinical outcomes are lacking for ICIs. The exploration of effective biomarkers is therefore needed to optimize patient benefit in the treatment of ESCC.
Methods
Sixty‐nine patients with advanced ESCC enrolled at one center from two prospective trials were consecutively analyzed. NLR was dynamically collected and high‐resolution HLA‐I genotyping were performed on genomic DNA. Overall response rate (ORR), median progression‐free survival (mPFS) and median overall survival (mOS) were investigated.
Results
Thirty‐three (47.8%) of 69 patients with baseline NLR ≥4 demonstrated significantly worse clinical outcomes (ORR 9.1% vs. 36.1%, p = 0.018; mPFS 1.8 vs. 3.2 months, hazard ratio [HR] 1.79, p = 0.026; mOS 7.4 vs. 11.0 months, HR 2.28, p = 0.008). An NLR decrease ≥20% at the first radiological evaluation was associated with longer OS (median, 14.0 vs. 7.9 months, p = 0.038). Eleven (15.9%) patients with HLA‐I homozygosity presented poorer clinical outcomes (ORR 0 vs. 27.6%, p = 0.056; mPFS 1.8 vs. 2.4 months, HR 3.37, p = 0.010; mOS 5.6 vs. 10.5 months, HR 3.97, p = 0.004). Patients with baseline NLR ≥4 and HLA‐I homozygosity had the worst outcome (ORR 0; mPFS 1.4 months; mOS 1.8 months) among all. The association between NLR, HLA‐I genotyping and clinical outcomes was independent of programmed death receptor ligand‐1 expression.
Conclusions
NLR and HLA‐I genotyping could have predictive and prognostic value in patients with advanced ESCC receiving camrelizumab, and the combination of biomarkers may help to identify more patient benefit from immunotherapy.
Keywords: camrelizumab, esophageal squamous cell carcinoma (ESCC), human leukocyte antigen (HLA)‐I genotyping, neutrophil‐to‐lymphocyte ratio (NLR)
Our study evaluated the predictive and prognostic value of NLR and HLA‐I genotype in advanced esophageal squamous cell carcinoma patients treated with an immune checkpoint inhibitor. Patients enrolled at one center from two prospective trials and treated with camrelizumab were consecutively analyzed. The patients with baseline NLR ≥4 or HLA‐I homozygosity demonstrated worse clinical outcomes and the combination of biomarkers may help to identify patients most likely to benefit from immunotherapy.
INTRODUCTION
Esophageal cancer is the seventh most common malignancy in incidence and the sixth most common leading cause of cancer death worldwide and esophageal squamous cell carcinoma (ESCC) is the predominant subtype in Asia. 1 The prognosis of patients with advanced ESCC remained poor, with an overall survival of 8–11 months, 2 , 3 , 4 suggesting an urgent need for novel effective therapeutic strategies.
Recently, immune checkpoint inhibitors (ICIs) have dramatically altered the therapeutic pattern in several tumors. 5 , 6 , 7 In the second‐line treatment of advanced ESCC, ICIs have become the standard of care attributing to the superior efficacy over traditional chemotherapy. 8 , 9 , 10 Although clinical responses to ICIs are enriched in patients with programmed death receptor‐ligand 1 (PD‐L1) expression positive tumors, there are still responders in patients with negative PD‐L1 expression, appealing for the strong need of more effective biomarkers.
In the past few years, the recognition of inflammation as one of the hallmarks of cancer and its potential role in promoting tumor progression 11 , 12 , 13 has sparked increasing interest in the research of neutrophils in the tumor immune microenvironment. Recently, the correlation of decreased overall survival (OS) and high neutrophil to lymphocyte ratio (NLR) has been observed in different tumors receiving ICIs. 14 , 15 , 16 , 17 However, studies on NLR in ESCC are limited. 18 , 19 , 20 , 21 A few studies have attempted to identify the association between NLR and survival in patients with ESCC treated with ICI‐containing regimens; however, the findings are inconsistent and they all have common limitations. First, the retrospective nature limited the quality of response evaluation and follow‐up, so none of these studies reported the complete panorama of responses and survivals as well as the continuous tracking of NLR at each evaluation; second, patients enrolled in these studies were treated with heterogenous therapeutic strategies, including different single‐agent ICIs, dual blockade of PD‐1 and CTLA‐4, or ICIs combined with surgery, radiotherapy or chemotherapy. Therefore, a high‐quality analysis based on a relatively homogeneous population treated with a specific ICI monotherapy with complete follow‐up data is needed.
In addition to the immune function of inflammatory cells, the role of antigen recognition and presentation are also of vital importance in the response to ICIs. Human leukocyte antigen (HLA) is expressed on somatic as well as immune cells and plays an important role in T lymphocyte‐based antitumor immune response. 22 HLA molecules are usually divided into two classes, HLA‐I and HLA‐II. It has been found that HLA‐I heterozygosity at all loci (A, B, and C) is associated with improved OS than HLA‐I homozygosity in at least one HLA locus in patients with melanoma or NSCLC, supporting the speculation that a more diverse HLA class I repertoire would lead to a better T lymphocyte‐based antitumor immune response. 23 , 24 , 25 , 26 However, the relationship between HLA‐I genotyping and clinical outcomes in ICI‐treated ESCC patients has not previously been investigated.
Therefore, this comprehensive analysis of two high‐quality prospective trials in patients with advanced (locally advanced or metastatic) ESCC treated with single‐agent camrelizumab was performed, which aimed to excavate the predictive and prognostic value of NLR and HLA‐I genotyping, and attempt to provide more clues for the biomarker network under immunotherapy.
METHODS
Study design and participants
This was a post‐hoc analysis of two prospective clinical trials; the Phase I trial of camrelizumab in patients with advanced solid tumor (ClinicalTrials.gov identifier: NCT02742935) and the randomized‐controlled Phase III ESCORT trial of camrelizumab in patients with advanced or metastatic ESCC (ClinicalTrials.gov identifier: NCT03099382). 8 , 27 All ESCC patients who received camrelizumab monotherapy enrolled in these two clinical trials at National Cancer Center/Cancer Hospital Chinese Academy of Medical Sciences were consecutively included in the analysis. The clinicopathological characteristics of all patients were collected from the electronic medical records, including age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), histological grade, previous treatment, and PD‐L1 expression. All the patients had signed the informed consent. The study was approved by independent ethics committees and conducted in accordance with local legal, regulatory requirements, the general principles of the International Ethical Guidelines for Biomedical Research Involving Human Subjects, and the International Conference on Harmonization guidelines on Good Clinical Practice and the Declaration of Helsinki.
Assessment
Tumor imaging assessment was performed by CT at baseline and every 8 weeks during the first 6 months, and every 12 weeks thereafter. Best overall response was assessed according to RECIST v1.1 for complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). PFS was defined as the time between the first administration of camrelizumab until PD or death due to any cause, and OS was defined as the time between the first dose until death due to any case. The ORR and DCR were defined as the percentage of patients with CR and PR, and patients with CR, PR and SD, respectively.
NLR calculation
The absolute number of neutrophils and lymphocytes were collected within 3 days before the first dose of camrelizumab, and when tumor imaging assessment was performed (within a window of ±7 days of the scheduled date). NLR was calculated by the division of the absolute number of neutrophils and lymphocytes, and the percentage change of NLR from baseline to first evaluation was calculated as 100% × ([first‐evaluation values − baseline value]/baseline value). The cutoff values of NLR‐high (NLR ≥4) and NLR‐low (NLR <4) was defined as 4, as previously reported in several studies. 28 , 29
PD‐L1 expression
Tumor cell PD‐L1 expression was measured by a central laboratory using a human PD‐L1 immunohistochemistry kit (6E8 antibody, Shuwen Biotech). PD‐L1 expression was quantified as tumor proportion score (TPS), which was defined as the percentage of viable tumor cells showing partial or complete membrane staining (≥1+), relative to all viable tumor cells present in the sample.
HLA genotyping and supertypes
Among all 69 patients included in the analysis, we performed whole exome sequencing in eight patients, and 509‐panel (CLIA‐certified hybridization‐capture based assay) sequencing in the other 61 patients to determine germline HLA Class I (A, B and C) genotype. The detailed sequencing methods are provided in the supplementary methods. Patients were defined as HLA heterozygosity if heterozygous for all HLA class I loci and homozygosity if homozygous for at least one HLA class I locus. 23 Genomic HLA‐A and HLA‐B alleles were classified into supertypes using the method described by Chowell et al. 23
Statistical analysis
Statistical analysis was performed using IBM SPSS software version 25.0 and GraphPad Prism 8.0. Survival was compared by log‐rank test using Kaplan–Meier methodology. Univariate and multivariate analysis for ORR and DCR were conducted using logistic regression. Univariate analysis for PFS and OS were conducted by Kaplan–Meier and log‐rank tests, and multivariate analysis was performed using Cox's regression model. Group comparisons of categorical data were performed using χ 2 or Fisher's exact test. Differences with two‐sided p‐values <0.050 were considered statistically significant.
RESULTS
Patient characteristics and overall clinical outcomes
A total of 69 patients with advanced ESCC enrolled in our center were consecutively analyzed, including 42 patients in the Phase I trial enrolled between May 11, 2016 and June 5, 2017, and 27 patients in the Phase III trial enrolled between May 10, 2017 and July 24, 2018. Patients were treated with camrelizumab at different dose levels, including three, 64 and two patients receiving a dose of 60, 200 and 400 mg every 2 weeks, respectively. The median baseline NLR was 3.9 (interquartile range [IQR] 2.6–6.0). A total of 33/69 (47.8%) of the patients had a baseline NLR ≥4, and 11/69 (15.9%) patients were detected with HLA‐I homozygosity. The baseline clinicopathological characteristics of patients are demonstrated in Table 1. The major baseline features between patients with NLR ≥4 and <4 were comparable.
TABLE 1.
Baseline patient characteristics
| Total (N = 69) | NLR <4 (N = 36) | NLR ≥4 (N = 33) | |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 61 [38–75] | 65 [50–75] | 62 [38–70] |
| <65 | 49 (71.0%) | 21 (58.3%) | 28 (84.8%) |
| ≥65 | 20 (29.0%) | 15 (41.7%) | 5 (15.2%) |
| Gender | |||
| Male | 64 (92.8%) | 33 (91.7%) | 31 (93.9%) |
| Female | 5 (7.2%) | 3 (8.3%) | 2 (6.1%) |
| ECOG PS | |||
| 0 | 47 (68.1%) | 28 (77.8%) | 19 (57.6%) |
| 1 | 22 (31.9%) | 8 (22.2%) | 14 (42.4%) |
| Smoking a | |||
| Never | 19 (27.5%) | 9 (25.0%) | 10 (30.3%) |
| Light | 15 (21.7%) | 7 (19.4%) | 8 (24.2%) |
| Heavy | 35 (50.7%) | 20 (55.6%) | 15 (45.5%) |
| Drinking | |||
| Yes | 52 (75.4%) | 28 (77.8%) | 24 (72.7%) |
| No | 17 (24.6%) | 8 (22.2%) | 9 (27.3%) |
| Disease stage | |||
| Locally advanced | 3 (4.3%) | 2 (5.6%) | 1 (3.0%) |
| Metastatic | 66 (95.7%) | 34 (94.4%) | 32 (97.0%) |
| Grade | |||
| Gx | 17 (24.6%) | 10 (27.8%) | 7 (21.2%) |
| G1 | 3 (4.3%) | 2 (5.6%) | 1 (4.3%) |
| G2 | 29 (42.0%) | 12 (33.3%) | 17 (51.5%) |
| G3 | 20 (29.0%) | 12 (33.3%) | 8 (24.2%) |
| Previous surgery | |||
| Palliative surgery | 3 (4.3%) | 1 (2.8%) | 2 (6.1%) |
| Radical esophagectomy | 26 (37.7%) | 16 (44.4%) | 10 (30.3%) |
| No | 40 (58%) | 19 (52.8%) | 21 (63.6%) |
| Previous radiotherapy | |||
| Yes | 46 (66.7%) | 20 (55.6%) | 26 (78.8%) |
| No | 23 (33.3%) | 16 (44.4%) | 7 (21.2%) |
| Line of prior chemotherapy | |||
| (0–1) | 44 (63.8%) | 24 (66.7%) | 20 (60.6%) |
| ≥2 | 25 (36.2%) | 12 (33.3%) | 13 (39.4%) |
| PD‐L1 TPS | |||
| ≥10% | 22 (31.9%) | 15 (41.7%) | 7 (21.2%) |
| <10% | 42 (60.9%) | 18 (50%) | 24 (72.7%) |
| NA | 5 (7.2%) | 3 (8.3%) | 2 (6.1%) |
| HLA‐I | |||
| Homozygosity | 11 (15.9%) | 7 (19.4%) | 4 (12.1%) |
| Heterozygosity | 58 (84.1%) | 29 (80.6%) | 29 (87.9%) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HLA‐I, human leukocyte antigen class I; NLR, neutrophil to lymphocyte ratio; PD‐L1, programmed death receptor ligand 1; TPS, tumor proportion score.
Classification of smoking history as follows: never, light (≤20 pack‐years), heavy (>20 pack‐years).
Among the 69 intention‐to‐treat (ITT) patients, a total of 64 patients had at least one post‐baseline tumor imaging assessment, and five patients were not evaluated due to rapid clinical deterioration. Among the ITT patients, the ORR and DCR were 23.2% (16/69) and 46.4% (32/69), respectively. By the time of data cutoff (October 31, 2021), two patients were still alive. The median PFS (mPFS) and median OS (mOS) were 1.9 (95% CI: 1.6–2.1) months and 8.9 (95% CI: 6.6–11.2) months, respectively, with the median follow‐up time of 48.6 (range 1.0–61.3) months (Table 2).
TABLE 2.
The response rates and survival outcomes stratified by different factors
| N | ORR | DCR | mPFS | (months) | mOS | (months) | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | 69 | 23.2% | 46.4% | 1.9 | 95% CI: 1.6–2.1 | 8.9 | 95% CI: 6.6–11.2 | ||
| Baseline NLR | |||||||||
| ≥4 | 33 | 9.1% | 39.4% | 1.8 |
HR 1.79 (1.07–3.00) p = 0.026 |
7.4 |
HR 2.28 (1.24–4.17) p = 0.008 |
||
| <4 | 36 | 36.1% | p = 0.018 | 52.8% | p = 0.265 | 3.2 | 11.0 | ||
| V1‐NLR | |||||||||
| ≥4 | 32 | 15.6% | 40.6% | 1.8 | HR 1.74 (1.03–2.93) | 7.4 | HR 2.38 (1.26–4.50) | ||
| <4 | 32 | 34.4% | p = 0.083 | 59.4% | p = 0.134 | 3.6 | p = 0.037 | 11.5 | p = 0.007 |
| Variation of NLR from baseline to V1 | |||||||||
| ≥ −20% | 45 | 22.2% | 48.9% | 2.0 | HR 1.38 (0.81–2.34) | 7.9 | HR 1.85 (1.03–3.31) | ||
| < −20% | 19 | 31.6% | p = 0.430 | 52.6% | p = 0.784 | 2.4 | p = 0.231 | 14.0 | p = 0.038 |
| HLA‐I | |||||||||
| Homo | 11 | 0 | 9.1% | 1.8 |
HR 3.37 (1.35–8.46) p = 0.010 |
5.6 |
HR 3.97 (1.56–10.12) p = 0.004 |
||
| Hetero | 58 | 27.6% | p = 0.056 | 53.4% | p = 0.018 | 2.4 | 10.5 | ||
| PD‐L1 TPS | |||||||||
| ≥10% | 22 | 36.4% | 63.6% | 4.0 | HR 0.47 (0.28–0.79) | 8.3 | HR 0.96 (0.54–1.71) | ||
| <10% | 42 | 14.3% | p = 0.042 | 33.3% | p = 0.020 | 1.8 | p = 0.004 | 9.6 | p = 0.883 |
| PD‐L1 TPS ≥10% | |||||||||
| NLR ≥ 4 | 7 | 14.3% | 57.1% | 3.7 | HR 1.35 (0.51–3.61) | 4.0 | HR 2.89 (0.92–9.07) | ||
| NLR < 4 | 15 | 46.7% | p = 0.193 | 66.7% | p = 1.000 | 4.4 | p = 0.545 | 8.4 | p = 0.069 |
| Homo | 4 | 0 | 0 | 1.8 | HR 12.6 (1.88–84.46) | 3.8 | HR 11.53 (1.80–73.95) | ||
| Hetero | 18 | 44.4% | p = 0.254 | 77.8% | p = 0.010 | 4.7 | p = 0.009 | 9.8 | p = 0.010 |
| PD‐L1 TPS <10% | |||||||||
| NLR ≥ 4 | 24 | 8.3% | 29.2% | 1.7 | HR 1.43 (0.76–2.68) | 7.4 | HR 2.45 (1.08–5.54) | ||
| NLR < 4 | 18 | 22.2% | p = 0.408 | 38.9% | p = 0.508 | 1.9 | p = 0.265 | 11.0 | p = 0.032 |
| Homo | 6 | 0 | 16.7% | 1.7 | HR 2.32 (0.74–7.26) | 5.6 | HR 1.72 (0.59–5.00) | ||
| Hetero | 36 | 16.7% | p = 0.569 | 36.1% | p = 0.640 | 1.8 | p = 0.147 | 9.9 | p = 0.322 |
| NLR + HLA‐I | |||||||||
| Homo & NLR ≥ 4 | 4 | 0 | 0 | 1.4 | 1.8 | ||||
| Homo & NLR < 4 | 7 | 0 | 14.3% | 1.8 | 6.5 | ||||
| Hetero & NLR ≥ 4 | 29 | 10.3% | 44.8% | 1.8 | 8.2 | ||||
| Hetero & NLR < 4 | 29 | 44.8% | * p = 0.005 | 62.1% | * p = 0.023 | 4.1 | * p < 0.001 | 13.4 | * p < 0.001 |
Abbreviations: CI, confidence interval; DCR, disease control rate; hetero, heterozygosity; HLA‐I, human leukocyte antigen class I; homo, homozygosity; PD‐L1, programmed death receptor ligand 1; mOS, median overall survival; mPFS, median progression‐free survival; NLR, neutrophil to lymphocyte ratio; ORR, overall response rate; TPS, tumor proportion score; V1, first tumor evaluation.
The comparison among four subgroups.
Impact of NLR on clinical outcomes
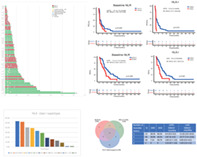
We first evaluated the association of patients' baseline NLR with their clinical outcomes. The 69 patients were categorized into two subgroups according to baseline NLR with a cutoff ratio of 4: NLR‐high (NLR‐H, 47.8%, 33/69) and NLR‐low (NLR‐L, 52.2%, 36/69). The response to ICI treatment and survival of NLR‐H patients was significantly worse than that of NLR‐L patients (ORR: 9.1% vs. 36.1%, p = 0.018; mPFS 1.8 vs. 3.2 months, HR 1.79, p = 0.026; mOS 7.4 vs. 11.0 months, HR 2.28, p = 0.008) (Figure 1a–c, Table 2).
FIGURE 1.
The impact of NLR on clinical outcomes. (a) The swim plot of time to progression of ITT patients during camrelizumab treatment (N = 69). (b) The PFS of ITT patients stratified by baseline NLR ≥4 or <4 (N = 69). (c) The OS of ITT patients stratified by baseline NLR ≥4 or <4 (N = 69). (d) The PFS of patients stratified by NLR ≥4 or <4 at first tumor evaluation (N = 64). (e) The OS of patients stratified by NLR ≥4 or <4 at first tumor evaluation (N = 64). (f) The variation of NLR from baseline to the first evaluation stratified by different responses. (g) The dynamic variation of NLR in patients with SD at first evaluation. BL, baseline; CI, confidence interval; CR, complete response; HR, hazard ratio; ITT, intent‐to‐treat; NLR, neutrophil to lymphocyte ratio; OS, median overall survival; PD, disease progression; PFS, median progression‐free survival; PR, partial response; SD, stable disease; V1, first tumor evaluation
We then investigated if on‐treatment changes in NLR correlated with clinical outcomes. In the 64 patients with tumor evaluation, the median NLR at first evaluation (V1‐NLR) was 3.9 (IQR 2.6–6.3), and 32/64 (50%) of the patients were V1‐NLR‐H with a cutoff value of 4. The PFS and OS were both significantly shorter in the V1‐NLR‐H subgroup (mPFS 1.8 vs. 3.6 months, HR 1.74, p = 0.037; mOS 7.4 vs. 11.5 months, HR 2.38, p = 0.007), and the response rate of patients in the V1‐NLR‐H subgroup was lower than that of the V1‐NLR‐L subgroup (Figure 1d,e and Table 2). A total of 19/64 (29.7%) patients had a pronounced decrease (≥20%) in NLR comprared with baseline, and these patients had significantly better OS compared with patients having an increase or only a modest decrease (<20%) of NLR (mOS 14.0 vs. 7.9 months, HR 0.54, p = 0.038) (Table 2). In addition, in the 32 patients with PD, the median NLR significantly increased from 4.3 (IQR 2.6–6.6) at baseline to 4.6 (IQR 2.9–6.3) (p = 0.039) at the first evaluation, and in the 23 patients who experienced SD at first evaluation, we observed a smooth fluctuation in those proceeding to response yet an obvious increase in those proceeding to progression (Figure 1f,g and Tables S1 and S2).
Impact of HLA‐I genotyping on survival and response
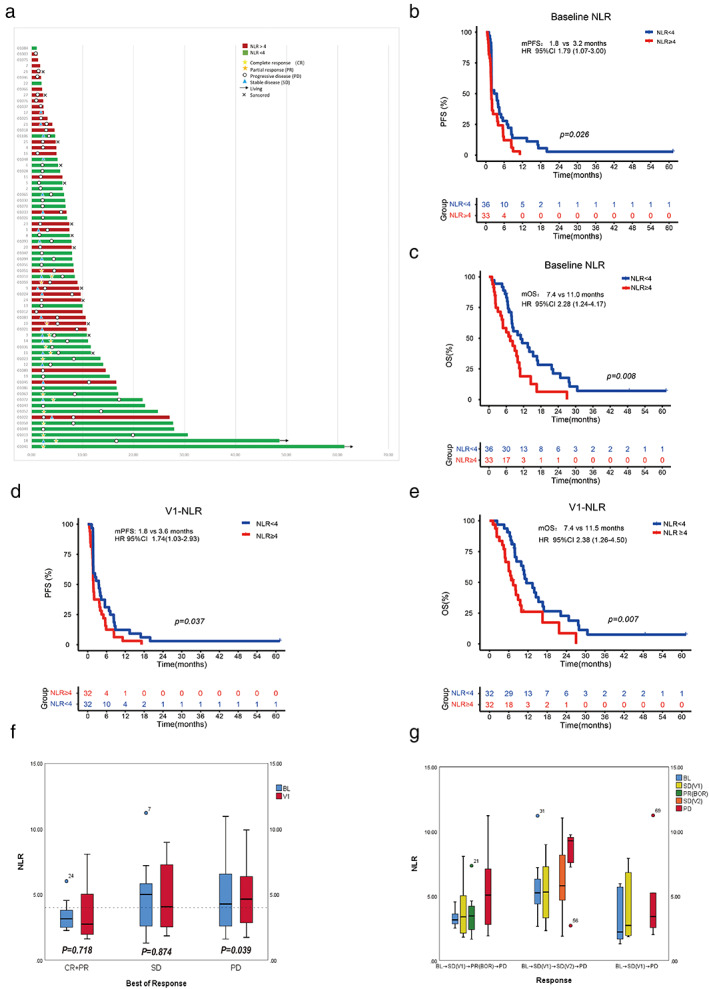
Homozygosity of HLA‐I was present in 11/69 (15.9%) patients, and 58/69 (84.1%) patients had HLA‐I heterozygosity. Eleven patients were HLA‐I homozygous, including five patients with HLA‐A homozygous, three patients with HLA‐B homozygous, and six patients with HLA‐C homozygous. Among them, one patient was homozygous for all three A, B, C loci, and another one patient was homozygous both in B and C loci. The ORR and DCR of patients with HLA‐I homozygosity were significantly lower than those with HLA‐I heterozygosity (ORR 0 vs. 27.6%, p = 0.056; DCR 9.1% vs. 53.4%, p = 0.018). Of note, no patients with HLA‐I homozygosity responded to the treatment. The PFS and OS were significantly shorter in patients with HLA‐I homozygosity (mPFS 1.8 vs. 2.4 months, HR 3.37, p = 0.010; mOS 5.6 vs. 10.5 months, HR 3.97, p = 0.004) (Figure 2a,b and Table 2).
FIGURE 2.
The impact of HLA‐I genotyping on clinical outcomes. (a) The PFS of ITT patients stratified by HLA‐I genotyping (N = 69). (b) The OS of ITT patients stratified by HLA‐I genotyping (N = 69). (c) The distribution of HLA‐I supertype in ITT patients (N = 69). CI, confidence interval; hetero, heterozygosity; HLA‐I, human leukocyte antigen class I; homo, homozygosity; HR, hazard ratio; ITT, intent‐to‐treat; mOS, median overall survival; mPFS, median progression‐free survival
In the 69 patients, the most common HLA‐I supertypes were A02 (53.6%), A03 (52.2%), B07 (40.6%) and B44 (39.1%) (Figure 2c). Among most supertypes, no significant differences were observed in patient response and survival, except that HLA‐I supertype B27 was associated with a significant longer OS (B27 vs. other supertypes: mOS 16.9 vs. 8.1 months, HR 0.53, p = 0.046) (Table S3). We further investigated the clinicopathological characteristics between patients with HLA‐I supertype B27 and patients with other HLA‐1 supertypes; however, no significant differences were observed (Table S4).
Impact of PD‐L1 expression on survival and response
A total of 64/69 (92.7%) patients provided adequate tumor tissues for PD‐L1 testing, and 22/69 (31.9%) had a PD‐L1 TPS ≥10%. The response rates and the mPFS was superior in the PD‐L1 TPS ≥10% subgroup (TPS ≥10% vs. <10%: ORR 36.4% vs. 14.3%, p = 0.042; DCR 63.6% vs. 33.3%, p = 0.020; mPFS 4.0 vs. 1.8 months, HR 0.47, p = 0.004) while the mOS did not exhibit significant differences (TPS ≥10% vs. <10% mOS: 8.3 vs. 9.6 months, HR 0.96, p = 0.883) (Table 2).
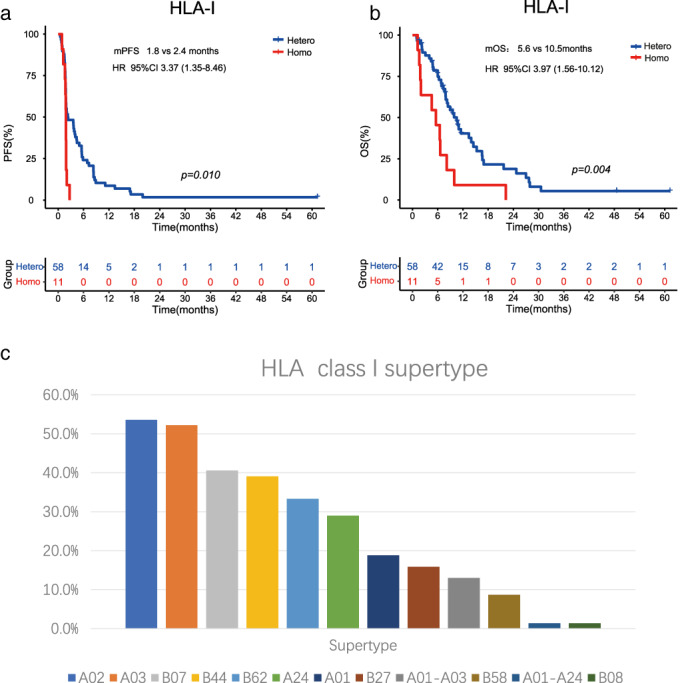
In patients with PD‐L1 TPS <10%, we found that baseline NLR‐H was significantly associated with poor OS (7.4 vs. 11.0 months, HR 2.45, p = 0.032, Figure 3a) compared with NLR‐L, while in patients with PD‐L1 TPS ≥10%, we observed a similar association between NLR‐H and poor clinical outcomes, although no statistical significance was observed.
FIGURE 3.
The impact of biomarker combination on clinical outcomes. (a) The OS of patients with PD‐L1 TPS <10% stratified by baseline NLR ≥4 or <4 (N = 42). (b) The PFS of patients with PD‐L1 TPS ≥10% stratified by HLA‐I genotyping (N = 22). (c) The OS of patients with PD‐L1 TPS ≥10% stratified by HLA‐I genotyping (N = 22). (d) The PFS of ITT patients stratified by the combination of baseline NLR (N = 69). (e) The OS of ITT patients stratified by the combination of baseline NLR (N = 69). (f) The Venn diagram of different combination of biomarkers and the clinical outcomes stratified by the number of favorable factors. CI, confidence interval; HLA‐I, human leukocyte antigen class I; HR, hazard ratio; ITT, intent‐to‐treat; mOS, median overall survival; mPFS, median progression‐free survival; NLR, neutrophil to lymphocyte ratio; PD‐L1, programmed death receptor ligand 1; TPS, tumor proportion score
In patients with PD‐L1 ≥10%, we found statistically significant differences in DCR, PFS and OS between patients with HLA‐I homozygosity and heterozygosity (DCR 0 vs. 77.8%, p = 0.010; mPFS 1.8 vs. 4.7 months, HR 12.6, p = 0.009; mOS 3.8 vs. 9.8 months, HR 11.53, p = 0.010), while in the subgroup with PD‐L1 TPS <10%, the patients with HLA‐I homozygosity all had worse response rates and survival outcomes compared to those with HLA‐I heterozygosity, although no statistical significance was observed (Figure 3b,c, Table 2).
Impact of biomarker combination on survival and response
We subsequently combined NLR status and HLA‐I genotyping for analysis, and found a significant improvement in clinical outcomes consecutively among these four groups: Group A (HLA‐I homozygosity and NLR‐H) versus Group B (HLA‐I homozygosity and NLR‐L) versus Group C (HLA‐I heterozygosity and NLR‐H) versus Group D (HLA‐I heterozygosity and NLR‐H) (ORR 0 vs. 0 vs. 10.3% vs. 44.8%, p = 0.005; DCR 0 vs. 14.3% vs. 44.8% vs. 62.1%, p = 0.023; mPFS 1.4 vs. 1.8 vs. 1.8 vs. 4.1 months, p < 0.001; mOS 1.8 vs. 6.5 vs. 8.2 vs. 13.4, p < 0.001) (Table 2, Figure 3d,e).
We also explored the association of clinical outcomes with the number of favorable factors (baseline NLR‐L, HLA‐I heterozygosity, PD‐L1 TPS ≥10%), and the results suggested that patients with more favorable factors had better response and survival (Figure 3f, Table S5). Notably, in the 12 patients with three favorable factors, 58.3% (7/12) of them responded to the treatment, and a total of 83.3% (10/12) experienced disease control. The mPFS was 5.0 months, and the mOS was 11.5 months, obviously longer than other patients with 0, 1 or 2 favorable factors.
Univariate and multivariate analysis
The univariate analysis on responses demonstrated that the baseline NLR‐H and PD‐L1 TPS <10% were significantly correlated with a lower ORR. Under multivariate analysis, baseline NLR‐H was an independent predictor of inferior ORR. The univariate analysis on survival demonstrated a worse ECOG score, nonsmokers, no previous surgery, HLA‐I homozygosity, NLR‐H at baseline, NLR‐H at first evaluation and PD‐L1 TPS <10% significantly correlated with a shorter PFS; and no previous surgery, ≥2 lines of prior chemotherapy, HLA‐I homozygosity, NLR‐H at baseline, NLR‐H at first evaluation correlated with a shorter OS. Under multivariate analysis, baseline NLR status was the independent predictor of OS, and HLA‐I genotyping was the independent predictor of both PFS and OS (Table 3)
TABLE 3.
The univariate and multivariate analysis of ORR, DCR, PFS and OS
| Variable | Objective response rate | Disease control rate | Progression‐free survival | Overall survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| Age (years) | 1.06 (0.97–1.16) | 0.228 | 1.03 (0.95–1.11) | 0.499 | 0.97 (0.94–1.00) | 0.075 | 0.98 (0.94–1.02) | 0.263 | ||||||||
| ECOG PS (1 vs 0) | 4.24 (0.87–20.64) | 0.111 | 1.06 (0.38–2.92) | 0.916 | 2.35 (1.26–4.41) | 0.007 | 1.45 (0.81–2.61) | 0.211 | 1.68 (0.83–3.4) | 0.150 | ||||||
| Smoking (yes vs no) | 2.05 (0.51–8.15) | 0.474 | 5.33 (1.55–18.3) | 0.011 | 4.98 (1.20–20.7) | 0.027 | 0.53 (0.28–0.99) | 0.046 | 0.65 (0.34–1.22) | 0.181 | 1.01 (0.55–1.86) | 0.980 | ||||
| Gender (male vs. female) | 1.22 (0.13–11.81) | 1.000 | 0.55 (0.09–3.53) | 0.866 | 1.41 (0.57–3.48) | 0.454 | 1.16 (0.44–3.07) | 0.763 | ||||||||
| Disease stage (locally advanced vs. metastatic) | 1.70 (0.14–20.07) | 0.674 | 0.57 (0.05–6.53) | 0.647 | 0.82 (0.23–2.94) | 0.767 | 0.89 (0.19–3.93) | 0.850 | ||||||||
| Previous radiotherapy (yes vs. no) | 0.56 (0.18–1.75) | 0.313 | 1.56 (0.56–4.30) | 0.393 | 0.95 (0.58–1.60) | 0.857 | 1.03 (0.59–1.82) | 0.908 | ||||||||
| Previous surgery (yes vs. no) | 2.98 (0.94–9.49) | 0.058 | 1.50 (0.37–5.97) | 0.569 | 3.04 (1.13–8.20) | 0.026 | 1.32 (0.39–4.44) | 0.659 | 0.57 (0.35–0.94) | 0.029 | 0.94 (0.53–1.65) | 0.823 | 0.52 (0.29–0.92) | 0.026 | 0.58 (0.32–1.04) | 0.069 |
| Prior chemo‐lines (0–1 vs ≥2) | 1.97 (0.56–6.93) | 0.441 | 1.16 (0.43–3.12) | 0.765 | 0.86 (0.51–1.45) | 0.577 | 0.49 (0.27–0.88) | 0.018 | 1.31 (0.69–2.46) | 0.410 | ||||||
| PD‐L1 TPS (≥vs. <10%) | 3.43 (1.01–11.68) | 0.042 | 2.83 (0.71–11.32) | 0.142 | 3.5 (1.19–10.31) | 0.020 | 5.3 (1.4–19.35) | 0.014 | 0.47 (0.28–0.79) | 0.004 | 0.44 (0.26–0.77) | 0.004 | 0.96 (0.54–1.71) | 0.883 | ||
| Baseline NLR (≥4 vs.<4) | 0.18 (0.05–0.70) | 0.018 | 0.21 (0.05–0.86) | 0.030 | 0.58 (0.22–1.51) | 0.265 | 1.79 (1.07–3.00) | 0.026 | 1.69 (0.97–2.94) | 0.065 | 2.28 (1.24–4.17) | 0.008 | 2.50 (1.33–4.68) | 0.004 | ||
| HLA‐I (Homo vs. Hetero) | Not calculated a | 0.056 | Not calculated a | 0.999 | 0.09 (0.01–0.73) | 0.018 | 0.06 (0.01–0.64) | 0.020 | 3.37 (1.35–8.46) | 0.010 | 2.48 (1.20–5.14) | 0.015 | 3.97 (1.56–10.12) | 0.004 | 2.56 (1.26–5.22) | 0.009 |
Abbreviations: CI, confidence interval; DCR, disease control rate; ECOG PS, Eastern Cooperative Oncology Group performance status; HLA‐I, human leukocyte antigen class I; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; OR, odds ratio; ORR, overall response rate; OS, overall survival; PD‐L1, programmed death receptor ligand 1; PFS, progression‐free survival; TPS, tumor proportion score.
Not calculated:: OR could not be calculated when the objective response rate was 0% in patients with poor factors.
DISCUSSION
In this study, we unveiled that the baseline NLR and HLA‐I genotyping were associated with clinical outcomes of the advanced ESCC patients receiving camrelizumab monotherapy. To the best of our knowledge, this is the largest sample size study to evaluate the predictive and prognostic value of NLR in advanced ESCC patients treated with a single‐agent ICI. This is also the first study to explore the influence of HLA‐I genotyping in ESCC patients treated with ICI.
The exploration of novel and favorable biomarkers of immunotherapy in ESCC has constantly been investigated due to imperfections of existing biomarkers, 30 , 31 , 32 , 33 , 34 , 35 such as the high‐quality technical demands and tissue sample requirements for the analysis of tumor mutational burden (TMB), 32 the low incidence of microsatellite instability‐high (MSI‐H) among esophageal cancer patients, 33 the inconvenience in performing T cell‐inflamed gene‐expression profile (GEP) in routine clinical practice, 34 and the insufficient evidence with immune checkpoint coexpression. 35 PD‐L1 expression is a commonly used biomarker in patients treated with ICIs; however, its predictive value in ESCC patients remains controversial. 8 , 9 , 10 In our study, and the phase 3 ESCORT trial, higher PD‐L1 TPS was associated with higher response rates but not with prolonged OS. Together with the different anti‐PD‐L1 assays and scoring systems (TPS, CPS, etc.) utilized across different clinical trials, PD‐L1 expression is important as a biomarker, but far from adequate.
As a convenient and universal index, much attention has been drawn towards the predictive and prognostic value of NLR under immunotherapy which has been attributed to its ability to reflect an inflammatory microenvironment. Our study demonstrated that in patients with advanced ESCC treated with camrelizumab, high baseline NLR was associated with poor clinical outcomes regardless of PD‐L1 expression, suggesting NLR as a promising biomarker and that it could be supplementary to PD‐L1 expression in predicting benefit of immunotherapy in ESCC; in addition, the increase or a modest decrease of NLR from baseline to the first imaging assessment would reflect a worse OS. Moreover, the continuous monitoring of NLR also exhibited potentiality in predicting clinical outcomes and might help identify pseudoprogression.
Some key differences between our present study and previous studies on NLR in ESCC under immunotherapy should be highlighted. 18 , 19 , 20 , 21 First, our study reported the most comprehensive clinical outcomes, exhibiting significant differences in ORR, PFS and OS, while two of the four previous studies failed to exhibit the association between NLR (or derived NLR) with PFS, and one study did not analyze the results of OS and responses. Second, the cutoff values of NLR were different. The determination of our cutoff value was referenced by the median number and previous studies. 28 , 29 Third, the first imaging assessment was chosen as the timepoint to quantify the changes in NLR relative to baseline, instead of a fixed timepoint (3 or 6 weeks as previously‐reported), attributing to an easier real world compliance. Fourth, our study, for the first time, analyzed the impact of NLR in ESCC patients with different PD‐L1 expression levels. Lastly and most importantly, data from our study were of high quality and reliability which can be attributed to the fact that all patients with advanced ESCC were consecutively included from two prospective clinical trials without selection bias, and were all treated under the same single‐agent ICI with strict management and complete follow‐up.
Our analyses of HLA‐I genotyping in ESCC revealed an association between HLA‐I genotyping and clinical outcomes. We observed a significantly shorter OS in patients with HLA‐I homozygosity, consistent with the findings by Chowell et al. in patients with melanoma. 23 In addition, we demonstrated an inferior PFS, ORR and DCR in patients with HLA‐I homozygosity, which has not been comprehensively analyzed and reported in previous studies. 23 , 24 , 25 , 26 Supertype analysis found that supertype B27 might have an impact on OS without significant interactions with other clinicopathological features, suggesting the peptides presented by HLA‐I B27 might be important in ESCC, and this hypothesis warrants further verification. Also, HLA‐I homozygosity led to worse clinical outcomes regardless of PD‐L1 status, while the differences were especially remarkable in the subgroup with PD‐L1 TPS ≥10%. Therefore, HLA‐I homozygosity may have a profound impact on survival among patients with PD‐L1 TPS ≥10%, when treated with ICIs, which is consistent with the findings of Abed et al. 25
Due to the imperfection of all single biomarkers, the refined combination of biomarkers has become progressively important. The combination of NLR and HLA‐I may help to identify more patients who could benefit from immunotherapy. When PD‐L1 status was also taken into consideration, we were able to demonstrate distinctive clinical outcomes in patients with 3, 2, 1 or 0 favorable factors. Notably, only two patients with baseline NLR‐L, HLA‐heterozygosity, and PD‐L1 ≥10% presented with disease progression, and this might be explained by other underlying immune escape mechanisms we did not measure.
Our study had several limitations. First, this was a retrospective analysis with a relatively limited sample size, the statistical inferences were not robust and the results were preliminary. Second, we did not set control groups of other therapeutic strategies, such as chemotherapy or placebo to verify if these biomarkers were valid only in the context of immunotherapy. Third, other factors which might influence the clinical outcomes under immunotherapy were not analyzed, such as the TMB, MSI status, HLA‐I LOH status, etc. To make strong conclusions on the relationship between these biomarkers and clinical outcomes, a randomized, well‐controlled, prospective clinical study should be undertaken.
In conclusion, our study demonstrated that high baseline NLR and HLA‐I homozygosity correlated with worse clinical outcomes in patients with advanced ESCC under camrelizumab monotherapy, independent of PD‐L1 status, suggesting an intricate interaction of systemic inflammation and individual genotype with antitumor immunity in patients, in addition to alterations within the tumor microenvironment. A rational combination of biomarkers based on the results from our study could be established to select patients most likely to benefit from ICI treatment, and which warrant further refinement in the future.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
Supporting information
Appendix S1 Supporting information
Wang L, Zhu Y, Zhang B, Wang X, Mo H, Jiao Y, et al. Prognostic and predictive impact of neutrophil‐to‐lymphocyte ratio and HLA‐I genotyping in advanced esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitor monotherapy. Thorac Cancer. 2022;13(11):1631–1641. 10.1111/1759-7714.14431
Contributor Information
Jiachen Xu, Email: xujc@cicams.ac.cn.
Jing Huang, Email: huangjingwg@163.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Chen WW, Lin CC, Huang TC, Cheng AL, Yeh KH, Hsu CH. Prognostic factors of metastatic or recurrent esophageal squamous cell carcinoma in patients receiving three‐drug combination chemotherapy. Anticancer Res. 2013;33:4123–8. [PubMed] [Google Scholar]
- 3. Guo JC, Huang TC, Lin CC, Hsieh MS, Chang CH, Huang PM, et al. Postchemoradiotherapy pathologic stage classified by the American joint committee on the cancer staging system predicts prognosis of patients with locally advanced esophageal squamous cell carcinoma. J Thorac Oncol. 2015;10:1481–9. [DOI] [PubMed] [Google Scholar]
- 4. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–96. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 7. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five‐year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE‐001. Ann Oncol. 2019;30:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second‐line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open‐label, phase 3 study. Lancet Oncol. 2020;21:832–42. [DOI] [PubMed] [Google Scholar]
- 9. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:1506–17. [DOI] [PubMed] [Google Scholar]
- 10. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE‐181 study of Pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48. [DOI] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 12. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid‐derived suppressor cells. Semin Immunol. 2016;28:187–96. [DOI] [PubMed] [Google Scholar]
- 13. Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer. 2017;106:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil‐to‐lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan X, Wang D, Zhang W, Liu J, Liu C, Li Q, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti‐PD‐1 therapy. Front Cell Dev Biol. 2021;9:638312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lalani AA, Xie W, Martini DJ, et al. Change in neutrophil‐to‐lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo JC, Lin CC, Lin CY, et al. Neutrophil‐to‐lymphocyte ratio and use of antibiotics associated with prognosis in esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitors. Anticancer Res. 2019;39:5675–82. [DOI] [PubMed] [Google Scholar]
- 19. Wu X, Han R, Zhong Y, Weng N, Zhang A. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Cancer Cell Int. 2021;21:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JH, Ahn B, Hong SM, Jung HY, Kim DH, Choi KD, et al. Real‐world efficacy data and predictive clinical parameters for treatment outcomes in advanced esophageal squamous cell carcinoma treated with immune checkpoint inhibitors. Cancer Res Treat. 2022;54(2):505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S, Zou J, Liu C, Jiao X, Gong J, Li J, et al. Baseline derived neutrophil‐to‐lymphocyte ratio as a prognostic biomarker for non‐colorectal gastrointestinal cancer patients treated with immune checkpoint blockade. Clin Immunol. 2020;212:108345. [DOI] [PubMed] [Google Scholar]
- 22. Arasanz H, Gato‐Cañas M, Zuazo M, Ibañez‐Vea M, Breckpot K, Kochan G, et al. PD1 signal transduction pathways in T cells. Oncotarget. 2017;8:51936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Negrao M, Lam V, Reuben A, Rubin M, Landry L, Roarty E, et al. PD‐L1 expression, tumor mutational burden, and cancer gene mutations are stronger predictors of benefit from immune checkpoint blockade than HLA class I genotype in non‐small cell lung cancer. J Thorac Oncol. 2019;14:1021–31. [DOI] [PubMed] [Google Scholar]
- 25. Abed A, Calapre L, Lo J, Correia S, Bowyer S, Chopra A, et al. Prognostic value of HLA‐I homozygosity in patients with non‐small cell lung cancer treated with single agent immunotherapy. J Immunother Cancer. 2020;8:e001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee D, Park J, Choi H, Gim G, Cho S, Kim L, et al. Association of HLA class I homozygosity with unfavorable clinical outcomes in patients with non‐small cell lung cancer treated with chemo‐immunotherapy or immunotherapy as first‐line therapy. Heliyon. 2021;7:e07916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR‐1210, an anti‐PD‐1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–304. [DOI] [PubMed] [Google Scholar]
- 28. Zer A, Sung MR, Walia P, Khoja L, Maganti M, Labbe C, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD‐1 Axis inhibitors in patients with advanced non‐small‐cell lung cancer. Clin Lung Cancer. 2018;19:426.e1–34.e1. [DOI] [PubMed] [Google Scholar]
- 29. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst. 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 30. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prasad V, Kaestner V, Mailankody S. Cancer drugs approved based on biomarkers and not tumor type‐FDA approval of Pembrolizumab for mismatch repair‐deficient solid cancers. JAMA Oncologia. 2018;4:157–8. [DOI] [PubMed] [Google Scholar]
- 32. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD‐1 inhibition. N Engl J Med. 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability‐high as a predictor for anti‐PD‐1/PD‐L1 immunotherapy efficacy. J Hematol Oncol. 2019;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ott PA, Bang YJ, Piha‐Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T‐cell‐inflamed gene‐expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with Pembrolizumab across 20 cancers: KEYNOTE‐028. J Clin Oncol. 2019;37:318–27. [DOI] [PubMed] [Google Scholar]
- 35. Wang P, Chen Y, Long Q, Li Q, Tian J, Liu T, et al. Increased coexpression of PD‐L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma. J Immunother Cancer. 2021;9:e002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


