Abstract
Current treatments for obesity do not reliably reduce body weight over time. New interventional strategies, including chemogenetics, carry promise based on preclinical animal studies. Here, we focused on the ventral pallidum (VP) due to its clearly established role in eating behavior. Chronic inhibitory or excitatory chemogenetic activation was used to modulate the activity of VP-targeted neurons in rats on an obesogenic diet. Based on studies using acute VP manipulations, we hypothesized that VP inhibition would decrease weight gain, while VP stimulation would increase weight. Instead, both manipulations caused weight gain over time, and in a manner not clearly linked to consumption levels. We theorize that the complex reciprocal feedback between ventral striatal structures and metabolic centers likely underpin our unexpected findings. Regardless, this study suggests that the result of strategies to prevent obesity with chronic neuromodulation could be difficult to predict from prior preclinical studies that have used acute interventions.
Introduction
The prevalence of overweight and obesity (BMI ≥ 30 kg/m2) is increasing at a startling rate despite current treatment efforts, and is considered to be a global epidemic by the World Health Organization [1]. Up to 57.8% of the global adult population is predicted to become overweight or obese by 2030 [2]. Current treatments (e.g., lifestyle interventions, pharmacotherapy and surgical interventions) all suffer from the achieved weight loss not being sustained over the long term in many patients [3–5]. Because of the established morbidity associated with obesity (e.g., metabolic, endocrine and cardiovascular diseases) [6] there is a pressing need to find new and effective treatments [60].
Basal metabolic rate and behavioral actions (e.g., diet and exercise) are key factors in obesity, and are related to neural processes. Thus, interventions to directly manipulate these underlying neural processes are being actively pursued as potential therapeutic strategies. Networks involving reward and motivational processes (e.g., networks involving ventral striatal regions), and regions underpinning energy homeostatic processes (e.g., the hypothalamus and brainstem), have received particular attention. Manipulations of these areas to curb weight gain have included lesions, pharmacologic modulation, and molecular or electrical stimulation [7–9]. Unfortunately, the manipulation of hypothalamic activity to suppress appetite and/or increase energy expenditure have, like other clinical interventions, proven to be largely ineffective over the long term [10,11]. It has been hypothesized that modulating the mesolimbic reward network could overcome this limitation, and studies using network-based interventions (deep brain stimulation) in preclinical models and patients with obesity have demonstrated potential but with similar findings of time-limited effectiveness and variable effectiveness across individuals [10,12]. In short, long-term treatment options based on brain intervention remain elusive.
The newest generation of neuroscience technologies has allowed access to neural signaling mechanisms at very fine levels of detail, and many have shown promise in the realm of overeating and weight control [13,14, 60]. Among these, perhaps the most promising for human translation is a chemogenetic approach using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) [15]. DREADDs offer a naturalistic means of changing neuronal activity through endogenous intracellular signaling mechanisms and a relative non-invasiveness of delivering receptor ligands systemically once brain expression of the DREADD receptor has occurred [16–18]. As a result it has emerged as a highly attractive method for chronic brain manipulation with translational potential.
In most prior studies, acute chemogenetic manipulation produces a limited time window of effect (hours) and is tested on a behavior, such as an eating bout, that can be assessed acutely [16–18]. In contrast, treatment of chronic brain-based disorders (e.g., obesity and psychiatric illnesses) will likely require ongoing modulation on the order of months to years. However, only recently has research started to investigate the effectiveness of chronic chemogenetic manipulation of brain activity with longitudinal behavioral assessment [19,20]. Here, we report an initial attempt to assess the effects of chronic inhibition or activation of ventral pallidum (VP) targeted neurons.
Specifically, we evaluated the effect of chronic inhibitory or excitatory modulation of VP targeted neurons in rats on an obesogenic diet. The VP was selected as a target due to its critical role within the mesolimbic network in regulating motivation, effort, and hedonic processes [21–23]. Notably, drastic damage to VP activity can completely abolish how wanted and liked a palatable food is, the only such brain area to carry this function to our knowledge [21,24]. More subtle and acute inhibitions of the VP can reduce palatable food consumption, while other VP modulations such as those that stimulate the VP can elevate food consumption [21–23,25–28]. Moreover, DREADD-mediated inhibition of the VP can be effective at reducing motivational attraction to food cues [29] and motivation to seek other rewards [25,30,31]. Thus, the possibility of preventing diet-induced obesity through VP manipulations seemed likely.
Results
Based on the above background, we used an implantable osmotic minipump to deliver clozapine-N-oxide (CNO), a ligand for the DREADD receptor, continuously over ~1 month and monitored food and water consumption along with weight in rats with expression of hM4Di (Gi – inhibitory DREADD), rats with expression of hM3D (Gq – excitatory DREADD), and rats with expression of a control virus targeted to the VP. We hypothesized that chronic activation of inhibitory Gi receptors targeted to the VP would decrease food consumption and weight compared to the control group, while chronic excitatory Gq receptor activation would increase weight and food consumption.
Viral expression
All animals were evaluated for viral expression in the VP. One animal with expression outside the targeted area was excluded from analysis. Thus, analysis was run with group sizes as: 10 animals in Gi Group, 9 animals in Gq Group, and 10 animals in the Control Group. VP expression was defined by the Paxinos and Watson (2006) atlas, with the target coordinates being ventral to anterior commissure and lateral to the substantia innominata and lateral preoptic area but dorsal to magnocellular preoptic nucleus (coordinates above). Figure 1 plots per-rat expression areas with semi-transparent shading, with the areas of most consistent across-rat expression seen as darker (overlaid) coloration. The majority of Gi Group expression was most dense within our area of interest in the VP similar to what we have observed in previously published studies [29,40]. There was some minor/inconsistent spread anterior and medial to the target area (Fig. 1B, left). The Gq Group showed dense and mostly restricted expression in the VP, with some minor dorsal spreading (Fig. 1B, center). The Control Group exhibited more spread outside of the VP target (Fig. 1B, right). Some variance in expression area and spread across rats is to be expected with these procedures. Given that the area of consistent expression was centered in the VP in this study, with other areas receiving inconsistent and generally minor infiltration of the virus, we have confidence that the results reflect perturbation of the VP.
Figure 1. Chronic activation of Gi and Gq in ventral pallidum targeted neurons increases weight gain.
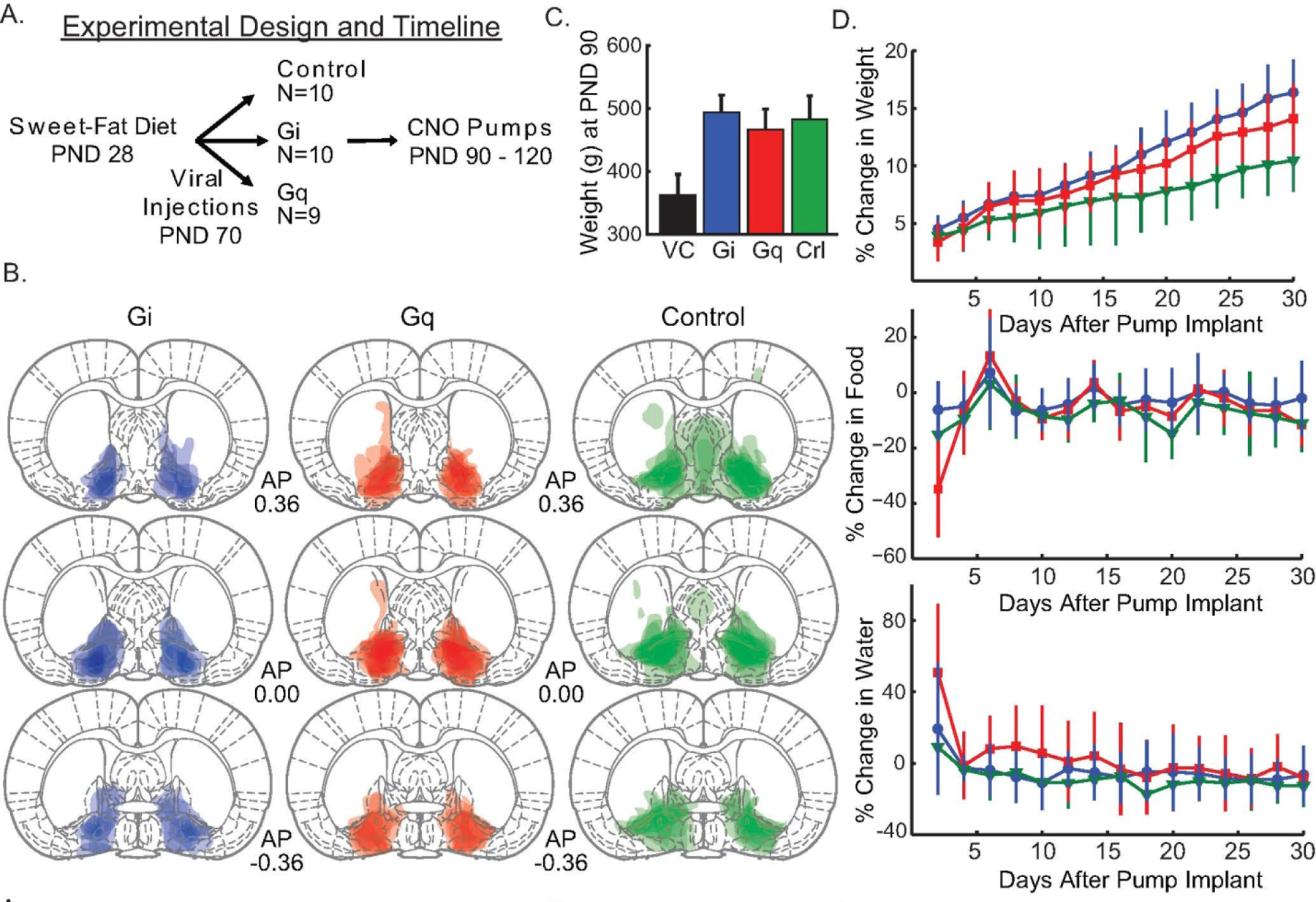
Panel A illustrates the experimental design and timeline. B. The expression of the fluorescent marker mCherry/eYFP for each animal is overlaid in three representative sections along the A-P axis (Bregma: 0.36, 0.00, −0.36) with a separate column and color for each group (Gi - Blue, Gq - Red, control - Green). C. Average weight (grams) of the three cohorts (Gi, Gq, and control [crl]) at postnatal day (PND) 90 ± 1 standard deviation. For comparison the average weight of rats on normal chow at PND 90 from the vendor (VC), Charles River, is shown in black. D. The effect of chronic CNO delivery on weight, food and water consumption is displayed with line color matching that of panel. E. Days are graphed as two-day averages. Error bars = ±1 standard deviation.
Percent change in weight from baseline
Fig. 1C illustrates that all three groups with ad libitum access to the sweet-fat diet had average weights that were both consistent with prior models of obesity at PND 90 [41], and, according to vendor data [42], likely weighed more than age matched rats from the vendor colony that were fed a standard rat chow. Both the Gi Group and the Gq Group differed in weight from the Control Group by PND 120 (pump removal). The effects on weight did not emerge until over a week into treatment, suggesting that the relevant changes in brain activity (in VP and wider circuitry) occurred over an extended time-course. Thus, chronic activation must have altered the network of networks connected to the VP in a different way then what was happening in the first 24–48 hours. In general, animals that ate more tended to weigh more, but water consumption levels did not vary with weight gain. While all animals gained weight over the course of the 30 days, the Gi Group and Gq Group gained w eight at a significantly faster rate than the control group. All groups received CNO.
To reveal this, a linear mixed model was constructed using individual rat percent change in weight from baseline (pre-pump) (%Δ weight; Fig. 1D, top) by fixed effects of Group assignment (Gi, Gq, Control), day (1–30), percent change in food consumption from baseline, and percent change in water consumption from baseline and random effects of slope (i.e. growth curves) and intercept (i.e. individual y-intercepts). There was a significant Group Gi by day interaction (est: 0.161 %Δ weight; CI: 0.06–0.25; SE: 0.049; p = 0.003). A similar difference was found for Group Gq, as the interaction between Group Gq and Group Control by day was significant (est: 0.112 %Δ weight; CI: 0.00–0.21; SE: 0.050; p = 0.033).
There was a significant main effect of day (est: 0.254 %Δ weight; CI: 0.19–0.33; SE: 0.035; p < 0.001) indicating that all animals displayed weight increases by the end of the study. A significant main effect of food consumption (est: 0.014 %Δ weight; CI: 0.01–0.02; SE: 0.003; p < 0.001) shows that animals who weighed more tended to eat more. However, percent change in weight did not vary in accordance with percent change in water consumption (est: −0.002 %Δ weight; CI: −0.01–0.00; SE: 0.003; p = 0.585), suggesting that there were not weight-associated differences in water drinking. The percent change in weight from baseline took time to diverge between the Gi and Gq Groups and the Control Group. Therefore, it was not surprising that neither the Gi Group (est: −0.216 %Δ weight; CI: −1.94–1.64; SE: 0.967; p = 0.825) nor the Gq group (est: −0.184 %Δ weight; CI: −1.84–1.87; SE: 0.993; p = 0.854) differed from the Control Group in percent change in weight measures (on average) across the 30 days.
We additionally broke down weight change data by virus serotype. The Gi group with the AAV5 serotype exhibited the greatest weight gain, but the AAV8 GI group gained more weight than controls as well. Specific weight gain percentages from the first day to the last day were: Control-AAV5: 10.62% weight gain; Gi-AAV5: 17.40% weight gain; Gi-AAV8: 13.54% weight gain; Gq-AAV8: 13.90% weight gain.
Percent change in food consumption
Even though the Gi and Gq Groups gained weight faster, the Groups did not differ in their food consumption compared to controls. Moreover, all animals lessened their overall daily consumption by the end of the study. Animals who ate more tended to drink more and weigh slightly more, though there was no effect of VP perturbation on this relationship. Thus, the elevated weight gain in Gi-and Gq-treated animals was not related to an elevation in the amount of food that they ate. The rats in the Gi Group did not display differences from rats in the Control Group in the amount of food consumed relative to baseline amounts over time as indicated by a non-significant group by day interaction (est: − 0.102 %Δ food; CI: −0.46–0.24; SE: 0.174; p = 0.905). The Gq Group also did not differ in consumption from Controls over time as seen in a non-significant group by day interaction (est: −0.175 %Δ food; CI: −0.58–0.17; SE: 0.173; p = 0.429). Further, on average across the 30 days, neither the Gi Group (est: 2.032 %Δ food; CI: −6.41–10.5; SE: 4.363; p = 0.645) nor the Gq Group (est: 2.185 %Δ food; CI: −7.05–11.6; SE: 4.450; p = 0.627) differed from the Control Group in the relative amount of food consumed compared to baseline.
A significant main effect of day (est: −0.456 %Δ food; CI: −0.75-(−0.15); SE: 0.142; p = 0.002) showed that all animals exhibited a relative decrease in food consumption compared to baseline by the end of the study. There was a significant main effect of weight (est: 1.248 %Δ food; CI: 0.64–1.86; SE: 0.302; p < 0.001), indicating that on days when animals had a larger percent change in weight, they also had corresponding relative changes in food consumption independent of Group. This correlation was also observed with water consumption (est: 0.125 %Δ food; CI: 0.06–0.19; SE: 0.031; p < 0.001), indicating that from day-to-day rats had changes in water consumption that related to changes in food consumption independent of Group.
Percent change in water consumption
Rats from all three Groups tended to drink less over time and water consumption did not fluctuate with weight gain. Again, however, water consumption and food consumption were related as animals tended to drink more if they ate more. The interaction between the Gi Group and Control Group by day was not significant (est: 0.126 %Δ water; CI: −0.27–0.56; SE: 0.236; p = 0.596). In addition, on average across the 30 days, the Gi Group did not significantly differ from the Control Group in relative water consumption levels compared to baseline (est: 0.713 %Δ water; CI: −12.6–12.9; SE: 7.28; p = 0.923). The interaction between the Gq Group and Control Group by day was significant (est: −0.589 %Δ water; CI: −1.04-(−0.14); SE: 0.236; p = 0.018), with Gq animals drinking less over time. However, on average across the 30 days, the Gq Group significantly differed from the Control Group in overall water consumption (est: 19.5 %Δ water; CI: 5.77–33.7; SE: 7.42; p = 0.014). Thus, the Gq Group had increased water consumption relative to baseline compared to the Control Group when averaged across the entire 30 days, despite a lot of variation in the data. However, the Gq Group tended to drink less as time went on compared to Controls in contrast to the time-course of relative weight change.
Daily water consumption had decreased relative to baseline consumption by the end of study as seen in a significant main effect of day (est: −0.391 %Δ water; CI: −0.72-(−0.01); SE: 0.192; p = 0.047). Water consumption did not fluctuate with weight gain as suggested by a non-significant main effect of weight (est: −0.012 %Δ water; CI: −0.83–0.79; SE: 0.402; p = 0.977). Food and water consumption were related with a significant main effect of food (est: 0.170 %Δ water; CI: 0.09–0.26; SE: 0.043; p < 0.001).
Discussion
The goal of this study was to evaluate the effects of chronic DREADD-based modulation of the VP on diet-induced weight gain. Our results indicate all animals gain weight over time when provided with a high-fat, high-sugar diet consistent with previous findings [34], but animals who received chronic inhibitory or excitatory modulation via DREADD receptors targeted to the VP gain weight at a faster rate than the Control Group. This result is not readily explained by food or water consumption differences between the experimental groups and the control group. The observed differences in weight trajectories that emerged without changes in water or caloric intake has been previously reported when chronic deep brain stimulation was targeted to either the nucleus accumbens [43] or the lateral hypothalamus [44] in models of obesity.
It was surprising that putatively inhibiting VP activity (with Gi DREADDs) did not cause reductions in food intake and/or weight because acute inhibition of the VP is effective at reducing rewarding food consumption, hedonic reactions to food, and motivation to procure food [21–23,25]. Unexpectedly, chronic Gq-mediated excitation did not increase food intake, even though acute VP excitation is closely linked to such changes [21,45]. Thus there were differences between the control group without DREADDs receiving CNO and those with DREADDs receiving CNO, meaning that the DREADD expression plus CNO, and not CNO alone, was responsible for altering weight gain without affecting food intake. While the cited acute manipulations involved behaviors with food rewards, they did not look at ad libitum access or weight over time; therefore, direct comparisons of acute and chronic DREADD manipulation cannot be made. Similarly, owing to the use of male subjects in this study, future work is required to parse out the acute and chronic effects of VP manipulations on weight regulation in the female population. In general, while our data is not ideal for making claims about differences between acute and chronic DREADD manipulations it is surprising that food consumption was not altered in either direction given the prior acute studies. Overall, this highlights the need for careful pre-clinical exploration of chronic brain manipulation where the premise may be based on acute interventions.
Our attempted intervention using chronic manipulation of the VP used the same DREADD receptors and CNO ligand that were used in previous acute DREADD manipulations that altered VP activity, changed food seeking and eating behavior [16–18]. It is possible that chronic activation caused unexpected changes in DREADD receptor function, CNO efficacy at the DREADD receptors over long periods of administration, CNO metabolism, and/or receptor/intracellular signaling adaptations to chronic agonist activity [46]. Of note, there is a potential for clozapine, CNO’s back-converted parent, to have contributed to results [47]. However, in rats of either sex, clozapine administration at relatively high doses in rats does not appear to affect weight gain, suggesting clozapine itself is unlikely to explain our effects [58]. Still, we allow for the possibility of an interaction between clozapine presence and DREADD-related neuronal manipulations to play a role here. Regardless of how the activity of VP targeted neurons was altered, the net result was an increase in weight with no change in caloric intake.
This surprising outcome could be the result of known reciprocal connectivity between regions within the ventral striatum (VP and nucleus accumbens) and the hypothalamus. The VP has both direct connections with the lateral hypothalamus [48], and indirect connections by way of the nucleus accumbens shell [49–51] that also has connection with the lateral hypothalamus [52,53]. Given the strong connectivity between the ventral striatal structures and the hypothalamus it is not surprising that chronic manipulation (deep brain stimulation - DBS) of either regions within the ventral striatum or the hypothalamus can produce similar effects on metabolism or motivated behavior. DBS of the nucleus accumbens shell or the lateral hypothalamus have both produced weight changes in rodent models of obesity without behavioral effects on food or water intake [43,44]. Moreover, Diepenbroek et al. showed that DBS of the nucleus accumbens shell could produce changes in peripheral glucose metabolism [54]. Both human ventral striatal DBS in a patient with obsessive compulsive disorder and optogenetic activation of dopamine D1 receptor neurons in nucleus accumbens of mice have been shown to increase glucose tolerance and insulin sensitivity [55]. Manipulation of lateral hypothalamic neuronal populations are known to alter both basal metabolic rate and spontaneous physical activity [56] and could be possible mediators of our DREADD manipulation by altering energy homeostatic processes that produced the observed weight changes. It is possible that more localized, cell type specific, or connectivity specific expression of DREADDS within the VP could produce translationally relevant changes in food consumption and weight [51]. Concerning neuronal type, the VP is a heterogenous structure, for example containing GABAergic projection and neurons and interneurons (some also co-producing enkephalin), corticopetal cholinergic neurons, and excitatory projection neurons that could all function differently for behavior [21, 23]. Cells of particular interest might be the glutamaterigic and ekephalinergic VP populations [62–65]. Clearly future work will need to parse out the contribution of different VP neurons. The promoter that we used was pan-neuronal, meaning that all neurons in the VP could have expressed the DREADD receptor, and the main precedent for this study is the above literature that similarly manipulates the VP, or records from VP neurons, in a similar non-neuronal-type-specific manner. Thus we sought to first test the VP ‘as a whole’ before proceeding with neuron-specific or pathway-specific manipulations.
Several features of the study design limit the breadth of conclusions that can be drawn. There were no cohorts fed a “control” rat chow diet, and prior work has shown that rats with ad libitum access to standard chow also develop obesity [57]. Future evaluation of a lower caloric, less palatable food would provide insight into the influence of food salience on the effect of chronic VP manipulation using DREADDs. Future studies could also explore changes in neuronal firing, DREADD receptor function and distribution, CNO binding or metabolism, intracellular signaling to establish the mechanisms underlying our surprising findings. While viral injections were targeted to the VP, and this was the structure with the highest density of expression, there was expression in surrounding brain regions that could also have contributed to the study outcome. It is important to note that the degree of off target expression within our Gi and Gq cohorts was similar to those observed in prior published studies from our group that have used acute DREADD manipulations [29,40]. Infiltration of the virus into areas like the septal area was very uncommon. Slight spread dorsal to the anterior commissure did occur across animals. However, given that the vast majority of viral expression both within and across animals was in the ventral pallidum, we are confident that the effects of the DREADD manipulations are related to the ventral pallidum. Finally, the exposure of rats to the stress of single housing could have contributed to the diet-induced weight gain that we report (i.e., isolation stress as a factor of weight gain in addition to the diet itself). This might mirror what is seen in clinical populations with the common comorbidity of stress and diet-related obesity [59,60] and potentially strengthen the translational relevance of the data.
To conclude, our results show that attempts to chronically modulate VP activity in rats on an obesogenic diet do not alter food consumption as hypothesized from studies that have used acute manipulations. Instead, chronic VP manipulation, whether inhibitory or excitatory, increased weight gain without changing food or water consumption. Our findings highlight the challenges of translating the findings from acute chemogenetic studies to those involving chronic manipulations of neural circuitry, thereby limiting the translational potential of the findings from acute manipulation studies.
Methods
Subjects
Male Sprague-Dawley rats (N=30) were purchased from Charles River (Shrewsbury, MA) at postnatal day (PND) 28 and individually housed using a 12 hour light/dark schedule with a palatable high-fat, high-sugar diet (“sweet-fat diet”), which contained 19% protein, 36.2% carbohydrates, and 44.8% fat by calories and 4.6 kcal/g (Teklad Diets 06415, South Easton, MA) and water available ad libitum. We used an early exposure to a palatable high calorie diet (starting after weaning from maternal milk) to mirror the US obesity epidemic, in which high-calorie food consumption can emerge in childhood and can lead to a lifetime of treatment resistant obesity [32–35]. Two cohorts of animals were run separately and the data was combined for final analysis. Food, water and weight (all in grams) were manually monitored daily. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised in 1996, and were approved by the Institutional Animal Care and Use Committee at Dartmouth College.
DREADD virus injection
Three groups of animals (N=10 each; PND 70) received bilateral VP viral vector infusions under anesthesia with isoflurane gas using a stereotaxic apparatus (Stoelting, Kiel, WI, USA). Surgery was conducted under aseptic conditions. A 5 µl, 33-gauge beveled needle-tipped syringe (World Precision Instruments, Sarasota, FL, USA) was lowered to the bilateral target sites, in mm: VP −0.12 AP, ± 2.4 ML, − 8.2 DV [36] and allowed to rest for 3 minutes prior to infusion. Viral vectors were infused at a rate of 0.15 µl/min. Dispersion of virus was allowed for 5 minutes post-infusion. The following viruses were used, each given at a total volume of 0.8 μl per hemisphere: 1) inhibitory Gi DREADD (AAV5-hSyn-hM4Di-mCherry; UNC Vector Core; n = 5; AAV8-hSyn-hM4Di-mCherry; Addgene; n = 5); excitatory Gq DREADD (AAV8-hSyn-hM3Gq-mCherry; Addgene; n = 10); and control construct (AAV5-hSyn-EYFP; UNC Vector Core; n = 10). Multiple virus serotypes were required due to supplier limitations. Alzet osmotic pumps (model 2ML4; Durect Corp., Cupertino, CA), were implanted subcutaneously (peri-scapular) at PND 90 to deliver CNO (~6 mg/kg/24 hours, dissolved acetic acid, and brought up to final volume in saline at PH 4) in all groups until removal at PND 120 (Fig. 1A). Animals received ketoprofen (3 mg/kg) following virus injection and pump implant surgery. Pumps were evaluated post-explant. We confirmed in each case that the CNO solution was evacuated from the pumps and that CNO had not precipitated out of solution within the pump.
Histology
After completion of the weight and intake measurement phase, rats were deeply anesthetized with 1–2 ml of Euthasol (phenobarbital) and perfused with 0.9% saline solution for approximately 6–8 min followed by 10% formalin in distilled H20 until fixture of head and neck tissue (approximately 3–4 min). Brain tissue was extracted, saturated with 20% sucrose (in distilled water) and frozen to −80°C. Brains were then sliced to 60 µm thick sections with a microtome, mounted to slides, and cover-slipped with Vectashield mounting medium containing DAPI. Fluorescent expression was imaged using a microscope (Olympus U-HGLGPS). For each brain, the area of neuronal fluorophore expression was manually transcribed onto blank, printed atlas pages [36] and then transcribed digitally via PowerPoint (Microsoft). Per-animal expression maps were then combined into group expression maps by digitally overlaying the expression areas at 34% transparency (Adobe Illustrator).
Weight and Intake Measurement and Analyses
Recordings of animal weights began on PND 28 immediately after weaning and continued through to the end of the study. Viral vector infusions occurred on PND 70 and CNO-containing pump implants were placed on PND 90 and per-animal weights, water consumption, and food consumption was measured for the following 30 days until pump removal at PND 120. Measurements were not taken on occasion, but never exceeded two consecutive days (i.e. there are never instances of 3 days passing without data collection). Linear mixed models (below) are well suited for longitudinal studies with missing observations and uneven measurement intervals [37] and thus were chosen for analysis. Analyses include both fixed effects (independent variables; i.e. group, day, and other covariates) as well as random effects (individual rat growth rates and individual starting values; i.e. slopes and y-intercepts). All dependent measures are reported as percent change (%Δ) from a 3-day averaged baseline that was established before pump implantation and chronic CNO administration.
In order to statistically analyze weight, food consumption and water consumption, a linear mixed model was used. The Group variable had three levels (Control, Gi, and Gq), was dummy coded, and compared each experimental group to the mutual control, such that the Gi Group was compared to the Control Group and the Gq Group was compared to the Control Group. All linear mixed models were fit by maximum likelihood and t-tests used Satterthwaite approximations of degrees of freedom (R; “lmerTest”) [38]. The reported statistics include parameter estimates (β values) in the units of the dependent variable, confidence intervals (CI; 95% bootstrapped confidence intervals around dependent variable), standard error of the parameter estimate (SE), and p-values. All statistical tests were carried out using R [39]. Graphs were created with Matlab (R2017b).
Chronic VP inhibition increases, rather than reduces, diet-induced weight gain
Chronic VP excitation also increases weight gain
Weight gain associated with VP manipulations is unrelated to food consumption level
Acknowledgements
The authors would like to thank Alyssa C. DiLeo and Alex R. Brown for their help with histology. This work was supported by funds from the Department of Psychiatry at the Geisel School of Medicine at Dartmouth (WD), NIH grant 1R01DA044199 (KSS), NSF grant IOS1557987 (KSS), NIH grant 1F31DA050369–01 (EBS), and the Dartmouth Clinical and Translational Science Institute under award number KL2TR001088 from the National Center for Advancing Translational Sciences (NCATS) of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James WP (2008) WHO recognition of the global obesity epidemic. Int J Obes (Lond) 32 Suppl 7: S120–126. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, et al. (2017) Obesity. Nat Rev Dis Primers 3: 17034. [DOI] [PubMed] [Google Scholar]
- 3.Christou NV, Look D, Maclean LD (2006) Weight gain after short-and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg 244: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, et al. (2016) Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 315: 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotthardt JD, Bello NT (2016) Can we win the war on obesity with pharmacotherapy? Expert Rev Clin Pharmacol: 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Heymsfield SB, Wadden TA (2017) Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med 376: 254–266. [DOI] [PubMed] [Google Scholar]
- 7.Teitelbaum P, Epstein AN (1962) The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev 69: 74–90. [DOI] [PubMed] [Google Scholar]
- 8.Anand BK, Brobeck JR (1951) Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med 77: 323–324. [DOI] [PubMed] [Google Scholar]
- 9.Nangunoori RK, Tomycz ND, Oh MY, Whiting DM (2016) Deep Brain Stimulation for Obesity: From a Theoretical Framework to Practical Application. Neural Plast 2016: 7971460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, et al. (2013) Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg 119: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, et al. (2008) Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 63: 119–123. [DOI] [PubMed] [Google Scholar]
- 12.Harat M, Rudas M, Zielinski P, Birska J, Sokal P (2016) Nucleus accumbens stimulation in pathological obesity. Neurol Neurochir Pol 50: 207–210. [DOI] [PubMed] [Google Scholar]
- 13.Krashes MJ, Kravitz AV (2014) Optogenetic and chemogenetic insights into the food addiction hypothesis. Front Behav Neurosci 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmiter RD (2017) Neural Circuits That Suppress Appetite: Targets for Treating Obesity? Obesity (Silver Spring) 25: 1299–1301. [DOI] [PubMed] [Google Scholar]
- 15.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104: 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016) DREADDS: Use and application in behavioral neuroscience. Behav Neurosci 130: 137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth BL (2016) DREADDs for Neuroscientists. Neuron 89: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett CJ, Krashes MJ (2016) Resolving Behavioral Output via Chemogenetic Designer Receptors Exclusively Activated by Designer Drugs. J Neurosci 36: 9268–9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer SM, Vesuna S, Ramakrishnan C, Huynh K, Young S, et al. (2016) Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep 6: 30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S, Munzberg H (2018) Testing Effects of Chronic Chemogenetic Neuronal Stimulation on Energy Balance by Indirect Calorimetry. Bio Protoc 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009) Ventral pallidum roles in reward and motivation. Behav Brain Res 196: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro DC, Cole SL, Berridge KC (2015) Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci 9: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Root DH, Melendez RI, Zaborszky L, Napier TC (2015) The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130: 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cromwell HC, Berridge KC (1993) Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res 624: 1–10. [DOI] [PubMed] [Google Scholar]
- 25.Chang SE, Smedley EB, Stansfield KJ, Stott JJ, Smith KS (2017) Optogenetic Inhibition of Ventral Pallidum Neurons Impairs Context-Driven Salt Seeking. J Neurosci 37: 5670–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, et al. (2018) Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula-Tegmental Circuitry and Constrain Reward Seeking. Biol Psychiatry 83: 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richard JM, Stout N, Acs D, Janak PH (2018) Ventral pallidal encoding of reward-seeking behavior depends on the underlying associative structure. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creed M, Ntamati NR, Chandra R, Lobo MK, Luscher C (2016) Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum. Neuron 92: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang SE, Todd TP, Bucci DJ, Smith KS (2015) Chemogenetic manipulation of ventral pallidal neurons impairs acquisition of sign-tracking in rats. Eur J Neurosci 42: 3105–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, et al. (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad AA, McNally GP (2016) Ventral Pallidum Output Pathways in Context-Induced Reinstatement of Alcohol Seeking. J Neurosci 36: 11716–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West DB, Diaz J, Woods SC (1982) Infant gastrostomy and chronic formula infusion as a technique to overfeed and accelerate weight gain of neonatal rats. J Nutr 112: 1339–1343. [DOI] [PubMed] [Google Scholar]
- 33.Morris MJ, Velkoska E, Cole TJ (2005) Central and peripheral contributions to obesity-associated hypertension: impact of early overnourishment. Exp Physiol 90: 697–702. [DOI] [PubMed] [Google Scholar]
- 34.Hariri N, Thibault L (2010) High-fat diet-induced obesity in animal models. Nutr Res Rev 23: 270–299. [DOI] [PubMed] [Google Scholar]
- 35.Cheng HS, Ton SH, Phang SCW, Tan JBL, Abdul Kadir K (2017) Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J Adv Res 8: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates 6th ed. Amsterdam ; Boston ; Oxford: Academic,. pp. 1 online resource (xxxi p.). [Google Scholar]
- 37.Gibbons RD, Hedeker D, DuToit S (2010) Advances in analysis of longitudinal data. Annu Rev Clin Psychol 6: 79–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuznetsova A, Brockhoff B, Christensen P (2016) lmerTest: test in linear mixed effects models. 2.0–33 ed. pp. R package [Google Scholar]
- 39.R-Core-Team (2016) R: a language and environment for statistical computing R Foundation for Statistical Computing. Vienna, Austria. [Google Scholar]
- 40.Chang SE, Todd TP, Smith KS (2018) Paradoxical accentuation of motivation following accumbens-pallidum disconnection. Neurobiol Learn Mem 149: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furnes MW, Zhao CM, Stenstrom B, Arum CJ, Tommeras K, et al. (2009) Feeding behavior and body weight development: lessons from rats subjected to gastric bypass surgery or high-fat diet. J Physiol Pharmacol 60 Suppl 7: 25–31. [PubMed] [Google Scholar]
- 42.Charles-River-Laboratories (2019). pp. SD Rat Growth Chart [Google Scholar]
- 43.Prinz P, Kobelt P, Scharner S, Goebel-Stengel M, Harnack D, et al. (2017) Deep brain stimulation alters light phase food intake microstructure in rats. J Physiol Pharmacol 68: 345–354. [PubMed] [Google Scholar]
- 44.Sani S, Jobe K, Smith A, Kordower JH, Bakay RA (2007) Deep brain stimulation for treatment of obesity in rats. J Neurosurg 107: 809–813. [DOI] [PubMed] [Google Scholar]
- 45.Stratford TR, Kelley AE, Simansky KJ (1999) Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res 825: 199–203. [DOI] [PubMed] [Google Scholar]
- 46.Mahler SV, Aston-Jones G (2018) CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology 43: 934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, et al. (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faget L, Zell V, Souter E, McPherson A, Ressler R, et al. (2018) Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun 9: 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahm DS, Brog JS (1992) On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50: 751–767. [DOI] [PubMed] [Google Scholar]
- 50.Zahm DS (2000) An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev 24: 85–105. [DOI] [PubMed] [Google Scholar]
- 51.Churchill L, Kalivas PW (1994) A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J Comp Neurol 345: 579–595. [DOI] [PubMed] [Google Scholar]
- 52.Huang XF, Han M, South T, Storlien L (2003) Altered levels of POMC, AgRP and MC4-R mRNA expression in the hypothalamus and other parts of the limbic system of mice prone or resistant to chronic high-energy diet-induced obesity. Brain Res 992: 9–19. [DOI] [PubMed] [Google Scholar]
- 53.Carus-Cadavieco M, Gorbati M, Ye L, Bender F, van der Veldt S, et al. (2017) Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature 542: 232–236. [DOI] [PubMed] [Google Scholar]
- 54.Diepenbroek C, van der Plasse G, Eggels L, Rijnsburger M, Feenstra MG, et al. (2013) Alterations in blood glucose and plasma glucagon concentrations during deep brain stimulation in the shell region of the nucleus accumbens in rats. Front Neurosci 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ter Horst KW, Lammers NM, Trinko R, Opland DM, Figee M, et al. (2018) Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci Transl Med 10. [DOI] [PubMed] [Google Scholar]
- 56.Zink AN, Bunney PE, Holm AA, Billington CJ, Kotz CM (2018) Neuromodulation of orexin neurons reduces diet-induced adiposity. Int J Obes (Lond) 42: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laaksonen KS, Nevalainen TO, Haasio K, Kasanen IH, Nieminen PA, et al. (2013) Food and water intake, growth, and adiposity of Sprague-Dawley rats with diet board for 24 months. Lab Anim 47: 245–256. [DOI] [PubMed] [Google Scholar]
- 58.Choi S, Disilvio B, Unangst J, Fernstrom JD (2007) Effect of chronic infusion of olanzapine and clozapine on food intake and body weight gain in male and female rats. Life Sci 81(12):1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF (2006) Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Eng J Med 355(8):763–78. [DOI] [PubMed] [Google Scholar]
- 60.Preguiça I, Alves A, Nunes S, Fernandes R, Gomes P, Viana SD, Reis F (2020) Diet-induced rodent models of obesity-related metabolic disorders—A guide to a translational perspective. Obes Rev 23(12) e13081. [DOI] [PubMed] [Google Scholar]
- 61.Gendelis S, Inbar D, Inbar K, Mesner S, Kupchik Y (2020). Metaplasticity in the ventral pallidum as a potential marker for the propensity to gain weight in chronic high-calorie diet. J Neurosci 40(50):9725–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farrell M, Esteban JSD, Faget L, Floresco SB, Hnasko TS, Mahler SV (2021) Ventral pallidum GABA mediate motivation underlying risky choice. J Neurosci 41(20):4500–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephenson-Jones M, Bravo-Rivera C, Ahrens S, Furlan A, Xiao X, Fernandes-Henriques C, Li B (2020) Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron 105(5):921–933.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, Yoo JH, Dulcis D, Hnasko TS (2018). Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun 9(1):849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heinsbroek JA, Bobadilla AC, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, Kalivas PW (2020). Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep 30(6):2018–2027.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


