Abstract
Background
In the phase III CASPIAN study, first-line durvalumab in combination with etoposide plus either cisplatin or carboplatin (EP) significantly improved overall survival (OS) versus EP alone in extensive-stage small-cell lung cancer (ES-SCLC). Durvalumab plus tremelimumab plus EP numerically improved OS versus EP, but did not reach statistical significance. Here we report updated OS in censored patients after median follow-up of >3 years.
Patients and methods
805 patients with treatment-naïve ES-SCLC were randomized 1 : 1 : 1 to durvalumab plus EP, durvalumab plus tremelimumab plus EP, or EP. The two primary endpoints were OS for durvalumab plus EP versus EP and for durvalumab plus tremelimumab plus EP versus EP.
Results
As of 22 March 2021 (median follow-up 39.4 months, 86% maturity), durvalumab plus EP continued to demonstrate improved OS versus EP: hazard ratio (HR) 0.71 [95% confidence interval (CI) 0.60-0.86; nominal P = 0.0003]; median OS was 12.9 versus 10.5 months, and 36-month OS rate was 17.6% versus 5.8%. Durvalumab plus tremelimumab plus EP continued to numerically improve OS versus EP: HR 0.81 (95% CI: 0.67-0.97; nominal P = 0.0200); median OS was 10.4 months, and 36-month OS rate was 15.3%. Twenty-seven and nineteen patients in the durvalumab plus EP and durvalumab plus tremelimumab plus EP arms, respectively, remained on durvalumab treatment at data cut-off.
Conclusions
Three times more patients were estimated to be alive at 3 years when treated with durvalumab plus EP versus EP, with the majority still receiving durvalumab at data cut-off, further establishing durvalumab plus EP as first-line standard of care for ES-SCLC.
Key words: durvalumab, tremelimumab, extensive-stage SCLC, CASPIAN, overall survival
Highlights
-
•
Durvalumab plus EP showed sustained OS benefit over EP with a well-tolerated safety profile after median follow-up >3 years.
-
•
Three times more patients were estimated to be alive at 3 years when treated with durvalumab plus EP versus EP alone.
-
•
These results further establish durvalumab plus EP as standard of care for first-line treatment of patients with ES-SCLC.
Introduction
After more than three decades of limited progress in treating extensive-stage small-cell lung cancer (ES-SCLC), the introduction of immune checkpoint inhibitors, particularly the programmed death ligand-1 (PD-L1) inhibitors durvalumab and atezolizumab, has provided significant improvements in overall survival (OS).1,2 Immune checkpoint inhibitors have shown impressive durable survival benefit in non-small-cell lung cancer;3, 4, 5 however, as immunotherapy trials in ES-SCLC are comparatively recent, the extent to which long-term survival can be improved in this setting is not yet established.
The phase III CASPIAN study assessed first-line durvalumab, with or without tremelimumab (anti-cytotoxic T-lymphocyte-associated antigen-4), in combination with etoposide plus either cisplatin or carboplatin (EP), in ES-SCLC. At the planned interim analysis after a median follow-up of 14.2 months [data cut-off (DCO) 11 March 2019; 63% maturity], one of the two primary endpoints was met when durvalumab plus EP significantly improved OS versus EP alone: hazard ratio (HR) 0.73 [95% confidence interval (CI) 0.59-0.91; P = 0.0047].1 At a subsequent analysis after a median follow-up of 25.1 months (DCO 27 January 2020; 82% maturity), OS benefit with durvalumab plus EP versus EP was sustained [HR 0.75 (95% CI 0.62-0.91; nominal P = 0.0032)]; durvalumab plus tremelimumab plus EP numerically improved OS versus EP [HR 0.82 (95% CI 0.68-1.00; P = 0.0451)], but the improvement did not reach statistical significance.6
Here, we report an updated analysis of OS from CASPIAN after >3 years of follow-up.
Methods
Study design
The design for the open-label phase III CASPIAN trial (NCT03043872) has been described previously.1,6 Briefly, patients with treatment-naïve ES-SCLC and World Health Organization (WHO) performance status 0/1 were randomized 1 : 1 : 1 to durvalumab plus EP, durvalumab plus tremelimumab plus EP, or EP. Patients in the immunotherapy arms received four cycles of EP plus durvalumab 1500 mg with or without tremelimumab 75 mg every 3 weeks, followed by maintenance durvalumab 1500 mg every 4 weeks until disease progression. Patients in the durvalumab plus tremelimumab plus EP arm received an additional tremelimumab dose after EP. Patients in the EP arm received up to six cycles of EP and optional prophylactic cranial irradiation. Treatment with immunotherapy beyond progression was permitted if there was evidence of benefit. Survival was assessed every 2 months following treatment discontinuation.
Endpoints and assessments
In this planned exploratory analysis (DCO 22 March 2021), we report long-term follow-up for the two primary endpoints of OS (time from randomization until death from any cause) for durvalumab plus EP versus EP and durvalumab plus tremelimumab plus EP versus EP; OS rates at 12, 18, 24, and 36 months; and OS in patient subgroups defined by prespecified baseline factors. Progression and response data were not collected beyond the previous DCO as progression-free survival (PFS) was sufficiently mature (87% data maturity6). Serious adverse events (SAEs) including adverse events (AEs) leading to death were analyzed, but other safety data were not collected after the previous DCO. A post-hoc analysis was carried out in the subset of patients who were ongoing treatment with durvalumab at the 22 March 2021 DCO to describe baseline characteristics, response and PFS (based on the earlier 27 January 2020 DCO), treatment exposure, and SAEs in these patients who had extended durvalumab exposure.
Statistical analysis
OS was analyzed in the intention-to-treat (ITT) population using a stratified log-rank test adjusting for planned platinum, with HRs and 95% CIs estimated using a stratified Cox proportional hazards model. Medians and landmark rates were derived using the Kaplan–Meier method. OS was analyzed in prespecified patient subgroups using an unstratified Cox proportional hazards model. AEs were assessed in all treated patients.
Ethics approval and consent to participate
The study was conducted in accordance with the International Conference on Harmonisation good clinical practice guidelines, the Declaration of Helsinki, and applicable local regulations with approval from an independent ethics committee or institutional review boards. The protocol and all modifications were approved by relevant ethics committees and regulatory authorities, and all patients provided written informed consent for participation.
Results
Patients
As previously reported,1 972 patients were screened between March 2017 and May 2018, of whom 805 were randomized to durvalumab plus EP (n = 268), durvalumab plus tremelimumab plus EP (n = 268), and EP (n = 269) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100408). Baseline characteristics were generally balanced across arms.1,6
Overall survival
At DCO, there were 695 deaths across all treatment arms (86% maturity): 47 new deaths since the previous analysis 14 months earlier. The median follow-up for OS in censored patients was 39.4 months (range 0.1-47.5 months). Forty-four (16%), 37 (14%), and 13 (5%) patients in the durvalumab plus EP, durvalumab plus tremelimumab plus EP, and EP arms, respectively, remained in survival follow-up.
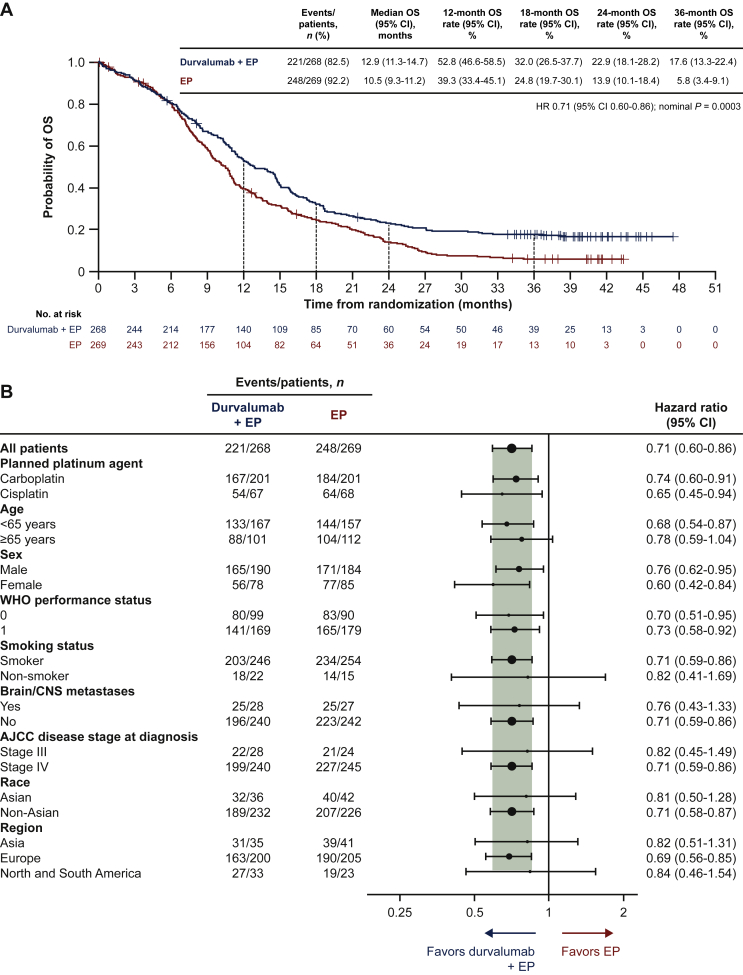
OS benefit with durvalumab plus EP versus EP was sustained: HR 0.71 (95% CI 0.60-0.86; nominal P = 0.0003; Figure 1A). Median OS was 12.9 months (95% CI 11.3-14.7 months) with durvalumab plus EP versus 10.5 months (95% CI 9.3-11.2 months) with EP, and 36-month OS rates were 17.6% (95% CI 13.3% to 22.4%) versus 5.8% (95% CI 3.4% to 9.1%). OS HRs consistently favored durvalumab plus EP versus EP across all prespecified patient subgroups (Figure 1B), as observed in prior analyses.1,6
Figure 1.
Overall survival in the intention-to-treat population: durvalumab plus EP versus EP.
(A) Kaplan–Meier graph of overall survival. (B) Subgroup analysis of overall survival.
Size of circle is proportional to the number of events across both treatment arms.
Smokers are defined as patients who currently use or have previously used cigarettes, cigarillos, cigars, pipe tobacco, or tobacco for smoking; non-smokers are defined as patients who have never used these products.
AJCC, American Joint Committee on Cancer; CI, confidence interval; CNS, central nervous system; EP, etoposide plus either cisplatin or carboplatin; HR, hazard ratio; OS, overall survival; WHO, World Health Organization.
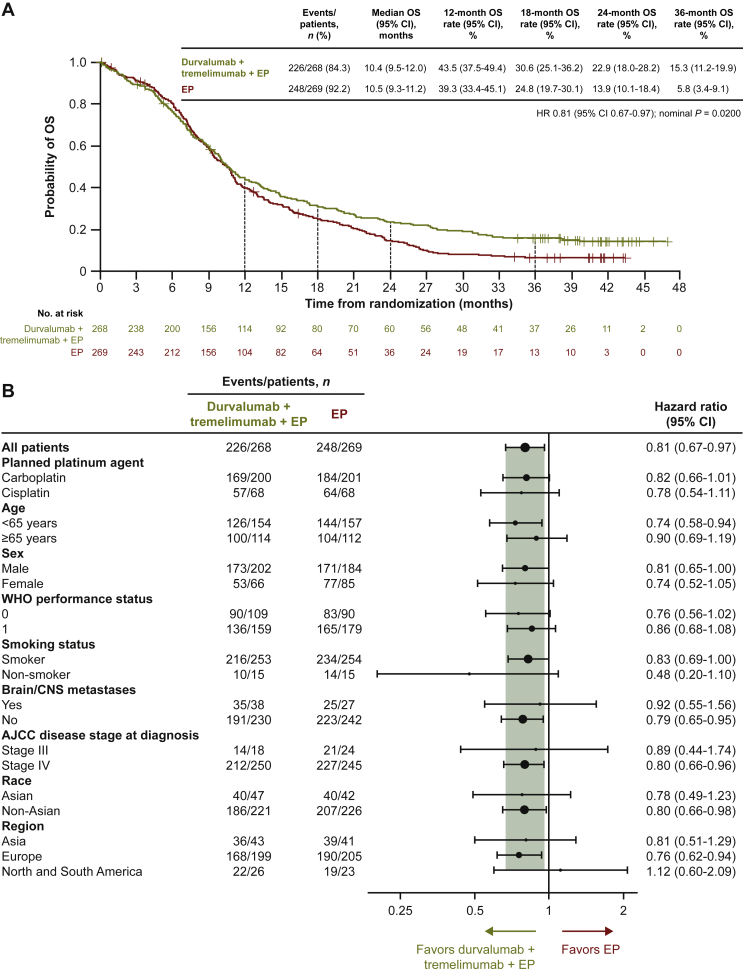
Numerical improvement in OS with durvalumab plus tremelimumab plus EP versus EP was maintained with additional follow-up: HR 0.81 (95% CI 0.67-0.97; nominal P = 0.0200; Figure 2A). Median OS was 10.4 months (95% CI 9.5-12.0 months) with durvalumab plus tremelimumab plus EP, and the 36-month OS rate was 15.3% (95% CI 11.2% to 19.9%). In line with the previous analysis,6 OS HRs for durvalumab plus tremelimumab plus EP versus EP across patient subgroups were generally consistent with the overall population (Figure 2B).
Figure 2.
Overall survival in the intention-to-treat population: durvalumab plus tremelimumab plus EP versus EP.
(A) Kaplan–Meier graph of overall survival. (B) Subgroup analysis of overall survival. Size of circle is proportional to the number of events across both treatment arms.
Smokers are defined as patients who currently use or have previously used cigarettes, cigarillos, cigars, pipe tobacco, or tobacco for smoking; non-smokers are defined as patients who have never used these products.
AJCC, American Joint Committee on Cancer; CI, confidence interval; CNS, central nervous system; EP, etoposide plus either cisplatin or carboplatin; HR, hazard ratio; OS, overall survival; WHO, World Health Organization.
Treatment exposure and subsequent therapy
As reported previously,6 the median total duration of durvalumab treatment and number of durvalumab doses received were higher in the durvalumab plus EP arm compared with the durvalumab plus tremelimumab plus EP arm (Table 1). Exposure to chemotherapy and tremelimumab was unchanged from the previous analysis.6
Table 1.
Durvalumab treatment exposure
| Durvalumab plus EP (n = 265) | Durvalumab plus tremelimumab plus EP (n = 266) | |
|---|---|---|
| Median number of durvalumab doses (range) | 7.0 (1-52) | 6.0 (1-46) |
| Total duration of durvalumab exposure, n (%) | ||
| ≥1 year | 54 (20.4) | 49 (18.4) |
| ≥2 years | 32 (12.1) | 30 (11.3) |
| ≥3 years | 24 (9.1) | 21 (7.9) |
| Median total duration of durvalumab, weeks (range) | 28.0 (0.3-198.7) | 23.1 (0.1-190.0) |
| Durvalumab dose delays, n (%) | 152 (57.4) | 157 (59.0) |
| Durvalumab dose interruptions, n (%) | 4 (1.5) | 4 (1.5) |
EP, etoposide plus either cisplatin or carboplatin.
Durvalumab was dosed for ≥2 and ≥3 years, respectively, in 12% and 9% of patients in the durvalumab plus EP arm and 11% and 8% in the durvalumab plus tremelimumab plus EP arm. At DCO, 27 (10%) and 19 (7%) patients remained on durvalumab in the durvalumab plus EP and durvalumab plus tremelimumab plus EP arms, respectively.
Six additional patients since the previous analysis6 received at least one subsequent systemic anticancer therapy (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100408). The incidence of subsequent immunotherapy was low in all arms.
Safety
SAEs occurred in 86 (32%), 126 (47%), and 97 (36%) patients in the durvalumab plus EP, durvalumab plus tremelimumab plus EP, and EP arms, respectively: six additional patients (one in the durvalumab plus EP arm and five in the durvalumab plus tremelimumab plus EP arm) since the previous analysis6 (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100408).
Any-cause AEs leading to death occurred in 14 (5%), 29 (11%), and 16 (6%) patients in the durvalumab plus EP, durvalumab plus tremelimumab plus EP, and EP arms, respectively (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100408). Four additional deaths resulting from AEs occurred since the previous analysis;6 none were considered treatment-related.
Patients ongoing durvalumab treatment at data cut-off
Among the 46 patients who were ongoing treatment with durvalumab at the 22 March 2021 DCO, there were some differences in baseline characteristics compared with the ITT population for their treatment arm (Table 2).1,6 Specifically, more patients had a WHO performance status of 0; fewer had brain or liver metastases; fewer were male (durvalumab plus EP arm); and the median age was lower (durvalumab plus tremelimumab plus EP arm).
Table 2.
Baseline characteristics in the 46 patients who remained on treatment with durvalumab at the 22 March 2021 data cut-off, compared with the ITT population
| Patients ongoing durvalumab at data cut-off |
CASPIAN ITT population1, 6a |
|||
|---|---|---|---|---|
| Durvalumab plus EP (n = 27) | Durvalumab plus tremelimumab plus EP (n = 19) | Durvalumab plus EP (n = 268) | Durvalumab plus tremelimumab plus EP (n = 268) | |
| Median age, years (range) | 61 (47-75) | 56 (46-73) | 62 (58-68) | 63 (58-68) |
| Age group, years, n (%) | ||||
| <65 | 19 (70.4) | 15 (78.9) | 167 (62.3) | 154 (57.5) |
| ≥65 | 8 (29.6) | 4 (21.1) | 101 (37.7) | 114 (42.5) |
| Sex, n (%) | ||||
| Men | 15 (55.6) | 14 (73.7) | 190 (70.9) | 202 (75.4) |
| Women | 12 (44.4) | 5 (26.3) | 78 (29.1) | 66 (24.6) |
| Race, n (%) | ||||
| White | 27 (100.0) | 17 (89.5) | 229 (85.4) | 215 (80.2) |
| Asian | 0 | 1 (5.3) | 36 (13.4) | 47 (17.5) |
| Other | 0 | 1 (5.3) | 3 (1.1) | 6 (2.2) |
| Disease stage, n (%) | ||||
| III | 4 (14.8) | 1 (5.3) | 28 (10.4) | 18 (6.7) |
| IV | 23 (85.2) | 18 (94.7) | 240 (89.6) | 250 (93.3) |
| WHO performance status, n (%) | ||||
| 0 | 12 (44.4) | 10 (52.6) | 99 (36.9) | 109 (40.7) |
| 1 | 15 (55.6) | 9 (47.4) | 169 (63.1) | 159 (59.3) |
| Histology, n (%) | ||||
| SCC (neuroendocrine) | 5 (18.5) | 5 (26.3) | 39 (14.6) | 39 (14.6) |
| SCC (combined)b | 22 (81.5) | 14 (73.7) | 229 (85.4) | 228 (85.1) |
| Other | 0 | 0 | 0 | 1 (0.4) |
| Metastases, n (%) | ||||
| Brain or CNS | 1 (3.7) | 1 (5.3) | 28 (10.4) | 38 (14.2) |
| Liver | 4 (14.8) | 4 (21.1) | 108 (40.3) | 117 (43.7) |
CNS, central nervous system; EP, etoposide plus either cisplatin or carboplatin; ITT, intention-to-treat; SCC, small-cell carcinoma; SCLC, small-cell lung cancer; WHO, World Health Organization.
Histology data not published previously.
Includes SCLC, SCC, SCC oat cell/intermediate/combined oat cell categories on the electronic case report form.
All 46 patients completed four cycles of chemotherapy (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100408); 8 of the 27 patients (29.6%) from the durvalumab plus EP arm and 7 of the 19 patients (36.8%) from the durvalumab plus tremelimumab plus EP arm received cisplatin. All 19 patients from the durvalumab plus tremelimumab plus EP arm received five cycles of tremelimumab (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100408). The 27 patients in the durvalumab plus EP arm received durvalumab treatment for at least 148 weeks (2.8 years) and the 19 patients in the durvalumab plus tremelimumab plus EP arm received durvalumab for at least 120 weeks (2.3 years) (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100408).
Patients still receiving durvalumab treatment at the 22 March 2021 DCO had previously experienced high rates of response: 42 of the 46 patients were responders, 23 (85.2%) in the durvalumab plus EP arm and 19 (100%) in the durvalumab plus tremelimumab plus EP arm (Table 3). PFS rates at 12 and 24 months for these 46 patients were 85.2% and 81.5% in the durvalumab plus EP arm and 84.2% and 78.9% in the durvalumab plus tremelimumab plus EP arm, respectively, (Table 3). Progression data were not collected after the 27 January 2020 DCO. Six patients in the durvalumab plus EP arm and four patients in the durvalumab plus tremelimumab plus EP arm who had experienced a progression event before that time were still being treated with durvalumab as of 22 March 2021 (Table 3).
Table 3.
Response and progression-free survival in the 46 patients who remained on treatment with durvalumab at the 22 March 2021 data cut-off
| Durvalumab plus EP (n = 27) | Durvalumab plus tremelimumab plus EP (n = 19) | |
|---|---|---|
| Best objective responsea | ||
| Responders, n (%) | 23 (85.2) | 19 (100.0) |
| Complete responseb | 6 (22.2) | 4 (21.1) |
| Partial responseb | 17 (63.0) | 15 (78.9) |
| Non-responders, n (%) | 4 (14.8) | 0 |
| Stable disease ≥6 weeks | 2 (7.4) | 0 |
| Progression | 2 (7.4) | 0 |
| PFSa | ||
| Progression events, n (%) | 6 (22.2) | 4 (21.1) |
| New lesions only | 2 (7.4) | 4 (21.1) |
| Target lesions only | 4 (14.8) | 0 |
| PFS rate at 12 months, % (95% CI)c | 85.2 (65.2-94.2) | 84.2 (58.7-94.6) |
| PFS rate at 24 months, % (95% CI)c | 81.5 (61.1-91.8) | 78.9 (53.2-91.5) |
CI, confidence interval; DCO, data cut-off; EP, etoposide plus either cisplatin or carboplatin; PFS, progression-free survival.
Objective response and PFS were investigator-assessed per Response Evaluation Criteria in Solid Tumors, version 1.1, and data are based on the earlier 27 January 2020 DCO, as progression and response data were not collected beyond this timepoint.
Defined as patients with complete response or partial response on at least one visit (unconfirmed responses).
Estimated using the Kaplan–Meier method.
Among the 46 patients still receiving durvalumab as of 22 March 2021, SAEs were experienced by 10 of the 27 patients (37.0%) in the durvalumab plus EP arm and 10 of the 19 patients (52.6%) in the durvalumab plus tremelimumab plus EP arm; these incidences are similar to those observed in the ITT population for their treatment arm (see Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100408). Patients ongoing durvalumab treatment at this DCO were more likely to have experienced a delay in durvalumab dosing (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100408) than the ITT population for their treatment arm (see Table 1).
Discussion
In this updated analysis of CASPIAN, three times more patients were estimated to be alive at 3 years in the durvalumab plus EP arm (17.6%) compared with the EP arm (5.8%), after the longest follow-up reported to date for a phase III trial of anti-PD-(L)1 combined with chemotherapy in ES-SCLC. OS favored durvalumab plus EP across all prespecified patient subgroups, including cisplatin- and carboplatin-treated patients, supporting the applicability of this regimen across a broad population. The sustained benefit with durvalumab plus EP has particular clinical relevance in this aggressive disease setting for which long-term prognosis has historically been dismal.7
Although durvalumab plus tremelimumab plus EP did not significantly improve OS in CASPIAN,6 the tail of the survival curve was similar to the durvalumab plus EP curve, with 15.3% of patients in the tremelimumab-containing arm estimated to be alive at 3 years, giving further support for the long-term clinical benefit that is possible with continuation of durvalumab maintenance treatment.
At DCO, 46 patients remained on durvalumab treatment, the majority of patients still in survival follow-up in the immunotherapy arms. A small proportion of these patients were still receiving durvalumab treatment more than a year after progression. These observations further demonstrate the long-term tolerability of durvalumab and suggest that long-term survival benefit was driven by first-line treatment with immunotherapy plus EP rather than subsequent treatment(s) received. A post-hoc exploratory analysis of these 46 patients revealed some differences in baseline characteristics that are known to be prognostic; this subset also had greater exposure to chemotherapy and immunotherapy, had higher cisplatin use, and achieved better response rates than the ITT population for their treatment arm.6 Since this analysis was post hoc and descriptive in nature, and based on a small number of patients, the results should be interpreted with caution. Nevertheless, understanding the features of patients who remain on long-term treatment in excess of 2 years may provide valuable insights for the future management of SCLC. Extended follow-up data from other randomized controlled trials in ES-SCLC would help to further characterize the extent to which immunotherapy treatments can impact long-term outcomes for patients in this setting.
It would be useful to establish a biomarker to predict long-term benefit from durvalumab plus EP treatment, and alternative combination strategies to bring long-term benefit to other patients. To date, no association has been found between outcomes with first-line immunotherapy and PD-L1 expression8, 9, 10 or tumor mutational burden10,11 in ES-SCLC. However, a post-hoc exploratory analysis of CASPIAN suggested a possible association of HLA genotype (specifically the HLA-DQB1∗03:01 allele) with longer OS in the durvalumab plus tremelimumab plus EP arm.12 Recently, there has been particular interest in potential combination strategies for four SCLC molecular subtypes with distinct therapeutic vulnerabilities, defined by differential expression of transcription factors.13, 14, 15
Durvalumab plus EP continued to be well tolerated with additional follow-up, with few SAEs reported since the previous analysis, suggesting no cumulative toxicity. As seen previously,6 the incidence of SAEs and AEs leading to death was higher with durvalumab plus tremelimumab plus EP than durvalumab plus EP or EP.
In conclusion, durvalumab plus EP demonstrated sustained OS benefit over EP with a well-tolerated safety profile after median follow-up of >3 years, consistent with previous analyses. Three times more patients were estimated to be alive at 3 years when treated with durvalumab plus EP versus EP alone, with the majority still receiving durvalumab at DCO, further establishing durvalumab plus EP as the standard of care for the first-line treatment of ES-SCLC.
Acknowledgements
The authors thank the patients, their families and caregivers, and all investigators involved in this study. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Helen Kitchen and Samantha Holmes of Ashfield MedComms (Macclesfield, UK), an Ashfield Health company, and was funded by AstraZeneca.
Funding
This work was supported by AstraZeneca (no grant number).
Disclosure
LPA reports receiving grants from AstraZeneca, Bristol-Myers Squibb, MSD, and Pfizer; consulting fees from Amgen, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Ipsen, Lilly, Merck, Mirati, MSD, Novartis, Pfizer, PharmaMar, Roche, Sanofi, and Servier; and honoraria from AstraZeneca, Janssen, Merck, Mirati, and Sanofi, and reports a leadership role with Genomica and Altum Sequencing, all outside the submitted work. YC reports receiving honoraria from Amgen, AstraZeneca, Bristol-Myers Squibb, Guardant Health, Jazz Pharmaceutical, Merck, Pfizer, and Takeda; and a research contract and support for meeting attendance/travel from Ipsen, all outside the submitted work. NR reports receiving honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Hoffmann-La Roche, Lilly, Merck, MSD, Pfizer, and Takeda; and consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Hoffmann-La Roche, Merck, MSD, Pfizer, and Takeda, all outside the submitted work. KH reports receiving grants and personal fees from AstraZeneca during the conduct of the study; grants from Bristol-Myers Squibb, Chugai, Lilly, and MSD outside the submitted work; and honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Lilly, MSD, Nippon Kayaku, Ono, Pfizer, Taiho, and Takeda outside the submitted work. MJH reports receiving speakers’ honoraria from AstraZeneca, Boehringer Ingelheim, MSD, Pfizer, Roche, and Takeda outside the submitted work. MCG reports receiving grants from AstraZeneca and Merck, and personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Roche, and Takeda, all outside the submitted work. EM reports receiving grants from AstraZeneca during the conduct of the study and grants from Roche outside the submitted work. HM and TD report full-time employment and stock ownership with AstraZeneca. HJ reports full-time employment and stock ownership with AstraZeneca, and a patent pending for the CASPIAN study trial design. JWG reports receiving research grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Genentech, and Merck; consulting fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, and Genentech; and support for travel from AstraZeneca all outside the submitted work. All other authors have declared no conflicts of interest.
Data sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supplementary data
References
- 1.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 2.Horn L., Mansfield A.S., Szczesna A., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 3.Garon E.B., Hellmann M.D., Rizvi N.A., et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H., Gettinger S., Vokes E.E., et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022 Feb 2:JCO2101308. doi: 10.1200/JCO.21.01308. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman J.W., Dvorkin M., Chen Y., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 7.Pietanza M.C., Byers L.A., Minna J.D., et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21(10):2244–2255. doi: 10.1158/1078-0432.CCR-14-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Ares L., Goldman J.W., Garassino M.C., et al. PD-L1 expression, patterns of progression and patient-reported outcomes (PROs) with durvalumab plus platinum-etoposide in ES-SCLC: results from CASPIAN. Ann Oncol. 2019;30(suppl 5):v928–v929. (abstr LBA89) [Google Scholar]
- 9.Rudin C.M., Awad M.M., Navarro A., et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S.V., Reck M., Mansfield A.S., et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133) J Clin Oncol. 2021;39(6):619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman J.W., Garassino M.C., Chen Y., et al. Durvalumab (D) ± tremelimumab (T) + platinum-etoposide (EP) in 1L ES-SCLC: characterization of long-term clinical benefit and tumour mutational burden (TMB) in CASPIAN. Ann Oncol. 2020;31(suppl 4):S1212–S1213. (abstr LBA86) [Google Scholar]
- 12.Garassino M, Shrestha Y, Lai MXZ, et al. Durvalumab ± tremelimumab + platinum-etoposide in 1L ES-SCLC: exploratory analysis of HLA genotype and survival in CASPIAN. J Thorac Oncol. 2021;16(suppl 10):S939 (abstr MA16.06).
- 13.Rudin C.M., Poirier J.T., Byers L.A., et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19(5):289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owonikoko T.K., Dwivedi B., Chen Z., et al. YAP1 expression in SCLC defines a distinct subtype with T-cell-Inflamed phenotype. J Thorac Oncol. 2021;16(3):464–476. doi: 10.1016/j.jtho.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay C.M., Stewart C.A., Park E.M., et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346–360.e7. doi: 10.1016/j.ccell.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.