Abstract
Carvacrol, a naturally occurring compound mainly present in the essential oil fraction of oregano and thyme, was studied for its effect on bioenergetic parameters of vegetative cells of the food-borne pathogen Bacillus cereus. Incubation for 30 min in the presence of 1 to 3 mM carvacrol reduced the viable cell numbers exponentially. Carvacrol (2 mM) significantly depleted the intracellular ATP pool to values close to 0 within 7 min. No proportional increase of the extracellular ATP pool was observed. Depletion of the internal ATP pool was associated with a change of the membrane potential (Δψ). At concentrations of 0.01 mM carvacrol and above, a significant reduction of Δψ was observed, leading to full dissipation of Δψ at concentrations of 0.15 mM and higher. Finally, an increase of the permeability of the cytoplasmic membrane for protons and potassium ions was observed (at 0.25 and 1 mM carvacrol, respectively). From this study, it could be concluded that carvacrol interacts with the membranes of B. cereus by changing its permeability for cations like H+ and K+. The dissipation of ion gradients leads to impairment of essential processes in the cell and finally to cell death.
Bacillus cereus is a spore-forming food-borne pathogen often associated with food products such as meat, vegetables, soup, rice, and milk and other dairy products. Between 1 and 20% of the total number of outbreaks of food infection in the world is caused by B. cereus (19). Growth of vegetative cells usually occurs within the temperature range of 10 to 50°C, with an optimum between 28 and 35°C. However, psychrotrophic variants of B. cereus, capable of growing at temperatures below 5°C, have been identified (6, 22). Although vegetative cells of B. cereus can easily be inactivated by heating, spores are considerably more resistant and can cause food spoilage after subsequent germination (6).
Mild preservation technologies are becoming more important in modern food industries. As a consequence, spore-forming microorganisms are likely to proliferate and hence become a serious food safety risk. Mild processes are often combined to obtain safe products with improved organoleptic quality. A novel way to reduce the proliferation of microorganisms is the use of essential oils. The antifungal and antibacterial effects of these components on different microorganisms have been described in several studies (5, 14, 16–18, 26–29). Among the diverse group of chemical components in essential oils, carvacrol exerts a distinct antimicrobial action. Carvacrol is the major component of the essential oil fraction of oregano (60 to 74% carvacrol) and thyme (45% carvacrol) (1, 20). In practice, carvacrol is added to different products, e.g., baked goods (15.75 ppm), nonalcoholic beverages (28.54 ppm/0.18 mM), chewing gum (8.42 ppm), etc. (8). However, not much is known about the mechanisms of action of this compound. A better knowledge of the mode of action is important regarding application in food systems. Recently, Ultee et al. (29) showed the antimicrobial effect of carvacrol on B. cereus. Hydrophobic compounds like carvacrol are likely to have an influence on biological membranes. The cytoplasmic membrane of bacteria has two principal functions: (i) barrier function and energy transduction, which allow the membrane to form ion gradients that can be used to drive various processes, and (ii) formation of a matrix for membrane-embedded proteins (such as the membrane-integrated F0 complex of ATP-synthase) (12, 24). In the present study, changes in the energy-transducing processes of B. cereus caused by carvacrol were studied in more detail. The effect of carvacrol on the intracellular ATP pool, the membrane potential, the pH gradient across the cytoplasmic membrane, and the potassium gradient was evaluated.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
B. cereus IFR-NL94-25 (obtained from the Institute of Food Research, Norwich, United Kingdom) was used throughout this study. Cells were grown in brain heart infusion (BHI) medium (Oxoid) supplemented with 0.5% (wt/vol) glucose (initial pH of 6.7) at 30°C. Cell cultures were maintained at −80°C in 15% glycerol as a cryoprotectant.
Chemicals.
Purified carvacrol was obtained from Fluka Chemie AG (Buchs, Switzerland). A stock solution (1 M) was made in 95% ethanol. The final ethanol concentration in the experiments was always kept below 2% (vol/vol).
Monitoring viability.
Vegetative cells of B. cereus were harvested by centrifugation, washed twice in a 50 mM potassium–HEPES buffer (pH 7.0) containing 1 mM MgSO4, and diluted to an optical density at 660 nm (OD660) of 0.1 (light path of 1 cm). Suspensions of 20 ml were kept at 20°C. Carvacrol was added to a final concentration between 1 and 2 mM. Samples were taken every 5 min during exposure (maximum exposure time, 40 min) and immediately diluted (102- to 105-fold) in peptone-physiological salt solution (1 g of peptone per liter and 8.5 g of NaCl per liter) to quench the influence of carvacrol. Serial dilutions were plated on BHI agar plates and incubated for 24 h at 30°C.
Determination of intra- and extracellular ATP concentrations.
Cells of an overnight culture were washed three times in a 25 mM potassium phosphate buffer (pH 7), and a suspension was prepared with an OD660 of 1 (light path of 1 cm). The experiment was started by adding glucose to a final concentration of 0.5% (wt/vol). Samples of 200 μl were taken every 2 min, added to Eppendorf tubes containing 200 μl of a mixture of silicon oil (AR200/AR20 ratio = 2:1) (Wacker Chemicals, Munich, Germany) on top of 100 μl of trichloroacetic acid-EDTA buffer (10% trichloroacetic acid and 2 mM EDTA), and centrifuged directly (5 min, 12,000 × g). The extracellular (upper layer) and the intracellular (lower layer) ATP concentrations were measured by using a 1243-107 ATP assay kit (Bio-Orbit, Turku, Finland). Luminescence was recorded with a model 1250 luminometer (Bio-Orbit).
Influence of carvacrol on the membrane potential (Δψ).
Cells of an overnight culture were washed twice in a 50 mM potassium–HEPES buffer (pH 7.0), containing 1 mM MgSO4. The cell pellet was diluted until an OD660 (light path of 1 cm) of 10 was reached. Exactly 30 μl of the cell suspension was diluted in 2 ml of buffer, containing 5 μM 3,3-dipropylthiacarbocyanine [DiSC3(5)] (Molecular Probes, Leiden, The Netherlands). The membrane potential (Δψ) was monitored with a Perkin-Elmer LS 50B spectrofluorometer at 20°C (excitation wavelength, 643 nm; emission wavelength, 666 nm). Following equilibration, 15 mM glucose was added to energize the cells. After a constant reading had been reached, 1 nM nigericin was added to diminish the pH gradient across the cytoplasmic membrane. After a steady fluorescence reading was reached, different concentrations of carvacrol (0.01 to 2 mM) were added. Valinomycin (1 nM) was added as a control.
Intracellular pH measurements.
The determination of the intracellular pH was based on the method described by Breeuwer et al. (3). Cells of an overnight culture were harvested, washed three times in 50 mM HEPES buffer (pH 7.0), and diluted to an OD660 (light path, 1 cm) of 1. Subsequently, cells were incubated in the presence of 1.5 μM carboxyfluorescein diacetate succinimidyl ester for 10 min at 30°C. Carboxyfluorescein diacetate succinimidyl ester is hydrolyzed to carboxyfluorescein succinimidyl ester in the cell and subsequently conjugated to aliphatic amines. After being washed with 50 mM potassium phosphate buffer (pH 5.81), cells were incubated with 10 mM glucose for 30 min at 30°C to eliminate nonconjugated carboxyfluorescein succinimidyl ester. In addition, cells were washed twice, resuspended in 50 mM potassium phosphate, and kept on ice until further use. The analysis was started by 30 μl of the cell suspension being added to a quartz cuvette containing 3 ml of a 50 mM phosphate buffer (pH 5.81), placed in a cuvette holder of a spectrofluorometer (Perkin-Elmer LS 50B), and stirred continuously. Fluorescence intensities were measured at excitation wavelengths of 490 nm (pH sensitive) and 440 nm (pH insensitive) by rapidly altering the monochromator between both wavelengths. The emission was determined at 525 nm, and the excitation and emission slit widths were set on 5 and 10 nm, respectively. The intracellular pH was calculated from the ratio of the emission at 490- and 440-nm excitation.
A calibration curve was determined in buffers with pH values ranging from 3 to 10. Buffers contained 50 mM glycine, 50 mM citric acid, 50 mM Na2HPO4 · 2H2O, and 50 mM KCl. pH values were adjusted with NaOH or HCl. The pHin and pHout (intracellular and extracellular pH, respectively) were equilibrated by addition of 1 μM valinomycin and 1 μM nigericin.
Determination of intra- and extracellular amounts of potassium.
Exponentially growing cells (overnight culture) were harvested and washed twice in a 50 mM sodium–HEPES buffer (pH 7.0). Cells were concentrated till an OD660 of 1 (light path of 1 cm) was reached. The extraction of potassium from the cells was carried out as described for the ATP determination. The potassium concentration was measured with a Flame Photometer (Jenway, Felsted, England) after diluting the samples in distilled water. Values were compared with a standard calibration curve of KCl.
Protein determination.
The determination of the amount of protein in the cells of B. cereus was carried out according to the work of Lowry et al. (21) with bovine serum albumin as a standard.
RESULTS
Viability of B. cereus in the presence of carvacrol.
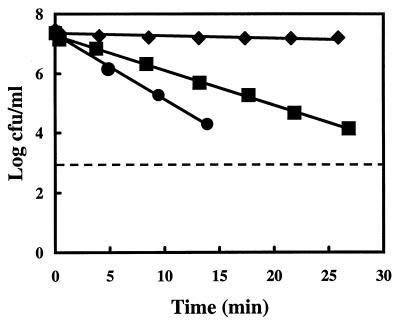
Carvacrol inhibits the growth of B. cereus effectively. Incubation and exposure temperatures significantly affect the death rate of B. cereus (29). The viability of B. cereus cells cultured at 30°C was determined under conditions (pH 7, 20°C) which were used throughout this study. Samples were taken every 5 min and plated on BHI plates to monitor the viable count (Fig. 1). A log-linear relationship was found between the time of exposure and the viable count of B. cereus. At 1 mM carvacrol, almost no reduction of the viable count was observed after 30 min, while 1.25 and 1.5 mM carvacrol resulted in a clear reduction of the viable counts. Therefore, it can be concluded that carvacrol is bactericidal toward B. cereus cells at 20°C when present at concentrations above 1 mM.
FIG. 1.
Viable count of B. cereus cells (log CFU/ml) at different time intervals after the addition of carvacrol. Cells were cultured in BHI (30°C), washed, and maintained in HEPES buffer at pH 7.0 (20°C). Carvacrol concentrations tested were 1 (⧫), 1.25 (■), and 1.5 (●) mM. The data represent mean values of triplicate measurements. The dashed line represents the detection limit.
Effect of carvacrol on ATP pools.
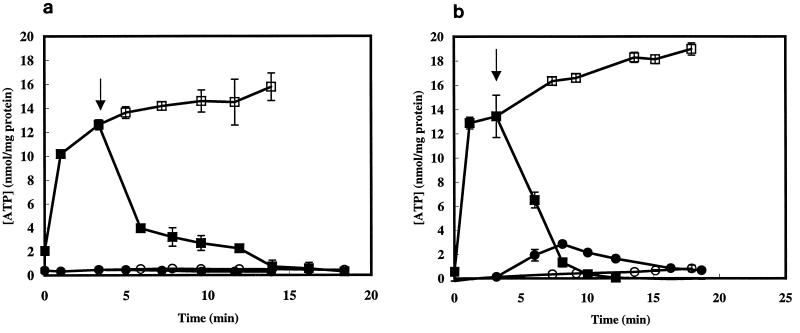
The bactericidal activity may lie in the disruption of the membrane integrity, since carvacrol is a lipophilic compound preferentially partitioning in this cell compartment. Cytoplasmic membrane disruption is expected to have a large impact on the membrane-associated energy-transducing system. Therefore, the effect of carvacrol on the intra- and extracellular ATP pools was studied. Addition of 1 mM carvacrol decreased the intracellular amount of ATP to values close to 0 within 14 min (Fig. 2a). No increase of the extracellular ATP pool was observed. Similar results were obtained with the addition of 2 mM carvacrol (Fig. 2b). The intracellular ATP pool was reduced to 0 within 10 min. A small increase of the extracellular ATP pool was observed, although this was not proportional to the decrease of the intracellular ATP pool.
FIG. 2.
Intracellular (squares) and extracellular (circles) ATP pools of glucose-energized vegetative cells of B. cereus in the absence (open symbols) or presence (closed symbols) of 1 (a) or 2 (b) mM carvacrol. Carvacrol was added at t = 4 min (indicated by arrow). Values represent the means of duplicate measurements. The standard errors of the means are indicated by the error bars.
Influence of carvacrol on membrane potential (Δψ).
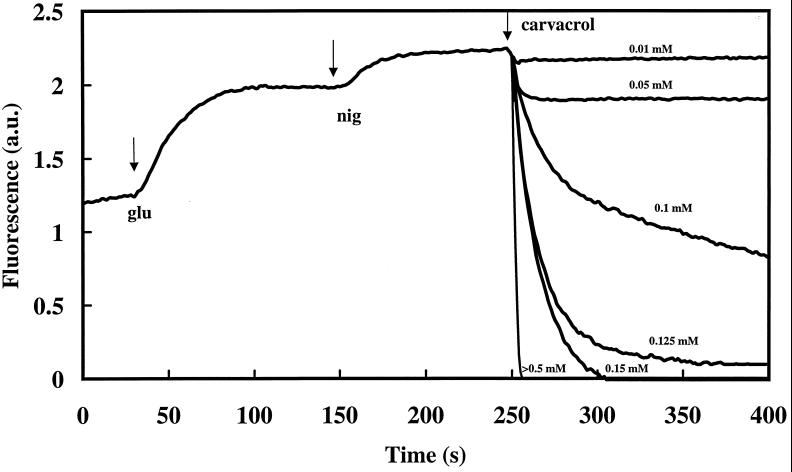
Depletion of the internal ATP pool by carvacrol may be associated with a reduced ATP synthesis. Therefore, we investigated the effect of carvacrol on the membrane potential, the driving force for ATP synthesis. Changes in the membrane potential can be visualized by changes in the fluorescence of a potentiometric dye. B. cereus cells were incubated in the presence of DiSC3(5). After the addition of glucose and nigericin, carvacrol was added (Fig. 3). Carvacrol reduced the membrane potential if present at 0.01 mM or higher. Increased concentrations caused a higher rate of reduction (0.01 and 0.25 U/s at 0.01 and 0.5 mM, respectively), and a lower steady-state membrane potential was reached. At concentrations higher than 0.15 mM, a complete dissipation of the membrane potential was observed.
FIG. 3.
Effect of carvacrol on the membrane potential of B. cereus in the presence of glucose (glu), nigericin (nig), and different concentrations of carvacrol. Exponentially growing cells were washed and maintained in HEPES buffer at pH 7.0 (20°C). Carvacrol was added at t = 250 s. The membrane potential was monitored by using the fluorescent probe DiSC3(5). a.u., arbitrary units.
Effect of carvacrol on intracellular pH.
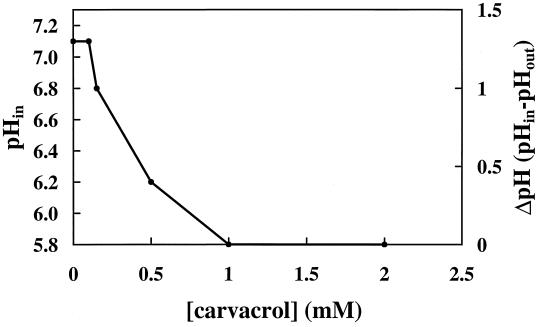
Depletion of intracellular ATP and dissipation of Δψ by carvacrol suggest effects on ion gradients across the cellular membrane. Therefore, the pHin was investigated in more detail. To rule out other essential gradients apart from proton gradients, all measurements were carried out in the presence of valinomycin. The pHin of B. cereus, cultured in BHI at pH 6.7 and washed in HEPES buffer (pH 5.81), was approximately 7.1.
Addition of glucose and valinomycin did not affect the pHin. As expected, nigericin dissipated the pH gradient across the membrane (data not shown). Exposure of the cells to carvacrol (Fig. 4) decreased pHin. In the presence of 0.25 mM carvacrol, the pH gradient across the cell membrane was reduced to 1 U. A further reduction of the ΔpH to 0.5 U was observed with 0.5 mM carvacrol. No effect on the ΔpH was observed at concentrations below 0.25 mM. Complete dissipation of the pH gradient was reached in the presence of 1 mM carvacrol or higher.
FIG. 4.
Effect of carvacrol on the pHin and ΔpH of vegetative cells of B. cereus. Cells were cultured in BHI, washed, and incubated in a 50 mM potassium phosphate buffer (pH 7) (see Materials and Methods).
Influence of carvacrol on potassium efflux.
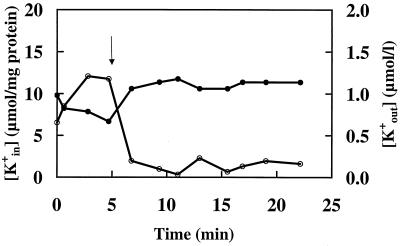
Carvacrol affects the intracellular ATP concentrations and the transmembrane electrical potential and dissipates the ΔpH. Subsequently, the effect of carvacrol on the permeability of the membrane toward potassium ions was investigated. Addition of glucose (to energize the cells) at t = 0 caused an approximately 100% increase of the intracellular potassium pools (K+in) (Fig. 5). Extracellular pools (K+out) decreased from 0.98 to 0.67 μmol/liter. Addition of 1 mM carvacrol after 5 min of incubation rapidly decreased the intracellular amount of K+. After 9 min of incubation, the intracellular amount of K+ was reduced from 12 μmol/mg of cell protein (at t = 5 min) to 0.99 μmol/mg of cell protein and the extracellular K+ was raised from 0.67 μmol/liter after 5 min to 1.14 μmol/liter at 9 min of incubation. The total amount of potassium (K+in + K+out) remained constant throughout the experiment.
FIG. 5.
Changes in intracellular (K+in) (○) and extracellular (K+out) (●) potassium pools of B. cereus cells during exposure to 1 mM carvacrol. Cells were cultured in BHI, washed, and maintained in Na-HEPES buffer (pH 7.0). Carvacrol was added at t = 5 min.
DISCUSSION
This study describes the effect of carvacrol on cells of the food-borne pathogen B. cereus. Carvacrol acts as a bactericidal compound, with its activity being dependent on the concentration and the time of exposure. Sikkema et al. (23) found that the accumulation of lipophilic compounds in the cell membrane (tested in liposomes prepared from Escherichia coli lipids) is proportional to the concentration in the aqueous phase and the membrane aqueous-phase partition coefficient. Enomoto et al. (7) observed a decrease of Δψ in liposomes during exposure to some fragrance compounds. These hydrophobic compounds dissolve in the membrane, and their activity was closely correlated with the membrane fluidity. Based on these studies, it is expected that more carvacrol dissolves in the membrane at higher concentrations.
Our study has shown that exposure to carvacrol leads to a decrease of the ATPin concentration. No proportional increase of the ATPout was observed. Therefore, it is concluded that carvacrol does not enhance the permeability of the membrane for ATP. Consequently, depletion of the internal ATP pool results from a reduced rate of ATP synthesis or increased ATP hydrolysis. A depletion of the ATP pool upon the addition of a lipophilic component has been observed in different studies (13, 15). In contrast to the present study, Helander et al. (11) observed a leakage of ATP from cells which were exposed to carvacrol (2 mM). However, this study was carried out with gram-negative bacteria, which have a different cell envelope.
The observation that already-low concentrations of carvacrol (>0.01 mM) cause a decrease of the membrane potential suggests that the membrane becomes more permeable for protons. This conclusion is supported by the observation that exposure of cells to carvacrol also causes dissipation of the proton gradient across the membrane. In accordance with these results, Sikkema et al. (25) showed an increased proton permeability of liposomal membranes during exposure to tetralin. Similarly, Cartwright et al. (4) described dissipation of the ΔpH in the presence of ethanol, due to an increased influx of protons.
Analysis of the intracellular and extracellular potassium pools revealed an increased permeability of the cell membrane for K+ upon exposure to carvacrol. K+ is the major cytoplasmic cation of growing bacterial cells, involved in several key functions of bacterial cells. This ion plays a role in the activation of cytoplasmic enzymes, the maintenance of turgor pressure, and possibly the regulation of the cytoplasmic pH (2). Different studies showed that an efflux of potassium ions is a first indication of membrane damage in bacteria (10, 24, 30). Δψ depends mainly on the potassium concentration in the cell (2). Heipieper et al. (9) showed a significant excretion of K+ to the external environment during exposure of Pseudomonas putida P8 to phenol. Gradients of solutes across the cytoplasmic membrane which use H+ as the coupling ion can also be affected by a dissipation of the proton motive force.
Although there was no immediate effect of carvacrol on the viability at concentrations of 1 mM and lower, clear effects on different bioenergetic parameters have been observed. Cells can probably cope with very low concentrations of carvacrol. Not only reduction of ATP synthesis by a dissipation of the proton motive force but also other (secondary) effects of carvacrol may result in the bactericidal or bacteriostatic action. For example, an inhibition of several enzymes due to leakage of essential ions, loss of turgor pressure, influence on DNA synthesis, reduced metabolic activities, and other processes in the cell can be a cause of the decreased viability during exposure to carvacrol. A loss of membrane integrity due to disturbance of hydrophobic interactions between lipids and proteins is often an important factor when considering the activity of toxic compounds (24). It can be concluded that the hydrophobic compound carvacrol interacts with the membranes of B. cereus by changing their permeability for cations like H+ and K+. The dissipation of ion gradients leads to impairment of essential processes in the cell and finally to cell death.
This study shows that carvacrol has biological effects at concentrations which are relevant for flavoring of foods (e.g., nonalcoholic beverages [0.18 mM/28.54 ppm] and baked goods [15.75 ppm]) (8). To products associated with outbreaks of B. cereus (e.g., rice, pasta, and soup), carvacrol could be applied both as an antimicrobial and as a flavoring compound.
ACKNOWLEDGMENTS
We thank R. A. Slump for his assistance with the viability tests and P. Breeuwer for his advice considering pHin measurements.
This work was financially supported by the Commission of the European Union through contract FAIR CT 96-1066.
REFERENCES
- 1.Arrebola M L, Navarro M C, Jiménez J, Ocaña F A. Yield and composition of the essential oil of Thymus serpylloides subsp. serpylloides. Phytochemistry. 1994;36:67–72. [Google Scholar]
- 2.Bakker E, Mangerich W E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981;147:820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breeuwer P, Drocourt J L, Rombouts F M, Abee T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol. 1996;62:178–183. doi: 10.1128/aem.62.1.178-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartwright C P, Juroszek J R, Beavan M J, Ruby M S, de Morais S M F, Rose A H. Ethanol dissipates the proton-motive force across the plasma membrane of Saccharomyces cerevisiae. J Gen Microbiol. 1986;132:369–377. [Google Scholar]
- 5.Conner D E. Naturally occurring compounds. In: Davidson P M, Branen A L, editors. Antimicrobials in foods. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 441–468. [Google Scholar]
- 6.Dufrenne J, Soentoro P, Tatini S, Day T, Notermans S. Characteristics of Bacillus cereus related to safe food production. Int J Food Microbiol. 1994;23:99–109. doi: 10.1016/0168-1605(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto S, Kashiwayanagi M, Kurihara K. Liposomes having high sensitivity to odorants. Biochim Biophys Acta. 1991;1062:7–12. doi: 10.1016/0005-2736(91)90327-5. [DOI] [PubMed] [Google Scholar]
- 8.Fenaroli G. Fenaroli’s handbook of flavor ingredients. 3rd ed. Boca Raton, Fla: CRC Press, Inc.; 1995. [Google Scholar]
- 9.Heipieper H J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heipieper H J, Meulenbeld G, Oirschot Q, de Bont J A M. Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl Environ Microbiol. 1996;62:6665–6670. doi: 10.1128/aem.62.8.2773-2777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helander I K, Alakomi H L, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid E J, von Wright A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem. 1998;46:3590–3595. [Google Scholar]
- 12.Hensel M, Achmus H, Deckers-Hebestreit G, Altendorf K. The ATP synthase of Streptomyces lividans: characterization and purification of the F1F0 complex. Biochim Biophys Acta. 1996;1274:101–108. [Google Scholar]
- 13.Jenkins R O. Production of toluene cis-glycol by Pseudomonas putida in glucose fed-batch culture. Biotechnol Bioeng. 1987;29:873–883. doi: 10.1002/bit.260290709. [DOI] [PubMed] [Google Scholar]
- 14.Juven B J, Kanner J, Schved F, Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol. 1994;76:626–631. doi: 10.1111/j.1365-2672.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 15.Kennicutt M C. ATP as an indicator of toxicity. Water Res. 1980;14:325–328. [Google Scholar]
- 16.Kim J M, Marshall M R, Cornell J A, Preston III J F, Wei C I. Antibacterial activity of carvacrol, citral, and geraniol against Salmonella typhimurium in culture medium and on fish cubes. J Food Sci. 1995;60:1364–1374. [Google Scholar]
- 17.Kim J M, Marshall M R, Wei C I. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 1995;43:2839–2845. [Google Scholar]
- 18.Knobloch K, Weigand H, Weis N, Schwarm H M, Vigenschow H. Action of terpenoids on energy metabolism. In: Brunke E J, editor. Progress in essential oil research. Berlin, Germany: Walter de Gruyter; 1986. pp. 429–445. [Google Scholar]
- 19.Kramer J M, Gilbert R J. Bacillus cereus and other Bacillus species. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 21–70. [Google Scholar]
- 20.Lagouri V, Blekas G, Tsimidou M, Kokkini S, Boskou D. Composition and antioxidant activity of essential oils from oregano plants grown wild in Greece. Z Lebensm-Unters -Forsch. 1993;197:20–23. [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Rusul G, Yaacob N H. Prevalence of Bacillus cereus in selected foods and detection of enterotoxin using TECRA-VIA and BCET-RPLA. Int J Food Microbiol. 1995;25:131–139. doi: 10.1016/0168-1605(94)00086-l. [DOI] [PubMed] [Google Scholar]
- 23.Sikkema J, de Bont J A M, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;11:8022–8028. [PubMed] [Google Scholar]
- 24.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikkema J, Poolman B, Konings W N, de Bont J A M. Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J Bacteriol. 1992;174:2986–2992. doi: 10.1128/jb.174.9.2986-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivropoulou A, Papanikolaou E, Nikolaou C, Kokkini S, Lanaras T, Arsenakis M. Antimicrobial and cytotoxic activities of origanum essential oils. J Agric Food Chem. 1996;44:1202–1205. [Google Scholar]
- 27.Thompson D P. Influence of pH on the fungitoxic activity of naturally occurring compounds. J Food Prot. 1990;53:428–429. doi: 10.4315/0362-028X-53.5.428. [DOI] [PubMed] [Google Scholar]
- 28.Thompson D P. Inhibition of growth of mycotoxigenic Fusarium species by butylated hydroxyanisole and/or carvacrol. J Food Prot. 1996;59:412–415. doi: 10.4315/0362-028X-59.4.412. [DOI] [PubMed] [Google Scholar]
- 29.Ultee A, Gorris L M G, Smid E J. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J Appl Microbiol. 1998;85:211–218. doi: 10.1046/j.1365-2672.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- 30.Uribe S, Ramirez J, Pena A. Effects of β-pinene on yeast membrane functions. J Bacteriol. 1985;161:1195–1200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]