Abstract
Bambusa arundinacea (RETZ.) Willd. is distributed in tropical regions of Pakistan, India, and China. It has been used for a long time as a folk remedy for cirrhosis, urinary tract ailments, and various other abdominal cavity disorders. It has antioxidant, free-radical-scavenging, and anti-inflammatory effects. The aims and objectives of this study were to validate the folkloric uses of Bambusa arundinacea and to evaluate its nephroprotective potential on scientific grounds. Gentamycin (G.M, 40 mg/kg) was used to induce nephrotoxicity in the animal model. Two doses of the methanolic extract of Bambusa arundinacea (MEBA; 300 and 500 mg/kg) were utilized in addition to silymarin (200 mg/kg/d). Treatments were administered once daily for 14 days. After 14 days, the blood was collected and the kidneys were removed. The antioxidant potential of the standardized MEBA was evaluated using the total phenolic content, the total flavonoid content, and the DPPH scavenging activity. The plant extract was rich with flavonoid content. The DPPH scavenging activity was 65% as compared to butylated hydroxy toluene (96%), with IC50 values 31.65 and 7.80 μg/mL, respectively. The phytochemical analysis was performed using HPLC, and MEBA was found to contain various phytoconstituents such as quercetin, caffeic acid, vanillic acid, gallic acid, chlorogenic acid, and cinnamic acid. Antioxidant enzymes such as superoxide dismutase and catalase were assayed, and MEBA exhibited significantly improved CAT and SOD levels. The levels of renal function markers such as serum creatinine, serum urea, blood urea nitrogen, serum urea, and serum uric acid levels also evaluated, and a significant retrieval was found in a dose-dependent fashion. Good improvement was also made in various hematological parameters. Statistical analysis was done using analysis of variance to determine the significance of differences among the data. In conclusion, the standardized methanolic extract of Bambusa arundinacea was able to alleviate gentamicin-induced nephrotoxicity by enhancing the antioxidant defensive mechanisms of renal tubular cells.
1. Introduction
Kidneys are vital organs. Their main function is to maintain the volume, composition, and acid–base balance of the total fluid. Many environmental xenobiotics and drugs influence these functions.1 Aminoglycosides produce nonoliguric acute renal failure in 10–25% of therapeutic courses. This type of renal failure manifests as a decreased urine concentrating capacity, tubular proteinuria, lysosomal enzymuria, mild glycosuria, and alterations in the electrolyte balance. Moreover, it also reduces ammonium excretion, depresses the glomerular filtration rate, and increases the amount of serum creatinine and blood urea nitrogen.2
The cellular mechanisms of gentamicin (G.M)-induced nephrotoxicity are still poorly understood. Reactive oxygen species (ROS) have an important role in the pathogenesis of this toxicity. The production and accumulation of ROS results in the induction of apoptosis and tubular necrosis as well as the increased infiltration of leukocytes.3 Gentamicin has been showed to increase the generation of reactive oxygen species such as superoxide anions,4−6 hydroxyl radicals, and hydrogen peroxide in kidneys, and all of these may lead to renal issues.7,8 Moreover, gentamicin reduces the efficiency of kidney antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione (GSH).9 Free radicals have also been implicated in both glomerular diseases10,11 and neutrophil-mediated glomerular diseases.12,13 The drug is nephrotoxic because a small but sizable proportion (about 5%) of the administered dose is retained in the epithelial cell linings of proximal renal tubules.14
Drugs that can mitigate the toxicity of aminoglycoside always remain a fertile area of research. In the scientific literature, we found that various drugs, such as deferoxamine, methimazole, vitamin E, vitamin C, diethyldithiocarbamate, l-histidinol, and thymoquinone, have been used to prevent G.M-induced nephrotoxicity.15 Howeverall, all these drugs are far from ideal in practice. Phytotherapy research is an area that in the recent times has been proven to successfully mange several morbidities.
Bans (Bamboo) are the common name for Bambusa arundinacea (RETZ.) Willd. Bamboo is one of the world’s most valuable plant resources. It has contributed significantly to human development and continues to do so for the approximately 2 billion people residing in the tropical and subtropical regions of Asia, Latin America, and Africa. The root (burnt root) is applied to treat ringworm, bleeding gums, and arthritis.16 The bark is used for skin eruptions. The leaf has antileprotic and anticoagulation properties that can be used in hemoptysis.17 The seeds are acrid laxatives said to be beneficial in the treatment of strangury and urinary discharges.18 The leaves of Pleioblastus amarus, a tall bamboo that grows in Southern China, have a slightly bitter and pungent taste and are used to treat fever, fidgeting, and lung inflammation.19 They are powerful nutrient sources that contain various phytochemicals, such as quercetin, gallic acid, caffeic acid, vanillic acid, chlorogenic acid, synergic acid, p-coumaric acid, and m-coumaric acid. Quercetin is a flavonoid that has powerful antioxidant properties.20−22 Gallic acid, among various polyphenols, has also emerged as a strong antioxidant.23 Cinnamic acid has antioxidant and antimicrobial properties.24p-Coumaric acid also plays a role as an anti-inflammatory compound.25 Tetrahydrocurcumin is a molecule that has anti-inflammatory properties and can help to avoid cisplatin-induced nephrotoxicity.26 Inflammation and ROS play significant roles in the pathophysiology of nephrotoxicity;27 therefore, the administration of compounds with antioxidant and anti-inflammatory properties induces ameliorative effects. The present study was designed to investigate the effect of the methanolic extract of B. arundinacea (MEBA) in a rat model of G.M-induced nephrotoxicity.
2. Material and Methods
2.1. Plant Material and Extraction Method
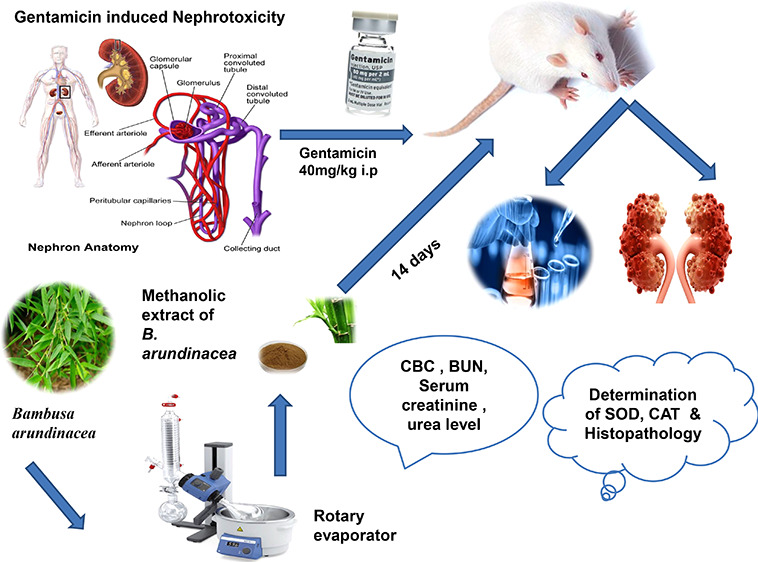
The fresh leaves of the plant of Bambusa aruandinacaea were collected from local market of Faisalabad and identified by taxonomist prof. Dr. Mansoor Ahmed at the Department of Botany, University of Agriculture Faisalabad, Faisalabad, Pakistan; voucher number: 21–2505–01 was submitted in the concerned herbarium for future reference. The fresh leaves were dried in the shade at room temperature. The dried plant was converted into a powder and soaked in methanol for 14 days. After soaking, the mixture was filtered with a muslin cloth, followed by whatman filter paper 1. The filtrate was evaporated using a rotary evaporator and stored at room temperature in a container. Moreover, the extract was mixed with normal saline and stored for experimental use.
2.2. Experimental Animals
Wistar Rats of either sex weighing 150−200 g were selected, and 25 rats were obtained from the animal house of the University of Veterinary and Animal Sciences, Lahore, Pakistan, and kept in separate cages in the animal house of the Faculty of Pharmaceutical sciences, Government College University Faisalabad, Faisalabad, Pakistan. Environmental conditions of temperature, a 12 h light–dark cycle, and humidity were provided. All the in vivo experiments were conducted according to guidelines of animal use for experimental purposes recommended by the European Union on Animal Care, and advance approval was obtained from the Institutional Review Board (IRB) of Government College University Faisalabad. Over the course of the experiment, the rats were provided with a proper diet. Water was given for all 24 h of the day.
2.3. Experimental Design
The experimental protocol was designed for 14 days. Rats of either sex were divided into five groups, and each group was comprised of five rats. Rats in every group were given the oral preparations with the feeding tube. The dosage calculation was done on the basis of body weight. Group I was maintained as the normal control group, which was given 1 mL/kg normal saline daily.28 Group II received 40 mg/kg G.M intraperitoneally (i.p.) at the same time for 14 days.29 Group III animals were treated with 200 mg/kg silymarin per oral (p.o.) and served as the standard group. Group IV and group V animals were treated with two different doses of the methanolic extract of B.arundinacea (MEBA), namely 300 and 500 mg/kg (p.o.). Groups III, IV, and V were intoxicated with gentamicin (40 mg/kg; i.p.) 3 h after the administration of silymarin or the extracts for 14 days. After 24 h of fasting following the last dose, all the animals were euthanized on the 15th day. The blood was collected in eppendorf tubes. The kidney was dissected, cleaned of extraneous tissues, and preserved in a 10% formalin solution for the histopathological examination.30
2.4. Sample Collection and Hematological Assay Parameters
The direct cardiac puncture method was adopted to acquire blood samples, which were stored in EDTA in hematological analysis gel clot vials for biochemical assays such the blood cell count. Blood samples were centrifuged at 2500 rpm for 10 min, and serum was taken to perform serum tests. The serum was stored at −20 °C for future testing. A fully automated hematological analyzer, Methic 18 Vet/Orphee/France, was used to find a complete blood profile.31
2.5. Biochemical Analysis
Blood samples were centrifuged at 2500 rpm for 10 min, and serum was taken to perform serum tests, e.g., urea, uric acid, blood urea nitrogen, superoxide dismutase (SOD), catalase (CAT), and serum creatinine. The laboratory determinations were carried out by automated chemical analyzer in our laboratory using an Olympus AU 600 analyzer. Specifically, serum samples were analyzed using electrodes ion-sensitive for sodium. The glutamate dehydrogenase (GLDH) method was used to determine urea levels, and the uricase/PAP method (an enzymatic color test) was used to determine uric acid levels.32 The serum creatinine level was determined using a creatinine calorimetric kit (Invitrogen creatinine detection kit). Creatinine and an alkaline solution react with picrate to form a colored complex. The color of the complex was measured at 492 nm. BUN was measured in blood samples with an automatic biochemistry analyzer (Hitachi 7600–020/7170A; Hitachi High-Technologies Corp., Tokyo, Japan).33
2.6. Analysis of Catalase Peroxidase (CAT) and Superoxide Dismutase (SOD)
SOD enzyme activity was determined using a RANSOD kit, which was based on the method of McCord and Fridovich. Xanthine and xanthine oxidase were used to generate superoxide anion radicals, which react with phenyl tetrazolium chloride to form a red formazan dye. The absorbance was measured at 325 nm. SOD inhibits the reaction by converting the superoxide radical into oxygen.34 The catalase activity was used to determine the presence of catalase, an enzyme that breaks down the harmful substance hydrogen peroxide into water and oxygen. An organism can produce catalase, which produces bubbles of oxygen when hydrogen peroxide is added to it. The CAT activity was determined by adding H2O2 to the sample, and the absorbance was measured at 240 nm. One unit of CAT activity was defined as an absorbance change of 0.01 U/min.35
2.7. DPPH Assay
A solution of 0.004% DPPH (1 mL) was used to assess the radical scavenging activity (RSA) of DPPH (freshly prepared). This solution was mixed with 3 mg of the plant extract; a higher concentration of the extract could be added later. After mixing, the solution was placed in dark area for 30 min. The absorbance was measured at 517 nm. Higher radical scavenging activity led to less absorbance in the reaction mixture. The antioxidant activities of ascorbic acid and butylated hydroxyl toluene (BHT) were marked as benchmarks. The control solution did not have plant extract. The experiment was repeated three times.36
2.8. Total Phenolic Content (TPC)
The total phenolic content of B. arundinacea was determined using Singleton and Rossi’s technique.100 The calibration curve was made by combining a methanolic gallic acid solution (1 mL; 0.025–0.400 mg/mL) with 5 mL of the Folin–Ciocalteu reagent (diluted 10-fold). Before the addition of sodium carbonate (4 mL, 0.115 mg/mL), the mixture was incubated for 5 min. The absorbance was measured at 765 nm after the final mixing. All the experiments were performed in triplicate. Gallic acid equivalents (GAE, mg/g) of the dry extract were used to calculate the total amount of the phenolic components in the extract.36
2.9. Total Flavonoid Content (TFC)
The total flavonoid content was determined using the method of Tian and colleagues.37 Distilled water (2 mL) was mixed with 0.5 mg of the extract and 0.15 mL of a sodium nitrite (5%) solution. This solution was incubated for 6 min. Furthermore, to the solution was added aluminum chloride (10% of 0.15 mL) was added to that solution, followed by 4% sodium hydroxide. The mixture was incubated for 6 min. Reaction mixture volume was made up to 5 mL by adding 95% methanol. After 15 min of incubation, the absorbance was recorded by a spectrophotometer at 510 nm.
2.10. Histopathological Examination
The isolated kidney was cut into small pieces, preserved, and fixed into 10% formalin for two days. The kidney pieces were the washed to remove formalin, followed by dehydration with isopropyl alcohol solutions of increasing strength (70%, 80% and 90%) for 12 h each. Then, the final dehydration was done in absolute alcohol for 12 h. Further alcohol was removed using xylene. Subsequently, the kidney pieces were subjected to paraffin infiltration in an automatic tissue processing unit.38 Hard paraffin was liquefied and transferred into square-shaped blocks. The kidney pieces were then placed in the block containing paraffin and allowed to cool.
The blocks were then cut using microtome to get sections with a thickness of 5 μM. The sections were taken on a microscopic slide onto which a sticky substance, egg albumin, had been applied. The sections were placed in an oven at 60 °C for 1 h. Subsequently, the paraffin melts and egg albumin denatured, thereby fixing the tissue slide. Staining involved the use of eosin, an acid stain that stained all the basic cell constituents pink, and hematoxylin, a basic stain that stained the entire acidic cell components blue. The slide was immersed in the hematoxylin stain for 1–2 min and then in eosin dye for 30 s. The tissue was dehydrated with the successive use of 80%, 90%, and 100% isopropyl alcohol and finally with xylene for 20–30 min. The coverslip was placed on the slides using one drop of desterene dibutyl phthalate xylene (DPX). Care was taken not to leave air bubbles, and samples were then left to dry overnight to make the permanent slide.39,40 All slides were observed for changes in histopathological characteristics, and photographs were taken by using an Accuscope 3000 microscope at 40× resolution. The purpose was to determine how much tubular necrosis, epithelial cell damage, and inflammation was present in cells due to reactive oxidative stress.
2.11. Statistical Analysis
All data are presented as the mean ± SEM. One-way analysis of variance (ANOVA) was used to analyze the data using Graph Pad Instat software package ver. 7.2. The difference was considered significant if the if P value was <0.05.
3. Results
3.1. Phytochemical Analysis of the Methanolic Extract of B.arundinacea (MEBA)
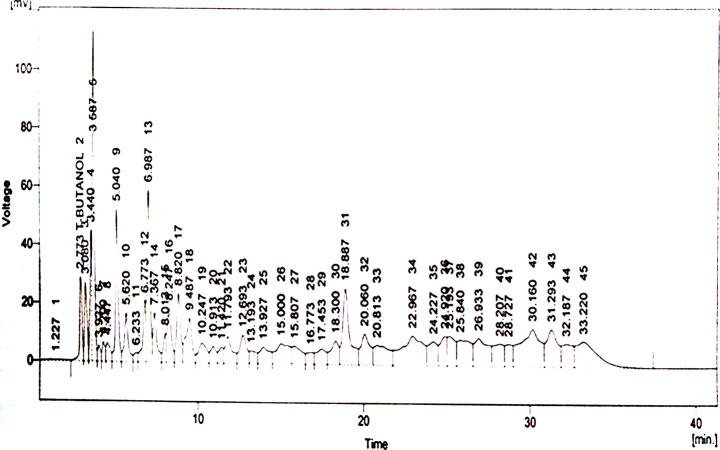
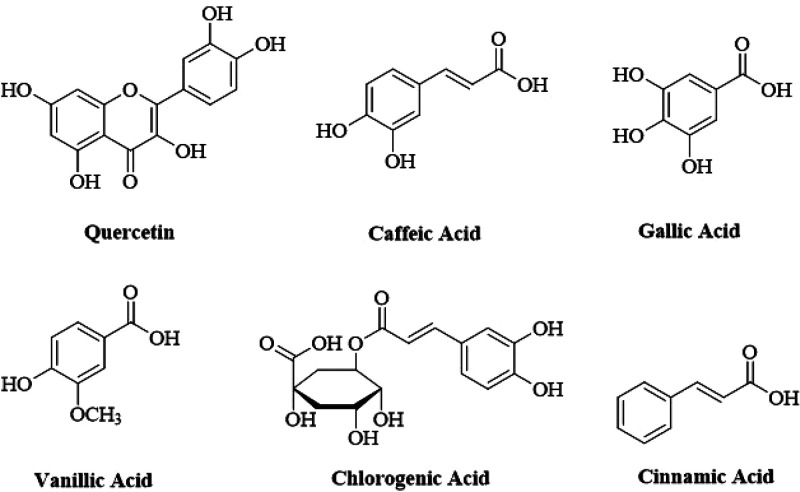
In the HPLC (model SPD-10AV with a UV–visible detector made by Shimadza, Japan) analysis, the methanolic extract of B. arundinacea (MEBA) displayed major compounds quercetin, gallic acid, vanillic acid, caffeic acid, chlorogenic acid, synergic acid, p-coumaric acid, m-coumarin acid, and cinnamic acid. Quercetin is only flavonoid, and all others are phenolic compounds. Results are shown in parts per million (ppm). The HPLC profile displays major and minor peaks detected at 230 nm. The HPLC analysis chromatograph is shown in Figure 1, and the name of compounds identified by their retention times are shown in Table 1. Chemical structures of various phytoconstituents are given in Figure 2.
Figure 1.
HPLC chromatogram of the methanolic extract of B.arundinacea.
Table 1. HPLC Analysis of Phytochemicals in the Methanolic Extract of B. arundinacea (MEBA).
| compound | retention time | absorbance (ppm) |
|---|---|---|
| quercetin | 3.080 | 15.79 |
| gallic acid | 4.447 | 4.11 |
| caffeic acid | 12.693 | 12.76 |
| vanillic acid | 13.193 | 7.91 |
| chlorogenic acid | 15.807 | 20.02 |
| synergic acid | 16.773 | 3.15 |
| p-coumaric acid | 17.453 | 2.82 |
| m-coumaric acid | 20.060 | 4.75 |
| cinnamic acid | 25.193 | 11.55 |
Figure 2.
Structures of various phytoconstituents found in the methanolic extract of B.arundinacea.
3.2. Effect of the Methanolic Extract of B. arundinacea (MEBA) on Hematological Indices
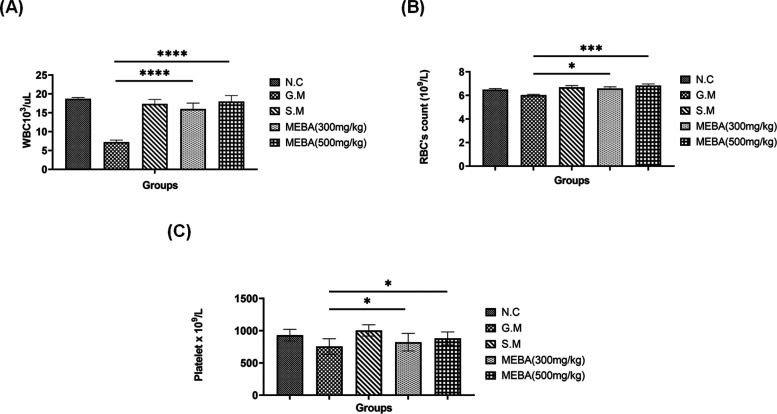
Gentamicin intoxication significantly reduced the WBC count, while MEBA at both doses (300 and 500 mg/kg) significantly (P < 0.001) increased the WBC count, which was comparable with that of the control group, in a dose-dependent fashion. Meanwhiole, gentamicin’s effect on other hematological indices such as RBCs and platelets was not so significant. However, the effect of MEBA and the standard drug silymarin were comparable, and both improved the RBC count and platelet count in a dose-dependent fashion. Results are shown in Figure 3 A–C.
Figure 3.
Effect of the methanolic extract of B. arundinacea (MEBA) on hematological indices. Effect of two different doses (300 and 500 mg/kg) of MEBA on (A) WBCs, (B) RBCs, and (C) platelets in the gentamicin disease-induced rat model. Data are presented as the mean ± SEM, n = 5. One-way analysis of variance (ANOVA) was used to evaluate the significant differences among all the groups. A statistically significant difference was found in data set A (***G.M vs MEBA), in data set B (***G.M vs 500 mg/kg MEBA and *G.M vs 300 mg/kg MEBA), and in data set C (*G.M vs MEBA). ***P < 0.001, highly significant; *P < 0.05, significant; N.C, normal control group; G.M, gentamicin (disease-induced group); and S.M, silymarin group.
3.3. Effect of the Methanolic Extract of B. arundinacea (MEBA) on Kidney Function Parameters
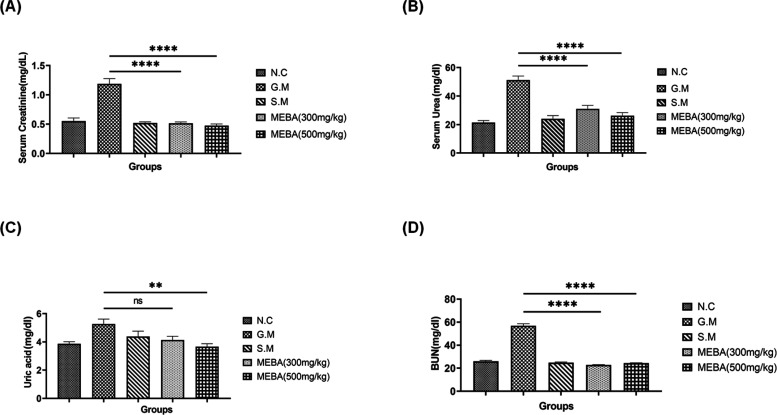
Gentamicin intoxication significantly (P < 0.001) increased the levels of serum creatinine, serum urea, uric acid, and blood urea nitrogen (BUN) as compared to those of the normal control (N.C). However, silymarin and both doses of MEBA (300 and 500 mg/kg) significantly (P < 0.001) improved all of these parameters in a dose-dependent fashion. The only exception is BUN, where the effects of the low dose are better than those of the higher dose. All the results are shown in Figure 4 A–D.
Figure 4.
Effect of the methanolic extract of B. arundinacea (MEBA) on the kidney function parameters (A) serum creatinine, (B) serum urea, (C) serum uric acid, and (D) blood urea nitrogen (BUN) in the rat model. Data are presented as the mean ± SEM, n = 5. One-way analysis of variance (ANOVA) was used to evaluate the significant differences among all the groups. A statistically significant difference was found in all data sets (***G.M vs MEBA) except data set c, where **G.M vs 500 mg/kg MEBA and ns G.M vs 300 mg/kg MEBA. ***P < 0.001, highly significant; **P < 0.01, more significant; ns, nonsignificant; N.C, normal control group; G.M: gentamicin (disease-induced group); and S.M, silymarin group.
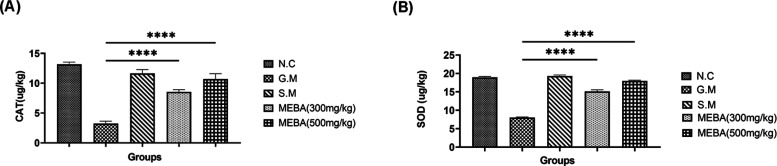
3.4. Effect of the Methanolic Extract of B. arundinacea (MEBA) on the Antioxidant Enzymes Catalase Peroxidase (CAT) and Superoxide Dismutase (SOD)
Gentamicin intoxication deteriorates the first-line antioxidant defensive system of kidney tubular cells. The levels of both the CAT and SOD were significantly (P < 0.001) reduced in the G.M intoxication group. Meanwhile, both does of MEBA (300 mg/kg and 500 mg/kg) improved the antioxidant potential by elevating the levels of CAT and SOD in a dose-dependent fashion, as shown in Figure 5 A and B, respectively.
Figure 5.
Effect of the methanolic extract of B. arundinacea (MEBA) on the antioxidant enzymes (A) catalase peroxidase (CAT) and (B) superoxide dismutase (SOD). Data are presented as the mean ± SEM, n = 5. One-way analysis of variance (ANOVA) was used to evaluate the significant differences among all the groups. A statistically highly significant difference was found for ***G.M vs MEBA in all data sets. ***P < 0.001, highly significant; N.C, normal control group; G.M, gentamicin (disease-induced group); and S.M, silymarin group.
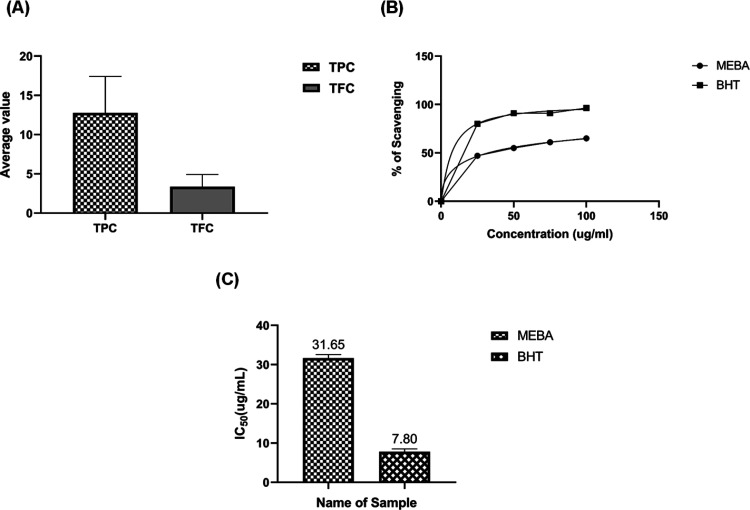
3.5. Total Flavonoid Content (TFC), Total Phenolic Content (TPC), and DPPH Radical Scavenging Potential of the Methanolic Extract of B. arundinacea (MEBA)
According to TFC and TPC analysis, MEBA was found to contain a good concentration of flavonoids. At the fixed concentration of 100 μg/mL MEBA, the radical scavenging potential was 65% as compared with that of butylated hydroxy toluene (BHT) (96%). The IC50 value of BHT was 7.80 ± 1.09 μg/mL, while that of MEBA was 31.65 ± 0.53 μg/mL. Results are shown in Figure 6.
Figure 6.
Determination of the total flavonoid content (TFC), the total phenolic content (TPC), and the DPPH radical scavenging potential of the methanolic extract of B. arundinacea (MEBA). (A) Determination of TFC and TPC. (B) determination of the radical scavenging potential. (C) IC50 values of MEBA and BHT. The experiment was performed in triplicate. Data are expressed as the mean ± SEM (n = 3, P < 0.001).
3.6. Histopathological Assay
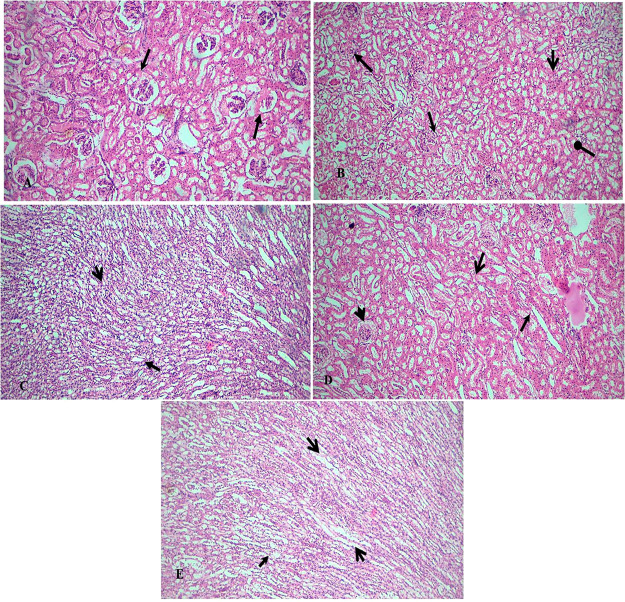
To evaluate the effect of MEBA on the histological changes in the kidney, H&E staining was performed (Figure 7). Histopathological sections from kidney tissues of G.M-treated rats showed degeneration, desquamation, and necrosis in tubules in addition to swelling in glomerulus. There was also intertubular hemorrhage and acute leukocyte infiltrations in the inter tubular region. In comparison, the coadministration of MEBA and G.M reduced these parameters in renal tissues in a dose–response manner compared to the G.M group. The administration of silymarin enhanced the regenerative activity and reduced the tubular epithelial changes, and reductions in degenerative and necrotic changes were seen in some cases. A significant change in morphologic appearance, recovered tubular epithelial cell damage, and a normal morphological view of medulla were observed with 500 mg/kg MEBA.
Figure 7.
Effect of the methanolic extract of B. arundinacea (MEBA) on gentamicin-induced nephrotoxicity. The magnification of
the microscope was 40×. (A) Healthy kidney structure was observed
in the Control group. The glomerulus and tubules (open arrow and arrow)
are in good condition. (B) In the G.M group, the kidney damage is
significant. There was acute tubular necrosis (arrow), widespread
tubular degeneration (open arrow), intertubular hemorrhage (oval arrow),
and acute leukocyte infiltrations in the intertubular region (stealth
arrow). (C) The S.M group was normal in size and shape and had normal
renal convoluted tubules and glomerulus (open arrow and arrow). The
regenerative activity is the maximum. (D) In the 300 mg/kg MEBA group,
mild tubular epithelial changes and slight tubular degenerative and
necrotic changes (open arrow and arrows) were seen. In some cases,
light leukocyte infiltrations could still be detected (stealth). (E)
The 500 mg/kg MEBA group exhibited a significant change in morphologic
appearance. The tubular epithelial cell damage was recovered, and
a normal morphological view of the medulla was observed (open arrow
and arrows). Leukocyte infiltrations were recovered (stealth arrow).
G.M, gentamicin intoxication; SM, silymarin group;  , arrow;
, arrow;  , open arrow;
, open arrow;  , stealth arrow; and
, stealth arrow; and  , oval arrow).
, oval arrow).
4. Discussion
The major mechanism behind the gentamicin-induced nephrotoxicity is the production of ROS and inflammatory mediators. Thus, it is postulated that the use of antioxidant and anti-inflammatory substances can minimize the occurrence of this toxicity. Herbal drugs are thought to be multifunctional because of the presence of multiple phytoconstituents that have antioxidant and anti-inflammatory activities. Thus, by probing the scientific literature, we can find a long list of various plant, such as Pistacia vera, Bauhinia purpurea, Ferulago angulata, grape seed, and Zingiber officinales, that have a vital roles in gentamicin-induced nephrotoxicity. Examples of herbal plants used as kidney protective agents are Urticadioica L, soybean (Glycine max), Chrysanthemum indicum, Glycyrrhizia glabra, and many more.29,39
The plant B. arundinacea of family Poaceae used for this research is traditionally used for a number of remedies.18 The plant has been used in homeopathy as herbal diuretics. In the present study, B. arundinacea was tested to validate its folkloric use as a nephroprotective agent in kidney ailments.16 Gentamicin-induced nephrotoxicity is distinguished by increased levels of urea, uric acid, creatinine, and blood urea nitrogen in plasma as well as urine. Over 14 days, a 40 mg/kg dose of gentamicin significantly reduced the WBC count and amount of antioxidant enzymes, as discussed in the Results (Figures 3–5). Two plants extract doses (300 and 500 mg/kg) are critically observed and compared with the gentamicin group. The plant extracts significantly improved both the WBC count and the activity of antioxidant enzymes. The plant extracts decreased the levels of serum toxicity biomarkers such as serum creatinine, serum urea, and uric acid.
Several compounds with antioxidant activity, such as quercetin, caffeic acid, and gallic acid, have been successfully used to prevent or ameliorate gentamicin-induced nephrotoxicity.25 In the present study, the role of ROS in G.M-induced nephrotoxicity was assessed using the antioxidant agent MEBA and the levels of the biochemical indicators of oxidative stress, mainly SOD and CAT, were evaluated. It has been reported that under normal conditions ROS generated during cellular functions are eliminated by intrinsic antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase.25 Thus, a DPPH assay was also performed to evaluate the antioxidant potential of MEBA. The DPPH scavenging activity of MEBA was found to be 65% as compared to BHT (96%), with IC50 values of 31.65 and 7.80 μg/mL, respectively. (Figure 7). Therefore, as an ROS scavenger with antioxidant molecules, MEBA may have the capacity to partially reduce or eliminate the deleterious effects induced by gentamicin. As already mentioned, this antioxidant potential is might be due to the presence of various phytoconstituents, the structures of which are shown in Figure 2.
Our study results are in accordance with the earlier published reports explaining the beneficial effect of B. arundinacea against reactive oxygen species.39 In earlier studies, some investigators demonstrated in their observations the histopathological and structural changes in renal tissue after the administration of gentamicin.39 We have reported the histopathological view of renal sections in the gentamicin-treated group (Figure 7B), which compared to the control group showed degeneration, desquamation, and necrosis in tubules as well as swelling in the glomerulus (Figure 7A). Similarly, in histopathological examination in the present work, we observed damage in the structures of the kidneys of gentamicin-treated rats. Glomerular and tubular epithelial changes were considerably mild in the groups treated with the methanolic extract of Bambusa arundinacea (300 and 500 mg/kg), and the restoration of normal histopathology was also observed. Two weeks of treatment with G.M and the methanolic extract at a dose of 300 mg/kg showed reductions in tubular necrosis and tubular degeneration, as was observed from histopathology; however, slight leukocyte infiltrations in the intratubular area were present. In case of animals treated with G.M and methanolic extract at a dose of 500 mg/kg, the regeneration of tubular epithelial cells was observed, and there was no sign of necrosis, degeneration, or mild inflammation (Figure 7D and E).
It can be concluded that morphological changes in kidneys were caused by the G.M injection, but these changes were considerably mild in the G.M plus methanolic extract (500 mg/kg)-treated animals. The extract dose of 300 mg/kg seems to normalize kidney parameters less effectively as compared to 500 mg/kg MEBA. A plausible explanation is that this plant shows nephroprotective activity attributed to its phytochemical constituents quercetin, caffeic acid, vanillic acid, gallic acid, chlorogenic acid, and cinnamic acid in a dose-dependent fashion. In short, the data indicated that G.M-induced nephrotoxicity might be related to oxidative damage. The coadministration of Bambusa arundinacea diminished the negative effects of G.M-induced nephrotoxicity, possibly by inhibiting a free-radical-mediated process. Further investigation of these promising protective effects of Bambusa aundinacea against G.M-induced renal injury may have a considerable impact on the development of clinically feasible strategies to treat patients with renal failure. There is also emerging trend of modernizing techniques for formulation development,42,43 which can also be implemented for this type of standardized plant extract for better compliance and bioavailability in future.
Therefore, it could be concluded that the methanolic extract of B. arundinacea possessed nephroprotective activity against gentamicin-induced toxicity, hence validating its traditional use. The result of the study confirms the nephroprotective activity of B. arundinacea in experimental animal models. Further research is definitely needed to isolate the compound accountable for such activity and to establish the mechanism of action.
5. Conclusion
In conclusion, it might be postulated that the methanolic extract of B. arundinacea (MEBA) significantly improved the gentamicin-induced nephrotoxicity in animal models, which might provide the rationale for the folkloric uses of B. arundinacea. The protective effects of the plant extract were due the presence of various phytoconstituents, as already discussed in the prior section Thus, this study validates the traditional uses of this plant and opens a new era for carrying out further molecular level research after the isolation and purification of various known and unknown compounds.
Acknowledgments
Authors are thankful to Department of Pharmacology, Government College University Faisalabad, for providing laboratory and animal house facilities.
No funding was provided.
The authors declare no competing financial interest.
References
- Begg E. J.; Barclay M. L. Aminoglycosides--50 years on. Br. J. Pharmacol. 1995, 39, 597–603. [PMC free article] [PubMed] [Google Scholar]
- Kaloyanides G. J.; Pastoriza-Munoz E. Aminoglycoside nephrotoxicity. Kidney Int. 1980, 18, 571–582. 10.1038/ki.1980.175. [DOI] [PubMed] [Google Scholar]
- Martínez-Salgado C.; Eleno N.; Tavares P.; Rodríguez-Barbero A.; García-Criado J.; Bolaños J. P.; López-Novoa J. M. Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int. 2002, 62, 1682–1692. 10.1046/j.1523-1755.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- Li J.; Li Q.-x.; Xie X.-f.; Ao Y.; Tie C.-r.; Song R.-j. Differential roles of dihydropyridine calcium antagonist nifedipine, nitrendipine and amlodipine on gentamicin-induced renal tubular toxicity in rats. Eu. J. Pharmacol. 2009, 620, 97–104. 10.1016/j.ejphar.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Yaman İ.; Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp. Toxicol. Pathol. 2010, 62, 183–190. 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Nitha B.; Janardhanan K. Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food Chem. Toxicol. 2008, 46, 3193–3199. 10.1016/j.fct.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Balakumar P.; Chakkarwar V. A.; Kumar V.; Jain A.; Reddy J.; Singh M. Experimental models for nephropathy. J. Renin Angiotensin Aldosterone Syst. 2008, 9, 189–195. 10.1177/1470320308098343. [DOI] [PubMed] [Google Scholar]
- Walker P. D.; Barri Y.; Shah S. V. Oxidant mechanisms in gentamicin nephrotoxicity. Renal Failure 1999, 21, 433–442. 10.3109/08860229909085109. [DOI] [PubMed] [Google Scholar]
- Polat A.; Parlakpinar H.; Tasdemir S.; Colak C.; Vardi N.; Ucar M.; Emre M. H.; Acet A. Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochemica. 2006, 108, 365–371. 10.1016/j.acthis.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Effect of enzymatically generated reactive oxygen metabolites on the cyclic nucleotide content in isolated rat glomeruli. J. Clin. Investig. 1984, 74, 393–401. 10.1172/JCI111434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V.; Baricos W. H.; Basci A. Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase (s) by reactive oxygen metabolites. J. Clin. Investig. 1987, 79, 25–31. 10.1172/JCI112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan A.; Wiggins R. C.; Kunkel R. G.; Till G. O.; Johnson K. J. Glomerular injury and proteinuria in rats after intrarenal injection of cobra venom factor. Evidence for the role of neutrophil-derived oxygen free radicals. Am. J. Pathol. 1986, 123, 57–66. [PMC free article] [PubMed] [Google Scholar]
- Rehan A.; Johnson K. J.; Kunkel R. G.; Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985, 27, 503–511. 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq M.-P.; Tulkens P. M. Aminoglycosides: nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43, 1003–1012. 10.1128/AAC.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: some recent research. Food Chem. Toxicol. 2003, 41, 1447–1452. 10.1016/S0278-6915(03)00186-8. [DOI] [PubMed] [Google Scholar]
- Soni V.; Jha A. K.; Dwivedi J.; Soni P. Traditional uses, phytochemistry and pharmacological profile of Bambusa arudinacea Retz. Cell. Med. 2013, 3, 20.1–20.6. 10.5667/tang.2013.0011. [DOI] [Google Scholar]
- Indian medicinal plants: An illustrated dictionary; Khare C. P., Ed.; Springer Science & Business Media, 2007. [Google Scholar]
- Chopra R. N., Indigenous drugs of India: Their medicinal and economic aspecta; The Art Press: Kolkata, India, 1933. [Google Scholar]
- Vanithakumari G.; Manonayagi S.; Padma S.; Malini T. Antifertility effect of Bambusa arundinacea shoot extracts in male rats. J. Ethnopharmacol. 1989, 25, 173–180. 10.1016/0378-8741(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Swarts S. G.; Yin L.; Liu C.; Tian Y.; Cao Y.; Swarts M.; Yang S.; Zhang S. B.; Zhang K.. et al. Antioxidant properties of quercetin. In Oxygen transport to tissue XXXII; LaManna J. C.; Puchowicz M. A.; Xu K.; Harrison D. K.; Bruley D. F., Eds.; Springer, 2011; pp 283–289. [DOI] [PubMed] [Google Scholar]
- Hussain L.; Akash M. S. H.; Tahir M.; Rehman K.; Ahmed K. Z. J. Hepatoprotective effects of methanolic extract of Alcea rosea against acetaminophen-induced hepatotoxicity in mice. Bangladesh J. Pharmacol. 2014, 9, 322–327. 10.3329/bjp.v9i3.19068. [DOI] [Google Scholar]
- Manan M.; Saleem U.; Akash M. S. H.; Qasim M.; Hayat M.; Raza Z.; Ahmad B. J. A. o. Antiarthritic Potential of Comprehensively Standardized Extract of Alternanthera bettzickiana: In Vitro and In Vivo Studies. ACS Omega 2020, 5, 19478–19496. 10.1021/acsomega.0c01670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhani B.; Sharma N.; Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. Rsc Adv. 2015, 5, 27540–27557. 10.1039/C5RA01911G. [DOI] [Google Scholar]
- Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. 10.2174/138955712801264792. [DOI] [PubMed] [Google Scholar]
- Kannan R. R. R.; Arumugam R.; Thangaradjou T.; Anantharaman P. Phytochemical constituents, antioxidant properties and p-coumaric acid analysis in some seagrasses. Food Res. Int. 2013, 54, 1229–1236. 10.1016/j.foodres.2013.01.027. [DOI] [Google Scholar]
- Heidari-Soreshjani S.; Asadi-Samani M.; Yang Q.; Saeedi-Boroujeni A. Phytotherapy of nephrotoxicity-induced by cancer drugs: an updated review. J. Nephropathol. 2017, 6, 254. 10.15171/jnp.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafieian-Kopaei M.; Nasri H. Re: Erythropoietin ameliorates oxidative stress and tissue injury following renal ischemia/reperfusion in rat kidney and lung. Med. Princ. Pract. 2014, 23, 95. 10.1159/000350842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan S.; Vijayarathna S.; Jothy S. L.; Ping K. Y.; Latha L. Y. Hepatoprotective potential of Elaeis guineensis leaf against paracetamol induced damage in mice: A serum analysis. Int. Proc. Chem. Biol. Environ. Eng. 2012, 39, 231–234. [Google Scholar]
- Rad A. K.; Mohebbati R.; Hosseinian S. Drug-induced nephrotoxicity and medicinal plants. Iran. J. Kidney Dis. 2017, 11, 169. [PubMed] [Google Scholar]
- Ghaznavi H.; Mehrzadi S.; Dormanesh B.; Tabatabaei S. M. T. H.; Vahedi H.; Hosseinzadeh A.; Pazoki-Toroudi H.; Rashidian A. Comparison of the protective effects of melatonin and silymarin against gentamicin-induced nephrotoxicity in rats. J. Evid. Based Complementary Altern. Med. 2016, 21, NP49–NP55. 10.1177/2156587215621672. [DOI] [PubMed] [Google Scholar]
- Hadi M. A.; Almamoori A. M.; Al-Hassnawi A. T.; Hameedi E. H. Oxidative response associated with treatment of male Albino rats with Eruca sativa Mill leaves extract and correlations with complete blood picture. J. Pharm. Sci. Res. 2017, 9, 2278–2285. [Google Scholar]
- Milionis H. J.; Kakafika A. I.; Tsouli S. G.; Athyros V. G.; Bairaktari E. T.; Seferiadis K. I.; Elisaf M. S. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. Am. Heart J. 2004, 148, 635–640. 10.1016/j.ahj.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Tan F.; Chen Y.; Yuan D.; Gong C.; Li X.; Zhou S. Dexmedetomidine protects against acute kidney injury through downregulating inflammatory reactions in endotoxemia rats. Biomed. Rep. 2015, 3, 365–370. 10.3892/br.2015.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assady M.; Farahnak A.; Golestani A.; Esharghian M. Superoxide dismutase (SOD) enzyme activity assay in Fasciola spp. parasites and liver tissue extract. Iran. J. Parasitol. 2011, 6, 17–22. [PMC free article] [PubMed] [Google Scholar]
- Khan R. A.; Khan M. R.; Sahreen S. CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern. Med. 2012, 12, 178. 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissedre P. L.; Frankel E. N.; Waterhouse A. L.; Peleg H.; German J. B. Inhibition of In vitro human LDL oxidation by phenolic antioxidants from grapes and wines. J. Sci. Food Agric. 1996, 70, 55–61. . [DOI] [Google Scholar]
- Singleton V. L.; Rossi J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 3, 144–158. [Google Scholar]
- Tian W.; Chen G.; Gui Y.; Zhang G.; Li Y. Rapid quantification of total phenolics and ferulic acid in whole wheat using UV–Vis spectrophotometry. Food Control 2021, 123, 107691. 10.1016/j.foodcont.2020.107691. [DOI] [Google Scholar]
- Tamri P.; Hemmati A.; Amirahmadi A.; Zafari J.; Mohammadian B.; Dehghani M.; Allahyari N. Evaluation of wound healing activity of hydroalcoholic extract of banana (Musa acuminata) fruit’s peel in rabbit. Pharmacol. Online 2016, 3, 203–208. [Google Scholar]
- Boroushaki M. T.; Fanoudi S.; Mollazadeh H.; Boroumand-Noughabi S.; Hosseini A. Reno-protective effect of Rheum turkestanicum against gentamicin-induced nephrotoxicity. Iran. J. Basic Med. Sci. 2019, 22, 328–333. 10.22038/ijbms.2019.31552.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain L.; Ikram J.; Rehman K.; Tariq M.; Ibrahim M.; Akash M. S. H. Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turk. J. Biol. 2014, 38, 396–402. 10.3906/biy-1312-32. [DOI] [Google Scholar]
- Kim T. Y.; Nam Y. R.; Park J. H.; Lee D.-E.; Kim H.-S. J. A. o. Site-specific lipidation of a small-sized protein binder enhances the antitumor activity through extended blood half-life. ACS Omega 2020, 5, 19778–19784. 10.1021/acsomega.0c02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baati T.; Njim L.; Jaafoura S.; Aouane A.; Neffati F.; Ben Fradj N.; Kerkeni A.; Hammami M.; Hosni K. J. A. o. Assessment of Pharmacokinetics, Toxicity, and Biodistribution of a High Dose of Titanate Nanotubes Following Intravenous Injection in Mice: A Promising Nanosystem of Medical Interest. ACS Omega 2021, 6, 21872–21883. 10.1021/acsomega.1c01733. [DOI] [PMC free article] [PubMed] [Google Scholar]