Abstract
Pulmonary hypertension (PH), which includes pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH), is a progressive condition with significant morbidity and mortality due to right heart failure if left untreated. Riociguat is a soluble guanylate cyclase (sGC) stimulator and is the only treatment approved for both PAH and CTEPH. The objectives of this review are to describe the epidemiology and pathophysiology of PAH and CTEPH; synthesize the pharmacology, efficacy, safety, and utilization of riociguat; and discuss the role of the pharmacist in managing patients with these conditions. Data presented in this review is supported by peer reviewed literature, using PubMed and key words including pulmonary hypertension, pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, and riociguat. The review draws on key studies and review articles that discuss the pathophysiology of PAH and CTEPH, as well as articles discussing the safety and efficacy of riociguat. The overall goal in the treatment of PAH and CTEPH is to improve long-term survival. Treatment planning depends on the type of PH, treatment goals, comorbidities, and risk profiles. Pharmacists serve a valuable role as part of the multidisciplinary team in the care of patients with PH, many of whom may have comorbidities that contribute to high costs and resource utilization. Riociguat is a first-in-class medication and the only approved treatment for both PAH and CTEPH. In clinical trials, riociguat has demonstrated favorable efficacy and tolerability. Riociguat is a valuable addition to the armamentarium of options for treating patients with PH.
Keywords: pulmonary hypertension (PH), pulmonary arterial hypertension (PAH), chronic thromboembolic pulmonary hypertension (CTEPH), and riociguat
Introduction
Pulmonary hypertension (PH) is a progressive condition of multifactorial etiology. 1 PH is associated with markedly decreased exercise capacity and reduced quality of life. 2 Despite the availability of treatments, the natural history and prognosis are variable and patients with PH may progress to right heart failure and death.1,3 Five groups have been defined for clinical classification that categorize PH based on the underlying cause of elevated pulmonary pressures.4,5 Pulmonary arterial hypertension (PAH) represents Group 1 and includes the following subgroups: idiopathic, heritable, drug- and toxin-induced, persistent PH of the newborn, and associated PAH. 5 Most recently, 2 additional subgroups have been added to Group 1 to account for differences in treatment considerations—PAH long-term responders to calcium channel blockers and PAH with overt features of venous involvement. 4 PH due to pulmonary artery obstruction represents Group 4 and includes chronic thromboembolic pulmonary hypertension (CTEPH).
This article reviews the epidemiology and pathophysiology of these 2 groups—PAH and CTEPH—as well as the pharmacology, pharmacokinetics, dosing, and clinical efficacy and safety of riociguat; the utilization of riociguat in treating patients with PH; and the role of the pharmacist in the appropriate and safe use of therapies for PAH and CTEPH.
Pathophysiology and Epidemiology
The complex pathophysiology of PAH (Group 1 PH) is characterized by the remodeling of small pulmonary arteries via proliferation of smooth muscle and endothelial cells. 6 This leads to a reduction in the size of the pulmonary artery lumen and reactivity of the vascular bed, an increase in pulmonary vascular resistance (PVR), and elevated pressure in the pulmonary circulation. 3 Treatments approved for PAH target key pathways of endothelial dysfunction involving endothelin-1, nitric oxide, and prostacyclin.6,7
PAH is rare, with prevalence estimates in the range of 10.6 to 52 per million people.8-10 PAH predominantly affects women (female: male ratio of 4:1). 11 PH is a common complication of connective tissue diseases (CTDs), with prevalence as high as 12% in patients with systemic sclerosis (SSc). 12 Survival rates have improved considerably compared with the National Institutes of Health registry cohort for patients with a diagnosis of PAH in the 1980s.1,13 The median survival has been estimated to be 85% at 1 year and 58% at 5 years. 1 In fact, 30% of SSc-related deaths are due to PAH, which remains the leading cause of morbidity and mortality in this population. 12 Although overall mortality rates in patients with PAH have improved, the morbidity and mortality associated with the condition remain unacceptably high.
Although the exact pathogenesis is uncertain, CTEPH (Group 4 PH) primarily occurs after an acute pulmonary embolism (PE) and is thought to be the result of single or recurrent PE arising from venous thrombosis.6,14,15 CTEPH may also be caused by in situ thrombosis in the lung as a result of primary arteriopathy and endothelial dysfunction, similar to PAH. Ultimately, CTEPH results from mechanical obstruction of the pulmonary arteries. This obstruction is caused by the presence of organized fibrotic thrombi tightly attached to the medial layer of the elastic pulmonary arteries, replacing the normal intima. 16 The consequences of this occlusion include restricted blood flow and increased PVR.6,17 Progressive pulmonary vascular remodeling (eg, thrombi organization, fibrous stenosis, microvascular changes) obstructs pulmonary arteries, leading to PH and right heart failure.3,14
The epidemiology of CTEPH is not well established, but the incidence of CTEPH after symptomatic acute PE is reported to range from 0.4% to 6.2%; however, the true incidence of CTEPH may be higher, because the disease is often underdiagnosed due to variable rates of prior acute PE and non-specific symptoms.18-21 The prognosis is especially poor in patients with untreated CTEPH, with 3-year mortality rates approaching 90%. 22
Clinical Management and Treatment Guidelines
The overall goal in the treatment of PH (including PAH and CTEPH) is to improve risk status and long-term outcomes.23,24 Additional goals of therapy may include improvements in exercise tolerance, delay in clinical worsening, and factors related to quality of life. Management considerations include type of PH, treatment goals, presence and type of comorbidities, risk profiles, and patient preference for treatment.24,25
PAH
Historically, individual indicators of prognosis, such as World Health Organization Functional Class (WHO FC) (Table 1), have been used to guide decisions about appropriate therapy; however, additional considerations now include a risk-based approach, with the development of multiple risk stratification strategies. 24 The current European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines recommend that treatment decisions should be guided by a comprehensive, multiparameter risk assessment. 5 These guidelines provide a summary of risk determinants based on prognostic relevance, which includes clinical signs of right heart failure, progression of symptoms, syncope, 6-minute walking distance (6MWD), cardiopulmonary exercise testing, biomarkers, imaging, and hemodynamics. 5 According to this assessment, the patient can be classified as having low, intermediate, or high risk for estimated 1-year mortality of <5%, 5% to 10%, or >10%, respectively. 5 This approach is supported by recently published recommendations from the 6th World Symposium on Pulmonary Hypertension (WSPH). 26
Table 1.
WHO FC.
| WHO FC | Description |
|---|---|
| I | No limitation of physical activity; ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near syncope |
| II | Slight limitation of physical activity, but no symptoms at rest; ordinary physical activity causes undue dyspnea or fatigue, chest pain, or near syncope |
| III | Marked limitation of physical activity, but no symptoms at rest; less than ordinary physical activity causes undue dyspnea or fatigue, chest pain, or near syncope |
| IV | Inability to perform any physical activity without discomfort; symptoms may be present at rest; signs of right-sided heart failure present |
Abbreviation: WHO FC, World Health Organization Functional Class.
Source: Galiè N, et al (2015). 24
Treatment goals for patients with PAH include attaining low-risk status to achieve improvements in exercise capacity, quality of life, and right ventricular (RV) function, as well as lowering mortality risk. 5 Multiparameter goals of treatment have been proposed based on a set of prognostic indicators (ie, improvement in FC [I or II]; normal/near-normal RV size and function; hemodynamic parameters showing normalization of RV function; 6MWD >380 to 440 m; cardiopulmonary exercise testing, including peak oxygen consumption >15 mL/min/kg and ventilatory equivalent for CO2 [EqCO2] <45 L/min; and normal brain natriuretic peptide [BNP] levels). 23
The 2015 ESC/ERS guidelines offer recommendations for pharmacologic management of PAH based on WHO FC. 5 Oral monotherapies for patients with WHO FC II symptoms with an endothelin receptor antagonist (ERA) (ie, bosentan, ambrisentan, or macitentan), a phosphodiesterase-5 inhibitor (PDE-5i; ie, sildenafil or tadalafil), a soluble guanylate cyclase (sGC) stimulator (ie, riociguat), or a selective IP receptor agonist (ie, selexipag) all receive Class I recommendations. For patients with WHO FC III symptoms, additional Class I recommendations include prostacyclin analogues (ie, IV epoprostenol, inhaled iloprost, or inhaled or SC treprostinil). 5 In addition to recommendations for monotherapy, the guidelines include recommendations for both up-front and sequential combination therapies. Up-front combination therapy with ambrisentan and tadalafil received a Class I recommendation in patients with WHO FC II or III symptoms. Several sequential combination therapies for WHO FC II and III received Class I recommendations, including macitentan added to sildenafil, riociguat added to bosentan, and selexipag added to an ERA and/or to a PDE-5i. Sildenafil added to epoprostenol also has a Class I recommendation for WHO FC III patients only. 5
The 6th WSPH, held in Nice, France, in 2018, updated recommendations for patients with PAH, including medical therapy. 27 A treatment algorithm provided an overview of initial strategies based on the severity of PAH, assessed by a multiparametric risk stratification approach. The task force recommends that after confirmation of the diagnosis of PAH, the initial approach and initial monotherapy for treatment-naïve patients should follow the 2015 ESC/ERS PH guidelines. 26 Based on these guidelines, further treatment escalation is required if low-risk status is not achieved in scheduled follow-up assessments. 26
The 2018 American College of Chest Physicians (CHEST) guidelines and expert panel report on pharmacotherapy also provide recommendations on pharmacologic therapy for PAH. 28 These guidelines suggest that management should be based on WHO FC and previous exposure to PAH-targeted therapies, with additional consideration for prognosis. In addition, patients should be evaluated at a PH center before therapy is initiated. A new recommendation from the expert panel suggests that treatment-naïve patients with WHO FC II or III symptoms should be started on first-line oral combination therapy with ambrisentan and tadalafil. For those unwilling or unable to tolerate combination therapy, up-front oral monotherapy is recommended as an alternative with an ERA (ie, bosentan, ambrisentan, or macitentan), a PDE-5i (ie, sildenafil or tadalafil), or an sGC stimulator (ie, riociguat). 28 Initiation of a parenteral prostanoid is suggested for patients with WHO FC III symptoms, evidence of rapid progression, and markers of poor prognosis. 28 Referral to an expert center for management is also recommended. For patients with WHO FC IV symptoms, initiation of therapy with a parenteral prostanoid is recommended; intravenous epoprostenol is generally regarded as the therapy of choice. 28 The second new recommendation states that patients with stable or symptomatic PAH on background therapy with ambrisentan should have tadalafil added. 28
CTEPH
CTEPH is the only potentially curable form of PH, through pulmonary endarterectomy (PEA) surgery.5,29 For patients with CTEPH who are eligible for PEA, the goal of treatment is to cure the disease. For this reason, all patients with CTEPH should be evaluated for surgery, and PEA should be offered to those who are eligible.5,21 These are both class 1 recommendations according to the 2015 ESC/ERS guidelines. 5 Unfortunately, with up to 50% of CTEPH patients ineligible for surgery because of comorbidities or inaccessible distal location of pulmonary vascular obstructions, a substantial need for medical therapy remains.30,31 According to the ESC/ERS guidelines, riociguat has a class 1 recommendation for use in symptomatic patients with persistent/recurrent CTEPH after PEA, or for patients with inoperable CTEPH as determined by a specialist team that includes at least 1 experienced PEA surgeon. 5
For patients who are ineligible for PEA, balloon pulmonary angioplasty (BPA) has emerged as an alternative; however, BPA should be reserved for use at expert centers. 21 A large UK national cohort study reported that 51% of patients had mean pulmonary artery pressure (mPAP) ≥25 mmHg 3 months to 6 months following PEA. 32 In patients who are not candidates or who have persistent or recurrent PH symptoms following PEA, targeted medical therapy—with or without BPA—is recommended.5,29 Additionally, all patients should be treated with lifelong anticoagulation therapy, regardless of surgical candidacy.
Role of Riociguat in Therapy
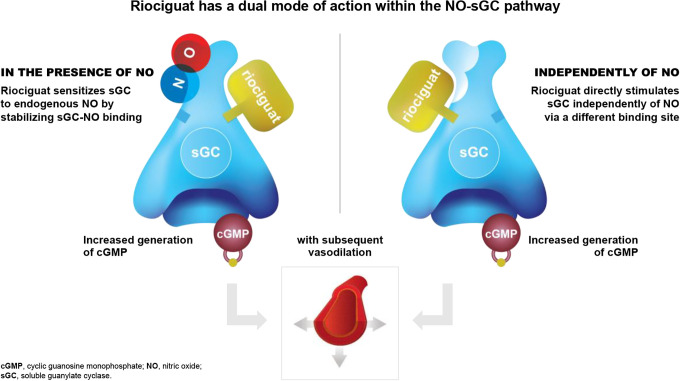
Riociguat was approved by the FDA in October 2013 as the first medication from the novel class of sGC stimulators and is the only treatment approved for both PAH and CTEPH. As an sGC stimulator with a dual mode of action, riociguat acts in synergy with endogenous nitric oxide and directly stimulates sGC independently of nitric oxide availability. This increased production of cyclic guanosine monophosphate (cGMP) results in vasorelaxation and antiproliferative and antifibrotic effects33,34 (Figure 1).
Figure 1.
Riociguat mechanism of action.33,34 Riociguat has a dual mode of action and sensitizes, soluble guanylate cyclase (sGC) to endogenous nitric oxide (NO) in the presence of NO (left) and acts independently of NO to directly stimulate SGC via a different binding site (right).
Clinical Trials
PATENT-1/-2
In a 12-week, multicenter, double-blind, placebo-controlled Phase 3 trial (PATENT-1), patients with symptomatic PAH (n = 443) were randomized in a 2:4:1 ratio to receive placebo, riociguat titrated up to 2.5 mg 3 times daily (TID), or riociguat titrated up to 1.5 mg TID, respectively. 35 Patients who were treatment-naïve and those on background therapy with an ERA or non-intravenous prostanoids were eligible for inclusion. Notably, patients receiving a PDE-5i were ineligible. 35 The primary outcome was the change from baseline to week 12 in 6MWD. Secondary endpoints included PVR, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, WHO FC, and time to clinical worsening. 35 There were no significant differences in baseline characteristics between the 2.5 mg group and the placebo group. Most patients were WHO FC II and III (42% and 53%, respectively). At 12 weeks, the 2.5 mg group showed a mean increase in 6MWD of 30 m as compared with a reduction of 6 m in the placebo group (P < 0.001). Improvements in 6MWD were observed both in treatment-naïve patients and in those on background ERA (n = 167) or prostanoid (n = 24). 36 Riociguat significantly improved PVR, NT-proBNP levels, WHO FC, and time to clinical worsening. The most common serious adverse event in the 2.5 mg-maximum group and in the placebo group was syncope (1% and 4%, respectively). 35 An open-label extension study (PATENT-2) included 396 patients who had completed PATENT-1. Improvements in 6MWD, WHO FC, and NT-proBNP concentrations were maintained after 2 years of treatment. 37
Given the relatively high prevalence of PAH in CTD, a subgroup from the PATENT studies was analyzed. Patients with PAH-CTD demonstrated improvements in 6MWD that were maintained at 2 years.38,39 At 2 years, WHO FC had improved in 36%, stabilized in 59%, and worsened in 6% of the PAH-CTD population treated with riociguat. 39
RESPITE
As outlined in the 2015 ESC/ERS guidelines, PDE-5i is a first-line option for PAH FC II-III; however, a proportion of patients fail to reach treatment goals. The objective of the RESPITE trial was to investigate the possible benefit of switching patients from PDE-5i to riociguat. Results of the 24-week, Phase 3b, prospective, open-label, single-arm trial demonstrated potential benefit of riociguat as a second-line agent after PDE-5i treatment failure in patients with PAH. 40 Patients with symptomatic PAH who had an insufficient response to treatment with tadalafil or sildenafil were included. Patients could continue background therapy with ERAs. Exploratory outcome measures included 6MWD, WHO FC, and NT-proBNP, as well as hemodynamic parameters, such as PVR and cardiac index. 40 Of the 61 patients enrolled, 51 completed the trial. Clinical improvement in all outcome measures was observed in patients with PAH who switched from PDE-5i treatment to riociguat. 40 A post-hoc analysis of RESPITE showed that switching to riociguat improved RV function in patients who had insufficient PDE-5i response. 41 Further study of this strategy may be warranted before it is routinely used in clinical practice. Two drug-related serious adverse events (SAEs) were reported from this study—RV failure and asthenia. 40 No SAEs occurred during the PDE-5i treatment-free period, and only 2 patients reported SAEs during the first 2 weeks of riociguat treatment. 40
CHEST-1/-2
In a 16-week, multicenter, double-blind, placebo-controlled Phase 3 study, patients (n = 261) with inoperable CTEPH or persistent/recurrent CTEPH after PEA were randomized 2:1 to receive riociguat titrated up to 2.5 mg TID or placebo. Patients who had received treatment with an ERA, a prostacyclin analogue, a PDE-5i, or nitric oxide donors within 3 months of enrollment were excluded. 42 The primary outcome was change in 6MWD. Secondary outcomes included changes in cardiopulmonary hemodynamics, NT-proBNP, WHO FC, and time to clinical worsening. By week 16, riociguat significantly improved the primary endpoint of 6MWD, as well as the key secondary endpoints of PVR, NT-proBNP, and WHO FC. 42 The riociguat group showed a mean increase in 6MWD of 39 m, compared to a reduction of 6 m in the placebo group (P < 0.001). Improvements in WHO FC were consistent across predefined subgroups. 43 RV failure was the most common serious adverse event, occurring in 3% of patients in each group, followed by syncope (in 2% of the riociguat group and 3% of the placebo group). 42 An open-label extension study (CHEST-2) included 237 patients who had completed CHEST-1. The probability of survival was 97% at 1 year and 94% at 2 years. 43 Results of the CHEST-1/-2 studies showed that riociguat was well tolerated in patients with inoperable or persistent CTEPH. 43
Ongoing Clinical Trials
Riociguat is also being explored in the settings of PEA and BPA, which are options for selected patients with inoperable CTEPH or as rescue therapy after surgery. Sequential therapy with riociguat and BPA has been investigated in patients with inoperable CTEPH and has shown benefit in a prospective cohort study. 44 A PEA bridging therapy study is currently under way to evaluate riociguat in patients with operable CTEPH before PEA. 45 The primary endpoint is the 90-day change from baseline in PVR pre-PEA. Key secondary endpoints include change from baseline in PVR to 6 months post PEA, all-cause death between randomization and 6 months post PEA, intraoperative circulatory arrest time, and frequency of intraoperative surgery-related complications. 45
Pharmacotherapy Considerations for Riociguat
Pharmacokinetics
Riociguat is absorbed orally, and its bioavailability is not affected by food. Absolute bioavailability is approximately 94%, and peak plasma concentrations occur within 1.5 hours after oral administration. 43 Riociguat is a substrate of P-glycoprotein (P-gp) and a breast cancer resistance protein and is mainly cleared through metabolism by several cytochrome P450 (CYP) enzymes (eg, CYP1A1, CYP3A4/5, CYP2J2). However, formation of the major active metabolite, M1, is catalyzed by CYP1A1. 43 Riociguat is eliminated in the urine (40%) and feces (53%), primarily as metabolites. The terminal elimination half-life is about 12 hours in patients with PAH and 7 hours in healthy subjects. 43
Dosing Considerations
The starting dose of riociguat is 1 mg orally TID. 43 For patients who are at risk for hypotension and those taking strong CYP3A4 and P-gp/BCRP inhibitors, a lower starting dose of 0.5 mg TID may be considered. 43 Riociguat is available in 5 tablet sizes: 0.5 mg, 1 mg, 1.5 mg, 2 mg, and 2.5 mg. 43 The dose is increased no more than once every 2 weeks by a minimum of 0.5 mg TID, as tolerated, to a maximum dose of 2.5 mg TID. 43 Dose increases may occur only if systolic blood pressure remains greater than 95 mmHg, with no symptoms of hypotension. Doses should be taken approximately 6 to 8 hours apart. 43 The dose should be decreased by 0.5 mg TID for symptomatic hypotension. 43 For patients who smoke, plasma concentrations are reduced by 50% to 60% compared with non-smokers; a maximum dose above 2.5 mg TID may be necessary. The safety and efficacy of dosing above 2.5 mg TID have not been established. If smoking cessation is successful, a dose reduction may be required. 43 In the case of treatment interruption of 3 or more days, re-titration is required. 43 For patients with swallowing difficulties and those receiving medications via gastric feeding tubes, tablets may be crushed and mixed with water or soft foods for administration. 43
Drug-Drug Interactions
Drug-drug interactions associated with riociguat include increased risk of hypotension with concomitant administration of nitrates, nitric oxide donors, and PDE-5i. Concurrent use with any drug in these classes is contraindicated. When transitioning from a PDE-5i, sildenafil and tadalafil should be discontinued for a minimum of 24 hours and 48 hours, respectively, before riociguat is initiated. Additional important drug-drug interactions are summarized in Table 2. 43
Table 2.
Drug Interactions.
| Drug class | Interaction |
|---|---|
| Nitrates | Coadministration of riociguat with nitrates or nitric oxide donors in any form is contraindicated because of hypotension |
| PDE Inhibitors | Coadministration of riociguat with specific PDE-5i (eg, sildenafil, tadalafil, vardenafil) and nonspecific PDE inhibitors (eg, dipyridamole, theophylline) is contraindicated because of hypotension Do not administer within 24 hours of sildenafil Do not administer 24 hours before or within 48 hours after tadalafil |
| Strong CYP and P-gp/BCRP Inhibitors | Concomitant use of riociguat with strong CYP inhibitors and P-gp/BCRP inhibitors, such as azole antimycotics (eg, ketoconazole, itraconazole) or HIV protease inhibitors (eg, ritonavir), increases riociguat exposure and may result in hypotension Consider a starting dose of 0.5 mg TID when initiating riociguat and monitor for signs and symptoms of hypotension A dose reduction should be considered in patients who may not tolerate the hypotensive effect of riociguat |
| Strong CYP3A Inducers | Strong inducers of CYP3A (eg, rifampin, phenytoin, carbamazepine, phenobarbital, St. John’s wort) may significantly reduce riociguat exposure Data are not available to guide dosing of riociguat when strong CYP3A inducers are coadministered |
| Antacids | Antacids, such as aluminum hydroxide/magnesium hydroxide, decrease riociguat absorption and should not be taken within 1 hour of riociguat administration |
Source: Adempas [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2018. 43
Special Populations
Riociguat carries a boxed warning against use in pregnancy owing to embryofetal toxicity. 43 Negative pregnancy tests are required before initiation, monthly during treatment, and 1 month after treatment discontinuation in females of reproductive potential (FRP). Riociguat is contraindicated in pregnant women and in patients with PH associated with idiopathic interstitial pneumonias (PH-IIP). Nursing mothers should be advised not to breastfeed during treatment with riociguat. Riociguat is not recommended in patients with renal impairment who have creatinine clearance <15 mL/min (calculated using the Cockcroft Gault formula) 46 or are on dialysis, nor in patients with severe (Child Pugh C) hepatic impairment. 43
Risk Evaluation and Mitigation Strategy Program
Because of the risk of embryofetal toxicity, specific requirements have been established to demonstrate compliance with the riociguat risk evaluation and mitigation strategy (REMS) program (Table 3). 43 Riociguat is available only to females through this program. All females, regardless of their reproductive potential, must be enrolled in the REMS program; prescribers and certified pharmacies must also be enrolled in the program. FRP must be able to comply with pregnancy testing and contraception requirements of the program. FRP include girls who have entered puberty and all females who have a uterus and have not passed through menopause. Reliable contraception must be used during treatment and for 1 month following discontinuation. 43 Males are not required to enroll in the REMS program.
Table 3.
Summary of Riociguat Rems Program Requirements.
| Requirement | Female of reproductive potential | Pre-pubertal | Post-menopausal or other medical reasons for permanent irreversible infertility |
|---|---|---|---|
| Prescriber enrolls female patients into the riociguat (Adempas) REMS program | X | X | X |
| Counseling with the Adempas REMS Guide for Females Who Can Get Pregnant | X | ||
| Counseling with the Riociguat (Adempas) Medication Guide, including the risk of teratogenicity | X | X | |
| Prescriber must order and review pregnancy tests before starting treatment, monthly during treatment, and for 1 month after stopping treatment | X | ||
| Prescriber must verify reproductive status annually by completing the “Riociguat (Adempas) REMS Change in Reproductive Potential Status and Pre-pubertal Annual Verification Form” for females who are at least 8 years of age | X | ||
| Prescriber must complete the “Riociguat (Adempas) REMS Change in Reproductive Potential Status and Pre-pubertal Annual Verification Form” upon becoming aware of any changes or misclassification in reproductive status | X | X | X |
Abbreviation: REMS, risk evaluation and mitigation strategy.
Source: Adempas [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2018. 43
The Role of the Pharmacist
Pharmacists are well positioned to ensure appropriate use of therapies for PAH and CTEPH. 47 It is essential for pharmacists to understand the management of these conditions, which requires knowledge of the drugs in each class, their roles, and considerations for use, such as adverse effects, drug-drug interactions, and administration issues. These topics are highly relevant for patient education and engagement to help promote treatment adherence and improve outcomes.
Most pharmacotherapies used to treat PH are classified as specialty medications and are accessible only through specialty pharmacies. Specialty medicines are often associated with higher costs, sensitive requirements for administration, and intensive clinical monitoring. 47 In many cases, important considerations for medication access may include determination of medical necessity, financial assessment for affordability, prior authorization, and REMS program enrollment (for physicians, pharmacies, and female patients). From an inpatient perspective, pharmacists play a key role in formulary management and assurance of medication safety. Formulary decisions must be made based on frequency of admission of patients on PH medications and cost considerations in selecting dosage forms and strengths for each medication. Pharmacists are involved in creating and maintaining the use of electronic order sets that are designed to provide clinical decision support to ensure the safe ordering and administration of these therapies. These order set features may include requirements for input of safety information to comply with REMS program requirements, automated calculations, and dosing limits. Pharmacists are also involved in therapy transitions in cases where alternate routes of administration are required or for management of adverse effects, dosing regimen simplification, escalation of therapy, or minimization of medication costs.
Discussion
PAH and CTEPH are progressive conditions that, if left untreated, result in progressive right heart failure and death. The importance of multidisciplinary care is highlighted in current treatment guidelines, which recommend early referral to expert centers to provide timely and accurate diagnosis, as well as prompt initiation of therapy. 5 A multidisciplinary team consisting of physicians, pharmacists, advanced practice providers, nurses, and psychologists with social work support operates collaboratively to provide emergency care, diagnostics, and intensive therapy. Key functions of expert centers include assessing underlying causes of PH, managing PAH-specific therapies, and working collectively with other healthcare providers to optimize patient outcomes. 48
Pharmacists serve a valuable role as part of the multidisciplinary team in the care of patients with PH, many of whom may have comorbidities that contribute to high costs and resource utilization. 48 To help meet the complex needs of this population, pharmacists ensure appropriate and safe use of therapies, manage formulary decision-making, and provide clinical decision support. In light of limited data supporting a single, first-line approach to treatment of PH, familiarity with the data supporting available recommended therapies and their use can help ensure optimal care based on clinical guidelines and other evidence-based protocols. Pharmacists are uniquely positioned to communicate evidence both to the multidisciplinary healthcare team and to patients as part of a shared decision-making process for therapy management.
Riociguat is a first-in-class medication that is the only approved treatment for both PAH and CTEPH. In clinical trials, riociguat has demonstrated favorable efficacy and is well tolerated. Riociguat is a valuable addition to the armamentarium of options for treating patients with PH.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Marianne Kenny was contracted as a consultant at Bayer during the preparation of this manuscript but employed by Yoh Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for editorial assistance was provided by Bayer Healthcare Pharmaceuticals.
References
- 1.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest. 2012;142(2):448–456. [DOI] [PubMed] [Google Scholar]
- 2.Fowler RM, Gain KR, Gabbay E. Exercise intolerance in pulmonary arterial hypertension. Pulm Med. 2012;2012:359204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society Inc; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A. 2015 ESC/ERs guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology [ESC] and the European Respiratory Society [ERS]: Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur Respir Rev. 2010;19(115):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahay S, Humbert M, Sitbon O. Medical treatment of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2017;38(5):686–700. [DOI] [PubMed] [Google Scholar]
- 8.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. [DOI] [PubMed] [Google Scholar]
- 9.Peacock AJ, Murphy NF, McMurrey JJV, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30(1):104–109. [DOI] [PubMed] [Google Scholar]
- 10.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 suppl):D51–D59. [DOI] [PubMed] [Google Scholar]
- 11.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aithala R, Alex AG, Danda D. Pulmonary hypertension in connective tissue diseases: an update. Int J Rheum Dis. 2017;20(1):5–24. [DOI] [PubMed] [Google Scholar]
- 13.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35(5):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113(16):2011–2020. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev. 2015;24(136):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadinnapola C, Goplan D, Jenkins DP. Diagnosing chronic thromboembolic pulmonary hypertension: current perspectives. J Vasc Diagn. 2014;2(2):75–83. [Google Scholar]
- 18.CADTH. Common drug review. mifepristone and misoprostol. Can Agency Drugs Technol Heal. 2016;(7):2016. Accessed April 11, 2019. https://www.cadth.ca/mifepristone-and-misoprostol
- 19.Pengo V, Lensing A, Prins M, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S, Helmersen D, Provencher S, et al. Diagnostic evaluation and management of chronic thromboembolic pulmonary hypertension: a clinical practice guideline. Can Respir J. 2010;17(6):301–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(1):1801915. doi:10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenoy V, Anton J, Collard CD, Youngblood SC. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Anesthesiology. 2014;120(5):1255–1261. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D73–D81. [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975 [DOI] [PubMed] [Google Scholar]
- 25.Frantz RP. Treatment considerations for pulmonary arterial hypertension and assessment of treatment response. Adv Pulm Hypertens. 2018;16(3):120–124. [Google Scholar]
- 26.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galiè N, Mclaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53(1):1802148. doi:10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–586. [DOI] [PubMed] [Google Scholar]
- 29.Kim NH, Mayer E. Chronic thromboembolic pulmonary hypertension: the evolving treatment landscape. Eur Respir Rev. 2015;24(136):173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madani M, Ogo T, Simonneau G. The changing landscape of chronic thromboembolic pulmonary hypertension management. Eur Respir Rev. 2017;26(146):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Lopez J, Channick RN. Medical therapy for chronic thromboembolic pulmonary hypertension. Adv Pulm Hypertens. 2014;12(4):193–198. [Google Scholar]
- 32.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom National Cohort. Circulation. 2016;133(18):1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33(4):785–792. [DOI] [PubMed] [Google Scholar]
- 34.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123(20):2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghofrani HA, Galiè N, Grimminger F, Ekkehard G, Humbert M, Jing Z-C. Riociguat for the treatment of pulmonary hypertension. N Engl J Med. 2013;369(4):330–340. [DOI] [PubMed] [Google Scholar]
- 36.Hambly N, Granton J. Riociguat for the treatment of pulmonary hypertension. Expert Rev Respir Med. 2015;9(6):679–695. [DOI] [PubMed] [Google Scholar]
- 37.Ghofrani H, Grimminger F, Ekkehard G, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4(5):361–371. [DOI] [PubMed] [Google Scholar]
- 38.Thakkar V, Lau EMT. Connective tissue disease-related pulmonary arterial hypertension. Best Pract Res Clin Rheumatol. 2016;30(1):22–38. [DOI] [PubMed] [Google Scholar]
- 39.Humbert M, Coghlan JG, Ghofrani HA, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. 2017;76(2):422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeper MM, Simonneau G, Corris PA, Klinger JR, Langleben D, Naeije R. Respite: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J. 2017;50(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benza R, Corris P, Klinger J, Langleben D, Naeije R, Simonneau G. Switching from PDE5i to riociguat in the RESPITE study: effect on right heart function. J Heart Lung Transplant. 2018;37(4 suppl 1):S152. [Google Scholar]
- 42.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;36945(4):319–329. [DOI] [PubMed] [Google Scholar]
- 43.Adempas [Package Insert]. Bayer HealthCare Pharmaceuticals Inc; 2018. [Google Scholar]
- 44.Wiedenroth CB, Ghofrani HA, Adameit MSD, et al. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ. 2018;8(3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. https://ClinicalTrials.gov . Riociguat in patients with operable CTEPH prior to pulmonary endarterectomy (PEA bridging study). Published 2018. Accessed October 8, 2020. https://clinicaltrials.gov/ct2/show/NCT03273257
- 46.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Muck W. Assessment of the effects of renal impairment and smoking on the pharmacokinetics of a single oral dose of the soluble guanylate cyclase stimulator riociguat (BAY 63-2521). Pulm Circ. 2016; 6(suppl 1):S15–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodard T, Kim C, Siao F. Review of the diagnosis and management of pulmonary arterial hypertension. US Pharm. 2018; 43(3):HS–10-HS–15. Accessed October 8, 2020. https://www.uspharmacist.com/article/review-of-the-diagnosis-and-management-of-pulmonary-arterial-hypertension [Google Scholar]
- 48.Hill NS, Cawley MJ, Heggen-Peay CL. New therapeutic paradigms and guidelines in the management of pulmonary arterial hypertension. J Manag Care Spec Pharm. 2016;22(3 suppl A):S3–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]