Abstract
Background
Over-reliance on short-acting β2-agonists (SABAs) is associated with poor asthma outcomes. However, the extent of SABA use in Turkey is unclear owing to a lack of comprehensive healthcare databases. Here, we describe the demographics, disease characteristics and treatment patterns from the Turkish cohort of the SABA use IN Asthma (SABINA) III study.
Methods
This observational, cross-sectional study included patients aged ≥ 12 years with asthma from 24 centres across Turkey. Data on sociodemographics, disease characteristics and asthma treatments were collected using electronic case report forms. Patients were classified by investigator-defined asthma severity (guided by the 2017 Global Initiative for Asthma [GINA]) and practice type (primary/specialist care). The primary objective was to describe SABA prescription patterns in the 12 months prior to the study visit.
Results
Overall, 579 patients were included (mean age [standard deviation; SD]: 47.4 [16.1] years; 74.3% female), all of whom were treated by specialists. Most patients had moderate-to-severe asthma (82.7%, GINA steps 3–5), were overweight or obese (70.5%), had high school or university/post-graduate education (51.8%) and reported fully reimbursed healthcare (97.1%). The mean (SD) asthma duration was 12.0 (9.9) years. Asthma was partly controlled/uncontrolled in 56.3% of patients, and 46.5% experienced ≥ 1 severe exacerbation in the preceding 12 months. Overall, 23.9% of patients were prescribed ≥ 3 SABA canisters in the previous 12 months (considered over-prescription); 42.9% received no SABA prescriptions. As few patients had mild asthma, only 5.7% were prescribed SABA monotherapy. Therefore, most patients (61.5%) were prescribed SABA in addition to maintenance therapy, with 42.8% receiving ≥ 3 SABA canisters in the previous 12 months. Inhaled corticosteroids (ICS), ICS + a long-acting β-agonist fixed-dose combination and oral corticosteroids were prescribed to 14.5%, 88.3% and 28.5% of all patients, respectively. Additionally, 10.2% of patients purchased SABA over the counter, of whom 27.1% purchased ≥ 3 canisters in the preceding 12 months.
Conclusions
Despite all patients being treated by specialists and most receiving fully reimbursed healthcare, nearly a quarter of patients received prescriptions for ≥ 3 SABA canisters in the previous 12 months. This highlights a public health concern and emphasizes the need to align clinical practices with the latest evidence-based recommendations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-022-02008-9.
Keywords: Asthma, Exacerbations, Turkey, Prescriptions, Short-acting β2-agonists
Background
Asthma, a chronic inflammatory disease of the airways affecting approximately 339 million patients worldwide [1], is associated with a significant social and economic burden [2]. Although information on the epidemiology of asthma in the Middle East, including Turkey, remains limited, results from the SNAPSHOT observational study conducted in five countries in the Gulf cluster reported that the adjusted prevalence of asthma in the general adult population aged > 18 years was 4.4% in Turkey [3]. Despite the availability of updated international and national guidelines and an effective array of medications, poor adherence to treatment guidelines and suboptimal asthma control continue to be reported globally, including in Turkey [4–6]. For example, results from the 2006 Asthma Insights and Reality in Turkey (AIRET) study in 400 patients with asthma surveyed across 15 cities indicated that guideline-based asthma control was achieved in only 1.3% of participants. Moreover, daily use of anti-inflammatory therapy, including inhaled corticosteroids (ICS), was low in those with persistent asthma [6]. Unsurprisingly, according to the 2018 Global Asthma Report, Turkey reported the highest rate of hospital admissions for asthma (all ages) among 30 European countries between 2011 and 2015 [1]. In addition, results from two cost-of-illness studies in Turkey highlighted that the management of asthma exerts a significant economic burden on the country in terms of annual direct medical costs, predominantly drug treatments and hospitalisations [7, 8].
Adherence to evidence-based treatment guidelines is essential to achieve improved clinical outcomes for patients with asthma [9]. However, many patients rely on short-acting β2-agonists (SABAs) for rapid symptom relief, even though they have no inherent anti-inflammatory activity and SABA use without concomitant ICS may be pro-inflammatory [10, 11]. Since SABA overuse (≥ 3 canisters/year) is associated with poor disease control and an increased risk of exacerbations, hospitalisations and mortality [12–16], the Global Initiative for Asthma now recommends as-needed low-dose ICS-formoterol as the preferred reliever for adults and adolescents with mild asthma and for those with moderate-to-severe asthma who are prescribed ICS-formoterol maintenance therapy [9].
An examination of the prevalence of SABA use is required to help clinicians and healthcare policymakers fully understand the extent of SABA overuse and ensure that treatment practices align with the latest evidence-based treatment recommendations, particularly in light of updated GINA treatment recommendations [9]. However, to date, there is a paucity of data on trends in asthma medication use in Turkey, primarily due to a lack of comprehensive healthcare databases. Accordingly, Turkey was included in the SABA use IN Asthma (SABINA) III pillar of the SABINA Programme to describe the global extent of SABA use and its clinical consequences through a series of real-world observational studies applying a harmonised approach to data collection, evaluation and interpretation [17]. SABINA III, conducted in 23 countries across the Asia–Pacific, Africa, the Middle East, Latin America and in Russia, used electronic case report forms (eCRFs) to overcome the lack of robust national healthcare databases in many of the participating countries [18]. Here, we report on results from SABINA Turkey to provide real-world evidence on asthma management practices in this country.
Methods
Study design
Detailed methodology for SABINA III has been published previously [18]. In brief, this was an observational, cross-sectional study conducted at 24 centres in Turkey, with patient recruitment from September 2019 to January 2020. The study sites were selected using purposive sampling with the aim of obtaining a sample representative of asthma management within each participating site.
The objectives of this study were to describe the demographics and clinical features of the asthma population by asthma severity and to estimate both SABA and ICS prescriptions per patient and within the different SABA and ICS groups. At each site, during a single study visit, prespecified patient data were collected by healthcare providers (HCPs) and collated into a centrally designed eCRF.
Study population
Patients aged ≥ 12 years with a documented diagnosis of asthma, ≥ 3 consultations with the HCP or practice and medical records containing data for ≥ 12 months prior to the study visit were included. Patients with a diagnosis of other chronic diseases such as chronic obstructive pulmonary disease (COPD) or an acute or chronic condition that, in the opinion of the investigator, would limit their ability to participate in the study were excluded from the study. Signed informed consent was collected from participating patients or legal guardians of patients aged < 18 years.
Variables and outcomes
As previously described [18], patients were categorised by their SABA and ICS prescriptions in the 12 months prior to the study visit. SABA prescriptions were categorised as 0, 1–2, 3–5, 6–9, 10–12 and ≥ 13, with over-prescription defined as ≥ 3 SABA canister prescriptions per year [12, 19, 20]. ICS canister prescriptions during the previous 12 months were recorded and categorised according to the prescribed average daily dose as low, medium or high [21].
Other variables included sociodemographic characteristics (age, number of comorbid conditions, body mass index [BMI], smoking status, educational level [primary or secondary school, high school or university and/or post-graduate education], medication reimbursement status [not reimbursed, partially reimbursed or fully reimbursed] and practice type [primary or specialist care]). Patients were also characterised based on investigator-classified asthma severity, guided by GINA 2017 treatment steps (GINA steps 1–2, mild asthma; GINA steps 3−5, moderate-to-severe asthma) [21]. The time since asthma diagnosis was recorded, together with the number of severe exacerbations in the preceding 12 months, which was based on the American Thoracic Society/European Respiratory Society recommendations, and defined as a worsening of asthma symptoms requiring hospitalisation, an emergency room visit or the need for intravenous corticosteroids or oral corticosteroids (OCS) for ≥ 3 days or a single intramuscular corticosteroid dose [22].
In addition, data on prescriptions for asthma medications, including ICS, fixed-dose combinations of ICS with long-acting β2-agonists (LABAs), OCS burst treatment, OCS maintenance treatment and antibiotics prescribed for asthma in the preceding 12 months were collected. Data on pharmacy purchases of over-the-counter (OTC) SABA without a prescription were also recorded. Asthma symptom control was evaluated using the GINA 2017 assessment of asthma control [21] and categorised as well controlled, partly controlled or uncontrolled.
Statistical analysis
Descriptive analyses were used to characterise patients according to baseline demographics and clinical characteristics. Continuous variables were summarised as the number of non-missing values, mean, standard deviation (SD), median and range. Categorical variables were summarised as frequency counts and percentages.
Results
Patient disposition and clinical and sociodemographic characteristics
Patient disposition
Overall, 588 patients were enrolled in the study. However, nine patients were excluded due to an asthma duration of < 12 months. Subsequently, 579 patients were included in the overall analysis (Fig. 1). All patients were treated by specialists, including pulmonologists/respiratory physicians, immunologists, allergists and paediatricians. However, five patients were erroneously allocated to ‘Primary Care’, and six patients had missing values for ‘Practice Type’. Therefore, data on overall disease characteristics and treatment patterns are reported for 579 patients, whereas data on asthma severity (‘mild’ versus ‘moderate-to-severe’) are reported for 568 patients.
Fig. 1.
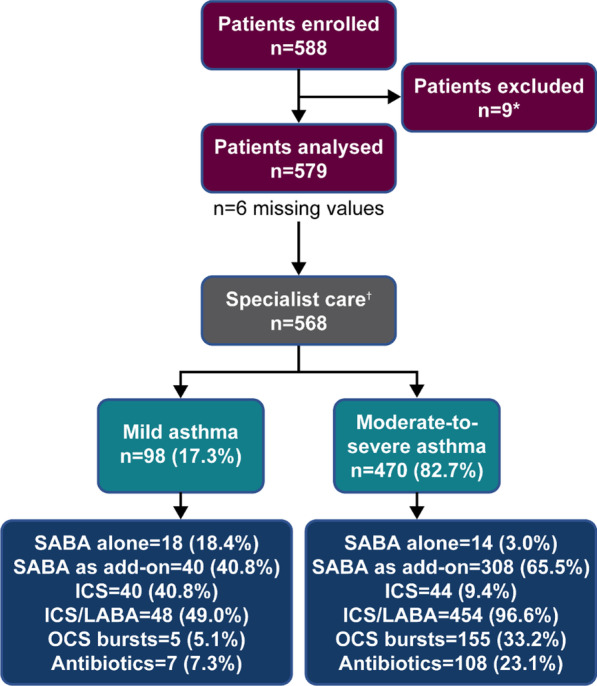
Patient disposition and study population by investigator-classified asthma severity in the SABINA III Turkey Cohort. *Patients excluded had a history of asthma < 12 months. †Asthma severity data are reported for 568 patients and not for the six patients with missing data and the five patients erroneously categorised under primary care. ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; OCS, oral corticosteroids; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma
Patient characteristics
The mean (SD) age of patients was 47.4 (16.1) years, with more than half of patients (57.2%) aged 18–54 years. The majority of patients were female (74.3%) and had never smoked (68.6%; Table 1). The mean (SD) BMI of patients was 28.4 (5.9) kg/m2 and as per the World Health Organization classification criteria [23], 26.6% of patients had normal BMI, 35.1% were overweight and 35.4% were obese. Overall, just over half of patients had received high school education (25.4%) or obtained university and/or post-graduate education (26.4%). In line with the system of Universal Health Insurance (Genel Sağlık Sigortası) in Turkey [24–26], almost all patients (97.1%) reported fully reimbursed healthcare.
Table 1.
Sociodemographic and clinical characteristics according to investigator-classified asthma severity in the SABINA III Turkey Cohort
| All (N = 579) | Patients classified by asthma severity (n = 568)* | ||
|---|---|---|---|
| Investigator-classified mild asthma (n = 98) | Investigator-classified moderate-to-severe asthma (n = 470) | ||
| Age (years) | |||
| Mean (SD) | 47.4 (16.1) | 40.7 (19.2) | 48.8 (15.0) |
| Age group (years), n (%) | |||
| 12–17 | 32 (5.5) | 13 (13.3) | 18 (3.8) |
| ≥ 18–54 | 331 (57.2) | 58 (59.2) | 268 (57.0) |
| ≥ 55 | 216 (37.3) | 27 (27.6) | 184 (39.1) |
| Sex, n (%) | |||
| Female | 430 (74.3) | 61 (62.2) | 362 (77.0) |
| BMI (kg/m2)† | |||
| Mean (SD) | 28.4 (5.9) | 26.4 (5.1) | 28.7 (6.0) |
| BMI groups (kg/m2)†, n (%) | |||
| < 18.5 | 17 (2.9) | 7 (7.1) | 10 (2.1) |
| 18.5–24.9 | 154 (26.6) | 26 (26.5) | 128 (27.2) |
| 25–29.9 | 203 (35.1) | 46 (46.9) | 151 (32.1) |
| ≥ 30 | 205 (35.4) | 19 (19.4) | 181 (38.5) |
| Education level, n (%) | |||
| Primary and/or secondary school | 266 (46.0) | 37 (37.8) | 222 (47.2) |
| High school | 147 (25.4) | 28 (28.6) | 116 (24.7) |
| University and/or post-graduate education | 153 (26.4) | 33 (33.7) | 119 (25.3) |
| Not established | 13 (2.2) | 0 (0.0) | 13 (2.8) |
| Healthcare insurance/medication funding, n (%) | |||
| Not reimbursed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partially reimbursed | 11 (1.9) | 2 (2.0) | 9 (1.9) |
| Fully reimbursed | 562 (97.1) | 95 (96.9) | 456 (97.0) |
| Not established | 6 (1.0) | 1 (1.0) | 5 (1.1) |
| Smoking status history, n (%) | |||
| Active smoker | 64 (11.1) | 9 (9.2) | 54 (11.5) |
| Former smoker | 118 (20.4) | 19 (19.4) | 97 (20.6) |
| Never smoker | 397 (68.6) | 70 (71.4) | 319 (67.9) |
BMI, body mass index; SABINA, SABA use IN Asthma; SD, standard deviation; WHO, World Health Organization
*Asthma severity data are reported for 568 patients and not for the six patients with missing data or the five patients erroneously categorised under primary care
†BMI categorisation is based on WHO classification [23]
Disease characteristics
Patients had a median (minimum, maximum) asthma duration of 9.0 (1.0, 56.0) years (Table 2). Most patients (82.7%) had investigator-classified moderate-to-severe asthma (GINA treatment steps 3‒5), whereas 17.3% had mild asthma (GINA treatment steps 1‒2). Most patients were at GINA treatment step 4 (32.3%) or step 5 (32.0%). Patients reported a mean (SD) of 1.2 (1.8) severe asthma exacerbations and 46.5% experienced ≥ 1 severe asthma exacerbation in the 12 months preceding study initiation. A greater proportion of patients with moderate-to-severe asthma experienced ≥ 1 severe exacerbation in the previous 12 months compared with those with mild asthma (52.6% vs 17.3%). Overall, 67.0% of patients had ≥ 1 comorbidity; a higher percentage of patients with moderate-to-severe asthma had ≥ 1 comorbidity compared with those with mild asthma (69.8% vs 52.0%). The level of asthma symptom control was assessed as well controlled in 43.7%, partly controlled in 32.5% and uncontrolled in 23.8% of patients. Compared with patients with moderate-to-severe asthma, a higher proportion of patients with mild asthma had well-controlled asthma (67.3% vs 38.7%).
Table 2.
Asthma characteristics according to investigator-classified asthma severity in the SABINA III Turkey Cohort
| All (N = 579) | Patients classified by asthma severity (n = 568)* | ||
|---|---|---|---|
| Investigator-classified mild asthma (n = 98) | Investigator-classified moderate-to-severe asthma (n = 470) | ||
| Asthma duration (years) | |||
| Mean (SD) | 12.0 (9.9) | 8.6 (7.7) | 12.6 (10.2) |
| Median (min, max) | 9.0 (1.0, 56.0) | 6.0 (1.0, 40.0) | 10.0 (1.0, 56.0) |
| Number of severe asthma exacerbations 12 months before the study visit | |||
| Mean (SD) | 1.2 (1.8) | 0.3 (0.8) | 1.3 (1.9) |
| Number of severe asthma exacerbations 12 months before the study visit by group, n (%) | |||
| 0 | 310 (53.5) | 81 (82.7) | 223 (47.4) |
| 1 | 105 (18.1) | 8 (8.2) | 96 (20.4) |
| 2 | 61 (10.5) | 6 (6.1) | 53 (11.3) |
| 3 | 48 (8.3) | 2 (2.0) | 46 (9.8) |
| > 3 | 55 (9.5) | 1 (1.0) | 52 (11.1) |
| GINA classification, n (%) | |||
| Step 1 | 39 (6.7) | 39 (39.8) | 0 (0.0) |
| Step 2 | 60 (10.4) | 59 (60.2) | 0 (0.0) |
| Step 3 | 108 (18.7) | 0 (0.0) | 105 (22.3) |
| Step 4 | 187 (32.3) | 0 (0.0) | 184 (39.1) |
| Step 5 | 185 (32.0) | 0 (0.0) | 181 (38.5) |
| Level of asthma symptom control, n (%) | |||
| Well controlled | 253 (43.7) | 66 (67.3) | 182 (38.7) |
| Partly controlled | 188 (32.5) | 25 (25.5) | 159 (33.8) |
| Uncontrolled | 138 (23.8) | 7 (7.1) | 129 (27.4) |
| Number of comorbidities, n (%) | |||
| None | 191 (33.0) | 47 (48.0) | 142 (30.2) |
| 1–2 | 300 (51.8) | 36 (36.7) | 258 (54.9) |
| 3–4 | 78 (13.5) | 14 (14.3) | 61 (13.0) |
| ≥ 5 | 10 (1.7) | 1 (1.0) | 9 (1.9) |
GINA, Global Initiative for Asthma; max, maximum; min, minimum; SABINA, SABA use IN Asthma; SD, standard deviation
*Asthma severity data are reported for 568 patients and not for the six patients with missing data or the five patients erroneously categorised under primary care
Prescribed asthma treatment
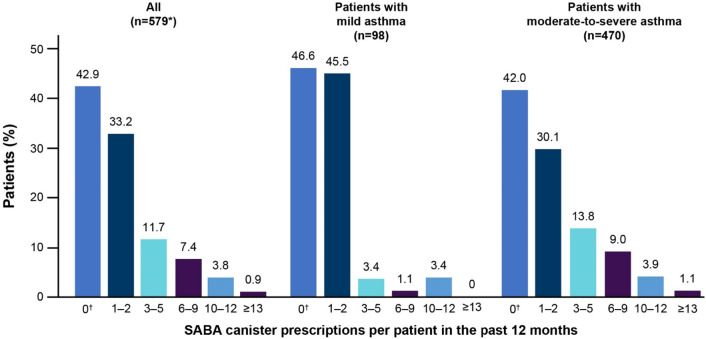
Among patients prescribed SABA monotherapy or SABA in addition to maintenance therapy, 23.9% were prescribed ≥ 3 SABA canisters and 4.7% were prescribed ≥ 10 SABA canisters in the 12 months prior to study entry; 42.9% of patients were not prescribed any SABA (Fig. 2). A higher proportion of patients with moderate-to-severe asthma were prescribed both ≥ 3 (27.9%) and ≥ 10 SABA canisters (5.1%) than those with mild asthma (8.0% and 3.4%, respectively) in the previous 12 months.
Fig. 2.
SABA prescriptions by investigator-classified asthma severity in the 12 months before the study visit in the SABINA III Turkey Cohort. *Asthma severity data are reported for 568 patients and not for the six patients with missing data or the five patients erroneously categorised under primary care. †The category of patients classified as having 0 SABA canister prescriptions included patients using non-SABA relievers, non-inhaler forms of SABA and/or SABA purchased OTC. Missing data for the overall population: n = 136; mild asthma: n = 11; moderate-to-severe asthma: n = 125. OTC, over the counter; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma
SABA monotherapy
Overall, 5.7% of patients were prescribed SABA monotherapy, with a median (minimum, maximum) of 2.0 (1.0, 12.0) canisters in the previous 12 months. Of these, 35.5% of patients were prescribed ≥ 3 SABA canisters and 16.1% were prescribed ≥ 10 SABA canisters in the preceding 12 months (Table 3).
Table 3.
SABA prescriptions in the 12 months before the study visit in the SABINA III Turkey Cohort
| All (N = 579) | Patients classified by asthma severity (n = 568)* | ||
|---|---|---|---|
| Investigator-classified mild asthma (n = 98) | Investigator-classified moderate-to-severe asthma (n = 470) | ||
| Patients prescribed SABA monotherapy, n (%) | |||
| Yes | 33 (5.7) | 18 (18.4) | 14 (3.0) |
| No | 546 (94.3) | 80 (81.6) | 456 (97.0) |
| Number of SABA canisters or inhalers per patient prescribed 12 months before the study visit | |||
| Number of patients | 31 | 17 | 13 |
| Mean (SD) | 4.2 (3.9) | 2.7 (3.5) | 5.9 (3.8) |
| Median (min, max) | 2.0 (1.0, 12.0) | 2.0 (1.0, 12.0) | 6.0 (2.0, 12.0) |
| Number of SABA canisters or inhalers (as categories) per patient prescribed 12 months before the study visit, n (%) | |||
| 0–2 | 20 (64.5) | 15 (88.2) | 5 (38.5) |
| 3–5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6–9 | 6 (19.4) | 0 (0.0) | 5 (38.5) |
| 10–12 | 5 (16.1) | 2 (11.8) | 3 (23.1) |
| ≥ 13 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of missing values | 2 | 1 | 1 |
| Total | 31 | 17 | 13 |
| Patients prescribed SABA in addition to maintenance therapy, n (%) | |||
| Yes | 356 (61.5) | 40 (40.8) | 308 (65.5) |
| No | 223 (38.5) | 58 (59.2) | 162 (34.5) |
| Number of SABA canisters or inhalers per patient prescribed 12 months before the study visit | |||
| Number of patients | 222 | 30 | 189 |
| Mean (SD) | 3.5 (3.2) | 2.2 (2.3) | 3.7 (3.3) |
| Median (min, max) | 2.0 (1.0, 16.0) | 2.0 (1.0, 12.0) | 2.0 (1.0, 16.0) |
| Number of SABA canisters or inhalers per patient prescribed 12 months before the study visit in categories, n (%) | |||
| 0–2 | 127 (57.2) | 25 (83.3) | 101 (53.4) |
| 3–5 | 52 (23.4) | 3 (10.0) | 48 (25.4) |
| 6–9 | 27 (12.2) | 1 (3.3) | 25 (13.2) |
| 10–12 | 12 (5.4) | 1 (3.3) | 11 (5.8) |
| ≥ 13 | 4 (1.8) | 0 (0.0) | 4 (2.1) |
| Number of missing values | 134 | 10 | 119 |
| Total | 222 | 30 | 189 |
Max, maximum; min, minimum; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma; SD, standard deviation
*Asthma severity data are reported for 568 patients and not for the six patients with missing data or the five patients erroneously categorised under primary care
SABA in addition to maintenance therapy
Overall, 61.5% of patients were prescribed SABA in addition to their maintenance therapy, with a mean (SD) of 3.5 (3.2) canisters in the previous 12 months. Among the 222 patients for whom data were available, 42.8% were prescribed ≥ 3 SABA canisters and 7.2% were prescribed ≥ 10 SABA canisters in the preceding 12 months (Table 3). A higher proportion of patients with moderate-to-severe asthma were prescribed SABA in addition to maintenance therapy compared with those with mild asthma (65.5% vs 40.8%).
SABA purchase
Overall, 10.2% of patients purchased SABA OTC, of whom 27.1% purchased ≥ 3 SABA canisters in the previous 12 months (Table 4). A higher proportion of patients with moderate-to-severe asthma than those with mild asthma purchased ≥ 3 SABA canisters OTC without a prescription (29.4% vs 12.5%).
Table 4.
Patients receiving SABA without a prescription in the 12 months before the study visit in the SABINA III Turkey Cohort
| All (N = 579) | Patients classified by asthma severity (n = 568)* | ||
|---|---|---|---|
| Investigator-classified mild asthma (n = 98) | Investigator-classified moderate-to-severe asthma (n = 470) | ||
| Number of patients who received SABA without a prescription 12 months before the study visit, n (%) | |||
| Yes | 59 (10.2) | 8 (8.2) | 51 (10.9) |
| No | 514 (88.8) | 90 (91.8) | 413 (87.9) |
| Unknown | 6 (1.0) | 0 (0.0) | 6 (1.3) |
| Number of SABA canisters or inhalers per patient obtained without a prescription in categories, n (%) | |||
| 1–2 | 43 (72.9) | 7 (87.5) | 36 (70.6) |
| 3–5 | 15 (25.4) | 1 (12.5) | 14 (27.5) |
| 6–9 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10–12 | 1 (1.7) | 0 (0.0) | 1 (2.0) |
| ≥ 13 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not applicable✝ | 0 (0.0) | 0 (0.0) | 0 (0.0) |
eCRF, electronic case report form; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma
*Asthma severity data are reported for 568 patients and not for the six patients with missing data or the five patients erroneously categorised under primary care
✝‘Not applicable’ could be selected in the eCRF when patients purchased non-canister forms of SABA (e.g. oral or nebulised SABA) without a prescription
Asthma maintenance medications
Overall, 14.5% of patients were prescribed ICS, with a mean (SD) of 2.2 (2.0) canisters in the preceding 12 months. Of these patients, 52.4% were prescribed low-dose ICS, 30.5% medium-dose ICS and 17.1% high-dose ICS (Table 5). As expected, a higher proportion of patients with mild asthma (40.8%) received ICS compared with those with moderate-to-severe asthma (9.4%).
Table 5.
Other asthma treatments prescribed in the 12 months before the study visit in the SABINA III Turkey Cohort
| All (N = 579) | Patients classified by asthma severity (n = 568)* | ||
|---|---|---|---|
| Investigator-classified mild asthma (n = 98) | Investigator-classified moderate-to-severe asthma (n = 470) | ||
| Patients prescribed ICS, n (%) | |||
| Yes | 84 (14.5) | 40 (40.8) | 44 (9.4) |
| No | 495 (85.5) | 58 (59.2) | 426 (90.6) |
| Total prescribed daily ICS dose, n (%) | |||
| Low dose | 43 (52.4) | 24 (63.2) | 19 (43.2) |
| Medium dose | 25 (30.5) | 12 (31.6) | 13 (29.5) |
| High dose | 14 (17.1) | 2 (5.3) | 12 (27.3) |
| Number of missing values | 2 | 2 | 0 |
| Total | 82 | 38 | 44 |
| Number of ICS canisters or inhalers per patient prescribed 12 months before the study visit | |||
| Number of patients | 84 | 40 | 44 |
| Mean (SD) | 2.2 (2.0) | 2.4 (2.1) | 2.0 (1.9) |
| Patients prescribed ICS/LABA (fixed-dose combination), n (%) | |||
| Yes | 511 (88.3) | 48 (49.0) | 454 (96.6) |
| No | 68 (11.7) | 50 (51.0) | 16 (3.4) |
| Total prescribed daily ICS dose, n (%) | |||
| Low dose | 97 (19.1) | 28 (59.6) | 67 (14.8) |
| Medium dose | 244 (47.9) | 15 (31.9) | 226 (49.9) |
| High dose | 168 (33.0) | 4 (8.5) | 160 (35.3) |
| Number of missing values | 2 | 1 | 1 |
| Total | 509 | 47 | 453 |
| Patients prescribed OCS burst/short course, n (%) | |||
| Yes | 164 (28.5) | 5 (5.1) | 155 (33.2) |
| No | 412 (71.5) | 93 (94.9) | 312 (66.8) |
| Number of missing values | 3 | 0 | 3 |
| Total | 576 | 98 | 467 |
| Patients prescribed OCS maintenance treatment, n (%) | |||
| Yes | 18 (3.1) | 0 (0.0) | 17 (3.6) |
| No | 561 (96.9) | 98 (100.0) | 453 (96.4) |
| Patients prescribed antibiotics for asthma, n (%) | |||
| Yes | 118 (20.5) | 7 (7.3) | 108 (23.1) |
| No | 457 (79.5) | 89 (92.7) | 360 (76.9) |
| Number of missing values | 4 | 2 | 2 |
| Total | 575 | 96 | 468 |
ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; OCS, oral corticosteroids; SABINA, SABA use IN Asthma; SD, standard deviation
*Asthma severity data are reported for 568 patients and not for the six patients with missing data or the five patients erroneously categorised under primary care
ICS/LABA fixed-dose combination as maintenance therapy was prescribed to 88.3% of patients. Of these patients, 19.1% were prescribed low-dose ICS, 47.9% medium-dose ICS and 33.0% high-dose ICS (Table 5). A greater proportion of patients with moderate-to-severe asthma were prescribed an ICS/LABA fixed-dose combination compared with those with mild asthma (96.6% vs 49.0%). In patients with mild asthma who received an ICS/LABA fixed-dose combination, 70.8% were prescribed a formoterol-containing ICS/LABA (38.2% of whom received beclometasone/formoterol and 61.8% budesonide/formoterol; Additional file 1).
Oral corticosteroids
In the 12 months prior to study entry, OCS burst treatment was prescribed to 28.5% of patients, with a higher percentage of patients with moderate-to-severe asthma prescribed an OCS burst compared with those with mild asthma (33.2% vs 5.1%; Table 5).
Antibiotics
Overall, 20.5% of patients were prescribed antibiotics for asthma (Table 5). Prescriptions of antibiotics were higher in patients with moderate-to-severe asthma than in those with mild asthma (23.1% vs 7.3%).
Overall, 42% of patients were prescribed leukotriene receptor antagonists and 10.9% of patients were prescribed monoclonal antibodies in the 12 months before the study visit. Additionally, long-acting muscarinic antagonists, xanthines and short-acting muscarinic antagonists were prescribed to 7.9%, 2.9% and 1.7% of patients, respectively.
Asthma treatments and exacerbations
When patients were stratified by treatments prescribed in the previous 12 months, most patients who were prescribed an OCS burst experienced ≥ 1 severe exacerbation (93.3%), followed by those prescribed antibiotics (82.2%) and SABA in addition to maintenance treatment (60.1%).
Discussion
This nationwide cross-sectional study conducted as part of the SABINA III study to characterise the asthma patient population and describe the extent of SABA prescriptions provides valuable insights into asthma treatment practices in Turkey. Notably, 23.9% of patients for whom data were available were prescribed SABA in excess of current treatment recommendations (≥ 3 SABA canisters/year). Moreover, the burden of asthma was high, with 46.5% of patients experiencing ≥ 1 severe exacerbation in the previous 12 months.
In general, the baseline sociodemographics and disease characteristics of patients from Turkey were consistent with those of the SABINA III cohort [18]. Reflecting the rapid increase in obesity in Turkey over the past 20 years [27], 35.1% of patients were classified as overweight (BMI, 25–29.9 kg/m2) and 35.4% as obese (BMI, ≥ 30 kg/m2). More than one-third of patients who had moderate-to-severe asthma were obese; however, this finding is not unexpected, given that obesity increases the prevalence of asthma and is associated with poorer outcomes [28–30]. Strikingly, and in keeping with the results from the SNAPSHOT study in five Middle Eastern countries, including Turkey [3], 67% of patients from this Turkish cohort had ≥ 1 comorbidity. This highlights the need for careful assessment of comorbidities as part of routine clinical care, particularly because the presence of asthma-related comorbidities has been shown to negatively impact asthma control [31]. Furthermore, since Turkey provides comprehensive health coverage to its citizens [24–26], almost all patients (97.1%) received fully reimbursed healthcare; this is in stark contrast to the overall SABINA III population, wherein only 47.2% of patients were fully reimbursed [18]. Although all study sites were intended to be representative of healthcare practices across the country, all patients in this Turkish cohort were treated by specialists, resulting in most patients being classified with moderate-to-severe asthma (82.7%). This was likely due to inherent challenges commonly encountered in conducting clinical trials at a primary care level [32] as well as those observed in conducting real-world studies [33]. Consequently, this cohort of patients from Turkey represents a ‘better case scenario’, with virtually all patients receiving fully reimbursed healthcare and all patients receiving treatment under specialist care.
Overall, only 5.7% of patients, most of whom were classified with mild asthma, were prescribed SABA monotherapy in the preceding 12 months; this was expected, given that only 6.7% of patients were diagnosed as GINA step 1. However, 61.5% of patients were prescribed SABA in addition to maintenance therapy, with SABA prescriptions more common in patients with moderate-to-severe asthma than in those with mild asthma. Moreover, 42.8% of patients were prescribed ≥ 3 SABA canisters with maintenance therapy in the previous 12 months, which is considered over-prescription; worryingly, 7.2% of patients were prescribed ≥ 10 SABA canisters. This is of concern since overall findings from SABINA III, which included 8351 patients across 24 countries, indicated an association between high SABA prescriptions and poor clinical outcomes, with prescriptions of 3–5, 6–9, 10–12 and ≥ 13 SABA per year (vs 1 − 2) associated with increasingly lower odds of controlled or partly controlled asthma and higher rates of severe exacerbations across treatment steps and clinical care settings [18]. Nevertheless, overall prescriptions of ≥ 3 SABA canisters 12 months prior were considerably lower in Turkey (23.9%) than in the overall SABINA III population (38.0%) [18]. One possible explanation for this finding is that all patients in Turkey were treated at secondary and tertiary centres by specialists, who are likely more familiar with current asthma treatment guidelines and have the expertise to provide optimal care for patients with asthma [34], whereas SABINA III included patients also treated under primary care [18]. Hence, SABA over-prescription may have been higher if primary care physicians, who may be less familiar with updated guideline treatment recommendations, had participated in this study. Moreover, results on SABA prescriptions in Turkey may have been impacted by the relatively large proportion of missing data; therefore, SABA over-prescription in Turkey is potentially higher than documented. The omission of SABA prescription data highlights the importance of maintaining accurate medical records since excess SABA inhalers should be identified as a signal for poorly controlled asthma [35]. Taken together, these findings clearly indicate that there is a considerable proportion of patients with asthma across Turkey who are currently not optimally treated according to current GINA recommendations despite being under specialist care.
Importantly, not all SABAs were obtained with prescriptions. In fact, 10.2% of patients purchased SABA OTC in the previous 12 months, with over a quarter of patients (27.1%) purchasing ≥ 3 SABA canisters. This highlights patients’ over-reliance on SABA therapy and their willingness to self-manage worsening asthma symptoms [36–39]. This is particularly concerning because many patients who purchased SABA OTC likely did so in addition to their SABA prescriptions. However, this finding is not unexpected as it is common for patients to purchase medications OTC in Turkey. Indeed, results from a national survey of community pharmacists on their role in asthma management in Turkey indicated that 91.0% of pharmacists provided rescue medication without a prescription [40]. Unsurprisingly, therefore, recent studies in Turkey have reported that although pharmacists play a vital role in asthma care in Turkey, further education and training are required to improve both their knowledge of asthma and asthma control in Turkey [40, 41]. Similarly, there is a need to educate patients on self-management of asthma and drive policy changes to regulate SABA purchase without prescriptions, particularly because SABA purchase is associated with low rates of consultation with family practitioners and specialists, low use of prescription-only medication (particularly ICS) and undertreatment of asthma [39, 42, 43].
The majority of patients were prescribed either ICS or ICS/LABA fixed-dose combinations as maintenance medication. Overall, 14.5% of patients were prescribed ICS, which was in alignment with the fact that only 10.4% of patients were diagnosed as GINA step 2. However, a mean of only 2.2 canisters was prescribed in the previous 12 months, suggesting potential ICS underuse as one canister per month is considered good clinical practice. This finding is also a matter of concern because together with over-prescription of SABAs, insufficient provision of ICS-containing treatments has been identified as a preventable cause of death from asthma [35]. Interestingly, almost half of all patients with mild asthma were prescribed an ICS/LABA fixed-dose combination, which was not in alignment with GINA recommendations at the time this study was conducted [44, 45]. This observation may be indicative of prescription behaviour and the fact that physicians may be more comfortable prescribing an ICS/LABA fixed-dose combination instead of ICS to maximise patient adherence and ensure optimal symptom control. Moreover, some physicians may be reluctant to step down asthma treatment, even in patients with well-controlled asthma. However, this finding aligns with the results of a recent evaluation of inhaler therapies in respiratory diseases conducted in Turkey between 1998 and 2015, which reported that following the introduction of ICS/LABA fixed-dose combinations in 2002, their use has increased and they have become the most commonly used treatments in Turkey [46]. Notably, although over 80% of patients in this Turkish cohort were classified with moderate-to-severe asthma, only 10.9% of patients were prescribed a monoclonal antibody, a finding likely attributable to their high cost [8, 47]. However, a high proportion of patients (over 40%) were prescribed a LTRA, which may be due to their ease of administration and reassuring safety profile [48]. Alternatively, patients may have been prescribed a LTRA to treat common asthma comorbidities such as allergic rhinitis [49] or nasal polyps [50] or for better asthma control in patients with predominantly moderate-to-severe asthma [51].
More than a quarter of patients (28.5%), predominantly those with moderate-to-severe asthma, were prescribed OCS burst treatment. This was presumably to treat exacerbations because 93.3% of patients who were prescribed OCS burst treatment experienced ≥ 1 severe exacerbation, further emphasizing that patients in this Turkish cohort had suboptimal asthma control. However, this high percentage of patients prescribed OCS burst treatment may also reflect the fact that physicians often prescribe OCS as rescue medication in the event of worsening asthma symptoms or as part of a written asthma action plan. Furthermore, despite the introduction of two main antimicrobial stewardship programmes in Turkey [52], and a significant reduction in overall antibiotic prescriptions between 2011−2018 following governmental interventions at a national level [53], 20.5% of patients were prescribed antibiotics for asthma, indicating a lack of familiarity with asthma guidelines that do not support the routine use of antibiotics unless there is strong evidence of a lung infection [45]. Indeed, results from a Cochrane Database Systematic Review found limited evidence that antibiotics administered at the time of an asthma exacerbation may improve symptoms and peak expiratory flow rate at follow-up compared with standard care or placebo [54]. In addition, results from a real-life comparative effectiveness study concluded that the routine addition of antibiotics to OCS in the management of asthma exacerbations provided little clinical benefit [55].
Notably, even though all patients were treated by specialists and the majority reported full healthcare reimbursement, less than half of patients (43.7%) had well-controlled asthma and 23.8% of patients had uncontrolled asthma. These findings are aligned with those from the SABINA III cohort, where 17.2% of patients were recruited by primary care physicians, and just 43.2% of patients had well-controlled asthma [18] Likewise, results from SABINA Turkey are consistent with those from a cross-sectional multicentre survey in 2,336 patients with asthma recruited from seven geographical districts in Turkey, which reported that only 51.5% of patients had controlled asthma, as assessed by the Turkish version of the Asthma Control Test [56]. Consequently, the burden of asthma in SABINA Turkey was high, with 46.5% of patients experiencing at least one severe asthma exacerbation in the previous 12 months. Even though a greater proportion of patients with moderate-to-severe asthma experienced ≥ 1 severe asthma exacerbation in the previous year, 17.3% of patients with mild asthma also reported ≥ 1 severe asthma exacerbation, suggesting the potential underestimation of patients with milder disease or the inappropriate management of patients with mild asthma, resulting in poor symptom control. Taken together, these findings highlight the need for educational initiatives targeting both patients and physicians to optimise asthma care. To this end, it is anticipated that the Global Alliance against Chronic Respiratory Diseases (GARD) Turkey project, a voluntary alliance launched by the Turkish Ministry of Health on chronic airway disease (asthma and COPD), will improve asthma outcomes through the creation of working groups focussed on monitoring chronic respiratory diseases, advocacy and awareness of the programme, disease prevention as well as early detection and effective treatment and prevention of complications, including educating patients and HCPs about appropriate treatment [57].
Results from this study should be interpreted in light of several limitations. Firstly, this study used purposive sampling that can be highly prone to research bias. SABA prescriptions were used as a proxy for SABA use; thus, SABA prescriptions may not reflect actual SABA use or treatment adherence. In addition, because data entry into the eCRF relied on the physician’s assessment, findings may have been impacted by misinterpretation of instructions and recall bias. All patients were recruited from specialist care, leading to a greater number of patients with moderate-to-severe asthma being recruited into the study. Thus, the study population may not be truly representative of the overall asthma patient population or reflect the way asthma is currently being managed in Turkey. Consequently, additional studies are required at the primary care level to gain a more comprehensive understanding of treatment practices across Turkey. Moreover, data on the use of alternative relievers such as ICS/formoterol combinations was not recorded. Similarly, the percentage of patients prescribed a maintenance and reliever therapy regimen was also not captured. Finally, the large amount of missing data on SABA prescriptions in the 12 months before the study visit precluded a thorough and accurate assessment of the extent of SABA use in Turkey.
In summary, to the best of our knowledge, this is the first study specifically designed to examine the extent of SABA prescriptions in Turkey. Crucially, the use of a standardised threshold for defining SABA over-prescription enabled an assessment of SABA use not only across Turkey but also globally across regions and countries, whilst the centralised eCRF can be a source of public health data. The collection of these real-world data on SABA over-prescription will enable policymakers and clinicians to make targeted changes in clinical practices to improve asthma outcomes in Turkey.
Conclusion
Despite treatment under specialist care and fully reimbursed healthcare, results from the Turkish cohort of the SABINA III study indicated SABA over-prescription (≥ 3 canisters in the previous 12 months) in nearly a quarter of all patients. Furthermore, 10.2% of patients purchased SABA OTC without a prescription. Overall, less than half of all patients had well-controlled asthma (43.7%) and 46.5% of patients experienced ≥ 1 severe asthma exacerbation in the preceding year. These findings highlight that SABA over-prescription is a public health issue in Turkey, necessitating the need for HCPs and policymakers to work together to promote educational initiatives and ensure that clinical practices align with the latest evidence-based recommendations.
Supplementary Information
Additional file 1. Maintenance medication categorised by asthma severity in the 12 months before the study visit in the SABINA III Turkey Cohort.
Acknowledgements
Medical writing support was provided by Niraj Babu, PhD, of Cactus Life Sciences (part of Cactus Communications), Mumbai, India, in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and funded by AstraZeneca.
SABINA Turkey Study Group: Deniz Kızılırmak1, Nejat Altıntaş9, İsmet Bulut10, Tülin Çağatay11, Bilun Gemicioğlu12, Özgür İnce13, Kıvılcım Oğuzülgen14, Füsun Kalpaklıoğlu15, Ayşe Baççıoğlu15, Funda Aksu2, Murat Altuntaş16, Ferda Öner Erkekol17, Gül Karakaya18, Ali Fuat Kalyoncu18, Ebru Damadoğlu18, İsmail Hanta19, Ersoy Altunok19, Adviye Özer20, Demet Polat Yuluğ3, Gazi Gülbaş21, Mecit Süerdem22, Burcu Yormaz22, Emel Ceylan23, Duygu Erge23, Aykut Çilli24, Berat Celil Doğan24, Fuat Erel25, Can Sevinç8, Ceyda Anar26, Gülseren Pekbak4, Müge Erbay4.
1Manisa Celal Bayar University Faculty of Medicine, Department of Pulmonology, Manisa, Turkey; 2University of Health Sciences Atatürk Chest Diseases and Chest Surgery Education and Research Hospital, Division of Immunology and Allergy, Department of Chest Diseases, Ankara, Turkey; 3Mersin University Faculty of Medicine, Department of Pulmonology, Mersin, Turkey; 4Bursa Uludağ University Faculty of Medicine, Department of Pulmonology, Division of Allergy and Immunology, Bursa, Turkey; 8Dokuz Eylül Üniversitesi Tıp Fakültesi, İzmir, Turkey; 9Namık Kemal Üniversitesi Tıp Fakültesi Hastanesi, Turkey; 10Süreyyapaşa Göğüs Hastalıkları ve Göğüs Cerrahisi EAH, Turkey; 11İstanbul Üniversitesi İstanbul Tıp Fakültesi, Turkey; 12İstanbul Üniversitesi Cerrahpaşa—Cerrahpaşa Tıp Fakültesi, Turkey; 13Medicana Samsun Hastanesi, Turkey; 14Gazi Üniversitesi Tıp Fakültesi, Turkey; 15Kırıkkale Üniversitesi Tıp Fakültesi Hastanesi, Turkey; 16Zonguldak Uzunmehmet Göğüs ve Meslek Hastalıkları Hastanesi, Turkey; 17Ankara Şehir Hastanesi, Turkey; 18Hacettepe Üniversitesi Tıp Fakültesi, Turkey; 19Çukurova Üniversitesi Tıp Fakültesi, Turkey; 20Adana Çukurova Devlet Hastanesi, Turkey; 21İnönü Üniversitesi Tıp Fakültesi Turgut Özal Tıp Merkezi, Turkey; 22Selçuk Üniversitesi Tıp Fakültesi, Turkey; 23Adnan Menderes Üniversitesi Tıp Fakültesi, Turkey; 24Akdeniz Üniversitesi Tıp Fakültesi Hastanesi, Turkey; 25Balıkesir Üniversitesi Tıp Fakültesi Hastanesi, Turkey; 26İzmir Dr. Suat Seren Göğüs Hastalıkları ve Göğüs Cerrahisi EAH
Abbreviations
- AIRET
Asthma Insights and Reality in Turkey
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- eCRF
Electronic case report form
- GARD
Global alliance against chronic respiratory diseases
- GINA
Global initiative for asthma
- HCP
Healthcare provider
- ICS
Inhaled corticosteroid
- LABA
Long-acting β-agonist
- OCS
Oral corticosteroids
- OTC
Over the counter
- SABA
Short-acting β2-agonist
- SABINA
SABA use IN Asthma
- SD
Standard deviation
Author contributions
AY and UG served as SABINA Trial country coordinators for this study. Study design, data collection, data analysis, data interpretation and writing (AY, UG, MB); data collection, data analysis, data interpretation and writing (KA, SAN, DE, DM, MB); data verification (KA, SAN, DE, DM, UG, MB). All authors reviewed and approved the final manuscript.
Funding
AstraZeneca funded the SABINA studies and was involved in designing the programme, developing the study protocol, conducting the studies and performing the analyses. AstraZeneca was given the opportunity to review the manuscript before submission and funded medical writing support.
Availability of data and materials
The data that support the findings of this study are available from AstraZeneca but restrictions apply to the availability of these data (https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure), which were used under license for the current study, and so are not publicly available. Data are however available from Dr. Arzu Yorgancıoğlu upon reasonable request and with permission of AstraZeneca.
Declarations
Ethics approval and consent to participate
The study was conducted in compliance with the study protocol, local ethics committees and the Declaration of Helsinki. The study protocol was approved by Celal Bayar University Ethics Committee and Turkish Ministry of Health ethics committee. Signed informed consent was collected from participating patients or legal guardians of patients aged < 18 years.
Consent for publication
Not applicable.
Competing interests
AY has received research grants from MSD, AstraZeneca and Sanofi as a principal investigator/collaborator, received honoraria from AstraZeneca, Abdi İbrahim, GSK, Novartis Sandoz, Sanofi, and Chiesi and served as a consultant/advisory member for AstraZeneca, GSK, Novartis, Sanofi, Chiesi, and Deva. KA has received research support from Sanofi, AstraZeneca, Health Institutes of Turkey (TUSEB) and received consulting or scientific speaking fees from AstraZeneca, GlaxoSmithKline, Novartis, Sandoz, Chiesi, İbrahim Etem, Abdi İbrahim, Deva and Acino. SAN has received research grants from AstraZeneca, Bayer, GSK, Novartis, Sanofi as a principal investigator, received honoraria from AstraZeneca, Chiesi, GSK and Novartis Sandoz and served as consultant advisory board member Astra-Zeneca, Chiesi, Deva, GSK and Novartis Sandoz. DE served as speaker for Novartis, GSK, AstraZeneca, Vem ilaç and as a clinical research investigator for MSD, AstraZeneca, Sanofi, GSK. DE is also ad-board member at Novartis and GSK. DM received grants from AstraZeneca and Sanofi as a principal investigator, received honoraria from Abdi İbrahim, GSK, Novartis and served as a consultant/advisory member for ALK, Chiezi, GSK and Novartis. UG is an employee of AstraZeneca. MJHIB was an employee of AstraZeneca when this study was conducted and has shares in AstraZeneca.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arzu Yorgancıoğlu, Email: arzuyo@hotmail.com.
SABINA Turkey Study Group:
Deniz Kızılırmak, Nejat Altıntaş, İsmet Bulut, Tülin Çağatay, Bilun Gemicioğlu, Özgür İnce, Kıvılcım Oğuzülgen, Füsun Kalpaklıoğlu, Ayşe Baççıoğlu, Funda Aksu, Murat Altuntaş, Ferda Öner Erkekol, Gül Karakaya, Ali Fuat Kalyoncu, Ebru Damadoğlu, İsmail Hanta, Ersoy Altunok, Adviye Özer, Demet Polat Yuluğ, Gazi Gülbaş, Mecit Süerdem, Burcu Yormaz, Emel Ceylan, Duygu Erge, Aykut Çilli, Berat Celil Doğan, Fuat Erel, Can Sevinç, Ceyda Anar, Gülseren Pekbak, and Müge Erbay
References
- 1.Global Asthma Network (GAN), The Global Asthma Report. 2018. Available from: http://www.globalasthmareport.org/. Accessed 21 Apr 2021.
- 2.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1701103. doi: 10.1186/s40733-016-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarraf H, Aydin O, Mungan D, Albader M, Mahboub B, Doble A, et al. Prevalence of asthma among the adult general population of five Middle Eastern countries: results of the SNAPSHOT program. BMC Pulm Med. 2018;18(1):68. doi: 10.1186/s12890-018-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemicioglu B, Bayram H, Cimrin A, Abadoglu O, Cilli A, Uzaslan E, et al. Asthma control and adherence in newly diagnosed young and elderly adult patients with asthma in Turkey. J Asthma. 2019;56(5):553–61. doi: 10.1080/02770903.2018.1471707. [DOI] [PubMed] [Google Scholar]
- 5.Yildiz F, Mungan D, Gemicioglu B, Yorgancioglu A, Dursun B, Oner Erkekol F, et al. Asthma phenotypes in Turkey: a multicenter cross-sectional study in adult asthmatics; PHENOTURK study. Clin Respir J. 2017;11(2):210–23. doi: 10.1111/crj.12326. [DOI] [PubMed] [Google Scholar]
- 6.Sekerel BE, Gemicioglu B, Soriano JB. Asthma insights and reality in Turkey (AIRET) study. Respir Med. 2006;100(10):1850–4. doi: 10.1016/j.rmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Turktas H, Bavbek S, Sekerel B, Oksuz E, Malhan S. Economic burden of adult asthma in Turkey: a cost of illness study from payer perspective. J Respir Med. 2018;2(1):6. doi: 10.5152/TurkThoracJ.2019.19025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bavbek S, Malhan S, Mungan D, Misirligil Z, Erdinc M, Gemicioglu B, et al. Economic burden of severe asthma in Turkey: a cost of illness study from payer perspective. Eur Ann Allergy Clin Immunol. 2021;53(3):128–37. doi: 10.23822/EurAnnACI.1764-1489.149. [DOI] [PubMed] [Google Scholar]
- 9.Global initiative for asthma. Global strategy for asthma management and prevention. 2022. Available from: http://ginasthma.org/. Accessed 1 June 2022.
- 10.O'Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017;50(3):1701103. doi: 10.1183/13993003.01103-2017. [DOI] [PubMed] [Google Scholar]
- 11.Aldridge RE, Hancox RJ, Robin Taylor D, Cowan JO, Winn MC, Frampton CM, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med. 2000;161(5):1459–64. doi: 10.1164/ajrccm.161.5.9906052. [DOI] [PubMed] [Google Scholar]
- 12.Bloom CI, Cabrera C, Arnetorp S, Coulton K, Nan C, van der Valk RJP, et al. Asthma-related health outcomes associated with short-acting beta2-agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther. 2020;37(10):4190–208. doi: 10.1007/s12325-020-01444-5. [DOI] [PubMed] [Google Scholar]
- 13.Nwaru BI, Ekstrom M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4). [DOI] [PMC free article] [PubMed]
- 14.FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–40. doi: 10.1016/j.rmed.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Stanford RH, Shah MB, D'Souza AO, Dhamane AD, Schatz M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403–7. doi: 10.1016/j.anai.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Tomisa G, Horváth A, Szalai Z, Müller V, Tamási L. Prevalence and impact of risk factors for poor asthma outcomes in a large, specialist-managed patient cohort: a real-life study. J Asthma Allergy. 2019;12:297–307. doi: 10.2147/JAA.S211246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrera CS, Nan C, Lindarck N, Beekman MJHI, Arnetorp S, van der Valk RJP. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J. 2020;55(2):1901858. doi: 10.1183/13993003.01858-2019. [DOI] [PubMed] [Google Scholar]
- 18.Bateman ED, Price DB, Wang HC, Khattab A, Schonffeldt P, Catanzariti A, et al. Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Resp J. 2021;59(5):2101402. [DOI] [PMC free article] [PubMed]
- 19.Janson C, Menzies-Gow A, Nan C, Nuevo J, Papi A, Quint JK, et al. SABINA: an overview of short-acting β2-agonist use in asthma in European countries. Adv Ther. 2020;37(3):1124–35. doi: 10.1007/s12325-020-01233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Global initiative for asthma. Global strategy for asthma management and prevention. 2017. Available from: http://ginasthma.org/. Accessed 30 Jan 2021.
- 22.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Body Mass Index - BMI. Available from: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed 28 Mar 2021.
- 24.Gürsoy K, editor. An overview of Turkish healthcare system after health transformation program: main successes, performance assessment, further challenges, and policy options. Sosyal Güvence. 2015;0(7):83–112.
- 25.Universal Health Insurance. Genel Sağlık Sigortası, Republic of Turkey. Available from: http://www.globalasthmareport.org/. Accessed 22 Dec 2021.
- 26.Yilmaz V. Rethinking universal health coverage: a qualitative study of patient organisation perspectives on the Turkish health-care system. Sociol Health Illn. 2021;43(6):1372–87. doi: 10.1111/1467-9566.13306. [DOI] [PubMed] [Google Scholar]
- 27.Erem C. Prevalence of overweight and obesity in Turkey. IJC Metab Endocr. 2015;8(3):38–41. doi: 10.1016/j.ijcme.2015.07.002. [DOI] [Google Scholar]
- 28.Mohan A, Grace J, Wang BR, Lugogo N. The effects of obesity in Asthma. Curr Allergy Asthma Rep. 2019;19(10):49. doi: 10.1007/s11882-019-0877-z. [DOI] [PubMed] [Google Scholar]
- 29.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122(3):507–11.e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63(1):14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz, F, ASIT Study Group. Factors influencing asthma control: results of a real-life prospective observational asthma inhaler treatment (ASIT) study. J Asthma Allergy. 2013;6:93–101. [DOI] [PMC free article] [PubMed]
- 32.Taft T, Weir C, Kramer H, Facelli JC. Primary care perspectives on implementation of clinical trial recruitment. J Clin Transl Sci. 2019;4(1):61–8. doi: 10.1017/cts.2019.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudrapatna VA, Butte AJ. Opportunities and challenges in using real-world data for health care. J Clin Invest. 2020;130(2):565–74. doi: 10.1172/JCI129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cloutier MM, Salo PM, Akinbami LJ, Cohn RD, Wilkerson JC, Diette GB, et al. Clinician agreement, self-efficacy, and adherence with the guidelines for the diagnosis and management of Asthma. J Allergy Clin Immunol Pract. 2018;6(3):886–94.e4. doi: 10.1016/j.jaip.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royal College of Physicians. Why asthma still kills? National Review of Asthma Deaths. Available from: https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills. Accessed 28 Mar 2021.
- 36.Douglass JA, Goeman DP, McCarthy EA, Sawyer SM, Aroni RA, Stewart K, et al. Over-the-counter β2-agonist purchase versus script: a cross-sectional study. Respir Med. 2012;106(2):223–9. doi: 10.1016/j.rmed.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell D, Ruffin R, Wilson D, Farmer E, Wakefield M, Chittleborough C. Over-the-counter purchase of inhaled beta-2 agonists: a qualitative study of asthma patients. Health Promot J Austr. 2000;10:38–42. [Google Scholar]
- 39.Reddel HK, Ampon RD, Sawyer SM, Peters MJ. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open. 2017;7(9):e016688. doi: 10.1136/bmjopen-2017-016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dizdar EA, Civelek E, Sekerel BE. Community pharmacists' perception of asthma: a national survey in Turkey. Pharm World Sci. 2007;29(3):199–204. doi: 10.1007/s11096-006-9071-1. [DOI] [PubMed] [Google Scholar]
- 41.Gemicioglu B, Borekci S, Can G. Investigation of knowledge of asthma and inhaler devices in pharmacy workers. J Asthma. 2014;51(9):982–8. doi: 10.3109/02770903.2014.928310. [DOI] [PubMed] [Google Scholar]
- 42.Gibson P, Henry D, Francis L, Cruickshank D, Dupen F, Higginbotham N, et al. Association between availability of non-prescription beta 2 agonist inhalers and undertreatment of asthma. BMJ. 1993;306(6891):1514–8. doi: 10.1136/bmj.306.6891.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry DA, Sutherland D, Francis L. The use of non-prescription salbutamol inhalers by asthmatic patients in the Hunter Valley, New South Wales. Newcastle Retail Pharmacy Research Group. Med J Aust. 1989;150(8):445–9. [PubMed]
- 44.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2019. Available from: https://ginasthma.org/. Accessed 26 Aug 2020.
- 45.Global initiative for asthma. Global strategy for asthma management and prevention. 2020. Available from: http://ginasthma.org/. Accessed 29 Feb 2021.
- 46.Bayar B, Kutkan NU, Şafak Yılmaz E, Tolun Ç, Doğan E, Atikeler EK, et al. Cost evaluation of inhaler therapies used in respiratory diseases: 1998–2015 period in Turkey. Value Health Reg Issues. 2017;13:31–8. doi: 10.1016/j.vhri.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Hale A, Kurtuluş A, Özdedeoğlu Ö, Akdoğan BB, Kalkan İK, Köycü G, et al. Direct cost analysis for patients with severe asthma receiving omalizumab treatment. J Health Sci Med. 4(5):735–40.
- 48.Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;2012(5):Cd002314. [DOI] [PMC free article] [PubMed]
- 49.Hossenbaccus L, Linton S, Garvey S, Ellis AK. Towards definitive management of allergic rhinitis: best use of new and established therapies. Allergy Asthma Clin Immunol. 2020;16(1):1–17. doi: 10.1186/s13223-020-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wentzel JL, Soler ZM, DeYoung K, Nguyen SA, Lohia S, Schlosser RJ. Leukotriene antagonists in nasal polyposis: a meta-analysis and systematic review. Am J Rhinol Allergy. 2013;27(6):482–9. doi: 10.2500/ajra.2013.27.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kittana N, Hattab S, Ziyadeh-Isleem A, Jaradat N, Zaid A-N. Montelukast, current indications and prospective future applications. Exp Rev Respirat Med. 2016;10(9):943–56. doi: 10.1080/17476348.2016.1207533. [DOI] [PubMed] [Google Scholar]
- 52.Isler B, Keske Ş, Aksoy M, Azap ÖK, Yilmaz M, Yavuz SŞ, et al. Antibiotic overconsumption and resistance in Turkey. Clin Microbiol Infect. 2019;25(6):651–3. doi: 10.1016/j.cmi.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Aksoy M, Isli F, Kadi E, Varimli D, Gursoz H, Tolunay T, et al. Evaluation of more than one billion outpatient prescriptions and eight-year trend showing a remarkable reduction in antibiotic prescription in Turkey: A success model of governmental interventions at national level. Pharmacoepidemiol Drug Saf. 2021;30(9):1242–9. doi: 10.1002/pds.5311. [DOI] [PubMed] [Google Scholar]
- 54.Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A. Antibiotics for exacerbations of asthma. Cochrane Database Syst Rev. 2018;6(6):CD002741-CD. [DOI] [PMC free article] [PubMed]
- 55.Murray CS, Lucas SJ, Blakey J, Kaplan A, Papi A, Paton J, et al. A real-life comparative effectiveness study into the addition of antibiotics to the management of asthma exacerbations in primary care. Eur Respir J. 2021:2003599. [DOI] [PubMed]
- 56.Turktas H, Mungan D, Uysal MA, Turkish Asthma Control Survey Study Group. Determinants of asthma control in tertiary level in Turkey: a cross-sectional multicenter survey. J Asthma. 2010;47(5):557–62. [DOI] [PubMed]
- 57.Yorgancıoğlu A, Gemicioglu B, Ekinci B, Özkan Z, Bayram H, Ergan B, et al. Asthma in the context of global alliance against respiratory diseases (GARD) in Turkey. J Thorac Dis. 2018;10(3):2052–8. doi: 10.21037/jtd.2018.03.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Maintenance medication categorised by asthma severity in the 12 months before the study visit in the SABINA III Turkey Cohort.
Data Availability Statement
The data that support the findings of this study are available from AstraZeneca but restrictions apply to the availability of these data (https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure), which were used under license for the current study, and so are not publicly available. Data are however available from Dr. Arzu Yorgancıoğlu upon reasonable request and with permission of AstraZeneca.