Abstract
Background
Probiotics have proven beneficial in a number of immune-mediated and allergic diseases. Several human studies have evaluated the efficacy and safety of probiotics in allergic rhinitis; however, evidence for their use has yet to be firmly established.
Objective
We undertook a systematic review and meta-analysis aiming to address the effect and safety of probiotics on allergic rhinitis.
Methods
We systematically searched databases [MEDLINE (PubMed), Embase, and the Cochrane Central Register of Controlled Trials] from inception until June 1, 2021. Qualified literature was selected according to inclusion and exclusion criteria, the data were extracted, and a systematic review and meta-analysis was conducted.
Results
Twenty-eight studies were included. The results showed that probiotics significantly relieved allergic rhinitis symptoms (standardized mean difference [SMD], −0.29, 95% confidence interval (CI) [−0.44, −0.13]; p = 0.0003, I 2 = 89%), decreased Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) scores compared with the control group (SMD, −0.64, 95% CI [−0.79, −0.49], p < 0.00001, I 2 = 97%), and increased T helper cell 1(Th1)/Th2 ratio (mean difference [MD], −2.47, 95% CI [−3.27, −1.68], p < 0.00001, I 2 = 72%). There was no significant change in overall or specific IgE levels between probiotic-treated and placebo-treated subjects (SMD, 0.09, 95% CI [−0.16, 0.34], I 2 = 0%, and SMD, −0.03, 95% CI [−0.18, 0.13], p = 0.72, I 2 = 0%, respectively).
Conclusions
To sum up, probiotic supplement seems to be effective in ameliorating allergic rhinitis symptoms and improving the quality of life, but there is high heterogeneity in some results after subgroup analysis and clinicians should be cautious when recommending probiotics in treating allergic rhinitis.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO/, PROSPERO (CRD42021242645).
Keywords: probiotics, allergy rhinitis, allergy, systematic review, meta-analysis
Introduction
Allergic rhinitis (AR) is characterized by a nasal sensitive inflammation, which is estimated to already affect 10%–40% of the worldwide population (1, 2). Common symptoms of AR are nasal itching, sneezing, rhinorrhea, and nasal congestion. In addition, some patients experience symptoms of allergic rhinoconjunctivitis, such as watery or itchy or red eyes. Severe AR can affect the quality of life, sleep, and work performance (1).
In 1989, Strachan found that the number of siblings was inversely related to the prevalence of hay fever among peers in the UK. Then, he proposed the “Hygiene hypothesis” (3), that the changed intestinal microbiota due to the lack of contact with infectious sources, parasites, and symbiotic microorganisms affects the normal development of immune system. The “Hygiene hypothesis” extends to the “Old Friends” and the “Microflora hypothesis” (4, 5). The “Microflora hypothesis” believes that a diverse gut microbiota plays an important role in shaping host immune development and that disruption or dysbiosis of the normal gut microbiota contributes to the development of immune disorders such as allergic diseases (6, 7). Host–microbes symbiosis plays a cardinal role in maintaining health and immune homeostasis. Changes in the intestinal flora are considered to be one of the most important indicators of allergic diseases (8, 9). Probiotics are live bacteria that colonize the gastrointestinal tract and they provide a health benefit to the host when administered in adequate amounts (10). Recent studies have shown that probiotics are non-pharmaceutical agents that can increase the production of systemic IFN, IL10, and IL12, improve the pre-Th1 immune response, and reduce Th2 cytokines (11), and thus have been proposed as modulators of the allergic response and advocated as therapeutic and preventive interventions for allergic disease (12, 13).
Probiotics include the Lactobacillus group (L. rhamnosus GG, L. sporogenes, L. reuteri RC-14, L. plantarum 299v, L. acidophilus, and L. lactis), the Bifidobacterium group (B. bifidum, B. longum, and B. infantis), the Streptococcus group (S. thermophilus, S. lactis, and S. fecalis), and non-bacterial organisms (non-pathogenic yeast Saccharomyces boulardii). The most common probiotics are the Lactobacillus and Bifidobacterium groups (14). Many studies have attempted to assess the role of probiotics in the treatment of AR with inconsistent findings. While some have found a protective effect of probiotics on AR (15–18), several others have found no association (19, 20). Given that there have been further published studies, we undertook a systematic review and meta-analysis aiming to address the effect and safety of probiotics on AR, and meanwhile, we attempted to explore the possible causes of between-study heterogeneity via subgroup.
Methods and Analysis
Study Registration
The protocol of this systematic review and meta-analysis has been registered on the PROSPERO platform with an assigned registration number CRD42021242645, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols statement guidelines. This research was conducted based on this protocol.
Database Search
We have performed a search in MEDLINE (PubMed), Embase, and the Cochrane Central Register of Controlled Trials. Additional studies will be sought by manually checking the references of included studies and relevant reviews. Searches will be restricted to publications appearing from inception to June 1, 2021. We used subject (“Rhinitis, Allergic”, “Rhinitis, Allergic, Seasonal”, “Rhinitis, Allergic, perennial”, “prebiotics”,” probiotics”) and free words (“Seasonal Allergic Rhinitis”, “Pollen Allergy” “Pollinosis”, “Hay Fever”, “allergic rhinitis”, “Perennial Allergic Rhinitis”, “prebiotics”,” probiotics”) to search in the databases aforementioned. The search strategy was as follows, taking PubMed as an example:
(1) (Seasonal Allergic Rhinitis [MeSH Terms]) OR (Perennial Allergic Rhinitis [MeSH Terms]) OR (Allergic Rhinitides, Seasonal) OR (Allergic Rhinitis, Seasonal) OR (Rhinitides, Seasonal Allergic) OR (Rhinitis, Seasonal Allergic) OR (Seasonal Allergic Rhinitides) OR (Pollen Allergy)) OR (Allergies, Pollen) OR (Allergy, Pollen)) OR (Pollen Allergies) OR (Pollinosis)) OR (Pollinoses) OR (Hay Fever)) OR (Fever, Hay) OR (Perennial Allergic Rhinitis) OR (Allergic Rhinitis, Perennial).
(2) (Probiotics [MeSH Terms]) OR (Prebiotics [MeSH Terms]) OR(Probiotics) OR (Prebiotics).
(3) (1) AND (2).
Eligible Criteria
Studies were included if they met all of the following criteria (1): study design: experimental (randomized and quasi-randomized controlled trials) studies (2); study participants: participants with AR (3); intervention: the intervention group/s should receive probiotics supplementation in any dosage, or regimen as decided by the trialists of the respective trials (4); comparator(s)/control: the participants in the comparison group/s might receive a placebo or other drugs (5); if other drugs were used in the treatment group, they must also be used in the control group in the same way; and (6) language: articles published in the English language.
Articles were excluded if they were published in the form of conference abstract, case report, case series, letter to the editor, correspondence, editorial, narrative reviews, systematic reviews, and meta-analyses.
Study Selection and Data Extraction
Two investigators independently reviewed titles, abstracts, and full-text articles according to the aforementioned inclusion and exclusion criteria. Disagreement was resolved through discussion or a third investigator. The same two investigators extracted the following data from each selected study: literature characteristics (the first author’s name, journal, year of publication, and study design); participant information (age and sample size); intervention information (intervention duration and comparison group components); outcome (AR and related adverse events); and conclusion.
Risk of Bias Assessment
The risk of bias assessment was conducted through The Cochrane Risk of Bias Tool Version 1 (21) in Review manager 5.3.4 software by CL and ML. Any disagreement was settled through consultation with the author SP.
Statistical Analyses
Statistical analyses were completed using Review Manager 5.3.4 software (RevMan; Version 5.3.4. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We chose the mean difference (MD) and standardized mean difference (SMD) for continuous outcomes. MD is the difference between the two means, which eliminates the influence of the absolute value between multiple studies. SMD can be simply understood as the quotient of the difference between the two means divided by the combined standard deviation, which not only eliminates the influence of the absolute value of multiple studies, but also eliminates the different effects of multiple study measurement units. Statistical heterogeneity was judged using the inconsistency index (I 2), and significant heterogeneity was reported if the I 2 is over 50%. The fixed-effect model was be used in this meta-analysis because larger sample studies will receive greater weight and provide greater contributions to pooled effects. Subgroup analyses were conducted to explore the source of heterogeneity. Publication bias assessment was conducted through funnel plots if more than 10 trials were included. Sensitivity analysis was used to explore the stability of the results. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group was used to assess the evidence quality for outcomes across studies.
Results
Database Search Results
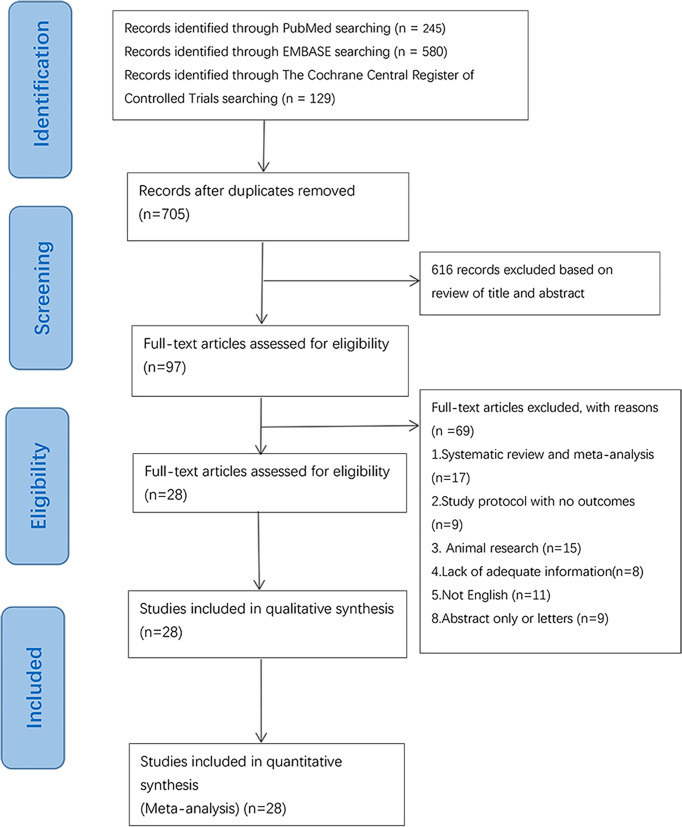
The initial search was completed on June 1, 2021. We have identified 245 potentially relevant publications from PubMed, 580 from Embase, and 129 from The Cochrane Central Register of Controlled Trials. Endnote was used to eliminate duplicate publications, resulting in 97 records for review. After excluding publications that did not meet the inclusion or the exclusion criteria, we included 28 studies for systematic review and meta-Analysis. A flow diagram illustrating the exclusion of articles with specific reasons is shown in Figure 1 (PRISMA flowchart).
Figure 1.
Flowchart of database searching and study identification.
Study Characteristics
Twenty-eight trials were included in the systematic analysis and meta-analysis. The main characteristics of the individual studies are shown in Table 1 . Overall, one of these RCTs was a multicenter study (42). Twenty-eight studies included patients from 2 to 65 years of age. Fifteen studies included adults (age > 18 years old) (15–17, 25, 27, 28, 30, 32, 33, 36, 37, 40–42, 44), and eleven studies included children or teenagers (age < 18 years old) (18, 20, 23, 24, 26, 29, 34, 35, 38, 39, 43), and two studies included adults and children (22, 31). Fourteen studies included patients with seasonal allergic rhinitis (SAR) (15–18, 22, 25, 27, 29, 32, 33, 37, 39, 40, 44). Eleven studies included patients with perennial allergic rhinitis (PAR) (20, 23, 24, 28, 31, 32, 34, 36, 38, 41, 42) and three studies included patients with SAR and PAR (26, 30, 43). The intervention group of fourteen studies used Lactobacillus strains (17, 20, 22–24, 26–28, 30, 32, 33, 35, 37, 44), and four studies used Bifidobacterium strains (16, 25, 36, 39). Three studies used both Bifidobacterium strains and Lactobacillus strains (18, 40, 42). The other three studies used Tetragenococcus halophilus Th22 (31), E. coli Nissle 1917 (15), and Broncho-Vaxom (41), respectively. Three studies used probiotics combined with antihistamines (29, 34, 38). One study used Bifidobacterium strains and Enterococcus faecium (43). The treatment time of probiotics ranged from 6 weeks to 6 months.
Table 1.
Study characteristics.
| Study | Country | Type | Sample size | Participator characteristics | Type of allergic rhinitis | Intervention | Control | Intake of intervention from/until | Outcome | Conclusions | Adverse events/side effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Helin et al. (22) | Finland | RCT | 38 | Young adults and teenagers (age 14–36 years old) allergic to birch pollen | Seasonal (birch pollen) | Lactobacillus rhamnosus (at least 5×109CFUs/capsule) (2 capsules twice a day) | Placebo (microcrystalline cellulose)(2 capsules twice a day) | 5.5 months | 1. RTSS (nasal, eye, lung, any symptom) medication use 2. Oral apple challenge |
No indication of a beneficial treatment effect in this study | Not mentioned whether any adverse events occurred |
| Wang et al. (23) | China | RCT | 80 | Patients(age < 18 years old, mean 15.4 years) had been diagnosed as having perennial allergic rhinitis for more than 1 year | Perennial (Dp) | Lactobacillus paracasei-33 (LP-33) (1×107 CFUs/ml) (yogurt/200 ml/day) | Placebo (yogurt) (200 ml/day) |
30 days | 1. Modified PRQLQ | LP-33-fortified fermented milk can effectively and safely improve the quality of life of patients with allergic rhinitis | No obvious adverse events were found |
| Peng et al. (24) | China | RCT | 90 | Children (age > 5 years) old, mean, 15.7 years) with perennial allergic rhinitis characterized by intermittent or continuous nasal symptoms for more than 1 year | Perennial (Dp) | Live or heat-killed Lactobacillus paracasei (LP-33) (5×109 CFUs/capsule) two capsules per day | Placebo (two capsules per day) | 30 days | 1. Modified PRQLQ | 1. Heat-killed LP-33 can effectively improve the overall quality of life; 2. The efficacy of the heat-killed LP33 was not inferior to the live variant |
No obvious adverse events were found |
| Xiao et al (16) | Japan | RCT | 40 | Adult volunteers (age 22–61 years old) with a clinical history of Japanese cedar pollinosis | Seasonal (JCP) | Yogurt with Bifidobacterium longum BB536 (approximately 5×1010 CFUs/2 g) twice daily |
Placebo (yogurt) twice daily | 18 weeks | 1. Nasal, eye, and throat symptom score, eye drops, and mask wearing 2. Blood sample for total IgE, JCP-specific IgE, IFN-γ, IL-10, or eosinophil rate |
BB536-supplementation may relieve JCPsis symptoms | No obvious adverse events were found |
| Xiao et al. (25) | Japan | RCT | 44 | Adult volunteers (age 22 57 years old) with a clinical history of Japanese cedar pollinosis | Seasonal (JCP) | Yogurt with Bifidobacterium longum BB536 powder [approximately 5× 1010 colony-forming units (CFUs)/2 g] twice daily | Placebo (yogurt) twice daily | 13 weeks | 1. Symptom scores for sneezing, rhinorrhea, nasal blockage, nasal itching, eye, and throat 2. Blood sample for total IgE, JCP-specific IgE, IFN-γ, IL-10, or eosinophil rate |
The efficacy of BB536 in relieving JCPsis symptoms through the modulation of Th2-skewed immune response | No obvious adverse events were found |
| Giovannini et al. (26) | France | RCT | 187 | Children (age 2–5 years old) with allergic rhinitis or asthma | Perennial and seasonal | Fermented milk containing Lactobacillus casei (LcS) (1×108 cfu/ml) 100 ml/day | Placebo (milk) (100 ml/day) | 12 months | 1. The time free from episodes of asthma/rhinitis 2. Total serum IgA, IgE, IgG, and IgM |
Long-term consumption of fermented milk containing Lactobacillus casei may improve the health status of children with allergic rhinitis | Abdominal symptoms, diarrhea, and fever episodes |
| Tamura et al. (27) | Japan | RCT | 120 | Adults (age >18 years old, mean, 39 years) with allergic rhinitis | Seasonal (JCP) | Fermented milk with Lactobacillus casei strain Shirota (LcS)(4×1010 CFU/80 ml/day); | Placebo (fermented milk) (80 ml/day) | 8 weeks | 1. Symptom-medication score, medical (SEM) examination of nasal cavity 2. Blood examination (anti-JCP IgE; eosinophil number; Th1/Th2 relative ratio) |
Fermented milk containing LcS does not prevent allergic symptoms in patients sensitive to JCP | No obvious adverse events were found |
| Ishida et al. (28) | Japan | RCT | 52 | Adults (age >18 years old, mean, 35.4 years) with perennial allergic rhinitis and high concentrations of anti-house dust IgE or anti house dust mite IgE | Perennial (house dust and mite) | Acidified milk with Lactobacillus acidophilus strain L-92 (L-92) (3 × 1010 counts/100 ml/day | Placebo (acidified milk) (100 ml/day) | 8 weeks | 1. Symptom-medication score (SMS) (nasal, ocular) 2. Score of nasal cavity findings 3. Blood sample (total IgE and sIgE levels, Th1/Th2 ratio in blood, eosinophils) |
L-92 can alleviate the symptoms of perennial allergic rhinitis | No obvious adverse events were found |
| Ciprandi et al. (29) | Italy | RCT | 20 | Children (age 12–15 years old, mean 13.4 years) with allergic rhinitis | Seasonal | Bacillus clausii at the dosage schedule of three vials+ levocetirizine (5 mg/day) | Levocetirizine (5 mg/day) | 3 weeks | 1. Total nasal symptom scores (TNSS) 2. Medication use |
B. clausii may exert a modulatory effect on allergic response as documented by reduced eosinophil infiltration | Not mentioned whether any adverse events occurred |
| Ivory et al. (30) | England | RCT | 20 | AR sufferers (age 18–45 years old) with a history of seasonal allergic rhinoconjuctivitis | Perennial and seasonal | Probiotic drinks contain Lactobacillus casei Shirota (LcS) (6.5 ×109 LcS/65 ml/day) | Placebo (placebo drinks/65ml/day) | 5 months | 1. Blood examination (IL-1b, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IFN-g, and TNF-a) | Probiotic supplementation modulates immune responses in allergic rhinitis | Not mentioned whether any adverse events occurred |
| Nishimura et al. (31) | Japan | RCT-DB | 45 | Subjects (age 16–60 years old) with perennial allergic rhinitis and had a history of PAR of more than 3 years | Perennial (house dust or mites) | Tetragenococcus halophilus Th22 (high-dose tablets that contain 10 mg/tablet, 6 tablets/day; low-dose tablets that contain 3.4 mg/tablet, 6 tablets/day) | Placebo (6 tables/day) | 8 weeks | 1. Total nasal symptom scores (TNSS) (combination of sneezing, rhinorrhea, and nasal obstruction) 2. Serum total IgE and sIgE levels, eosinophil count, nasal eosinophil, and neutrophil counts |
Th221 can be expected to safely improve the symptoms of PAR | No obvious adverse events were found |
| Kawase et al. (32) | Japan | RCT | 40 | Adults (age >18 years old, mean, 36.9 years) with a clinical history of Japanese cedar pollinosis | Seasonal (JCP) | Fermented milk contains usual bacteria and Lactobacillus GG and L. gasseri TMC0356 (110 g/day) | Placebo (yogurt contains the usual bacteria) (110 g/day) | 10 weeks | 1. Symptom score (sneezing, rhinorrhea, itching) 2. Symptom-medication score 3. Blood examination (total IgE, sIgE, Th1/Th2 ratio, TARC, CRP, eosinophils) |
The fermented milk prepared with LGG and TMC0356 might be beneficial in JCP | Not mentioned whether any adverse events occurred |
| Ouweh et al. (18) | Sweden | RCT | 47 | Children (age 4–13 years old) with clinically and immunologically documented and physician-verified birch pollen allergy | Seasonal(birch pollen); | A combination of Lactobacillus acidophilus and Bifidobacterium lactis (5x109 CFU/capsules/day) | Placebo (one capsule/day) | 4 months | 1. Presence of nasal, respiratory, or ocular symptoms; 2. Serum sIgE level, blood 3. Nasal eosinophil counts, cytokines IL-4, IL-5, IL-6, IL-10, TNF-α, TGF-β2, soluble CD14 4. Fecal microbiota, calprotectin, and IgA |
1. Probiotics prevent the infiltration of eosinophils into the nasal mucosa; 2. Probiotics reduce nasal symptoms |
Not mentioned whether any adverse events occurred |
| Yonekura et al. (17) | Japan | RCT | 126 | Patients (age 20–50 years old) with Japanese cedar pollinosis | Seasonal (JCP) | Lactobacillus paracasei strain KW3110 (1×1012–3×1012 CFU/g/day) | Placebo (dextrin) (1 g/day) | 3 months | 1. Nasal symptoms (sneezing, runny nose, stuffy nose) 2. Quality-of-life score 3. Blood examination (total IgE, sIgE, serum eosinophil count and ECP, Th1/Th2 ratio); |
1. KW3110 can significantly reduce nasal symptoms and the serum level of eosinophil cationic protein 2. KW3110 can improve quality-of-life scores when pollen scattering was low |
Loose stools; diarrhea |
| Nagata et al. (33) | Japan | RCT-DB | 35 | Female college students (age 18–27 years old) with seasonal allergic diseases | Seasonal (JCP) |
Lactobacillus plantarum No. 14 (LP14) (8.7 ×108CFU/0.5 g) (0.5 g/day) |
Placebo (branched dextrin) (0.5 g/day) | 6 weeks | 1. Scores for ocular SME, itchy eyes, and medicine taking 2. Total IgE, anti-JCP IgE, eosinophil count, CRP; and Th1 percentage, Th2 percentage, and Th1/Th2 ratio, antiragweed, anti-house dust mite IgE, fecal microbiota |
LP14 strongly induced the gene expression of Th1-type cytokines, which indicates the clinical effects of LP14 on seasonal allergic rhinitis | No obvious adverse events were found |
| Jan et al. (20) | China | RCT-DB | 240 | Patients (age < 18 years old, mean: 8 years) with history of perennial allergic symptoms for at least 3 years | Perennial (Dp, Df, or dust) | Lactobacillus rhamnosus (4×109 CFU/g) (1 g/day) | Placebo (microcrystalline cellulose) (1 g/day) | 12 weeks | 1. Nasal, eye, lung symptom clinical score 2. Blood cell counts, total IgE, and blood eosinophil counts |
L. rhamnosus treatment neither reduced rhinitis symptom scores nor altered immunological parameters in symptomatic children | Not mentioned whether any adverse events occurred |
| Lue et al. (34) | Sweden | RCT | 63 | Children (age 7–12 years old) with moderate-to-severe perennial allergic rhinitis | Perennial(house dust mite); | Levocetirizine (5 mg/day)with Lactobacillus johnsonii EM1 (Lj EM1) (1×1010 CFU/capsule/day) | Levocetirizine (5 mg/day) | 12 weeks | 1. Daily diary of total symptom score and sleep quality 2. The Pediatric Rhinoconjunctivitis Quality of Life (PRQLQ) 3. Nasal peak expiratory flow rate 4. Nasal smear 5. Peripheral blood eosinophils, total serum IgE, mite-specific IgE, ECP, resistin, IL4, IL-10, IFN-g, and TGF-b |
Levocetirizine plus Lj EM1 was more effective for perennial allergic rhinitis than levocetirizine and that this difference persisted for at least 3 months after discontinuation of Lj EM1 | No obvious adverse events were found |
| Lin et al. (35) | Sweden | RCT-DB | 199 | Children (6–12 years old) have a history of perennial allergic symptoms for at least 3 years | Perennial (Dp, Df, or dust) | Lactobacillus salivarius PM-A0006 (4×109 CFUs/g) (500 mg/day) | Placebo (500 mg/day) | 12 weeks | 1. Specific symptom scores for eye, nose, lung, medicine 2. Eosinophil count, total IgE level |
Lactobacillus salivarius treatment reduces rhinitis symptoms and drug usage in children with allergic rhinitis | Not mentioned whether any adverse events occurred |
| Singh et al. (36) | Switzerland | RCT-DB | 20 | Adult subjects (age 20–65 years old) with clinical history of SAR and positive skin prick test to grass pollen | Perennial (house dust and mite) | Bifidobacterium lactis NCC2818 (2×109CFU/day) 2 g/day | Placebo (2 g/day) | 8 weeks | 1. TNSS 2. IL-2, IL-5, IL-10, IFN-γ, IL-13, IL-1, and TNF-1β in whole-blood cell cultures; total IgE and sIgE level |
Oral administration of the probiotic NCC2818 mitigates immune parameters and allergic symptoms during seasonal exposure | No obvious adverse events were found |
| Dölle et al. (15) | Germany | RCT-DB | 34 | Subjects (age 18–65 years old) with grass pollen-dependent allergic rhinoconjunctivitis | Seasonal (JCP) | 2.5–25 billion viable bacteria of the strain E. coli Nissle 1917 (1 capsule daily over the first 4 days, 2 capsules daily until the end of treatment) | Placebo (1 capsule daily over the first 4 days, 2 capsules daily until the end of treatment) | 6 months | 1. SMS during grass-pollen season 2. Skin-prick test, conjunctival provocation test, RQLQ, total IgE, sIgE, sIgA levels |
6 months of coseasonal nonspecific immunomodulation by EcN is not sufficient to achieve clinical efficacy in grass pollen-allergic subjects | Diarrhea, abdominal pain, flatulence |
| Costa et al. (37) | France | RCT-DB | 425 | Subjects (age 18–60 years old) with persistent AR, symptomatic during the grass pollen season, and a positive skin test or specific immunoglobulin E to grass pollens | Seasonal (grass) | Lactobacillus paracasei subsp. (paracasei LP-33) 2.0×109 CFU/capsule/day + loratadine (10 mg/day) | Placebo (one capsule/day) + loratadine(10 mg/day) | 5 weeks | 1. The RQLQ global score 2. Nasal and ocular symptoms |
LP-33 improves the quality of life of subjects with persistent AR who are currently being treated with an oral H1-antihistamine. Whereas nasal symptoms had not changed, ocular symptoms had consistently improved | No obvious adverse events were found |
| Lin et al. (38) | China | RCT | 60 | Children (age 6–13 years old) had perennial allergic rhinitis for more than 1 year | Perennial (house dust mites); | Levocetirizine (8 weeks) +Lactobacillus paracasei (HF.A00232) (4 weeks); | Levocetirizine (8 weeks) +placebo (4 weeks) | 12 weeks | 1. PRQLQ 2. sIgE, IL-4, IFN-γ, IL-10, TGF-β |
Dietary supplementation with LP (HF.A00232) provided no additional benefit when used with regular levocetirizine in treating AR in the initial 8 weeks, but there was a significant improvement in individual symptoms of sneezing, itchy nose, and swollen eyes, after discontinuing regular levocetirizine treatment | No obvious adverse events were found |
| Nembrini et al. (19) | England | RCT-DB | 131 | Grass pollen allergic subjects (age 18–65 years old) | Seasonal (grass pollen) | A probiotic blend containing 5 × 109 CFU Lactobacillus paracasei NCC 2461 (5 g/day) | Placebo (maltodextrin);(5 g/day) | 8 weeks | 1. TNSS 2. RQLQ 3. Medication score |
Oral administration of NCC 2461 did not show a beneficial effect on allergic rhinitis | No obvious adverse events were found |
| Delgiudice et al. (39) | Italy | RCT-DB | 40 | Patients (age 4–17 years old) with allergic rhinitis and intermittent asthma due to Parietaria officinalis pollen | Seasonal (Parietaria officinalis pollen) | A mixture powder composed of three bifidobacteria Bifidobacterium Longum BB536 (3 billion units) + Bifidobacterium infantis M-63 (1 billion units) + Bifidobacterium breve M-16 V (1 billion units) (0.5 ml per os all days for 2 months) | Placebo (0.5 ml per os all days for 2 months) | 2 months | 1. RTSS 2. Quality of life (QoL) |
A bifidobacteria mixture was capable of significantly improving AR symptoms and QoL in children with pollen-induced AR and intermittent asthma | No obvious adverse events were found |
| Dennis-wall et al. (40) | America | RCT-DB | 173 | Participants (age 18–60 years old) who typically receive a global score of ≥2 on the MRQLQ during peak allergy season |
Seasonal | Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and B. longum MM-2 (1.5 billion CFU/capsule) (2 capsules/day, 1.5 billion colony-forming units/capsule) | Placebo (348 mg potato starch) twice a day | 8 weeks | 1. Rhinoconjunctivitis-specific quality of life (MRQLQ) 2. Gastrointestinal function 3. Immune markers |
Probiotic improved rhinoconjunctivitis-specific quality of life during allergy season for healthy individuals with self-reported seasonal allergies | No obvious adverse events were found |
| Meng et al. (41) | China | RCT-DB | 60 | Patients (age > 18 years, mean, 31.34 years) with moderate to severe perennial AR for >2 years | Perennial | Broncho-Vaxom (BV) (7 mg/day); | Placebo (7 mg/day) | 3 cycles (10 consecutive days followed by a 20-day resting period/cycle) | 1. Individual nasal symptom score (INSS) 2. Total nasal symptom score (TNSS) 3. IL-4, IL-13, and interferon (IFN)-γ |
Oral administration of BV may be considered as an alternative therapeutic strategy for patients with persistent AR | Slight abdominal pain (adverse events were spontaneously alleviated without drug treatment) |
| Kang et al. (42) | South Korea | Multicenter randomized controlled study | 95 | Subjects (age 19–65 years old) with persistent rhinitis symptoms for at least two consecutive years | Perennial (Dp, Df, cat, dog, and cockroach) | Probiotic NVP-1703 (a mixture of Bifidobacterium longum and Lactobacillus plantarum) [1.0 × 1010 CFU/day (2 g/stick pack)] | Placebo (maltodextrin) (2 g/stick pack) | 4 weeks | 1. TNSS(nasal congestion, rhinorrhea, nasal itching, and sneezing) 2. RCAT 3. Blood eosinophil count 4. Allergen-specific IgE, and immunological parameters in serum (IL-4, IL-5, IL-10, IL-13, IFN-γ); |
NVP-1703 can be treatment option for perennial AR | No obvious adverse events were found |
| Anania et al. (43) | Italy | RCT-DB | 250 | Children (age 6–17 years) with allergic rhinitis, undergoing treatment with conventional AR therapies [antihistamines (oral)+corticosteroids (local)] | Perennial (dust);and seasonal (grass pollen) | Bifidobacterium animalis subsp. Lactis BB12 and Enterococcus faecium L3 (2 × 109 CFUs/2.5 g/ sachet) (one sachet per day) | Placebo (maltodextrin) (one sachet per day) | 3 months | 1. Nasal symptoms score 2. Pharmacological treatment of AR |
A mixture of BB12 and L3 statistically decreased signs and symptoms of AR and reduced significantly the need of conventional therapy | No obvious adverse events were found |
Total nasal symptom scores (TNSS), rhinoconjunctivitis total symptom score (RTSS), rhinitis control assessment test (RCAT), Mini Rhinoconjunctivitis Quality of Life Questionnaire (MRQLQ), colony-forming units (CFUs), Dermatophagoides pteronyssinus (Dp), and Dermatophagoides farinae (Df).
Risk of Bias Assessment
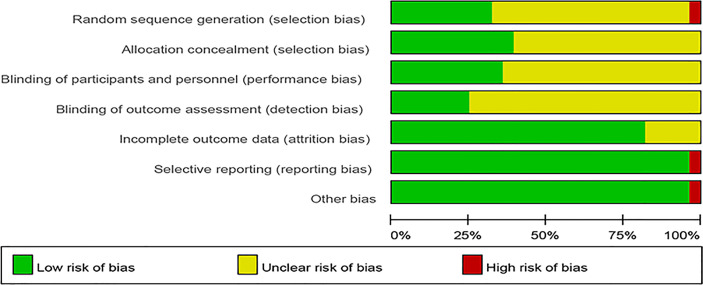
The risk of bias assessment is presented in Figures 2 , 3 . Most studies did not clearly show how to generate random sequences, nor did they clearly state whether association obfuscation was performed. In terms of masking method, most of the studies have insufficient information to permit judgment of “Low risk” or “High risk”. We assessed three trials having high risk of bias for different reasons. One of the trials did not report all the pre-specified primary outcome indicators (30). The random allocation method in one of the studies was incorrect (The patients were randomized according to the birth date) (41). Since Nagata reported that participants were all female college students from the same university in the trial (33), it was marked as “high risk” in other bias.
Figure 2.
Risk of bias.
Figure 3.
Summary of risk of bias.
Overall Analyses
Allergic Rhinitis Symptoms Score
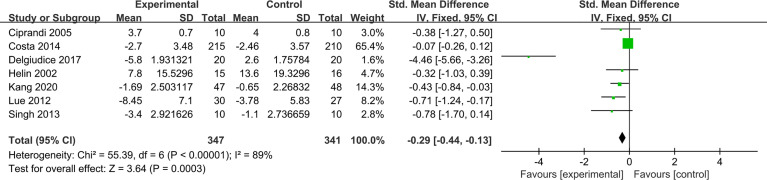
AR symptoms score included rhinoconjunctivitis total symptom score (RTSS) and total nasal symptom scores (TNSS). RTSS includes five individual AR symptoms (nasal congestion, sneezing, rhinorrhea, nasal pruritus, and eye itching) noted from 0 (no symptom) to 3 (severe symptom). TNSS were expressed as the sum of the scores for the four symptoms (nasal congestion, rhinorrhea, nasal itching, and sneezing) noted from 0 (no symptom) to 3 (severe symptom). Seven trials reported pre- and post-treatment data of AR symptoms score available for meta-analysis. Compared with placebo, probiotics significantly improved AR symptoms score (SMD, −0.29, 95% CI [−0.44, −0.13]). There was high heterogeneity in the result (p = 0.0003, I 2 = 89%) ( Figure 4 ). Sensitivity analysis indicates that the result is robust ( Supplementary Material 13 ). Due to the significantly statistical heterogeneity encountered in the analysis, several subgroup analyses were conducted separately according to the classification of AR, combination of drugs, and intervention of treatment group.
Figure 4.
Forest plot for allergic rhinitis symptoms score.
With regard to classification of AR, probiotics can significantly relieve symptoms in patients with SAR (SMD −0.56, 95% CI [−0.87, −0.25]; p = 0.0003, I 2 = 0%), and there was significant benefit that probiotics supplementation relieved PAR symptoms score (SMD,−0.19, 95% CI [−0.37, −0.01]; p = 0.03, I 2 = 94%) ( Supplementary Material 1 ). Subgroup analysis according to the combination of drugs again found some evidence of a protective effect of probiotics (monotherapy) in relieving AR symptoms compared with placebo (SMD, −0.73, 95% CI [−1.05, −0.42]; p < 0.00001, I 2 = 93%). Compared with antihistamines, probiotics combined with antihistamines (combination therapy) have no significant relief of AR symptoms (SMD, −0.15, 95% CI [−0.32, 0.03]; p = 0.10, I 2 = 61%) ( Supplementary Material 2 ). The results of subgroup analysis showed that probiotics (single) compared with placebo cannot significantly relieve symptoms (SMD, −0.49, 95% CI [−1.05, 0.07], p = 0.09). Similarly, probiotics combined with antihistamines compared with antihistamines have no significant relief of AR symptoms (SMD, −0.15, 95% CI [−0.32, 0.03], p = 0.10, I 2 = 61%). Probiotics (mixed) compared with placebo have significant relief of AR symptoms (SMD, −0.85, 95% CI [−1.23, −0.46], p < 0.0001, I 2 = 97%) ( Supplementary Material 3 ) ( Table 2 ).
Table 2.
Subgroup analysis for outcomes.
| Number of comparisons | Results | p-value for overall effect | I 2 | p-value for subgroup difference | |
|---|---|---|---|---|---|
| Std. Mean Difference (95%) | |||||
| Allergic Rhinitis Symptoms Score | |||||
| All comparisons | 7 | −0.29 [−0.44, −0.13] | p = 0.0003 | 89% | |
| Classification of allergic rhinitis | p = 0.04 | ||||
| Perennial allergic rhinitis (PAR) | 4 | −0.19 [−0.37, −0.01] | p = 0.03 | 94% | |
| Seasonal allergic rhinitis (SAR) | 3 | −0.56 [−0.87, −0.25] | p = 0.0003 | 0% | |
| Combination of drugs | p = 0.02 | ||||
| Monotherapy | 4 | −0.73 [−1.05, −0.42] | p < 0.00001 | 93% | |
| Combined (probiotics combined with antihistamines) | 3 | −0.15 [−0.32, 0.03] | p = 0.10 | 61% | |
| Intervention of treatment group | p = 0.004 | ||||
| Probiotics combined with antihistamines | 3 | −0.15 [−0.32, 0.03] | p = 0.10 | 61% | |
| Mixed probiotics | 2 | −0.85 [−1.23, 0.46] | p < 0.0001 | 97% | |
| Single probiotic | 2 | −0.49 [−1.05, −0.07] | p = 0.09 | 0% | |
| Std. Mean Difference (95%) | |||||
| Rhino-conjunctivitis Quality of Life Questionnaire Score | |||||
| All comparisons | 7 | −0.64 [−0.79, −0.49] | p < 0.00001 | 97% | |
| Classification of allergic rhinitis | p < 0.00001 | ||||
| Perennial allergic rhinitis (PAR) | 4 | −2.10 [−2.45, −1.74] | p < 0.00001 | 97% | |
| Seasonal allergic rhinitis (SAR) | 3 | −0.32 [−0.49, −0.15] | p = 0.0002 | 96% | |
| Combination of drugs | p < 0.00001 | ||||
| Monotherapy (probiotics) | 5 | −1.74 [−2.03, −1.46] | p < 0.00001 | 97% | |
| Combined (probiotics combined with antihistamines) | 2 | −0.21 [−0.39, −0.03] | p = 0.02 | 0% | |
| Intervention of treatment group | p < 0.00001 | ||||
| Probiotics combined with antihistamines | 2 | −0.21 [−0.39, −0.03] | p = 0.02 | 0% | |
| Mixed probiotics | 1 | −5.16 [−6.50, −3.81] | p < 0.00001 | NA | |
| Single probiotic (IL-33) | 3 | −3.81 [−4.29, −3.32] | p < 0.00001 | 0% | |
| Std. Mean Difference (95%) | |||||
| Total IgE | |||||
| All comparisons | 9 | −0.03 [−0.18, 0.13] | p = 0.72 | 0% | |
| Classification of allergic rhinitis | 0.34 | ||||
| Perennial allergic rhinitis and Seasonal allergic rhinitis (PAR and SAR); | 1 | −0.19 [0.48, 0.10] | – | NA | |
| Perennial allergic rhinitis(PAR); | 5 | 0.07 [−0.13, 0.27] | p = 0.50 | 8% | |
| Seasonal allergic rhinitis (SAR) | 3 | −0.09 [0.48, 0.30] | p = 0.65 | 0% | |
| Combination of drugs | p = 0.82 | ||||
| Monotherapy (probiotics) | 8 | −0.03 [−0.19, 0.13] | p = 0.69 | 0% | |
| Combined (probiotics combined with antihistamines) | 1 | 0.03 [−0.49, 0.55] | – | NA | |
| Std. Mean Difference (95%) | |||||
| sIgE | |||||
| All comparisons | 6 | 0.09 [−0.16, 0.34] | p = 0.49 | 0% | |
| Classification of allergic rhinitis | 0.40 | ||||
| Perennial allergic rhinitis (PAR) | 2 | −0.03 [−0.41, 0.34] | p = 0.86 | 0% | |
| Seasonal allergic rhinitis (SAR) | 4 | 0.18 [−0.15, 0.51] | p = 0.28 | 0% | |
| Combination of drugs | 0.12 | ||||
| Monotherapy (probiotics) | 5 | 0.00 [−0.27, 0.27] | p = 0.99 | 0% | |
| Combined (probiotics combined with antihistamines) | 1 | 0.55 [−0.08, 1.18] | p = 0.09 | NA | |
| Mean Difference (95%) | |||||
| Th1/Th2 ratio | |||||
| All comparisons | 4 | −2.01 [−3.94, −0.08] | p = 0.04 | 72% | |
| Classification of allergic rhinitis | p = 0.02 | ||||
| Perennial allergic rhinitis (PAR) | 1 | −1.50 [−2.63, −0.37] | p = 0.01 | NA | |
| Seasonal allergic rhinitis (SAR) | 3 | −3.42 [−4.54, −2.30] | p = 0.04 | 72% | |
| Combination of drugs | p = 0.002 | ||||
| Monotherapy (probiotics) | 3 | −1.34 [−2.41, −0.28] | p = 0.01 | 0% | |
| Combined (probiotics combined with antihistamines) | 1 | −3.9 [−5.10, −2.70] | p < 0.00001 | NA |
NA, not applicable.
Rhinoconjunctivitis Quality of Life Questionnaire Score
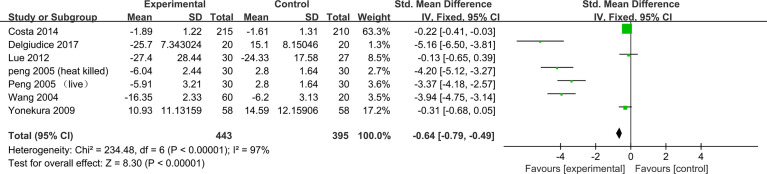
Seven trials reported pre- and post-treatment data of Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) scores available for meta-analysis. The results combined with the fixed-effect model showed a significant decrease in RQLQ scores in the probiotic group compared with the control group (−0.64, 95% CI [−0.79, −0.49], p < 0.00001, I 2 = 97%) ( Figure 5 ). Sensitivity analysis indicates that the result is stable ( Supplementary Material 13 ).
Figure 5.
Forest plot for Rhinoconjunctivitis Quality of Life Questionnaire Score.
Subgroup analysis according to the classification of AR found some evidence of a significant decrease in RQLQ scores for SAR in the probiotic group compared with the control group (SMD, −0.32, 95% CI [−0.49, −0.15], p = 0.0002, I 2 = 96%), and a greater beneficial effect in PAR (SMD, −2.10, 95% CI [-2.45, −1.74], p < 0.00001, I 2 = 97%) ( Supplementary Material 4 ). Subgroup analysis according to the combination of drugs again found some evidence of a protective effect of probiotics (monotherapy) in relieving AR symptoms compared with placebo (SMD, −1.74, 95% CI [−2.03, −1.46]; p < 0.00001, I 2 = 97%). Compared with antihistamines, probiotics combined with antihistamines (combination therapy) have a significant relief of AR symptoms (SMD, −0.21, 95% CI [−0.39, −0.03]; p = 0.02, I 2 = 0%) ( Supplementary Material 5 ). The results of subgroup analysis showed that probiotics (single) comparing with placebo can significantly relieve symptoms (SMD, -3.81,95% CI [-4.29, -3.32], p<0.00001, I2=0%). Similarly, probiotics combined with antihistamines (combination therapy) compared with antihistamines showed significant improvement in RQLQ scores (SMD, −0.21, 95% CI [−0.39, −0.03], p = 0.02, I 2 = 0%) ( Supplementary Material 6 ) ( Table 2 ).
Immunologic Parameters
Total IgE
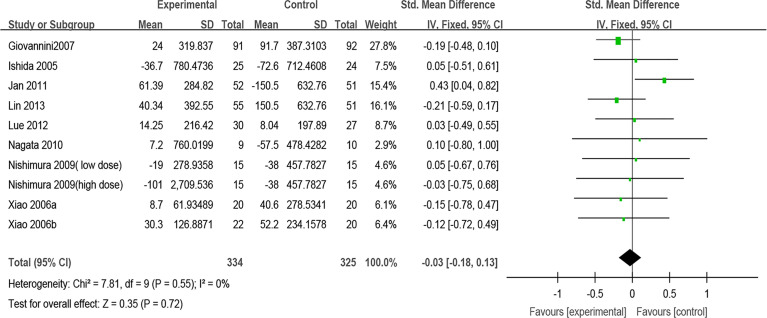
Nine trials reported the effect of probiotics on total IgE. After pooling nine estimates, there was no difference found in total IgE between the probiotic group and the control group (SMD, −0.03, 95% CI [−0.18, 0.13], p = 0.72, I 2 = 0%) ( Figure 6 ). Sensitivity analysis indicates that the result is stable ( Supplementary Material 13 ). Subgroup analyses were conducted according to the classification of AR and combination of drugs. The results of subgroup analysis showed that the effect of probiotics on total IgE could not be affected by the classification of AR (PAR or SAR) or combined with other drugs ( Supplementary Materials 7 and 8 ) ( Table 2 ).
Figure 6.
Forest plot for Total IgE.
Specific IgE
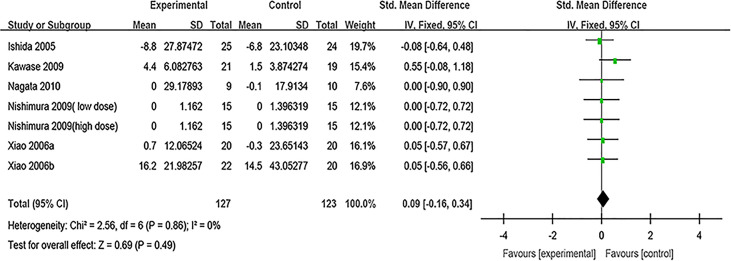
Specific IgE was evaluated in six studies. After pooling six estimates, there was no difference found in sIgE between the probiotic group and the control group (SMD, 0.09, 95% CI [−0.16, 0.34], p = 0.49, I 2 = 0%) ( Figure 7 ). Sensitivity analysis indicates that the result is stable ( Supplementary Material 13 ). Subgroup analyses were conducted according to the classification of AR and combination of drugs. The results of subgroup analysis showed that the effect of probiotics on sIgE could not be affected by the classification of AR (PAR or SAR) or combined with other drugs ( Supplementary Materials 9 and 10 ) ( Table 2 ).
Figure 7.
Forest plot for sIgE.
Th1/Th2 ratio
Four trials reported enough data to allow meta-analysis for the Th1/Th2 ratio. The results showed that the Th1/Th2 ratio was lower in the control group when the effect estimates from four trials were pooled (MD, −2.47, 95% CI [−3.27, −1.68], p < 0.00001, I 2 = 72%) ( Figure 8 ). Sensitivity analysis indicates that the result is stable ( Supplementary Material 13 ). Subgroup analyses were conducted according to the classification of AR. The results of subgroup analysis showed that the effect of probiotics on the Th1/Th2 ratio could not be affected by the classification of AR (PAR or SAR) or treatment plan (monotherapy/combined) ( Supplementary Materials 11 and 12 ) ( Table 2 ).
Figure 8.
Forest plot for Th1/Th2 ratio.
Adverse Events
Of the twenty-eight studies included, seventeen RCTs mentioned that no obvious adverse events were found during the research, while seven RCTs did not mention whether any adverse events occurred. Four RCTs have reported adverse events including diarrhea, abdominal pain, flatulence, and fever episodes. One study reported that loose stools and diarrhea were observed in the active and placebo groups, which had no significant differences in adverse events between the two groups (chi-square test, p < 0.4) (17). Another study showed that subjects with these adverse drug reactions (diarrhea, abdominal pain, and flatulence) recovered within a few days. In this study, it was found that one subject’s adverse reaction was almost certainly related to the drug (15). One study reported slight abdominal pain in probiotic groups and all of the adverse events were spontaneously alleviated without drug treatment (41). One study revealed that abdominal symptoms (abdominal symptoms, diarrhea, and fever episodes) were reported in 56.5% versus 64.2% of children in intervention and control groups, respectively (p = 0.282) (26).
GRADE Evidence Quality Evaluation
The quality of evidence applied for each outcome is summarized in Table 3 . The quality of evidence on the Allergic Rhinitis Symptoms Score, Rhinoconjunctivitis Quality of Life Questionnaire Score, Total IgE, Antigen-specific IgE, and Th1/Th2 ratio was rated as very low, very low, low, low, and very low, respectively ( Table 3 ).
Table 3.
GRADE assessment.
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control | |||||
| Allergic Rhinitis Symptoms Score | The mean RTSS global score in the intervention groups was 0.29 standard deviations lower
(0.44 to 0.13 lower) |
SMD −0.29 (−0.44 to −0.13) | 688 (7 studies) |
⊕⊝⊝⊝ Very low 1,2,3 |
|
| Rhino-conjunctivitis Quality of Life Questionnaire Score | The mean RQLQ global score in the intervention groups was 2.38 standard deviations lower
(3.58 to 1.19 lower) |
SMD −2.38 (−3.58 to −1.19) | 838 (7 studies) |
⊕⊝⊝⊝ Very low 1,2,3 |
|
| Total IgE | The mean total IgE in the intervention groups was 0.03 standard deviations lower
(0.18 lower to 0.13 higher) |
SMD −0.03 (−0.18 to 0.13) | 659 (10 studies) |
⊕⊕⊝⊝ Low 1,3 |
|
| Antigen-specific IgE | The mean antigen-specific IgE in the intervention groups was 0.09 standard deviations higher
(0.16 lower to 0.34 higher) |
SMD 0.09 (−0.16 to 0.34) | 250 (7 studies) |
⊕⊕⊝⊝ Low 1,3 |
|
| Th1/Th2 | The mean Th1/Th2 in the intervention groups was 2.47 lower
(3.27 to 1.68 lower) |
MD −2.47 [−3.27, −1.68] | 238 (4 studies) |
⊕⊝⊝⊝ Very low 1,3 |
|
*The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI, Confidence interval.
GRADE Working Group grades of evidence.
High quality, Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality, Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality, Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality, We are very uncertain about the estimate.
1In some studies, random sequence generation, allocation concealment, and blinding of participants and personnel are not described.
2There is a significant heterogeneity (I2 > 50%).
3PICO is not exactly the same.
Discussion
In this study, the clinical evidence of probiotics in the treatment of AR was systemically collated and analyzed so as to provide a better guidance for clinical practice. Our results showed that probiotics supplementation for patients with AR can ameliorate AR symptoms and improve the quality of life. Probiotics supplementation can correct the Th1/Th2 balance. There was no significant change in overall or antigen-specific IgE levels between probiotic-treated and placebo-treated subjects. The results of this study have significant heterogeneity, and the source of heterogeneity was explored by subgroup analysis. The results of subgroup analysis showed that probiotics can significantly relieve AR symptoms in patients with SAR. Subgroup analysis according to combination of drugs again found some evidence of a protective effect of probiotics (monotherapy) in relieving AR symptoms compared with placebo. Compared with antihistamines, probiotics combined with antihistamines (combination therapy) have no significant relief of AR symptoms. Subgroup analyses of these outcomes failed to find out the source of heterogeneity. The different doses, durations, and strains of probiotics may be the sources of heterogeneity. With regard to RQLQ score, the results of subgroup analysis according to combination of drugs showed that probiotics (single probiotic strain) compared with placebo can significantly improve the quality of life. Similarly, probiotics combined with antihistamines (combination therapy) compared with antihistamines showed a significant decrease in RQLQ scores, which means an improvement in the quality of life. As we all know, helper T cells play a key role in the adaptive immune response. Human T helper cells can be divided into two main subtypes, Th1 and Th2. The significant trend of immune response to Th2 lineage may lead to allergic diseases. Immunoglobin E (IgE)-mediated allergic inflammation is the main pathophysiological mechanism of AR and drives T helper 2 (Th2) cell polarized immune reactions (45).
The balance Th1/Th2 is associated with AR. Th2 induces the activation of B cells and IgE class switching, which leads to B-cell differentiation into plasma cells that produce allergen-specific IgE. IgE enters the circulation and binds through its Cϵ3 domain to the high-affinity IgE receptor (FcϵRI) on the surface of mast cells and basophils (46). Activated mast cells and basophils release inflammatory mediators (e.g., histamine and leukotrienes) that cause symptoms such as nasal itching, sneezing, and runny nose. At the same time, these inflammatory mediators lead to a predominance of Th2 immune responses, further exacerbating inflammation. Therefore, the predominance of Th2 and its related cytokines correlates with the severity of AR. The Th1/Th2 ratio can reflect the effect of improving allergy symptoms by drugs to a certain degree.
Our meta-analysis demonstrated that probiotics supplementation can correct the Th1/Th2 balance, which indicates that probiotic supplementation can ameliorate AR by regulating the balance of Th1/Th2. However, only four of the included studies reported the Th1/Th2 ratio.
The purpose of most systematic reviews or meta-analyses is to explore the preventive effect of probiotic supplementation on allergic diseases (47–50). There are less systematic reviews or meta-analyses to explore the therapeutic effect of probiotics on AR. A systematic review and meta-analysis of probiotics in the treatment of AR published in 2015 has shown that probiotics may be beneficial in improving symptoms and quality of life in patients with AR (51). One meta-analysis showed that probiotics have beneficial effects in the treatment of AR, especially with SAR and LP-33 strains (52). However, previous systematic reviews failed to explore the causes of heterogeneity as much as possible. Compared with previous systematic reviews and meta-analyses, our meta-analysis conducted subgroup analysis according to types of AR (PAR/SAR) and treatment plan (single probiotic strain/mixed probiotic strains/probiotics combined with antihistamines; monotherapy/combined). We found that a single probiotic strain (LP-33) can significantly improve the quality of life of patients with AR from the meta-analysis of three studies. Two studies used mixed probiotic strains. One study demonstrated that a Bifidobacteria mixture (B. longum BB536, B. infantis M-63, and B. breve M-16 V) was able to significantly improve AR symptoms and quality of life in children with pollen-induced AR and intermittent asthma (39). Another study showed that probiotic NVP-1703 (a mixture of B. longum and L. plantarum) relieves AR symptoms by prompting Treg cells to release IL-10 (42). However, there was a high heterogeneity from meta-analysis of two studies, which may be related to the use of different probiotics. The different strains of probiotics, doses, and durations may be the sources of heterogeneity. To date, no serious adverse events have been observed for probiotic treatment; thus, it appears to be safe.
To sum up, probiotic supplement seems to be effective in ameliorating AR symptoms and improving the quality of life, but there is high heterogeneity in some results after subgroup analysis, and clinicians should be cautious when recommending probiotics in treating AR.
There are some limitations in this meta-analysis. First, the sample size of some included RCTs was small. Second, airborne pollen concentrations are associated with symptom severity and recovery in patients with SAR. The pollen concentrations varied due to different regions in different trials. This is a source of clinical heterogeneity.
Conclusion
This study found that in spite of the positive results of some outcomes, there is weak evidence that probiotics have a potential benefit in the treatment of AR. More RCTs using specific probiotic strains and consistent outcome measures are also needed in the future to investigate efficacy and safety.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
CL, X-DA, and ML were involved in the methodological design of the systematic review, and conducted the acquisition of data, analyses, and interpretation. SP directed and organized the systematic review and the methodologist team, was involved in the initial concept and methodological design of the systematic review, and conducted data acquisition and interpretation. ZL was involved in the initial concept and methodological design of the systematic review, conducted data interpretation, and provided substantial feedback on the drafted manuscript. CL wrote the manuscript and SP revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by Foundation of Chengdu Science and Technology Bureau (No. 2021-YF05-01940-SN). The sponsors are not involved in the design, execution, or writing of the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.848279/full#supplementary-material
Subgroup analysis according to classification of allergic rhinitis for allergic rhinitis symptoms score.
Subgroup analysis according to combination of drugs for allergic rhinitis symptoms score.
Subgroup analysis according to intervention of treatment group for allergic rhinitis symptoms score.
Subgroup analysis according to classification of allergic rhinitis for rhinoconjunctivitis quality of life questionnaire score.
Subgroup analysis according to combination of drugs for rhinoconjunctivitis quality of life questionnaire score.
Subgroup analysis according to intervention of treatment group for rhinoconjunctivitis quality of life questionnaire score.
Subgroup analysis according to classification of allergic rhinitis for total IgE.
Subgroup analysis according to combination of drugs for total IgE.
Subgroup analysis according to classification of allergic rhinitis for sIgE.
Subgroup analysis according to combination of drugs for sIgE.
Subgroup analysis according to classification of allergic rhinitis for Th1/Th2 ratio.
Subgroup analysis according to combination of drugs for Th1/Th2 ratio.
References
- 1. Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines-2016 Revision. J Allergy Clin Immunol (2017) 140(4):950–8. doi: 10.1016/j.jaci.2017.03.050 [DOI] [PubMed] [Google Scholar]
- 2. Chong SN, Chew FT. Epidemiology of Allergic Rhinitis and Associated Risk Factors in Asia. World Allergy Organ J (2018) 11(1):17. doi: 10.1186/s40413-018-0198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strachan DP. Hay Fever, Hygiene, and Household Size. BMJ (Clinical Res ed) (1989) 299(6710):1259–60. doi: 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and Other Environmental Organisms as Immunomodulators for Immunoregulatory Disorders. Springer Semin immunopathol (2004) 25(3-4):237–55. doi: 10.1007/s00281-003-0148-9 [DOI] [PubMed] [Google Scholar]
- 5. Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The Hygiene Hypothesis: Current Perspectives and Future Therapies. ImmunoTargets Ther (2015) 4:143–57. doi: 10.2147/ITT.S61528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noverr MC, Huffnagle GB. The 'Microflora Hypothesis' of Allergic Diseases. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2005) 35(12):1511–20. doi: 10.1111/j.1365-2222.2005.02379.x [DOI] [PubMed] [Google Scholar]
- 7. Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking Bigger: How Early-Life Environmental Exposures Shape the Gut Microbiome and Influence the Development of Asthma and Allergic Disease. Allergy (2019) 74(11):2103–15. doi: 10.1111/all.13812 [DOI] [PubMed] [Google Scholar]
- 8. West CE, Jenmalm MC, Prescott SL. The Gut Microbiota and Its Role in the Development of Allergic Disease: A Wider Perspective. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2015) 45(1):43–53. doi: 10.1111/cea.12332 [DOI] [PubMed] [Google Scholar]
- 9. McCoy KD, Köller Y. New Developments Providing Mechanistic Insight Into the Impact of the Microbiota on Allergic Disease. Clin Immunol (Orlando Fla) (2015) 159(2):170–6. doi: 10.1016/j.clim.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nations F, Organization WH. Probiotics in Food - Health and Nutritional Properties and Guidelines for Evaluation: Probiotics in Food - Health and Nutritional Properties and Guidelines for Evaluation. Food and Agriculture Organization of the United Nations. (2006). [Google Scholar]
- 11. Hajavi J, Esmaeili SA, Varasteh AR, Vazini H, Atabati H, Mardani F, et al. The Immunomodulatory Role of Probiotics in Allergy Therapy. J Cell Physiol (2019) 234(3):2386–98. doi: 10.1002/jcp.27263 [DOI] [PubMed] [Google Scholar]
- 12. Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: Effects on Immunity. Am J Clin Nutr (2001) 73Suppl:444s–50s. doi: 10.1093/ajcn/73.2.444s [DOI] [PubMed] [Google Scholar]
- 13. Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle RJ, Cocco R, et al. Clinical Use of Probiotics in Pediatric Allergy (CUPPA): A World Allergy Organization Position Paper. World Allergy Organ J (2012) 5(11):148–67. doi: 10.1097/WOX.0b013e3182784ee0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Björkstén B. The Intrauterine and Postnatal Environments. J Allergy Clin Immunol (1999) 104(6):1119–27. doi: 10.1016/S0091-6749(99)70002-3 [DOI] [PubMed] [Google Scholar]
- 15. Dölle S, Berg J, Rasche C, Worm M. Tolerability and Clinical Outcome of Coseasonal Treatment With Escherichia Coli Strain Nissle 1917 in Grass Pollen-Allergic Subjects. Int Arch Allergy Immunol (2014) 163(1):29–35. doi: 10.1159/000356328 [DOI] [PubMed] [Google Scholar]
- 16. Xiao JZ, Kondo S, Yanagisawa N, Takahashi N, Odamaki T, Iwabuchi N, et al. Probiotics in the Treatment of Japanese Cedar Pollinosis: A Double-Blind Placebo-Controlled Trial. Clin Exp Allergy (2006) 36(11):1425–35. doi: 10.1111/j.1365-2222.2006.02575.x [DOI] [PubMed] [Google Scholar]
- 17. Yonekura S, Okamoto Y, Okawa T, Hisamitsu M, Chazono H, Kobayashi K, et al. Effects of Daily Intake of Lactobacillus Paracasei Strain KW3110 on Japanese Cedar Pollinosis. Allergy Asthma Proc (2009) 30(4):397–405. doi: 10.2500/aap.2009.30.3256 [DOI] [PubMed] [Google Scholar]
- 18. Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific Probiotics Alleviate Allergic Rhinitis During the Birch Pollen Season. World J Gastroenterol (2009) 15(26):3261–8. doi: 10.3748/wjg.15.3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nembrini C, Singh A, Vissers Y, Mercenier A, Nutten S, Blanchard C. Oral Administration of Lactobacillus Paracasei NCC2461 for the Modulation of Grass Pollen Allergic Rhinitis: A Randomized, Placebo-Controlled Study During the Pollen Season. Allergy: Eur J Allergy Clin Immunol (2015) 70:119–20. doi: 10.1186/s13601-015-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jan RH, Chen CJ, Chen LK, Wen SH, Lin TY. Is the Effect of Probiotics on Allergic Rhinitis Confined to Dermatophagoides Farinae, Dermatophagoides Pteronyssinus, or Dust-Sensitive Children? A Randomized Prospective Double-Blind Controlled Trial. Tzu Chi Med J (2011) 23(2):51–4. doi: 10.1016/j.tcmj.2011.05.003 [DOI] [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clinical Res ed) (2011) 343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helin T, Haahtela S, Haahtela T. No Effect of Oral Treatment With an Intestinal Bacterial Strain, Lactobacillus Rhamnosus (ATCC 53103), on Birch-Pollen Allergy: A Placebo-Controlled Double-Blind Study. Allergy: Eur J Allergy Clin Immunol (2002) 57(3):243–6. doi: 10.1034/j.1398-9995.2002.1s3299.x [DOI] [PubMed] [Google Scholar]
- 23. Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of Perennial Allergic Rhinitis With Lactic Acid Bacteria. Pediatr Allergy Immunol (2004) 15(2):152–8. doi: 10.1111/j.1399-3038.2004.00156.x [DOI] [PubMed] [Google Scholar]
- 24. Peng GC, Hsu CH. The Efficacy and Safety of Heat-Killed Lactobacillus Paracasei for Treatment of Perennial Allergic Rhinitis Induced by House-Dust Mite. Pediatr Allergy Immunol (2005) 16(5):433–8. doi: 10.1111/j.1399-3038.2005.00284.x [DOI] [PubMed] [Google Scholar]
- 25. Xiao JZ, Kondo S, Yanagisawa N, Takahashi N, Odamaki T, Iwabuchi N, et al. Effect of Probiotic Bifidobacterium Longum BBS36 in Relieving Clinical Symptoms and Modulating Plasma Cytokine Levels of Japanese Cedar Pollinosis During the Pollen Season. A Randomized Double-Blind, Placebo-Controlled Trial. J Investigational Allergol Clin Immunol (2006) 16(2):86–93. [PubMed] [Google Scholar]
- 26. Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A Randomized Prospective Double Blind Controlled Trial on Effects of Long-Term Consumption of Fermented Milk Containing Lactobacillus Casei in Pre-School Children With Allergic Asthma and/or Rhinitis. Pediatr Res (2007) 62(2):215–20. doi: 10.1203/PDR.0b013e3180a76d94 [DOI] [PubMed] [Google Scholar]
- 27. Tamura M, Shikina T, Morihana T, Hayama M, Kajimoto O, Sakamoto A, et al. Effects of Probiotics on Allergic Rhinitis Induced by Japanese Cedar Pollen: Randomized Double-Blind, Placebo-Controlled Clinical Trial. Int Arch Allergy Immunol (2007) 143(1):75–82. doi: 10.1159/000098318 [DOI] [PubMed] [Google Scholar]
- 28. Ishida Y, Nakamura F, Kanzato H, Sawada D, Hirata H, Nishimura A, et al. Clinical Effects of Lactobacillus Acidophilus Strain L-92 on Perennial Allergic Rhinitis: A Double-Blind, Placebo-Controlled Study. J dairy scie (2005) 88(2):527–33. doi: 10.3168/jds.S0022-0302(05)72714-4 [DOI] [PubMed] [Google Scholar]
- 29. Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus Clausii Effects in Children With Allergic Rhinitis. Allergy (2005) 60(5):702–3. doi: 10.1111/j.1398-9995.2005.00722.x [DOI] [PubMed] [Google Scholar]
- 30. Ivory K, Chambers SJ, Pin C, Prieto E, Arqués JL, Nicoletti C. Oral Delivery of Lactobacillus Casei Shirota Modifies Allergen-Induced Immune Responses in Allergic Rhinitis. Clin Exp Allergy (2008) 38(8):1282–9. doi: 10.1111/j.1365-2222.2008.03025.x [DOI] [PubMed] [Google Scholar]
- 31. Nishimura I, Igarashi T, Enomoto T, Dake Y, Okuno Y, Obata A. Clinical Efficacy of Halophilic Lactic Acid Bacterium Tetragenococcus Halophilus Th221 From Soy Sauce Moromi for Perennial Allergic Rhinitis. Allergol Int (2009) 58(2):179–85. doi: 10.2332/allergolint.O-08-548 [DOI] [PubMed] [Google Scholar]
- 32. Kawase M, He F, Kubota A, Hiramatsu M, Saito H, Ishii T, et al. Effect of Fermented Milk Prepared With Two Probiotic Strains on Japanese Cedar Pollinosis in a Double-Blind Placebo-Controlled Clinical Study. Int J Food Microbiol (2009) 128(3):429–34. doi: 10.1016/j.ijfoodmicro.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 33. Nagata Y, Yoshida M, Kitazawa H, Araki E, Gomyo T. Improvements in Seasonal Allergic Disease With Lactobacillus Plantarum No. 14. Biosci biotechnol Biochem (2010) 74(9):1869–77. doi: 10.1271/bbb.100270 [DOI] [PubMed] [Google Scholar]
- 34. Lue KH, Sun HL, Lu KH, Ku MS, Sheu JN, Chan CH, et al. A Trial of Adding Lactobacillus Johnsonii EM1 to Levocetirizine for Treatment of Perennial Allergic Rhinitis in Children Aged 7-12 Years. Int J Pediatr otorhinolaryngol (2012) 76(7):994–1001. doi: 10.1016/j.ijporl.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 35. Lin TY, Chen CJ, Chen LK, Wen SH, Jan RH. Effect of Probiotics on Allergic Rhinitis in Df, Dp or Dust-Sensitive Children: A Randomized Double Blind Controlled Trial. Indian pediatrics (2013) 50(2):209–13. doi: 10.1007/s13312-013-0068-2 [DOI] [PubMed] [Google Scholar]
- 36. Singh A, Hacini-Rachinel F, Gosoniu ML, Bourdeau T, Holvoet S, Doucet-Ladeveze R, et al. Immune-Modulatory Effect of Probiotic Bifidobacterium Lactis NCC2818 in Individuals Suffering From Seasonal Allergic Rhinitis to Grass Pollen: An Exploratory, Randomized, Placebo-Controlled Clinical Trial. Eur J Clin Nutr (2013) 67(2):161–7. doi: 10.1038/ejcn.2012.197 [DOI] [PubMed] [Google Scholar]
- 37. Costa DJ, Marteau P, Amouyal M, Poulsen LK, Hamelmann E, Cazaubiel M, et al. Efficacy and Safety of the Probiotic Lactobacillus Paracasei LP-33 in Allergic Rhinitis: A Double-Blind, Randomized, Placebo-Controlled Trial (GA2LEN Study). Eur J Clin Nutr (2014) 68(5):602–7. doi: 10.1038/ejcn.2014.13 [DOI] [PubMed] [Google Scholar]
- 38. Lin WY, Fu LS, Lin HK, Shen CY, Chen YJ. Evaluation of the Effect of Lactobacillus Paracasei (HF.A00232) in Children (6-13 Years Old) With Perennial Allergic Rhinitis: A 12-Week, Double-Blind, Randomized, Placebo-Controlled Study. Pediatr Neonatol (2014) 55(3):181–8. doi: 10.1016/j.pedneo.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 39. Del Giudice MM, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium Mixture (B Longum BB536, B Infantis M-63, B Breve M-16V) Treatment in Children With Seasonal Allergic Rhinitis and Intermittent Asthma. Ital J Pediatrics (2017) 43(1):25. doi: 10.1186/s13052-017-0340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dennis-Wall JC, Culpepper T, Nieves C, Jr, Rowe CC, Burns AM, Rusch CT, et al. Probiotics (Lactobacillus Gasseri KS-13, Bifidobacterium Bifidum G9-1, and Bifidobacterium Longum MM-2) Improve Rhinoconjunctivitis-Specific Quality of Life in Individuals With Seasonal Allergies: A Double-Blind, Placebo-Controlled, Randomized Trial. Am J Clin Nutr (2017) 105(3):758–67. doi: 10.3945/ajcn.116.140012 [DOI] [PubMed] [Google Scholar]
- 41. Meng Q, Li P, Li Y, Chen J, Wang L, He L, et al. Broncho-Vaxom Alleviates Persistent Allergic Rhinitis in Patients by Improving Th1/Th2 Cytokine Balance of Nasal Mucosa. Rhinology (2019) 57(6):451–9. doi: 10.4193/Rhin19.161 [DOI] [PubMed] [Google Scholar]
- 42. Kang MG, Han SW, Kang HR, Hong SJ, Kim DH, Choi JH. Probiotic NVP-1703 Alleviates Allergic Rhinitis by Inducing IL-10 Expression: A Four-Week Clinical Trial. Nutrients (2020) 12(5):1427. doi: 10.3390/nu12051427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anania C, Di Marino VP, Olivero F, De Canditiis D, Brindisi G, Iannilli F, et al. Treatment With a Probiotic Mixture Containing Bifidobacterium Animalis Subsp. Lactis BB12 and Enterococcus Faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients (2021) 13(4):1315. doi: 10.3390/nu13041315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nembrini C, Singh A, De Castro CA, Mercenier A, Nutten S. Oral Administration of Lactobacillus Paracasei NCC 2461 for the Modulation of Grass Pollen Allergic Rhinitis: A Randomized, Placebo-Controlled Study During the Pollen Season. Clin Trans Allergy (2015) 5(1). doi: 10.1186/s13601-015-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baraniuk JN. Pathogenesis of Allergic Rhinitis. J Allergy Clin Immunol (1997) 99(2):S763–72. doi: 10.1016/S0091-6749(97)70125-8 [DOI] [PubMed] [Google Scholar]
- 46. Palomares O, Akdis M, Martín-Fontecha M, Akdis CA. Mechanisms of Immune Regulation in Allergic Diseases: The Role of Regulatory T and B Cells. Immunol Rev (2017) 278(1):219–36. doi: 10.1111/imr.12555 [DOI] [PubMed] [Google Scholar]
- 47. Du X, Wang L, Wu S, Yuan L, Tang S, Xiang Y, et al. Efficacy of Probiotic Supplementary Therapy for Asthma, Allergic Rhinitis, and Wheeze: A Meta-Analysis of Randomized Controlled Trials. Allergy Asthma Proc (2019) 40(4):250–60. doi: 10.2500/aap.2019.40.4227 [DOI] [PubMed] [Google Scholar]
- 48. Sestito S, D'Auria E, Baldassarre ME, Salvatore S, Tallarico V, Stefanelli E, et al. The Role of Prebiotics and Probiotics in Prevention of Allergic Diseases in Infants. Front Pediatrics (2020) 8. doi: 10.3389/fped.2020.583946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meirlaen L, Levy EI, Vandenplas Y. Prevention and Management With Pro-, Pre and Synbiotics in Children With Asthma and Allergic Rhinitis: A Narrative Review. Nutrients (2021) 13(3):934. doi: 10.3390/nu13030934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amalia N, Orchard D, Francis KL, King E. Systematic Review and Meta-Analysis on the Use of Probiotic Supplementation in Pregnant Mother, Breastfeeding Mother and Infant for the Prevention of Atopic Dermatitis in Children. Australas J Dermatol (2020) 61(2):e158–73. doi: 10.1111/ajd.13186 [DOI] [PubMed] [Google Scholar]
- 51. Zajac AE, Adams AS, Turner JH. A Systematic Review and Meta-Analysis of Probiotics for the Treatment of Allergic Rhinitis. Int Forum Allergy rhinol (2015) 5(6):524–32. doi: 10.1002/alr.21492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Güvenç IA, Muluk NB, Mutlu F, Eşki E, Altıntoprak N, Oktemer T, et al. Do Probiotics Have a Role in the Treatment of Allergic Rhinitis? A Comprehensive Systematic Review and Meta-Analysis. Am J rhinol Allergy (2016) 30(5):157–75. doi: 10.2500/ajra.2016.30.4354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis according to classification of allergic rhinitis for allergic rhinitis symptoms score.
Subgroup analysis according to combination of drugs for allergic rhinitis symptoms score.
Subgroup analysis according to intervention of treatment group for allergic rhinitis symptoms score.
Subgroup analysis according to classification of allergic rhinitis for rhinoconjunctivitis quality of life questionnaire score.
Subgroup analysis according to combination of drugs for rhinoconjunctivitis quality of life questionnaire score.
Subgroup analysis according to intervention of treatment group for rhinoconjunctivitis quality of life questionnaire score.
Subgroup analysis according to classification of allergic rhinitis for total IgE.
Subgroup analysis according to combination of drugs for total IgE.
Subgroup analysis according to classification of allergic rhinitis for sIgE.
Subgroup analysis according to combination of drugs for sIgE.
Subgroup analysis according to classification of allergic rhinitis for Th1/Th2 ratio.
Subgroup analysis according to combination of drugs for Th1/Th2 ratio.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.