Summary
Background
Low-density and asymptomatic Plasmodium vivax infections remain largely undetected and untreated and may contribute significantly to malaria transmission in the Amazon.
Methods
We analysed individual participant data from population-based surveys that measured P vivax prevalence by microscopy and polymerase chain reaction (PCR) between 2002 and 2015 and modelled the relationship between parasite density and infectiousness to vectors using membrane feeding assay data. We estimated the proportion of sub-patent (i.e., missed by microscopy) and asymptomatic P vivax infections and examined how parasite density relates to clinical manifestations and mosquito infection in Amazonian settings.
Findings
We pooled 24,986 observations from six sites in Brazil and Peru. P vivax was detected in 6·8% and 2·1% of them by PCR and microscopy, respectively. 58·5% to 92.6% of P vivax infections were asymptomatic and 61·2% to 96·3% were sub-patent across study sites. P vivax density thresholds associated with clinical symptoms were one order of magnitude higher in children than in adults. We estimate that sub-patent parasite carriers are minimally infectious and contribute 12·7% to 24·9% of the community-wide P vivax transmission, while asymptomatic carriers are the source of 28·2% to 79·2% of mosquito infections.
Interpretation
Asymptomatic P vivax carriers constitute a vast infectious reservoir that, if targeted by malaria elimination strategies, could substantially reduce malaria transmission in the Amazon. Infected children may remain asymptomatic despite high parasite densities that elicit clinical manifestations in adults.
Funding
US National Institutes of Health, Fundação de Amparo à Pesquisa do Estado de São Paulo, and Belgium Development Cooperation.
Keywords: malaria, Plasmodium vivax, asymptomatic infections, sub-patent infections, fever threshold, Amazon
Research in context.
Evidence before this study
Amazonians are exposed to infection with Plasmodium vivax (that accounts for 80% of the local malaria burden) and P falciparum. Asymptomatic parasite carriers do not seek treatment and may remain infectious for weeks or months. To identify previous studies that have quantified the relative contribution of asymptomatic and sub-patent infections to P vivax transmission, we searched the PubMed and SciELO databases for publications in English, Spanish, Portuguese, or French that appeared until Aug 1, 2021. We used the search terms “plasmodium” AND “vivax” AND (“membrane feeding” OR “skin feeding” OR “direct membrane feeding” OR “mosquito feeding” OR “infectious reservoir”) and retrieved four studies that addressed the relative infectiousness of asymptomatic vs symptomatic P vivax carriers to malaria vectors.
Empirical estimates of infectiousness vary widely across studies. None of the 24 asymptomatic carriers of P vivax (20 of them with sub-patent parasitaemia) was able to infect Anopheles dirus via standard membrane feeding in Thailand, while 49·3% of the mosquitoes fed on symptomatic P vivax carriers (n=70) became infected. In Ethiopia, individuals with symptomatic and patent P vivax infections (n=29) were 4-fold more infectious to An arabiensis (46·5% of the mosquitoes fed on them were infected) than asymptomatic carriers of patent parasitaemia (n=24; 12·0% of the mosquitoes infected) and 58-fold more infectious than asymptomatic carriers of sub-patent parasitaemia (n=53; 0·8% of the mosquitoes infected). Patent and asymptomatic P vivax infections accounted for 76·2% of the infectious reservoir.
Two studies compared the relative infectiousness of asymptomatic vs symptomatic P vivax infections in South America. On the Pacific Coast of Colombia, symptomatic carriers of patent P vivax infection (n=16) were found to be 14-fold more infectious to An albimanus than asymptomatic carriers (n=14), with 57% vs 4% of mosquitoes infected. In Brazil, symptomatic individuals (n=42) with patent P vivax infection were 29-fold more infectious to laboratory-reared An aquasalis, a malaria vector of coastal areas, than asymptomatic carriers (n=24), with 41% vs 1.4% of mosquitoes infected. In Thailand, the proportion of An dirus mosquitoes that were infected by membrane feeding reached 50% at a parasite density of approximately 100 blood stages per microliter.
Added value of this study
Our pooled analysis of 34 population-based cross-sectional surveys in riverine villages and farming settlements in Brazil and Peru showed that 70·9% of P vivax infections are asymptomatic and 69·7% are sub-patent. Importantly, young children remain asymptomatic at P vivax density levels that usually elicit clinical symptoms in adults. This is the first evidence of age-specific parasite density thresholds associated with clinical illness in populations naturally exposed to P vivax.
An darlingi infection rates are very low at sub-patent parasite densities but reach 50% at 2,300 parasites/μL. Asymptomatic carriers of P vivax are estimated to be the source of 28·2% to 79·2% mosquito infections across study sites, while sub-patent infections are responsible for smaller proportions of the community-wide transmission, estimated at 12·7% to 24·9% across study sites.
Implications of all the available evidence
Asymptomatic carriers constitute a critical yet neglected infectious reservoir that fuels residual P vivax transmission in the Amazon. Malaria elimination policies in the region require active case detection strategies. Importantly, parasite densities that contribute significantly to P vivax transmission are typically above the microscopy detection threshold and may not require more complex and expensive molecular methods to be routinely detected.
Alt-text: Unlabelled box
Introduction
Malaria transmission has declined in Latin America and the Caribbean over the past two decades, but 120 million people remain exposed to some risk of infection across the region.1 Plasmodium vivax accounts for 72% of the regional malaria burden, estimated at 800,000 cases in 2019, 90% of them occurring in the Amazon.2 P vivax causes less severe disease compared with P falciparum, but its distinctive biology poses major challenges for current malaria elimination strategies focused on early diagnosis and prompt treatment of clinically apparent infections.3
Not all malaria infections elicit clinical manifestations and can be detected by conventional microscopy or rapid diagnostic tests.4,5 Asymptomatic and sub-patent (i.e., missed by microscopy) infections are common across the Amazon,6, 7, 8, 9, 10, 11 suggesting that individuals exposed to malaria rates far below those in holoendemic Africa can also develop immunity that efficiently limits parasite growth and reduces the risk of disease. Importantly, carriers of P vivax densities below the microscopy detection limit of 100 parasites per μL of blood are often asymptomatic in Amazonians. Asymptomatic infections remain undiagnosed and untreated, and may contribute to transmission over several weeks or months.10,12,13 Mature gametocytes are present early in nearly all P vivax blood-stage infections.10,14
Cost-effective strategies are crucially needed to address the human reservoir of P vivax transmission in the Amazon, which comprises symptomatic and patent, asymptomatic and patent, and asymptomatic and sub-patent infections.12 To define priority targets for more intensive interventions, individuals who contribute disproportionally to malaria transmission to local vectors must be identified.14 The present pooled analysis of individual data aims to determine how cumulative exposure to malaria (using age as a proxy) relates to the proportion of P vivax infections that are sub-patent and asymptomatic and how parasite density relates to the risk of clinical manifestations and of human-to-mosquito P vivax transmission across the Amazon.
Methods
Study area
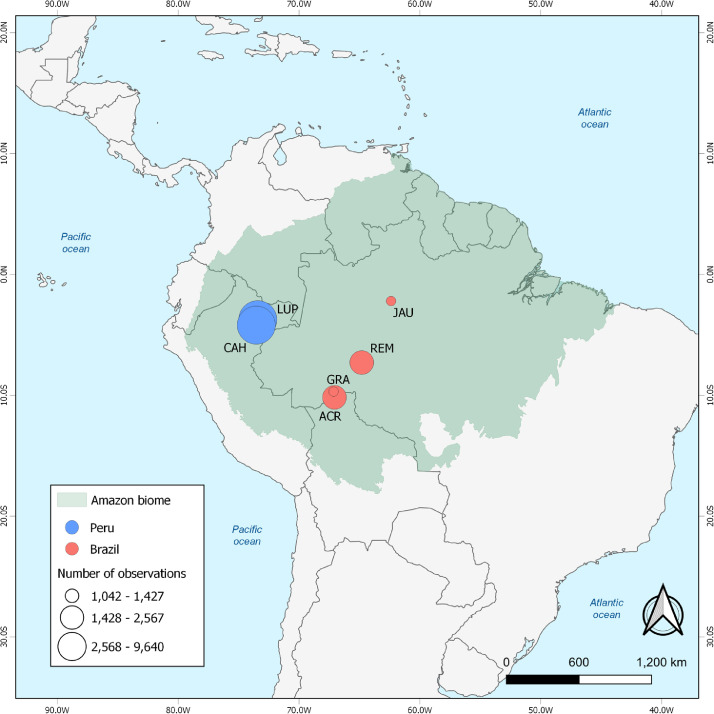
The Amazon extends over nine countries and territories in northern South America (Figure 1). Anopheles (Nyssorhynchus) darlingi is the primary malaria vector in the region.15 Most malaria transmission occurs in riverine villages, farming settlements, gold mining camps, and Amerindian reserves, but also within and near urban centers.1,2 Amazonian Brazil and Peru together account for 25% of the current annual burden of malaria in the Americas.2
Figure 1.
Map showing the Amazon Basin in South America and the study sites in Brazil and Peru that provided data for the present analyses. The locations of the six study sites (CAH and LUP in Peru; and ACR, GRA, JAU, and REM in Brazil) are indicated.
Literature data
The study protocol was registered in the PROSPERO database (CRD42020194174) and the search strategy is described in the appendix (pp 1–2). We retrieved anonymised individual participant information regarding age, sex, presence of symptoms, and P vivax diagnosis (by both microscopy and qualitative or quantitative PCR) from 34 population-based cross-sectional surveys that described 24,986 observations from six study sites in the Amazon Basin of Brazil and Peru. We pooled the results by study site and only included study participants with microscopy and PCR data and information on age and symptoms. The study sites (Figure 1) were: the villages of Cahuide (CAH) and Lupuna (LUP) in Peru (11 surveys from Mar, 2013, through Sep, 2015);11,13 farming settlements in Acrelândia (ACR; four surveys between Jan and Jul, 2013)12 and Granada (GRA; four surveys from Mar, 2004, through Nov, 2006),16 both in Brazil; traditional riverine villages in Jaú National Park, Brazil (JAU; five surveys from Nov, 2002 through Jul, 2003);8 and farming settlements in Remansinho, Brazil (REM; 10 surveys from Mar, 2010, through Oct, 2014).10 Study populations are further described in the appendix, p 3–5 and 16.
We additionally retrieved individual peripheral-blood parasite density estimates (sexual plus asexual stages combined), which were obtained with quantitative real-time PCR assays. Data were available from 25 cross-sectional surveys in four sites (CAH, LUP, ACR, and REM), corresponding to 1,680 PCR-confirmed P vivax infections with known parasite density. Quantitative PCR protocols targeted species-specific domains of the 18S rRNA genes of P vivax.10,17 Parasite density estimates were obtained in a similar way in all studies; amplicon numbers were interpolated from standard curves prepared with serial dilutions of the target sequence (appendix p 5–6).10,17
Standard membrane-feeding assays
Standardised membrane-feeding assays were carried out as described.18 Female mosquitoes were fed on infected blood through a membrane and subsequently examined for oocysts by microscopy of dissected mosquito midguts. Blood donors with a range of parasite densities were adults >18 years of age who had P vivax blood-stage infection diagnosed by microscopy during cross-sectional surveys in CAH and LUP. Experiments were carried out with 3-day old mosquitoes from a laboratory-established colony in Iquitos, Peru,19 that were exposed for 30 min to 1 mL of heparinized blood from 87 volunteers.18 Importantly, laboratory-reared mosquitoes did not differ from wild mosquitoes in biting activity and average susceptibility to infection.19 Engorged mosquitoes were dissected 7 to 9 days after blood feeding to determine the presence of oocysts. The proportion (%) of mosquitoes infected was calculated as 100 × number of mosquitoes with at least one oocyst/total dissected. Study protocols were approved by the Ethics Review Boards of the Regional Health Direction of Loreto (R-157-13-14) and Universidad Peruana Cayetano Heredia, Peru (SIDISI 59751), and by the Human Subjects Protection Program of the University of California, San Diego, USA (approval number, 120652). Written informed consent was obtained from all study participants. Experimental mosquito infection data were used to determine how P vivax densities in humans relate to infection rates in blood-fed An darlingi.
Data analysis
We used Stata 15.1 (StataCorp, College Station, TX) and R 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) in statistical analyses.
We extracted data from cross-sectional surveys carried out in six sites to estimate: (a) the age-related frequency of patent and sub-patent (i.e., not detected by microscopy) P vivax infections in each site; (b) the age-related frequency of P vivax infections that were symptomatic and asymptomatic in each site; (c) the age-related frequency of P vivax infections that are sub-patent and asymptomatic in each sites; and (d) the age-related proportion of P vivax infections that were both asymptomatic and sub-patent among infected individuals in each site. We used the following standardised age groups: 0–5 years, 6–15 years, 16–40 years, and >40 years. Sub-patent infections were defined as those diagnosed by PCR but missed by microscopy, regardless of the parasite density. PCR positivity rate was defined as the proportion of infections that were diagnosed by PCR in each study site, regardless of the microscopy results. Asymptomatic infections were defined as in the original studies, considering different combinations of the following malaria-related symptoms: measured or reported fever, chills, headache, profuse sweating, weakness, myalgia, arthralgia, abdominal pain, nausea, and vomiting. The symptom-free period used to define asymptomatic parasite carriage was at least 7 days before sample collection in CAH, LUP, ACR and REM, at least 30 days before and 30 days after blood collection in GRA, and at least 30 days before and 15 days after blood collection in JAU. Because we pooled data from successive surveys, denominators correspond to the total of observations per study site; the same individual may have experienced several malaria episodes.
We extracted individual parasite density data from single-species P vivax infections that were PCR-diagnosed during cross-sectional surveys in four sites to estimate: (a) the distribution of parasite densities in patent vs sub-patent infections and symptomatic vs asymptomatic infections; (b) the proportion of infected individuals with parasite densities above certain thresholds of interest; (c) the probability of clinical manifestations of malaria at varying parasite densities; and (d) the relative contribution of each infected individual to community-wide P vivax transmission. A uniform criterion was used to define asymptomatic parasite carriage in this participant subset: absence of self-reported or measured fever and headache within 7 days prior to blood collection for malaria diagnosis. As above, we pooled data from cross-sectional surveys and the denominators are the total numbers of PCR-diagnosed infections per study site rather than numbers of individuals. We fitted separate logistic regression models20 to each dataset to describe: (a) the relationship between PCR-determined parasite density and the detectability of infection by microscopy and (b) the relationship between PCR-determined parasite density and the risk of malaria-related symptoms (fever or headache) within the past 7 days.
We fitted a Hill function to membrane feeding data to describe the probability of mosquito infection following a blood meal given the host′s parasitaemia. Because older individuals are more likely to be bitten due to their greater body surface,21,22 we applied an age-dependent force of infection normalised from 0 to 1.23 We multiplied by the probability of mosquito bite given host′s age to obtain individual estimates of infectiousness (appendix p 5–6). Importantly, the age-related variation in mosquito biting assumed by our model23 closely recapitulates estimates from empirical data.22 The community-wide human-to-mosquito infection rate corresponds to the sum of all individual infectiousness estimates.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Low-density and asymptomatic parasitemia across age groups
Overall, 1696 P vivax infections were diagnosed by PCR and 514 by microscopy, with higher PCR positivity rates compared to microscopy in all study sites. The overall proportion of PCR-diagnosed P vivax infections that were also detected by microscopy, or microscopy detection rate, was 30·3% (range across sites, 3·7% in ACR to 38.8% in REM).
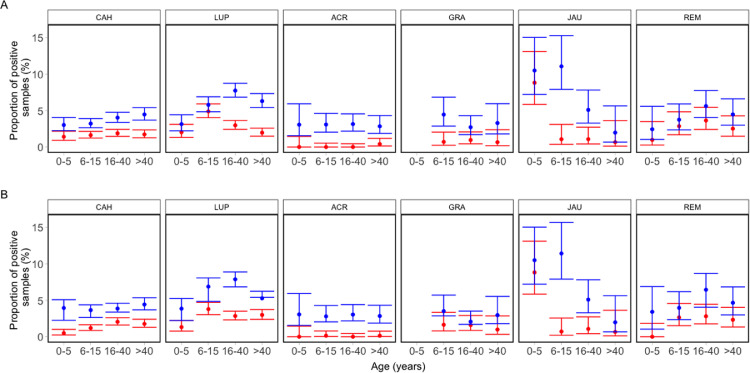
The prevalence of P falciparum infection decreases with age in holoendemic Africa, presumably due to partial anti-parasite immunity.4,24 We found no similar age-related variation in parasite positivity rates in our settings from Peru and Brazil where P vivax predominates, most likely because overall transmission levels are one order of magnitude lower in the Amazon and only a minority of highly exposed individuals appear to develop significant anti-parasite immunity.23 The P vivax infection rates, regardless of self-reported symptoms or the diagnostic method used, did not decrease with age except for the remote riverine villages of the Jaú National Park of Amazonas, Brazil (Figure 2 and Appendix pp 6–7). Sub-patent P vivax infections predominated over patent infections in all age groups in all six study sites analysed (Figure 2A).
Figure 2.
Age-specific relative frequency of patent vs. sub-patent and symptomatic vs. asymptomatic Plasmodium vivax infections diagnosed by polymerase chain reaction across six studies in the Amazon. Patent (red symbols) and sub-patent (blue symbols) infections are shown in A; symptomatic (red symbols) and asymptomatic (blue symbols) P vivax infections are shown in B. Error bars indicate 95% confidence intervals of proportions We pooled data from cross-sectional surveys carried out in six sites. CAH: villages of Cahuide, La Habana, and Doce de Abril, all in Loreto, Peru.11,14 LUP: villages of Lupuna, Santa Rita, and San Pedro, all in Loreto, Peru.11,14 ACR: 7 farming settlements in Acrelândia, Acre, Brazil.12 GRA: Granada farming settlement, Acre, Brazil.17 JAU: 14 riverine villages in Jaú National Park, Amazonas, Brazil.8 REM: Remansinho farming settlement, Amazonas.10 The number of observations in each age group was as follows: CAH, 0–5 years, 1,421; 6–15 years, 3,017; 16–40 years, 3,082; 41 years and older, 2,261; total, 9,781. LUP, 0-5 years, 986; 6–15 years, 2,006; 16–40 years, 3,021; 41 years and older, 2,443; total, 8,456. ACR, 0–5 years, 261; 6–15 years, 715; 16–40 years, 856; 41 years and older, 737; total, 2,569. GRA, 6–15 years, 427; 16–40 years, 627; 41 years and older, 304; total, 1,358. (Under-five children from GRA were removed, as this age group has not been systematically sampled during the community-wide surveys.) JAU, 0–5 years, 238; 6–15 years, 280; 16–40 years, 373; 41 years and older, 152; total, 1,043. REM, 0–5 years, 205; 6–15 years, 454; 16–40 years, 605; 41 years and older, 515; total, 1,779. The symptom-free period used to define asymptomatic parasite carriage was at least 7 days before sample collection in CAH, LUP, ACR and REM, at least 30 days before and 30 days after blood collection in GRA, and at least 30 days before and 15 days after blood collection in JAU. Error bars indicate 95% confidence intervals of proportions.
We examined how the prevalence of symptomatic vs asymptomatic infections relates to age across six studies. Overall, asymptomatic infections accounted for 70·9% of all episodes of the 1696 P vivax carriage confirmed by PCR during cross-sectional surveys (range across sites, 58·5% in GRA to 92.6% in ACR) and were shown to predominate over symptomatic infections in all age groups across all study sites (Figure 2B).
Infections that are both sub-patent and asymptomatic were similarly frequent across age groups in most sites (Appendix p 8–9). The exceptions were LUP (higher positivity rate of sub-patent and asymptomatic infections in the 16–40 years group) and JAU (higher positivity rate in the 0–5 and 6–15 years groups). The proportion of P vivax infections that were asymptomatic and sub-patent did not increase with age, contrary to what is expected from the development of strong clinical immunity in adolescents and adults. The only exception was JAU, where fewer asymptomatic and sub-patent infections were diagnosed in under-five children compared to the 6–15 and 16–40 years age groups (Appendix pp 6–9).
Parasite densities and clinical manifestations in Plasmodium vivax malaria
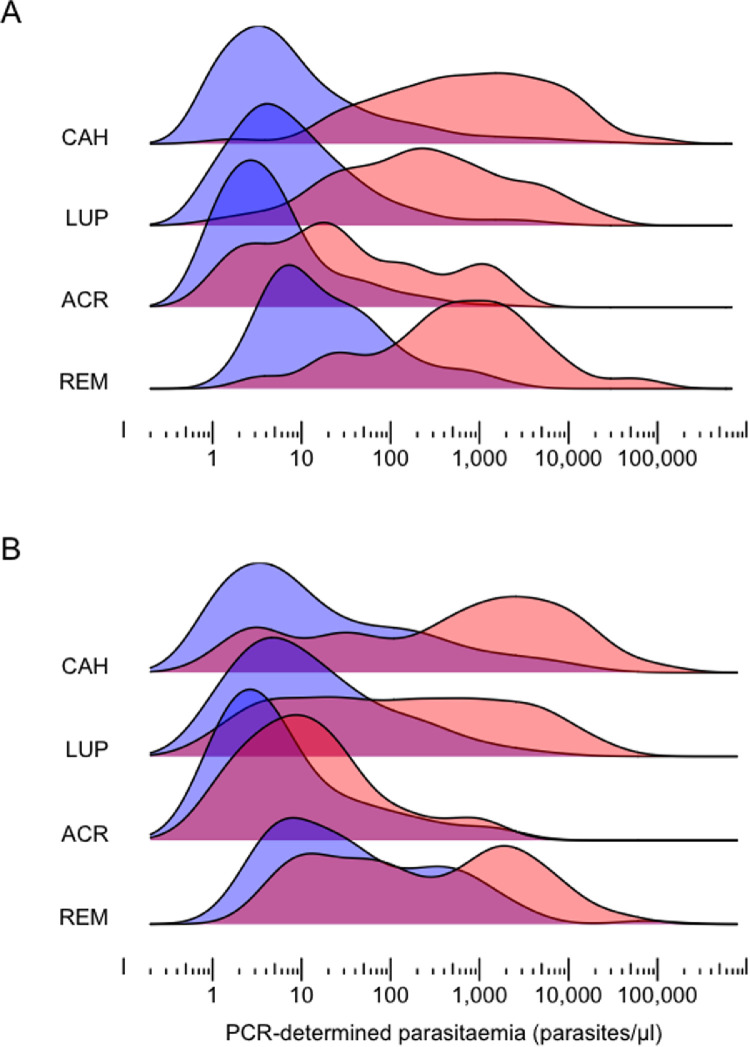
We analysed parasite densities measured by quantitative PCR in 491 P vivax infections diagnosed during cross-sectional surveys in CAH (average PCR positivity rate, 5·4%), 736 infections from LUP (average PCR positivity rate, 9·3%), 216 infections from ACR (average PCR positivity rate, 3·1%), and 128 infections from REM (average PCR positivity rate, 7·2%). Low levels of parasitaemia predominated in all settings, with a median of 11 (interquartile range [IQR], 2 to 290) parasites/μL in CAH, 12 (IQR, 3 to 112) parasites/μL in LUP, 5 (IQR, 2 to 17) parasites/μL in ACR, and 47 (IQR, 10 to 483) parasites/μL in REM. Most infections (68·2% in CAH, 71·3% in LUP, 88·0% in ACR and 61·2% in REM) were below the usual detection threshold of microscopy under field conditions, estimated at 100 parasites/μL.5 There was a substantial overlap in the distribution of levels of parasitaemia that were detected vs missed by microscopy (Figure 3A) and, more strikingly, between those from symptomatic vs asymptomatic individuals (Figure 3B). The parasite density thresholds above which >50% of P vivax infections were detected by microscopy were estimated at 156 (95% confidence interval [CI], 109 to 231) parasites/μL in CAH, 82 (95% CI, 62 to 110) parasites/μL in LUP, 67 (95% CI, 33 to 201) parasites/μL in ACR and 153 (95% CI, 86 to 298) parasites/μL in REM (appendix p 11).
Figure 3.
Distribution of total Plasmodium vivax densities (parasites/μl) estimated by quantitative polymerase chain reaction in four Amazonian settings. Kernel density function estimates are shown for (A) patent (red) vs sub-patent (blue) P vivax infections and (B) symptomatic (red) vs asymptomatic (blue) P vivax infections in the CAH (Cahuide; n=491) and LUP (Lupuna; n=736) study sites in Peru,11,14 and in the ACR (Acrelândia; n=216)12 and REM (Remansinho; n=128)10 study sites in Brazil. Individuals reporting no fever or headache within at least 7 days prior to sample collection were defined as asymptomatic.
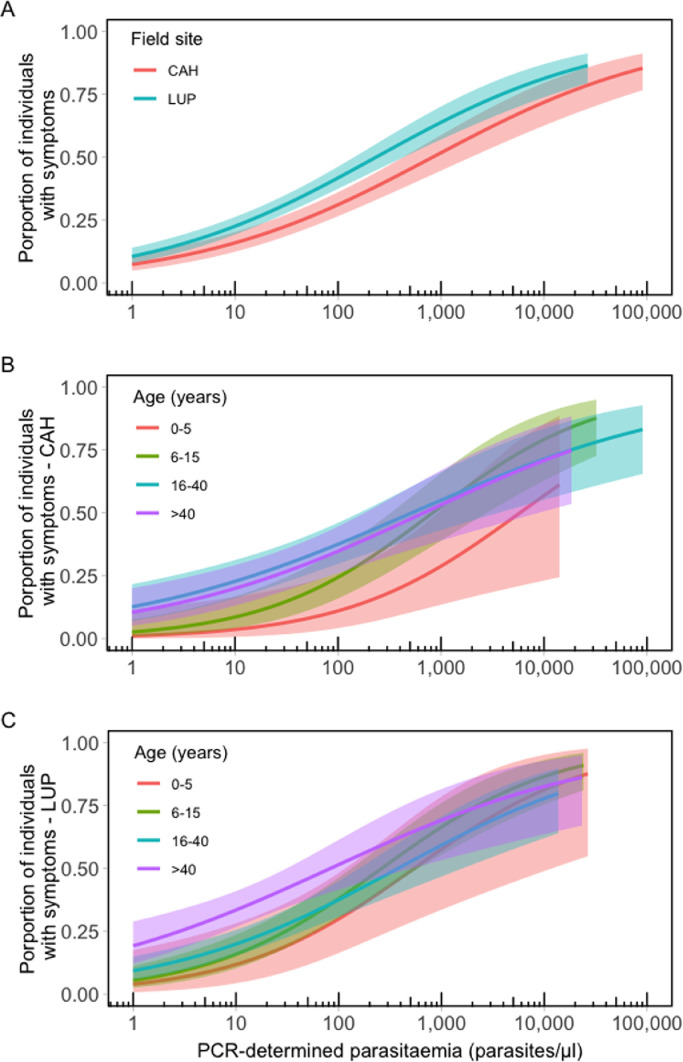
We next examined how the risk of clinical disease relates to P vivax blood-stage density. Logistic model fits in Figure 4A indicate that >50% of individuals are expected to have malaria-related symptoms (fever or headache) at parasite densities above 817 (95% CI, 426 to 1867) parasites/μL in CAH and 233 (95% CI, 143 to 428) parasites/μL in LUP. Results for sites in Brazil, where fewer infections were analysed, are presented in the appendix (p 12). Clinical thresholds are substantially higher than microscopy detection thresholds, implying that many P vivax infections below the clinical threshold are detectable by routine microscopy.
Figure 4.
Proportion of individuals with clinical manifestations of vivax malaria by parasite density measured by quantitative polymerase chain reaction. Separate univariate logistic regression models were fitted to empirical data from Cahuide (CAH; n=491) and Lupuna (LUP; n=736) using the stats R package: (A) total population of CAH and LUP; (B) age-specific model fits for CAH; (C) age-specific model fits for LUP. Age groups considered were: 0–5 years, 6–15 years, 16–40 years, and 41 years and older. The shaded area indicates the 95% confidence intervals.
Importantly, the risk of clinical symptoms per parasite density increased with age in both CAH (Figure 4B) and LUP (Figure 4C). We estimated that, at parasite densities of 14,692 (95% CI, 1,938 to 141,432) parasites/μL and 740 (95% CI, 145 to 4,177) parasites/μL in CAH and LUP, respectively, more than 50% of under-five children experience symptoms. These thresholds decreased to 483 (95% CI, 166 to 1638) parasites/μL and 59 (95% CI, 28 to 136) parasites/μL in study participants aged >40 years from the same sites. Sample sizes were too small for age-stratified analyses in ACR and REM. We conclude that age-dependent clinical thresholds for P vivax exist in low-endemicity settings, consistent with the “pyrogenic thresholds” that differ between children and adults exposed to intense P falciparum transmission in Sub-Saharan Africa.25,26
Parasite density and mosquito infection rate
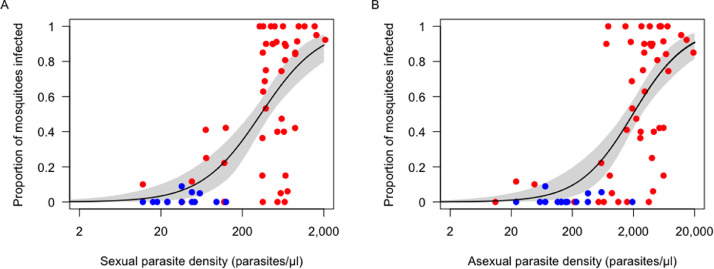
Figure 5 describes the relationship between experimental mosquito infection rates and the microscopy-determined density of sexual and asexual parasites in blood donors. Importantly, gametocytaemia and asexual parasitaemia were linearly correlated across the range of parasite densities observed in P vivax-infected blood donors (r2=0·673, P<0·001; appendix p 13). Very few mosquitoes were infected at low gametocyte densities, but mosquito infection rates increased rapidly and reached 50% at 337 (95% CI, 252 to 448) gametocytes/μL (Figure 5A) or at 1,973 (95% CI, 1,377 to 2,737) asexual blood stages/μL (Figure 5B), which corresponds to a total parasitaemia of approximately 2,300/μL. Few study participants harboured >2,300 parasites/μL during population-based cross-sectional surveys: 12·1% in CAH, 6·5% in LUP, none in ACR and 8·5% in REM. Gametocytaemia and asexual parasitaemia were both linearly correlated with the mean oocyst counts found in mosquito midguts (Appendix, p 14)
Figure 5.
Proportion of Anopheles darlingi mosquitoes infected with Plasmodium vivax in membrane feeding experiments in relation to parasite density measured by microscopy. Data are from 87 independent membrane feeding assays with 10 to 64 (mean, 37•3) laboratory-reared An darlingi mosquitoes examined for oocysts per experiment. Parasite density estimates are presented separately for sexual (A) and asexual (B) blood stages. Data from symptomatic and asymptomatic blood donors are represented by red and blue dots, respectively. The continuous line shows the Hill function (appendix p 2) fitted to the data and the grey shading indicates the 95% confidence intervals.
Low-density and asymptomatic infections and onward Plasmodium vivax transmission
Substantial heterogeneity has been documented in mosquito-to-human P falciparum transmission in Africa, where 20% of the children are estimated to receive 80% of all infectious mosquito bites in the community.27 Here, we suggest that human-to-mosquito P vivax transmission may also conform to the “20/80 rule” in the Amazon. The top-20% spreaders are estimated to originate between 78·8% and 92·9% of all P vivax transmission events in each site (Appendix, p 15). Considering individual infectiousness estimates on the basis of subjects′ age and parasite density, we calculate that sub-patent carriers contribute only 12·7% to 24·9% of the overall P vivax transmission although they account for 60·9% to 73·6% of all human infections with this parasite across study sites (Table 1). Carriers of total (sexual and asexual) parasitaemia <100/μL are very slightly, or not at all infectious to mosquitoes (Figure 5), further suggesting that sub-patent infections constitute a small fraction of the infectious reservoir in low-endemicity Amazonian settings. Most parasite carriers were asymptomatic, but their estimated contribution to onward transmission varies widely across studies (table). Infections that are both sub-patent and asymptomatic are estimated to be responsible for 9·6% to 24·5% of the overall P. vivax transmission across sites. As a group, individuals >15 years of age originate more mosquito infections than children.
Table 1.
Relative contribution of different population strata to community-wide Plasmodium vivax transmission across four sites in the Amazon.
| Study site |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cahuide, Peru (n = 491) |
Lupuna, Peru (n = 736) |
Acrelândia, Brazil (n = 216) |
Remansinho, Brazil (n = 128) |
|||||
| Type of parasite carrier | No. (%) | % Contributiona | No. (%) | % Contributiona | No. (%) | % Contributiona | No. (%) | % Contributiona |
| Patent | 168 (34.2) | 82.3 (70.5, 94.0) | 256 (34.8) | 80.0 (68.2, 91.8) | 57 (26.4) | 75.1 (38.7, 100) | 50 (39.1) | 87.3 (63.8, 100) |
| Sub-patent | 323 (65.8) | 17.7 (11.4, 24.1) | 480 (65.2) | 20.0 (13.7, 26.3) | 159 (73.6) | 24.9 (5.9, 44.0) | 78 (60.9) | 12.7 (6.9, 18.5) |
| Parasitaemia ≥100/μl | 171 (34.8) | 96.8 (85.6, 100) | 209 (28.4) | 93.2 (82.4, 100) | 26 (12.0) | 86.1 (54.2, 100) | 50 (39.1) | 95.4 (73.7, 100) |
| Parasitaemia <100/μl | 320 (65.2) | 3.2 (2.7, 3.7) | 527 (71.6) | 6.8 (6.0, 7.5) | 190 (88.0) | 13.9 (10.5, 17.3) | 78 (60.9) | 4.6 (3.5, 5.7) |
| Symptomatic | 139 (28.3) | 66.9 (55.5, 78.3) | 239 (32.5) | 71.8 (59.6, 83.9) | 39 (18.1) | 20.8 (3.3, 38.3) | 41 (32.0) | 58.1 (36.2, 79.9) |
| Asymptomatic | 352 (71.7) | 33.1 (24.6, 41.6) | 497 (67.5) | 28.2 (21.7, 34.8) | 177 (81.9) | 79.2 (39.6, 100) | 87 (68.0) | 41.9 (24.0, 59.9) |
| Patent and asymptomatic | 70 (14.3) | 19.5 (13.9, 25.1) | 117 (15.9) | 14.1 (10.6, 17.5) | 26 (12.0) | 54.7 (23.3, 86.2) | 27 (21.1) | 32.4 (17.0, 47.7) |
| Sub-patent and asymptomatic | 282 (57.3) | 13.6 (8.1, 19.1) | 380 (51.6) | 14.2 (8.8, 19.5) | 151 (69.9) | 24.5 (5.5, 43.5) | 60 (46.9) | 9.6 (4.5, 14.7) |
| Age ≤15 years | 192 (39.1) | 28.6 (21.4, 35.9) | 251 (34.1) | 40.4 (32.2, 48.6) | 89 (41.2) | 19.3 (5.8, 32.8) | 37 (28.9) | 23.3 (11.9, 34.7) |
| Age >15 years | 299 (60.9) | 71.4 (57.1, 85.6) | 485 (65.9) | 59.6 (47.1, 72.1) | 127 (58.8) | 80.7 (40.0, 100) | 91 (71.1) | 76.7 (49.4, 100) |
Individual contributions to transmission are defined as the probability of mosquito infection following a blood meal given the host′s parasitaemia measured by PCR (Figure 5) multiplied by the the probability of mosquito bite given host′s age. The community-wide human-to-mosquito infection rate corresponds to the sum of all individual infectiousness estimates. The relative contributions of each population stratum to overall infection rate corresponds to the ratio between the average infection rate of individuals in the stratum (with its respective 95% confidence interval [CI]) to the community-wide infection rate. Numbers in parentheses are the 95% CIs of the relative contribution of each population stratum to overall transmission, derived from the average individual infectiousness of study participants in each stratum.
Discussion
Over the past decade, we have systematically investigated the main challenges for malaria control and elimination across the Amazon.9 We currently focus on residual malaria transmission in Brazil10,12,16 and resurgent malaria transmission in riverine villages in Peru,11,14 where a vast clinically silent human reservoir of P vivax infection that remains largely unaddressed by regional malaria control efforts.9 Here, we estimate the relative contribution of low-density and asymptomatic infections to P vivax transmission in the Amazon.
In total, 69·7% of PCR-diagnosed P vivax infections were missed by microscopy and 70·9% were asymptomatic in 34 population-based surveys from across the region, with proportions of patent vs sub-patent and asymptomatic vs symptomatic infections varying little across age groups in most settings. In contrast, P falciparum infections in older individuals are more likely to be sub-patent in Africa, a finding that is usually attributed to the gradual development of anti-parasite immunity.20,24
We show that young Amazonian children may be asymptomatic when harbouring P vivax densities that typically elicit clinical manifestations in older individuals (Figure 4B, C). To our knowledge, this is the first evidence of age-specific parasite density thresholds associated with clinical illness in populations naturally exposed to P vivax. How the interplay between parasite virulence and host′s innate and adaptative immunity regulates the clinical expression of vivax malaria across age groups has yet to be investigated. One can speculate that, once exposed to the parasite, older adults develop stronger inflammatory responses that cause malaria-related symptoms, compared to young children. The public health consequences of these findings are clear: asymptomatic children harbouring relatively high levels of parasitaemia may contribute disproportionally to malaria transmission in the community in the absence of active case detection.
We infer that sub-patent parasite carriers contribute little to transmission (Table), except for the residual malaria site with the lowest PCR prevalence in Brazil,12 although we note that parasite densities may fluctuate over time.5 Similarly, sub-patent parasite carriers were estimated to originate only 13·6% of the P vivax infections to local An arabiensis mosquitoes in hypoendemic Ethiopia.28 Our results have major practical implications for regional malaria elimination strategies. They suggest that conventional microscopy or rapid diagnostic tests may suffice to identify the vast majority of infections that contribute significantly to human-to-mosquito transmission of P vivax in the Amazon and possibly in other low-endemicity settings. The widespread use of ultrasensitive molecular techniques may not be required, at the present stage, as a core component of malaria elimination programs in the region.
We assume that the relative infectiousness of asymptomatic carriers depends primarily on their parasite densities and age. We did not compare the infection potential of symptomatic vs asymptomatic carriers of similar P vivax gametocytaemias, because there were relatively few data from mosquito feeding experiments on asymptomatic volunteers (Figure 5). We note, however, that asymptomatic P falciparum carriers appear to be relatively more infectious to An. gambiae mosquitoes than patients with clinical symptoms, after adjusting for gametocyte density, possibly due to gametocyte inactivation during and shortly after malaria paroxysms by circulating inflammatory cytokines and reactive intermediates.29 In contrast, asymptomatic P vivax carriers appear to be little infectious to local An dirus mosquitoes in Thailand, although the estimated transmissibility threshold for that vector30 was one order of magnitude lower than that estimated for An darlingi (Figure 5). Our results imply that asymptomatic carriers, many of them harbouring patent parasitaemia, constitute a critical infectious reservoir of P vivax in the Amazon that should be targeted by active case detection strategies. In residual malaria settings of Brazil12 and Ethiopia,28 chronic asymptomatic P vivax infections may be responsible for as much as 79% to 92% of all human-to-mosquito transmission events.
Our study provides new insights into the relationship between P vivax blood-stage density, clinical symptoms, and mosquito infection that are critical to malaria elimination in the Amazon. However, it had some limitations. For example, we did not consider sources of heterogeneity in infectiousness other than host′s parasite density and age, such as individual attractiveness to vectors and use of protective measures. Our transmissibility threshold estimates were derived from membrane feeding assay data and we acknowledge that direct skin feeding experiments on individuals might yield somewhat different results. Moreover, only adults were included in membrane feeding experiments; the relative infectiousness of children vs adults warrants further investigation. We considered single time-point measurements of parasite density and infectiousness to mosquitoes and do not consider temporal variation in these parameters. Finally, parasite density data were obtained with two slightly different quantitative PCR methods,10,18 although both diagnostic protocols target the same P vivax gene and applied the same standardized way of measuring parasitaemia (Appendix, p 5).
In conclusion, we offer quantitative evidence that asymptomatic carriers of P vivax, although not necessarily those with very low levels of parasitaemia, constitute a significant source of human-to-mosquito infection at the community level that must be specifically targeted by malaria control and elimination strategies in low-endemicity settings across the Amazon.
Contributors
MUF, DG, ARU, and JMV conceived and designed the study. MUF, RMC, ICJ, JHK, ARA, SLA, and ARU accessed and verified the data. MUF, MM, ARA, SLA, JEC, ALC, DG, ARU, and JMV collected the data, and MUF, RMC, ICJ, JHK, and ARU analysed the data. MUF, RMC, ICJ, JHK, and ARU drafted the first version of the manuscript. All authors saw the draft of the manuscript, contributed to data interpretation, and provided input on writing. All authors critically reviewed the manuscript, had full access to study data, and had the final responsibility to submit for publication.
Data sharing
Original data from the cross-sectional surveys in Cahuide (CAH) and Lupuna (LUP), Peru (https://clinepidb.org/ce/app/record/dataset/DS_a885240fc4), and those from Remansinho (REM), Brazil (https://clinepidb.org/ce/app/record/dataset/DS_8e1f623542), are available through the clinical epidemiology database resource ClinEpiDB. Original data for the surveys in Acrelândia (ACR) can be found online (https://doi.org/10.1371/journal.pntd.0005221.s006). Original data from the cross-sectional surveys in Granada and Jaú are available at the University of São Paulo data repository (https://uspdigital.usp.br/repositorio/). Researchers who are interested in potential collaboration should contact the corresponding author (muferrei@usp.br).
Editorial disclaimer
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
MUF receives a research fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, and was supported by a visiting scientist fellowship from the Casa da América Latina and Fundação Millennium BCP, Portugal. RMC was supported by a PhD scholarship from CNPq. ARA is supported by a postdoctoral scholarship from the Fonds de la Recherche Scientifique (FNRS), Belgium. All other authors declare no competing interests.
Acknowledgments
This work was supported by the National Institutes of Health as part of the International Centres of Excellence in Malaria Research program (U19 AI089681); the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (2013/02764-8, 2016/18740-9, and 2019/23139-0); and the Belgium Development Cooperation (DGD) (FA3-III, 2014–2016, FA4 2017–2021). We thank our staff at the University of São Paulo, Brazil, and the Universidad Peruana Cayetano Heredia, Peru, for their administrative and laboratory support; and the staff of the ICEMR Amazonia insectary in Iquitos, Peru, for the invaluable help with membrane feeding assays.
Footnotes
Funding: US National Institutes of Health, Fundação de Amparo à Pesquisa do Estado de São Paulo, and Belgium Development Cooperation.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100169.
Appendix. Supplementary materials
References
- 1.Ferreira MU, Castro MC. Malaria situation in Latin America and the Caribbean: residual and resurgent transmission and challenges for control and elimination. Methods Mol Biol. 2019;2013:57–70. doi: 10.1007/978-1-4939-9550-9_4. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World Malaria Report 2020. Geneva, World Health Organization; 2020. Available at: https://www.who.int/publications/i/item/9789240015791.
- 3.Price RN, Commons RJ, Battle KE, Thriemer K, Mendis K. Plasmodium vivax in the era of the shrinking P. falciparum map. Trends Parasitol. 2020;36:560–570. doi: 10.1016/j.pt.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–636. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 5.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- 6.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66:641–648. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- 7.Roshanravan B, Kari E, Gilman RH, et al. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2003;69:45–52. [PubMed] [Google Scholar]
- 8.Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–459. [PubMed] [Google Scholar]
- 9.da Silva-Nunes M, Moreno M, Conn JE, et al. Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012;121:281–291. doi: 10.1016/j.actatropica.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa S, Gozze AB, Lima NF, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8:e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas-Aguirre A, Guzman-Guzman M, Chuquiyauri R, et al. Temporal and micro-spatial heterogeneity in transmission dynamics of co-endemic Plasmodium vivax and Plasmodium falciparum in two rural cohort populations in the Peruvian Amazon. J Infect Dis. 2021;223:1466–1477. doi: 10.1093/infdis/jiaa526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontoura PS, Finco BF, Lima NF, et al. Reactive case detection for Plasmodium vivax malaria elimination in rural Amazonia. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovira-Vallbona E, Contreras-Mancilla JJ, Ramirez R, et al. Predominance of asymptomatic and sub-microscopic infections characterizes the Plasmodium gametocyte reservoir in the Peruvian Amazon. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinka ME, Rubio-Palis Y, Manguin S, et al. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva NS, da Silva-Nunes M, Malafronte RS, et al. Epidemiology and control of frontier malaria in Brazil: lessons from community-based studies in rural Amazonia. Trans R Soc Trop Med Hyg. 2010;104:343–350. doi: 10.1016/j.trstmh.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Mangold KA, Manson RU, Koay ES, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno M, Tong-Rios C, Orjuela-Sanchez P, et al. Continuous supply of Plasmodium vivax sporozoites from colonized Anopheles darlingi in the Peruvian Amazon. ACS Infect Dis. 2018;4:541–548. doi: 10.1021/acsinfecdis.7b00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno M, Tong C, Guzmán M, et al. Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am J Trop Med Hyg. 2014;90:612–616. doi: 10.4269/ajtmh.13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slater HC, Ross A, Felger I, et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun. 2019;10:1433. doi: 10.1038/s41467-019-09441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith T, Killeen G, Lengeler C, Tanner M. Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am J Trop Med Hyg. 2004;71:80–86. [PubMed] [Google Scholar]
- 22.Gonçalves BP, Kapulu MC, Sawa P, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun. 2017;8:1133. doi: 10.1038/s41467-017-01270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corder RM, Ferreira MU, Gomes MGM. Modelling the epidemiology of residual Plasmodium vivax malaria in a heterogeneous host population: a case study in the Amazon Basin. PLoS Comput Biol. 2020;16 doi: 10.1371/journal.pcbi.1007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogier C, Commenges D, Trape JF. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg. 1996;54:613–619. doi: 10.4269/ajtmh.1996.54.613. [DOI] [PubMed] [Google Scholar]
- 26.Dollat M, Talla C, Sokhna C, Diene Sarr F, Trape JF, Richard V. Measuring malaria morbidity in an area of seasonal transmission: pyrogenic parasitemia thresholds based on a 20-year follow-up study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadesse FG, Slater HC, Chali W, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018;66:1883–1891. doi: 10.1093/cid/cix1123. [DOI] [PubMed] [Google Scholar]
- 29.Barry A, Bradley J, Stone W, et al. Increased gametocyte production and mosquito infectivity in chronic versus incident Plasmodium falciparum infections. medRxiv 2020.04.08.20057927 [Preprint] Apr 11, 2020. [cited 2021 Aug 25]. Available from: 10.1101/2020.04.08.20057927. [DOI] [PMC free article] [PubMed]
- 30.Kiattibutr K, Roobsoong W, Sriwichai P, et al. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int J Parasitol. 2017;47:163–170. doi: 10.1016/j.ijpara.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.