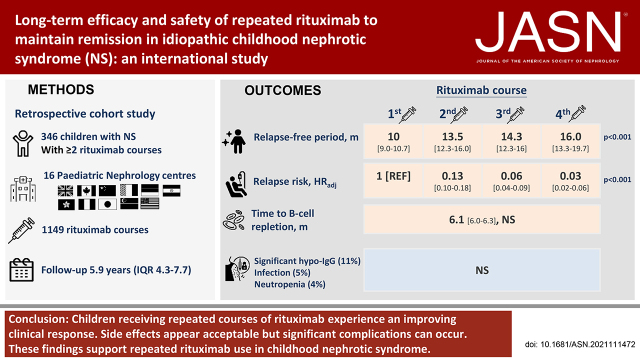
Significance Statement
Children with frequently relapsing, steroid-dependent nephrotic syndrome (FRSDNS) often require multiple courses of rituximab. However, long-term effects from repeated treatments remain unknown. In this international, multicenter study of 346 children receiving 1149 courses of rituximab, the risk of relapse decreased and relapse-free survival significantly improved with repeated treatments. Important side effects, including hypogammaglobulinemia, neutropenia, and infections, were mostly mild, but significant adverse events could occur. The incidence of side effects did not increase with more treatment courses nor a higher cumulative dose of rituximab. These findings suggest that repeating rituximab therapy is an effective and reasonably safe approach for most children with FRSDNS.
Keywords: rituximab, nephrotic syndrome, hypogammaglobulinemia, neutropenia, children, biologics
Visual Abstract
Abstract
Background
Long-term outcomes after multiple courses of rituximab among children with frequently relapsing, steroid-dependent nephrotic syndrome (FRSDNS) are unknown.
Methods
A retrospective cohort study at 16 pediatric nephrology centers from ten countries in Asia, Europe, and North America included children with FRSDNS who received two or more courses of rituximab. Primary outcomes were relapse-free survival and adverse events.
Results
A total of 346 children (age, 9.8 years; IQR, 6.6–13.5 years; 73% boys) received 1149 courses of rituximab. A total of 145, 83, 50, 28, 22, and 18 children received two, three, four, five, six, and seven or more courses, respectively. Median (IQR) follow-up was 5.9 (4.3–7.7) years. Relapse-free survival differed by treatment courses (clustered log-rank test P<0.001). Compared with the first course (10.0 months; 95% CI, 9.0 to 10.7 months), relapse-free period and relapse risk progressively improved after subsequent courses (12.0–16.0 months; HRadj, 0.03–0.13; 95% CI, 0.01 to 0.18; P<0.001). The duration of B-cell depletion remained similar with repeated treatments (6.1 months; 95% CI, 6.0 to 6.3 months). Adverse events were mostly mild; the most common adverse events were hypogammaglobulinemia (50.9%), infection (4.5%), and neutropenia (3.7%). Side effects did not increase with more treatment courses nor a higher cumulative dose. Only 78 of the 353 episodes of hypogammaglobulinemia were clinically significant. Younger age at presentation (2.8 versus 3.3 years; P=0.05), age at first rituximab treatment (8.0 versus 10.0 years; P=0.01), and history of steroid resistance (28% versus 18%; P=0.01) were associated with significant hypogammaglobulinemia. All 53 infective episodes resolved, except for one patient with hepatitis B infection and another with EBV infection. There were 42 episodes of neutropenia, associated with history of steroid resistance (30% versus 20%; P=0.04). Upon last follow-up, 332 children (96%) had normal kidney function.
Conclusions
Children receiving repeated courses of rituximab for FRSDNS experience an improving clinical response. Side effects appear acceptable, but significant complications can occur. These findings support repeated rituximab use in FRSDNS.
Idiopathic childhood nephrotic syndrome (NS) is an uncommon disease characterized by edema, nephrotic-range proteinuria, and hypoalbuminemia. Although most children attain remission after corticosteroids, about 30%–50% of patients develop frequent relapses or become steroid dependent.1,2 Steroid-sparing agents are used either sequentially or in combination among children with frequently relapsing, steroid-resistant NS (FRSDNS). A subgroup of children, however, continue to relapse and become multidrug dependent.2,3 Rituximab, a chimeric anti-CD20 mAb, has emerged as an effective treatment for FRSDNS to sustain long-term remission.4–6
Our previous work showed that disease severity, rituximab dose, and maintenance immunosuppression all affect treatment outcomes.7,8 Nonetheless, the drug effect is not permanent and most patients eventually relapse, requiring multiple courses to maintain remission.7 Therefore, it is crucial to understand the long-term efficacy and safety after repeated rituximab use. Although rituximab appears safe,4,6,9–15 young children appear more susceptible to complications, such as neutropenia and hypogammaglobulinemia.16–18
In this study, we performed an international, multicenter study to determine the long-term efficacy and safety of repeated use of rituximab in children with FRSDNS.
Methods
We conducted an international, multicenter, retrospective cohort study at 16 tertiary pediatric nephrology centers from ten countries in Asia, Europe, and North America, between March 1, 2020 and March 31, 2021. We included children and young people (aged 1–18 years at disease onset) with frequently relapsing, steroid-resistant, or multidrug-dependent NS who received two or more courses of rituximab between 2005 and 2020. All patients were steroid sensitive and in remission at the time of first rituximab treatment. Patients were followed for at least 24 months after the second course of rituximab.
Standard definitions were used for NS, remission, and relapses (Supplemental Table 1).19 Patients with multidrug-dependent NS required at least two immunosuppressants to maintain remission, including a corticosteroid, mycophenolate mofetil (MMF), and/or calcineurin inhibitor (CNI).14,20 Children with a history of initial or late steroid resistance who became steroid responsive after additional immunosuppressant treatment,3,21 notably CNIs, were also included, provided they were steroid responsive at first treatment with rituximab. Redose indications included B-cell repopulation, relapse, and/or prophylactic rituximab administration. The latter might include redosing on the basis of patients’ previous clinical response, significant toxicity from other medications, or social reasons. Children who were given repeated courses of rituximab at a regular interval, as per departmental policy (e.g., every 6 months),22 irrespective of B-cell population, relapse, or previous clinical response, were excluded. Further exclusion criteria were multidrug refractory NS that failed to attain remission, congenital or infantile NS, secondary NS (e.g., genetic podocytopathy, lupus nephritis, IgA nephropathy), and age >21 years at first rituximab administration.
Data collected included baseline demographics, renal histology, previous therapies, rituximab dose and concomitant immunosuppression, relapses, adverse events, total B-cell counts, and IgG levels. Anonymized data were recorded on standardized forms. This study was performed in accordance with the Declaration of Helsinki and the Declaration of Istanbul, and was approved by institutional review boards as required at each participating center.
Therapy
Both the original rituximab product and its biosimilars were administered, according to institutions’ choices. Depending on local polices, rituximab dosing ranged from 375 to 1500 mg/m2 per course, i.e., 375 mg/m2 for one to four infusion(s) or 750 mg/m2 for one to two infusion(s). Two infusions of rituximab would be considered as two different courses if they were given >4 weeks apart. Cumulative dose referred to the total rituximab received over all courses of treatment. The duration of concomitant immunosuppression was individualized and defined as use of one or more agents, including corticosteroid, MMF, and/or CNI. Rituximab protocol, immunosuppression titration, and monitoring policies were at the discretion of individual centers.
Outcomes
The primary outcomes were relapse-free survival after each course of rituximab, defined as time to relapse, and adverse events. Children who did not relapse were censored at additional course or last follow-up, whichever occurred earlier. The last treatment course would not be evaluated if the observation period was <12 months. Adverse events included death, severe infusion reactions, hypogammaglobulinemia, neutropenia, infections, benign neoplasia, malignancy, and cardiovascular, neurologic, cutaneous, pulmonary, and other complications. Secondary outcomes included the status of B-cell population, long-term remission (relapse-free period ≥2 years), rituximab resistance (relapse-free period <3 months), and the development of CKD or kidney failure. B-cell depletion and repletion were defined as CD19+ B cells forming <1% and ≥1% of the total lymphocyte population, respectively. Neutropenia and agranulocytosis were defined as absolute neutrophil count <1.5 × 109 and 0.5 × 109 per liter, respectively. Hypogammaglobulinemia was defined according to the individual laboratory’s reference for age. Hypogammaglobulinemia was considered significant if episodes had a very low IgG level of <200 mg/dl or required intravenous Ig (IVIG) replacement. Episodes were also regarded as significant if the patients developed concomitant infection with an IgG level <500 mg/dl.
Statistical Analyses
The Kaplan–Meier method with clustered log-rank test was used to compare the relapse-free survival and B-cell depletion survival with repeated courses of rituximab. Median survival time, with 95% CIs derived using the complementary log-log method, was calculated. All potential factors were included in a multiple mixed effects Cox regression model with a subject-specific intercept (random effect) to determine their relationship with relapse. These factors included the number of the course of rituximab, dosing regimen and cumulative dose at each rituximab episode, duration of concomitant immunosuppression, sex, ethnicity, age at NS diagnosis, age at each rituximab episode, history of steroid resistance, disease severity, and number of previous immunosuppression treatments. Multicollinearity in the mixed model was evaluated using the variance inflation factor. The mixed effects model with both subjects and centers as random intercept (subjects nested within centers) was also examined and compared with the nested model using a likelihood ratio test. We also performed two sensitivity analyses, restricting to individuals receiving repeated courses of rituximab exclusively due to disease relapse (sensitivity analysis 1), and children with the same total number of rituximab courses (sensitivity analysis 2). The Pearson chi-squared test, Fisher exact test, independent t test, and Mann–Whitney U test were used to compare the baseline characteristics among patients with and without adverse events. Effects of rituximab regimen, age at rituximab episodes, concomitant immunosuppression, and duration of adverse events were investigated using the likelihood ratio test by comparing the generalized linear mixed effects model with a fixed effect and subject-specific intercept (random effect) to the nested model with random effect only. For missing data, no imputation was used. Statistical analyses were performed using R version 4.1.1, with the survival, survminer, coxme, lme4, and performance packages. Statistical significance was set at P<0.05.
Results
Patient Population
A total of 346 children (253 male) treated with 1149 courses of rituximab were included (Table 1). Median (interquartile range [IQR] age at presentation and first rituximab therapy were 3.1 (2.2–4.8) and 9.8 (6.6–13.5) years, respectively. A total of 145 (42%), 83 (24%), 50 (15%), 28 (8%), 22 (6%), and 18 (5%) children received two, three, four, five, six, and seven or more courses of rituximab, respectively. Eighty percent of children were multidrug dependent, and 21% had a history of steroid resistance. A total of 246 children (71%) underwent kidney biopsies, and minimal change disease (n=169, 69%) was the predominant histologic diagnosis. Median (IQR) follow-up duration was 5.9 (4.3–7.7) years. The baseline characteristics and details of rituximab regimen are presented in Table 2 and Supplemental Tables 2 and 3.
Table 1.
Patients and rituximab treatments eligible for analysis
| Patient Enrollment Details | Subjects (n) | Courses of Rituximab (n) |
|---|---|---|
| Initial enrollment | 410 | 1332 |
| Excluded | 64 | 183 |
| First rituximab at age >21 yr | 2 | 9 |
| Less than two courses of rituximab | 36 | 36 |
| <24 mo of observation after second course | 25 | 50 |
| <12 mo of observation at last course | — | 85 |
| Inadequate patient data for analysis | 1 | 3 |
| Included for analysis | 346 | 1149 |
Table 2.
Baseline characteristics and details of treatment regimen in 346 children receiving 1149 courses of rituximab
| Variable | Value |
|---|---|
| Baseline characteristics of patients (n=346) | |
| Sex, n (%) | |
| Female | 93 (26.9) |
| Male | 253 (73.1) |
| Race and ethnicity, n (%) | |
| White | 138 (39.9) |
| East Asian | 124 (35.8) |
| South Asian | 36 (10.4) |
| Black | 22 (6.4) |
| Others | 26 (7.5) |
| Age at presentation (yr), median (IQR) | 3.1 (2.2–4.8) |
| Age at first rituximab treatment (yr), median (IQR) | 9.8 (6.6–13.5) |
| Disease severity, n (%) | |
| Multidrug dependence | 276 (79.8) |
| Steroid dependence with or without frequent relapsing | 64 (18.5) |
| Frequent relapsing alone | 6 (1.7) |
| History of steroid resistance, n (%) | 72 (20.8) |
| Initial steroid resistance | 28 (8.1) |
| Late steroid resistance | 44 (12.7) |
| Renal biopsy, n (%) | 246 (71.1) |
| Minimal change disease | 169 (68.7) |
| Focal segmental glomerulosclerosis | 58 (23.6) |
| Othersa | 19 (7.7) |
| Previous immunosuppression, n (%) | |
| CNI | 297 (85.8) |
| MMF | 172 (49.7) |
| Cyclophosphamide | 153 (44.2) |
| Levamisole | 51 (14.7) |
| Others | 58 (16.8) |
| Number of steroid-sparing agents before rituximab, n (%) | |
| 0 | 18 (5.2) |
| 1 | 86 (24.9) |
| 2 | 114 (32.9) |
| 3 | 97 (28.0) |
| 4 | 29 (8.4) |
| ≥5 | 2 (0.6) |
| Cumulative rituximab dose at last follow-up (mg/m2), median (IQR) | 1500 (1125–2625) |
| Treatment regimen: rituximab course (n=1149) | |
| Rituximab dose (per course), n (%) | |
| Low (375 mg/m2) | 746 (64.9) |
| Medium (750 mg/m2) | 230 (20.0) |
| High (1125–1500 mg/m2) | 173 (15.1) |
| Average dose per course (mg/m2), median (IQR) | 375 (375–750) |
| Use of immunosuppression after each rituximab treatment, n (%)b | 1070 (93.1) |
| Discontinuation within 6 months, n (%) | 495 (46.3) |
| Duration (mo), median (IQR) | 2.0 (1.5–3.4) |
| Any immunosuppression ≥6 months, n (%) | 574 (53.6) |
| Duration (mo), median (IQR) | 13.0 (8.8–21.4) |
| Type of immunosuppression used after each course of rituximab | |
| Corticosteroid, n (%)c | 971 (84.5) |
| Duration (mo), median (IQR) | 2.3 (1.5–6.0) |
| MMF, n (%) | 474 (41.3) |
| Duration (mo), median (IQR) | 11.7 (6.3–20.6) |
| CNI, n (%) | 455 (39.6) |
| Duration (mo), median (IQR) | 6.4 (3.0–11.9) |
| Indications for repeating rituximab treatment (n=803), n (%) | |
| Relapse | 665 (82.8) |
| B-cell repletion | 109 (13.6) |
| Prophylactic administrationd | 29 (3.6) |
Included idiopathic mesangial proliferative GN (n=11), IgM nephropathy (n=4), c1q nephropathy (n=3), and idiopathic glomerulosclerosis (n=1).
Two instances of missing data for the use of concomitant immunosuppression and one instance of missing data for the duration of immunosuppression use.
Corticosteroid included prednisolone and prednisone.
Rituximab was given prophylactically on the basis of previous clinical response (n=24), the presence of CNI-associated nephrotoxicity (n=4), and social reasons (n=1).
Primary Outcomes
Long-Term Efficacy
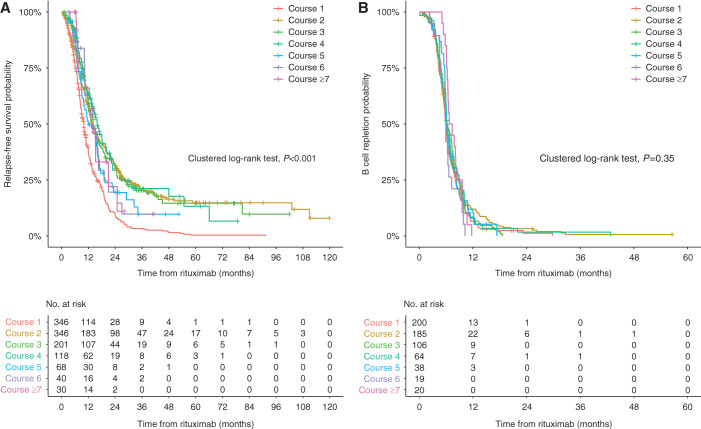
A total of 873 relapses (76%) were observed after 1149 courses of rituximab. The overall median relapse-free period was 12.5 months (95% CI, 12.0 to 13.2 months). Median relapse-free survival was different between repeated courses (clustered log-rank test P<0.001; Figure 1A, Table 3). The median relapse-free period was shortest with first rituximab treatment at 10.0 months (95% CI, 9.0 to 10.7 months), which progressively extended to 16.0 months (95% CI, 13.3 to 19.7 months) after the fourth course. This improvement in response was more substantial within individual subgroups of children receiving the same number of courses of rituximab (Table 4, Supplemental Figure 1, B–F).
Figure 1.
Relapse-free survival was different between repeated courses of rituximab, despite comparable duration of B-cell depletion. (A) Probability of relapse-free survivals in 346 children after repeated courses of rituximab (n=1149). The relapse-free survivals differed by treatment courses (clustered log-rank test P<0.001) and was shortest after the first course of rituximab. (B) Kaplan–Meier curves for the total B-cell recovery in 632 courses of rituximab with B-cell monitoring.
Table 3.
Relapse-free survival and B-cell repletion after repeated courses of rituximab
| Rituximab Course | Relapse | B-Cell Repletion | ||||
|---|---|---|---|---|---|---|
| Total (n) | Events (n) | Median Time (mo) (95% CI) | Total (n) | Events (n) | Median Time (mo) (95% CI) | |
| All courses | 1149 | 873 | 12.5 (12.0 to 13.2) | 632 | 622 | 6.1 (6.0 to 6.3) |
| Course 1 | 346 | 308 | 10.0 (9.0 to 10.7) | 200 | 196 | 6.0 (5.7 to 6.2) |
| Course 2 | 346 | 265 | 13.5 (12.3 to 16) | 185 | 182 | 6.0 (5.6 to 6.5) |
| Course 3 | 201 | 139 | 14.3 (12.3 to 16) | 106 | 106 | 6.2 (5.7 to 7.3) |
| Course 4 | 118 | 72 | 16.0 (13.3 to 19.7) | 64 | 62 | 6.2 (5.9 to 7.0) |
| Course 5 | 68 | 49 | 12.0 (9.0 to 16.0) | 38 | 37 | 6.2 (5.8 to 7.4) |
| Course 6 | 40 | 24 | 13.4 (10.5 to 16.5) | 19 | 19 | 6.0 (4.7 to 6.6) |
| Course ≥7 | 30 | 16 | 14.0 (9.0 to 21) | 20 | 20 | 7.0 (6.1 to 8.4) |
Table 4.
Relapse-free survival among individual subgroups of patients receiving the same total number of rituximab courses
| Rituximab Course | Subjects with Two Courses (n=145) | Subjects with Three Courses (n=83) | Subjects with Four Courses (n=50) | Subjects with Five Courses (n=28) | Subjects with Six Courses (n=22) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event (n) |
Median Time, (mo) (95% CI) |
Event (n) |
Median Time, (mo) (95% CI) |
Event (n) |
Median Time, (mo) (95% CI) |
Event (n) |
Median Time, (mo) (95% CI) |
Event (n) |
Median Time, (mo) (95% CI) |
|
| Course 1 | 128 | 10.7 (9.0 to 12.7) | 76 | 11.0 (8.4 to 12) | 42 | 9.0 (7.7 to 12.6) | 27 | 7.8 (5.7 to 9.3) | 21 | 7.9 (5.7 to 10) |
| Course 2 | 86 | 27.1 (22.2 to 36.9) | 76 | 13.0 (10.5 to 15) | 45 | 11.0 (7.5 to 12.7) | 25 | 8.0 (5.0 to 9.1) | 18 | 7.6 (4.8 to 10) |
| Course 3 | — | — | 44 | 24.0 (16.0 to NA) | 44 | 12.8 (10.5 to 17) | 22 | 11.8 (9.1 to 14.8) | 17 | 8.9 (6.5 to 12.0) |
| Course 4 | — | — | — | — | 26 | 21.8 (16.5 to NA) | 21 | 15.1 (10.2 to 18.1) | 14 | 9.3 (7.1 to 15.9) |
| Course 5 | — | — | — | — | — | — | 16 | 22.7 (15.6 to 34.2) | 16 | 11.5 (6.9 to 17.5) |
| Course 6 | — | — | — | — | — | — | — | — | 13 | 16.3 (10.3 to 25) |
| P Value | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 | |||||
Multivariate analysis demonstrated that, compared with the first course, the relapse risk progressively decreased with subsequent courses during the observation period (adjusted hazard ratio, 0.03–0.13; 95% CI, 0.01 to 0.18; P<0.001; Table 5). The treatment effect plateaued after course 4 and further treatment episodes (Supplemental Table 4). To limit potential bias, two sensitivity analyses were performed, restricted to the 262 children who were redosed exclusively due to disease relapse (sensitivity analysis 1), and those receiving the same total number of rituximab courses (sensitivity analysis 2). The same qualitative trends of improving treatment response with repeated rituximab treatment reported in the main cohort were demonstrated in both sensitivity analyses (Table 6, Supplemental Figure 1).
Table 5.
Association with relapse in 1146 courses of rituximab
| Variables | Adjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|
| No. of rituximab course | ||
| Course 1 | 1 (reference) | |
| Course 2 | 0.13 (0.10 to 0.18) | <0.001a |
| Course 3 | 0.06 (0.04 to 0.09) | <0.001a |
| Course 4 | 0.03 (0.02 to 0.06) | <0.001a |
| Course 5 | 0.04 (0.02 to 0.07) | <0.001a |
| Course 6 | 0.03 (0.01 to 0.06) | <0.001a |
| Course ≥7 | 0.03 (0.01 to 0.06) | <0.001a |
| Rituximab dose per course | ||
| Low (375 mg/m2) | 1 (reference) | |
| Medium (750 mg/m2) | 0.90 (0.69 to 1.18) | 0.44 |
| High (1125–1500 mg/m2) | 1.01 (0.65 to 1.57) | 0.95 |
| Cumulative dose, per 375 mg/m2 | 0.95 (0.89 to 1.01) | 0.12 |
| Concomitant immunosuppression duration (mo) | 0.91 (0.90 to 0.92) | <0.001a |
| Sex | ||
| Female | 1 (reference) | |
| Male | 1.02 (0.76 to 1.37) | 0.92 |
| Race and ethnicity | ||
| White | 1 (reference) | |
| East Asian | 1.52 (1.09 to 2.13) | 0.01a |
| South Asian | 1.66 (1.03 to 2.65) | 0.03a |
| Black | 1.73 (0.99 to 3.02) | 0.05a |
| Others | 1.56 (0.93 to 2.63) | 0.09 |
| Age at diagnosis (yr) | 1.00 (0.96 to 1.06) | 0.86 |
| Age at each rituximab episode (yr) | 0.92 (0.89 to 0.96) | <0.001a |
| History of steroid resistance | ||
| No | 1 (reference) | |
| Initial steroid resistance | 1.06 (0.63 to 1.78) | 0.81 |
| Late steroid resistance | 1.53 (1.02 to 2.30) | 0.03a |
| Disease severity | ||
| Multidrug dependent | 1 (reference) | |
| Frequent relapsing alone | 0.18 (0.06 to 0.53) | 0.001a |
| Steroid dependence with or without frequent relapsing | 0.79 (0.55 to 1.12) | 0.17 |
| No. previous immunosuppression treatments | 1.20 (1.03 to 1.39) | 0.02a |
Multiple mixed effects Cox regression analyses. Three rituximab courses were not included in the analysis due to missing data (duration on concomitant immunosuppression).
P<0.05.
Table 6.
Sensitivity analyses for the association with relapse
| Rituximab Course | Sensitivity Analysis 1 | Sensitivity Analysis 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Redose Exclusively on the Basis of Disease Relapse (262 patients; 784 courses) | Individuals Receiving the Same Total No. of Courses of Rituximab | |||||||
| Two Courses (145 patients; 290 courses) | Three Courses (83 patients; 249 courses) | Four Courses (50 patients; 200 courses) | ||||||
| HRadj (95% CI) | P Value | HRadj (95% CI) | P Value | HRadj (95% CI) | P Value | HRadj (95% CI) | P Value | |
| Course 1 | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — | 1 (reference) | — |
| Course 2 | 0.12 (0.09 to 0.17) | <0.001 | 0.12 (0.06 to 0.24) | <0.001 | 0.18 (0.10 to 0.33) | <0.001 | 0.13 (0.06 to 0.29) | <0.001 |
| Course 3 | 0.05 (0.03 to 0.08) | <0.001 | 0.05 (0.02 to 0.12) | <0.001 | 0.04 (0.01 to 0.11) | <0.001 | ||
| Course 4 | 0.03 (0.01 to 0.06) | <0.001 | 0.01 (0.00 to 0.02) | <0.001 | ||||
| Course 5 | 0.03 (0.01 to 0.06) | <0.001 | ||||||
| Course 6 | 0.03 (0.01 to 0.07) | <0.001 | ||||||
| Course ≥7 | 0.02 (0.01 to 0.07) | <0.001 | ||||||
Multiple mixed effects Cox regression model. Adjusted for dosing regimen, cumulative dose, duration of concomitant immunosuppression, sex, race and ethnicity, age at diagnosis, age at each rituximab episode, history of steroid resistance, disease severity, and number of previous immunosuppression treatments. HRadj, adjusted hazard ratio.
Use of concomitant immunosuppression for each month was associated with a 9% reduction in relapse risk (adjusted hazard ratio, 0.91; 95% CI, 0.90 to 0.92; P<0.001). Rituximab dose for each course and cumulative dose did not affect treatment outcomes. Increasing age at treatment episodes and milder disease severity, as reflected by absence of steroid dependency and less previous immunosuppression, were significant protective factors against relapse. In contrast, patients with late steroid resistance were at higher risk of relapse. Black, East Asian, and South Asian children, compared with White children, were more susceptible to a relapse. Data pertaining to these predictive factors are presented in Table 5.
Long-Term Safety Profiles
Overall, 469 adverse events were recorded during the 1149 courses of rituximab. Among all treatment episodes, hypogammaglobulinemia (n=353 in 693 monitored courses, 51%) accounted for majority of adverse events, followed by infections (n=53, 5%) and neutropenia (n=42 in 1120 monitored courses, 4%). Most side effects were mild and are presented in Table 7. No patients died in the cohort. Severe infusion reactions (n=9), neurologic (n=3) and cardiovascular (n=1) complications, malignancy (n=1), and other complications were uncommon (Supplemental Table 5). These events did not appear to be increased with higher courses of rituximab. One patient developed abdominal rhabdomyosarcoma and required resection. This patient remained in cancer remission at last follow-up. There was no other reported case of malignancy.
Table 7.
Adverse events in 1149 courses of rituximab
| Adverse Events | No. (%) or Median (IQR) | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All Courses (n=1149) | Course 1 (n=346) | Course 2 (n=346) | Course 3 (n=201) | Course 4 (n=118) | Course 5 (n=68) | Course 6 (n=40) | Course ≥7 (n=30) | ||
| Hypogammaglobulinemia | |||||||||
| Courses of rituximab with IgG monitoring | 693 | 177 | 207 | 138 | 76 | 46 | 26 | 23 | |
| Hypogammaglobulinemia (all) | 353 (50.9) | 85 (48.0) | 103 (49.8) | 64 (46.4) | 38 (50) | 26 (56.5) | 19 (73.1) | 18 (78.3) | 0.46 |
| Onset time after each course (mo)a,b | 1.6 (0.3–6.7) | 1.3 (0.3–5.9) | 1.8 (0.3–8.4) | 1.1 (0.3–6.6) | 2.1 (0.3–10.8) | 2.8 (0.7–4.9) | 0.8 (0.3–11.0) | 2.7 (1.4–5.5) | |
| Lowest IgG value (mg/dl) | 348 (238–462) | 341 (205–467) | 351 (264–495) | 334 (196–452) | 355 (256–510) | 376 (282–451) | 295 (246–410) | 316 (224–373) | 0.40 |
| Prolonged episodes (>6 months) | 67 (9.7) | 19 (10.7) | 15 (7.2) | 14 (10.1) | 7 (9.2) | 6 (13.0) | 2 (7.7) | 4 (17.4) | 0.17 |
| Recovery time (mo)c | 11.5 (8.1–21.8) | 17.6 (9.1–44.5) | 11.3 (7.8–15.9) | 8.7 (6.9–11.4) | 10.2 (8.6–27.1) | 10.8 (9.9–11.7) | 24.6 (19.5–29.7) | 14.8 (11.9–19.4) | 0.73 |
| Significant hypogammaglobulinemiad | 78 (11.3) | 21 (11.9) | 19 (9.2) | 19 (13.8) | 9 (11.8) | 5 (10.9) | 3 (11.5) | 2 (8.7) | 0.75 |
| Onset time after each course (mo) | 5.8 (0.6–10.6) | 2.6 (0.6–9.5) | 10.9 (6.3–15.5) | 2.2 (0.5–7.0) | 9 (0.3–11.3) | 4.3 (3.2–5.5) | 4.7 (2.6–7.6) | 8.0 (7.0–8.9) | |
| Very low IgG levels (<200 mg/dl) | 66 (9.5) | 19 (10.7) | 15 (7.2) | 16 (11.6) | 8 (10.5) | 3 (6.5) | 3 (11.5) | 2 (8.7) | 0.48 |
| Concomitant infection | 8 (1.2) | 1 (0.6) | 4 (1.9) | 2 (1.4) | 1 (1.3) | 0 | 0 | 0 | — |
| Need of IVIG replacement | 25 (3.6) | 6 (3.4) | 9 (4.3) | 6 (4.3) | 0 | 3 (6.5) | 1 (3.8) | 0 | — |
| Isolated hypogammaglobulinemia | 17 | 5 | 5 | 3 | 0 | 3 | 1 | 0 | |
| Severe infection | 3 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | |
| Recurrent infections | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Additional immunomodulation | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Unknown | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Neutropeniae | |||||||||
| Courses of rituximab with count monitoring | 1120 | 337 | 339 | 197 | 116 | 66 | 38 | 27 | |
| Neutropenia (all) | 42 (3.8) | 18 (5.3) | 11 (3.2) | 4 (2.0) | 4 (3.4) | 2 (3.0) | 1 (2.6) | 2 (7.4) | 0.06 |
| Onset time after each course (mo)b | 2.9 (1.7–4.6) | 3.5 (1.4–4.5) | 2.4 (1.7–4.6) | 2.0 (1.5–2.8) | 2.5 (1.4–4.2) | 2.8 (2.7–2.8) | 15.7 | 4.9 (4.0–5.7) | |
| Lowest value (mg/dl) | 0.7 (0.2–1.2) | 0.4 (0.2–1.2) | 1.0 (0.6–1.2) | 1.1 (0.7–1.2) | 0.7 (0.4–0.9) | 0.5 (0.2–0.7) | 0.5 | 0.4 (0.2–0.5) | 0.51 |
| Concomitant infection | 9 (0.8) | 5 (1.5) | 1 (0.3) | 1 (0.5) | 0 | 1 (1.5) | 0 | 1 (3.7) | — |
| Agranulocytosisf | 18 (1.6) | 10 (3.0) | 3 (0.9) | 1 (0.5) | 1 (0.9) | 1 (1.5) | 1 (2.6) | 1 (3.7) | 0.001 |
| Onset time after each course (mo)b | 2.9 (1.7–4.5) | 3.7 (2–4.7) | 1.8 (1.7–2.3) | 1.7 | 0.5 | 2.7 | 15.7 | 3.2 | |
| Other adverse events | |||||||||
| Any other adverse events | 74 (6.4) | 20 (5.8) | 27 (7.8) | 12 (6.0) | 7 (5.9) | 3 (4.4) | 3 (7.5) | 2 (6.7) | 0.90 |
| Infection | 53 (4.6) | 15 (4.3) | 19 (5.5) | 9 (4.5) | 4 (3.4) | 3 (4.4) | 1 (2.5) | 2 (6.7) | 0.92 |
| Severe infusion reactiong | 9 (0.8) | 4 (1.2) | 2 (0.6) | 1 (0.5) | 2 (1.7) | 0 | 0 | 0 | — |
| Neurologic | 3 (0.3) | 0 | 2 (0.6) | 0 | 0 | 0 | 1 (2.5) | 0 | — |
| Cutaneous | 2 (0.2) | 0 | 1 (0.3) | 0 | 0 | 0 | 1 (2.5) | 0 | — |
| Benign tumor | 1 (0.1) | 0 | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | — |
| Cardiovascular | 1 (0.1) | 0 | 0 | 0 | 0 | 0 | 1 (2.5) | 0 | — |
| Malignancy | 1 (0.1) | 0 | 0 | 1 (0.5) | 0 | 0 | 0 | 0 | — |
| Others | 5 (0.4) | 1 (0.3) | 2 (0.6) | 1 (0.5) | 1 (0.8) | 0 | 0 | 0 | — |
Data are expressed as n (%) or median (IQR), as appropriate. P values were derived from the likelihood ratio test.
Thirty episodes of hypogammaglobulinemia lasted for more than one course of rituximab.
Missing onset time for hypogammaglobulinemia, neutropenia, and agranulocytosis in a total of eight rituximab episodes.
Seven episodes of hypogammaglobulinemia had not resolved at last follow-up.
Clinically significant hypogammaglobulinemia was defined as episodes with very low IgG levels (<200 mg/dl), associated with concomitant infections and/or requiring IVIG replacement.
Neutropenia was defined as an absolute neutrophil count <1.5 × 109 per liter.
Agranulocytosis was defined as an absolute neutrophil count <0.5 × 109 per liter.
Severe infusion reactions that required termination of the rituximab treatment.
Hypogammaglobulinemia
IgG levels were monitored in 250 children and 693 treatment courses (Table 7). A total of 147 patients developed 353 episodes (51%) of hypogammaglobulinemia at a median (IQR) of 1.6 (0.3–6.7) months from the rituximab infusion. The first episode was detected after two (IQR, one to three) courses of rituximab. Sixty-seven episodes were prolonged (>6 months) and lasted for 11.5 (IQR, 8.1–21.8) months. Only seven episodes of hypogammaglobulinemia persisted until last follow-up. A total of 78 hypogammaglobulinemia episodes were considered significant among 54 patients, and were either severe (<200 mg/dl; n=66), associated with concomitant infections (n=8), and/or requiring IVIG replacement (n=25). Fifteen children (28%) had recurrent significant hypogammaglobulinemia during subsequent treatment courses (two episodes, n=9; three episodes, n=3; four episodes, n=3). The proportions of any hypogammaglobulinemia and significant hypogammaglobulinemia were comparable across treatment courses. Younger age at presentation (2.8 [IQR, 2.2–3.8] versus 3.3 [IQR, 2.3–5.1] years; P=0.05), younger age at first rituximab treatment (8.0 [IQR, 5.5–11.8] versus 10.0 [IQR, 6.9–13.8] years; P=0.01), and a history of steroid resistance (27.8% versus 17.3%; P=0.01) were associated with significant hypogammaglobulinemia (Supplemental Table 6). Other factors, such as dose, cumulative dose, and immunosuppression use, were not associated with hypogammaglobulinemia.
Neutropenia and Agranulocytosis
Neutrophils were monitored in 342 patients and 1120 treatment episodes (Table 6). A total of 42 (3.8%) and 18 (1.6%) episodes of neutropenia and agranulocytosis were observed in 33 and 15 patients, respectively (Table 6). These episodes occurred at a median (IQR) of 2.9 (1.7–4.6) months after rituximab treatment. Rates of neutropenia were comparable with repeated courses. Concurrent MMF at the onset of neutropenia and agranulocytosis was present in 53% and 59% episodes, respectively. There were nine episodes of concomitant infections (gingivostomatitis, n=3; non-specific febrile neutropenia, n=2; viral infection, n=2; viral gastroenteritis, n=1; Pneumocystis pneumonia, n=1). All episodes resolved with interventions, including conservative measures (n=3), granulocyte colony-stimulating factor (n=3), and withholding causative medications such as MMF/cotrimoxazole (n=5). Another seven patients received granulocyte colony-stimulating factor for isolated neutropenia. Although history of steroid resistance was associated with neutropenia (30% versus 20%; P=0.04), no specific factor was associated with agranulocytosis (Supplemental Table 6).
Infection
A total of 49 patients developed 53 infections during rituximab treatment, and 25 episodes (47%) required hospitalization. The overall incidence of infection was 4.6%, and this did not increase with more courses of rituximab (Table 6). Of the infections, 61% occurred in the presence of concurrent immunosuppression. Forty-five percent of children received cotrimoxazole as primary prophylaxis against Pneumocystis. The most common infections were pneumonia (n=10, 19%), upper respiratory tract infection (n=8, 15%), chickenpox (n=8, 15%), gingivostomatitis (n=5, 9%), and herpes zoster (n=3, 6%). The majority of these infections were mild. One patient with influenza pneumonitis required intensive care for noninvasive ventilation. In addition, one patient developed Pneumocystis pneumonia, and two patients had new-onset hepatitis B infections. All infections resolved over a median (IQR) duration of 10 (5–14) days, except in one patient with chronic hepatitis B infection, who required tenofovir, and another with chronic Epstein–Barr virus infection.
Secondary Outcomes
Total B-Cell Population
B cells were depleted after 97% of courses of rituximab (n=815 of 839). Total B cells were subsequently monitored in 632 treatment courses, and recovery was observed in 622 courses. The median duration of B-cell depletion was 6.1 months (95% CI, 6.0 to 6.3 months). This was comparable between repeated courses, ranging from 6.0 to 6.2 months (clustered log-rank test P=0.35; Figure 1B, Table 3). After B-cell repletion, relapses were observed in 435 courses of rituximab (70%), with a median difference of 2.6 months between relapse and B-cell repopulation (Supplemental Table 7). Thirty percent of children did not relapse after B-cell repopulation at last follow-up.
Long-Term Remission
Of the 1149 courses of rituximab, 203 treatment episodes attained long-term remission (≥2 years). The mean±SD number of course received to attain first long-term remission was 2.4±1.3. Eight percent, 28%, and 22% of children attained long-term remission after course 1, 2, and 3, respectively. A total of 102 treatment episodes (50%) eventually relapsed, with a median (IQR) time to relapse of 29.1 (26.0–38.2) months.
Poor Treatment Response and Development of CKD or Kidney Failure
A total of 56 patients (16%) had a poor response to rituximab (relapse-free period <3 months) after 1.8±1.1 rituximab treatments. Ten children did not receive further rituximab treatment. Of the 46 patients who received subsequent courses, 40 had an improved response, with a median (IQR) relapse-free period of 11.4 (6.6–19.5) months. Only six patients relapsed within 3 months again. After 2.4±1.4 courses, seven patients (2%) developed multidrug-refractory, steroid-resistant relapses that failed to remit despite multiple treatments, including rituximab therapy. Anti-rituximab antibodies were evaluated in only two of these patients and were positive in one. At last follow-up, 332 children (96%) had normal kidney function. Ten patients had CKD (history of steroid resistance, n=4; multidrug refractory relapse, n=1; history of CNI use, n=10), and another four patients with refractory relapses developed kidney failure requiring kidney replacement therapy.
Discussion
Our study shows a novel finding that treatment response progressively improves with repeated courses of rituximab. The adverse event profile remains acceptable and does not increase with more courses nor a higher cumulative dose. These data suggest repeated use of rituximab is both an effective and safe approach in FRSDNS. This observation may reflect a disease-modifying effect, resulting in improved clinical response and long-term remission in a subset of patients.
A significant proportion of children with FRSDNS relapse 1 year after rituximab treatment.7,23 Many of these children continue to have frequent relapses or steroid dependency, necessitating reinitiation of intensive immunosuppression and/or additional rituximab.23 Similar to our findings, the most common redose indication for rituximab was disease relapse in up to 87% of children with complicated FRSDNS.7 A different strategy is to monitor the B-cell response and repeat rituximab when they repopulate, because several reports have shown that relapses occur after B-cell reconstitution.13,24–26 Sellier-Leclerc et al.27 reported that, after 15 months of B-cell depletion through single or multiple courses of rituximab, 60% of patients remained in long-term remission. In our study, there was no consistent, temporal relationship between relapse and B-cell population, and relapse was not observed in 30% of our population, even after B-cell recovery. In addition, some patients were offered prophylactic rituximab administration on the basis of their past clinical history; clinical status, such as steroid toxicity; and social reasons. B-cell reconstitution and prophylactic treatments accounted for 15% of the redose indications in our patient cohort; this may result in an underestimation of the treatment efficacy because patients received further rituximab even before disease relapse. Therefore, we performed a sensitivity analysis restricted to individuals who were redosed strictly due to disease relapse, which also demonstrated an improving efficacy with repeated rituximab. Finally, some centers adopted regular therapy to induce persistent B-cell depletion.22 These children were excluded from this study.
In our previous smaller, single-center study, we reported the outcomes of 58 children with FRSDNS who had multiple courses of rituximab.28 Although most patients received only up to two or three courses, there was no change in relapse-free period between treatments. Our current data showed both relapse-free survival and relapse risk improved significantly and progressively with repeated rituximab therapies. This effect appears genuine because all children received at least two courses of rituximab. The change in response was also substantial even when comparing individual subgroups exposed to the same total number of treatment courses. Improvement in response does not appear to be dose dependent, but more related to repeated drug exposures. One potential confounder is that patients with an unfavorable response at first treatment with rituximab were not offered a second course. Nonetheless, as demonstrated in our data, there was no consistent relationship between an initially suboptimal response to rituximab and outcomes upon subsequent treatments. Although childhood NS usually improves with age,2 age at each course of rituximab was included as an adjustment factor in our regression model. Our data also showed that duration of B-cell depletion remained constant with multiple courses of treatment. Similar findings were reported in a study where 90% children with NS had a comparable duration of B-cell depletion during subsequent rituximab treatments.29 We suggest that, even after total B-cell recovery, there is an ongoing and cumulative effect on subsets of B cells in other locations with repeated treatments. Colucci et al.17 reported that, after complete repopulation of total, transitional, and mature-native B cells, a sustained and significant reduction of total memory and switched memory B cells was still observed in most children after rituximab treatment. Importantly, in multivariate analysis, only reconstitution of switched memory B cells was predictive of a relapse, but evaluation of this factor is only available in research settings.17 Finally, the improved efficacy appeared to plateau after the fourth course of rituximab. This may reflect a subset of patients who were the most difficult to treat, with suboptimal response to rituximab and failure to attain long-term remission.
Various patient factors account for a diverse response to rituximab.8 Although there is great variability in steroid responsiveness by race and ethnicity, a racial and ethnic effect on the response to rituximab has not been previously described.30 Our results, however, showed that South Asian, East Asian, and Black children showed a less favorable response to rituximab than White children. Concurring with published reports, patients with a more severe disease were also more prone to relapse after rituximab treatment. These patients included those with multidrug dependence, steroid resistance, previous immunosuppression, and younger age at treatment episodes.7,24,25,31,32 Our previous work also showed that maintenance immunosuppression had a positive effect on treatment outcomes.7 This work better defines that relapse risk. The relapse risk was reduced by 9% with each additional month’s use of concurrent immunosuppression. Iijima et al.33 reported that the use of MMF as maintenance therapy after rituximab reduced relapse, although the relapse-preventing effect disappeared after drug discontinuation. This observation echoes our findings that duration of immunosuppression use contributes to the protective effect on disease relapse. However, immunosuppression use should be balanced against potential adverse events, such as infection, especially in the presence of additional risk factors, such as hypogammaglobulinemia and neutropenia.34 Of note, histology has not been identified as a predictive factor of relapse.6,31,32 Because 100 patients did not undergo kidney biopsy in our cohort, histology was not included in adjustment in the regression model.
Overall, rituximab is safe in children, but serious complications, such as fatal hepatitis reactions and fulminant myocarditis, do occur.4,6,9–15,35–40 Previous studies with fewer patients and shorter follow-up did not suggest that higher cumulative dose or increasing treatment courses led to more side effects.7,14,18 Our data support these findings, with hypogammaglobulinemia, neutropenia, and infection being the three most common adverse events. Kamei et al.16 reported agranulocytosis in 9.6% patients after rituximab treatment; agranulocytosis was more prevalent among young children. We showed a much lower incidence of neutropenia (3.7%) and agranulocytosis (1.6%), and younger age at rituximab treatment was not associated with this complication. All adverse events had favorable outcomes and resolved with interventions, such as antibiotics and granulocyte colony-stimulating factor. Infections did occur, but they were mostly self-limiting. The episodes of hepatitis reactivation and Pneumocystis pneumonia highlight the importance of identifying chronic hepatitis carriers with appropriate antiviral coverage, and of considering cotrimoxazole prophylaxis for a period of 3–6 months.21,35 There is currently no evidence suggesting increased malignancy potential.41–43 One of our patients developed rhabdomyosarcoma, but its relationship with rituximab could not be substantiated. One particular concern of rituximab use is the vulnerability to coronavirus disease 2019 (COVID-19) during the pandemic. Fortunately, the risk of acquiring COVID-19, symptomatic disease, and dismal outcomes do not appear to be increased among children receiving rituximab for NS, compared with the general pediatric population.44,45 Nonetheless, vaccination is strongly recommended to protect susceptible patients against COVID-19, and additional vaccine doses may be warranted to achieve adequate immunogenicity.46
Persistent hypogammaglobinemia is another important long-term sequela from rituximab.17 Parmentier et al.18 reported that 30% of children developed hypogammaglobulinemia after rituximab, and half of these episodes persisted beyond 1 year after B-cell recovery. We reported a higher rate (51%) of this complication, but only one fifth of the episodes were considered clinically significant. This rate may be under-reported due to discrepant IgG monitoring policies among participating centers. Because the assessment of significant infection was consistent across sites, most of these episodes of hypogammaglobulinemia were likely insignificant episodes such that physicians did not offer follow-up monitoring. It should be noted that baseline IgG levels before rituximab treatment were not available in this study. Preexisting hypogammaglobulinemia is common among children with NS due to urinary loss and immunosuppression use, and tends to persist after rituximab therapy.34 Because >80% of rituximab redoses were due to relapse, the high prevalence of hypogammaglobulinemia reported might partly be due to the aforementioned factors rather than rituximab alone. The development of hypogammaglobulinemia was not related to cumulative dose,18 rituximab regimen, nor maintenance immunosuppression. Younger age at first rituximab treatment, similar to published data, was associated with hypogammaglobulinemia.17,18 In concurrence with the report by Inoki et al.,47 history of steroid resistance was associated with hypogammaglobulinemia. This is not surprising because these patients are treatment refractory, with protracted nephrotic states and constant exposure to intensive immunosuppression. Only 1% of all episodes of hypogammaglobulinemia were associated with infection, and some required IVIG replacement. IVIG was mostly administered for isolated low IgG levels without clinical manifestations, although published guidelines do not recommend an absolute level for substitution.48
Although the safety profiles of repeated rituximab treatment appear acceptable in this study, careful interpretation is required because children with severe complications might not be offered further courses of rituximab. The cumulative risk may also be increased with repeated exposures. The risks of neutropenia and hypogammaglobulinemia are not negligible and serious complications can occur. This concern is particularly true among the youngest children, whose treatment is often challenging and susceptible to significant complications.
With this in mind, we suggest the use of rituximab after all other treatment options have been exhausted, with cautious use among the youngest patients.8 The decision to repeat rituximab treatment should preferably be due to disease relapse. We do not recommend routinely redosing rituximab on the basis of B-cell reconstitution because a significant proportion of patients may be subjected to excessive or unnecessary treatments. Prophylactic administration, according to B-cell repopulation or previous response, may be considered in selected patients with history of severe relapse or significant steroid toxicity. If this approach is to be used, clinicians should withhold rituximab and carefully reassess after every two to three treatment cycles because some patients may develop long-term disease remission. A single infusion of rituximab at 375 mg/m2 is a reasonable regimen for redosing, with or without the use of concomitant immunosuppression to extend the relapse-free period. Thorough consideration and family counseling is also necessary for individuals with a history of serious complications. Because most children remain asymptomatic despite hypogammaglobulinemia, it may not be necessary to withhold further rituximab. Clinicians should monitor Ig levels and white cell counts regularly, before and after rituximab treatment. The use of antibiotic prophylaxis and Ig replacement to prevent infection remains controversial and requires an individualized approach after evaluating potential risk factors, such as patient age, history of severe infection, and concurrent immunosuppression.34
Our study provides long-term data in a multiethnic, multiracial, pediatric cohort from around the world. It has several limitations typical of a retrospective study, such as selection and reporting bias. These factors were minimized by well-defined inclusion criteria and an adequate observation period for each patient and treatment episode. However, it should be noted that the definition used for steroid-resistant NS was variable in different countries. This lack of a uniform definition may, therefore, limit the scope of our results and conclusions. Second, there was a risk of bias in the results among patients for which rituximab was repeated prophylactically or on the basis of B-cell repopulation. Third, treatment policies varied between centers, including redose indications, rituximab regimen, and titration of immunosuppression. In particular, monitoring practices for B cells and Ig levels were inconsistent. Fourth, both the original rituximab product and its biosimilars were used, according to local policies. There are currently no clinical data demonstrating equivalent treatment efficacy and safety profiles for the biosimilars. Finally, only 6% of patients were Black, and this may affect the generalization of our findings. African patients may respond differently to rituximab, because they are known to have higher rates of steroid resistance and an underlying renal histology other than minimal change disease.49
In conclusion, children receiving repeated courses of rituximab experience an improving clinical response and may attain long-term remission. However, the duration of B-cell depletion remains constant. Side effect profiles appear acceptable even after several courses of rituximab. These reassuring findings clearly support repeated rituximab use in children with FRSDNS, which are often required. In addition, maintenance immunosuppression is a viable option to extend relapse-free remission. Prospective trials are needed to examine the immunologic changes and identify the optimal treatment approach in this patient population.
Disclosures
O. Boyer reports serving in an advisory or leadership role for Alexion, Alnylam, Chiesi, Takeda, and Travere; having consultancy agreements with Biocodex; and participating in a board for rituximab use in lupus for Roche in 2020. M. Colucci reports having other interests in, or relationships with, Associazione per la Cura del Bambino Nefropatico ONLUS, Fondazione Bambino Gesù, and Italian Ministry of Health for Institutional Research (Ricerca Corrente). D.S. Gipson reports serving as a consultant, through her University of Michigan employer, for AstraZeneca, Boehringer-Ingelheim, Genentech/Roche, Goldfinch Bio, Travere, and Vertex; serving in an advisory or leadership role for AstraZeneca, Goldfinch Bio, and Vertex; receiving research funding (to the University of Michigan) from Boehringer-Ingelheim, Goldfinch Bio, Novartis, Reata, and Travere; and having other interests in, or relationships with, the Nephrotic Syndrome Patient Reported Outcome Consortium (public-private partnership, with GlaxoSmithKline, Goldfinch Bio, Pfizer, NephCure Kidney International, and Zyversa). R. Hamada reports receiving honoraria from Alexion Pharmaceuticals Inc., Asahi Kasei Pharma Corporation, Chugai Pharmaceutical Co. Ltd., and Teijin Pharma Ltd.; and receiving research grants from Chugai Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Takeda Pharmaceutical Co., and Teijin Pharma Ltd. J. Hogan reports receiving honoraria from Alnylam, Chiesi, and Travere; and receiving research funding from CareDx. K. Ishikura reports receiving honoraria from Asahi Kasei Pharma Corporation, Chugai Pharmaceutical Co. Ltd., Novartis International AG, Otsuka Pharmaceutical Co. Ltd., Pfizer Inc., Teijin Pharma Limited, and Vifor (International) AG, and Zenyaku Kogyo Co. Ltd.; receiving research funding from Asahi Kasei Pharma Corporation, Japan Blood Products Organization, JCR Pharmaceuticals Co. Ltd., Novartis International AG, Otsuka pharmaceutical Co. Ltd., Shionogi Co. Ltd., Teijin Pharma Limited, and The Morinaga Foundation for Health and Nutrition; and receiving grants and lecture fees from Chugai Pharmaceutical Co. Ltd. and Zenyaku Kogyo Co. Ltd. K. Kamei reports receiving research funding from Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co., Otsuka Pharmaceutical Co. Ltd., Public Foundation of Vaccination Research Center, Shionogi Co. Ltd., Taiju Life Social Welfare Foundation, Teijin Pharma Ltd., and Terumo Foundation for Life Sciences and Arts; and receiving honoraria from Baxter Ltd., Tanabe Mitsubishi Pharma, and Zenyaku Kogyo Co. Ltd. M. Kemper reports receiving honoraria from Alynam, and serving in an advisory or leadership role for Pediatric Nephrology. R.S. Parekh reports serving in an advisory or leadership role for Bishop Strachan School (board member), CJASN (as associate editor), Conference of Independent Schools of Ontario (board member), Coramed and SpineFx (via spouse, who is officer), and International Society of Nephrology (council); receiving research funding from the Canadian Institute of Health Research, National Institutes of Health, and Ontario Ministry; having patents or royalties (via spouse) with Coramed, IZI, and SpineFx; and having ownership interest in Coramed (stock), SpineFx, and Synaptive (stock). S. Radhakrishnan reports being employed by, and having ownership interest in, Aurora Cannabis. K. Tullus reports having consultancy agreements with, receiving honoraria from, and serving in an advisory or leadership role for Travere. M. Vivarelli reports having consultancy agreements with Achillion Pharmaceuticals, Alexion Pharmaceuticals, Apellis Pharmaceuticals, BioCryst Pharmaceuticals, Novartis Pharmaceuticals, Roche Pharmaceuticals, and Travere Pharmaceuticals; receiving research funding from Alexion Pharmaceuticals and Chemocentrix (for clinical studies), Bayer, and Novartis (for participation in clinical studies); having other interests in, or relationships with, the 2022 European Society of Pediatric Nephrology Meeting (as chair of the scientific committee), the European Rare Kidney diseases Network (as chair), European Society of Pediatric Nephrology Working Group on Glomerular Disease (as chair), National Kidney Foundation (as cochair of a white paper on atypical hemolytic uremic syndrome nomenclature), and Working Group on Immune Glomerulopathies; and serving on the editorial boards of Frontiers in Pediatrics – Pediatric Nephrology, Kidney International, and Pediatric Nephrology. H.K. Yap reports having other interests in, or relationships with, the National Kidney Foundation, Singapore. All remaining authors have nothing to disclose.
Funding
None. Costs of rituximab were covered by the involved hospitals or patients.
Supplementary Material
Acknowledgments
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
The Paediatric Nephrology Centre in Hong Kong Children’s Hospital and Department of Paediatric Nephrology in Great Ormond Street Hospital served as the study coordinating centers. A. Angeletti, Z. Arslan, B. Basu, O. Boyer, C.-Y. Chan, E.Y.-H. Chan, M. Colucci, G. Dorval, C. Dossier, S. Drovandi, G.M. Ghiggeri, D.S. Gipson, R. Hamada, J. Hogan, K. Ishikura, K. Kamei, M. Kemper, A.L.-t. Ma, R. Parekh, S. Radhakrishnan, P. Saini, Q. Shen, R. Sinha, C. Subun, S. Teo, M. Vivarelli, H. Webb, H. Xu, H.K. Yap, and E.L.M. Yu reviewed and edited the manuscript; A. Angeletti, Z. Arslan, B. Basu, O. Boyer, C.-Y. Chan, E.Y.-H. Chan, M. Colucci, G. Dorval, C. Dossier, S. Drovandi, G.M. Ghiggeri, D.S. Gipson, R. Hamada, J. Hogan, K. Ishikura, K. Kamei, M. Kemper, A.L.-t. Ma, R. Parekh, S. Radhakrishnan, P. Saini, Q. Shen, R. Sinha, C. Subun, S. Teo, M. Vivarelli, H. Webb, H. Xu, and H.K. Yap were responsible for data curation; E.Y.-H. Chan and K. Tullus conceptualized the study, wrote the original draft, and were responsible for investigation and project administration; E.Y.-H. Chan, K. Tullus, and E.L.M. Yu were responsible for formal analysis, methodology, and validation; K. Tullus provided supervision; and E.Y.-H. Chan, K. Tullus, and E.L.M. Yu were responsible for visualization.
Data Sharing Statement
All data is included in the manuscript and/or supporting materials.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021111472/-/DCSupplemental.
Supplemental Figure 1. Kaplan-Meier analysis in subgroups of children receiving repeated courses of rituximab.
Supplemental Table 1. Definitions of nephrotic syndrome in children.
Supplemental Table 2. Time intervals between courses of rituximab according to different re-dose indications.
Supplemental Table 3. Dosing regimen used in 1149 courses of rituximab.
Supplemental Table 4. Association with relapse in 1146 courses of rituximab: Multiple mixed effects Cox regression model with different course as reference group.
Supplemental Table 5. Details of miscellaneous adverse events following repeated courses of rituximab.
Supplemental Table 6. Clinical characteristics of (A) patients and (B) rituximab episodes with and without significant hypogammaglobulinaemia, neutropenia and agranulocytosis.
Supplemental Table 7. Clinical outcomes following B-cell repletion.
References
- 1.Webb NJA, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. ; PREDNOS Collaborative Group : Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: Phase III randomised controlled trial and economic evaluation. BMJ 365: l1800, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter SA, Mistry S, Fitzpatrick J, Banh T, Hebert D, Langlois V, et al. : Prediction of short-and long-term outcomes in childhood nephrotic syndrome. Kidney Int Rep 5: 426–434, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iijima K, Sako M, Nozu K: Rituximab for nephrotic syndrome in children. Clin Exp Nephrol 21: 193–202, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. ; Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group : Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384: 1273–1281, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, et al. : Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 26: 2259–2266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, et al. ; Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group : Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25: 850–863, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan EY, Webb H, Yu E, Ghiggeri GM, Kemper MJ, Ma AL, et al. : Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int 97: 393–401, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Chan EY, Tullus K: Rituximab in children with steroid sensitive nephrotic syndrome: In quest of the optimal regimen. Pediatr Nephrol 36: 1397–1405, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Fujinaga S, Sakuraya K, Yamada A, Urushihara Y, Ohtomo Y, Shimizu T: Positive role of rituximab in switching from cyclosporine to mycophenolate mofetil for children with high-dose steroid-dependent nephrotic syndrome. Pediatr Nephrol 30: 687–691, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Webb H, Jaureguiberry G, Dufek S, Tullus K, Bockenhauer D: Cyclophosphamide and rituximab in frequently relapsing/steroid-dependent nephrotic syndrome. Pediatr Nephrol 31: 589–594, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, et al. : Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 27: 1910–1915, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Prytuła A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, et al. : Rituximab in refractory nephrotic syndrome. Pediatr Nephrol 25: 461–468, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, et al. : Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: A randomized clinical trial. JAMA Pediatr 172: 757–764, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonanni A, Calatroni M, D’Alessandro M, Signa S, Bertelli E, Cioni M, et al. : Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 84: 1238–1249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamei K, Ogura M, Sato M, Ito S, Ishikura K: Infusion reactions associated with rituximab treatment for childhood-onset complicated nephrotic syndrome. Pediatr Nephrol 33: 1013–1018, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, et al. : Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: Case series and review of literature. Nephrol Dial Transplant 30: 91–96, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Colucci M, Carsetti R, Serafinelli J, Rocca S, Massella L, Gargiulo A, et al. : Prolonged impairment of immunological memory after anti-CD20 treatment in pediatric idiopathic nephrotic syndrome. Front Immunol 10: 1653, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmentier C, Delbet J-D, Decramer S, Boyer O, Hogan J, Ulinski T: Immunoglobulin serum levels in rituximab-treated patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 35: 455–462, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 20.Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM: Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11: 710–720, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trautmann A, Vivarelli M, Samuel S, Gipson D, Sinha A, Schaefer F, et al. ; International Pediatric Nephrology Association : IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 35: 1529–1561, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T, Okamoto T, Sato Y, Yamazaki T, Hayashi A, Aoyagi H, et al. : Periodically repeated rituximab administrations in children with refractory nephrotic syndrome: 2-Year multicenter observational study. Pediatr Nephrol 34: 87–96, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Kamei K, Ishikura K, Sako M, Aya K, Tanaka R, Nozu K, et al. ; Rituximab for Childhood-Onset Refractory Nephrotic Syndrome (RCRNS) Study Group : Long-term outcome of childhood-onset complicated nephrotic syndrome after a multicenter, double-blind, randomized, placebo-controlled trial of rituximab. Pediatr Nephrol 32: 2071–2078, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Hogan J, Dossier C, Kwon T, Macher M-A, Maisin A, Couderc A, et al. : Effect of different rituximab regimens on B cell depletion and time to relapse in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 34: 253–259, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Fujinaga S, Hirano D, Mizutani A, Sakuraya K, Yamada A, Sakurai S, et al. : Predictors of relapse and long-term outcome in children with steroid-dependent nephrotic syndrome after rituximab treatment. Clin Exp Nephrol 21: 671–676, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Park E, Hyun HS, Cho MH, Ahn YH, Choi HJ, et al. : Long-term repeated rituximab treatment for childhood steroid-dependent nephrotic syndrome. Kidney Res Clin Pract 36: 257–263, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sellier-Leclerc A-L, Baudouin V, Kwon T, Macher M-A, Guérin V, Lapillonne H, et al. : Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood--follow-up after CD19 recovery. Nephrol Dial Transplant 27: 1083–1089, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Subun C, Suwannahitatorn P, Webb H, Tullus K: Rituximab in childhood steroid-sensitive nephrotic syndrome: Are multiple subsequent courses safe and effective? Arch Dis Child 106: 815–818, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Delbet JD, Leclerc G, Ulinski T: Idiopathic nephrotic syndrome and rituximab: May we predict circulating B lymphocytes recovery? Pediatr Nephrol 34: 529–532, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Chanchlani R, Parekh RS: Ethnic differences in childhood nephrotic syndrome. Front Pediatr 4: 39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamei K, Ogura M, Sato M, Sako M, Iijima K, Ito S: Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol 31: 89–95, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Sinha A, Bhatia D, Gulati A, Rawat M, Dinda AK, Hari P, et al. : Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant 30: 96–106, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Iijima K, Sako M, Oba M, Tanaka S, Hamada R, Sakai T, et al. ; Japanese Study Group of Kidney Disease in Children : Mycophenolate mofetil after rituximab for childhood-onset complicated frequently-relapsing or steroid-dependent nephrotic syndrome. J Am Soc Nephrol 33: 401–419, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan EY, Ma AL, Tullus K: Hypogammaglobulinaemia following rituximab therapy in childhood nephrotic syndrome [published online ahead of print January 9, 2022]. Pediatr Nephrol 10.1007/s00467-021-05345-9 [DOI] [PubMed] [Google Scholar]
- 35.Tsutsumi Y, Kanamori H, Mori A, Tanaka J, Asaka M, Imamura M, et al. : Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf 4: 599–608, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Chaumais M-C, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, et al. : Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24: 1753–1755, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Boren EJ, Cheema GS, Naguwa SM, Ansari AA, Gershwin ME: The emergence of progressive multifocal leukoencephalopathy (PML) in rheumatic diseases. J Autoimmun 30: 90–98, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Gea-Banacloche JC: Rituximab-associated infections. Semin Hematol 47: 187–198, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Sellier-Leclerc A-L, Belli E, Guérin V, Dorfmüller P, Deschênes G: Fulminant viral myocarditis after rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol 28: 1875–1879, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Ito S, Ogura M, Kamei K, Miyairi I, Miyata I, et al. : Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol 28: 145–149, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Peuvrel L, Chiffoleau A, Quéreux G, Brocard A, Saint-Jean M, Batz A, et al. : Melanoma and rituximab: An incidental association? Dermatology 226: 274–278, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Velter C, Pagès C, Schneider P, Osio A, Brice P, Lebbé C: Four cases of rituximab-associated melanoma. Melanoma Res 24: 401–403, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, et al. : Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: A 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol 29: 814–824, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Marlais M, Wlodkowski T, Al-Akash S, Ananin P, Bandi VK, Baudouin V, et al. : COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child 106: 798–801, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha R, Marlais M, Sarkar S, Obukhova V, Lucchetti L, Vasudevan A, et al. : Impact of COVID-19 pandemic on use of rituximab among children with difficult nephrotic syndrome [published online ahead of print September 21, 2021]. Pediatr Res 10.1038/s41390-021-01744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma AL-T, Leung D, Chan EY, Chim S, Cheng S, Ho FT, et al. : Antibody responses to 2 doses of mRNA Covid vaccine in pediatric renal patients [published online ahead of print February 26, 2022]. Kidney Int 10.1016/j.kint.2022.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoki Y, Kamei K, Nishi K, Sato M, Ogura M, Ishiguro A: Incidence and risk factors of rituximab-associated hypogammaglobulinemia in patients with complicated nephrotic syndrome [published online ahead of print October 4, 2021]. Pediatr Nephrol 10.1007/s00467-021-05304-4 [DOI] [PubMed] [Google Scholar]
- 48.Wijetilleka S, Jayne DR, Mukhtyar C, Ala A, Bright PD, Chinoy H, et al. : Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases. Rheumatology (Oxford) 58: 889–896, 2019 [DOI] [PubMed] [Google Scholar]
- 49.Wine R, Vasilevska-Ristovska J, Banh T, Knott J, Noone D, Gbadegesin R, et al. : Trends in the epidemiology of childhood nephrotic syndrome in Africa: A systematic review. Glob Epidemiol 3: 100061, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.