Significance Statement
Current recommendations suggest discontinuation of immunosuppressants 1 year after kidney transplant failure. In this first prospective multicenter study of 269 patients with kidney transplant failure in 16 Canadian centers, most patients were prescribed immunosuppressants for longer than 2 years. Continued use of immunosuppressants was not associated with an increased risk of death or hospitalized infection. However, the continued use of immunosuppressants did not prevent rejection of the failed allograft or an increase in anti-HLA antibodies, possibly due to inadequate drug exposure. The findings challenge current recommendations and highlight the need for a controlled trial of immunosuppressant use in patients with transplant failure.
Keywords: kidney transplantation, rejection, survival, transplant nephrectomy
Abstract
Background
Patients with kidney transplant failure have a high risk of hospitalization and death due to infection. The optimal use of immunosuppressants after transplant failure remains uncertain and clinical practice varies widely.
Methods
This prospective cohort study enrolled patients within 21 days of starting dialysis after transplant failure in 16 Canadian centers. Immunosuppressant medication use, death, hospitalized infection, rejection of the failed allograft, and anti-HLA panel reactive antibodies were determined at 1, 3, 6, and 12 months and and then twice yearly until death, repeat transplantation, or loss to follow-up.
Results
The 269 study patients were followed for a median of 558 days. There were 33 deaths, 143 patients hospitalized for infection, and 21 rejections. Most patients (65%) continued immunosuppressants, 20% continued prednisone only, and 15% discontinued all immunosuppressants. In multivariable models, patients who continued immunosuppressants had a lower risk of death (hazard ratio [HR], 0.40; 95% confidence interval [CI], 0.17 to 0.93) and were not at increased risk of hospitalized infection (HR, 1.81; 95% CI, 0.82 to 4.0) compared with patients who discontinued all immunosuppressants or continued prednisone only. The mean class I and class II panel reactive antibodies increased from 11% to 27% and from 25% to 47%, respectively, but did not differ by immunosuppressant use. Continuation of immunosuppressants was not protective of rejection of the failed allograft (HR, 0.81; 95% CI, 0.22 to 2.94).
Conclusions
Prolonged use of immunosuppressants >1 year after transplant failure was not associated with a higher risk of death or hospitalized infection but was insufficient to prevent higher anti-HLA antibodies or rejection of the failed allograft.
In 2018, over 7000 patients returned to dialysis after a failed allograft in the United States making transplant failure the fourth leading individual cause of dialysis initiation.1,2 Patients with transplant failure have a higher risk of death and hospitalization predominantly due to infection and cardiovascular disease.3–9
The optimal immunosuppressant management of patients with transplant failure remains uncertain and there is wide variation in clinical practice.10,11 Retention of the failed allograft may require immunosuppressant medications to prevent rejection, graft intolerance syndrome, and development of anti-HLA antibodies. These considerations must be weighed against the risk of infection, cardiovascular disease, and malignancy associated with the use of immunosuppressants. Nephrectomy may allow discontinuation of immunosuppressants, but is not a benign surgical procedure and removal of the failed allograft may paradoxically increase anti-HLA antibodies limiting opportunities for repeat transplantation.12–15 Current opinion-based recommendations from the American Society of Transplantation kidney pancreas community of practice suggest gradual withdrawal of all immunosuppressant drugs in repeat transplant candidates in the first year after transplant failure while monitoring patients for changes in sensitization and symptoms of graft intolerance syndrome.16 The British Transplant Society recommends all immunosuppressants except steroids be stopped immediately after transplant nephrectomy and continuation of immunosuppression in patients with an in situ allograft when there is a possibility of repeat transplantation within 1 year after transplant failure.17
Previous observational studies have been limited by their retrospective design and limited sample size.18–21 Similarly, registry analyses are limited by the absence of information about immunosuppressant medication use in dialysis-treated patients with transplant failure.4,5,14,22,23 The objective of this study was to determine the association between immunosuppressant use and the outcomes of death, hospitalized infection, rejection of the failed allograft, and development of anti-HLA antibodies.
Methods
Study Population
This was a prospective multicenter cohort study of patients with transplant failure in Canada between July 2011 and April 2016. The study included adult patients (≥18 years) who initiated maintenance dialysis treatment after failure of a first kidney-only transplant. Study participants were enrolled within 21 days of starting maintenance dialysis treatment after transplant failure in 16 transplant centers. All patients provided written informed consent. The study protocol was approved by the research ethics board at each site as well as the coordinating center (Ottawa Hospital Research Ethics Board #2009747-01H).
Data Collection
Study visits were conducted at baseline and at 1, 3, 6, and 12 months after the initiation of maintenance dialysis, and then every 6 months until the patient left the study, died, or underwent repeat transplantation, or end of study. At each study visit a blood sample was obtained for the measurement of panel reactive antibodies (PRAs). Demographic characteristics including self-reported race and ethnicity (collected for this study to inform generalizability), medical history, self-reported functional status, immunosuppressive medication use, and pertinent laboratory data were recorded at baseline. Immunosuppressant medication use was determined by patient interview and review of medical and pharmacy records. Functional status was ascertained by a nonexternally validated study-specific questionnaire that asked how far they could walk at their own pace before needing to stop because of weakness, shortness of breath, chest pain, or leg pain. Information on hospitalizations, medication use, functional status, allograft nephrectomy, and acute rejection was ascertained at each follow-up visit.
Outcomes
The study outcomes included death, in-patient hospitalization for infection, rejection of the failed allograft, and PRAs. Death was identified through monthly contact with study coordinators at treating dialysis centers. Hospitalizations for infection were identified through patient interviews and medical record review. Hospitalized infection was included as an outcome if infection was the primary or secondary discharge diagnosis, and confirmatory laboratory/imaging evidence of infection (e.g., positive blood culture, chest radiograph indicating pneumonia) was present. Rejection of the failed allograft was identified through patient interview and medical record review. Rejections required documentation of compatible signs/symptoms (e.g., allograft tenderness) that began after maintenance dialysis was initiated and either an increase in prescribed immunosuppressants was needed or a transplant nephrectomy was performed. Serum samples for PRAs were sent to the University of Alberta Hospital histocompatibility laboratory for testing of both class I and class II anti-HLA antibodies. Samples were tested by FlowPRA (One Lambda, West Hills, CA) as per the manufacturer’s instructions. Beads were run on BD FACS Canto II. Results were reported as class I and II PRAs.
Statistical Analyses
Descriptive statistics were used to summarize the data. A Cox proportional hazards model was used to determine the association between immunosuppressant medication use and death. Immunosuppressant medication use was treated as a time-varying independent variable. “Discontinuation of immunosuppressants” was defined as not taking any immunosuppressant medications. “Continuation of prednisone only” was defined as not taking any immunosuppressant except prednisone. “Continued immunosuppression” was defined as taking an immunosuppressant other than prednisone alone (e.g., cyclosporine, tacrolimus, mycophenolate mofetil, sirolimus). Time zero was defined as the date the patient entered the study which occurred within 21 days of initiating maintenance dialysis after transplant failure. The proportional hazard assumptions were verified by fitting interaction terms with time and studying the graphs of the Schoenfeld residuals. The multivariable analyses controlled for age, duration of transplant survival, and presence of diabetes at baseline. Patients were censored at repeat transplantation, lost to follow-up, or end of study. For the outcomes of hospitalized infection and rejection of the failed allograft, a competing risk analysis was performed using a cause-specific hazards model, estimating hazards with Cox regression and censoring patients with a competing event (death) at the time of occurrence. In a sensitivity analysis of hospitalization for infection, we did not censor follow-up at the first event and used an Andersen–Gill model for multiple events.
Nonparametric spline regression was used to study the association between PRA and immunosuppressant use over time. Spline regression allows for a flexible model without making any assumptions about its shape (e.g., linear, quadratic, piecewise function). The smoothness of the curve is influenced mostly by the number of knots and its position. In these models, we used natural splines with restricted maximum likelihood estimation, using two quantile-spaced knots, to allow flexible spline without overfitting the data. In a sensitivity analysis, we repeated the spline regression stratified by PRA above or below 20% at baseline. SAS Software Institute version 9.4 was used for the survival analysis and R Software 3.6.1 (R foundation, Vienna, Austria) was used to perform the splines analysis, with the packages lme4, lmerTest, and splines.
Results
Among the 580 eligible patients, 269 (46%) provided informed consent and were enrolled in the study. The reason for patient exclusion was patient refusal due to perceived burden of study participation. In addition, there was one patient who regained allograft function. Baseline characteristics of the study patients are shown in Table 1 and the number of eligible and enrolled patients in each of the 16 Canadian transplant centers is shown in Supplemental Table 1. The mean (SD) age was 52 (15) years, 170 (63%) were male and 167 (62%) of the patients were white. A majority (76%) had mild or no limitation of normal activities at baseline (Supplemental Figure 1) and a minority, 27%, had a history of cardiac disease. The median duration of transplant survival was 10.8 (interquartile range [IQR], 4.9–16.1) years. Fifteen patients had transplant survival of <1 year. The most frequent cause of allograft failure was interstitial fibrosis and tubular atrophy (27%). The study cohort included eight patients with primary nonfunction, 30 patients with transplant failure attributed to acute T cell or acute humoral rejection, and 49 patients with chronic humoral rejection/transplant glomerulopathy. The first dialysis treatment after allograft failure occurred during a hospital admission for 135 (50%) patients. Peritoneal dialysis was the initial dialysis modality for 46 (17%) patients and this proportion increased to 20% by the end of the study (Supplemental Figure 2). At the time of allograft failure, 136 (51%) patients had a central venous catheter for dialysis access, but this proportion decreased over time as the prevalence of fistula use increased (Supplemental Figure 3).
Table 1.
Baseline patient characteristics
| Baseline Characteristic | Entire Cohort (n=269) |
|---|---|
| Age, yr, mean (SD) | 51.8 (15.4) |
| Male, n (%) | 170 (63.2) |
| Race or ethnicity, n (%) | |
| Aboriginal | 13 (4.8) |
| Asian | 25 (9.3) |
| Black | 25 (9.3) |
| Indian subcontinent | 16 (5.9) |
| Other including multiethnic/preference not to report | 23 (8.6) |
| White | 167 (62.1) |
| Donor source, n (%) | |
| Deceased | 191 (71) |
| Living | 78 (29) |
| Duration of allograft survival before transplant failed | |
| Median (IQR), yr | 10.8 (4.9–16.1) |
| Transplant duration, n (%) | |
| <1 yr | 15 (5.6) |
| >1 yr | 254 (94.4) |
| Transplant year, n (%) | |
| Before 1990 | 16 (5.9) |
| 1990–2000 | 92 (34.2) |
| 2001–2010 | 126 (46.8) |
| 2011–2016 | 35 (13) |
| Primary cause of allograft failure, n (%) | |
| Interstitial fibrosis and tubular atrophy (chronic allograft nephropathy) | 73 (27.1) |
| Other | 51 (19) |
| Chronic antibody-mediated rejection/transplant glomerulopathy | 49 (18.2) |
| Recurrent GN | 24 (8.9) |
| Acute T cell–mediated rejection | 23 (8.6) |
| Unknown | 16 (5.9) |
| Primary nonfunction | 8 (3.0) |
| Acute antibody-mediated rejection | 7 (2.6) |
| Polyomavirus nephropathy | 6 (2.2) |
| Calcineurin inhibitor toxicity | 4 (1.5) |
| Arterial or venous thrombosis | 3 (1.1) |
| De novo GN | 3 (1.1) |
| Obstruction | 2 (0.7) |
| Current immunosuppressive medications, n (%)a | |
| Prednisone | 233 (86.6) |
| Tacrolimus | 142 (52.8) |
| Mycophenolate mofetil | 76 (28.3) |
| Cyclosporine | 53 (19.7) |
| Mycophenolate sodium | 31 (11.5) |
| Azathioprine | 27 (10) |
| Sirolimus | 12 (4.5) |
| Other | 3 (1.11) |
| Treatment for acute rejection any time before to allograft failure, n (%) | |
| Yes | 116 (43.1) |
| No | 109 (40.5) |
| Unknown | 44 (16.4) |
| Comorbid conditions, n (%) | |
| Cancer | 58 (21.6) |
| Cardiovascular disease | 72 (26.8) |
| Diabetes mellitus | 87 (32.3) |
| Smoking, n (%) | |
| Current | 39 (14.5) |
| Former | 83 (30.9) |
| Never | 147 (54.6) |
| Cause of ESKD, n (%) | |
| Other | 95 (35.3) |
| GN | 81 (30.1) |
| Diabetes mellitus | 38 (14.1) |
| Polycystic kidney disease | 24 (8.9) |
| Unknown | 18 (6.7) |
| Hypertension | 13 (4.8) |
| Laboratory parameters, mean (SD) | |
| Hemoglobin, g/L | 95.8 (16.9) |
| Parathyroid hormone, pmol/L | 65.5 (97.5) |
| Serum albumin, g/L | 33.6 (5.7) |
| Calcium (total uncorrected), mmol/L | 2.2 (0.2) |
| Phosphorus, mmol/L | 1.6 (0.6) |
Proportions may total >100% as multiple selections were accepted for this field.
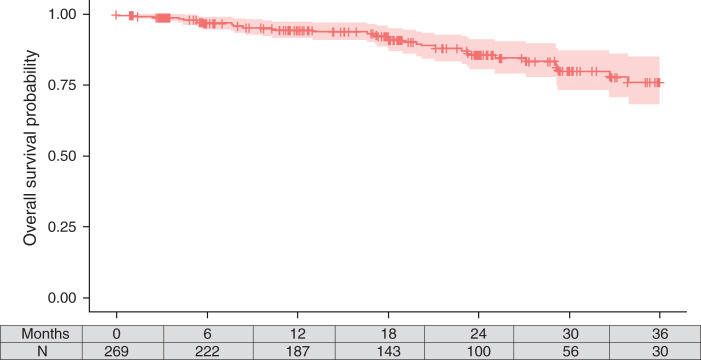
The median study follow-up was 558 days (IQR, 298–875). Only 38 (14%) patients underwent repeat transplantation after a median of 496 (IQR, 307–716) days after study enrollment, 33 (12%) died (n=13 died in the first year after transplant failure), 13 were lost to follow-up, and seven withdrew from the study. Patient survival is shown in Figure 1; the 1-year survival probability was 94% (95% confidence interval [CI], 92 to 98). The unadjusted death rate was 7.51 (95% CI, 5.17 to 10.5) per 100 patient-years. The leading causes of death were cardiac (18%), sepsis (18%), and other (33%). There were no deaths in the 15 patients with transplant survival <1 year.
Figure 1.
Kaplan–Meier estimate of survival among study cohort.
One hundred and seventy-two (64%) patients had 412 hospitalizations during the study follow-up period. The overall hospitalization rate was 93.7, (95% CI, 84.9 to 103.3) per 100 patient-years. There were 143 hospitalizations for infection (35%) followed by 56 hospitalizations for cardiovascular disease (14%). The 143 hospitalizations for infection occurred among 91 patients. The majority of hospitalized infections (n=78) occurred within the first year of follow-up and the median time to hospitalized infection was 271 days (IQR, 64–472). There were 21 rejection events in 18 patients. The median time to rejection was 271 days (IQR, 86–445). Of the 18 patients with rejection, nine required allograft nephrectomy. An additional 12 patients underwent nephrectomy for reasons other than rejection. There were 20 cancer diagnoses involving 18 patients.
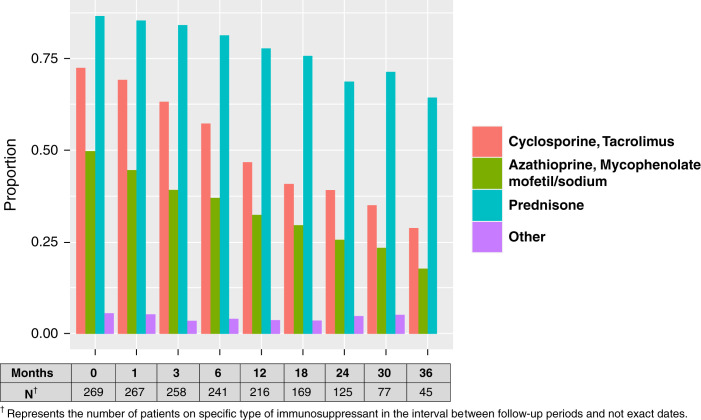
Figure 2 shows the use of individual immunosuppressant drugs during follow-up. At 2 years after allograft failure, over 60% of patients were still taking prednisone, nearly 40% were taking a calcineurin inhibitor (either cyclosporine or tacrolimus) and 25% were taking an antiproliferative agent (mycophenolate or azathioprine). At study entry, 97% (n=262) of patients were taking immunosuppressants. During follow-up, 15% of patients discontinued all immunosuppressants after a median of 361 days (IQR, 96–548). At study entry 8% of patients had discontinued all immunosuppressant medication except prednisone and this proportion increased to 20% during follow-up. Most patients (65%) were taking immunosuppressant medications other than prednisone alone. The median daily doses of cyclosporine, tacrolimus, mycophenolate mofetil, mycophenolate sodium, azathioprine, and prednisone are shown in Supplemental Figure 4. The characteristics of patients grouped by immunosuppressant usage during follow-up is shown in Supplemental Table 2.
Figure 2.
Proportion of patients taking individual immunosuppressant medications over a 36-month follow-up period.
Patient Survival
Continuation of immunosuppressants was associated with a lower risk of death (adjusted hazard ratio [HR], 0.4; 95% CI, 0.17 to 0.93) compared with discontinuation of all immunosuppressants or use of prednisone only. The full model results are shown in Supplemental Table 3. In a separate time-dependent multivariable model, allograft nephrectomy was not associated with lower risk of death (adjusted HR, 0.88; 95% CI, 0.20 to 3.81).
Hospitalization for Infection
Continuation of immunosuppressants was not associated with higher risk of hospitalized infection (adjusted HR, 1.81; 95% CI, 0.80 to 4.0) compared with discontinuation of immunosuppressants or use of prednisone only. A sensitivity analysis which included all episodes of hospitalized infection, not just the first episode per patient, did not change these findings (adjusted HR, 1.31; 95% CI, 0.75 to 2.29).
In a separate time-dependent multivariable model, allograft nephrectomy (adjusted HR, 0.54; 95% CI, 0.19 to 1.56) was also not associated with a lower risk of hospitalized infection. A sensitivity analysis which included all episodes of hospitalized infection, not just the first episode per patient, produced similar findings (adjusted HR, 0.71; 95% CI, 0.28 to 1.82).
Acute Rejection
Continued use of immunosuppressants was not associated with a lower risk of rejection (adjusted HR, 0.81; 95% CI, 0.22 to 2.94) compared with discontinuation of all immunosuppressants or use of prednisone only.
Changes in PRAs after Allograft Failure
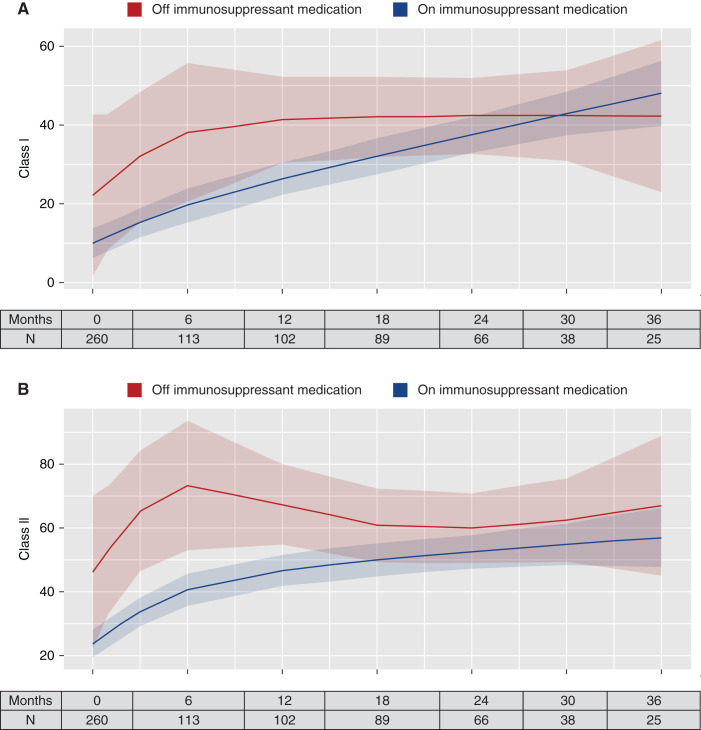
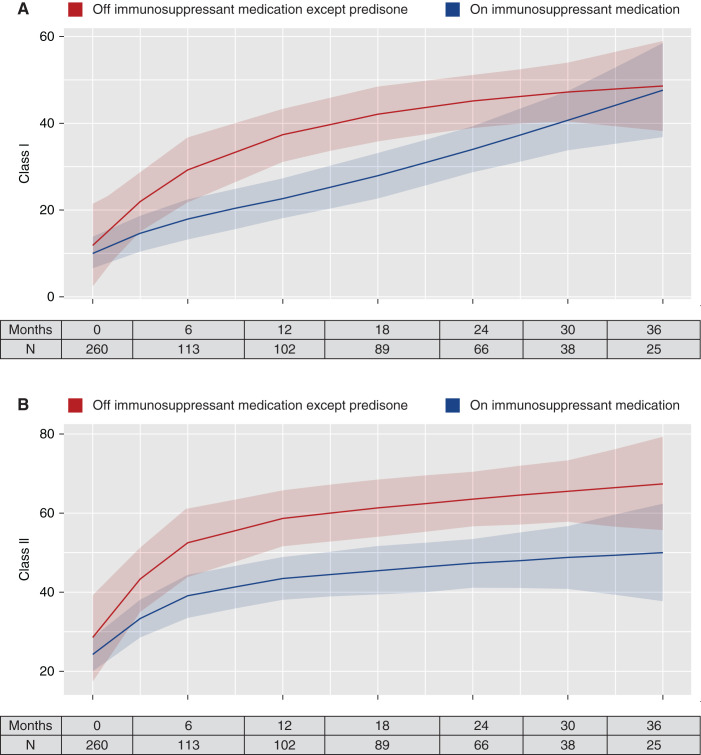
Anti-HLA antibodies increased during follow-up. The mean (SD) percentage of class I PRAs at baseline was 11% (20%) and at the end of study was 27% (37%). The mean (SD) percentage of class II PRAs at baseline was 25% (32%) and at the end of study was 47% (40.6%). Figures 3 and 4 display the temporal changes of PRAs over time. The predicted class I PRAs (Figure 3A) and class II PRAs (Figure 3B) were both lower for patients who continued immunosuppressants compared with those who discontinued all immunosuppressants. The differences, however, were not statistically significant. A similar pattern was seen for patients who discontinued all immunosuppression except low-dose prednisone. The predicted class I PRA (Figure 4A) and class II PRA (Figure 4B) percentages were lower for those who continued immunosuppressants compared with patients who discontinued immunosuppressants or continued prednisone only; however, the differences were not statistically significant. Similar results were obtained after stratifying patients based on PRAs above or below 20% at baseline (Supplemental Figures 5–8).
Figure 3.
Temporal change in PRAs for those who discontinued all immunosuppressants. (A) Class I PRAs; (B) class II PRAs. Solid lines represent the predicted PRA level at the time point in the x axis, estimated using nonparametric spline regression using the restricted maximum likelihood criteria with two quantile-spaced knots. The shaded areas are 95% CIs. The tables represent the number of patients who had a value at the time point in the x axis.
Figure 4.
Temporal change in PRAs for those who discontinued all immunosuppressants except low-dose prednisone. (A) Class I PRAs; (B) class II PRAs. Solid lines represent the predicted PRA level at the time point in the x axis, estimated using nonparametric spline regression using the restricted maximum likelihood criteria with two quantile-spaced knots. The shaded areas are 95% CIs. The tables represent the number of patients who had a value at the time point in the x axis.
Discussion
In this prospective study of adult Canadian patients with transplant failure, we found that continuation of all immunosuppressant medications was associated with a lower risk of death potentially due to suppression of inflammation after allograft failure. Continued use of immunosuppressant medications was not associated with higher risk of hospitalized infection or protective of rejection of the failed allograft. Anti-HLA antibodies increased during follow-up but were not associated with immunosuppressant use. The findings challenge current recommendations to discontinue immunosuppressant medications 1 year after allograft failure and paradoxically raise concerns that the current prolonged use of immunosuppressants after transplant failure in Canadian patients may be of insufficient intensity to prevent rejection and sensitization. The observational study findings do not provide definitive evidence to extend the duration of treatment with immunosuppressant medication after transplant but do bring into question current recommendations and indicate the need for a prospective controlled trial.
The death rate in this contemporary patient cohort (7.51 per 100 patient-years) was higher than that reported in a previous registry-based study of patients with transplant failure in Canada between 1994 and 2000 (5.14 per 100 patient-years) and similar to that reported in a study of patients with transplant failure in the United States between 1988 and 1998 and in a more recent study from the province of Alberta (7.3 per 100 patient-years).4,5,9 The higher rate of death may reflect more complete ascertainment of patient death in dialysis-treated patients than in the previous registry-based Canadian analysis.5 Consistent with previous studies, mortality and hospitalizations were highest in the first year after allograft failure. The lower risk of death associated with continued use of immunosuppressant medications may be due to suppression of chronic inflammation. Alternatively, the association may be the result of residual confounding by indication, with immunosuppressants preferentially continued in healthier patients who may be considered repeat transplant candidates. There were some differences in the characteristics of patients with different immunosuppressant use during follow-up and our multivariable models were limited by the number of events to include adjustment for patient age, diabetes, and duration of allograft survival. Continued use of immunosuppressants was not associated with a statistically significant increased risk of hospitalization but the results may be of clinical significance. It is important to recognize that hospitalization practices may vary and therefore this observation may not be generalizable beyond this study population.
A unique strength of this study is the prospective documentation of immunosuppressant use. Most patients were prescribed immunosuppressants beyond the first year after transplant failure with 40% still prescribed a calcineurin inhibitor and 25% still prescribed azathioprine or mycophenolate mofetil 2 years after transplant failure. The continuation of immunosuppressants beyond the first year differs from recent recommendations.16 The continued use of immunosuppressant medications in this study beyond the first year may be influenced by the fact that most study patients were likely repeat transplant candidates and by continued health insurance coverage of immunosuppressant medications after transplant failure in Canada. Despite the continued use of immunosuppressants beyond the first year after transplant failure, study patients still experienced rejection episodes and sensitization. These findings are somewhat unexpected considering the relatively long duration of transplant survival and low proportion of patients with early allograft failure in whom the risk rejection and sensitization may be greater. The finding that continuation of immunosuppressants was not protective of rejection of the failed allograft, along with the finding that anti-HLA antibodies were nonsignificantly lower in patients who continued immunosuppressants, suggests that the dosage of immunosuppressant medication in patients with transplant failure may be insufficient to prevent rejection and development of anti-HLA antibodies.
Importantly, because the patients were under the care of primary nephrologists, the reasons why immunosuppressant medications were or were not continued could not be determined. Because measurement of drug levels was not mandated in this observational study and it is challenging to obtain timed drug levels in patients on dialysis, there was missing data on drug levels in clinical records. We also did not directly measure medication adherence making it impossible to determine the actual drug exposure in study patients. Nonadherence is a known contributor to allograft failure and may be a particularly relevant consideration in patients with transplant failure. To address this limitation, we ascertained the prescribing practices of transplant nephrologists in the participating centers. This end-of-study survey found that all centers recommend discontinuation of antimetabolite drugs and continuation of low-dose prednisone with variable recommendations regarding the use of calcineurin inhibitors in the first year after transplant failure. In centers recommending continuation of calcineurin inhibitors, the majority recommended therapeutic levels lower than during the period of allograft function (data not shown).
Current practice recommendations to discontinue immunosuppressants in the first year were in part informed by a large retrospective cohort study of patients with transplant failure between 1972 and 1996 that showed an increased risk of infection and mortality with continuation of immunosuppressants after transplant failure.24 Further, patients in that study were routinely offered elective nephrectomy potentially leading to sicker patients who elected against nephrectomy to be maintained on immunosuppressants. This study’s findings suggest potential harm with discontinuation of immunosuppressant medications in contrast to recent recommendations.16
Patients who discontinued all immunosuppressant medications or who used low-dose prednisone only had higher levels of sensitization than patients who continued immunosuppressants, but the group differences were not statistically different. The study findings differ from retrospective studies showing that continuation of immunosuppressant medication prevents sensitization.18 Retrospective analyses may be subject to bias as only repeat transplant candidates with available anti-HLA antibody testing are included. In the study by Augustine and colleagues, the majority of patients who continued immunosuppression had a functioning pancreas transplant and were likely maintained levels of immunosuppression sufficient to suppress sensitization.18 Tacrolimus has been shown to protect against sensitization and a tacrolimus level >3 ng/ml provided some degree of protection from broad sensitization.20 This is consistent with data from patients with allograft function showing that a tacrolimus level of 6 ng/ml is associated with a lower risk of sensitization.25 Prospective studies are needed to define the level of immunosuppression required to prevent sensitization after transplant failure.
This study also failed to find an association between allograft nephrectomy and survival or hospitalized infection but this analysis is limited by the small number of nephrectomy events. Allograft nephrectomy is associated with a lower risk of death in patients with transplant failure.14,26 In Canada, allograft nephrectomy is generally reserved for patients with ongoing rejection or graft intolerance syndrome resistant to medical therapy and the use of allograft nephrectomy is lower than that reported in the United States.14 The relatively small number of patients who underwent allograft nephrectomy precludes further analyses to determine changes in PRAs or inform immunosuppressant management in the setting of nephrectomy.
To our knowledge this is the first multicenter prospective study of patients with transplant failure. The strengths of the study include a relatively large well-characterized study cohort, protocol driven collection of prescribed immunosuppressant medications supplemented by patient interview, rigorous ascertainment of study outcomes, and prospective collection of serum samples and centralized determination of PRAs using state-of-the-art methods. Some of the associations demonstrated may be clinically significant and may have reached statistical significance in a larger study or with a longer patient follow-up. The multicenter patient enrollment strengthens the generalizability of the study finding within Canada but may not be applicable outside Canada. Further, a significant proportion of eligible patients did not consent to participate due to perceived difficulty with completing the study protocol, which may in part be related to the stress of adjusting to the reality of transplant failure and the transition to maintenance dialysis. Because patients were of varied transplant vintage, we were unable to access pretransplant donor and recipient HLA typing to determine the presence of donor-specific antibodies. The study was not designed to address access to repeat transplantation and in the absence of national referral and waitlisted data in Canada, we can only report repeat transplantation events.27 Finally, the study sample size was too limited to determine differences in other potentially clinical relevant outcomes such as malignancy.
In summary, mortality in contemporary patients with transplant failure in Canada continues to remain high in contrast to secular improvements in the survival of patients on dialysis.2 Most patients continued immunosuppressant medication beyond the first year after transplant failure. We did not find evidence of an increased risk of death or hospitalized infection with continued use immunosuppressants. The continued use of immunosuppressant medications beyond the first post-transplant year did not prevent rejection of the failed allograft or sensitization as measured by an increase in anti-HLA antibodies, suggesting that higher dosing, closer monitoring of patient adherence, and consideration of individualized dosing based on the degree of HLA mismatch, duration of allograft survival, and cause of allograft failure should be further studied. Importantly, although this prospective study is a higher level of evidence than previous retrospective studies examining the association of immunosuppressant medication use with patient outcomes, the findings are based on observational data and therefore subject to bias. The study findings not only indicate the need for a randomized controlled trial but provide information to inform the design of and sample size for such a trial.
Disclosures
D. Baran reports honoraria from Alexion. P. Campbell reports consultancy with Paladin Labs; and advisory or leadership roles (all unpaid) with the American Society for Histocompatibility and Immunogenetics (ASHI) (past president, 2019–2020), the National Clinical Affairs Committee for ASHI (past chair, 2020–2021), the National HLA advisory committee (past chair) and the Canadian Blood Services (2020 to present). D. Fergusson reports advisory or leadership role for Clinical Trials and Transfusion Medicine Reviews. M. Hébert reports patents or royalties from Thermo Fisher One Lambda; and reports advisory or leadership role with Mila (Quebec Artificial Intelligence Institute), Institute of Research in Vegetal Biology, and Sainte-Justine Hospital. A. House reports honoraria from AstraZeneca Canada and Baxter Healthcare; and is on the speakers bureau for Baxter Healthcare for continuous renal replacement therapy. O. Johnston reports research funding from Astellas, CSL Behring, and Merck; and reports honoraria from Paladin Labs. J. Kim reports advisory or leadership role with Canadian Organ Replacement Register, Canadian Society of Transplantation, Scientific Registry of Transplant Recipients Technical Advisory Committee, Organ Procurement and Transplantation Network/United Network for Organ Sharing Data Advisory Committee, Canadian Blood Services, Health Canada, and Eledon Pharmaceuticals (Data Monitoring Committee member for phase 1b trial, paid). G. Knoll is on the editorial board of Canadian Journal of Kidney Health and Disease. J. Perl reports consultancy with AstraZeneca, Baxter Healthcare Canada, Bayer, Davita Healthcare Partners, Fresenius Medical Care, LiberDi, and Otsuka; reports research funding and salary support from Agency for Healthcare Research and Quality and Arbor Research Collaborative For Health; reports honoraria from AstraZeneca, Baxter Healthcare USA/Canada, Davita Healthcare Partners, DCI, Fresenius Medical Care, and US Renal Care; and is on the speakers bureau for Baxter Healthcare and Fresenius Medical Care. C. White reports an advisory or leadership role with Canadian Journal of Kidney Health and Disease. All remaining authors have nothing to disclose. Because J. Gill is an editor of JASN, he was not involved in the peer review process for this manuscript. A guest editor oversaw the peer review and decision-making process for this manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research (grant FRN 102732).
Supplementary Material
Acknowledgments
The authors would like to thank all members of the research teams and patients at study sites for their contribution to this study. The authors would also like to acknowledge Paule-Marjolaine Bodson-Clermont and Kip Brown, University of Montreal Hospital Research Centre, for their assistance with statistical analyses.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
D. Baran, M.-J. Hébert, I. Houde, A. House, O. Johnston, M. Karpinski, J. Kim, R. Mainra, L. Senecal, D. Treleaven, L.A. Tibbles, and C. White were responsible for data curation and reviewed and edited the manuscript; P. Campbell was responsible for conceptualization, investigation, methodology, resources, and reviewed and edited the manuscript; M. Chassé was responsible for formal analysis, investigation, and reviewed and edited the manuscript; D. Fergusson was responsible for formal analysis, and reviewed and edited the manuscript; J. Gill and G. Knoll were responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing the original draft, and reviewed and edited the manuscript; P. Karnabi was responsible for formal analysis, writing the original draft, and reviewed and edited the manuscript; G. Knoll was responsible for resources and supervision; J. Perl was responsible for data curation and project administration; and T. Ramsay was responsible for methodology and reviewed and edited the manuscript.
Data Sharing Statement
All data is included in the manuscript and/or supporting materials.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021121642/-/DCSupplemental.
Supplemental Figure 1. Proportion of patients with functional status over a 36-month follow-up period.
Supplemental Figure 2. Proportion of patients on hemodialysis and peritoneal dialysis over a 36-month follow-up period.
Supplemental Figure 3. Proportion of patients using different dialysis access over a 36-month follow-up period.
Supplemental Figure 4. Median daily dose of cyclosporine, tacrolimus, mycophenolate mofetil, mycophenolate sodium, azathioprine, and prednisone.
Supplemental Figure 5. Temporal change in PRAs for those with PRAs >20% at baseline and discontinued all immunosuppressants. (A) Class I PRAs; (B) class II PRAs.
Supplemental Figure 6. Temporal change in PRAs for those with PRA <20% at baseline and discontinued all immunosuppressants. (A) Class I PRAs; (B) class II PRAs.
Supplemental Figure 7. Temporal change in PRAs for those with PRAs >20% at baseline and discontinued all immunosuppressants except low-dose prednisone. (A) Class I PRAs; (B) class II PRAs.
Supplemental Figure 8. Temporal change in PRAs for those with PRAs <20% at baseline and discontinued all immunosuppressants except low-dose prednisone. (A) Class I PRAs; (B) class II PRAs.
Supplemental Table 1. Study recruitment by center.
Supplemental Table 2. Baseline characteristics by immunosuppressant prescription during follow-up.
Supplemental Table 3. Cox multivariable model for the outcome of death.
References
- 1.Kochar GS, Langone AJ: How should we manage renal transplant patients with failed allografts who return to dialysis? Blood Purif 49: 228–231, 2020 [DOI] [PubMed] [Google Scholar]
- 2.System USRD: USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020. Available at: https://adr.usrds.org/2020/. Accessed on March 26, 2022 [Google Scholar]
- 3.Gill JS, Abichandani R, Khan S, Kausz AT, Pereira BJ: Opportunities to improve the care of patients with kidney transplant failure. Kidney Int 61: 2193–2200, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Kaplan B, Meier-Kriesche HU: Death after graft loss: An important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Knoll G, Muirhead N, Trpeski L, Zhu N, Badovinac K: Patient survival following renal transplant failure in Canada. Am J Transplant 5: 1719–1724, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Mourad G, Minguet J, Pernin V, Garrigue V, Peraldi MN, Kessler M, et al. : Similar patient survival following kidney allograft failure compared with non-transplanted patients. Kidney Int 86: 191–198, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Gill JS, Rose C, Pereira BJ, Tonelli M: The importance of transitions between dialysis and transplantation in the care of end-stage renal disease patients. Kidney Int 71: 442–447, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Johnston O, Zalunardo N, Rose C, Gill JS: Prevention of sepsis during the transition to dialysis may improve the survival of transplant failure patients. J Am Soc Nephrol 18: 1331–1337, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lam NN, Boyne DJ, Quinn RR, Austin PC, Hemmelgarn BR, Campbell P, et al. : Mortality and morbidity in kidney transplant recipients with a failing graft: A matched cohort study. Can J Kidney Health Dis 7: 2054358120908677, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayliss GP, Gohh RY, Morrissey PE, Rodrigue JR, Mandelbrot DA: Immunosuppression after renal allograft failure: A survey of US practices. Clin Transplant 27: 895–900, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Alhamad T, Lubetzky M, Lentine KL, Edusei E, Parsons R, Pavlakis M, et al. : Kidney recipients with allograft failure, transition of kidney care (KRAFT): A survey of contemporary practices of transplant providers. Am J Transplant 21: 3034–3042, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Nimmo AMSA, McIntyre S, Turner DM, Henderson LK, Battle RK: The impact of withdrawal of maintenance immunosuppression and graft nephrectomy on HLA sensitization and calculated chance of future transplant. Transplant Direct 4: e409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Bello A, Congy-Jolivet N, Sallusto F, Guilbeau-Frugier C, Cardeau-Desangles I, Fort M, et al. : Donor-specific antibodies after ceasing immunosuppressive therapy, with or without an allograft nephrectomy. Clin J Am Soc Nephrol 7: 1310–1319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS: Nephrectomy after transplant failure: Current practice and outcomes. Am J Transplant 7: 1961–1967, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ghyselen L, Naesens M: Indications, risks and impact of failed allograft nephrectomy. Transplant Rev (Orlando) 33: 48–54, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Lubetzky M, Tantisattamo E, Molnar MZ, Lentine KL, Basu A, Parsons RF, et al. : The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Am J Transplant 21: 2937–2949, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Andrews PA; Standards Committee of the British Transplantation Society : Summary of the British Transplantation Society Guidelines for Management of the Failing Kidney Transplant. Transplantation 98: 1130–1133, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Augustine JJ, Woodside KJ, Padiyar A, Sanchez EQ, Hricik DE, Schulak JA: Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation 94: 738–743, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Casey MJ, Wen X, Kayler LK, Aiyer R, Scornik JC, Meier-Kriesche HU: Prolonged immunosuppression preserves nonsensitization status after kidney transplant failure. Transplantation 98: 306–311, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucisano G, Brookes P, Santos-Nunez E, Firmin N, Gunby N, Hassan S, et al. : Allosensitization after transplant failure: The role of graft nephrectomy and immunosuppression - a retrospective study. Transpl Int 32: 949–959, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Schrezenmeier E, Lehner LJ, Merkel M, Mayrdorfer M, Duettmann W, Naik MG, et al. : What happens after graft loss? A large, long-term, single-center observation. Transpl Int 34: 732–742, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Brar A, Markell M, Stefanov DG, Timpo E, Jindal RM, Nee R, et al. : Mortality after renal allograft failure and return to dialysis. Am J Nephrol 45: 180–186, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Bonani M, Achermann R, Seeger H, Scharfe M, Müller T, Schaub S, et al. : Dialysis after graft loss: A Swiss experience. Nephrol Dial Transplant 35: 2182–2190, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Smak Gregoor PJ, Zietse R, van Saase JL, op de Hoek CT, IJzermans JN, Lavrijssen AT, et al. : Immunosuppression should be stopped in patients with renal allograft failure. Clin Transplant 15: 397–401, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Wiebe C, Rush DN, Nevins TE, Birk PE, Blydt-Hansen T, Gibson IW, et al. : Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol 28: 3353–3362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS: Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol 21: 374–380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SJ, Gill JS, Knoll G, Campbell P, Cantarovich M, Cole E, et al. : Referral for kidney transplantation in Canadian provinces. J Am Soc Nephrol 30: 1708–1721, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.