Kidney physiology and the gut microbiome are intricately linked. The kidneys remotely sense changes in microbial metabolism.1 In advanced CKD, retained microbial metabolites contribute to the syndrome of uremia.2 Experimental models of CKD exhibit changes in the gut microbiome3 and gut metabolome, and these are replicated in individuals with CKD.4,5 The bidirectional metabolic and immunologic crosstalk, referred to as the gut-kidney axis, is enormously complex and only partly understood. In this issue of JASN, Linh and coworkers6 provide additional intriguing evidence that intestinal bacterial translocation contributes to the pathophysiology of diabetic nephropathy.
The mitochondrial antiviral signaling (MAVS) protein was originally identified as part of a protein network involved in the detection of intracellular double-stranded RNA viruses (hence the name). Linh et al.6 explored the role of MAVS in diabetic nephropathy, using MAVS knockout (MAVS−/−) mice with or without streptozotocin-induced diabetic nephropathy. MAVS−/− mice developed higher levels of albuminuria, augmented interstitial inflammation, and elevated levels of kidney injury marker-1 (Figure 1).6
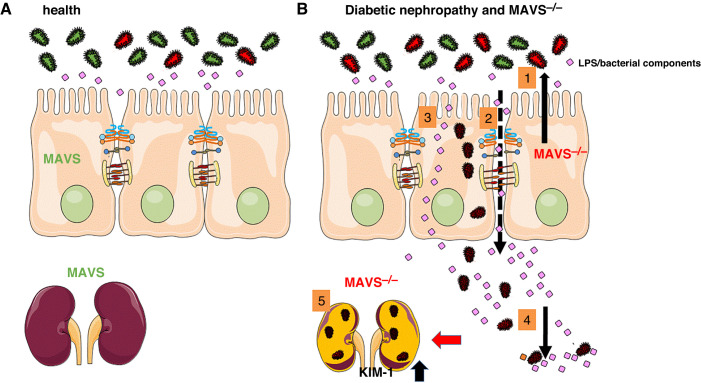
Figure 1.
The leaky gut and MAVS knockout contribute to the pathophysiology of diabetic nephropathy. (A) In normal conditions, the intestinal barrier function is intact, and luminal microbiota and endotoxins are confined to the lumen. (B) In diabetic nephropathy, deregulation of MAVS signaling in epithelial cells and the kidney alters the distribution of the microbiota and endotoxins because of (1) the presence of dysbiosis, and (2) increased paracellular, and (3) transcellular permeability. (4) Then, viable bacteria, such as Klebsiella oxytoca, can enter the systemic circulation and reach other organs, (5) including the kidney, leading to increased production of kidney injury molecule-1 (KIM-1) and renal dysfunction.
The most straightforward explanation for these results would be that the absence of MAVS in tubular and glomerular cells increases the deleterious effects of hyperglycemia, or that MAVS confers a protective effect against hyperglycemia. MAVS expression increased in tubuli of the experimental diabetic nephropathy MAVS+/+ group.6 Upregulation of a mitochondrial protein in diabetes is possibly econdary to hyperglycemia. Mitochondria play an essential role in cellular respiration, i.e., in the transformation of oxygen and glucose into carbon dioxide, water, plus ATP as the cellular energy carrier. Interestingly, MAVS was found to interact with hexokinase II.7 The hexokinase isozymes transform glucose to glucose-6-phosphate, initiating glycolysis. MAVS knockdown and knockout resulted in reduced hexokinase-II activity, and decreased pyruvate and lactate production.7 However, whereas the hexokinase-II isozyme is predominantly expressed in (cardio)myocytes and adipose tissue,8 hexokinase I is the dominant isozyme in kidney cells. Interactions between MAVS and hexokinase I await further exploration, and could shed more light on the role of MAVS in kidney tissue.
Because MAVS is ubiquitously expressed, we also have to consider systemic effects. MAVS−/− models exhibit clear differences in gut microbial taxonomic composition, without changes in the overall richness and diversity.9 MAVS−/− mice have a reduced gut barrier integrity, whereas targeted engagement of the STING (stimulator of IFN genes) pathway promotes gut barrier integrity. Also in Linh et al.’s study, MAVS−/− mice had increased intestinal permeability as compared with the control group. Hyperglycemia in cell culture experiments interfered with the intestinal barrier, in line with other experimental models.
A strength of Linh et al.’s study is that they also used culturomics to assess bacterial translocation into different organs. Increased circulating 16s ribosomal RNA copy numbers were found in animals with experimentally induced diabetic nephropathy (both MAVS−/− and wild type), whereas copy numbers in the sham MAVS−/− animals were low and did not differ from wild-type animals. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was used to identify specific microbial species. In animals with experimentally induced diabetic nephropathy (both MAVS−/− and wild type), several species were isolated from blood, lymph nodes, spleen, and kidney,6 with Klebsiella oxytoca present in all studied organs. MAVS−/− was found to modulate the taxonomy of invasive species identified using tissue culturomics, in line with previous observations of the gut microbiome taxonomy.9
What can these models teach us about diabetes and diabetic nephropathy? Evidently, these data warrant confirmation in human disease. Preliminary unpublished data from the same group are suggestive of bacterial translocation in patients with diabetic nephropathy. A sizable number of large human observational studies have shown alterations in the gut microbiome10 and gut permeability in diabetes mellitus; these are mostly cross-sectional association studies. As Linh et al.’s study suggests, increased intestinal permeability to bacteria is in the causal chain of additional kidney stress. Longitudinal observational and interventional studies could shed more light on this putative disease mechanism.
An additional important question is whether these findings are specific to diabetic nephropathy, or whether gut barrier dysfunction occurs in response to CKD irrespective of the underlying pathology. In the study by Linh et al.,6 streptozotocin-induced diabetes was combined with uninephrectomy, thereby moderately reducing the GFR. Animal and cell culture experiments provide evidence that the tight junctions, sealing together the colonocytes and maintaining barrier integrity, are affected by the uremic environment.11 Collagen type 4a3–deficient mice spontaneously develop CKD, resembling the phenotype of Alport disease. In this normoglycemic CKD model, 60% of liver samples showed culturable live bacteria.3 Short-chain fatty acids, a group of metabolites derived from the metabolism of fiber by the gut microbiota and generally proven to promote health and intestinal barrier function, are also significantly lowered in CKD. These and other experimental studies suggest that loss of kidney function affects the intestinal barrier.
To date, the expanding evidence in animal models is not matched by human data. One of the challenges is that in vivo measures of intestinal permeability have not been validated in CKD. Most of these assays are influenced by changes in GFR.12 Moreover, patients with CKD and preclinical models show reduced intestinal motility, potentially influencing the assessment of the intestinal permeability in vivo. In diseases with a compromised kidney and/or intestinal motility function, ex vivo assessment of the intestinal permeability seems mandatory.
Linh et al. provide another piece of evidence suggesting a prime role of the gut in kidney disease. Their finding that MAVS, a mitochondrial protein contributing to innate immunology, plays a modulatory role definitely is intriguing, yet leaves room for speculation on underlying mechanism(s). As the number of animal studies on the “leaky gut” in association with CKD is expanding, human studies remain scarce. If human studies in individuals with CKD, be it diabetic nephropathy or other causes of CKD for that matter, would confirm an increased intestinal permeability and influx of live bacteria, this would open a whole new field of research and, possibly, novel therapeutic targets.
Disclosures
B. Meijers reports having consultancy agreements with AstraZeneca, Baxter, Bayer, Fresenius, Nipro, Novartis, and Vifor Fresenius; serving on a speakers bureau for Baxter; receiving honoraria from Baxter, Fresenius, Nipro, and Vifor; receiving research funding from Bayer, Ionis, and Nipro; and serving on the editorial boards of BMC Nephrology and Toxins. All remaining authors have nothing to disclose.
Funding
B. Meijers is a senior clinical investigator of the Fonds Wetenschappelijk Onderzoek (grant 1800820N) and received support from KU Leuven via grants 3M190551 and C14/21/103.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Intestinal Bacterial Translocation Contributes to Diabetic Kidney Disease,” on pages 1105–1119.
Author Contributions
S. Dejongh, R. Farré, and B. Meijers reviewed and edited the manuscript; and R. Farré and B. Meijers wrote the original draft and were responsible for visualization.
References
- 1.Jansen J, Jansen K, Neven E, Poesen R, Othman A, van Mil A, et al. : Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc Natl Acad Sci USA 116: 16105–16110, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meijers B, Evenepoel P, Anders HJ: Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol 15: 531–545, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Andersen K, Kesper MS, Marschner JA, Konrad L, Ryu M, Kumar Vr S, et al. : Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol 28: 76–83, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poesen R, Windey K, Neven E, Kuypers D, de Preter V, Augustijns P, et al. : The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol 27: 1389–1399, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joossens M, Faust K, Gryp T, Nguyen ATL, Wang J, Eloot S, et al. : Gut microbiota dynamics and uraemic toxins: One size does not fit all. Gut 68: 2257–2260, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linh HT, Iwata Y, Senda Y, Sakai-Takemori Y, Nakade Y, Oshima M, et al. : Intestinal bacterial translocation contributes to diabetic kidney disease. J Am Soc Nephrol 33: 1105–1119, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, et al. : Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178: 176–189.e15, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heikkinen S, Suppola S, Malkki M, Deeb SS, Jänne J, Laakso M: Mouse hexokinase II gene: Structure, cDNA, promoter analysis, and expression pattern. Mamm Genome 11: 91–96, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Plantamura E, Dzutsev A, Chamaillard M, Djebali S, Moudombi L, Boucinha L, et al. : MAVS deficiency induces gut dysbiotic microbiota conferring a proallergic phenotype. Proc Natl Acad Sci USA 115: 10404–10409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. ; MetaHIT consortium : Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528: 262–266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, et al. : Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol 36: 438–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terpstra ML, Singh R, Geerlings SE, Bemelman FJ: Measurement of the intestinal permeability in chronic kidney disease. World J Nephrol 5: 378–388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]