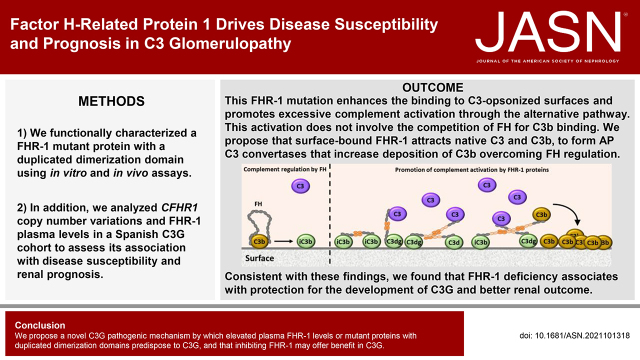
Significance Statement
Mutations in factor H–related protein 1 (FHR-1) that result in duplication of its dimerization domain associate with the chronic renal disease C3 glomerulopathy (C3G), which is characterized by complement dysregulation. The molecular basis for this association is only partially understood. The authors show that these FHR-1 mutations enhance FHR-1’s binding to C3-activated fragments on opsonized surfaces and promote an excessive complement activation that overcomes FH regulation. They also show that elevated levels of FHR-1 associate with poor renal prognosis for patients with C3G, whereas a genetic deficiency of FHR-1 offers protection against C3G development. These findings advance our understanding of C3G pathogenesis and suggest that inhibition of FHR-1 may have therapeutic potential in C3G.
Keywords: C3 glomerulopathy, complement, factor H-related protein 1, glomerular disease, disease susceptibility, prognosis, genetic renal disease
Visual Abstract
Abstract
Background
C3 glomerulopathy (C3G) is a heterogeneous group of chronic renal diseases characterized predominantly by glomerular C3 deposition and complement dysregulation. Mutations in factor H–related (FHR) proteins resulting in duplicated dimerization domains are prototypical of C3G, although the underlying pathogenic mechanism is unclear.
Methods
Using in vitro and in vivo assays, we performed extensive characterization of an FHR-1 mutant with a duplicated dimerization domain. To assess the FHR-1 mutant’s association with disease susceptibility and renal prognosis, we also analyzed CFHR1 copy number variations and FHR-1 plasma levels in two Spanish C3G cohorts and in a control population.
Results
Duplication of the dimerization domain conferred FHR-1 with an increased capacity to interact with C3-opsonized surfaces, which resulted in an excessive activation of the alternative pathway. This activation does not involve C3b binding competition with factor H. These findings support a scenario in which mutant FHR-1 binds to C3-activated fragments and recruits native C3 and C3b; this leads to formation of alternative pathway C3 convertases, which increases deposition of C3b molecules, overcoming FH regulation. This suggests that a balanced FHR-1/FH ratio is crucial to control complement amplification on opsonized surfaces. Consistent with this conceptual framework, we show that the genetic deficiency of FHR-1 or decreased FHR-1 in plasma confers protection against developing C3G and associates with better renal outcome.
Conclusions
Our findings explain how FHR-1 mutants with duplicated dimerization domains result in predisposition to C3G. They also provide a pathogenic mechanism that may be shared by other diseases, such as IgA nephropathy or age-related macular degeneration, and identify FHR-1 as a potential novel therapeutic target in C3G.
C3 glomerulopathy (C3G) is a heterogeneous group of rare and severe kidney diseases characterized by predominant deposition of C3 in the glomeruli.1,2 Patients with C3G have a poor renal prognosis, with up to 40%–50% of patients reaching ESKD, and recurrences after renal transplantation are common. C3G is associated with dysregulation of the complement alternative pathway (AP), with both complement pathogenic mutations and/or autoantibodies against complement components identified in many patients.3–5
The factor H (FH) protein family is composed of FH, its splice variant FHL-1, and five FH-related proteins (FHR1–5), which are evolutionary and structurally related to FH.6 FH is the main regulator of the AP, both in the fluid phase and on surfaces. It acts as a cofactor of factor I for the proteolytic cleavage of C3b, competes with factor B (FB) for C3b binding, and accelerates the dissociation of the AP C3 convertase.7–9 Among the FHRs, FHR-1 is the best-characterized molecule. It interacts with C3-activated fragments and carbohydrate molecules, but, in contrast to FH, no regulatory activities at the level of C3b and the AP C3 convertase have been found.6,10 It was reported that FHR-1 inhibits the C5 convertase and the formation of the membrane attack complex, but this activity is still controversial.11 In addition, it was proposed that FHR-1 promotes complement activation by competing with FH binding to surface-bound C3b, a term called FH deregulation activity,11 and that surface-bound FHR-1 supports the formation of the AP C3 convertase through the binding of fluid-phase C3b.12
Variations in the genes encoding the FHRs are associated with common and rare diseases, including C3G. A common deletion leading to the deficiency of FHR-1 and FHR-3 (ΔCFHR3-CFHR1) is associated with protection against age-related macular degeneration (AMD) and IgA nephropathy (IgAN), whereas it confers increased risk for SLE.13–15 Genomic rearrangements involving the CFHRs that lead to either hybrid genes or to internal gene duplications predispose to atypical hemolytic uremic syndrome (aHUS) and C3G.16–20 Among these, mutations in the carboxy-terminal (C-terminal) of FHR-1 are prototypical of aHUS, whereas mutations leading to the duplication of the dimerization domains of FHR-1, FHR-2, and FHR-5 are exclusively found in C3G.
The term FH deregulation activity of the FHRs was coined when the dimeric nature of the FHRs was initially described,11 and was later used to explain the association of the FHR-1 and FHR-5 mutants with C3G and aHUS.6 However, recent functional and structural data on wild-type FHR-1 and aHUS-associated FHR-1 mutants suggest this pathogenic mechanism of FH deregulation may be limited to the aHUS-associated FHR-1 mutants that have acquired the capacity to bind sialic acids.10 These recent data also proposed that a physiologic role of FHR-1 is binding to iC3b and C3dg on C3-opsonized surfaces to promote further complement activation, likely by attracting native C3 to the cell surface.10 Because of the implications of these novel data to the pathogenesis of C3G, we have reanalyzed the consequences of the C3G-associated FHR-1 mutations.
In this study, we structurally and functionally characterized a novel FHR-1 mutant protein with a duplicated dimerization domain (SCR1 and SCR2). We show that this mutant displays an enhanced ability to promote complement activation on C3-opsonized surfaces, and that this mechanism does not involve the competition with FH for C3b binding. Moreover, we report that ΔCFHR3-CFHR1 is a protective factor against the development of C3G and that FHR-1 levels determine disease prognosis. Altogether, we propose a novel C3G pathogenic mechanism that explains why elevated plasma FHR-1 levels, or mutant proteins with duplication of the dimerization domains, predispose to C3G.
Methods
Generation and Purification of Recombinant FHR-1 Proteins
To generate recombinant FHR-1 wild-type protein (rFHR-1), a pEZ-M02 vector containing the CFHR1 cDNA was obtained from GeneCopoeia (catalog number EX-Z0243-M02) and the cDNA was cloned into a modified version of pCAGGS plasmid21 using an In-Fusion strategy (In-Fusion HD Cloning; Takara Bio USA). The recombinant mutant FHR-1 allele with the internal duplication (rFHR-1mut) and a monomeric version of FHR-1 (rFHR-1mon) were also generated using an In-Fusion strategy. rFHR-1mon was generated by mutating three key amino acids within the dimerization motif (Tyr34Ser, Ser36Tyr, and Tyr39Glu), using the same strategy described to generate a monomeric FHR-5.11 The recombinant proteins were transiently expressed in FreeStyle 293-F cells following the manufacturer’s instructions (Life Technologies). The culture supernatant was filtered using a 0.22-mm, vacuum-driven, bottle-top filter and purified using an affinity column coupled with the in-house anti–FHR-1, anti–FHR-2, and anti–FHR-5 (2C6, also named MBC125) mAb (developed by Dr. Claire Harris, Cardiff University, Cardiff, United Kingdom). Fractions containing the purified FHRs were pooled and dialyzed against PBS.
Analytic Ultracentrifugation Assay
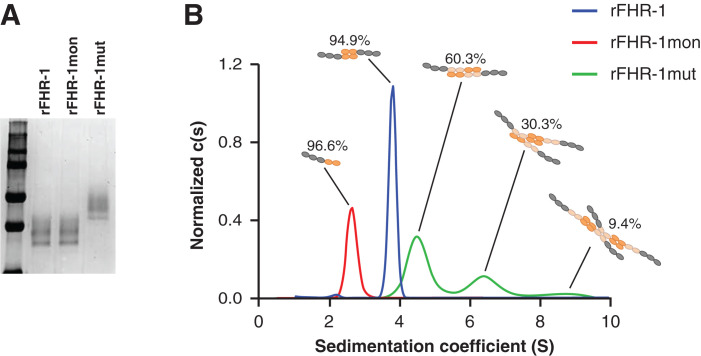
Purified recombinant FHR-1 proteins, with an OD280 nm of 0.2, in PBS were used in the assays (Figure 1A). Velocity sedimentation experiments were performed on a Beckman Optima XL-A analytic ultracentrifuge at 20°C and 48,000 rpm for 2 hours. Sedimentation data were processed using SEDFIT 16.1c with a confidence level (F-ratio) of 0.68, and the sedimentation coefficients and apparent molecular masses were calculated according to the theoretic molecular mass of wild-type FHR-1 (FHR-1wt; 38 kD) and FHR-1mut (52k Da).
Figure 1.
FHR-1 mutant with duplicated dimerization domains forms multimers. (A) Coomassie-stained SDS-PAGE gel of purified rFHR-1, FHR-1mon, and FHR-1mut. (B) Continuous sedimentation coefficient distribution analysis c(s) of velocity sedimentation experiments of rFHR-1, rFHR-1mut, and rFHR-1mon, performed by analytic ultracentrifugation, revealed single peaks for rFHR-1 and rFHR-1mon with coefficients 3.8S and 2.7S, which are compatible with a dimer and a monomer, respectively. In contrast, rFHR-1mut displayed several peaks at 4.6S, 6.5S, and 8.7S, compatible with dimers, trimers, and tetramers, respectively.
Complement Reagents
Purified complement components, such as FH, factor I, factor D (FD), C3, C3b, and C3dg, were purchased from Complement Technologies (Tyler, TX).
C3b, C3dg, and C3 Binding Assays
C3b, C3dg, and C3 (5 µg/ml) were immobilized in a microtiter plate overnight (O/N) at 4°C. After blocking with 1% BSA in Tris–Tween 20, serial dilutions (0.63–40 nM) of the different recombinant FHR-1 preparations were incubated in triplicates for 1 hour. The bound FHR-1 proteins were detected using the mAb 2C6 as primary antibody and a horseradish peroxidase–conjugated goat anti-mouse IgG as secondary antibody (catalog number 31430; Dako, Glostrup, Denmark). The binding of the FHR-1 proteins to different amounts of deposited C3dg (0.31–10 µg/ml) was performed as above, by incubating a single 40 nM concentration of the FHR-1 proteins.
Flow Cytometry C3b-Binding FH Competition Assay in Sheep Erythrocytes
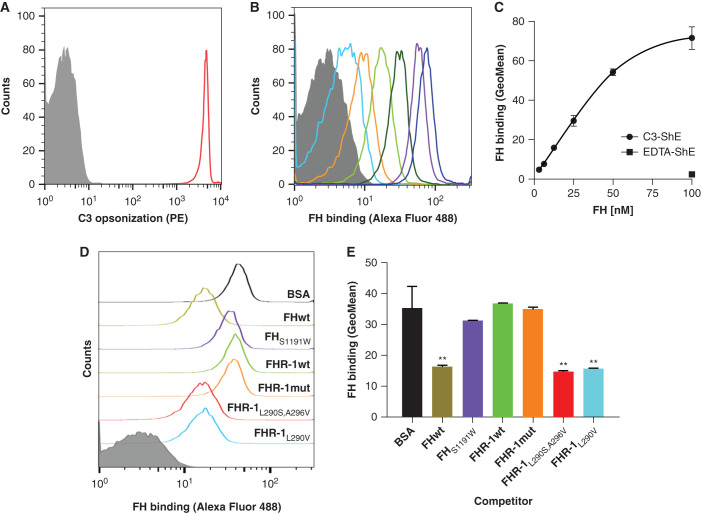
Sheep erythrocytes (ShEs; Real Laboratory, Durviz) in EDTA-CFD buffer were sensitized using a 1:1600 dilution of rabbit anti-sheep erythrocyte antiserum (catalog number ORLC25, Siemens Amboceptor), incubated for 30 minutes at 37°C, and then washed twice with EDTA-CFD buffer. Sensitized ShEs, at 0.5 OD414 nm in CFD, were then opsonized with a 1:20 dilution of normal human serum (NHS) depleted of FH, FHR-1, FHR-2, FHR-5 and FB and 20 µg/ml of OmCI for 30 minutes at 37°C. As a negative control, opsonization was also performed in the presence of EDTA-CFD. The reactions were stopped with 2 ml of EDTA-CFD and cells were washed twice in PBS. Cell opsonization was analyzed by flow cytometry experiments with an in-house mouse monoclonal anti-human C3 antibody (12.17)22 and a phycoerythrin-labeled, goat anti-mouse IgG secondary antibody (catalog number 12-4010-82; eBiosciences). The binding of FH to either opsonized or nonopsonized ShE was then titrated. To do so, increasing amounts (3.25–100 nM) of FH labeled with Alexa Fluor 488 (FHAF488; Molecular Probes catalog number A10235; Invitrogen) were incubated with the cells, for 1 hour at 4°C, in PBS containing 0.1% BSA. FHAF488 binding was then analyzed by flow cytometry. For the FH binding competition assays, 50 nM FHAF488 was incubated for 1 hour at 4°C with increasing amounts (25, 50, or 100 nM) of the competitors (wild-type FH [FHwt], FHS1191W, FHR-1wt, FHR-1mut, FHR-1L290S,A296V, FHR-1L290V) before incubating FHAF488 with the opsonized ShEs. As a negative control for the competition, 50, 100, or 200 nM of BSA was also incubated with FHAF488. A molecule is considered a competitor of the FH binding to opsonized ShEs when the FHAF488 geometric mean determined by flow cytometry in the presence of that molecule is significantly reduced, as compared with the geometric mean obtained for the negative control (i.e., FHAF488 binding in the presence of BSA).
C3 Convertase Formation and Complement Activation Assays
AP C3 convertases were formed in microtiter plates coated with the different FHR-1 proteins and BSA (negative control). Assays were conducted in Tris-buffered saline (TBS), pH 7.4, containing 2 mM calcium chloride and 5 mM magnesium chloride. FHR-1 (1 µM) and BSA were coated O/N at 4°C. After blocking with 3% BSA (wt/vol) in TBS for 2 hours at room temperature (RT), 10 μg/ml C3b was incubated for 30 minutes at 37°C. After washing, FB (10 μg/ml), FD (0.1 μg/ml), and factor P (2 μg/ml) were incubated for 1 hour at 37°C. C3 convertase formation was followed by double detection using an in-house rabbit anti-hFB polyclonal antibody (provided by P.S.-C.) and an in-house rabbit anti-hC3 polyclonal antibody (provided by S.R.d.C.), which were incubated for 1 hour at RT. The functioning of this convertase was demonstrated by adding 10 µg/ml of purified C3 for 1 hour at 37°C, followed by the quantification of the generated C3a in the supernatant using a C3a ELISA kit (Quidel). For the complement activation assays with NHS, a variation of the above assay was performed by incubating the immobilized FHR-1 proteins with 10% NHS in TBS with either 5 mM EGTA or 5 mM EDTA, or with 10% NHS supplemented with FH at different concentrations (0.32, 0.64, and 1.28 µM) for 30 minutes at 37°C. C3 deposition on the plate was detected using an in-house anti-hC3 biotinylated mAb (12.1722) provided by Mercedes Domínguez (Instituto de Investigación Carlos III, Madrid) and a peroxidase-conjugated streptavidin (catalog number 016-030-084; Jackson ImmunoResearch Europe Ltd.).
Immunofluorescence Studies on Mouse Kidney Sections
Binding of the rFHR-1 proteins to glomeruli in vivo was performed by injecting 20 µg of the proteins intravenously into wild-type (C57BL/6N), FH-deficient (Cfh−/−), or C3-deficient (C3−/−) mice. Two hours later, mice were euthanized and kidneys were removed for analysis. Snap-frozen kidney sections were cryostat cut at 5 μm, acetone fixed for 10 minutes, and stored at −80°C until use. For FHR-1 binding experiments ex vivo, Cfh−/− kidney sections were incubated with different concentrations of the FHR-1 proteins in PBS for 1 hour at RT. After blocking with Biotin Blocking System (catalog number X0590; Dako), we incubated the 2C6 biotinylated antibody at 1:50 for 1 hour at RT, and the secondary antibody streptavidin–Alexa 488 (1:2000) was incubated for 1 hour at RT. Sections were mounted using Vectashield. Alternatively, tissue sections were pretreated with Clostridium perfringens neuroaminidase (1:10 in PBS 1×) for 90 minutes at 37°C before incubation with the FHR-1 proteins.
Binding of FHR-1 Proteins and C3 Deposition on Human Renal Epithelial Cells
Human renal epithelial cells (HREpiCs; P10665; Innoprot) were cultured in Epithelial Cell Medium (catalog number 4101; Sciencell) supplemented with 10% FBS, 5% epithelial cell growth supplement (catalog number 4152; Sciencell) and penicillin/streptomycin antibiotics. Cells were incubated in a humidified incubator set at 5% carbon dioxide and 37°C. HREpiCs were seeded (5000 cells per well) on a precoated poly-l-lysine cover glass (catalog number 177372; Thermofisher) in Epithelial Cell Medium for 48 hours. The cells were then incubated with 20% NHS that was previously supplemented with 0.44 µM of either the rFHR-1mut or rFHR-1wt for 1 hour at 37°C. After two washes with PBS, cells were fixed with 4% paraformaldehyde in PBS for 10 minutes at RT. After blocking with 2% BSA in PBS for 1 hour at RT, cells were incubated for 1 hour at RT with a mixture of primary antibodies containing the 2C6 mAB and the in-house anti-hC3 12.17 biotinylated mAb for the detection of bound FHR-1 proteins and C3 deposition, respectively. Subsequently, a mixture of the corresponding secondary anti-mouse IgG Alexa Fluor 647 antibody (Thermo Fisher) and streptavidin 488 (Thermo Fisher) was also incubated for 1 hour at RT. After washing, cover glasses were mounted using ProLong Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (Thermo Fisher). Images were taken with a confocal laser scanning microscope (Olympus FV 1200), using a 60× immersion objective with Z stacking of 1 µm each, and analyzed with ImageJ. Colocalization analysis and Pearson coefficients were obtained using the ImageJ JACoP plugin. C3 quantification was measured in each Z stack by multiplying the area and the mean gray value.
FH-Dependent Hemolytic Assays
The capacity of the different FHR-1 proteins to promote complement activation on cellular surfaces was assessed in two different hemolytic assays using either guinea pig erythrocytes (GpEs) or ShEs. In brief, GpEs (0.5% packed cell volume; TCS Biosciences) in AP buffer, consisting of veronal buffer saline (2.5 mM barbital, 1.5 mM sodium barbital, 144 mM sodium chloride, pH 7.4) with 5 mM magnesium chloride, 8 mM EGTA, and 0.1% gelatin, were incubated with 15% NHS in AP buffer and increasing amounts of FH (0–1.28 µM) for 30 min at 37°C. Reactions were stopped using AP buffer containing 20 mM EDTA. After centrifugation, supernatants were read at 414 nm. Erythrocytes diluted in AP buffer were used as blanks for spontaneous lysis, and serum without added FH was considered the reference for 100% lysis. To set the experimental conditions in which increasing amounts of FHR-1 proteins were analyzed, we chose the minimal concentration of FH resulting in 20% lysis. For the sheep hemolytic assay, ShEs in AP buffer were incubated with different amounts of NHS previously depleted of 75% of FH (NHSΔFH75%). Erythrocytes in water were considered 100% lysis, and erythrocytes diluted in AP buffer were used as blanks for spontaneous lysis. The amount of NHSΔFH75% giving a 20% lysis was chosen to set the baseline experimental conditions in which the addition of different amounts of FHR-1 proteins was analyzed.
Patient and Healthy Control Cohorts
Spanish patients with C3G, diagnosed according to the 2013 consensus guidelines criteria,23 were included in this study. Patients with C3G from cohort 1 (n=89) were obtained from a well-defined and previously reported cohort of patients belonging to the Spanish Group for the Study of Glomerular Diseases.24 Only White patients were included in this study. The demographic data and the baseline clinical characteristics of C3G cohort 1 are detailed in Table 1. Spanish patients with C3G (n=217) from cohort 2 and the healthy control population (n=188) were recruited from the Spanish registry of patients with aHUS and C3G (https://www.ahusc3g.es/).
Table 1.
Baseline clinical characteristics of C3G patients from cohort 1
| Variable | Value (n=89) |
|---|---|
| Age at diagnosis (yr), mean±SD | 26±21 |
| Male sex, n (%) | 48 (54) |
| C3GN/DDD, n (%) | 74/15 (83/17) |
| Autoantibodies, n (%) | |
| C3 nephritic factor | 20 (22) |
| Anti-FH | 8 (9) |
| Antecedent infection, n (%) | 22 (25) |
| Hypertension at diagnosis, n (%) | 55 (62) |
| Serum creatinine (mg/dl), median (IQR) | 1.4 (0.7–3.1) |
| eGFR at diagnosis (ml/min per 1.73 m2), median (IQR) | 55 (20–120) |
| Albumin (g/dl), mean±SD | 3±0.8 |
| Proteinuria (g/24 hr), median (IQR) | 3 (1.5–6.4) |
| Serum C3 (mg/dl), mean±SD | 63±39 |
| Serum C4 (mg/dl), mean±SD | 24±9 |
Continuous variables are presented as mean±SD, or median (IQR). DDD, dense deposit disease; IQR, interquartile range.
Quantification of FHR-1 in Plasma Samples of C3G Patients and Controls
FHR-1 plasma levels were measured by sandwich ELISA, as previously described.25 This assay uses two in-house antibodies: the polyclonal anti-human FH 34 + 35 rabbit antibody, which recognizes FHR-1 but not FHR-2 and FHR-5 (developed by P.S.-C. and S.R.d.C.; Centro de Investigaciones Biológicas, Madrid, Spain), and the monoclonal 2C6 (also named MBC125) mouse antibody, which recognizes an epitope in SCR1/2 of human FHR-1 (developed by Dr. Claire Harris). In brief, 96-well microtiter plates were coated with the 34 + 35 antibody in 1× PBS, pH 7.3, O/N at 4°C. After washing, plates were blocked at RT with PBS, pH 7.3, 0.2% Tween 20, and 1% BSA (blocking buffer), and then incubated for 1 hour at RT with plasma samples diluted in blocking buffer. A plasma sample, whose FHR-1 levels were previously determined in an ELISA assay using purified FHR-1 as a reference, was used as a standard curve. FHR-1 was detected with the 2C6 mAb followed by a horseradish peroxidase–conjugated goat anti-mouse IgG antibody in blocking buffer (P0447; Dako). Bound antibody was detected using an o-phenylenediamine dihydrochloride substrate, and the reaction was stopped with 0.1 M sulfuric acid. Absorbance was measured at 492 nm. This FHR-1 ELISA is robust and specific for measuring FHR-1 plasma levels because FHR-1–deficient samples were completely negative (Supplemental Figure 1).
Statistical Analysis
The difference in the binding of FHR-1 proteins to immobilized C3b, C3dg, and native C3 was analyzed by one-way ANOVA. t Tests were used to compare the formation of the AP C3 convertases or complement deposition on the immobilized FHR-1 and BSA proteins, and in the analysis of ShE competition assays. The allele frequency of ΔCFHR3-CFHR1 in C3G and healthy control cohorts was compared, and P values, odds ratios, and 95% CIs were calculated using the two-sided Fisher exact test. In all cases, P values <0.05 were considered significant. GraphPad Prism software was used for the analyses.
Study Approval
The studies herein described received institutional review board approval (CEIC Hospital Clínico San Carlos and Comisión de Bioética, Consejo Superior de Investigaciones Científicas); written informed consent was obtained for all participants.
Supplemental Methods are available in Supplemental Appendix 1.
Results
Identification of a Novel Rearrangement in a Patient with Recurrent C3G
Copy number variation analysis of the CFH-CFHR1 loci in a patient (GN185) from our Spanish C3G cohort identified a novel genomic rearrangement in CFHR1. A description of the main findings and a clinical synopsis of the patient are provided in Supplemental Appendices 1 and 2, respectively. The novel CFHR1 allele encodes a FHR-1 mutant protein, in which the dimerization domains SCR1 and SCR2 are duplicated (Supplemental Figure 3F). Notably, we previously reported a similar FHR-1 mutant protein with an internal duplication of SCR1–4 associated with familial C3G, emphasizing the relevance of the duplication of the dimerization domains in FHR-1 in the pathogenesis of C3G.26 To characterize the novel mutant protein, rFHR-1, rFHR-1mut, and rFHR-1mon were generated in HEK293 cells and purified from the culture supernatants by affinity chromatography (Figure 1A).
Duplication of Dimerization Domains Allows Multimerization of the Mutant FHR-1 Protein
We previously postulated that the duplication of the dimerization domains in FHR-1 and FHR-5 leads to the multimerization of the proteins.11,26 To provide a detailed characterization of this multimerization, we analyzed the solution structure of the proteins by analytic ultracentrifugation sedimentation velocity experiments. Sedimentation coefficients of rFHR-1 (3.8S) and rFHR-1mon (2.7S) illustrate that these proteins fit best with a model of a moderately elongated dimer and a monomer, respectively (Figure 1B). Interestingly, the sedimentation profile of rFHR-1mut showed a complex mixture of different species. Up to 60.3% of the protein had a sedimentation coefficient of 4.6S, 30% presented a coefficient of 6.5S, and the remaining 9.4% had a coefficient of 8.7S, which—according to the theoretic mass of the protein—are compatible with a slightly elongated dimer, a trimer, and a tetramer, respectively (Figure 1B). These data demonstrate that the mutant FHR-1 forms significant numbers of higher order complexes under these experimental conditions.
Increased Binding of FHR-1 Mutant Protein to Surface-Bound C3 Activated Fragments and Native C3
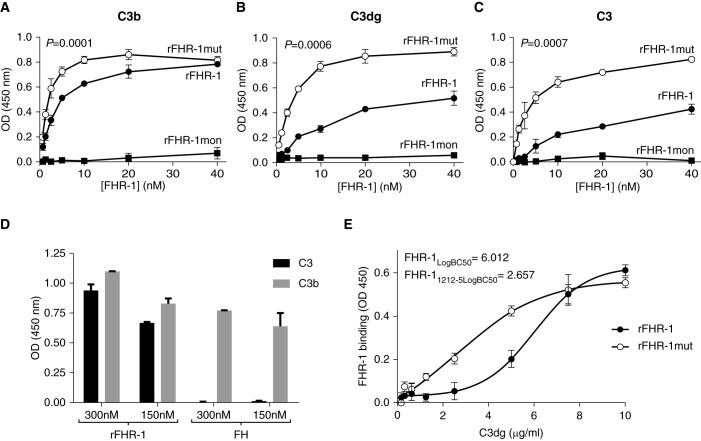
We next explored if the duplication of the dimerization domains in FHR-1 influences the interaction with surface-bound C3b and C3dg in microtiter plate assays. In parallel, we also investigated the binding of FHR-1 to native C3, because we recently reported this interaction and discussed its potential physiologic relevance.10 In all cases, rFHR-1mut bound more efficiently than rFHR-1 to C3, C3b, and C3dg, whereas rFHR-1mon binding was not observed in these conditions (Figure 2, A–C). The lack of FH binding to immobilized C3 allowed us to exclude the presence of any traces of C3b or C3(H2O) in the C3 preparation (Figure 2D). In addition, the binding of FHR-1 to C3 was also confirmed in a reverse setting (i.e., binding of fluid-phase C3 to immobilized FHR-1) to exclude any possible C3 conformational changes due to the immobilization (Supplemental Figure 6).
Figure 2.
The duplication of the dimerization domain in FHR-1 increases the binding of the protein to surface-bound C3 activated fragments and native C3. (A–C) The binding of increasing amounts of rFHR- 1, rFHR-1mut, and rFHR-1mon to C3b-, C3dg-, and C3-coated microtiter plates illustrate an enhanced binding capacity of the mutant compared with the wild type, whereas binding of rFHR-1mon cannot be observed in this setting. In all panels, a representative experiment of a minimum of two independent experiments is shown. Data are shown as means±SD of triplicates. P values resulted from a one-way ANOVA analysis. (D) Binding of rFHR-1 and FH at two different concentrations (150 and 300 nM) to immobilized C3b and C3 (5 µg/ml). The lack of FH binding to C3 rules out any contamination of C3(water) in the C3 preparation. (E) Binding of rFHR-1 and rFHR-1mut to microtiter plates coated with different densities of C3dg molecules. The values of the interpolated C3dg density required for 50% protein binding (LogBC50) illustrated that rFHR-1mut requires less than half the density of C3dg on the surface than rFHR-1. A representative experiment of three independent experiments is shown. Data are shown as means±SD of triplicates.
To test whether the enhanced binding ability of the mutant protein provides an advantage in the context of surfaces with low density of C3-activated products, we coated C3dg at different concentrations on microtiter plates and assayed the binding of rFHR-1 and rFHR-1mut proteins. Notably, the binding of rFHR-1mut was significantly higher than that of rFHR-1 at lower C3dg concentrations and, as expected, this difference disappeared under saturated C3dg conditions (Figure 2E).
FHR-1 Binding to Glomeruli In Vivo is C3 Dependent and Enhanced by Multimerization
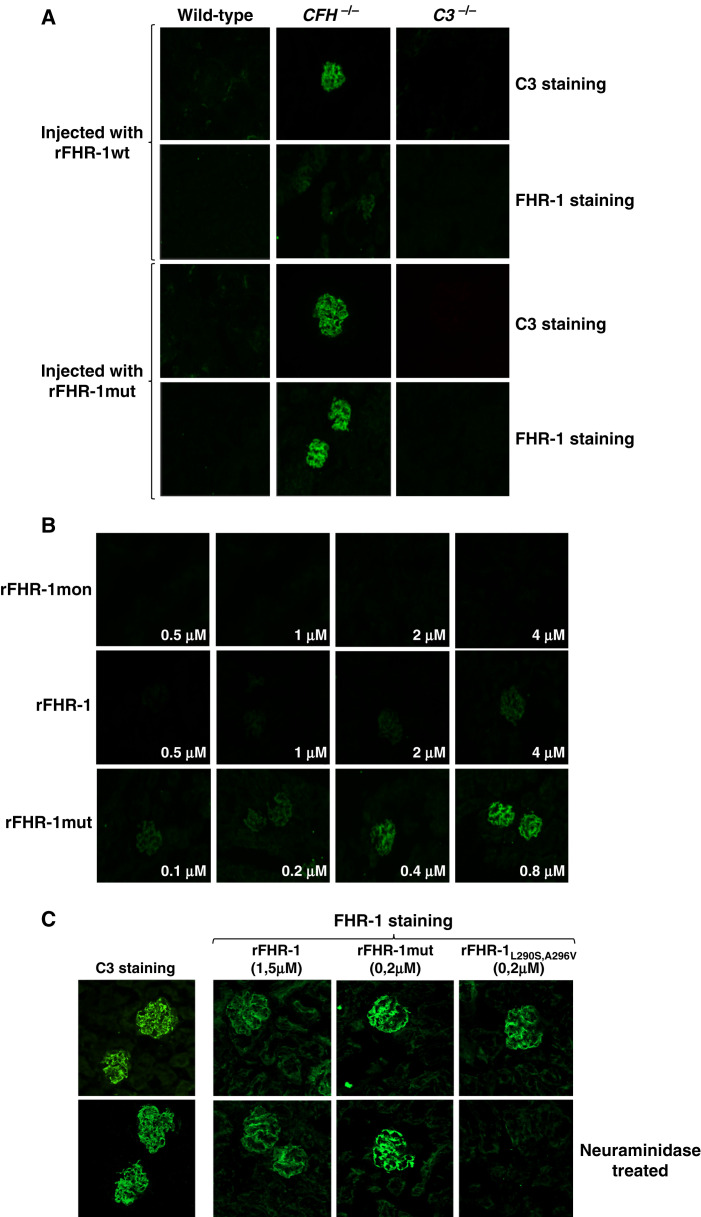
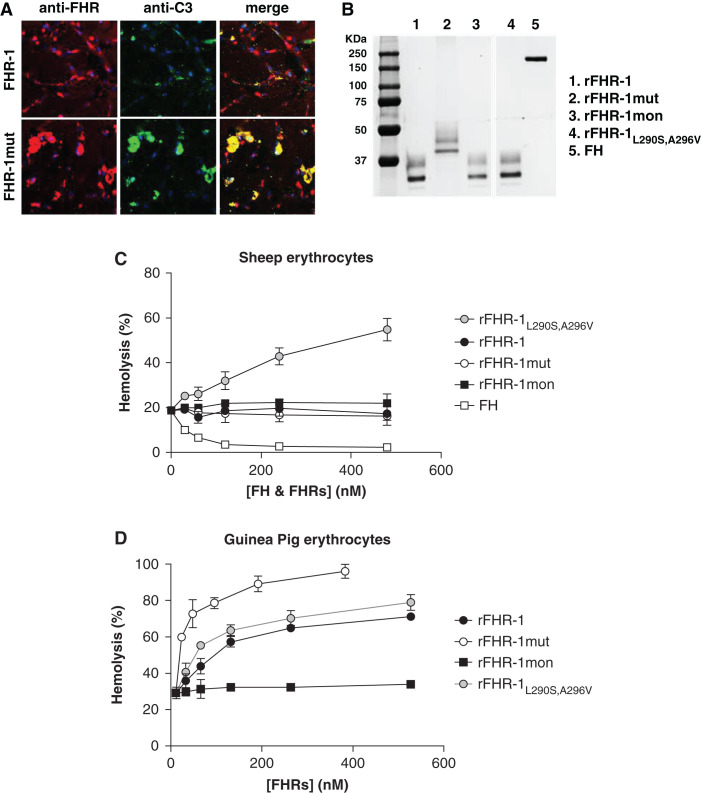
To study the binding of FHR-1 to C3 molecules in vivo, the different rFHR-1 preparations were injected into wild-type, C3-deficient (C3−/−), and FH-deficient (Cfh−/−) mice. Immunofluorescence experiments in kidney sections of the injected mice showed that rFHR-1 and rFHR-1mut, but not rFHR-1mon, bound only to the glomeruli of Cfh−/− mice, and that the binding of the mutant protein was stronger (Figure 3A). To quantify these differences, we performed ex vivo experiments, incubating kidney sections of Cfh−/− mice with different amounts of the proteins, and found that rFHR-1mut binds about ten times more efficiently than rFHR-1 (Figure 3B). As expected, the binding of these proteins to the glomeruli was dependent on the presence of C3-activated fragments, and it was not observed in wild-type or C3−/− animals (Figure 3A). Importantly, removing sialic acid from the kidney section using neuraminidase did not affect the binding of rFHR-1 or rFHR-1mut (Figure 3C).
Figure 3.
Binding of FHR-1 to the glomeruli is dependent on C3 deposition and independent of the presence of sialic acid on the surface. (A) Immunofluorescence analysis of renal C3 and FHR-1 proteins in kidney sections of Cfh−/−, wild-type, and C3−/− mice injected with identical amounts of either rFHR-1wt or rFHR-1mut. A significantly higher binding of rFHR-1mut compared with rFHR-1wt was observed in the Cfh−/− mice. This binding was dependent on C3 deposition because it was not observed in wild-type or C3−/− mice, which are negative for C3 staining. (B) Ex vivo, dose-dependent binding of FHR-1 proteins to kidney sections of Cfh−/− animals. Increasing amounts of rFHR-1mon (1–8 µg), rFHR-1 (1–8 µg), and rFHR-1mut (0.25–2 µg) were incubated with kidney sections of Cfh−/−mice, and the binding of these proteins to the surface was detected with the anti-FHR-1 2C6 mAB. (C) Ex vivo binding experiments of FHR-1 proteins to cryostat kidney sections of Cfh−/− mice untreated or treated with neuraminidase. The binding of the rFHR-1wt and rFHR-1mut to the glomeruli is not affected by treatment with neuraminidase. In contrast, binding of the aHUS-associated FHR-1L290S,A296V mutant protein is greatly affected, as previously described.10 This protein was used as a positive control to confirm the removal of sialic acids from the surface. Original magnification, ×40.
Wild-Type and Mutant FHR-1 Proteins Do Not Compete with FH for Binding to C3b Deposited in a Physiologic Surface
Because FHR-1 shares the C-terminal C3b-binding domain with FH, it has been assumed that wild-type FHR-1 can outcompete FH binding to surface-bound C3b. This assumption has traditionally been supported by competition assays in C3b-coated ELISA plates.11 However, the physiologic relevance of this competition assay is questionable because we now know that FHR-1 does not bind sialic acids, which should hamper a putative competition with FH for binding to C3b deposited in physiologic surfaces, where FH binding is dramatically enhanced by the presence of sialic acids.10 To provide a formal demonstration that FHR-1 proteins do not compete with FH for C3b binding at sialic acid–containing surfaces, we developed a competition assay using ShEs, in which ShEs were opsonized with C3b and incubated with increasing amounts of FHAF488. The detection of either C3 or FH on the ShE was then analyzed by flow cytometry, which showed that the binding of FHAF488 to C3b-opsonized ShEs is dose dependent (Figure 4, A–C). To prove the presence of sialic acid in the ShE is also critical for the binding of FH to the surface, we competed with the binding of FHAF488 by adding either FHwt or an FH mutant protein (FHS1191W) unable to bind sialic acids.10,27 As expected, FHAF488 binding of was markedly reduced in the presence of FHwt, but was not affected by FHS1191W (Figure 4, D and E, and Supplemental Figure 7). In this setting, addition of FHR-1wt or FHR-1mut did not affect the FHAF488 binding either, illustrating that none of these FHR-1 proteins compete with the binding of FH to C3b in the context of sialic acid–containing physiologic surface (Figure 4, D and E, Supplemental Figure 7). In contrast, the addition of the aHUS-associated FHR-1 mutant proteins FHR-1L290S,A296V and FHR-1L290V, which bind sialic acids,10,28,29 significantly reduced binding of FHAF488 (Figure 4, D and E, Supplemental Figure 7).
Figure 4.
FH binding to C3b deposited in a physiological surface is not competed by wild-type FHR-1 nor the mutant protein with duplicated dimerization domains. (A) First, antibody-sensitized ShEs were C3b opsonized by incubating cells with NHS depleted of FH and FB (NHSΔFH/FB) in the presence of OmCI to prevent C5 activation. As a negative control, ShEs incubated with NHSΔFH/FB in the presence of EDTA (EDTA-ShE) were used. Opsonization of the cells (C3-ShE, red curve) was confirmed with an in-house monoclonal mouse anti-human C3 antibody (12.17) and a goat anti-mouse IgG phycoerythrin-conjugated antibody (PE) and, as expected, EDTA-ShE (gray curve) were not stained. (B and C) Second, the binding of increasing amounts (3.125–100 nM) of FHAF488 to the ShE was analyzed and showed a FHAF488 dose–dependent binding to the opsonized C3-ShE, whereas the binding to nonopsonized EDTA-ShE was absent at the maximum protein concentration. Competitive binding of 50 nM FHAF488 to C3-ShE was then performed using equimolar amounts of FHwt, FHS1191W (a mutant that does not have the capacity to bind sialic acids), FHR-1wt, FHR-1mut, and the aHUS-associated mutant proteins FHR-1L290S,A296V and FHR-1L290V. Equimolar amounts of BSA were used as a negative control and, hence, represent the binding of FHAF488 to C3-ShE when there is no competition. As can be seen by (D) the flow cytometry curves or (E) by the geometric mean (GeoMean) representations, neither FHR-1wt, FHR-1mut, nor FHS1191W compete with the binding of FHAF488 to cells. Only the aHUS-associated FHR-1 mutant proteins or FHwt significantly displace the FHAF488 from the C3-ShE. Representative experiments of a minimum of two independent experiments are shown in all cases. Data in (E) correspond to the mean±SD of triplicates. **P<0.01, t test.
Surface-Bound FHR-1 Allows the Formation of the C3 Convertase and Increases Local Complement Deposition through the AP
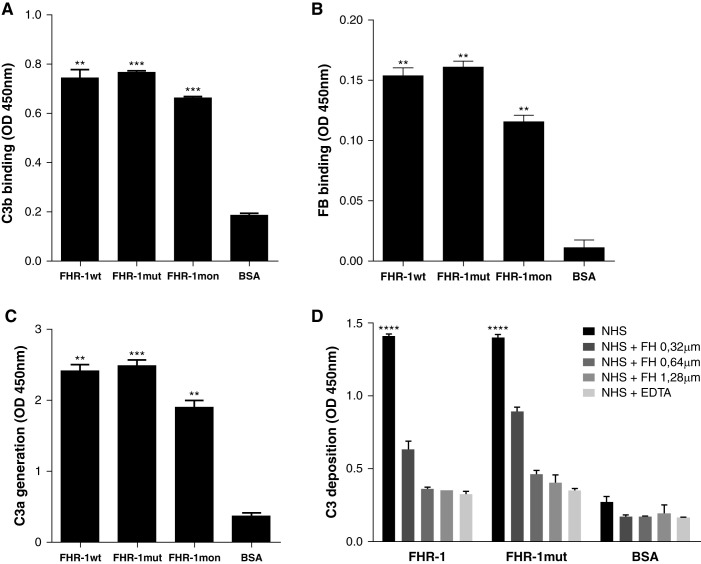
It was previously shown that the binding of C3b to surface-bound FHR-1, FHR-4, and FHR-5 does not interfere with the assembly of a fully active C3bBb convertase.12,30,31 To confirm that this is also true for FHR-1mut, we immobilized the different rFHR-1 preparations (wild-type, mutant, and monomer) on microtiter plates and, after incubation with C3b, FB, and FD, measured the formation of the convertase by detection of C3b and FB on the surface. As can be seen in Figure 5, the three FHR-1 proteins bound C3b and FB and formed a functional C3 convertase (Figure 5, A–C).
Figure 5.
Surface-bound FHR-1 proteins promote complement activation through the AP by supporting the formation of the C3 convertase. The sequential addition of purified C3b, FB, and FD on a microtiter plate coated with rFHR-1 proteins (wild type, mutant, and monomer) or BSA led to the formation of a C3 convertase only on rFHR-1 proteins, as illustrated by the detection of both (A) C3b and (B) FB. (C) The generation of a functional C3 convertase on FHR-1–coated surfaces is illustrated by the detection of C3a in supernatants after incubating C3 with the C3b-FB complexes previously formed, like in (A and B). (D) Deposition of C3 on microtiter plates coated with either rFHR-1 proteins or BSA and incubated with 10% NHS in the presence of buffer containing either EGTA or EDTA, or supplemented with increasing amounts of FH. Deposition of C3 was detected with the anti-C3 12.17 antibody. In all panels, a representative experiment of a minimum of two independent experiments is shown. Means±SD of triplicates are depicted. Statistical differences between the FHR-1 proteins and the BSA control were calculated. **P<0.01, *** P<0.001, ****P<0.0001, t test.
To further investigate the ability of surface-bound FHR-1 to activate the complement through the AP, microtiter plates coated with rFHR-1 and rFHR-1mut were incubated with NHS in the presence of EGTA or EDTA, and C3 deposition was measured. For both FHR-1 proteins, deposition of C3-activated fragments was observed in the presence of EGTA, and this deposition was abolished in the presence of EDTA, indicating FHR-1–coated surfaces promote complement activation through the AP (Figure 5D). Importantly, the addition of increasing amounts of FH prevented the deposition of C3-activated fragments.
FHR-1 Mutant Enhances Complement Activation and C3 Deposition on Cell Surfaces
Taking advantage of the fact that FHR-1 binds to primary HREpiCs (Supplemental Figure 8), we also investigated if the presence of the mutant protein in NHS enhances complement deposition on these cell surfaces. To test this, HREpiCs were incubated with 20% NHS supplemented with rFHR-1 or rFHR-1mut, and were subsequently stained for C3 and FHR-1. Although both FHR-1 proteins bind to the HREpiCs, the mutant displayed stronger binding and enhanced C3 deposition (Figure 6A). Importantly, rFHR-1mut colocalized with C3 on the HREpiCs (Pearson correlation coefficient r=0.76), indicating the binding of the mutant to the cell surface promotes local complement activation.
Figure 6.
Complement regulation on surfaces can be overcome by the binding of FHR-1 proteins, and it is dependent on the surface context. (A) HREpiCs seeded on a cover glass were incubated with 20% NHS supplemented with either rFHR-1 (0.44 µM) or mutant rFHR-1 (0.44 µM). The deposition of FHR proteins (red staining) or C3 (green staining) was detected with the antibodies 2C6 and 12.17, respectively. Representative images of n=2 experiments are shown in each case. Original magnification, ×60. (B) Coomassie-stained SDS- PAGE gel of the recombinant FHR-1 proteins used in the FH-dependent hemolytic assays using either (C) ShEs or (D) GpEs. Before the addition of increasing concentrations of different rFHR-1 proteins, the conditions of the hemolytic assays were set to a basal lysis of 20%–30% (see Methods). rFHR-1L290S,A296V was used as a positive control for lysis. Conversely, FH was used as a control for the prevention of lysis. Percent hemolysis calculated as the mean±SD of three independent experiments.
We evaluated the ability of the various FHR-1 proteins (Figure 6B) to promote complement activation on different cell surfaces with two different FH-dependent hemolytic assays that used either ShEs or GpEs. With ShEs, none of the wild-type, mutant, or monomeric FHR-1 proteins had any effect on cell lysis (Figure 6C). However, the addition of an aHUS-associated FHR-1 mutant protein that has an identical C-terminal domain to FH (FHR-1L290S,A296V) increased hemolysis in this setting. In contrast, in GpE hemolytic assays, all proteins except rFHR-1mon were able to promote complement activation, with FHR-1mut having a stronger effect (Figure 6D), further demonstrating the effect of multimerization in promoting complement activation in this setting.
The FHR-1 Deficiency Protects from C3G Development and a Bad Prognosis
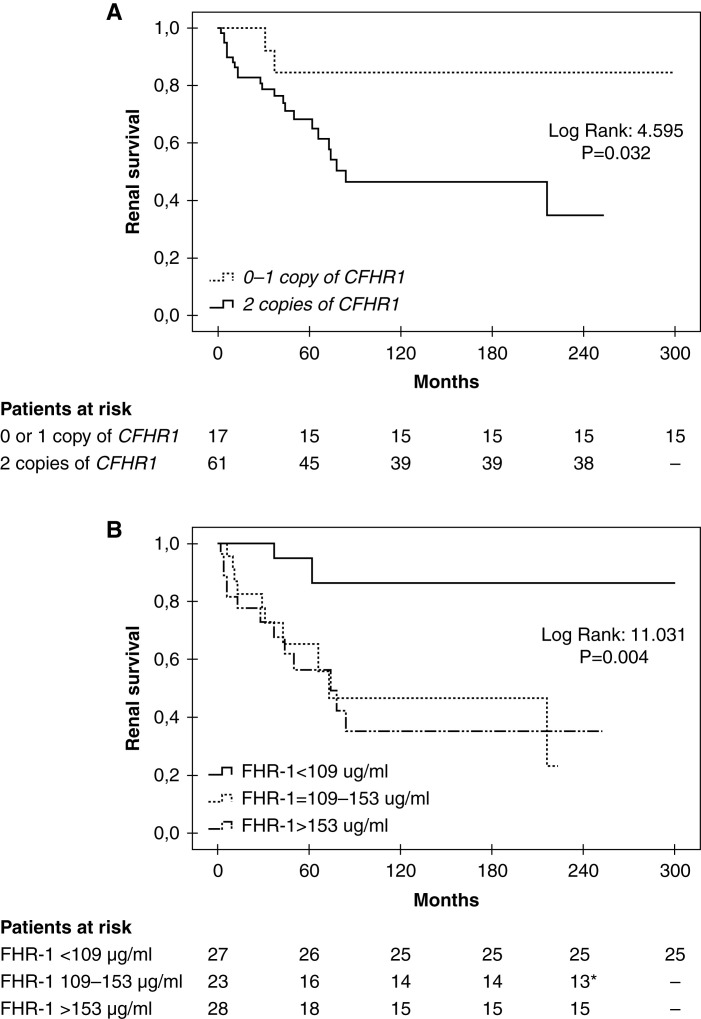
Knowing that enhanced FHR-1 activity is pathogenic in C3G, we then postulated that FHR-1 deficiency should be a protective factor for the development of the disease. To test this, we investigated whether the genomic deletion of CFHR3 and CFHR1 (ΔCFHR3-CFHR1) is negatively associated with C3G in a well-characterized Spanish C3G cohort (n=89, cohort 1).24 Patients’ baseline characteristics for this C3G cohort are shown in Table 1. The ΔCFHR3-CFHR1 allele frequency in C3G was significantly reduced compared with that of a Spanish control population (n=188), suggesting a decreased level of FHR-1 is a protective factor for the development of C3G (0.146 versus 0.229; P=0.03; odds ratio, 0.578; 95% CI, 0.36 to 0.93; Table 2). This finding was further confirmed in an independent and larger C3G cohort (n=217, cohort 2) obtained from the Spanish registry of patients with aHUS and C3G (Table 2). Interestingly, the deficiency of CFHR1 also had a positive effect on renal survival because patients either homozygous or heterozygous for the deletion of CFHR1 (considering the ΔCFHR3-CFHR1 and the deletion of CFHR1–CFHR4) presented with a lower rate of ESKD compared with patients without the deletion (Figure 7A). This effect was also observed when patients with pathogenic complement gene variants were excluded (Supplemental Figure 9). Consistently, patients with lower plasma FHR-1 levels at diagnosis were also associated with a better renal outcome (Figure 7B).
Table 2.
Genetic association of ΔCFHR3-CFHR1 with C3G in the Spanish population
| ΔCFHR3-CFHR1 status | Control (n=188) | C3G Cohort 1 (n=89) | C3G Cohort 2 (n=217) | ||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | P Value | n (%) | OR (95% CI) | P Value | |
| Genotype ΔCFHR3-CFHR1 | |||||||
| No deletion (%) | 113 (60) | 67 (75) | 2.02 (1.15 to 3.55) | 0.01 | 158 (73) | 1.78 (1.17 to 2.70) | 0.008 |
| Heterozygous (%) | 64 (34) | 18 (20) | 0.49 (0.27 to 0.89) | 0.02 | 52 (24) | 0.61 (0.40 to 0.94) | 0.03 |
| Homozygous (%) | 11 (6) | 4 (5) | 0.76 (0.23 to 2.45) | 0.64 | 7 (3) | 0.54 (0.20 to 1.41) | 0.23 |
| AF ΔCFHR3-CFHR1 | 0.229 | 0.146 | 0.58 (0.36 to 0.93) | 0.03 | 0.152 | 0.61 (0.42 to 0.86) | 0.007 |
AF, allele frequency; OR, odds ratio.
Figure 7.
Kaplan–Meier curves illustrate that lower number of CFHR1 copies or lower levels of plasma FHR-1 at diagnosis are associated with better renal survival. ESKD was considered as the event and a follow-up of 300 months is depicted. (A) The number of CFHR1 copies was established considering the presence of ΔCFHR3-CFHR1 and the deletion of CFHR1–CFHR4. (B) Plasma FHR-1 levels were determined in patient samples at diagnosis. Patients with a significant deterioration of renal function at diagnosis and who developed ESKD within the first month were excluded from the analysis. *A patient reached ESKD by month 216 and the maximum follow-up time for this group of patients was 223 months.
Discussion
The identification and characterization of a novel C3G-associated FHR-1 mutant protein with duplicated dimerization domains (FHR-1mut) provided us with novel insights into C3G pathogenesis. We demonstrate that FHR-1mut is a gain-of-function mutant with an enhanced capacity to bind to C3-opsonized surfaces and to promote complement activation through the AP. This advantage of the mutant FHR-1 over the wild-type protein stems from the mutant’s ability to form multimeric complexes. Importantly, the promotion of AP activation by FHR-1 does not involve competition with FH for binding to C3b. Instead, it is the attraction of fluid-phase C3 and C3b by excessive surface-bound FHR-1, leading to formation of the AP C3 convertase, that sustains complement activation and generation of additional surface-bound C3-activated fragments, overcoming FH regulation and resulting in complement dysregulation. The FH/FHR-1 ratio is, therefore, crucial to control complement amplification on opsonized surfaces. In agreement with these ideas, we found that the common polymorphism involving the genomic deletion ΔCFHR3-CFHR1 is a protective factor against the development of C3G, and that it is also associated with better renal outcomes in patients. Conversely, high plasma FHR-1 levels at diagnosis associate with a worse renal outcome.
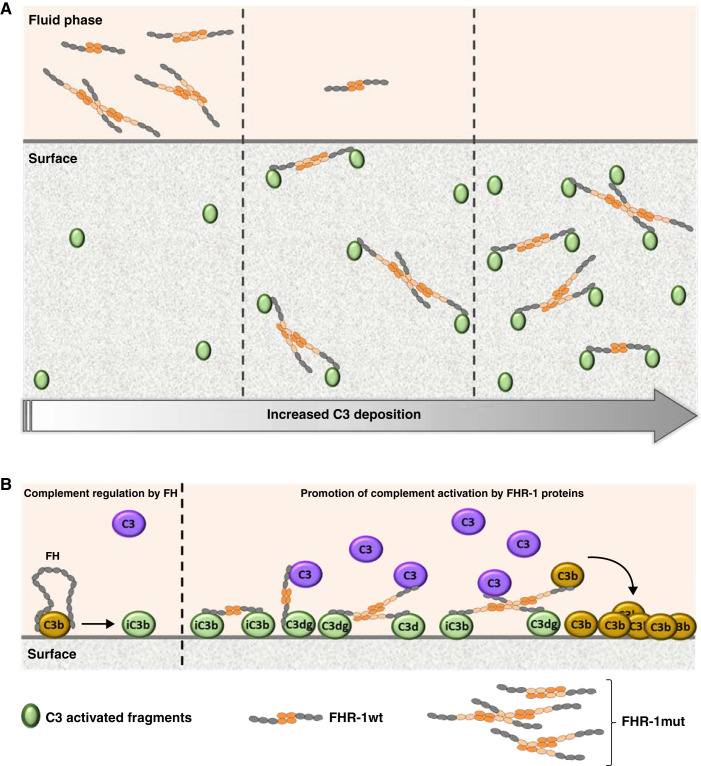
In this study, we performed analytic ultracentrifugation sedimentation velocity experiments to characterize the structural organization in solution of different FHR-1 proteins, and showed that FHR-1mut presents significant numbers of trimers and tetramers in addition to the dimers that characterize the wild-type protein. As a result, FHR-1mut presents an increased capacity to bind surface-bound C3, C3b, and C3dg compared with the wild-type protein. Importantly, similar findings were corroborated in vivo by injecting the different FHR-1 preparations into Cfh−/− mice, a well-known model of C3G with prominent glomerular C3 deposition.32 Of note, the presence of C3 deposits (i.e., iC3b, C3dg, and C3d) is an absolute requirement for the binding of FHR-1 proteins to glomeruli. Two nonmutually exclusive mechanisms explain the advantage of FHR-1mut in binding the different C3 molecules. First, the multimeric FHR-1 species have an enhanced avidity for the molecules because of an increased number of ligand interaction sites. On the other hand, duplication of the dimerization domain itself results in a larger FHR-1 molecule, which may provide an advantage in reaching C3 ligands in a surface when their density is low (Figure 8A). In fact, our data clearly show that FHR-1mut requires less C3 deposition to bind to a surface compared with the wild-type protein.
Figure 8.
Proposed mechanistic model for the promotion of complement activation by the C3G-associated FHR-1 mutations with duplicated dimerization domains. A two-step model is proposed on the basis of the main functional differences found between FHR-1wt and FHR-1mut. (A) First, FHR-1mut displays an enhanced avidity to bind C3-activated fragments, which translates into an advantage compared with the wild-type protein to bind to a surface when the density of C3 fragments is lower, as illustrated by the different scenarios with different degrees of C3 deposition. These different scenarios may be due to different time points but may also be related to different surface contexts. (B) Second, FHR-1 proteins bound to a C3-opsonized surface recruit C3 (predominantly) or C3b molecules from the fluid phase, increasing the concentration of potential components to form the AP C3 convertase, promoting an excessive complement activation and deposition of additional C3-activated fragments that overcome FH regulation. In this context, the multimeric forms of the mutant protein may also have an advantage compared with the wild-type protein to simultaneously bind to the C3-opsonized surface and recruit the C3 molecules from the fluid phase.
The biologic role of FHR-1 is controversial because both complement regulatory and activating functions have been described.11,12,26,33 The evidence reported here, showing that, in the presence of FHR-1 proteins, there is increased C3 deposition on cell surfaces and increased lysis in hemolytic assays, indicates that FHR-1 proteins promote complement activation on surfaces rather than prevent it. This promotion of complement activation was first interpreted as a consequence of the ligand competition between FH and FHR-1 for C3b binding, thus avoiding complement regulation by FH and allowing further activation (FH deregulation activity).6,11,26 In this context, FHR mutant proteins with duplicated dimerization domains were thought to be better complement deregulators because their increased avidity for C3b molecules would outcompete FH more efficiently. An important conclusion of this report is that C3b ligand competition between FHR-1 and FH is restricted to the FHR-1 mutants associated with aHUS that have acquired the capacity to bind sialic acids, and that wild-type FHR-1 and the mutants with duplicated dimerization domains do not compete with FH binding to C3b on physiologic surfaces. This is important because it provides a mechanistic explanation for the strict correlation that is observed between C-terminal FHR-1 mutants and aHUS, and between FHR-1 mutants with duplicated dimerization domains and C3G.6
Sialic acids, present in all mammalian cell surfaces, enhance the interaction of FH with surface-bound C3b and are the basis for the protection of host surfaces by FH from nonspecific AP complement activation.34 Wild-type FHR-1 lacks the capacity to bind sialic acids, which eliminates the potential competition with FH for surface-bound C3b and preserves regulation by FH in host surfaces.10 aHUS-associated FHR-1 mutant proteins are pathogenic because they acquire the capacity to bind sialic acids. This qualitative change in the molecule allows an efficient competition with FH for surface-bound C3b, a situation that compromises the regulation by FH of complement activation in the endothelium.10 We show here that, in contrast to aHUS, the C3G-associated FHR-1 mutants do not compete with FH for binding surface-bound C3b, despite their increased avidity for surface-bound C3 activated fragments. As a consequence, aHUS and C3G dysregulate the complement at different locations—aHUS-associated mutants at endothelial surfaces and C3G-associated mutants at C3-opsonized surfaces. Interestingly, this implies that, in C3G, the presence of FHR-1 exacerbates a previous condition that has resulted in the formation of an opsonized surface.
We also demonstrated that surface-bound FHR-1mut, like FHR-1 and the FHR-1 mutant associated with aHUS, supports the formation of the C3 convertase upon its interaction with fluid-phase C3b, promoting complement activation and further C3b deposition through the AP. Notably, this effect was counterbalanced by the regulatory activity of FH, illustrating that the balance between FHR-1/FH levels would determine the outcome of complement activation/regulation. FHR-1 also binds native C3. Because the concentration of C3 in plasma (approximately 1.2–1.5 mg/ml) largely exceeds that of circulating C3b, we expect the preferential ligand of FHR-1 would initially be C3. This C3 might then be activated, either spontaneously or through proteases, increasing the local concentration of the components of the C3 convertase. Hence, we propose that the pathogenic mechanism of C3G-associated FHR-1 mutations is based on an increased avidity for C3-opsonized surfaces, which increases promotion of complement activation at these sites and overcomes FH regulation (Figure 8).
This mechanistic understanding of the C3G-associated mutants implies that increased FHR-1 levels could also contribute to the development of C3G. This is supported by the identification of heterozygous duplications of CFHR-1 and high plasma FHR-1 levels in patients with C3G from our and other registries.35,36 Following this reasoning, we postulated that the deficiency of FHR-1 should be a protective factor against the development of the disease, a hypothesis that is consistent with the observation, reported here, that the allele frequency of ΔCFHR3-CFHR1 is significantly reduced in our Spanish C3G population. This finding corroborates a previous report that variation in CFHR3-CFHR1 copy number is associated with the C3G phenotype. We also report here that patients carrying one copy or no copies of CFHR1 or having lower plasma FHR-1 levels are associated with better renal outcomes, as compared with those carrying two copies of CFHR1 or having higher plasma FHR-1 levels. This illustrates that levels of FHR-1 also have an effect on renal survival in patients with C3G. Although FHR-1 levels are known to increase with renal function decline,25,37 our genetic data indicate that FHR-1 level is an independent prognostic factor of renal survival in patients with C3G. This is interesting because ΔCFHR3-CFHR1 has previously been associated with protection against the development of AMD and IgAN,13,14,25 and elevated plasma levels of FHR-1 are associated with advanced AMD and disease progression in IgAN,25,37,38 giving further support for the idea that the underlying pathogenic mechanisms leading to AMD and IgAN share common features with C3G.
In summary, our findings explain how the FHR-1 mutant proteins with duplicated dimerization domains predispose individuals to C3G and provide a pathogenic mechanism for C3G that may be extrapolated to other diseases, such as IgAN or AMD. Our findings also highlight the relevance of the FH/FHR-1 ratio and FHR-1 levels in C3G, further supporting the potential therapeutic benefit of inhibiting FHR-1.
Disclosures
F. Caravaca-Fontán reports receiving research funding from, and serving on a speakers bureau for, Novartis Pharmaceuticals. S. Elías reports receiving research funding from Mutua Madrileña. G. Fernández-Juarez reports receiving honoraria from GlaxoSmithKline, and receiving research funding from Instituto Salud Calos III. E. Goicoechea de Jorge reports receiving honoraria from Alexion Pharmaceuticals and Astellas. M.C. Pickering reports having consultancy agreements with Alexion Pharmaceuticals, Apellis Pharmaceuticals, and Gyroscope Pharmaceuticals; receiving honoraria from Alexion Pharma (consultancy fees), Apellis Pharmaceuticals (consultancy fees), and Gyroscope (for scientific advisory board fees); and serving on the scientific advisory board of Gyroscope Pharmaceuticals. M. Praga reports having consultancy agreements with Alexion, AstraZeneca, GlaxoSmithKline, Novartis, Silence, and Travere; receiving honoraria from Alexion, GlaxoSmithKline, Novartis, and Travere; and receiving research funding from Novartis. S. Rodríguez de Córdoba reports receiving research funding and honoraria from Alexion Pharmaceuticals, and having ownership interest in Secugen S.L. All remaining authors have nothing to disclose.
Funding
E. Goicoechea de Jorge is supported by Ministerio de Ciencia e Innovación grant RTI2018-095955-B-100 and the European Union’s Horizon 2020 Framework Programme grant 899163. J. Gutiérrez-Tenorio is supported by Ministerio de Ciencia e Innovación grant BES-2015-073833. L. Lucientes Continente is supported by the Autonomous Region of Madrid grant S2017/BMD-3673. G. Fernández-Juarez, P. Sánchez-Corral, B. Márquez-Tirado, and M. Praga are supported by the Instituto de Salud Carlos III and the European Union’s European Regional Development Fund grants PI19/01695, PI19/00970, and PI19/01624, respectively. M.C. Pickering is a Wellcome Trust Senior Fellow in Clinical Science (212252/Z/18/Z). S. Rodríguez de Córdoba is supported by the Ministerio de Economía y Competitividad grant PID2019-104912RB-100 and Autonomous Region of Madrid grant S2017/BMD-3673.
Supplementary Material
Acknowledgments
We are indebted to the patients with C3G, and their relatives, participating in this study, who form part of the aHUS/C3G national registry (http://www.ahusc3g.es/). We are also thankful to Emilia Arjona Bolaños for assisting and providing information related to the registry. We are also grateful to Héctor Martín-Merinero for providing the purified aHUS-associated mutant proteins FHR-1L290S,A296V, FHR-1L290V, and FHS1191L.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
F. Caravaca-Fontán, S. Elías, G. Fernández-Juarez, E. Goicoechea de Jorge, M.C. Pickering, M. Praga, S. Rodríguez de Córdoba, and A. Saiz Gonzalez were responsible for resources; E. Goicoechea de Jorge and S. Rodríguez de Córdoba conceptualized the study; E. Goicoechea de Jorge, J. Gutiérrez-Tenorio, L. Lucientes Continente, T.H. Malik, B. Márquez-Tirado, P. Sánchez-Corral, and A. Tortajada were responsible for investigation; E. Goicoechea de Jorge, B. Márquez-Tirado, R. Roldán Montero, and A. Saiz Gonzalez were responsible for methodology; E. Goicoechea de Jorge and M.C. Pickering provided supervision; E. Goicoechea de Jorge, S. Rodríguez de Córdoba, and P. Sánchez-Corral reviewed and edited the manuscript; and E. Goicoechea de Jorge wrote the original draft and was responsible for formal analysis, funding acquisition, and project administration.
Data Sharing Statement
All data is included in the manuscript and/or supporting materials.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021101318/-/DCSupplemental.
Supplemental Appendix 1. Description of main findings in a case of recurrent C3 glomerulopathy.
Supplemental Appendix 2. Clinical synopsis of patient GN185.
Supplemental Appendix 3. Supplemental Methods.
Supplemental Table 1. Patient’s complement profile during the third relapse.
Supplemental Figure 1. Measurement of plasma FHR-1 by sandwich enzyme-linked immunosorbent assay (ELISA).
Supplemental Figure 2. Patient’s clinical history and renal histology findings.
Supplemental Figure 3. An internal duplication in CFHR1 segregates with C3G in pedigree GN185.
Supplemental Figure 4. Immunohistochemistry of complement components in the patient’s renal tissue.
Supplemental Figure 5. Patient’s renal function evolution and treatments and evaluation of C5 inhibition by eculizumab.
Supplemental Figure 6. Binding of C3 to surface-bound FHR-1.
Supplemental Figure 7. FH C3b binding competition assay in sheep erythrocytes (ShE).
Supplemental Figure 8. FHR-1 binding to human renal epithelial (HREpi) cells.
Supplemental Figure 9. Kaplan-Meier curves for kidney survival according to the presence of CFHR1 copy number in patients without complement pathogenic variants.
Supplemental Appendix 4. References.
References
- 1.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, et al. : C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RJH, Appel GB, Blom AM, Cook HT, D’Agati VD, Fakhouri F, et al. : C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat Rev Nephrol 15: 129–143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Meyer NC, Wang K, Nishimura C, Frees K, Jones M, et al. : Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol 7: 265–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corvillo F, Okrój M, Nozal P, Melgosa M, Sánchez-Corral P, López-Trascasa M: Nephritic factors: An overview of classification, diagnostic tools and clinical associations. Front Immunol 10: 886, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donadelli R, Pulieri P, Piras R, Iatropoulos P, Valoti E, Benigni A, et al. : Unraveling the molecular mechanisms underlying complement dysregulation by nephritic factors in C3G and IC-MPGN. Front Immunol 9: 2329, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Józsi M, Tortajada A, Uzonyi B, Goicoechea de Jorge E, Rodríguez de Córdoba S: Factor H-related proteins determine complement-activating surfaces. Trends Immunol 36: 374–384, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Pangburn MK, Schreiber RD, Müller-Eberhard HJ: Human complement C3b inactivator: Isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med 146: 257–270, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiler JM, Daha MR, Austen KF, Fearon DT: Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A 73: 3268–3272, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whaley K, Ruddy S: Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med 144: 1147–1163, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin Merinero H, Subías M, Pereda A, Gómez-Rubio E, Juana Lopez L, Fernandez C, et al. : Molecular bases for the association of FHR-1 with atypical hemolytic uremic syndrome and other diseases. Blood 137: 3484–3494, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, et al. : Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A 110: 4685–4690, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csincsi AI, Szabó Z, Bánlaki Z, Uzonyi B, Cserhalmi M, Kárpáti É, et al. : FHR-1 binds to C-reactive protein and enhances rather than inhibits complement activation. J Immunol 199: 292–303, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, et al. : Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U: A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet 38: 1173–1177, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, et al. : Association of genetic variants in complement factor h and factor h-related genes with systemic lupus erythematosus susceptibility. PLoS Genet 7: e1002079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Wiesener M, Eberhardt HU, Hartmann A, Uzonyi B, Kirschfink M, et al. : Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest 124: 145–155, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, et al. : Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schramm EC, Roumenina LT, Rybkine T, Chauvet S, Vieira-Martins P, Hue C, et al. : Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood 125: 2359–2369, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao X, Ghossein C, Tortajada A, Zhang Y, Meyer N, Jones M, et al. : Familial C3 glomerulonephritis caused by a novel CFHR5-CFHR2 fusion gene. Mol Immunol 77: 89–96, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Corral P, Pouw RB, López-Trascasa M, Józsi M: Self-damage caused by dysregulation of the complement alternative pathway: Relevance of the factor H protein family. Front Immunol 9: 1607, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu S, Narita M, Tsujimoto Y: Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Subías Hidalgo M, Yébenes H, Rodríguez-Gallego C, Martín-Ambrosio A, Domínguez M, Tortajada A, et al. : Functional and structural characterization of four mouse monoclonal antibodies to complement C3 with potential therapeutic and diagnostic applications. Eur J Immunol 47: 504–515, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Hou J, Markowitz GS, Bomback AS, Appel GB, Herlitz LC, Barry Stokes M, et al. : Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 85: 450–456, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Caravaca-Fontán F, Díaz-Encarnación MM, Lucientes L, Cavero T, Cabello V, Ariceta G, et al. ; Spanish Group for the Study of Glomerular Diseases GLOSEN : Mycophenolate mofetil in C3 glomerulopathy and pathogenic drivers of the disease. Clin J Am Soc Nephrol 15: 1287–1298, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tortajada A, Gutiérrez E, Goicoechea de Jorge E, Anter J, Segarra A, Espinosa M, et al. : Elevated factor H-related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int 92: 953–963, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, et al. : C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest 123: 2434–2446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín Merinero H, Zhang Y, Arjona E, Del Angel G, Goodfellow R, Gomez-Rubio E, et al. : Functional characterization of 105 factor H variants associated with aHUS: Lessons for variant classification. Blood 138: 2185–2201, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dopler A, Stibitzky S, Hevey R, Mannes M, Guariento M, Höchsmann B, et al. : Deregulation of factor H by factor H-related protein 1 depends on sialylation of host surfaces. Front Immunol 12: 615748, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goicoechea de Jorge E, Tortajada A, García SP, Gastoldi S, Merinero HM, García-Fernández J, et al. : Factor H competitor generated by gene conversion events associates with atypical hemolytic uremic syndrome. J Am Soc Nephrol 29: 240–249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csincsi AI, Kopp A, Zöldi M, Bánlaki Z, Uzonyi B, Hebecker M, et al. : Factor H-related protein 5 interacts with pentraxin 3 and the extracellular matrix and modulates complement activation. J Immunol 194: 4963–4973, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebecker M, Józsi M: Factor H-related protein 4 activates complement by serving as a platform for the assembly of alternative pathway C3 convertase via its interaction with C3b protein. J Biol Chem 287: 19528–19536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, et al. : Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31: 424–428, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse HM, Schirmer S, et al. : Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 114: 2439–2447, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T: Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol 11: 77–82, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Loeven MA, Maciej-Hulme ML, Yanginlar C, Hubers MC, Kellenbach E, de Graaf M, et al. : Selective binding of heparin/heparan sulfate oligosaccharides to factor H and factor H-related proteins: Therapeutic potential for C3 glomerulopathies. Front Immunol 12: 676662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piras R, Breno M, Valoti E, Alberti M, Iatropoulos P, Mele C, et al. : CFH and CFHR copy number variations in C3 glomerulopathy and immune complex-mediated membranoproliferative glomerulonephritis. Front Genet 12: 670727, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H, Willicombe M, McLean AG, Brookes P, et al. : Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int 92: 942–952, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorés-Motta L, van Beek AE, Willems E, Zandstra J, van Mierlo G, Einhaus A, et al. : Common haplotypes at the CFH locus and low-frequency variants in CFHR2 and CFHR5 associate with systemic FHR concentrations and age-related macular degeneration. Am J Hum Genet 108: 1367–1384, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.