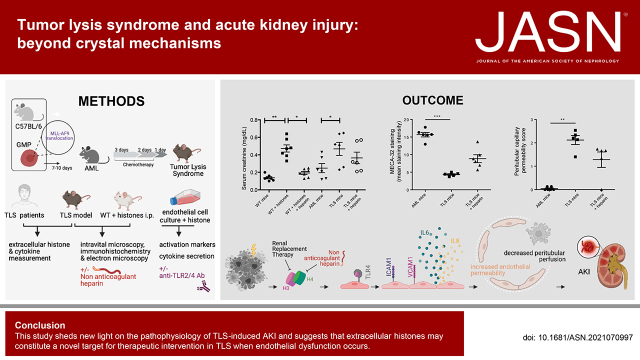
Significance Statement
The pathophysiology of AKI during tumor lysis syndrome (TLS) is not fully understood. We aimed to decipher crystal-dependent and crystal-independent mechanisms of TLS-induced AKI. Analyzing urine and blood from patients with TLS provided data on crystal-independent mechanisms of the pathogenesis of AKI during TLS. We also explored mechanisms of TLS-induced AKI in vitro and in vivo in a murine model of TLS (syngeneic mice with acute myeloid leukemia receiving chemotherapy). We found that extracellular histones released in huge amounts during TLS profoundly alter the endothelium. Nonanticoagulant heparin mitigated AKI in this model.
Keywords: acute renal failure, endothelium, histones, tumor lysis syndrome
Visual Abstract
Abstract
Background
The pathophysiology of AKI during tumor lysis syndrome (TLS) is not well understood due to the paucity of data. We aimed to decipher crystal-dependent and crystal-independent mechanisms of TLS-induced AKI.
Methods
Crystalluria, plasma cytokine levels, and extracellular histones levels were measured in two cohorts of patients with TLS. We developed a model of TLS in syngeneic mice with acute myeloid leukemia, and analyzed ultrastructural changes in kidneys and endothelial permeability using intravital confocal microscopy. In parallel, we studied the endothelial toxicity of extracellular histones in vitro.
Results
The study provides the first evidence that previously described crystal-dependent mechanisms are insufficient to explain TLS-induced AKI. Extracellular histones that are released in huge amounts during TLS caused profound endothelial alterations in the mouse model. The mechanisms of histone-mediated damage implicates endothelial cell activation mediated by Toll-like receptor 4. Heparin inhibits extracellular histones and mitigates endothelial dysfunction during TLS.
Conclusion
This study sheds new light on the pathophysiology of TLS-induced AKI and suggests that extracellular histones may constitute a novel target for therapeutic intervention in TLS when endothelial dysfunction occurs.
Tumor lysis syndrome (TLS) is a life-threatening complication of cancer treatment in patients with proliferative and/or chemosensitive malignancies with high tumor burden. TLS occurs either as a consequence of diverse anticancer treatments or, less frequently, spontaneously. Cancers with high potential for cell lysis include acute leukemias, high-grade lymphomas, and other rapidly proliferating tumors.1,2 Tumor cell lysis results in the release of intracellular ions and metabolites into the circulation, thus leading to hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. Clinical TLS is present when laboratory TLS is accompanied by an increased creatinine level, seizures, cardiac dysrhythmia, or even multiple organ failure and death.1 TLS sometimes mimics sepsis, presenting as systemic inflammatory response syndrome with multiple organ failures.3 TLS-induced AKI, occurring in up to 64% of patients with TLS, strongly reduces the likelihood of complete remission of the underlying malignancy and is associated with a higher mortality rate.4 Crystal-induced kidney injury occurs in TLS when calcium phosphate, uric acid, and xanthine precipitate in renal tubules. Nowadays, most patients prone to development of TLS receive rasburicase. Moreover, urine alkalinization that may increase calcium-phosphate precipitation is no longer recommended. The pathologic mechanisms underlying TLS-induced AKI are not well defined because of a scarcity of data. Studies published so far consist mainly of individual case reports.5,6 Indeed, case reports that described renal calcium-phosphate crystals during TLS were published in 1979 and 1984, when alkalinization was largely used in the management of TLS.5,6 Moreover, in these case reports, patients had extreme phosphatemia levels (>5 mmol/L) that are rarely seen during TLS because most patients would undergo dialysis before reaching such levels. Many patients with TLS experience AKI without any evidence of hyperphosphatemia or hyperuricemia-induced crystals, especially because rasburicase, a recombinant urate oxidase, is considered a standard of care treatment for patients at high risk of TLS.7 Darmon et al.4 have shown, in a study focusing on high-risk patients with TLS and AKI in the rasburicase era, that 28.3% of patients with TLS experienced AKI, 53% of them requiring RRT. These results are similar to those of previous studies of TLS undertaken before the widespread use of rasburicase, when allopurinol and alkalinization were mainly used, with AKI occurring in up to one third of patients with TLS.8,9
Extracellular histones are increasingly recognized for their cytotoxic effects. Histones consist of five cationic proteins (H1, H2A, H2B, H3, and H4) in the nucleus of eukaryotic cells. When cell death is extensive, high levels of unchained extracellular histones are released that convey cytotoxic effects.10 The vascular endothelium forms the active interface between blood and tissues and can thereby potentiate multiple organ failure. In this context, some authors have suggested that extracellular histones may contribute to endothelial dysfunction during sepsis or trauma.10 To date, extracellular histones have not been examined in the context of the pathophysiology of TLS.
In this study, we aimed to decipher crystal-dependent and -independent mechanisms of TLS- induced AKI. To this end, we studied crystalluria and the effect of crystals on AKI in patients with TLS. We developed a model of TLS in syngeneic mice with acute myeloid leukemia (AML), analyzed ultrastructural changes in kidneys, and found profound endothelial alterations, leading us to interrogate the role of extracellular histones in endothelial dysfunction during TLS.
Methods
Patients
Plasma samples were obtained from two cohorts of patients.
First Cohort
Frozen plasma samples were obtained from an existing and available biobank of 736 patients with hematologic malignancies admitted to the intensive care unit (ICU). The study was carried out in 17 ICUs participating in a research network, initiated in 2005, in France and Belgium.11 From January 2010 to May 2011, consecutive patients with hematologic malignancies admitted to the ICUs for any reason were included. Exclusion criteria were complete cure of the malignancy for >5 years and age <18 years. In each center, investigators used a standardized electronic case report form to collect the study data. Clinical data, biologic data, and plasma were collected prospectively. A total of 94 patients were diagnosed as having TLS at admission. Laboratory TLS and clinical TLS were defined on the basis of the daily recorded laboratory and clinical values, using the Cairo–Bishop criteria (Supplemental Table 1).12 Definition and staging of AKI were defined on the basis of serum creatinine and urine output criteria, according to Kidney Disease Improving Global Outcomes guidelines (Supplemental Table 2).13
Peripheral blood samples were collected within 24 hours of ICU admission and processed within 2 hours of sampling. Specimens were centrifuged at 1500 × g for 10 minutes at 20°C, and the plasma was stored as 500-μl aliquots at −80°C.
Urine samples were unavailable in this cohort.
Second Cohort
In parallel, a prospective reliability cohort of 55 patients with TLS (hospitalized in the Department of Intensive Care Medicine, Hôpital Saint Louis) were analyzed. All patients included in this cohort presented with laboratory or clinical TLS on admission. Uricemia was assessed every 6 hours during the first 72 hours of TLS, and then every 12 hours until day 7. Urine and plasma samples were collected prospectively at day 1, 3, 5, and 7.
Plasma from healthy blood donors were obtained in accordance with institutional regulations from the Etablissement Français du Sang, Hôpital Saint Louis, Paris, France.
Clinical and Biologic Data
The following clinical and biologic data were recorded: demographic parameters, medical history, presenting symptoms and treatments. Liver enzyme levels, lactate dehydrogenase (LDH) levels, calcemia, uricemia, and phosphatemia were recorded at day 1, 2, 3, and 7. Serum creatinine was recorded daily throughout the patients’ stay in the ICU and at 3 months. The Sequential Organ Failure Assessment score was computed at admission then daily throughout the patient’s stay in the ICU, as previously described.14 We assessed patients’ vital status at ICU discharge, hospital discharge, and at 3 and 6 months.
Crystalluria
Crystalluria was analyzed at day 1, 3, 5, and 7 using a phase contrast microscope, with a polarized light device (Laboratoire d’Explorations Fonctionnelles, Hôpital Tenon, Paris, France). Urine samples brought to the laboratory within 2 hours of voiding were kept at room temperature and were processed rapidly. Urine-specific gravity and pH were measured. Undiluted urine was then homogenized by gentle shaking and rotation (neither centrifuged nor filtered) and 10 mm3 was immediately placed in a Malassez cell (CML, Nemours, France) for examination by light microscopy using a polarizing microscope (Optiphot-2; Nikon, Champigny-sur-Marne, France). The entire cell was examined at 200× magnification to localize crystals and aggregates, and then at 400× magnification (high power field). All crystals and aggregates were counted on the entire cell and their size determined using the included micrometric scale. The results were expressed as number of crystals per millimeter cubed. Only one Malassez cell was examined in each instance. Examiners of crystalluria were blinded to the clinical status of the patients.
ELISA
A multiplex assay was used to measure soluble factors in patients with TLS, including IL-6, IL-8, TNF-α, soluble intercellular adhesion molecule 1 (sICAM-1), IL-1β, IL-10, and IFN-γ (V-PLEX Proinflammatory Panel 1, Meso Scale Discovery).
The concentrations of cytokines and chemokines, including IL-6 and IL-8, in culture supernatants were determined using ELISA kits (Human ELISA MAX; BioLegend). The manufacturer’s instructions were followed, and the concentrations were determined by spectrophotometry at 450 nm (Molecular Devices).
Extracellular Histone Measurements
We measured the levels of extracellular histones in the plasma of patients and mice using a Cell Death Detection ELISA kit (Roche Applied Science, Penzberg, Germany). Purified mixed calf thymus histones were used to generate standard curves.
Mice
Animal Experiments
C57BL/6 mice (8 weeks old) from Envigo were housed and studied under sterile conditions at the University Institute of Hematology (Hôpital Saint Louis).
AML Mice
For the in vivo model of TLS, we used a mouse model of AML driven by retroviral expression of a single mutational hit, the MLL-AF9 gene fusion, as previously described. 15,16 Expression of MLL-AF9 in normal granulocyte macrophage progenitor cells (GMPs) is sufficient to generate an aggressive, transplantable myeloid leukemia in syngeneic, immunocompetent C57BL/6 mice, with a clonal leukemic stem cell population that displays an immunophenotype similar to that of normal GMPs (Linlow, Sca-1, c-Kit+, CD16/32high, and CD34high). We further enriched for stem cell activity by serially transplanting the MLL-AF9–driven leukemias through secondary, tertiary, and quaternary recipients, generating leukemias with 100% penetrance in about 3–4 weeks. We established that cytarabine, given at 100 mg/kg daily for 5 days, in combination with doxorubicin, given at 1 mg/kg daily for the first 3 days, was the maximum tolerated chemotherapeutic regimen in this model. This treatment clears about 60%–70% of leukemic blasts from bone marrow. TLS occurs 24 hours after the end of the chemotherapeutic regimen. In the control group, mice received 150 µl of vehicle Hanks' balanced salt solution (HBSS). In mice who received heparin, we injected 3 mg/kg heparin intraperitoneally (i.p.) twice a day before and during the administration of chemotherapy.
Toll-Like Receptor 4 Knockout Mice
Toll-like receptor 4 (TLR4)–deficient female mice (Tlr4−/−, C57BL/6 background) and the corresponding wild-type (WT) mice were provided by Prof. Shizuo Akira (Osaka University, Osaka, Japan).
Injection of Recombinant Histones
C57BL/6 female mice were injected with recombinant histones (25 mg/kg body wt, i.p. injection) or vehicle (0.9% saline) (single bolus). Previously published data on mice using different doses of histones have shown that doses >50mg/kg were lethal.10 Other authors used 10–50 mg/kg according to studies and evidenced toxic effects of extracellular histones.17–19 Therefore, we chose an intermediate dose of 25 mg/kg i.p. in our study.
Clinical Chemistry Analyses in Mice
Serum creatinine was measured using a high-performance liquid chromatography method.20 BUN and LDH in serum were measured using commercially available kits and an automatic analyzer. Data are expressed as serum BUN in milligrams per deciliter and serum LDH concentration in international units per liter.
Intravital Microscopy Imaging
Intravital microscopy imaging was performed using two different techniques: an inverted Zeiss Axiovert fluorescent microscope (Zeiss, Paris, France) and confocal microscopy.
Intravital Confocal Microscopy
To visualize kidney microvascular perfusion and permeability in vivo, confocal microscopy was performed using an Olympus Inverted Microscope IX83 equipped with DSD2 system attached to an Andor Zyla camera (Andor Technology) and a 37°C heated chamber. Briefly, the left kidney of anesthetized mice was exposed by flank incision and gently pulled to allow insertion of a slit plate at the level of the renal artery and vein. This system allows the physical separation of the organ from the rest of the body. At that time, a total volume of 200 μl of albumin-FITC (100 μg/g body wt; 50 mg/ml stock solution; Sigma), was intravenously injected in the eye sinus. The animal was then flipped over to position the kidney on a window plate at the microscope stage, and image acquisition (one image every 5 seconds for 30 minutes) could start instantaneously.
Masked playback analysis of videotaped images were performed offline by a blind examinator. Leakage of albumin-FITC from peritubular capillaries into the interstitium (outside the vessels) was analyzed every 1 minute during the first 15 minutes of each video, using a semiquantitative score (from one to three) for each mouse.
Renal Microvascular Perfusion
Renal microvascular perfusion was analyzed in WT mice injected with recombinant histones or vehicle. Intravital video microscopy was performed as described previously.21 The left kidney was exposed by a flank incision and positioned on a glass stage above an inverted Zeiss Axiovert 200M fluorescent microscope equipped with an Axiocam HSm camera (Zeiss). Videos of 10 seconds (approximately 30 frames per second) at 200× magnification were acquired from five randomly selected, nonoverlapping fields of view. Body temperature was maintained at 35°C–37°C with a warming lamp. Approximately 150 capillaries were randomly selected and analyzed from these fields of view from the kidney of each animal. Capillaries were categorized as “continuous” if red blood cell movement was uninterrupted, “intermittent” if red blood cell movement stopped or reversed, or “no flow” if no red blood cell movement was observed. Data were expressed as the percentage of vessels in each of the three categories.
Morphologic Analysis
Kidneys were rapidly excised after euthanasia. Kidneys were partly fixed in alcohol-formalin– acetic acid solution, dehydrated, embedded in paraffin, and further processed for Masson trichrome staining or Periodic acid–Schiff staining (5-μm sections). Histologic changes in the cortex and in the outer stripe of the outer medulla were assessed by quantitative measurements of tissue damage, as previously described.22 Tubular damage was defined as tubular epithelial swelling, loss of brush border, vacuolar degeneration, necrotic tubules, cast formation, and desquamation. The degree of kidney damage was estimated at ×200 magnification using five randomly selected fields for each animal by the following criteria: zero, normal; one, areas of damage <25%; two, damage involving 25%–50% of tubules; three, damage involving 50%–75% of tubules; four, damage involving 75%–100% of tubules.
Immunohistochemical Analyses
Renal fragments embedded in paraffin were cut into 3-μm sections. Kidneys were immunostained with purified rat anti-mouse panendothelial cell antigen (MECA-32; BD Biosciences) (for peritubular capillary density), a purified rat anti-mouse CD54 antibody (ICAM-1; Biolegend), and a purified rabbit anti-cleaved PARP (Asp214) antibody (c-PARP; Ozyme) (for apoptosis analysis). Results were visualized using the Anti-Rat HRP-DAB Cell & Tissue Staining Kit (R&D Systems). Density of peritubular capillaries and staining intensity were determined on pictures at 200× magnification using ImageJ Fiji software, as previously described.23
Transmission Electron Microscopy
For scanning electron microscopy, kidneys were extracted and fixed for 24 hours in paraformaldehyde. Sections were postfixed in 1% buffered osmium tetroxide for 1 hour, dehydrated in an ascending series of ethanol with 1% uranyl acetate included in 70% ethanol, and flat embedded in epoxy mixture (Spurr resin). Ultrathin sections were cut using a Reichert Jung ultramicrotome (Leica, Nussloch, Germany) and collected on copper grids. Sections were stained on drops of lead citrate for 3 minutes and examined in a Hitachi H-7100 electron microscope (Hitachi, Tokyo, Japan). Digital images were acquired using a charge-coupled device camera (Hamamatsu AMT 540.3, Hamamatsu, Japan). The number of capillary fenestrations was semiquantitatively graded and expressed as a score between zero and four, with a score of zero representing 0%–5%, one representing 6%–25%, two representing 26%–50%, three representing 51%–75%, and four representing 76%–100% of the total endothelial capillary circumference having fenestrations (i.e., a score of four being the finding in healthy kidneys). The areas with the endothelial nucleus were omitted, and at least ten micrographs of capillaries per mouse taken at an original magnification of ×10,000 were evaluated. All analyses were performed in a blinded manner.
In Vitro Experiments
Cell Lines and Culture Reagents
Primary human renal glomerular endothelial cells (HRGECs) were purchased from Innoprot (Spain) and cultured in Endothelial Cell Medium (ScienCell), as previously described.24 The human dermal microvascular endothelial cell line (HMEC-1) was cultured in supplemented MCDB-131 medium (Thermo Fisher Scientific, Watham, MA), as previously described.24,25 For experiments using nonanticoagulant heparin, we used N-acetyl heparin sodium (Sigma-Aldrich).
Stimulation Assays
Highly-purified human recombinant histones H3 (New England BioLabs Inc., Ipswich, MA) were used for stimulatory experiments on HMEC-1s and HRGECs. For in vitro assays, 500,000 cells per well seeded in six-well plates were stimulated with 5–40 μg/ml of histones in cell culture media supplemented with 1% FCS. Unstimulated cells were used as negative control. Experiments were performed in triplicate, at a minimum.
Immunofluorescence Confocal Laser Microscopy
HMEC-1s were incubated with extracellular histones for 4 hours. A rabbit polyclonal antibody to histone H3 (1791; Abcam) was used as primary antibody, and a Cy3-conjugated anti-rabbit IgG antibody was used as secondary antibody. An anti-TLR4 primary antibody and a FITC-labeled secondary antibody were used to visualize TLR4, and 4′,6-diamidino-2-phenylindole (Vector) was used to stain the nuclei. Confocal imaging was performed with a Zeiss LSM 510 Confocal microscope.
Inhibition of TLR2 and TLR4
For TLR2 and TLR4 inhibition, endothelial cells (ECs) were preincubated with anti-TLR2 antibody (50 μg/ml; Thermo Fisher Scientific) or anti-TLR4 antibody (50 μg/ml; Thermo Fisher Scientific) for 1 hour before histone stimulation. Mouse IgG2a,κ antibody (50 μg/ml; Thermo Fisher Scientific) was used as an isotype control. A specific TLR4 antagonist, TAK-242 (1 μM, Tocris Biotechne), was also used to confirm specific inhibition of TLR4.
Statistical Analyses
For statistical analyses, we used Prism (GraphPad software). All data are expressed as mean±SEM. All metric data were tested for normal distribution using the Shapiro–Wilks W test. Differences between groups were analyzed for statistical significance by t test or one-way ANOVA for repeated measures and subsequent Bonferroni post hoc test. Non-normally distributed single measurements were compared using the Mann–Whitney U test or the Kruskal–Wallis test with Dunn post hoc test. A P value <0.05 was accepted as statistically significant.
Study Approval
Experimental studies on mice were approved by the European ethical committee (Autorisation de Projet Utilisant des Animaux à des Fins Scientifiques #8909).
First Cohort
The study and plasma collection were approved by the appropriate ethics committees in France and Belgium. No data allowing patient identification were collected. All patients or relatives gave their informed consent to study participation (Programme Hospitalier de Recherche Clinique AOM 08235).
Second Cohort
This project was approved by the appropriate ethical committee (Hôpitaux Universitaires Paris Nord Val de Seine Institutional Review Board number 00006477, Paris 7 University, Assistance Publique-Hôpitaux de Paris).
Results
Characteristics of Patients with TLS
A total of 94 patients with hematologic malignancies were included in the study in the first cohort and 55 patients in the second cohort. All 149 patients were admitted to the ICU with TLS. Of the 149 patients, 129 (86.6%) developed AKI during ICU stay (patients with TLS-AKI). Their clinical characteristics, laboratory findings, treatments, and outcomes are shown in Table 1 and Table 2.
Table 1.
Clinical characteristics, laboratory findings, treatment, and outcome of patients at admission (first cohort)
| Characteristic | TLS (n=94) | Patients with TLS-AKI (n=83) | Patients with Non-AKI TLS (n=11) | P Value |
|---|---|---|---|---|
| Male sex, n (%) | 65 (69.1) | 58 (69.9) | 7 (63.6) | 0.42 |
| Age (yr), mean±SD | 58±12 | 61±14 | 50±20 | 0.67 |
| Past history of CKD, n (%) | 6 (6.4) | 5 (6.0) | 1 (9.0) | 0.69 |
| Baseline serum creatinine levels (μmol/L), median (IQR) | 88.0 (80.0–97.0) | 88.0 (80.0–97.0) | 97.0 (80.0–106.0) | 0.71 |
| Past history of hypertension, n (%) | 35 (37.2) | 33 (39.7) | 2 (18.2) | 0.16 |
| Chronic cardiac dysfunction, n (%) | 11 (11.7) | 11 (13.3) | 0 | 0.19 |
| Diabetes, n (%) | 12 (12.7) | 11 (13.3) | 1 (9.0) | 0.69 |
| Underlying malignancy, n (%) | ||||
| AML | 30 (31.9) | 27 (32.5) | 3 (27.3) | 0.73 |
| Acute lymphoid leukemia | 13 (13.8) | 10 (12.0) | 3 (27.3) | 0.17 |
| Lymphoma | 44 (46.8) | 39 (46.9) | 5 (45.5) | 0.92 |
| Chronic lymphocytic leukemia | 4 (4.3) | 4 (4.8) | 0 | 0.46 |
| Myeloma | 1 (1.1) | 1 (1.2) | 0 | 0.71 |
| Nephrotoxic drugs | 19 (20.2) | 19 (22.9) | 0 | 0.08 |
| Laboratory findings at admission | ||||
| Phosphates (mmol/L), median (IQR) | 2.1 (1.9–2.3) | 2.1 (1.9–2.3) | 2.2 (2.1–2.3) | 0.7 |
| Calcium (mmol/L), median (IQR) | 1.6 (1.2–2.1) | 1.6 (1.3–2.2) | 1.2 (0.9–1.5) | 0.02a |
| Potassium (mmol/L), median (IQR) | 4.2 (3.6–4.9) | 4.3 (3.6–5.1) | 4.1 (3.6–4.7) | 0.70 |
| LDH (× normal) at admission, median (IQR) | 4.0 (2.8–15.5) | 4.0 (3.3–8.0) | 20.0 (2.0–38.0) | >0.99 |
| Serum creatinine (μmol/L), median (IQR) | 157.5 (106.8–289.8) | 170.0 (124.0–298.0) | 78.0 (51.0–105.0) | <0.001a |
| Uric acid (mmol/L) (before rasburicase), median (IQR) | 503.0 (155.0–788.0) | 539.0 (165.8–862.0) | 200.0 (61.0–383.0) | 0.009a |
| Lactatemia (mmol/L), median (IQR) | 3.9 (1.8–9.2) | 4.2 (1.9–9.4) | 2.4 (1.6–3.1) | 0.39 |
| Diuresis (ml/24 h), median (IQR) | 665 (382–1125) | 750 (400–1300) | 1200 (1000–2370) | 0.007a |
| SOFA at admission, median (IQR) | 6 (3–10) | 6 (3–10) | 3 (1–3) | 0.0001a |
| AKI stage 1, n (%) | 16 (19.3) | |||
| AKI stage 2, n (%) | 11 (13.3) | |||
| AKI stage 3, n (%) | 56 (67.5) | |||
| Treatments | ||||
| Hypouricemic therapy, n (%) | 90 (95.7) | 80 (96.4) | 10 (90.9) | 0.39 |
| Need for RRT, n (%) | 67 (80.7) | 0 | <0.001a | |
| RRT duration (d), median (IQR) | 4 (2–13) | |||
| Need for vasopressors, n (%) | 33 (35.1) | 33 (39.8) | 0 | 0.009 |
| Need for mechanical ventilation, n (%) | 46 (48.9) | 44 (53.0) | 2 (18.2) | 0.03a |
| ICU mortality, n (%) | 34 (36.2) | 33 (39.8) | 1 (9.0) | 0.04a |
| 28-Day mortality, n (%) | 38 (40.4) | 37 (44.6) | 1 (9.0) | 0.02a |
P values are reported for comparisons across the two patient groups. IQR, interquartile range.
P<0.05.
Table 2.
Clinical characteristics, laboratory findings, crystalluria, treatment, and outcome of TLS patients (second cohort)
| Characteristics | TLS (n=55) | Patients with TLS-AKI (n=46) | Patients with Non-AKI TLS (n=9) | P Value |
|---|---|---|---|---|
| Male sex, n (%) | 40 (72.7) | 36 (78.3) | 4 (44.4) | 0.09 |
| Age (yr), mean±SD | 57±17 | 59±15 | 44±20 | 0.25 |
| Past history of CKD, n (%) | 1 (1.8) | 0 (0.0) | 1 (11.1) | 0.16 |
| Baseline serum creatinine levels (μmol/L), median (IQR) | 75.5 (61.0–90.8) | 76.5 (62.5–90.3) | 67.5 (58.3–94.5) | 0.71 |
| Past history of hypertension, n (%) | 15 (27.3) | 14 (30.4) | 1 (11.1) | 0.42 |
| Chronic cardiac dysfunction, n (%) | 2 (3.6) | 2 (4.3) | 0 (0.0) | >0.99 |
| Diabetes, n (%) | 8 (14.5) | 8 (17.4) | 0 (0.0) | 0.33 |
| Underlying malignancy, n (%) | ||||
| AML | 10 (18.2) | 9 (19.6) | 1 (11.1) | >0.99 |
| Acute lymphoid leukemia | 7 (12.7) | 5 (10.9) | 2 (22.2) | 0.59 |
| Lymphoma | 35 (63.6) | 30 (65.2) | 5 (55.5) | 0.71 |
| Chronic lymphocytic leukemia | 1 (1.8) | 1 (2.3) | 0 (0) | >0.99 |
| Others | 2 (3.6) | 1 (2.3) | 1 (11.1) | 0.30 |
| Nephrotoxic drugs | 25 (45.5) | 21 (45.7) | 4 (44.4) | >0.99 |
| Laboratory findings at admission | ||||
| Phosphates (mmol/L), median (IQR) | 4.4 (4.1–5.2) | 4.6 (4.1–5.2) | 4.2 (3.7–4.6) | 0.25 |
| Calcium (mmol/L), median (IQR) | 2.2 (2.0–2.3) | 2.2 (2.0–2.3) | 2.1 (1.9–2.3) | 0.34 |
| Potassium (mmol/L), median (IQR) | 4.4 (4.1–5.2) | 4.6 (4.1–5.2) | 4.2 (3.7–4.6) | 0.25 |
| LDH at admission (UI/L), median (IQR) | 2764 (1431–6750) | 2764 (1431–6750) | 2883 (1191–7344) | 0.95 |
| Serum creatinine (μmol/L), median (IQR) | 124.0 (89.0–190.5) | 124.0 (94.5–195.0) | 83.5 (64.5–138.0) | 0.04a |
| Uric acid (mmol/L) (before rasburicase), median (IQR) | 497.0 (226.0–615.0) | 520.0 (270.5–696.5) | 404.0 (220.9–511.3) | 0.27 |
| AKI stage 1, n (%) | 15 (32.6) | |||
| AKI stage 2, n (%) | 6 (13.0) | |||
| AKI stage 3, n (%) | 25 (54.4) | |||
| Delay between ICU admission and AKI (d), median (IQR) | 1 (0–1) | |||
| Positive crystalluria, n (%)b | 7 (12.7) | 5 (10.8) | 2 (22.2) | 0.46 |
| Weddellite crystals (calcium oxalate dihydrate) | 0 | 0 | 0 | >0.99 |
| Whewellite crystals (calcium oxalate monohydrate) | 2 (3.6) | 2 | 0 | >0.99 |
| Calcium-phosphate crystals | 1 (1.8) | 0 | 1 | 0.16 |
| Uric-acid crystals | 3 (5.5) | 2 | 1 | 0.42 |
| Struvite (magnesium ammonium phosphate hexahydrate) | 1 (1.8) | 1 | 0 | >0.99 |
| Urine pH (day 1), median (IQR) | 6.2 (5.7–6.5) | 6.2 (5.3–6.5) | 6.2 (5.9–6.3) | 0.94 |
| Calcium urinary excretion, median (IQR) | 0.1 (0.0–0.5) | 0.13 (0.0–0.5) | 0.1 (0.0–0.5) | 0.76 |
| Calcium-creatinine urinary ratio, mmol/mmol (day 1) | ||||
| Phosphate urinary excretion (Ph/creat), mmol/mmol (day 1) median (IQR) | 5.6 (3.2–7.2) | 4.9 (3.1–7.1) | 7.1 (3.4–7.9) | 0.39 |
| Uric acid excretion (Uric/creat), mmol/mmol (day 1) median (IQR) | 0.5 (0.1–0.9) | 0.4 (0.1–1.0) | 0.5 (0.0–0.7) | 0.49 |
| Uric acid supersaturation index (day 1), median (IQR) | 1.5 (0.1–4.3) | 1.3 (0.1–3.5) | 1.4 (0.1–2.6) | 0.94 |
| Calcium phosphate supersaturation index (day 1), median (IQR) | 0.21 (0.10–0.45) | 0.20 (0.08–0.47) | 0.28 (0.18–0.61) | 0.36 |
| Treatments | ||||
| Rasburicase use, n (%) | 46 (83.6) | 38 (82.6) | 8 (88.9) | >0.99 |
| Delay between ICU admission and first rasburicase administration (d), median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.82 |
| Allopurinol use, n (%) | 9 (16.4) | 8 (17.4) | 1 (11.1) | >0.99 |
| Need for RRT, n (%) | 25 (45.5) | 25 (54.4) | 0 | 0.03a |
| RRT duration (d), median (IQR) | 2 (1–4) | 2 (1–4) | ||
| Need for vasopressors, n (%) | 9 (16.4) | 8 (17.4) | 1 (11.1) | >0.99 |
| Need for mechanical ventilation, n (%) | 11 (20) | 10 (21.7) | 1 (11.1) | 0.67 |
| ICU mortality, n (%) | 9 (16.4) | 7 (15.2) | 2 (22.2) | 0.63 |
| 28-Day mortality, n (%) | 20 (36.4) | 16 (34.8) | 4 (44.4) | 0.71 |
P values are reported for comparisons across the two patient groups. IQR, interquartile range; Ca, calcium; creat, creatinine; Ph, phosphate; Uric, uric acid.
P<0.05.
Crystalluria is positive if at least one urine sample (day 1, 3, or 5) is positive. Crystalluria was negative at day 7 in all patients (49 patients had crystalluria analysis at day 7).
Urine Crystals Are Uncommon in Patients with TLS
Analysis of crystalluria on fresh urine samples was performed in 55 patients with TLS at day 1, 3, 5, and 7 after ICU admission. All patients received intravenous saline hydration. Ninety percent of patients had received urate oxidase, according to current guidelines, because of the high risk of developing TLS.7 Only three patients had uric-acid crystalluria, two in the TLS-AKI group and one in the non-AKI TLS group. Of note, these patients had not received urate oxidase. Only one patient had calcium-phosphate crystals and he did not develop AKI. The vast majority of patients with AKI did not have positive crystalluria (Table 2).
High-Dose Chemotherapy in AML Mice Induced TLS and Renal EC Dysfunction In Vivo
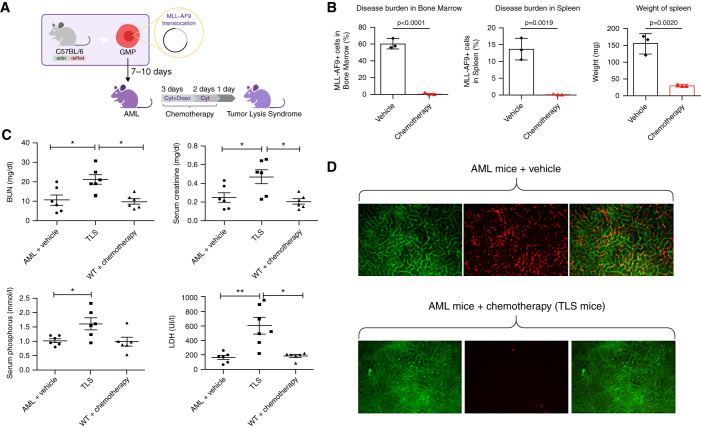
To further analyze AKI during TLS, we used a mouse model of AML driven by the MLL-AF9 gene fusion (Figure 1A).26 Treatment with a combination of an anthracycline (doxorubicin) and the antimetabolite cytarabine at the maximally tolerated dose, mimicking AML standard of care intensive chemotherapy,27 led to a sharp reduction in leukemic burden over an 8-day course in the blood, spleen, and bone marrow of treated mice, compared with vehicle control, and the induction of biologic TLS with increased LDH, BUN, phosphate, and creatinine levels 24 hours after the end of chemotherapy (Figure 1, B and C). In AML mice, in vivo intravital microscopy revealed leukemic cell infiltration of peritubular capillaries that was drastically reduced after chemotherapy (Figure 1D). In this in vivo model of TLS, we first analyzed histologic lesions using Masson trichrome and Periodic acid–Schiff staining. Despite the high degree of AKI in TLS mice, only mild tubular vacuolization was observed, with no significant difference between leukemic mice that had versus had not received chemotherapy (Supplemental Figure 1, A–C).
Figure 1.
Biochemical and microscopic analysis of a mouse model of AML mice show significant TLS after administration of chemotherapy. (A) Model of TLS in AML mice. (B) Disease (percent MLL-AF9+ blasts) in the bone marrow and spleen of MLL-AF9 mice after treatment with vehicle or with chemotherapy (24 hours after treatment). n=3 per group. (C) BUN, LDH, serum creatinine, and phosphate were measured in AML mice treated with vehicle (n=6) or chemotherapy (cytarabine + doxorubicin; n=6) and WT mice treated with chemotherapy (n=6) 24 hours after treatment. (D) In vivo renal intravital microscopy in MLL-AF9 mice that received vehicle or chemotherapy. MLL-AF9 leukemic blasts are DsRED positive and visible in renal peritubular capillaries. After chemotherapy, blast cell infiltration is no longer visible. All data are presented as mean±SEM *P<0.05, **P<0.01 versus vehicle, Mann–Whitney test.
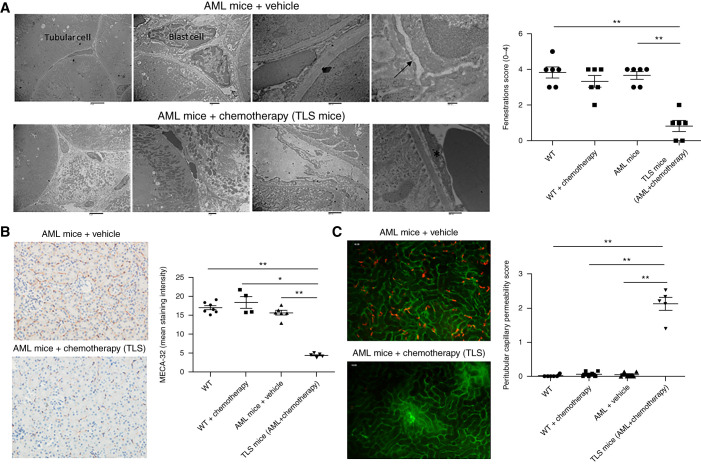
We then looked for renal ultrastructural changes by transmission electronic microscopy and observed profound alterations of peritubular ECs in TLS mice. Indeed, in healthy kidneys, transmission electron microscopy showed continuous fenestrations of the endothelium. Interestingly, TLS mice had profound alterations of the peritubular endothelium: the capillary shape was more irregular with swollen endothelium and lost fenestrations (Figure 2A). To confirm these alterations were not simply due to the toxicity of the chemotherapeutic drugs, we also analyzed the endothelium in WT mice (without leukemia) after chemotherapy. We did not detect any significant difference between the peritubular endothelium in these mice and that of WT mice (Supplemental Figure 2A). Moreover, we did not find any crystal deposits in TLS mice (as has been observed using transmission electron microscopy in other experimental models28) nor evidence of tubular injury.
Figure 2.
Peritubular endothelium is significantly altered in TLS leukemic mice. (A) Peritubular capillaries undergo ultrastructural alterations in TLS mice. Details of peritubular capillaries in transmission electron are shown for MLL-AF9 mice and TLS mice. Capillaries from MLL-AF9 kidneys had a flat endothelium with regular contours and multiple fenestrations (arrows). During TLS, the capillary shape became more irregular and the endothelium became swollen and lost fenestrations (asterisk). Leukemic blasts infiltrating the peritubular capillaries are seen in MLL-AF9 mice. A semiquantitative analysis showed that, compared with WT, MLL-AF9, and WT+chemotherapy kidneys, the capillary area with fenestrations was significantly decreased in TLS mice (n=6 per group). Scales bars, 6 μm in left, 1 μm in middle-left, 500 nm in middle-right, 200 nm in right panel. (B) Kidneys from WT mice treated with vehicle, WT mice receiving chemotherapy, and AML mice receiving chemotherapy (TLS mice) or vehicle were immunostained with a panendothelial cell antigen antibody (MECA-32). Decreased peritubular capillary staining was observed in TLS (peritubular capillary density, as quantified by computer image analysis of MECA-32 immunostaining). n=6 per group. Original magnification, 200×. (C) Peritubular capillary permeability in WT mice treated with vehicle, WT mice receiving chemotherapy, TLS mice, or AML mice. Analysis of interstitial leakage of albumin-FITC was performed using confocal videomicroscopy. Representative still images from the captured videos at 10 minutes in AML mice and TLS mice are presented in (C). Increased peritubular capillary permeability was observed after TLS. n=5–6 per group. *P<0.05, **P<0.01, Mann–Whitney test.
We confirmed renal endothelial alterations by immunohistochemical analysis. Indeed, renal MECA-32 staining was reduced in AML mice treated with chemotherapy compared with AML mice treated with vehicle (Figure 2B). Vascular permeability was assessed by confocal intravital microscopy. Increased vascular permeability was observed in TLS mice, detected by the leakage of albumin-FITC into the interstitium (Figure 2C, Supplemental Videos 1 and 2). Chemotherapy in WT mice did not decrease MECA-32 staining or alter capillary permeability (Figure 2, B and C, Supplemental Figure 2, B and C, Supplemental Video 3). There was no difference in terms of ICAM-1 staining in the TLS group when compared with AML mice treated with vehicle and WT mice (Supplemental Figure 3). Moreover, we did not observe significant apoptosis in vivo in TLS mice, as assessed by renal c-PARP staining (Supplemental Figure 4).
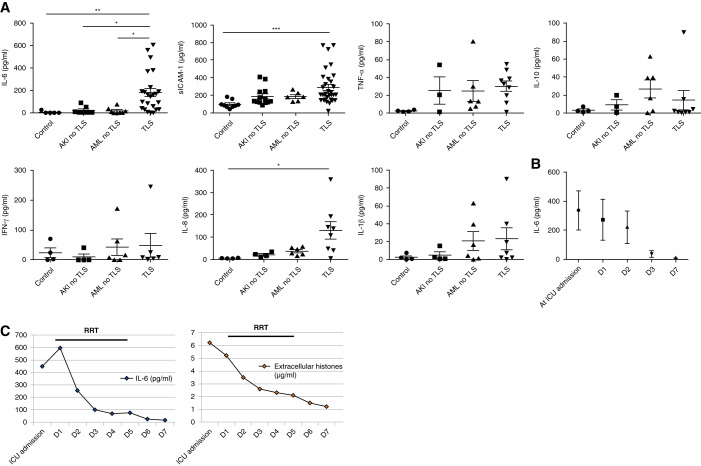
Circulating Histones Are Elevated during TLS and Correlate with Renal Outcome
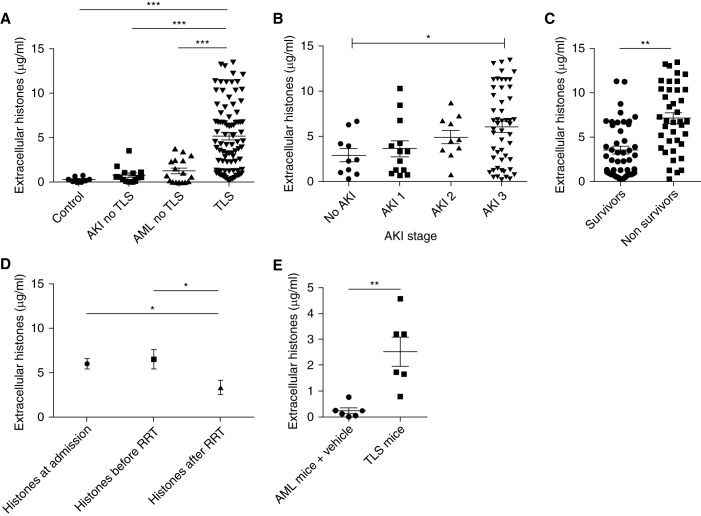
Because renal microvascular endothelial dysfunction appeared to be central to the pathophysiology of TLS-induced AKI, we then explored the mechanisms of endothelial dysfunction. Extracellular histones have previously been reported to induce endothelial cytotoxicity. The intracellular content is released after cell lysis in TLS, therefore, we hypothesized that extracellular histones may also be released during TLS. Indeed, assays of patient plasma demonstrated significantly elevated histones levels in patients with TLS (median [interquartile range (IQR)], 4.43 [1.34–7.64] μg/ml) compared with samples from patients with acute myeloid/lymphoid leukemia without TLS (median [IQR], 0.75 [0.03–2.46] μg/ml; P<0.001) or from patients with AKI due to other causes (0.58 [0.46–1.08] μg/ml; P<0.001) (Figure 3A).
Figure 3.
Circulating histones are elevated in patient plasma during TLS and in a mouse model of TLS. (A) Plasma histone concentrations were determined in four independent cohorts of patients. n=84 patients with TLS, n=8 controls, n=14 patients with AKI without TLS, n=17 patients with acute leukemia without TLS (Kruskal–Wallis test with Dunn post-test). (B) Plasma histone concentrations are shown according to AKI stage in patients with TLS-AKI (Kruskal–Wallis test with Dunn post-test). (C) Plasma histone concentrations in survivors and nonsurvivors at day 28 (paired t test). (D) Kinetic of plasma histones concentrations in patients who required RRT (hemodialysis; n=24) before and after a single session of 6 hours of RRT (Kruskal–Wallis test with Dunn post-test). (E) Extracellular histone concentrations in the AML mouse model with or without TLS. n=6 per group (Mann–Whitney test). All data are presented as mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
Moreover, levels of circulating histones significantly correlated with AKI severity and mortality (Figure 3, B and C). In patients who required RRT, levels of circulating histones were significantly decreased at the end of RRT (single session of 6 hours of hemodialysis): median (IQR) of 3.89 (2.23–11.93) μg/ml at the initiation of RRT versus 1.89 (1.02–4.72) μg/ml at the end of RRT (P<0.02; Figure 3D). In the mouse model of TLS (AML mice treated with chemotherapy), extracellular histones increased significantly 24 hours after high-dose chemotherapy administration (median levels of 0.17 μg/ml in vehicle-treated mice versus 2.47 μg/ml in chemotherapy-treated mice; P<0.002; Figure 3E).
Extracellular Histones Increase Renal Microvascular Dysfunction In Vivo
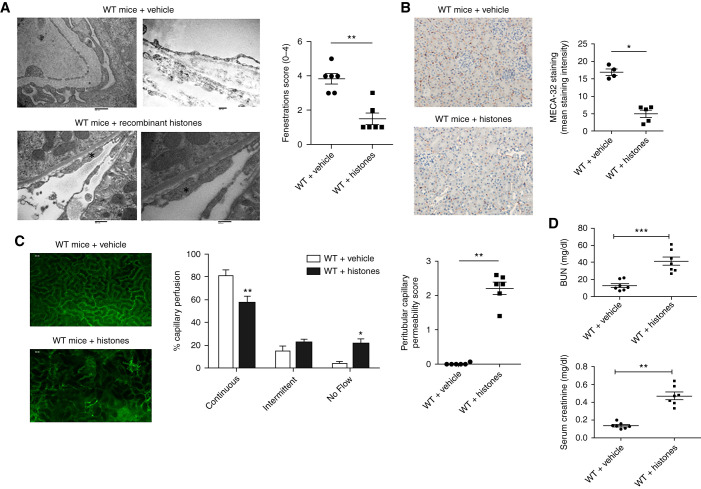
To establish the pathogenic role of extracellular histones on endothelial dysfunction in the context of TLS, we performed transmission electronic microscopy analysis in kidneys of WT mice injected with 25 mg/kg of recombinant histones. Median (IQR) plasma histones levels were 3.34 (2.56–9.3) μg/ml at 1 hour and 21.19 (20.32–21.85) μg/ml at 24 hours (Supplemental Figure 5). Twenty-four hours after injection, we observed similar ultrastructural changes compared with those observed in TLS mice (Figure 4A). After histone exposure, MECA-32 staining was also decreased (Figure 4B).
Figure 4.
Recombinant histones induce endothelial dysfunction in vivo. (A) Peritubular capillaries undergo ultrastructural alterations in mice injected with recombinant histones (25 mg/kg). Twenty-four hours after histone injection, the endothelium became swollen and lost fenestrations (asterisk). A semiquantitative analysis showed that, compared with WT mice, the capillary area with fenestrations was significantly decreased. n=6 per group. Scale bars, 600 nm in left panel, 400 nm in right panel. (B) Kidneys from WT mice receiving vehicle and histones were immunostained with anti-mouse panendothelial cell antigen antibody (MECA-32). Decreased peritubular capillary staining was observed 24 hours after recombinant histone injection. Peritubular capillary density was quantified by computer image analysis of MECA-32 immunostaining. n=5 per group. Original magnification, 200×. (C) Representative still images from the captured videos of peritubular capillaries in WT mice injected with vehicle or recombinant histones at 25 mg/kg. Analysis of interstitial leakage of albumin-FITC and capillary perfusion were performed using confocal videomicroscopy. Increased peritubular capillary permeability was observed 24 hours after histone exposure. Capillary perfusion also decreased in some areas of the kidney after histone exposure. n=6 per group. (D) BUN and serum creatinine were measured in WT mice treated with vehicle (n=7) or recombinant histones (n=7) 24 hours after treatment. *P<0.05, **P<0.01, ***P<0.001, compared with WT mice, Mann–Whitney test.
We then used intravital confocal microscopy to study whether histone exposure induced renal microvascular dysfunction. Leukocyte recruitment and renal microvascular permeability were evaluated by intravital microscopy after intravenous administration of 25 mg/kg of histones in C57BL/6 mice. We did not find any difference in leukocyte recruitment in mice treated with histones compared with control mice (Supplemental Figure 6). However, histone exposure significantly increased leakage of FITC-albumin from peritubular capillaries to the interstitial compartment in confocal microscopy (Figure 4C, Supplemental Videos 4–6). Capillary perfusion was then analyzed. We categorized the cortical distribution of peritubular capillary perfusion as continuous, intermittent, or no flow, as previously described.29 After 4 hours, mice injected with vehicle only had a high percentage of continuously perfused renal cortical capillaries (81%±5%), and only a small percentage of capillaries with no flow (4%±1.8%) (Figure 4C). In contrast, 4 hours after histone administration, the percentage of capillaries with continuous perfusion was reduced to 57%±6.2% (P<0.008 versus vehicle), and the percentage of capillaries with no perfusion was increased to 21%±4.6% (P<0.02 versus vehicle) (Figure 4C, Supplemental Video 7). After histone injection, renal function was altered with increased BUN and serum creatinine 24 hours after injection (Figure 4D).
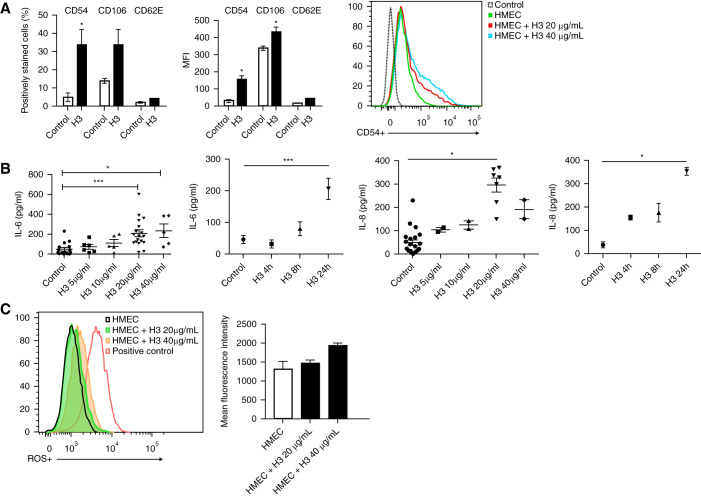
Extracellular Histones Activate ECs In Vitro and Mediate Cellular Damage
Extracellular histones induce EC activation with increased expression of CD54 (ICAM-1) and CD106 (vascular cell adhesion molecule-1) and increased secretion of IL-6 and IL-8 by ECs (Figure 5, A and B). Extracellular histones did not change CD62E (E-selectin) expression. At high concentrations (40 μg/ml), histones induced EC damage, as revealed by the production of reactive oxygen species in live cells (Figure 5C). Moreover, the proportion of annexin V–positive, 7AAD-negative cells was increased in histone-stimulated ECs compared with control cells (Supplemental Figure 7). Thus, high levels of extracellular histones can decrease the viability of microvascular ECs in vitro.
Figure 5.
Endothelial cells are activated by extracellular histones in vitro. (A) CD54, CD106, and CD62E expression were assessed in ECs activated with recombinant histones. Mean fluorescence intensity (MFI) and percentage of CD54+, CD106+, and CD62E+positive ECs nonactivated or activated with 20 μg/ml histones over 24 hours. Representative plots for CD54 expression are gated on microvascular ECs (HMECs). Mean±SEM is shown from four to six independent experiments. Mann–Whitney test. (B) IL-6 and IL-8 levels in the supernatant of HMEC treated with histones at the indicated concentrations (5, 10, 20, 40 μg/ml) and different time points at 20 μg/ml. Mean±SEM is shown from four to ten independent experiments. Kruskal–Wallis test with Dunn post-test. (C) Effect of histones on reactive oxygen species (ROS) production. HMECs were treated with histones at the indicated concentrations. Production of ROS by live cells was analyzed by flow cytometry using the CellROX™ Green Flow Cytometry Assay Kit. Tert-butyl hydroperoxide (THBP) solution, an inducer of ROS, was used as positive control. Kruskal–Wallis test with Dunn post-test. *P<0.05, ***P<0.001.
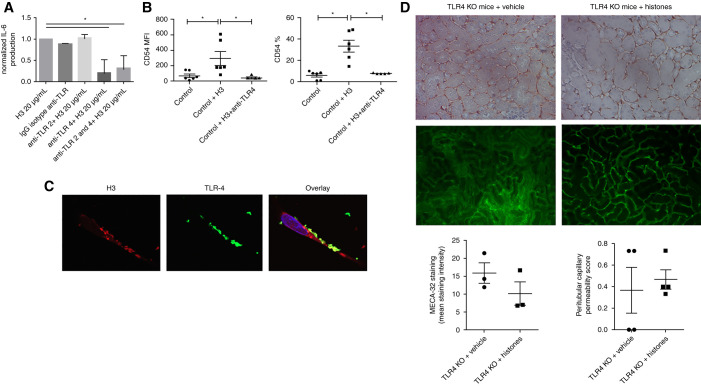
Histone Effect on Endothelial Phenotype Involved TLR4 but Not TLR2
On the basis of previous data showing extracellular histones have agonistic effects on TLRs (TLR2 and TLR4),30 we then asked if these receptors were involved in EC activation after exposure to histones. Neutralizing antibodies specific for either TLR2 or TLR4 were preincubated with ECs before histone stimulation in vitro. Anti-TLR4, but not anti-TLR2, strongly reduced histone-induced upregulation of IL-6 by ECs and their expression of CD54 (Figure 6, A and B). Moreover, confocal microscopy examination of ECs revealed colocalization of TLR4 and extracellular histones on the EC surface (Supplemental Figure 6C). In vivo, TLR4-knockout mice injected with recombinant histones were protected against renal endothelial dysfunction, as assessed by intravital microscopy and MECA-32 staining (Figure 6D, Supplemental Videos 8 and 9).
Figure 6.
TLR4, but not TLR2, was implicated in histone-mediated activation of ECs. (A) HMECs were preincubated with mouse isotype control IgG (Iso-IgG, 50 μg/ml), mouse anti-human TLR2 (anti-TLR2, 50 μg/ml), and/or anti-human TLR4 (anti-TLR4, 50 μg/ml) for 60 minutes, and then stimulated with 20 μg/ml histones (H3) for 24 hours. The secretion of IL-6 was then determined by ELISA. Data are presented as mean±SEM from four different experiments. (B) HMECs were preincubated with anti-TLR4 for 60 minutes (TAK-242, 1 μM) and stimulated with 20 μg/ml histones (H3) for 24 hours. Mean fluorescence intensity (MFI) and percentage of CD54-positive ECs after histones ±TAK-242 exposure. Data are presented as mean±SEM from six different experiments. (C) TLR4 and extracellular H3 colocalize in ECs. Confocal images showed the localization of histones H3 (red) and TLR4 (green) in ECs. 4′,6-Diamidino-2-phenylindole (blue) was used to stain the nuclei. (D) MECA-32 staining by immunohistochemistry and peritubular capillary permeability (intravital confocal microscopy) in TLR4 knockout (TLR4 KO) mice injected with vehicle or histones (24 hours after injection of 25 mg/kg of recombinant histones). No difference was observed between the two groups (Mann–Whitney test). n=4 per group. *P<0.05, Mann–Whitney test.
Patients with TLS Have Increased Endothelial Dysfunction Markers
Having observed the above modifications of human ECs in vitro, we then assessed plasma concentration of inflammatory markers in patients with TLS using a multiplex electrochemiluminescent assay of seven cytokines/chemokines (IL-6, IL-8, IL-10, TNF-α, IL-1β, IFN-γ, and sICAM-1). We found a significant increase of IL-6, IL-8, and sICAM-1 in patients with TLS, as compared with other groups (Figure 7A). The decrease of plasma IL-6 from ICU admission to day 7 paralleled the decrease of extracellular histone concentrations (Figure 7, B and C).
Figure 7.
Endothelial dysfunction markers were elevated in plasma of patients with TLS. (A) Plasma cytokine concentrations (multiplex analysis) in four independent cohorts of patients: n=7–27 patients with TLS, n=4–9 controls, n=3–13 patients with AKI without TLS, and n=6–8 patients with acute leukemia without TLS. Values are expressed as mean±SEM. (B) Time course of plasma IL-6 concentrations in nine patients with TLS. (C) Time course of IL-6 and extracellular histones concentrations. The left panel shows the kinetic of plasma IL-6 concentration in one patient with TLS from ICU admission to day 7 (D7) and the parallel decrease of extracellular histones concentrations from ICU admission to D7. *P<0.05, **P<0.01, ***P<0.001, Kruskal–Wallis test with Dunn post-test.
Nonanticoagulant Heparin Alleviates Histone-Induced Endothelial Cytotoxicity In Vitro and In Vivo
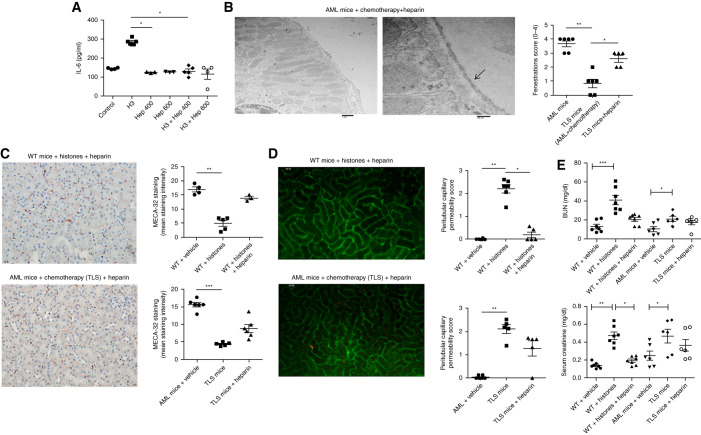
Heparin has a high negative charge density and a strong affinity for histones. Indeed, because histones are positively charged, heparin has been shown to neutralize extracellular histones.31 Heparin significantly decreased plasma histone levels in WT mice injected with recombinant histones (Supplemental Figure 5). We thus investigated the effects of nonanticoagulant heparin on ECs in vitro and in vivo during TLS. Administration of heparin decreased the activation of ECs after histone exposure (Figure 8A). In the mouse model of TLS, administration of heparin at 3 mg/kg twice a day, on the day before and during chemotherapy administration, was able to prevent renal endothelial dysfunction, as assessed by electron microscopy, MECA-32 immunostaining, and intravital microscopy (Figure 8, B–D, Supplemental Videos 10–12). Although nonanticoagulant heparin improved kidney function of WT mice injected with histones (Figure 8E), it was not able to significantly improve renal function in TLS mice (Figure 8E).
Figure 8.
Heparin mitigates the effect of extracellular histones and TLS on renal endothelial dysfunction. (A) IL-6 in the supernatant of ECs (HRGECs/HMECs) treated with 20 μg/ml histones and heparin at the indicated concentrations (400 and 600 μg/ml) for 24 hours. Mean±SEM is shown from four to ten independent experiments. *P<0.05, Mann–Whitney test. (B) Peritubular capillaries were examined by transmission electron microscopy in MLL-AF9 mice and TLS mice, having received chemotherapy and heparin 3 mg/kg twice a day 24 hours before and during the administration of chemotherapy. Capillaries from these mice have a flat endothelium with multiple fenestrations (arrows). A semiquantitative analysis showed that, compared with TLS mice without heparin, the capillary area with fenestrations was significantly increased. n=6 per group. Scale bars, 1 μm in left panel, 200 nm in right panel. *P<0.05, **P<0.01, Mann–Whitney test. (C) Kidneys from WT mice injected with histones and heparin (left panel) and TLS mice receiving heparin (right panel) were immunostained with panendothelial cell antigen antibody (MECA-32). Decreased peritubular capillary staining was observed after TLS or histone exposure, but not after heparin administration. Peritubular capillary density was quantified by computer image analysis of MECA-32 immunostaining. n=4–6 per group. Original magnification, 200×. **P<0.01, Mann–Whitney test. (D) Representative still images extracted after 10 minutes from videos of peritubular capillaries of WT mice injected with recombinant histones and heparin (left panel) and from TLS mice injected with heparin (right panel). Analysis of interstitial leakage of albumin-FITC and capillary perfusion were performed by confocal videomicroscopy. Increased peritubular capillary permeability was observed after TLS or exposure to histones, but not after heparin administration. n=5–6 per group. *P<0.05, **P<0.01, Mann–Whitney test. (E) BUN and serum creatinine were measured in WT mice treated with vehicle (n=7) or recombinant histones with (n=7) or without (n=7) heparin, in AML mice treated with vehicle (n=6), and TLS mice treated with (n=6) or without heparin (n=6). Nonanticoagulant heparin significantly decreased creatininemia of WT mice injected with histones. *P<0.005, **P<0.001, ***P<0.0001, Kruskal–Wallis test with Dunn post-test. Hep, heparin.
Discussion
In this study coupling a multicenter cohort of patients with TLS with an in vivo model of chemotherapy-induced TLS and in vitro studies, we demonstrate, for the first time, that uric-acid and calcium-phosphate crystals are rare during TLS-induced AKI in the rasburicase era and establish a new model for the pathophysiology of TLS-induced AKI, whereby (1) endothelial dysfunction participates in TLS-induced AKI; (2) plasma levels of extracellular histones are hugely increased during TLS, and this is associated with the occurrence and severity of AKI; (3) extracellular histones induce renal endothelial dysfunction in vitro and in vivo; and (4) nonanticoagulant heparin can neutralize extracellular histones and prevent endothelial damage.
The evidence for calcium-phosphate crystals during TLS relies on scarce data from relatively old case reports.5,6 Although we cannot exclude that crystal deposits may have been missed by electron microscopy in our experimental model, uric-acid and calcium-phosphate crystals were uncommon, despite the high prevalence of AKI in patients with TLS. This led us to question the role of additional crystal-independent mechanisms in the pathophysiology of TLS-induced AKI. Indeed, crystalluria is a sensitive method to predict the occurrence of crystal-induced nephropathy. Although calcium-phosphate crystal–induced nephropathy and uric-acid nephropathy are always preceded by crystalluria, the reverse is not true and crystalluria may occur without resulting in crystal-induced nephropathy or stone formation.32 Calcium-phosphate crystallization may result from high concentrations of calcium and phosphate, but the main factor increasing calcium-phosphate supersaturation in urine is high pH. Therefore, calcium-phosphate crystals occur when urinary alkalinization is performed. Because urine alkalinization is no longer recommended during TLS,7 this may also explain the absence of calcium-phosphate crystals in our study. Uric-acid nephropathies are also reported during TLS.1,33 However, the wide use of hypouricemic therapies (90% of the patients in our cohort) has probably decreased the risk of urate nephropathies. Despite these elements, AKI still develops during TLS. Our data concord with previous studies4 and AKI remains prevalent during TLS, despite wide use of rasburicase, use of saline for hydration, and close monitoring of patients with TLS in the centers involved in this study. Moreover, crystal-induced mechanisms do not explain sepsis-like syndromes with multiple organ failure that may be observed during TLS.3
In patients with TLS-induced AKI, we have found drastically higher levels of extracellular histones compared with patients experiencing postrenal AKI. However, although histones were significantly elevated in AKI stage 3, the increase of histones in AKI stage 1 and 2 was not significant. This may be due to a lack of statistical power of these results. Nevertheless, the association between AKI and histone elevation does not prove a causal effect of histones on AKI. The endothelial toxicity of extracellular histones has been previously described during sepsis or trauma.10,34 We show in this study that extracellular histones induce activation of microvascular ECs. In addition, injection of recombinant histones in vivo induces renal endothelial dysfunction with increased microvascular leakage. These results are in agreement with a previous study suggesting a role for histones in renal endothelial dysfunction in a model of endotoxin-induced AKI, in a TLR2/TLR4-dependent manner.30 Histones can bind EC membranes on TLR4 and increase permeability. Although TLR4 was involved in the modifying adhesion molecule expression and IL-6/IL-8 secretion by ECs, we did not find evidence of TLR2 being implicated in this model.
The cytokine production, including increased IL-6 release, observed in our patients, may participate in AKI. Indeed, emerging data show that local activation of IL-6 is implicated in renal endothelial dysfunction and AKI.35,36 Beyond AKI, extracellular histones have been linked to acute lung injury in the literature.10,37 However, although some patients experienced hemodynamic failure, neurologic failure, and AKI, and may have required mechanical ventilation, none of our patients with TLS experienced acute respiratory distress syndrome. In AML mice treated with vehicle and TLS mice, we did not detect any evidence of acute lung injury (Supplemental Figure 8).
Forming a dynamic interface between blood and tissues, the vascular endothelium may have implications in potentiating AKI and multiple organ failure. The role of microvascular dysfunction during TLS has not been previously explored. We set up an experimental model of MLL-AF9 AML to induce TLS and to allow in vivo studies. Indeed, patients with AML have a high risk of TLS because of the chemosensitivity and rapid proliferation rate in AML. Moreover, the expression of MLL-AF9 in normal GMPs is sufficient to generate an aggressive, transplantable myeloid leukemia with a high tumor burden. To be sure that the chemotherapy alone did not induce renal endothelial toxicity, we performed control experiments by administering chemotherapy in WT mice, and we did not observe any evidence of AKI or renal endothelial dysfunction. The chemotherapy regimen (doxorubicin and cytarabine) is currently used as the main chemotherapeutic regimen in patients with AML. Extremely rare case reports of acute rhabdomyolysis have been reported with cytarabine chemotherapy,38 but there are no reports of AKI induced by doxorubicin chemotherapy.
We confirmed in our model that TLS was associated with high circulating levels of histones and that renal endothelial dysfunction was present during AKI-induced TLS.
Our results do not exclude the involvement of other mechanisms involved in the pathophysiology of TLS-induced AKI. Indeed, other potentially toxic damage-associated molecular patterns, such as high mobility group box 1, produced by dying cells are released after chemotherapy.39 Moreover, other cancer-associated nephrotoxic insults may contribute to AKI40 and these mechanisms and extracellular histone release are not necessary mutually exclusive mediators of AKI in TLS. Nevertheless, the drastic increase of histones in the TLS mouse model and in patients with TLS, their association with AKI, and their endothelial toxicity suggest that extracellular histones are important mediators of TLS-induced endothelial dysfunction. Moreover, we found that heparin administration could prevent renal endothelial damage in the TLS model. Because of their electrostatic interactions, heparin neutralizes extracellular histones. In a murine model of septic AKI using cecal ligation and puncture, Wang et al.17 showed that heparin was able to alleviate renal inflammation through the neutralization of histones. Moreover, Kawai and colleagues41 demonstrated that heparin was able to improve multiple organ injuries and the survival rate of mice injected with a fatal dose of histones by inhibiting histone-EC binding. It is, however, possible that heparin may have other roles in AKI, independent of histone neutralization. For example, recent data showed that heparin binding protein (HBP) may promote macrophage activation and inflammation during sepsis-induced AKI,42,43 and elevated plasma levels of HBP have been associated with AKI during sepsis in humans.44 Different heparin derivatives were indeed able to abrogate the HBP-induced increased inflammatory response in vitro and in vivo.44 In our study, we used a nonanticoagulant heparin to maintain the neutralizing effect of heparin on histones, without increasing the risk of bleeding in patients with underlying hematologic malignancies who are already at high risk of spontaneous bleeding. Nevertheless, nonanticoagulant heparin did not significantly improve renal function in TLS mice, suggesting mechanisms other than histone-mediated AKI may be have been involved in our model.
Interestingly, we found a strong decrease of plasma extracellular histones after RRT in patients with TLS-AKI. Given the small size of extracellular histones (11–21 kD), we believe that RRT using membranes with a molecular mass cutoff around 20 kD may have removed extracellular histones. On the other hand, early initiation of RRT in patients with high-grade hematologic malignancies may result in underdosing chemotherapeutic drugs and may, therefore, decrease the chance of achieving complete remission. Nonanticoagulant heparin may then be of particular interest to delay the initiation of RRT. To date, there are no randomized clinical studies that have focused on the timing and indications of RRT in patients with TLS.
In conclusion, this study provides the first evidence that the previously described crystal-dependent mechanisms are insufficient to explain TLS-induced AKI.
Crystal-independent mechanisms of TLS-induced AKI involve extracellular histones and endothelial dysfunction. This study sheds new light on the pathophysiology of TLS-induced AKI and extracellular histones may, therefore, constitute a novel target for either diagnostic and/or therapeutic intervention in TLS when endothelial dysfunction occurs.
Disclosures
R. Itzykson reports consulting for Abbvie, Amgen, BMS/Celgene, Daiichi-Sankyo, Jazz Pharma, Karyopharm, Novartis, and Stemline Therapeutics; and receiving research funding from Novartis and Janssen; none of which is related to this work. E. Letavernier reports receiving consulting fees, and honoraria, from Biocodex; receiving research funding from Coloplast; and having patents or royalties via Inserm Transfert. N. Mooney reports receiving research funding from CSL-Behring. L. Zafrani reports receiving a Jazz Pharmaceuticals grant. All remaining authors have nothing to disclose.
Funding
This work was supported by a Jazz Pharmaceuticals grant, ESICM Basic Science Award, and Ministère de la Santé grant PHRC AOM 08235.
Supplementary Material
Acknowledgments
The Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique (GRRROH) members include: Elie Azoulay, Djamel Mokart, Frédéric Pène, Achille Kouatchet, Julien Mayaux, François Vincent, Martine Nyunga, Fabrice Bruneel, Pierre Perez, Anne-Pascale Meert, Dominique Benoit, Michael Darmon, and Virginie Lemiale.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Crystals or His(stones): Rethinking AKI in Tumor Lysis Syndrome,” on pages 1055–1057.
Authors Contributions
M. Arnaud¸ E. Arrii, J. Demonchy, C. Djediat, S. Fodil, P. Frère, E. Letavernier, M. Loiselle, M. Nacer, S. Placier, S. Pons, C. Vaganay, and L. Zafrani were responsible for investigation; M. Arnaud, C. Djediat, P. Frère, R. Itzykson, E. Letavernier, S. Placier, S. Pons, and L. Zafrani were responsible for methodology; M. Arnaud, P. Frère, R. Itzykson, E. Letavernier, N. Mooney, S. Pons, A. Puissant, and L. Zafrani were responsible for validation; M. Arnaud, R. Itzykson, M. Loiselle, N. Mooney, and L. Zafrani conceptualized the study; M. Arnaud, M. Loiselle, C. Vaganay, and L. Zafrani were responsible for data curation; M. Arnaud and L. Zafrani wrote the original draft and were responsible for formal analysis; C. Djediat, P. Frère, and S. Placier were responsible for software; C. Djediat, P. Frère, S. Placier, A. Puissant, C. Vaganay, and L. Zafrani were responsible for resources; C. Djediat, E. Letavernier, and L. Zafrani were responsible for visualization; R. Itzykson, E. Letavernier, J. Lion, N. Mooney, S. Placier, and A. Puissant provided supervision; R. Itzykson, E. Letavernier, N. Mooney, and A. Puissant reviewed and edited the manuscript; and L. Zafrani was responsible for funding acquisition and project administration.
Data Sharing Statement
All data is included in the manuscript and/or supporting materials.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021070997/-/DCSupplemental.
Supplemental Table 1. Cairo–Bishop definition of laboratory and clinical tumor lysis syndrome in adult.
Supplemental Table 2. AKI staging according to KDIGO guidelines.
Supplemental Figure 1. Standard kidney histology in AML mice with or without TLS.
Supplemental Figure 2. Endothelial analysis in WT mice receiving chemotherapy.
Supplemental Figure 3. ICAM-1 expression in WT, WT+chemotherapy, AML mice with or without TLS
Supplemental Figure 4. Renal expression of apoptosis in AML mice with or without TLS.
Supplemental Figure 5. Plasma histones concentrations in WT mice injected with recombinant histones +/- heparin.
Supplemental Figure 6. Analysis of leucocyte recruitment by intravital microscopy.
Supplemental Figure 7. Viability of endothelial cells after histone exposure.
Supplemental Figure 8. Lung tissue histology in AML and TLS mice.
Supplemental Video 1. Intravital confocal microscopy in AML mice.
Supplemental Video 2. Intravital confocal microscopy in TLS mice.
Supplemental Video 3. Intravital confocal microscopy in WT mice + chemotherapy.
Supplemental Video 4. Intravital confocal microscopy in WT mice + vehicle.
Supplemental Video 5. Intravital confocal microscopy in WT mice + histones 25mg/kg.
Supplemental Video 6. Intravital confocal microscopy in WT mice + histones 25mg/kg.
Supplemental Video 7. Intravital video microscopy.
Supplemental Video 8. Intravital confocal microscopy in TLR4KO mice + vehicle.
Supplemental Video 9. Intravital confocal microscopy in TLR4KO mice + histones 25mg/kg.
Supplemental Video 10. Intravital confocal microscopy in AML mice + histones + heparin.
Supplemental Video 11. Intravital confocal microscopy in TLS mice + histones + heparin.
Supplemental Video 12. Intravital confocal microscopy in WT mice + histones 25mg/kg + heparin.
References
- 1.Howard SC, Jones DP, Pui C-H: The tumor lysis syndrome. N Engl J Med 364: 1844–1854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafrani L, Canet E, Darmon M: Understanding tumor lysis syndrome. Intensive Care Med 45: 1608–1611, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Soares M, Feres GA, Salluh JIF: Systemic inflammatory response syndrome and multiple organ dysfunction in patients with acute tumor lysis syndrome. Clinics (São Paulo) 64: 479–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darmon M, Vincent F, Camous L, Canet E, Bonmati C, Braun T, et al. ; Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique (GRRR-OH) : Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique. Br J Haematol 162: 489–497, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Boles JM, Dutel JL, Briere J, Mialon P, Robasckiewicz M, Garre M, et al. : Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemia. Cancer 53: 2425–2429, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Kanfer A, Richet G, Roland J, Chatelet F: Extreme hyperphosphataemia causing acute anuric nephrocalcinosis in lymphosarcoma. BMJ 1: 1320–1321, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones GL, Will A, Jackson GH, Webb NJA, Rule S; British Committee for Standards in Haematology : Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol 169: 661–671, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Mato AR, Riccio BE, Qin L, Heitjan DF, Carroll M, Loren A, et al. : A predictive model for the detection of tumor lysis syndrome during AML induction therapy. Leuk Lymphoma 47: 877–883, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Montesinos P, Lorenzo I, Martín G, Sanz J, Pérez-Sirvent ML, Martínez D, et al. : Tumor lysis syndrome in patients with acute myeloid leukemia: identification of risk factors and development of a predictive model. Haematologica 93: 67–74, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. : Extracellular histones are major mediators of death in sepsis. Nat Med 15: 1318–1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. : Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 31: 2810–2818, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Cairo MS, Bishop M: Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol 127: 3–11, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group : Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Miller PG, Al-Shahrour F, Hartwell KA, Chu LP, Järås M, Puram RV, et al. : In Vivo RNAi screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell 24: 45–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pikman Y, Puissant A, Alexe G, Furman A, Chen LM, Frumm SM, et al. : Targeting MTHFD2 in acute myeloid leukemia. J Exp Med 213: 1285–1306, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Wang L, Cao C, Jin H, Zhang Y, Liu Y, et al. : Heparin attenuates histone-mediated cytotoxicity in septic acute kidney injury. Front Med (Lausanne) 7: 586652, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meara CHO, Coupland LA, Kordbacheh F, Quah BJC, Chang C-W, Simon Davis DA, et al. : Neutralizing the pathological effects of extracellular histones with small polyanions. Nat Commun 11: 6408, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT: Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 187: 2626–2631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen PST, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA: A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 286: F1116–F1119, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Holthoff JH, Seely KA, Pathak E, Spencer HJ 3rd, Gokden N, et al. : Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 180: 505–516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PST, Hewitt SM, et al. : Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: Acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69: 832–836, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe AR, Yue W: Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: An integrated protocol. Bio Protoc 9: e3465, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taflin C, Favier B, Baudhuin J, Savenay A, Hemon P, Bensussan A, et al. : Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A 108: 2891–2896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lion J, Taflin C, Cross AR, Robledo-Sarmiento M, Mariotto E, Savenay A, et al. : HLA class II antibody activation of endothelial cells promotes Th17 and disrupts regulatory T lymphocyte expansion. Am J Transplant 16: 1408–1420, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Roux B, Vaganay C, Vargas JD, Alexe G, Benaksas C, Pardieu B, et al. : Targeting acute myeloid leukemia dependency on VCP-mediated DNA repair through a selective second-generation small-molecule inhibitor. Sci Transl Med 13: eabg1168, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129: 424–447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouderlique E, Tang E, Perez J, Coudert A, Bazin D, Verpont M-C, et al. : Vitamin D and calcium supplementation accelerates Randall’s plaque formation in a murine model. Am J Pathol 189: 2171–2180, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR: Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int 81: 370–378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, et al. : Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 23: 1375–1388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildhagen KCAA, García de Frutos P, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, et al. : Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 123: 1098–1101, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Frochot V, Daudon M: Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Int J Surg 36: 624–632, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Kanwar YS, Manaligod JR: Leukemic urate nephropathy. Arch Pathol 99: 467–472, 1975 [PubMed] [Google Scholar]
- 34.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. : Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 187: 160–169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Lei C-T, Zhang C: Interleukin-6 signaling pathway and its role in kidney disease: An update. Front Immunol 8: 405, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, et al. : Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 94: 534–541, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Lefrançais E, Looney MR: Neutralizing extracellular histones in acute respiratory distress syndrome. A new role for an endogenous pathway. Am J Respir Crit Care Med 196: 122–124, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truica CI, Frankel SR: Acute rhabdomyolysis as a complication of cytarabine chemotherapy for acute myeloid leukemia: Case report and review of literature. Am J Hematol 70: 320–323, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. : Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 71: 4821–4833, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Rosner MH, Perazella MA: Acute kidney injury in patients with cancer. N Engl J Med 376: 1770–1781, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Kawai C, Kotani H, Miyao M, Ishida T, Jemail L, Abiru H, et al. : Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol 186: 829–843, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Lu Z, Li X, Yang P, Mu G, He L, Song C, et al. : Heparin-binding protein enhances NF-κB pathway-mediated inflammatory gene transcription in M1 macrophages via lactate. Inflammation 44: 48–56, 2021 [DOI] [PubMed] [Google Scholar]
- 43.Xing L, Zhongqian L, Chunmei S, Pingfa C, Lei H, Qin J, et al. : Activation of M1 macrophages in sepsis-induced acute kidney injury in response to heparin-binding protein. PLoS One 13: e0196423, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher J, Russell JA, Bentzer P, Parsons D, Secchia S, Mörgelin M, et al. : Heparin-binding protein (HBP): A causative marker and potential target for heparin treatment of human sepsis-induced acute kidney injury. Shock 48: 313–320, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.