ABSTRACT
This study aims to explore the role of fatty acid binding protein 4 (FABP4) in diabetic retinopathy (DR), and to elucidate the potential regulatory mechanism. We firstly developed a mouse model of DR by injection with streptozocin (STZ) into C57BL/6 male mice and a cell model of DR by induction of high glucose (HG) to ARPE-19 cells. BMS309403, an inhibitor of FABP4, was employed for treatment. The blood glucose in vivo was monitored and the histological changes of retinal tissues were observed by hematoxylin and eosin staining and Evans blue assay. The expression level of FABP4 was detected by western blot and Immunohistochemical staining. The critical factors related to lipid peroxidation and oxidative stress were detected using their commercial kits, respectively. Prussian blue staining, iron content assay and thiobarbituric acid-reactive substances (TBARS) assay were conducted to evaluate ferroptosis. As a result, FABP4 was elevated in retina and serum of STZ-induced mice and in HG-induced ARPE-19 cells. BMS309403 treatment notably alleviated reduced blood glucose, reduced histological damage, and vascular permeability. In addition, BMS309403 treatment inhibited lipid peroxidation, oxidative stress, and ferroptosis both in vivo and in vitro. Furthermore, BMS309403 promoted the activation of peroxisome proliferator-activated receptor γ (PPARγ). GW9662 (an inhibitor of PPARγ) or Erastin (an inducer of ferroptosis) partially weakened the suppressive effects of BMS309403 on HG-induced lipid peroxidation, oxidative stress and ferroptosis. Taken together, FABP4 inhibition alleviates lipid peroxidation and oxidative stress in DR by regulating PPARγ-mediated ferroptosis.
KEYWORDS: Fatty acid binding protein 4, diabetic retinopathy, lipid peroxidation, oxidative stress, ferroptosis, peroxisome proliferator-activated receptor γ
Graphical abstract
Introduction
Diabetic mellitus (DM) is a group of metabolic diseases characterized by chronic hyperglycemia, which can lead to chronic damage of kidney, blood vessels, heart, nerves, eyes and other organs. Diabetic retinopathy (DR) is one of the most common and major microangiopathic complications of diabetes mellitus, which can lead to retinal ischemia, retinal permeability changes and macular edema, eventually leading to visual impairment or even blindness [1,2]. According to statistical predictions, the number of DR patients worldwide will reach 191 million in 2040, which seriously endangers the physical and mental health of patients and places a heavy burden on families, society, and the global public health system [3]. Therefore, an in-depth understanding of the pathogenesis of DR and the exploration of effective targeted treatment methods are important to improve the diagnosis and treatment of DR.
Fatty acid binding protein 4 (FABP4) is a small molecular protein with a relative molecular mass of 14–15 kDa in the cytoplasm, which is a chaperone protein of free fatty acid (FFA). It plays an important role in maintaining glucose and lipid homeostasis. Studies have shown that elevated FABP4 is closely associated with the development of metabolism-related diseases, such as cardiovascular disease, atherosclerosis, and type 2 diabetes [4]. Notably, FABP4 is highly expressed in diabetic complications, such as diabetic nephropathy and gestational diabetes, and inhibition of FABP4 not only ameliorates high glucose-induced glomerular cell apoptosis by regulating endoplasmic reticulum stress, but also increases serum insulin concentrations and reduces pancreatic inflammation, thereby alleviating the progression of these diseases [5,6], suggesting that FABP4 is involved in and regulates the development of diabetes-related diseases. Recent clinical studies have found that serum FABP4 is positively correlated with the severity of DR and that FABP4 can be used as an independent prognostic marker in patients with type 2 diabetic retinopathy [7,8]. In addition, FABP4 also shows abnormally high expression in retinopathy, and FABP4 deficiency improves pathological retinal vascularization and provides some protection against retinopathy [9]. These studies suggest that FABP4 is closely related to diabetic retinopathy, and it is likely that FABP4 is involved in and regulates the development of DR, and its specific mechanism of action remains to be studied.
Therefore, this study aims to explore the specific role of FABP4 in DR, and attempts to elucidate its potentially regulatory mechanism during the progression of DR, providing a novel sight for the targeted treatment of DR.
Materials and methods
Animals study
Total 18 six-week-old C57BL/6 male mice were obtained from Model Animal Research Center of Nanjing University (Nanjing, China) and were housed in a controlled environment (constant temperature at 20 ± 2°C and humidity at 55 ± 5%, with a 12/12 h light/dark cycle) with free access to water and food. All animal study was approved by the Institutional Animal Care and Use Committees at the Tianjin Medical University Eye Hospital (Approval No. TJYY2022122053).
After acclimation for 1 week and fasting overnight, a single injection of 150 mg/kg streptozotocin (STZ, Sigma, St. Louis, MO) was administered intraperitoneally to establish a diabetic mouse model. Mice with randomized blood glucose levels greater than 19 mmol/L, polyuria, wasting and glycosuria were considered to have diabetes [10]. After 3 weeks of the onset of diabetes, mice were treated with oral administration with BMS309403 (a selective small-molecular inhibitor of FABP4; 40 mg/kg/d) for 3 weeks. During treatment, blood glucose level was monitored. After the mice were anesthetized, serum samples were collected for analysis. In addition, the retinal tissues were harvested, and one part of the tissues were fixed in 10% paraformaldehyde, while other parts were frozen and stored at −80°C for following experiments.
Serum biochemical parameters detection
The levels of triglyceride, total cholesterol and free fatty acid (FFA) in mice serum were detected using their corresponding commercial kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) strictly in line with the guide of the manufacturer. The level of FABP4 in mice serum was measured using mouse FABP4 enzyme-linked immunosorbent assay (ELISA) kit (ab277426; Abcam) in accordance with the protocol of the manufacturer.
Evans blue assay
Mice were anesthetized and injected with Evans blue dye (45 mg/kg) via tail vein for calculation of 2 h in accordance with the standard operation [11]. Mice were perfused via the left ventricle with 0.05 M citrate buffer to remove the left Evans blue dye in blood vessels. Afterward, the right eye of each mouse was enucleated and fixed with 50% formaldehyde/acetic acid/alcohol/saline (FAS) ophthalmic fixator (Servicebio) for 2 h. The tissue sections were imaged under a fluorescence microscope (Axio Observer A1; Carl Zeiss, Jena, Germany) with a red filter to check for Evans blue extravasation from retinal vessels.
Retinal morphological change
Retinal tissues were fixed in 10% paraformaldehyde for 24 h, dehydrated in gradient ethanol and transparently soaked in xylene, then embedded in paraffin wax and cut into 4 μm-thick paraffin sections with a microtome. The sections were then stained with hematoxylin and eosin (H&E; Servicebio, Wuhan, China) following the manufacturer’s instructions. Retinal pathological changes were observed under a light microscopy.
Immunohistochemical detection
The above sections were dewaxed in xylene, hydrated in gradient ethanol, added to sodium citrate buffer for microwave heating to repair the antigen, cooled at room temperature, and incubated with 3% H2O2 for 30 min to seal the nonspecific antigen. Subsequently, the sections were incubated with the primary antibody against FABP4 overnight at 4°C, followed by incubation with corresponding secondary antibody at room temperature for 10 min. Nuclear staining was performed using 4’,6-diamidino-2-phenylindole (DAPI) at room temperature for 30 min in the darkness. Finally, the slice was sealed and observed under a light microscope.
Prussian blue staining
The above tissue sections were dewaxed with xylene, hydrated with gradient ethanol, stained with 2% nuclear solid red for 10 min, treated with xylene soaking after gradient rinsing, sealed with neutral resin, and finally incubated with Prussian blue staining mixture for 10–20 min. Iron staining was observed under fluorescence microscope [12].
Measurement of iron level
The iron measurement was carried out referring to previous studies [13,14]. The retinal tissues were collected and homogenized in 4 ~ 10 volumes of Iron Assay Buffer using a Dounce homogenizer sitting at 4°C. Then, an Iron Assay Kit (ab83366, Abcam) was employed to assess the total iron in tissue homogenate. The absorbance at 593 nm was measured using a microplate reader. The standard curves were prepared simultaneously with known concentrations of iron solutions. The iron level was normalized to the protein concentration.
Western blot
Total proteins were extracted from tissues or cells using radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher, Markham, ON, Canada), the concentration of which was then determined by a bicinchoninic acid (BCA) kit (Pierce, Rockford, IL, USA). After adjustment, the same amount of proteins (30 μg/lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, MA, USA). The membranes were blocked at room temperature for 1 h in 5% skimmed milk and incubated with the corresponding primary antibodies at 4°C overnight. The next day, the horseradish peroxidase-conjugated secondary antibody was applied to incubate the membranes at room temperature for 30 min. Finally, the protein bands were visualized by an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotechnology, Amersham, UK) and quantified using ImageJ software version 1.46 (National Institute of Health).
Cell culture and treatments
Retinal pigmented epithelium cell line ARPE-19 (ATCC® CRL-2302™, Manassas, VA, USA) was cultured in a 1:1 mixture of Dulbecco’s Modified Eagles Medium and Ham’s F12 medium (DMEM/F12) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (all from Gibco, Waltham, MA, USA) at 37°C in 5% CO2. For experimental treatment, ARPE-19 cells were induced with high glucose (HG; 25 mM) for 48 h to simulate diabetes in vitro [15]. Meanwhile, ARPE-19 cells were also cultured in medium containing normal glucose (NG; 5.5 mM) for control and cultured in medium containing normal glucose and mannitol (MA; 22.5 mM) for negative control. In addition, ARPE-19 cells were pre-treated with 50 μM BMS309403 for 24 h before HG induction.
Thiobarbituric acid-reactive substances (TBARS) assay
Lipid peroxidation was assessed by TBARS method as described previously [16]. In brief, the supernatant of cell lysate was mixed with 0.1% thiobarbituric acid solution and vortexed, followed by incubation at 95°C for 30 min. Finally, the supernatant was collected and its absorbance at 532 nm was measured with a spectrophotometer (μQuant, Biotek, VT, USA)
Oil-red O staining
Cells were fixed in 4% paraformaldehyde for 10 min, washed with PBS for 1 min, and then rinsed with 50% isopropanol for 15 s. Afterward, cells were stained with Oil Red O dye solution (Beyotime, Shanghai, China) at 37°C for 1 min, followed by detainment with 60% isopropanol for 15 s. Finally, the cells were washed and imaged under an inverted microscope.
Measurement of oxidative stress factors
The content of malondialdehyde (MDA) and the activity of glutathione peroxidase (GSH-px) in each sample were measured using their corresponding commercial kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) in accordance with the instructions of the manufacturer, respectively. The level of reactive oxygen species (ROS) was measured by an oxidation-sensitive fluorescent probe DCFH-DA with a ROS Assay kit (Jianglaibio, Shanghai, China).
Statistical analysis
Data were analyzed using GraphPad 8.0 (GraphPad Software, San Diego, CA, USA) and presented as mean ± standard deviation (SD). Comparisons among groups were conducted using one-way ANOVA, followed by Tukey’s post hoc test. A p value less than 0.05 was indicated to be statistically significant.
Results
FABP4 inhibition attenuated retinal injury in a mouse model of DR
To investigate the effect of FABP4 inhibition on the progression of DR, we firstly developed a mouse model of DR by injection with STZ into C57BL/6 male mice, with or without BMS309403 administration. As shown in Figure 1a-b, the expression of FABP4 in retina tissue of diabetic mice was dramatically elevated, and BMS309403 administration could remarkably reduce the protein expression of FABP4. Meanwhile, an elevated level of serum FABP4 in diabetic mice and a declined serum FABP4 following BMS309403 administration were observed (Figure 1c). The monitored blood glucose revealed a high glucose level in STZ-induced mice, which was partially reduced after BMS309403 treatment (Figure 1d). In addition, H&E staining showed that the clear structure in each layer of mouse retina was destroyed in diabetic mouse, accompanied with a thinned retina, whereas these pathological changes were partially abolished by BMS309403 treatment (Figure 1e). What’s more, DR is one of the most common microvascular complications of DM, so the leakage of the Evans blue dye observed in retina of STZ-induced diabetic mice reflected a severe retinal injury, and the following reduction of this leakage in mice with BMS309403 administration indicated that FABP4 inhibition notably alleviated retinal vascular dysfunction in a mouse model of DR (figure 1f).
Figure 1.
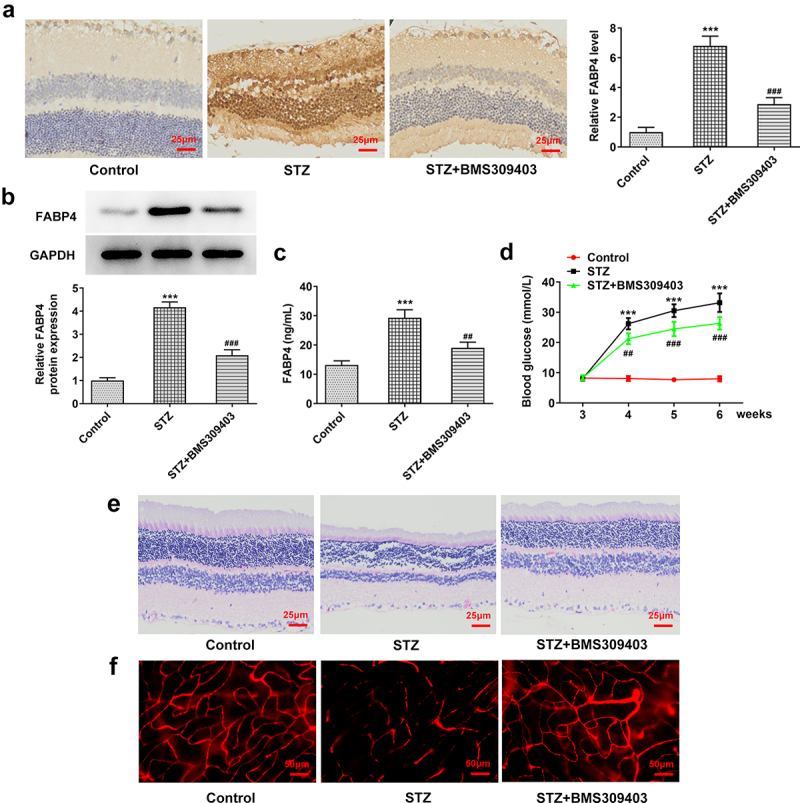
FABP4 inhibition attenuated retinal injury in a mouse model of DR.
A mouse model of DR was established by injection of 150 mg/kg STZ into C57BL/6 male mice. The diabetic mice were then treated with BMS309403, a selective small-molecular inhibitor of FABP4. The protein expression of FABP4 in retina tissue was detected by (A) immunohistochemical staining and (B) western blot. (C) The level of FABP4 in mice serum was measured using mouse FABP4 ELISA kit. (D) The blood glucose was monitored during the experiments. (E) The histological change of retina was assessed by H&E staining. (F) The vascular permeability was evaluated by Evans blue assay. ***p < 0.001 vs control; ##p < 0.01, ###p < 0.001 vs STZ.
FABP4 inhibition attenuated lipid peroxidation and oxidative stress in retinal tissues in a mouse model of DR
The retina is highly susceptible to lipid peroxidation and oxidative stress due to its high level of polyunsaturated fatty acids [17]. Here, we further explored the specific role of FABP4 during the progression of DR by evaluating its influence on lipid peroxidation and oxidative stress in diabetic mice. As shown in Figure 2a-c, the common lipid contents, including triglyceride, total cholesterol and FFA, were hugely elevated in blood of diabetic mice, which were then partly hindered by BMS309403 administration. MDA and ROS, the end products of lipid peroxidation, were found to be elevated in retinal tissues of diabetic mice, while GSH-Px, which can catalyze glutathione (GSH) to oxidized glutathione (GSSG) and protect the cells from oxide damage, was greatly downregulated in diabetic retina, indicating that oxidative stress occurred in DR. As expected, BMS309403 administration notably downregulated MDA and ROS levels and upregulated GSH-Px level (Figure 2d-f). These data suggested that FABP4 inhibition could partially weaken lipid peroxidation and oxidative stress in diabetic retina.
Figure 2.
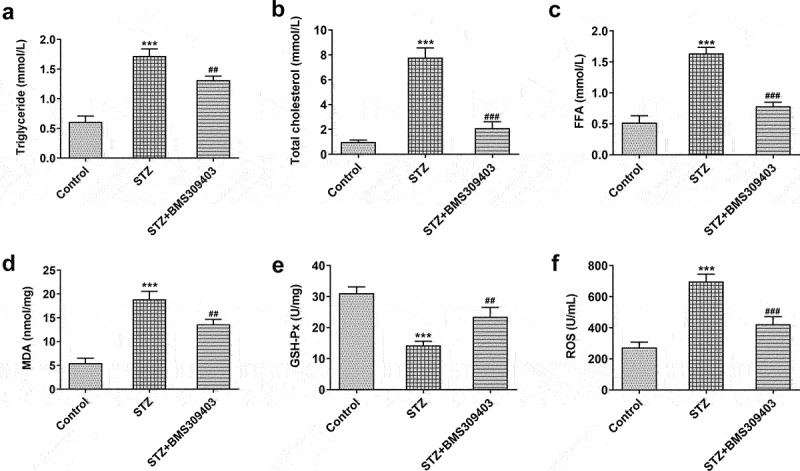
FABP4 inhibition attenuated lipid metabolism and oxidative stress in retinal tissues in a mouse model of DR.
The lipid contents of blood, including (A) triglyceride, (B) total cholesterol and (C) free fatty acid (FFA), were detected. The levels of (D) MDA, (E) GSH-Px and (F) ROS in retinal tissues of diabetic mice were measured. ***p < 0.001 vs control; ##p < 0.01, ###p < 0.001 vs STZ.
FABP4 inhibition reduced ferroptosis in retinal tissues in a mouse model of DR
Ferroptosis is characterized by the accumulation of lipid peroxidation products, Thus we next would like to uncover whether ferroptisis is involved in the regulatory role of FABP4 during DR progression. Firstly, we evaluated the iron deposition in retinal tissues. The results in Figure 3a-b displayed that in STZ-induced diabetic retina, the iron content was remarkably elevated, and BMS309403 treatment was able to abate the iron accumulation. SLC7A11 and glutathione peroxidase 4 (GPX4) are important indicators of ferroptosis. The results in Figure 3c that the downregulated protein expression of SLC7A11 and GPX4 in diabetic retina was partially reversed by BMS309403 treatment , further verifying that FABP4 inhibition has ability to reduce ferroptosis in DR.
Figure 3.
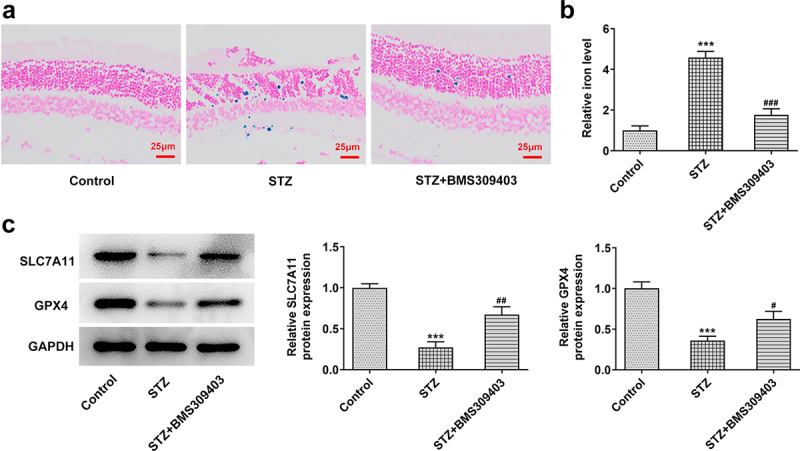
FABP4 inhibition reduced ferroptosis in retinal tissues in a mouse model of DR.
The iron accumulation in retinal tissues of diabetic mice was detected using (A) Prussian blue staining and (B) iron content kit assay. (C) The expression level of ferroptosis-related proteins, SLC7A11 and GPX4, was detected using western blot. ***p < 0.001 vs control; #p < 0.05, ##p < 0.01 vs STZ.
FABP4 inhibition promoted the activation of peroxisome proliferator-activated receptor γ (PPARγ) signaling in DR model in vivo and in vitro
To find out the further potential mechanism underlying the regulatory role of FABP4 in DR progression, PPARγ, a critical regulatory factor in DR and a primary target in the treatment of DR [18], was explored. The inactivation of PPARγ in DR was verified by the low protein expression of PPARγ in retinal tissues of STZ-induced diabetic mice, which was elevated by BMS309403 treatment (Figure 4a). In addition, ARPE-19 cells were stimulated with HG to mimic DR in vitro. The protein expression of FABP4 was elevated upon HG stimulation, consistent with the in vivo results, and BMS309403 treatment also reduced its expression in vitro (Figure 4b). In addition, HG stimulation also inhibited the activation of PPARγ in ARPE-19 cells, which was then partially reversed by BMS309403 treatment (Figure 4c).
Figure 4.
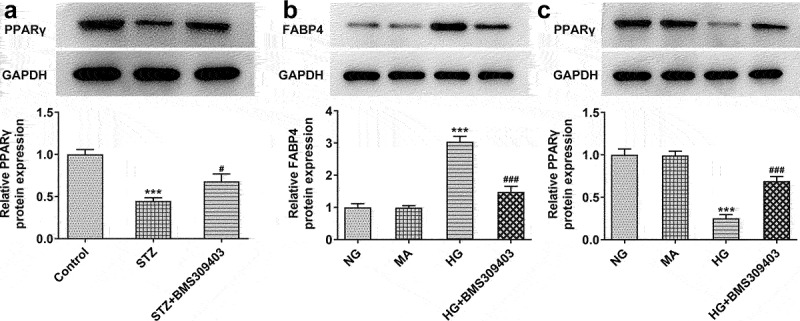
FABP4 inhibition promoted the activation of PPARγ signaling in DR model in vivo and in vitro.
(A) The protein expression of PPARγ in retinal tissues of diabetic mice was measured by western blot. ***p < 0.001 vs control; #p < 0.05 vs STZ. ARPE-19 cells were stimulated by HG to mimic DR in vitro. BMS3089403 was also used for treatment. The protein expression of (B) FABP4 and (C) PPARγ in cells of each group was measured by western blot. ***p < 0.001 vs MA; ###p < 0.001 vs HG.
FABP4 inhibition reduced ferroptosis by upregulating PPARγ activity in HG-induced ARPE-19 cells
To ensure the critical role of PPARγ underlying the regulatory role of FABP4 in DR, ARPE-19 cells were stimulated with HG, and treated with BMS309403 with or without GW9662, an inhibitor of PPARγ. As shown in Figure 5a-c, the high level of lipid peroxidation and iron were observed in HG-induced ARPE-19 cells, followed by the downregulated protein expression of SLC7A11 and GPX4, demonstrating the occurrence of ferroptosis in HG-induced ARPE-19 cells. However, the promoted ferroptosis was partly inhibited by BMS309403, as evidenced by the abolishment of these changes. What’s more, the inhibitory effect of BMS309403 on HG-induced ferroptosis was partly weakened by simultaneous treatment of GW9662, suggesting that FABP4 inhibition might suppress HG-induced ferroptosis in ARPE-19 cells through regulating PPARγ.
Figure 5.
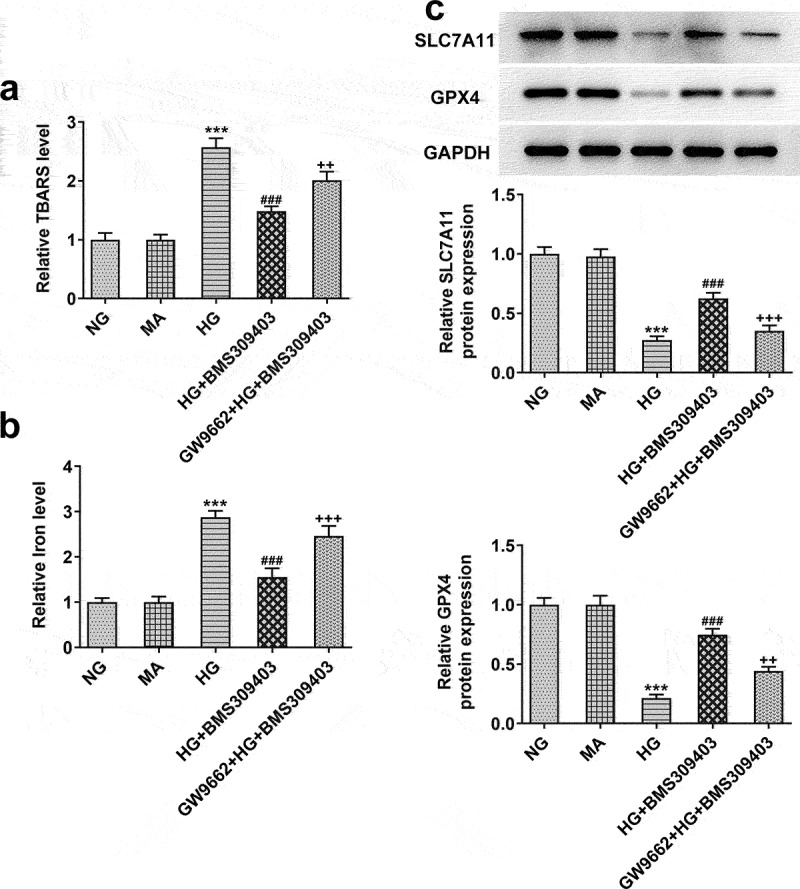
FABP4 inhibition reduced ferroptosis by upregulating PPARγ activity in HG-induced ARPE-19 cells.
ARPE-19 cells were stimulated with HG, and treated with BMS309403 with or without GW9662, an inhibitor of PPARγ. (A) The lipid peroxidation was evaluated using TBARS assay. (B) The iron content in cells was detected. (C) The expression level of ferroptosis-related proteins, SLC7A11 and GPX4, was detected using western blot. ***p < 0.001 vs MA; ###p < 0.001 vs HG; ++p < 0.01, +++p < 0.001 vs HG+BMS309403.
FABP4 inhibition alleviated HG-induced lipid peroxidation and oxidative stress by regulating PPARγ-mediated ferroptosis in ARPE-19 cells
As PPARγ-mediated ferroptosis exerts a critical function underlying the regulatory role of FABP4 in DR, we finally explored whether FABP4 modulated lipid peroxidation and oxidative stress in DR through PPARγ-mediated ferroptosis. ARPE-19 cells were stimulated with HG, and treated with BMS309403, accompanied with GW9662 (an inhibitor of PPARγ) or Erastin (an inducer of ferroptosis). As shown in Figure 6a, the results from oil-red O staining revealed a lipid accumulation upon HG stimulation, which was partly inhibited by BMS309403. Compared to HG+ BMS309403, additional GW9662 treatment or Erastin treatment got similar increase in lipid accumulation to some degree. Meanwhile, the protein expression of key enzymes for fatty acid oxidation, including carnitine palmityl transferase 1 (CPT1) and fatty acid synthetase (FAS), exhibited an elevation upon HG stimulation, which was then reduced by BMS309404 treatment. Consistently, the reduced protein expression of CPT1 and FAS upon BMS309404 treatment in HG-induced ARPE-19 cells was increased again by additional treatment of GW9662 or Erastin (Figure 6b), suggesting that FABP4 inhibition could inhibit lipid peroxidation in HG-induced ARPE-19 cells through activating PPARγ or inhibiting ferroptosis. Moreover, the inhibitory effects of BMS309403 on HG-induced elevation of ROS and MDA levels and decrease of GSH-Px level were partly abolished by the additional treatment of GW9662 or Erastin (Figure 6c-e), demonstrating that FABP4 inhibition could inhibit oxidative stress in HG-induced ARPE-19 cells through activating PPARγ or inhibiting ferroptosis.
Figure 6.
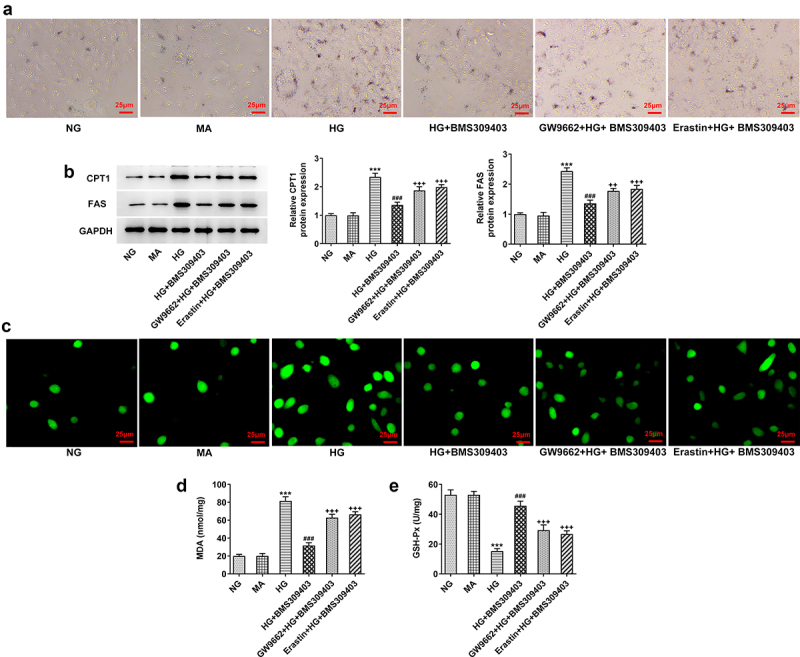
FABP4 inhibition alleviated HG-induced lipid metabolism and oxidative stress by regulating PPARγ-mediated ferroptosis in ARPE-19 cells.
ARPE-19 cells were stimulated with HG, and treated with BMS309403, accompanied with GW9662 (an inhibitor of PPARγ) or Erastin (an inducer of ferroptosis). (A) Oil-red O staining was performed to observe the lipid accumulation. (B) The protein expression of key enzymes for fatty acid oxidation, including CPT1 and FAS, was detected using western blot. (C) ROS level in cells were measured by fluorescent probe DCFH-DA. The level of (D) MDA and (E) GSH-Px was detected by their corresponding commercial kits, respectively. ***p < 0.001 vs MA; ###p < 0.001 vs HG; ++p < 0.01, +++p < 0.001 vs HG+BMS309403.
Discussion
DR has been recognized as one of the leading causes of blindness worldwide [19]. The specific pathogenesis of DR is complicated, and there is no definite conclusion. The retina is sensitive to oxidative stress and lipid peroxidation because of its rich blood supply and high content of unsaturated fatty acids [20]. Meanwhile, high blood glucose can induce a large amount of mitochondrial ROS production, resulting in a disruption of the oxidation-antioxidation balance in the body, leading to oxidative stress, which is one of the pathological mechanisms that cause many diabetic complications, including DR [21]. The existing evidence has revealed that the oxidative stress in DR patients gradually increases with the development of retinal lesions [22]. In recent decades, numerous studies have found that the intake of melatonin, flavonoids and other nutrients can effectively limit oxidative stress, thereby preventing retinal damage and preventing the occurrence of DR [23,24]. Therefore, the development of genes or agents targeting oxidative stress has become an attractive new strategy for DR treatment. In the present study, our study firstly disclosed BMS309404, an inhibitor of FABP4, could effectively attenuate the retinal injury in DR mice. Secondly, our study demonstrated that FABP4 inhibition exerted inhibitory effects on lipid peroxidation, oxidative stress and ferroptosis in STZ-induced diabetic mice in vivo and HG-induced ARPE-19 cells in vitro. Thirdly, the protective role of FABP4 inhibition in DR progression might be partly dependent on the crosstalk of PPARγ and PPARγ-mediated ferroptosis.
Ferroptosis is a recently discovered programmed cell death induced by iron-dependent oxidative damage. A key feature of the process of ferroptosis is the iron-dependent accumulation of lipid peroxides. High oxidative stress in diabetic patients leads to a disruption of the blood-brain barrier and the release of large amounts of divalent iron ions, leading to excessive production of ROS in a Fentonian reaction, which in turn oxidizes cell membrane lipids and contributes to ferroptosis [25,26]. Currently, a large number of studies have shown that ferroptosis exists in diabetic complications such as diabetes mellitus, diabetic nephropathy, and diabetic osteoporosis, which may be related to the tendency of sustained hyperglycemia to trigger oxidative stress, whereas the inhibition of ferroptosis have a mitigating effect on these diabetic complications [27–29]. However, up to date, only a recent research reported the phenomenon of ferroptosis in DR, and it was firstly discovered that ferroptosis served as a cell death pathway for retinal vascular endothelial cells in DR [30]. Meanwhile, ferroptosis induced by oxidative stress is an important cause of retinal pigment epithelial cell dysfunction, and ferrostatin-1, the inhibitor of ferroptosis, may reduce the inflammatory response and protect the structure and function of the retina [31,32]. In addition, recent studies reported that FABP4 could enhance lipid droplet formation in cancer cells by regulating oxidative stress-induced ferroptosis, revealing the involvement of ferroptosis in FABP4-mediated lipid accumulation and oxidative stress [33]. Consistently, our study not only disclosed inhibitory effects of FABP4 inhibition on lipid peroxidation, oxidative stress and ferroptosis in DR model in vivo and in vitro, but also illustrated that FABP4 inhibition alleviated lipid peroxidation and oxidative stress in DR by suppressing ferroptosis.
PPARγ is a nuclear receptor encoded by the PPARG gene, which is mainly distributed in adipose tissue and immune cells and is a key regulator of fatty acid storage and glucose metabolism. Studies have shown that PPARγ expression is suppressed in the retina in a high-glucose environment, and rosiglitazone, the PPARγ agonist, can delay the onset and progression of DR [34], suggesting that activation of PPARγ may be an effective way to improve DR. In addition, PPARγ has been widely recognized as a primary target in the treatment of DR [18]. Interestingly, the FABP4 gene has been reported to contain a peroxisome proliferation response element and is thus regulated by PPARγ [35]; on the other hand, FABP4 can directly interact with PPARγ, which in turn triggers PPARγ ubiquitination and subsequent proteasomal degradation [36]. The critical role of PPARγ in DR and the crosstalk between FABP4 and PPARγ prompt us further explore the involvement of PPARγ in FABP4-regulated DR progression. As expected, PPARγ was inactivated in diabetic mice or HG-induced ARPE-19 cells, and the activity of PPARγ was promoted by FABP4 inhibition. Further in vitro experiments disclosed that GW9662, an inhibitor of PPARγ, could partly abolish the protective effects of FABP4 inhibition against HG-induced lipid peroxidation and oxidative stress, suggesting that PPARγ might be the critical pathway through which FABP4 inhibition exerted its protective effects. In addition, our findings also uncovered that FABP4-mediated ferroptosis was PPARγ-dependent, as the ferroptosis was partly promoted by PPARγ inhibition.
Conclusion
The current work provided an evidence for the possible role of FABP4 as a therapeutic target during the progression of DR. Mechanically speaking, FABP4 inhibition could attenuate retinal injury in DR by inhibiting lipid peroxidation and oxidative stress through regulating PPARγ-mediated ferroptosis. Our data provide strong evidence that FABP4 inhibitor, BMS309404, has significant therapeutic potential for the clinical management of DR.
Acknowledgements
We would like to sincerely thank the reviewers for critical comments on this study.
Funding Statement
This study was supported by Free Exploration Nature Science Foundation of Shanxi (No. 202103021223014),Health Commission Foundation of Shanxi (No. 2020146), and Tianjin Research Innovation Project for Postgraduate Students (2021YJSB271).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Duh EJ, Sun JK, Stitt AW.. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14). DOI: 10.1172/jci.insight.93751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kralisch S, Fasshauer M. Adipocyte fatty acid binding protein: a novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia. 2013;56(1):10–21. [DOI] [PubMed] [Google Scholar]
- [5].Dong X, Yang L. Inhibition of fatty acid binding protein 4 attenuates gestational diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 2020;161:102179. [DOI] [PubMed] [Google Scholar]
- [6].Yao F, Li Z, Ehara T, et al. Fatty acid-binding protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol Cell Endocrinol. 2015;411:232–242. [DOI] [PubMed] [Google Scholar]
- [7].Itoh K, Furuhashi M, Ida Y, et al. Detection of significantly high vitreous concentrations of fatty acid-binding protein 4 in patients with proliferative diabetic retinopathy. Sci Rep. 2021;11(1):12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang XZ, Tu WJ, Wang H, et al. Circulating serum fatty acid-binding protein 4 levels predict the development of diabetic retinopathy in type 2 diabetic patients. Am J Ophthalmol. 2018;187:71–79. [DOI] [PubMed] [Google Scholar]
- [9].Saint-Geniez M, Ghelfi E, Liang X, et al. Fatty acid binding protein 4 deficiency protects against oxygen-induced retinopathy in mice. PLoS One. 2014;9(5):e96253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang P, Zhou YD, Tan Y, et al. Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HIF-1/VEGFA pathway and promoting PEDF expression. Int J Ophthalmol. 2021;14(5):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42(3):789–794. [PubMed] [Google Scholar]
- [12].Song Y, Wang B, Zhu X, et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37(1):51–64. [DOI] [PubMed] [Google Scholar]
- [13].Shen Q, Liang M, Yang F, et al. Ferroptosis contributes to developmental cell death in rice blast. New Phytol. 2020;227(6):1831–1846. [DOI] [PubMed] [Google Scholar]
- [14].Wang X, Zhang C, Zou N, et al. Lipocalin-2 silencing suppresses inflammation and oxidative stress of acute respiratory distress syndrome by ferroptosis via inhibition of MAPK/ERK pathway in neonatal mice. Bioengineered. 2022;13(1):508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu J, Hou Y, Lin L, et al. MicroRNA-5195-3p alleviates high glucoseinduced injury in human ARPE-19 cells by targeting GMFB. PLoS One. 2021;16(11):e0260071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stoppe C, Averdunk L, Goetzenich A, et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery. Sci Transl Med. 2018;10(441). DOI: 10.1126/scitranslmed.aan4886. [DOI] [PubMed] [Google Scholar]
- [17].Anderson RE, Rapp LM, Wiegand RD. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984;3(1):223–227. [DOI] [PubMed] [Google Scholar]
- [18].Costa V, Ciccodicola A. Is PPARG the key gene in diabetic retinopathy? Br J Pharmacol. 2012;165(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liew G, Wong VW, Ho IV. Mini review: changes in the incidence of and progression to proliferative and sight-threatening diabetic retinopathy over the last 30 years. Ophthalmic Epidemiol. 2017;24(2):73–80. [DOI] [PubMed] [Google Scholar]
- [20].Zhou T, Zhou KK, Lee K, et al. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia. 2011;54(2):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Q, Pfister F, Dorn-Beineke A, et al. Low-dose erythropoietin inhibits oxidative stress and early vascular changes in the experimental diabetic retina. Diabetologia. 2010;53(6):1227–1238. [DOI] [PubMed] [Google Scholar]
- [22].Zhang L, Yu J, Ye M, et al. Upregulation of CKIP-1 inhibits high-glucose induced inflammation and oxidative stress in HRECs and attenuates diabetic retinopathy by modulating Nrf2/ARE signaling pathway: an in vitro study. Cell Biosci. 2019;9(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ola MS, Al-Dosari D, Alhomida AS. Role of oxidative stress in diabetic retinopathy and the beneficial effects of flavonoids. Curr Pharm Des. 2018;24(19):2180–2187. [DOI] [PubMed] [Google Scholar]
- [24].Dehdashtian E, Mehrzadi S, Yousefi B, et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018;193:20–33. [DOI] [PubMed] [Google Scholar]
- [25].Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li D, Jiang C, Mei G, et al. Quercetin alleviates ferroptosis of pancreatic beta cells in type 2 diabetes. Nutrients. 2020;12(10):2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma H, Wang X, Zhang W, et al. Melatonin suppresses ferroptosis induced by high glucose via activation of the nrf2/ho-1 signaling pathway in type 2 diabetic osteoporosis. Oxid Med Cell Longev. 2020;2020:9067610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li S, Zheng L, Zhang J, et al. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435–449. [DOI] [PubMed] [Google Scholar]
- [30].Zhang J, Qiu Q, Wang H, et al. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res. 2021;407(2):112800. [DOI] [PubMed] [Google Scholar]
- [31].Tang W, Guo J, Liu W, et al. Ferrostatin-1 attenuates ferroptosis and protects the retina against light-induced retinal degeneration. Biochem Biophys Res Commun. 2021;548:27–34. [DOI] [PubMed] [Google Scholar]
- [32].Totsuka K, Ueta T, Uchida T, et al. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp Eye Res. 2019;181:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Luis G, Godfroid A, Nishiumi S, et al. Tumor resistance to ferroptosis driven by stearoyl-CoA desaturase-1 (SCD1) in cancer cells and fatty acid biding protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43:102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shen LQ, Child A, Weber GM, et al. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126(6):793–799. [DOI] [PubMed] [Google Scholar]
- [35].Damcott CM, Moffett SP, Feingold E, et al. Genetic variation in fatty acid-binding protein-4 and peroxisome proliferator-activated receptor gamma interactively influence insulin sensitivity and body composition in males. Metabolism. 2004;53(3):303–309. [DOI] [PubMed] [Google Scholar]
- [36].Garin-Shkolnik T, Rudich A, Hotamisligil GS, et al. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes. 2014;63(3):900–911. [DOI] [PubMed] [Google Scholar]


