Abstract
High-risk neuroblastoma has a poor prognosis and research studies have shown that increasing the intensity of therapy improves outcomes. Autologous hematopoietic cell transplant (aHCT) as consolidation therapy confers a significant survival advantage but is accompanied by significant morbidity. Transplant-associated thrombotic microangiopathy (TA-TMA) is a life-threatening complication caused by endothelial injury that often leads to hemolytic anemia, microthrombotic platelet consumption, and renal injury. Here we investigated the incidence, potential risk-factors, and sequelae of TA-TMA in patients with high-risk neuroblastoma. We conducted a retrospective chart review of all patients (n=141) with neuroblastoma in our institutions who underwent aHCT from 2000-2017. Ten patients (7%) developed TA-TMA. The patients in the TA-TMA group were similar to the rest of the subjects in demographics, disease burden, prior therapies, renal function, and timing of transplant. The type of conditioning regimen was the only statistically significant pre-transplant variable (p<0.001). Six of the 15 (40%) patients intended to receive tandem transplants (cyclophosphamide/thiotepa, then carboplatin/etoposide/melphalan (CEM)), four of the 68 (6%) patients who received conditioning with single CEM, and none of the 56 who received busulfan/melphalan were diagnosed with TA-TMA. Patients with TA-TMA were more likely to require ICU transfer, have a longer length of stay in the hospital, and experience a delay or change in their subsequent therapy. In our cohort overall, patients with a delay in therapy following transplant appeared to have a worse overall survival, although the difference was not statistically significant. Due to this high incidence and significant morbidity, we have implemented standardized screening for TA-TMA during and after transplant. We anticipate that screening will lead to earlier intervention and decreased severity of disease.
Keywords: Transplant-associated thrombotic microangiopathy, Pediatrics, Neuroblastoma, Autologous hematopoietic cell transplant, Tandem transplant
Introduction
Neuroblastoma is the most common extracranial solid tumor in children and accounts for a disproportionate amount of mortality.1, 2 Therapy for patients with high-risk disease has continued to increase in intensity over the years, and consolidation therapy with myeloablative chemotherapy and autologous hematopoietic stem cell transplant (aHCT) significantly improves event-free survival compared to standard dose chemotherapy.3-7 To capitalize on the impact of myeloablative therapy, pilot studies showed that tandem transplants were feasible.8,9 A recent randomized Children’s Oncology Group (COG) study showed significantly better outcomes with the tandem regimen of cyclophosphamide and thiotepa (Cy/Thio) followed approximately six weeks later with carboplatin, etoposide, and melphalan (CEM), compared to CEM alone.10 These findings have changed the standard of care in North America for high-risk neuroblastoma to tandem transplants with these conditioning regimens. Meanwhile, in a European study by the International Society of Pediatric Oncology European Neuroblastoma Group (SIOPEN), a single transplant using conditioning with CEM was compared to a single transplant using busulfan and melphalan (Bu/Mel). The latter was shown to have superior 3-year event-free survival (EFS), with comparable toxic death rates but fewer intensive care unit (ICU) admissions, making this the standard of care in Europe and the US for a period of time.11 To date, tandem transplant and single Bu/Mel transplant have not been compared directly in a randomized fashion. Regardless of the type of myeloablative regimen used, transplant is associated with potentially serious side effects with resulting morbidity and mortality.
Treatment-associated thrombotic microangiopathy (TA-TMA) is one possibly life-threatening complication seen in autologous and allogeneic bone marrow transplantation. Microangiopathic hemolytic anemia results in multi-organ tissue injury through endothelial damage, thought to be mediated by the activation of the complement pathway, and can result in sequelae of renal failure, encephalopathy, and other end-organ damage.12, 13 Rapid decreases in hemoglobin from hemolysis and platelet consumption due to microthrombi formation can be difficult to manage in post-transplant patients who are already transfusion dependent. Clinical presentation ranges from mild, with only modest increase in number of transfusions and high blood pressure, to quite severe, leading to renal injury requiring prolonged dialysis, or even death. This heterogeneity, along with other complications of transplant also impacting the renal and hematopoietic system, such as sinusoidal obstruction syndrome (SOS),14 can make the diagnosis of TA-TMA challenging.15
There is no clear consensus on the diagnostic criteria for this entity, and it is likely underrecognized. 15-21 With new treatment modalities available, such as eculizumab,22,23 patients would be expected to benefit from earlier diagnosis and treatment. In neuroblastoma, TA-TMA has been reported in as high as 20-30% of patients following conditioning with CEM.24-26 Here, we retrospectively describe the rates of TA-TMA at our institutions in all high-risk neuroblastoma patients undergoing aHCT, and compare the clinical characteristics and outcomes of patients with TA-TMA to those without.
Methods
Participants:
All patients who underwent aHCT for high-risk neuroblastoma at the University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland between the years 2000 to 2017 were included in this cohort (Supplementary Figure 1). Subjects were excluded if they had received a prior transplant (n=1) or if they did not complete planned conditioning chemotherapy for at least one transplant (n=1). When applicable, all transplant trials were approved by the institutional review board, and all patients/guardians gave informed consent for treatment. After approval of this retrospective review by the institutional review board, data were collected from electronic and paper medical records, and the Cancer Registry of the UCSF Helen Diller Family Comprehensive Cancer Center and stored in REDCap on a secure server. Clinical notes, demographic information, imaging reports, and laboratory results were reviewed. Race and ethnicity were self-reported. Stage was reported using the International Neuroblastoma Staging System.27 Pre-transplant glomerular filtration rate (GFR) was determined by nuclear medicine scan and normalized to body surface area.
Clinical features:
The following characteristics associated with TA-TMA were abstracted from the medical record when available: number of platelet and packed red blood cell transfusions, lactate dehydrogenase (LDH), bilirubin, D-dimer, haptoglobin, schistocytes on peripheral smear, creatinine, nuclear GFR, urinalysis (proteinuria and hematuria), anti-hypertensives used (other than specifically for diuresis), dialysis, and encephalopathy. To help rule out other causes of these findings besides TA-TMA, these values were also collected: direct (Coombs) antiglobulin test, prothrombin time, activated partial thromboplastin time, and liver ultrasound with Doppler reports. A patient was considered to have the diagnosis of TA-TMA if the diagnosis was made clinically in provider notes. If the diagnosis was not made clinically, the most widely accepted TA-TMA criteria in pediatrics was used, which requires five of the following: hypertension, thrombocytopenia, elevated LDH, proteinuria, anemia, schistocytes, or elevated soluble C5b9.18 Other supportive evidence, including decreased haptoglobin, elevated bilirubin, negative direct antiglobulin test, and normal coagulation studies were used to confirm the retrospective diagnosis. Anemia and thrombocytopenia were measured by the amount of transfusions required. Hematuria and proteinuria were defined as greater than “trace” on multiple urinalyses. Creatinine values were collected when available, but if the value was less than 0.3, the clinical laboratory reported it as “<0.3” without further quantification. Thus, this limited the calculation of renal dysfunction for patients with this low baseline. Laboratory results were collected during all transplant hospitalizations and in the outpatient setting, if available. No patients in our analytic cohort received a kidney biopsy. Treatments for TA-TMA were determined clinically and were noted if the therapy was given specifically for TA-TMA.
Transplant course:
Transfusions of blood products were reported as single transfusions given, regardless of volume or number of units. Transfusions were generally ordered if hemoglobin was less than 8 g/dL and for platelet counts less than 10-20 ×109/L, or higher for clinical bleeding or patients receiving defibrotide for treatment of SOS. These were determined from blood bank records and confirmed by changes in daily platelet counts and hemoglobin. They were recorded during the transplant hospitalization and in the eight weeks following transplant if outpatient data were available.
Infections were reported during the transplant hospitalization or afterwards if readmitted due to the infection. Viral infections were noted if reported as clinically significant in the notes or if treatment was given. Bacterial infections were noted if a blood culture was positive or if the diagnosis was made clinically in the chart and a full treatment course was given. Isolated upper respiratory infections and Clostridioides difficile colitis were excluded from this analysis. Fungal infections were recorded if documented by culture or presumed, usually with radiographic imaging correlation, and a full treatment course was given. Fever treated with antimicrobials in the setting of neutropenia without clinical suspicion for specific infection was not considered an infection in this study.
All calculations of times and durations were from the day of stem cell infusion (day 0). A delay in next therapy was defined as radiation starting greater than 42 days after day 0 of the final transplant or the planned second transplant being more than 10 weeks after the first transplant. Time periods were chosen generally to correspond with COG protocols during that time frame. Patients were enrolled on these studies when possible or they were treated using the study protocol as a guideline. From 2000-2006, patients were treated on study or following A3973, ANBL02P1, NANT1999-01, NANT1999-02 or NANT2001-02.4, 28-31 From 2007-2011, patients were treated on study or following ANBL0532 ( NCT00567567).10 From 2012-2017, patients were treated on study ANBL09P1 ( NCT01175356) or following ANBL12P1 ( NCT01798004) or ANBL0532.32,33
Statistical analysis:
Categorical data were reported as frequency and percent of patients with known data with comparisons made using the Fisher’s exact test. Continuous data were summarized using median and range with differences assessed using the Wilcoxon rank-sum test for two comparisons or the Kruskal-Wallis test for multiple comparisons. Kaplan-Meier survival analyses were evaluated for overall survival (OS) and cumulative incidence of relapse, with patients who died before relapse being censored at the time of death.32 Differences between curves were assessed using the log-rank test. A p-value of less than 0.05 was considered significant. All statistical analyses were completed using Stata, version 15 (College Station, TX) software.
Results
Incidence of TA-TMA:
One hundred forty-one patients underwent aHCT at UCSF Benioff Children’s Hospitals for high-risk neuroblastoma from 2000-2017 and were included in our analytic cohort. Patient characteristics are listed in Table 1. Sixty-eight (48%) patients underwent a single CEM transplant, 56 (40%) patients underwent a single Bu/Mel transplant, and 15 (11%) patients were intended to receive tandem transplant with Cy/Thio followed by CEM. Three of these patients were only able to tolerate the first transplant with Cy/Thio, with one not proceeding to a second transplant due to TA-TMA and two due to SOS.
Table 1:
Baseline characteristics of patients undergoing autologous hematopoietic cell transplant (aHCT) for neuroblastoma and association with development of transplant associated thrombotic microangiopathy (TA-TMA)
| Characteristics | TA-TMA (n=10) |
No TA-TMA (n=131) |
p-valuea |
|---|---|---|---|
| Age in years | 2.5 [1.8-5.2] | 4 [0.8-22.9] | 0.05 |
| Male sex | 6 (60%) | 80 (61%) | 1.0 |
| Race | 0.57 | ||
| • White | 5/10 (50%) | 79/124 (64%) | |
| • Black | 0/10 (0%) | 6/124 (5%) | |
| • Otherb | 5/10 (50%) | 39/124 (31%) | |
| Hispanic ethnicity | 2/10 (20%) | 26/126 (21%) | 1.0 |
| INSS stage 4 at diagnosis | 9 (90%) | 107 (82%) | 1.0 |
| Primary tumor site abdominal | 7 (70%) | 110 (84%) | 0.37 |
| Additional therapy beyond induction | |||
| chemotherapy and surgery prior to aHCT | |||
| Conditioningc | |||
| • None | 6 (60%) | 66 (50%) | 0.75 |
| • lrinotecan/temozolomide/chl4:18 | 0 (0%) | 5 (4%) | 1.0 |
| • 131I-MIBG therapy +/− chemotherapyd | 1 (10%) | 26 (20%) | 0.69 |
| • Local radiation | 3 (30%) | 23 (18%) | 0.39 |
| • Other cytotoxic chemotherapy | 3 (30%) | 38 (29%) | 1.0 |
| Disease status prior to transplant | 0.33 | ||
| • Complete response or very good partial response | 6 (60%) | 78 (60%) | |
| • Partial response | 3 (30%) | 30 (23%) | |
| • Stable disease | 0 (0%) | 18 (14%) | |
| • Progressive disease | 1 (10%) | 4 (3%) | |
| History of prior relapse | 1 (10%) | 7 (5%) | 0.45 |
| Nuclear GFR (ml/min/1.73m2) prior to transplant | 107.2 [76.6-128] (n=8) | 115.4 [63-234] (n=87) | 0.23 |
| GFR <100 ml/min/1.73m2 prior to transplant | 2/8 (25%) | 22/87 (25%) | 1.0 |
| Renal or renovascular involvement from neuroblastoma at diagnosis | 4/7 (57.1%) | 40/89 (45%) | 0.70 |
| Nephrectomy prior to transplant | 0/10 (0%) | 14/129 (11%) | 0.60 |
| Days from diagnosis to transplant | 187.5 [141-596] | 202 [138-1162] | 0.45 |
| Type of conditioning chemotherapy | <0.001 | ||
| • Single CEMd | 4 (40%) | 64 (49%) | |
| • Bu/Mel | 0 (0%) | 56 (43%) | |
| • Cy/Thio (intended for tandem) | 1 (10%) | 2 (2%) | |
| • Cy/Thio and CEM (tandem) | 5 (50%) | 7 (5%) | |
| • Other | 0 (0%) | 2 (2%) | |
| Transplant time period (years) | 0.35 | ||
| • 2000-2006 | 2 (20%) | 48 (37%) | |
| • 2007-2011 | 2 (20%) | 37 (28%) | |
| • 2012-2017 | 6 (60%) | 46 (35%) |
Data presented as number of patients (%) with denominator written if different than total sample size (n) for the column for categorical variables and as median [range] for continuous variables.
Continuous variables were compared using the Wilcoxon rank-sum test and categorical variables using the Fisher’s exact test.
The “other” patients were divided among Asian, Native Hawaiian or Pacific Islander, other or mixed.
Individual patients may be listed multiple times if they received multiple therapies prior to transplantation therefore we did not use a family-wise comparison.
This includes those patients (13) who received 131I-MIBG with single CEM as pre-transplant conditioning as part of a clinical trial.
Abbreviations: Bu/Mel- busulfan and melphalan, CEM-carboplatin, etoposide, melphalan, Cy/Thio- cyclophosphamide and thiotepa, GFR- glomerular filtration rate, INSS- International neuroblastoma staging system, MIBG- metaiodobenzylguanidine
Table 1 details the pre-transplant characteristics of patients in the TA-TMA and non TA-TMA groups. Ten patients (7%) had TA-TMA; seven were diagnosed clinically and three were diagnosed retrospectively upon chart review. The patients in the TA-TMA group were similar to the rest of the subjects in demographics, disease burden, prior therapies, renal function and renal involvement of neuroblastoma, and timing of transplant. The type of conditioning regimen, however, was the only statistically significantly different pre-transplant variable (p<0.001). Six of the 15 (40%) patients intended to receive tandem transplants, four of the 68 (6%) patients who received single CEM, and none of 56 who received Bu/Mel were diagnosed with TA-TMA (Supplementary Table 1).
Table 2 lists the most commonly used criteria18 used in the diagnosis of TA-TMA and the occurrence in patients with TA-TMA in our analytic cohort. Five of the TA-TMA patients were missing some measurements, so supplementary criteria were also listed. Mild renal dysfunction as measured by a 50% increase in creatinine was common during transplant (58/107, 54% of patients overall), but patients with TA-TMA had a higher frequency of more significant renal dysfunction when measured by a doubling of serum creatinine (71% vs. 21%, p=0.007) (Table 3).
Table 2:
Diagnostic criteria met by patients with transplant-associated thrombotic microangiopathy (TA-TMA)
| Jodele, et al. (ref 18) criteria for TA-TMA | Other supporting criteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN | HTN | ↑ Plt Tx | ↑ LDH | Proteinuria | ↑ RBC Tx | Schistocytes | ↑ sC5b9 | # of criteria meta |
DAT | ↓ haptoglobin | ↑ Cr | PT/PTT |
| 4 | Yes | Yes | Yes | Yes | Yes | Yes | No | 6 | -- | Yes | -- | Nl |
| 13c | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | -- | Yes | Yes | Nl |
| 36 | Yes | Yes | Yes | Yes | Yes | Yes | -- | 6 | Neg | -- | -- | Nl |
| 47 | Yes | Yes | Yes | Yes | Yes | No | No | 5 | Neg | Yes | No | Nl |
| 50 | Yes | Yes | Yes | Yes | Yes | Yes | No | 6 | -- | Yes | No | Nl |
| 51c | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | Pos | Yes | Yes | Abnl |
| 62 | Yes | Yes | Yes | Yes | No | Yes | -- | 5 | Neg | -- | Yes | Nl |
| 78d | Yesb | Yes | -- | Yes | Yes | Yes | -- | 5 | -- | -- | -- | Nl |
| 105c,d | Yes | Yes | -- | Yes | Yes | Yes | -- | 5 | Neg | -- | Yes | Abnl |
| 107d | Yes | Yes | Yes | Yes | Yes | Yes | -- | 6 | Neg | -- | Yes | Abnl |
Increased platelet and red blood cell transfusions defined as greater than the median. Elevated LDH is greater than the upper limit of normal for age and sex. Low haptoglobin is less than the lower limit of normal of 36mg/dL. Elevated soluble C5b9 is greater than the upper limit of normal of 244ng/ml. Renal dysfunction (↑ Cr) is defined by a doubling of serum creatinine (some patients are unknown due to lab reporting creatinine <0.3 so unable to calculate change in creatinine).
-- Indicates test not done or unknown.
The diagnosis of TA-TMA was made with five out of seven criteria.
Noted as being hypertensive, but no report of antihypertensive medications being used in the medical records.
These patients also had SOS.
These patients were diagnosed with TA-TMA retrospectively by chart review.
Abbreviations: Abnl- abnormal, Cr- creatinine, DAT- direct antiglobulin test (direct Coombs), HTN- hypertension, LDH- lactate dehydrogenase, Neg- negative, Nl- normal, Plt- platelet, Pos- positive, PT/PTT- prothrombin time/partial thromboplastin time, RBC- red blood cell, sC5b9- soluble C5b9, Tx- transfusions, SOS- sinusoidal obstruction syndrome, UPN- unique patient number
Table 3:
Clinical and laboratory criteria of transplant-associated thrombotic microangiopathy (TA-TMA) and complications after autologous hematopoietic cell transplant (aHCT)
| TMA (n= 10) |
No TA-TMA (n= 143) |
p-valuea | |
|---|---|---|---|
| Schistocytes present on peripheral smear | 9 (90%) | 24 (17%) | <0.001 |
| Number of RBC transfusions following aHCT | |||
| • Weeks 0-4 | 5.5 [2-10] | 2 [0-16] | 0.0003 |
| • Weeks 5-8 | 2 [0-5] | 0 [0-6] | <0.0001 |
| Number of platelet transfusions following aHCT | |||
| • Weeks 0-4 | 16.5 [9-43] | 7 [1-32] | 0.0002 |
| • Weeks 5-8 | 5.5 [0-15] | 0 [0-22] | <0.0001 |
| Proteinuria | 10/10 (100%) | 19/83 (23%) | <0.001 |
| Hemoglobinuria | 10/10 (100%) | 18/83 (22%) | <0.001 |
| Creatinine | |||
| • Increased by 50% | 5/7 (71%) | 53/100 (53%) | 0.45 |
| • Increased by 100% | 5/7 (71%) | 19/95 (21%) | 0.007 |
| Scheduled anti-hypertensives | 9/10 (90%) | 6/139 (4%) | <0.001 |
| Total bilirubin | 2.2 [1.1-16.3] | 0.9 [0.2-37.6] | 0.003 |
| SOS diagnosis | 3 (30%) | 35 (25%) | 0.71 |
| Infection during transplant hospitalization | |||
| • Viral | 3 (30%) | 20 (14%) | 0.18 |
| • Bacterial | 5 (50%) | 31 (22%) | 0.06 |
| • Fungal | 0 (0%) | 10 (7%) | 1.0 |
| ICU transfer | 5 (50%) | 19 (13%) | 0.009 |
| Initial hospital LOS (days) | 47.5 [27-0103] | 30 [6-101] | 0.005 |
| Delay or change in next therapy | 9/10 (90%) | 52/136 (39%) | 0.002 |
This table includes the total number of transplants, thus patients who underwent tandem transplants are included twice, with data from each transplant being reported as unique events. Data presented as number of patients (%) with denominator written if different than total sample size (n) for the column for categorical variables and as median [range] for continuous variables.
Continuous variables were compared using the Wilcoxon rank-sum test and categorical variables using the Fisher’s exact test.
Abbreviations: ICU- intensive care unit, LOS- length of stay, RBC- red blood cells, SOS- sinusoidal obstruction syndrome
Risk factors for TA-TMA:
Patients with TA-TMA had similar rates of SOS and infections as those without TA-TMA (Table 3). However, there was a trend towards higher viral and bacterial infection rates in patients with TA-TMA. Most of the infections occurred prior to the diagnosis of TA-TMA. Patients with TA-TMA were more likely to require intensive care unit (ICU) transfer (50% vs. 13%, p=0.009), and have a longer length of stay in the hospital during the transplant hospitalization (47.5 days vs. 30 days, p=0.005). Patients with TA-TMA were not readmitted at a higher rate (30% vs. 19%, p=0.42), but they were more likely to experience a delay or change in their subsequent therapy or planned second transplant (90% vs. 39%, p=0.002).
Clinical course and treatment of TA-TMA:
The clinical course and therapy of the ten patients with TA-TMA is shown in Table 4. TA-TMA was diagnosed at a median of day +24 from transplant (range 12-84 days). Patients were given the following therapies for their TA-TMA: eculizumab, a terminal complement inhibitor (6); steroids (4); and plasmapheresis (1). Some patients received multiple therapies. Three patients, who were diagnosed in retrospect, were not specifically treated. When given, treatment for TA-TMA lasted a median of 90 days (range 7-252 days). Only one patient required dialysis.
Table 4:
Clinical course of patients with transplant-associate thrombotic microangiopathy (TA-TMA)
| UPN | Conditioning | Days to TA-TMA diagnosisa |
Rx for TA- TMA and length of rx (days) |
Dialysis (days) |
Delay/change in next therapy |
TA-TMA course with further therapy |
Outcome (follow up time in months) |
|---|---|---|---|---|---|---|---|
| 4 | Cy/Thio and CEM | 18 | Eculizumab (51) Steroids (2) | No | Yes, RT started day + 54 | RT, IT, isotretinoin without complication | Alive, NED (34.1) |
| 13 | Cy/Thio and CEM | 15 | Eculizumab (252) Steroids (76) TPE (22) | Yes (140) | Yes, RT started day + 92, then after TA-TMA worsened during RT, no further therapy until relapse | TA-TMA worsened during RT (critically ill). After relapse, I/T/ch14:18 without complication | DOD (31.5) |
| 36 | Cy/Thio and CEM | 26 | Eculizumab (96) | No | Yes, RT started day + 53 | TA-TMA worsened with RT and delayed IT. IT then stopped due to hypersensitivity rxn and transient worsening of TA-TMA. Tolerated isotretinoin | Alive, NED (80.4) |
| 47 | Cy/Thio and CEM | 20 | Eculizumab (90) | No | Yes, RT started day + 90 | RT, IT, isotretinoin without complication | Alive, NED (18.9) |
| 50 | Cy/Thio | 34 | Eculizumab (72) Steroids (38) | No | Yes, therapy stopped for TA-TMA and NB due to progression and change of GOC | 1 cycle of I/T. TA-TMA still active at that time | DOD (4.1) |
| 51 | Cy/Thio and CEM | 34 | Eculizumab (174) | No | Yes, no RT or IT, proceeded with isotretinoin only after delay | Isotretinoin and DFMO without complication. After relapse, I/T/ch14:18 with possible recurrence of TA- TMA in lungs. | DOD (27.4) |
| 62 | Single CEM | 84 | Steroids (7) | No | No, TA-TMA not diagnosed until after RT. | No further therapy after TA-TMA with RT | DOD (4.5) |
| 78b | Single CEM | 12 | None | No | Yes, RT started day + 46 | Isotretinoin complicated by Evan’s syndrome so stopped early. After relapse, MIBG therapy x2 without complication | DOD (71.4) |
| 105b | Single CEM | 22 | None | No | Yes, RT started day + 110 | Prolonged isotretinoin due to multiple ganglioneuromas without complication | Alive, NED (208) |
| 107b | Single CEM | 36 | None | No | Yes, RT started day + 97 | No known therapy after RT | DOD (5.1) |
Time to TMA diagnosis is from transplant day 0
TMA diagnosed in retrospect on chart review
Abbreviations: AWD- alive with disease, CEM- carboplatin, etoposide, melphalan, Cy/Thio- cyclophosphamide and thiotepa, DFMO- difluoromethylornithine, DOD- dead of disease, GOC- goals of care, I/T- irinotecan and temozolomide, IT- immunotherapy (chl4:18/IL-2/GM-CSF), MIBG- 131I-Metaiodobenzylguanidine, NAC- N-acetylcysteine, NB- neuroblastoma, NED- No evidence of disease, RT- radiation therapy, Rx- treatment, rxn- reaction, TPE- therapeutic plasma exchange, UPN- unique patient number
Two patients received no further anti-tumor therapy after diagnosis of TA-TMA and subsequently died from progression of neuroblastoma (Table 4). One patient had no further therapy until their relapse. Eight patients underwent radiation therapy after transplant. Radiation triggered a new diagnosis of TA-TMA in one patient and worsened TA-TMA in two patients. Five patients received immunotherapy after TA-TMA, and one patient had worsening of their TA-TMA despite only receiving a small amount of the dose due to a hypersensitivity reaction. Another patient had a possible recurrence of TA-TMA in her lungs after immunotherapy, but this was not confirmed. Six patients received isotretinoin which did not lead to a flare of TA-TMA.
Outcomes of TA-TMA:
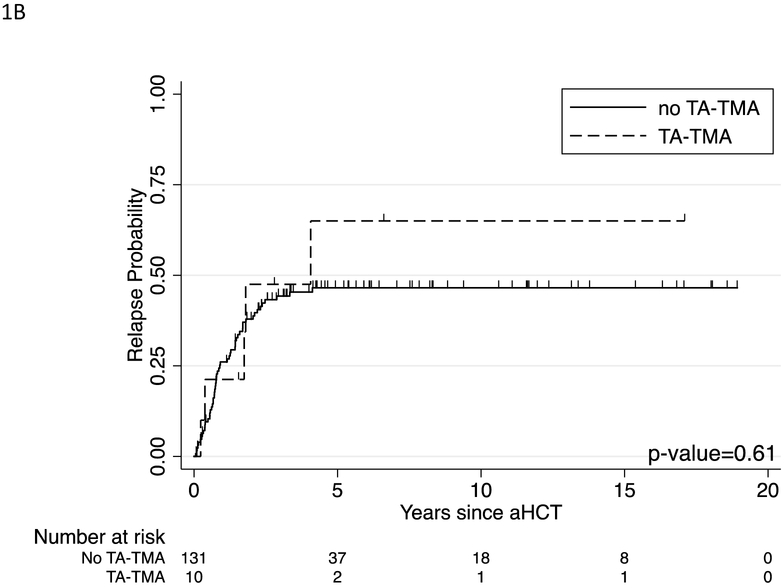
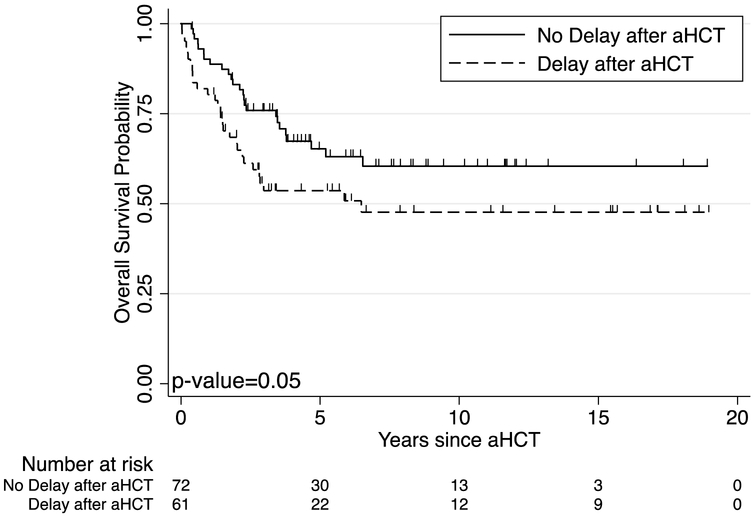
No patient died of TA-TMA in our cohort. However, three patients died from complications associated with SOS, all prior to 2013. The five-year OS for patients with TA-TMA was similar to those without TA-TMA, 47% (95% confidence interval [CI], 15% to 74%) vs. 61% (95% CI, 52% to 70%, p=0.23) (Figure 1A). The ten-year survival was also similar between these groups, 31% (95% CI, 6% to 62%) vs. 55% (95% CI, 45% to 64%, p=0.11) (Figure 1A). Cumulative incidence of relapse also showed no difference between patients with TA-TMA and those without (Figure 1B). Overall in our cohort, patients who experienced a delay or change in their therapy after transplant trended towards a worse 5-year overall survival of 54% (95% CI, 40% to 66%) vs 65% (95% CI, 52% vs 76%, p=0.06) as well as at 10 years 48% (95% CI, 33% to 61%) vs 60% (95% CI, 47% to 72%, p=0.05) (Figure 2).
Figure 1:
Outcome of patients with TA-TMA (n=10) compared to those without TA-TMA (n=131). (A) Ten-year overall survival in patients with TA-TMA compared to those without TA-TMA. (B) Cumulative incidence of relapse in patients with TA-TMA compared to those without TA-TMA. Patients who died prior to relapse were censored at the time of death. Groups werecompared using the log-rank test with p-values shown.
Figure 2:
Ten-year overall survival (OS) according to delays or changes in therapy after aHCT. Groups were compared using the log-rank test with p-value=0.05, indicating a trend for lower OS with delays or changes in therapy, but not statistically significant.
Discussion
We report that TA-TMA was a complication in 7% of patients with high-risk neuroblastoma who underwent aHCT in our analytic cohort. The only statistically significant pre-transplant risk factor emerging from our analysis was the type of conditioning regimen used, with 40% of patients who were intended to receive tandem transplant developing TA-TMA. For single transplants, 6% of patients treated with CEM developed TA-TMA whereas no patients who received Bu/Mel showed clinical signs of TA-TMA. Our overall incidence of TA-TMA was lower than the previously published rate of 22% (13 of 60 patients) in high-risk neuroblastoma transplants overall and 24% (10 of 41 patients) of single CEM transplants by Jodele, et al.26 However, the 40% incidence we observed in patients undergoing tandem transplants is similar to the 50% (3 of 6 patients) incidence reported by this same group.26 The reason for this difference among single transplants between institutions is not readily apparent, but there is agreement that tandem transplant confers higher risk of TA-TMA. The lower incidence of TA-TMA in single transplants in our study may be due in part to the retrospective nature of this study without consistent prospective screening for TA-TMA which may have decreased our sensitivity for detection of TA-TMA compared to Jodele et al. who had implemented clinical screening prior to their retrospective review.
TA-TMA is a serious complication that led to renal dysfunction, more time in the ICU and the hospital, and delay or omission of the next stage of therapy. Patients whose subsequent treatments were delayed after transplant, while not statistically significant, trended towards a worse overall survival. Thus, larger numbers of patients will likely be required to definitely determine the impact of TA-TMA on long-term survival. Beyond tandem aHCT, we were unable to identify other risk factors for TA-TMA, potentially because it is a rare entity. There was a trend towards TA-TMA patients being younger which, if confirmed, might suggest a role of different drug metabolism in younger patients.
TA-TMA and SOS are both endothelial damage syndromes and may represent a spectrum of conditioning-associated injury.14, 34, 35 Thus, the high frequency of SOS during transplant may confound the ability to correctly diagnose and treat TA-TMA due to the similarities in laboratory abnormalities and supportive care needs. Patients can have both disorders, and only with a high index of suspicion can the signs and symptoms of TA-TMA be separated from SOS. Monitoring for hypertension, elevated LDH, proteinuria, and schistocytes can help clarify whether TA-TMA is present, as these are not typically reported in SOS. In our cohort, SOS also led to delay or changes in the next stage of therapy as well as three deaths, indicating the serious impact of these endothelial damage-related complications. SOS has been reported more commonly after Bu/Mel transplants,36 although in our cohort there was no statistically significant difference in SOS among Bu/Mel, CEM, and tandem transplants (Supplementary Table 2). We have demonstrated in our relatively small cohort of tandem transplants, that the incidence of TA-TMA was significantly higher than in either Bu/Mel alone or CEM alone. This may suggest that there is cumulative endothelial damage with Cy/Thio followed by CEM. It is also worth noting that one of the patients that developed TA-TMA after single CEM did not clinically manifest the disease until after radiation, supporting the hypothesis of cumulative endothelial injury. The reason for this disparity in location and type of endothelial damage with these different conditioning regimens is unknown and warrants further investigation, as this could guide better preventative measures or earlier detection and treatment. Increased attention to the pharmacokinetics and pharmacogenomics of cyclophosphamide, thiotepa, carboplatin, and/or etoposide may also provide insight into which patients develop TA-TMA. Genetic susceptibility to complement activation and endothelial injury should also be the focus of further research, as this could potentially help individualize conditioning regimens.37-40
Our retrospective chart review was limited by incomplete data, since there was no prospective monitoring specifically for TA-TMA in patients with neuroblastoma at our institutions until recently. For example, patients often did not have complement or haptoglobin levels measured. There was likely some ascertainment bias due to our single CEM transplants being performed earlier in the study period, and thus we had less access to complete data in older medical record systems and paper charts. Patients were also not randomized to conditioning regimens, as these decisions were made clinically or on the basis of which clinical trial was open, which can confound results. The lack of a specific International Statistical Classification of Diseases and Related Health Problems (ICD) code or National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) classification contributed to the difficulty in identifying and reporting this disorder. In addition, despite this being the largest cohort of patients with neuroblastoma investigated for this outcome, it is a rare complication. The number of patients undergoing tandem transplant was relatively small and prevented more extensive analysis of the data in our limited sample size. More information on the incidence of TA-TMA may result from the recently completed COG randomized trial comparing CEM to tandem transplant ( NCT00567567).10
Increased awareness of TA-TMA has led our institutions to expand and standardize our screening efforts. For example, we obtain laboratory tests (including LDH and urinalysis) more frequently and have a higher index of suspicion of TA-TMA when evaluating patients, similar to the evaluation recommended by Jodele, et al.26 We anticipate that this will lead to earlier interventions and decreased severity of disease. We also have an ongoing pilot trial using prophylactic defibrotide to determine the feasibility, safety, and efficacy of defibrotide prophylaxis in a pediatric transplant population at high risk for TA-TMA, including patients with neuroblastoma receiving tandem transplants ( NCT03384693). With these combined efforts, we hope to decrease the incidence and severity of this life-threatening complication of aHCT in high-risk neuroblastoma patients.
Supplementary Material
Highlights.
Neuroblastoma patients undergoing autologous transplant have frequent complications
Incidence of transplant-associated thrombotic microangiopathy is significant
Tandem transplants have high risk of thrombotic microangiopathy (40%)
Morbidities include longer hospital stays, ICU care, and therapy delays
Therapy delays may negatively affect overall survival
Acknowledgements:
We specifically thank Dr. I. Elaine Allen and Dr. John Neuhaus within the UCSF CTSI for their statistical support.
Grant Support: This work was supported by the National Institutes of Health (NIH) T32 Research Training Grant in Childhood Cancer Grant Number 5T32CA128583 (VPT), Mildred V. Strouss Endowed Chair (KKM), Alex’s Lemonade Stand Foundation (KKM, KTV), Frank A. Campini Foundation (KTV), and Posey Family Foundation (KTV). This publication was also supported by the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI Grant Numbers KL2 TR001870 (KTV) and UL1 TR001872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: CCD has consulted for Jazz Pharmaceuticals and Alexion, Inc. No other authors have conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maris JM. Recent advances in neuroblastoma. The New England Journal of Medicine. 2010;362:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 6.Berthold F, Ernst A, Hero B, et al. Long-term outcomes of the GPOH NB97 trial for children with high-risk neuroblastoma comparing high-dose chemotherapy with autologous stem cell transplantation and oral chemotherapy as consolidation. Br J Cancer. 2018;119:282–290. 10.1038/s41416-018-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer. 2005;44:348–357. 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 8.Seif AE, Naranjo A, Baker DL, et al. A pilot study of tandem high-dose chemotherapy with stem cell rescue as consolidation for high-risk neuroblastoma: Children's Oncology Group study ANBL00P1. Bone Marrow Transplant. 2013;48:947–952. 10.1038/bmt.2012.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp SA, Stern JW, Bunin N, et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. 2000;18:2567–2575. 10.1200/JCO.2000.18.13.2567. [DOI] [PubMed] [Google Scholar]
- 10.Park JR, Kreissman SG, London WB, et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children's Oncology Group (COG) study. Journal of Clinical Oncology. 2016;34:LBA3–LBA3. 10.1200/JCO.2016.34.18_suppl.LBA3. [DOI] [Google Scholar]
- 11.Ladenstein R, Potschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–514. 10.1016/S1470-2045(17)30070-0. [DOI] [PubMed] [Google Scholar]
- 12.Khosla J, Yeh AC, Spitzer TR, Dey BR. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: current paradigm and novel therapies. Bone Marrow Transplant. 2018;53:129–137. 10.1038/bmt.2017.207. [DOI] [PubMed] [Google Scholar]
- 13.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadir Y, Brenner B. Thrombotic complications associated with stem cell transplantation. Blood Rev. 2012;26:183–187. 10.1016/j.blre.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 15.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion. 2004;44:294–304. [DOI] [PubMed] [Google Scholar]
- 16.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 17.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. 10.1097/TP.0b013e3181f24e8d. [DOI] [PubMed] [Google Scholar]
- 18.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–575. 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100. [DOI] [PubMed] [Google Scholar]
- 21.Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora's box. Bone Marrow Transplant. 2017. 10.1038/bmt.2017.39. [DOI] [PubMed] [Google Scholar]
- 22.Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20:518–525. 10.1016/j.bbmt.2013.12.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Fontbrune FS, Galambrun C, Sirvent A, et al. Use of Eculizumab in Patients With Allogeneic Stem Cell Transplant-Associated Thrombotic Microangiopathy: A Study From the SFGM-TC. Transplantation. 2015;99:1953–1959. 10.1097/TP.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 24.Laskin BL, Goebel J, Davies SM, et al. Early clinical indicators of transplant-associated thrombotic microangiopathy in pediatric neuroblastoma patients undergoing auto-SCT. Bone Marrow Transplant. 2011;46:682–689. 10.1038/bmt.2010.182. [DOI] [PubMed] [Google Scholar]
- 25.Jodele S, Dandoy CE, Myers KC, et al. Thrombotic Microangiopathy (TMA) and Organ Injury after Autologous Transplant is Higher with Carboplatin/Etoposide/Melphalan (CEM) Compared with Either Busulfan and Melphaln or Cyclophosphamide and Thiotepa in Patients with Neuroblastoma. Biology of Blood and Marrow Transplantation. 2017;23:S235–S236. 10.1016/j.bbmt.2016.12.462. [DOI] [Google Scholar]
- 26.Jodele S, Dandoy CE, Myers K, et al. High-dose Carboplatin/Etoposide/Melphalanincreases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. 2018;53:1311–1318. 10.1038/s41409-018-0159-8. [DOI] [PubMed] [Google Scholar]
- 27.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 28.Park JR, Scott JR, Stewart CF, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:4351–4357. 10.1200/JCO.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanik GA, Villablanca JG, Maris JM, et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biol Blood Marrow Transplant. 2015;21:673–681. 10.1016/j.bbmt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol. 2006;24:500–506. 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 31.Villablanca JG, Volchenboum SL, Cho H, et al. A Phase I New Approaches to Neuroblastoma Therapy Study of Buthionine Sulfoximine and Melphalan With Autologous Stem Cells for Recurrent/Refractory High-Risk Neuroblastoma. Pediatr Blood Cancer. 2016;63:1349–1356. 10.1002/pbc.25994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–481. Doi 10.2307/2281868. [DOI] [Google Scholar]
- 33.Weiss BY G; Naranjo A; Fitzgerald W; Shulkin B; Grupp S; Pater L; Mattei P; Park J; Matthay K A Safety and Feasibility Study of 131I-MIBG In Newly Diagnosed High-Risk Neuroblastoma: A Children’s Oncology Group (COG) Pilot. Advances in Neuroblastoma Meeting. 2018;Abstract #60. [Google Scholar]
- 34.Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–1502. 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- 35.Woywodt A, Haubitz M, Buchholz S, Hertenstein B. Counting the cost: markers of endothelial damage in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:1015–1023. 10.1038/sj.bmt.l704733. [DOI] [PubMed] [Google Scholar]
- 36.Schechter T, Perez-Albuerne E, Lin TF, et al. Veno-occlusive disease after high-dose busulfan-melphalan in neuroblastoma. Bone Marrow Transplant. 2018. 10.1038/s41409-018-0298-y. [DOI] [PubMed] [Google Scholar]
- 37.Jodele S, Zhang K, Zou F, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127:989–996. 10.1182/blood-2015-08-663435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ardissino G, Salardi S, Berra S, et al. Acquired Complement Regulatory Gene Mutations and Hematopoietic Stem Cell Transplant-Related Thrombotic Microangiopathy. Biol Blood Marrow Transplant. 2017;23:1580–1582. 10.1016/j.bbmt.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Rachakonda SP, Dai H, Penack O, et al. Single Nucleotide Polymorphisms in CD40L Predict Endothelial Complications and Mortality After Allogeneic Stem-Cell Transplantation. J Clin Oncol. 2018;36:789–800. 10.1200/JCO.2017.76.4662. [DOI] [PubMed] [Google Scholar]
- 40.Balassa K, Andrikovics H, Remenyi P, et al. The potential role of HLA-DRB1*11 in the development and outcome of haematopoietic stem cell transplantation-associated thrombotic microangiopathy. Bone Marrow Transplant. 2015;50:1321–1325. 10.1038/bmt.2015.161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.