Abstract
Background and Objectives
Neurofilament light (NfL) appears to be a promising fluid biomarker in repeat-expansion spinocerebellar ataxias (SCAs), with piloting studies in mixed SCA cohorts suggesting that NfL might be increased at the ataxic stage of SCA type 1 (SCA1). We here hypothesized that NfL is increased not only at the ataxic stage of SCA1, but also at its (likely most treatment-relevant) preataxic stage.
Methods
We assessed serum NfL (sNfL) and CSF NfL (cNfL) levels in both preataxic and ataxic SCA1, leveraging a multicentric cohort recruited at 6 European university centers, and clinical follow-up data, including actually observed (rather than only predicted) conversion to the ataxic stage. Levels of sNfL and cNfL were assessed by single-molecule array and ELISA technique, respectively.
Results
Forty individuals with SCA1 (23 preataxic, 17 ataxic) and 89 controls were enrolled, including 11 preataxic individuals converting to the ataxic stage. sNfL levels were increased at the preataxic (median 15.5 pg/mL [interquartile range 10.5–21.1 pg/mL]) and ataxic stage (31.6 pg/mL [26.2–37.7 pg/mL]) compared to controls (6.0 pg/mL [4.7–8.6 pg/mL]), yielding high age-corrected effect sizes (preataxic: r = 0.62, ataxic: r = 0.63). sNfL increases were paralleled by increases of cNfL at both the preataxic and ataxic stage. In preataxic individuals, sNfL levels increased with proximity to predicted ataxia onset, with significant sNfL elevations already 5 years before onset, and confirmed in preataxic individuals with actually observed ataxia onset. sNfL increases were detected already in preataxic individuals with SCA1 without volumetric atrophy of cerebellum or pons, suggesting that sNfL might be more sensitive to early preataxic neurodegeneration than the currently known most change-sensitive regions in volumetric MRI. Using longitudinal sNfL measurements, we estimated sample sizes for clinical trials with the reduction of sNfL as the endpoint.
Discussion
sNfL levels might provide easily accessible peripheral biomarkers in both preataxic and ataxic SCA1, allowing stratification of preataxic individuals regarding proximity to onset, early detection of neurodegeneration even before volumetric MRI alterations, and potentially capture of treatment response in clinical trials.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT01037777.
Classification of Evidence
This study provides Class III evidence that NfL levels are increased in both ataxic and preataxic SCA1 and are associated with ataxia onset.
Spinocerebellar ataxia (SCA) type 1 (SCA1) is a devastating neurodegenerative disease characterized by fast and irreversible decline of motor function already in midlife, caused by a translated CAG repeat expansion in the ATXN1 gene.1-3 The preataxic stage of SCA1 might provide a unique opportunity to delay or even prevent the neurodegenerative process by early therapeutic intervention, with targeted molecular therapies now coming into reach.3 Particularly, interventions with antisense oligonucleotides (ASOs) targeting mutated ATXN1 have shown first promising results in mitigating the molecular, pathologic, and behavioral phenotype in SCA1 mouse models.4,5 Such ASO interventions might allow prevention of the neurodegenerative process even before the occurrence of clinical symptoms.6,7 However, to pave the way for upcoming clinical trials of these therapies, objective and easily accessible biomarkers are needed for both the preataxic and ataxic stage of SCA1, particularly for stratification of preataxic individuals in proximity to future clinical onset, early detection of neuronal decay, and capture of the treatment response.
We here propose serum levels of neurofilament light (sNfL) as objective and easily accessible blood biomarkers for preataxic disease stratification and detection of early neuronal decay in SCA1. Neurofilaments are neuron-specific cytoskeletal proteins, released on neuronal damage and, with ultrasensitive assays, reliably quantifiable in peripheral blood.8-10 Previous monocentric studies in mixed cohorts of repeat-expansion SCAs, including small subsets of patients with SCA1,11,12 indicated that blood levels of neurofilament light (NfL) in multisystemic repeat-expansion SCAs are increased at the ataxic disease stage. However, these studies lacked detailed cohort-based assessment of the preataxic and conversion stage of SCA1, validation of blood NfL levels by CSF measurements of NfL (cNfL), and estimation of sample sizes for treatment trials with NfL used as outcome variable.
Leveraging a multicentric cohort of both preataxic and ataxic individuals with SCA1 with longitudinal follow-up assessments, including not only the predicted but also the actually observed ataxia onset, we tested the hypothesis that blood levels of NfL in SCA1 may serve as a peripheral biomarker of (1) proximity to clinical ataxia onset, (2) early neuronal decay at the preataxic stage, and (3) objective trial outcome variable allowing reduction of the required sample sizes in treatment trials. We expected increased sNfL and cNfL already at the preataxic stage, with the sNfL increase in preataxic individuals preceding first signs of brain atrophy in volumetric MRI and rising further in proximity to ataxia onset and even further NfL increase at the ataxic disease stage. The primary research questions were whether NfL levels are increased in ataxic and preataxic SCA1 and whether NfL levels are associated with proximity to ataxia onset.
Methods
Cohort
Our multicentric cohort comprised 40 individuals with SCA1 and 89 healthy controls, recruited within the framework of the European Integrated Project on Spinocerebellar Ataxias (EUROSCA) and Prospective Study of Individuals at Risk for Spinocerebellar Ataxia (RISCA) consortia (recruitment period 2008–2016) and additionally by the Department of Neurodegenerative Diseases, Center for Neurology, University of Tübingen (EUROSCA: 8 ataxic carriers; RISCA: 19 preataxic carriers, 19 controls; Tübingen: 4 preataxic carriers, 9 ataxic carriers, 70 controls).13-15 The recruitment included individuals with genetically confirmed SCA1 (ATXN1 repeat length ≥39), their first-degree relatives (i.e., siblings and children), and unrelated neurologically healthy controls. Carrier status was determined in all recruited relatives of mutation carriers. According to their score on the Scale for the Assessment and Rating of Ataxia (SARA),16 SCA1 mutation carriers were classified as either ataxic (SARA score ≥3, 17 individuals) or preataxic (SARA score <3, 23 individuals). Controls comprised mutation-negative first-degree relatives of SCA1 carriers and unrelated healthy individuals, all without symptoms or signs of neurodegenerative disease. Sample size calculation was based on a piloting study indicating that 8 ataxic individuals with SCA1 and 8 controls would suffice to detect significant differences of sNfL levels between groups (assuming α = 0.01, β = 0.01, equal group size, 2-tailed nonparametric test).12 However, we included all available individuals with SCA1 to also study associations of sNfL levels with clinical and genetic variables. Longitudinal blood samples were assessed in 17 individuals (11 ataxic, 3 preataxic, 3 controls; sampling interval median 2.7 years [interquartile range 2.0–3.4]). cNfL levels were available for another 6 individuals with SCA1 (5 ataxic participants and 1 preataxic individual, eTable 1, links.lww.com/WNL/B878 for details) and compared to cNfL levels of an independent cohort of 89 neurologically healthy controls (also recruited at the Department of Neurodegenerative Diseases, University of Tübingen). The repeat length of the expanded and normal alleles was determined by PCR-based fragment length analysis from 100 to 250 ng genomic DNA (CEQ8000 capillary sequencer, Beckman Coulter, Sharon Hill, PA). Demographic, clinical, and genetic characteristics of both cohorts are summarized in Table 1.
Table 1.
Baseline Characteristics of the Cohort With sNfL Measurements
Standard Protocol Approvals, Registrations, and Patient Consents
The ethics committee of the University of Tübingen approved the study (AZ 103/2017BO2). All participants provided written informed consent before participation according to the Declaration of Helsinki. The RISCA study was registered at ClinicalTrials.gov (NCT01037777).
NfL Quantification
Blood samples were centrifuged (4,000g, 10 minutes, at room temperature). Serum was frozen at −80°C within 60 minutes after collection, shipped, and analyzed without any previous thaw-freeze cycle. We measured sNfL levels in technical duplicates by single-molecule array (Simoa) technique on the Simoa HD-X analyzer (Quanterix, Billerica) using the NF-Light Advantage kit according to the manufacturer's instructions and reagents from a single lot (No. 502554) (dilution factor 1 in 4 in sample buffer).9 Serum was centrifuged at 14,000g for 4 minutes, and the upper 90% was transferred to the assay plates. All measurements had a coefficient of variation (CV) <20% (mean CV 4.6%). All concentrations were in the previously established range of quantification,9 with high stability of the assay (within-run CV <5.0%, between-run CVs <1.2%). The lower limit of quantification (LLoQ) was 0.5 pg/mL across all runs, defined as the lowest standard having a signal higher than the average signal of the blank plus 9 SDs and allowing a recovery in the range of 100 ± 20%. Longitudinal samples were measured in the same batch. We measured cNfL levels in technical duplicates by ELISA technique using the UmanDiagnostics NF-light kit (Umeå, Sweden) according to the manufacturer's instructions (dilution factor 1 in 2 in sample buffer, within-run CV <5%, between-run CV <10%, LLoQ 81 pg/mL).17 While the Simoa technique and ELISA approach are equally well suited to measure NfL in CSF (given their sufficiently low LLoQ and the previously reported correlation between both assay types of r = 1.09), ELISA was more attractive for measuring cNfL given its advantages in clinical applicability, cost-effectiveness, and technical robustness. Technicians were blinded to the genotypic and phenotypic status of the samples.
Brain Imaging
For quantification of pontine and cerebellar atrophy, which have been shown to represent the most change-sensitive volumetric MRI regions in SCA1,18,19 regional brain volumes were assessed in 12 preataxic carriers by semiautomated segmentation methods based on T1-weighted volumetric MRI scans, as previously described.15 Signs of pontine or cerebellar atrophy were considered to be present if the respective brain volumes were below a threshold defined as the lower boundary of the 90% CI (i.e., the 5% quantile) of the respective volumes measured in healthy age-matched controls (eFigure 1, links.lww.com/WNL/B878).
Statistical Analysis
Group Effects
We used Mann-Whitney U tests (2 sided, significance level p < 0.05, Bonferroni corrected for multiple comparisons) to compare NfL baseline levels between phenotypic groups (i.e., preataxic participants, ataxic participants, and controls). To correct the group effects for the age-related NfL increase observed in controls,20,21 we calculated the z score of each individual in relation to the NfL distribution in controls at the same age.22 For this, the difference between the measured level and the level predicted for controls at the same age was standardized relative to the distribution in controls at this age. Levels in controls were modeled by linear regression on the level of log-transformed data. If the assumption of normality was violated (assessed by inspection and Shapiro-Wilk test), we used log-transformed data for the statistical analysis after ensuring that the transformed data were normally distributed. If normality also was violated after transformation, nonparametric tests were applied. We reported the effect size, r, of the applied tests whenever possible. We analyzed the data in R (version 4.1, R Foundation for Statistical Computing, Vienna, Austria).
Association of sNfL Levels With Age and Repeat Length
We analyzed the association of sNfL levels with age and CAG repeat length in SCA1 carriers by linear regression. Specifically, we modeled sNfL levels (log transformed) in all SCA1 carriers (n = 40) with the predictors age and ATXN1 CAG repeat length. We centered age at 34 years (i.e., mean age of carriers) and CAG repeat length at 47 repeats (i.e., mean CAG repeat length), as in previous analogous analyses.22,23 We excluded 1 preataxic outlier (sNfL <5 pg/mL) to fulfill model assumptions of the regression model.
Association of sNfL With Proximity to Ataxia Onset
We analyzed sNfL levels in all preataxic individuals with SCA1 as a function of the time to the predicted onset of ataxia and, for the subset of 11 preataxic individuals converting to the ataxic stage during follow-up, as a function of the time to the actually observed onset of ataxia. For each preataxic individual with SCA1, we individually calculated the time to the predicted onset of ataxia from the CAG repeat count and the age at the time of assessment, as established previously.24 Thus, the estimate based on the repeat size is adjusted for the age that the individual has actually reached without developing ataxia, meaning that the older the preataxic individual is at the time of assessment, the higher the predicted age at onset is. Moreover, we assessed the conversion-free follow-up duration of preataxic individuals by Kaplan-Meier analyses (analogous to the event-free survival times in clinical trials, R packages survival and survminer). To test the hypothesis that baseline sNfL levels allow stratification of preataxic individuals regarding their time to phenoconversion, we used log-rank tests to compare the median time to conversion between preataxic individuals with high vs low sNfL levels (threshold defined by median split).
Sample Size Estimation for Intervention Trials
We estimated the sample sizes required in future treatment trials using the reduction of sNfL levels toward levels observed in healthy controls as the outcome measure.22,25 We estimated the total sample size required to detect a given control-adjusted relative reduction of sNfL levels (20%–80%) in the treatment arm, assuming that null mean change over time occurred in the placebo arm of the trial. We based the assumed interparticipant variability in the hypothetical trial on the measured intraparticipant variability in the change of analyte levels (from baseline to first follow-up) in our ataxic individuals. The estimation further assumed equal numbers of individuals in both study arms (i.e., allocation ratio 1:1), α = 0.01, β = 0.01, 2-tailed independent t tests, and the use of log-transformed biomarker levels. It was performed with GPower 3.1 software (Kiel, Germany).
Data Availability
The anonymized data of this article can be accessed on reasonable request addressed to the corresponding authors.
Results
Serum NfL Levels at the Preataxic and Ataxic Stages of SCA1
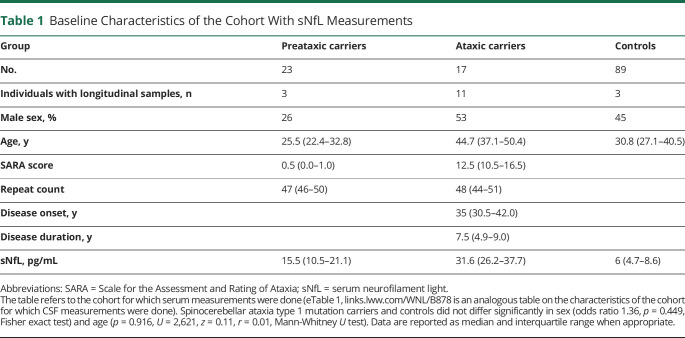
Our study included 40 individuals with SCA1 (23 preataxic individuals, including 11 converters; 17 ataxic individuals) and 89 healthy controls (Table 1). sNfL levels were significantly increased not only in ataxic individuals with SCA1 (median 31.6 pg/mL [interquartile range 26.2–37.7 pg/mL]) (U = 1,666, z = 6.51, p < 0.001, Bonferroni corrected for 3 groups) but also in preataxic individuals with SCA1 (15.5 pg/mL [10.5–21.1 pg/mL]) (U = 2091, z = 5.70, p < 0.001), each compared to controls (6.0 pg/mL [4.7–8.6 pg/mL]) (Figure 1, A and B). The effect size of the sNfL increase was strong at both the preataxic (r = 0.54) and ataxic (r = 0.63) stages. If corrected for the age-related sNfL increase by means of z transformation, the sNfL increase remained significant in both ataxic individuals (U = 1,663, z = 6.49, p < 0.001, Bonferroni corrected for 3 groups) and preataxic individuals (U = 2,208, z = 6.54, p < 0.001), again with high—and for preataxic individuals even higher—effect sizes (preataxic: r = 0.62, ataxic: r = 0.63) (Figure 1, C and D). Moreover, sNfL increased from the preataxic to the ataxic stage, as indicated by significant increases of both absolute levels (U = 301, z = 4.67, p < 0.001, r = 0.74) and age-corrected levels (U = 366, z = 2.89, p = 0.009, r = 0.46). sNfL levels differentiated preataxic and ataxic individuals with SCA1 with high accuracy (area under the curve 0.94, 95% CI 0.87–1.00). sNfL levels did not differ significantly between female and male individuals in either preataxic carriers (U = 194, W = 41, p = 0.516) or ataxic carriers (U = 70, W = 34, p = 0.888) or controls (U = 2,241, W = 1,016, p = 0.771).
Figure 1. Baseline sNfL Levels at the Preataxic and Ataxic Stages of SCA1.
(A) Serum levels of neurofilament light (sNfL) were measured in spinocerebellar ataxia type 1 (SCA1) mutation carriers at the preataxic (orange) and ataxic (red) disease stages and in mutation-negative controls (blue). (B) Boxes visualize median and lower and upper quartiles; whiskers extend to data within 1.5 interquartile range of the median. (C and D) To take into consideration the age-related increase of sNfL levels, the absolute levels were corrected for the age-related increase observed in controls by z transformation.
CSF NfL Levels at the Preataxic and Ataxic Stages of SCA1
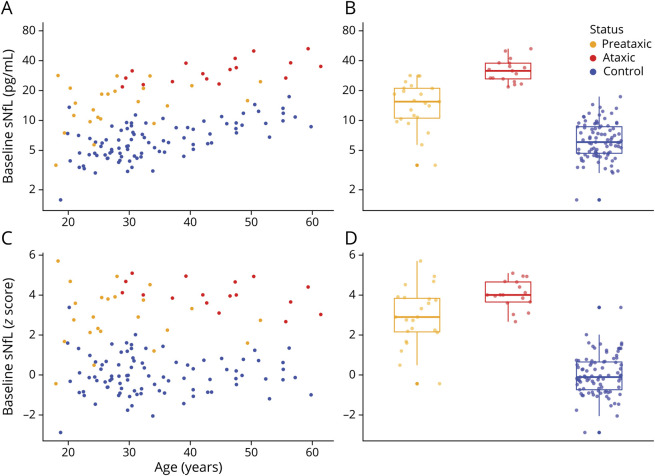
cNfL levels of each single preataxic (1,030 pg/mL) and ataxic (median 2,971 pg/mL, range 2,164–4,032 pg/mL) individual with SCA1 were clearly above the age-specific normal range of controls (Figure 2A), thus paralleling the NfL increases in blood. If compared to levels of controls of similar age (range ±5 years), the cNfL increases were significant in both preataxic (V = 0, p = 0.002) and ataxic (U = 150, z = 3.51, p < 0.001) individuals with SCA1, yielding high effect sizes (preataxic: r = 0.48, ataxic: r = 0.62).
Figure 2. cNfL Levels at the Preataxic and Ataxic Stages of SCA1 and Association of sNfL Levels With Age and CAG Repeat Count.
(A) Neurofilament light (NfL) levels in the CSF (cNfL) were increased in preataxic (orange) and ataxic spinocerebellar ataxia type 1 (SCA1) mutation carriers (red) compared to controls (blue). Dashed lines represent the 90% prediction interval of the cNfL levels in controls, modeled by linear regression of the log-transformed data. (B) We modeled serum levels of NfL (sNfL) (log transformed) across all SCA1 carriers (n = 40) by linear regression with the predictors age and ATXN1 repeat length. Both predictors proved significant and were used to generate the diagram. sNfL levels increased with age and, for a given age, each increase in the repeat length was associated with higher sNfL levels. (C) sNfL levels were higher in ataxic (red) than preataxic (orange) SCA1 carriers, also if stratified by ATXN1 repeat length.
Association of sNfL Levels in SCA1 With Repeat Length, Age, and Disease Stage
We modeled the baseline sNfL levels (log transformed) across all SCA1 carriers by linear regression with the predictors age and ATXN1 CAG repeat length using the pooled data of both preataxic and ataxic individuals (Figure 2B). Both age (p < 0.001) and repeat length (p < 0.001) were highly significant predictors of the sNfL level (F2,34 = 28.95, p < 0.001, adjusted R2 = 0.61). The model demonstrated that, for a given age, each increase in the CAG repeat count was associated with higher sNfL levels. For a given CAG repeat count, in turn, the sNfL level increased with age. Additional interaction terms and quadratic terms were not significant. Inclusion of disease stage (preataxic vs ataxic stage) as an additional predictor in the model further improved model fit (F3,33 = 22.50, p < 0.001, adjusted R2 = 0.64) and demonstrated that disease stage was a significant predictor of sNfL levels (p = 0.049) independently of the other significant predictors age (p < 0.001) and repeat length (p < 0.001) (Figure 2C).
Association of sNfL Levels at the Ataxic Stage With Disease Duration and Severity
The sNfL levels in ataxic individuals with SCA1 did not correlate with disease duration (ρ = 0.38, p = 0.169) (eFigure 2A, links.lww.com/WNL/B878) despite the presence of clinical progression (eFigure 3), supporting the notion that sNfL levels are biomarkers reflecting the rate of ongoing neuronal decay,11,22 whereby stable increases of sNfL levels might reflect stable progression at the ataxic stage of SCA1 (Figure 6B). In line with this notion, sNfL levels of ataxic individuals with SCA1 also did not correlate with clinical disease severity, as assessed by SARA (ρ = 0.08, p = 0.747) (eFigure 2B), suggesting that sNfL levels do not reflect clinical disease severity per se.
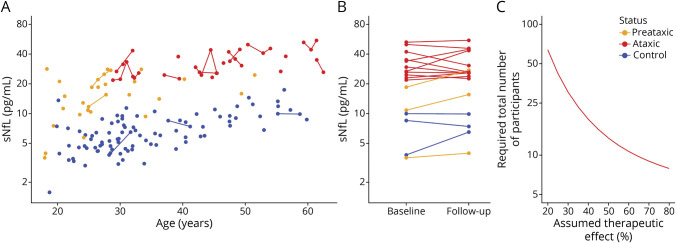
Figure 6. Longitudinal sNfL Levels in SCA1 and Sample Size Estimates for Treatment Trials.
(A) Longitudinal measurements of serum neurofilament light (sNfL) levels were available for a subset of preataxic (orange) and ataxic (red) spinocerebellar ataxia type 1 (SCA1) carriers and controls (blue). (B) Intraindividual stability of sNfL levels was assessed from baseline to first follow-up visit (median interval 2.7 years [interquartile range 2.0–3.4 years]). Lines link data of the same individuals. (C) Sample size estimations were performed for future intervention trials with the reduction of sNfL levels used as the outcome measure. The estimated total sample size (i.e., sum of individuals in both study arms) was plotted over the assumed therapeutic effect for lowering the NfL level in ataxic mutation carriers toward the levels observed in healthy controls.
Association of Increased sNfL Levels at the Preataxic Stage With Proximity to Ataxia Onset
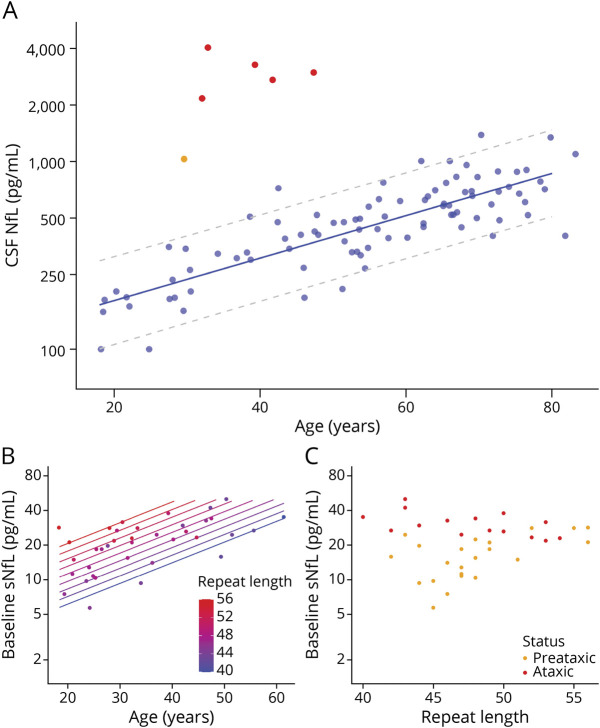
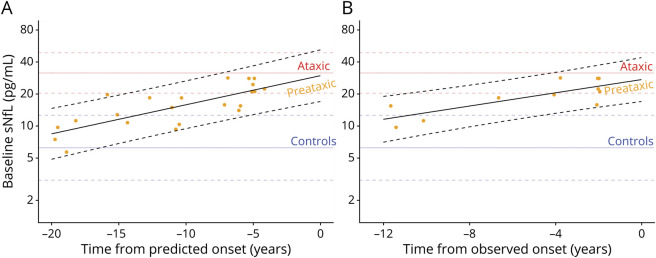
In preataxic individuals with SCA1, sNfL levels significantly increased with proximity to the individually predicted onset of ataxia, as revealed by linear regression (F1,20 = 28.67, p < 0.001, adjusted R2 = 0.57) (Figure 3A). We calculated the predicted age at ataxia onset from each individual’s CAG repeat length and their age at blood sampling.24 The increase of the sNfL levels with proximity to the predicted onset of ataxia remained significant if corrected for individuals’ age at blood sampling (F2,19 = 14.63, p < 0.001, adjusted R2 = 0.56). This finding was confirmed by analysis of the actually observed onset of ataxia of those preataxic individuals with SCA1 for whom conversion to the ataxic disease stage was actually clinically observed during longitudinal follow-up (converters n = 11). Here, sNfL levels significantly increased with proximity to the observed onset (F1,9 = 15.53, p = 0.003, adjusted R2 = 0.59) (Figure 3B). This sNfL increase with proximity to the observed onset of ataxia also remained significant if corrected for individuals’ ages (F2,8 = 6.82, p = 0.019, adjusted R2 = 0.54). Compared to controls, preataxic individuals with SCA1 showed significantly increased sNfL levels already 5 years before the predicted ataxia onset and 4 years before the observed ataxia onset, corresponding to the time point at which the prediction intervals of the sNfL levels in carriers and controls did not overlap any further (Figure 3, A and B).
Figure 3. Temporal Dynamics of sNfL Levels in Preataxic SCA1.
(A) Serum levels of neurofilament light (sNfL) baseline levels in preataxic individuals with spinocerebellar ataxia type 1 (SCA1) were plotted over the time to the individually predicted onset of ataxia, with negative time values corresponding to the preataxic disease stage. sNfL levels significantly increased with proximity to the predicted onset (F1,20 = 28.67, p < 0.001, adjusted R2 = 0.57), as visualized by the regression line and its 90% prediction band. (B) For the subset of preataxic individuals with SCA1 for whom conversion to the ataxic disease stage was actually clinically observed during follow-up, the sNfL levels at baseline were plotted over the time to the actually observed onset of ataxia. sNfL levels significantly increased with proximity to the observed onset (F1,9 = 15.53, p = 0.003, adjusted R2 = 0.59). To benchmark the levels of the preataxic individuals (orange points), the levels of controls (blue) and ataxic individuals with SCA1 (red) were visualized as horizontal lines (mean and 90% prediction band).
Stratifying Preataxic Individuals With SCA1 by sNfL Levels Regarding Time to Ataxia Onset
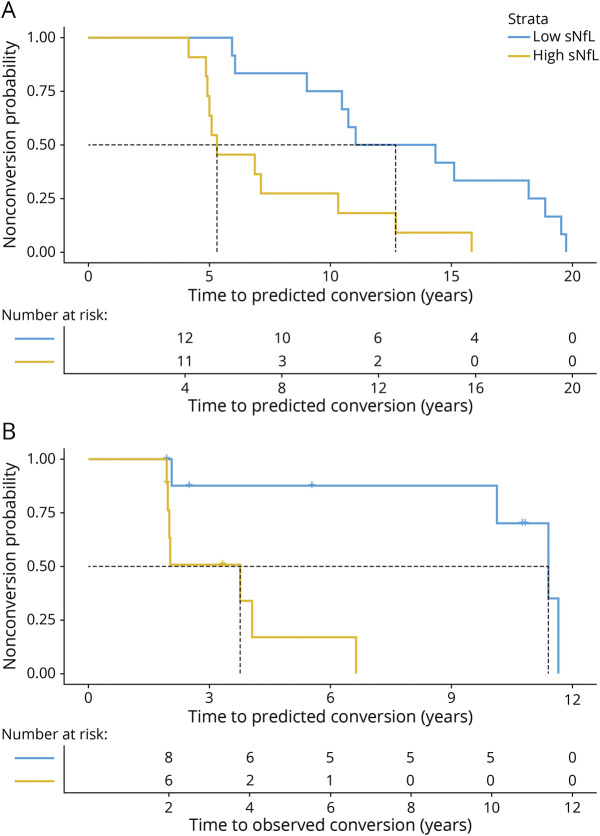
Baseline sNfL levels allowed stratification of preataxic individuals with SCA1 with regard to individual time to predicted ataxia onset, as revealed by Kaplan-Meier analyses (Figure 4A): The median time to predicted ataxia onset was significantly shorter in preataxic individuals with high sNfL levels (5.3 years, sNfL >15.5 pg/mL) than in those with low sNfL levels (12.7 years, sNfL <15.5 pg/mL, threshold for sNfL levels defined by median split) (Χ2 [1] = 8.0, p = 0.005, log-rank test). Accordingly, for preataxic individuals with high sNfL levels, the hazard of developing ataxia at any given time was ≈3 times as high as for individuals with low sNfL levels (hazard ratio 3.58 [95% CI 1.40–9.11], p = 0.008, Cox regression). These findings were confirmed in the subset of preataxic individuals with SCA1 for whom longitudinal follow-up information was available (n = 18), including 11 converters and 7 nonconverters (Figure 4B). Kaplan-Meier analyses showed that the median time to conversion was significantly shorter in individuals with high sNfL levels (3.8 years, sNfL >17.1 pg/mL) than in those with low sNfL levels (11.4 years, sNfL <17.1 pg/mL, threshold for sNfL levels defined by median split in the subset of preataxic individuals with follow-up data) (Χ2 [1] = 9.8, p = 0.002, log-rank test). For preataxic individuals with high sNfL levels, the hazard of conversion at any given time was ≈14 times as high as for individuals with low sNfL levels (14.40 [1.71–121], p = 0.014, Cox regression). These findings suggest that sNfL levels might be used to stratify preataxic SCA1 carriers with regard to proximity to ataxia onset.
Figure 4. Stratifying the Time to Conversion to the Ataxic Disease Stage by sNfL Levels.
(A) For all available preataxic spinocerebellar ataxia type 1 (SCA1) carriers (n = 23), the Kaplan-Meier diagram shows the share of the preataxic carriers remaining preataxic (i.e., the nonconversion probability) plotted over the time to the individually predicted ataxia onset, stratified by baseline serum neurofilament light (sNfL) level. We calculated the predicted age at ataxia onset from each individual’s repeat length and their age at blood sampling. We defined high vs low sNfL levels by median split (threshold 15.5 pg/mL). (B) For the subset of SCA1 carriers for whom longitudinal clinical follow-up information was available (n = 18), the diagram shows the nonconversion probability plotted over the time to the observed ataxia onset, again stratified by baseline sNfL level (median split, threshold 17.1 pg/mL). Vertical dashed lines represent the follow-up duration at which 50% of preataxic individuals (in each stratum) have converted to the ataxic disease stage (i.e., median event-free survival).
sNfL Levels in Preataxic Individuals With SCA1 Without Pontine or Cerebellar Atrophy
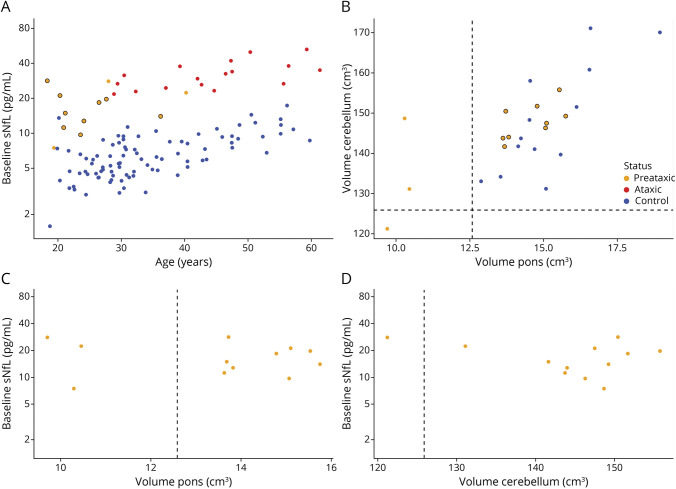
In the subset of preataxic SCA1 carriers of whom volumetric MRI data were available (n = 12), we identified a substantial subset of preataxic individuals (9 of 12 individuals, 75%) who did not have any evidence of pontine or cerebellar atrophy (Figure 5B). sNfL levels of these individuals (16.7 pg/mL [12.4–21.4 pg/mL]) were significantly higher than those of controls (6.0 pg/mL [4.7–8.6 pg/mL]) (U = 818, z = 4.58, p < 0.001, r = 0.46), thus suggesting that the sNfL increase in blood might precede not only clinical onset but also pontine and cerebellar volumetric atrophy, which are considered the earliest and most change-sensitive volumetric MRI changes in SCA1 (Figure 5A). In the preataxic individuals with SCA1 with available MRI data, the sNfL level was not associated with pontine (ρ = −0.07, p = 0.834) or cerebellar (ρ = −0.03, p = 0.921) volume, suggesting that the sNfL elevation in preataxic SCA1 was not driven by the volumetrically measurable brain atrophy (Figure 6, C and D) but that neuronal decay might start earlier.
Figure 5. sNfL Levels in Preataxic SCA1 in Relation to Pontine and Cerebellar Volumes.
(A) Serum neurofilament light (sNfL) levels of preataxic spinocerebellar ataxia type 1 (SCA1) carriers not showing any signs of pontine or cerebellar atrophy (orange point with black circle) are displayed compared to mutation-negative controls (blue), ataxic SCA1 carriers (red), and preataxic SCA1 carriers with pontine or cerebellar atrophy (orange point without black circle). (B) We considered signs of pontine or cerebellar atrophy present if the respective brain volumes were below a threshold defined as the lower boundary of the 90% CI (i.e., the 5% quantile) of the respective volumes measured in healthy age-matched controls, visualized by dashed lines. Preataxic individuals without signs of pontine or cerebellar atrophy are again visualized by orange points with black circles. (C and D) In preataxic SCA1 carriers, pontine and cerebellar volumes were not associated with the sNfL level.
sNfL Levels as Outcome Variable for Treatment Trials in SCA1
Our longitudinal measurements demonstrated that intraindividual sNfL levels are stable at the ataxic stage of SCA1 (Figure 6, A and B), with levels not differing between baseline and follow-up visit (t [10] = −0.07, p = 0.943, 2-sided paired t test). From the longitudinal measurements from baseline to first follow-up (interval 2.7 years [2.0–3.4 years]), we estimated sample sizes for future treatment trials using the reduction of sNfL levels as the outcome variable (log-transformed data). To detect a therapeutic effect size of 50%, the required total sample size would be 14 individuals at the ataxic stage (Figure 6C, also for visualization of other possible therapeutic effect sizes).
Classification of Evidence
This study provides Class III evidence that NfL levels are increased in both ataxic and preataxic SCA1 and are associated with ataxia onset.
Discussion
Leveraging a multicentric cohort of SCA1 mutation carriers, our study demonstrates increased blood levels of NfL in SCA1 (1) not only at the ataxic, but also already at the—likely most treatment-relevant—preataxic stage, (2) paralleled by increased NfL levels in the CSF, (3) rising in proximity to both predicted and actually observed onset, (4) preceding pontine and cerebellar atrophy on volumetric MRI at the preataxic stage, and (5) allowing small sample sizes in future treatment trials.
Our NfL findings from the ataxic stage of SCA1 extend previous findings from monocentric, mixed cohorts of polyglutamine SCAs11,12 by validating the sNfL increase in SCA1 within a multicentric setting, as essential for clinical trials in rare diseases like SCA1, and by demonstrating a parallel increase in the CSF, present even on a single-participant level. This parallel increase of NfL levels, which is in line with findings from other neurologic diseases,26,27 indicates that peripheral blood levels of NfL offer a valid peripheral blood biomarker for degeneration of the CNS at both the ataxic and preataxic stage of SCA1. However, given the small number of available CSF samples, larger SCA1 cohorts with longitudinal sNfL-cNfL pairs are needed to provide a more fine-grained map of the relative cNfL vs blood NfL dynamics across SCA1 diseases stages, particularly for the preataxic stage.
Moreover, our study suggests that the individual CAG repeat count, age, and disease stage (preataxic vs ataxic) are the major determinants of sNfL levels in SCA1, thus extending and specifying findings from other CAG repeat-expansion diseases.22,23 The observation that sNfL levels were not closely related to cross-sectional disease duration and disease severity (in line with previous findings12,22) indicates that sNfL in SCA1 is not primarily a biomarker of the current state of disease severity (i.e., disease severity biomarker) but rather might reflect the rate of ongoing neuronal decay (i.e., decay rate biomarker).11,22
This notion is further supported by the finding that sNfL levels increased from the preataxic to the ataxic stage but did not increase further in the ataxic stage during follow-up despite evidence of clinical progression. Thus, after the increase during the preataxic stage, sNfL levels plateau during the ataxic stage at a stable level. This finding might be best interpreted as evidence that neuronal decay rates increase during the preataxic stage and then remain stably elevated during the ataxic stage. While this notion of stage-dependent NfL dynamics warrants confirmation by larger longitudinal studies, it already receives support from findings in other multisystemic neurodegenerative diseases in which NfL levels peak around the time of clinical onset, subsequently stabilize at increased levels, and eventually possibly even decrease in late disease stages.28,29
Our study demonstrates that NfL levels are increased with high effect size not only at the ataxic stage of SCA1 but already at the preataxic stage with similarly high effect sizes. Significant increases compared to healthy controls occurred 5 years before the predicted ataxia onset, with levels rising further with temporal proximity to ataxia onset. This cross-sectional finding was further supported by intraindividual longitudinal sNfL increases in preataxic individuals. Moreover, the preconversion interval (i.e., the time to predicted ataxia onset) was significantly shorter in the preataxic individuals who had high sNfL levels than those with low levels.
An important point is that these associations of sNfL with proximity to predicted ataxia onset were validated by our findings on the associations of sNfL with the actually observed ataxia onset: while previous studies in the SCA field relied mostly only on the associations of a biomarker with the predicted onset of ataxia, a hypothetical proxy measure based on repeat length and the age reached without developing ataxia,24 our study also assessed the associations of sNfL with the actually observed onset of ataxia, leveraging longitudinal follow-up data of a sufficiently long time span to capture the actual disease conversion. These data show that sNfL levels indeed increase with proximity also to the actual onset, with higher sNfL levels heralding earlier phenoconversion, and with first significant increases occurring 4 years before the actual ataxia onset.
Taken together, these findings indicate that sNfL levels might serve as biomarkers for stratifying the highly treatment-relevant proximity-to-onset phase in SCA1 by allowing stratification of preataxic individuals according to their individual proximity to conversion. A biomarker-based stratification of this phase will be of high relevance for upcoming treatment trials to support the selection of candidate preataxic individuals for intervention trials because potentially burdensome treatments (e.g., intrathecally administered ASOs) should likely not be applied unnecessarily early or too late.3,22 However, sNfL might hereby be used not only as a stratification biomarker but also, pending further validation, possibly as a treatment-response biomarker for the preataxic stage. Given the increase of sNfL in the preataxic stage with proximity to clinical disease onset, a treatment-induced reduction of sNfL could serve as an objective biomarker surrogate endpoint for treatment trials at the preataxic stage to capture treatment-induced reduction of the SCA1-related neuronal decay, possibly even on a single-participant level. sNfL might hereby support individual management and counseling of preataxic mutation carriers regarding their individual biological disease stage and individual ongoing neuronal decay at the preclinical stage (when clinical symptoms are still absent) and, in combination with the CAG count, regarding their individual proximity to clinical onset, highlight the individual urgency for initiating possible disease-modifying treatments.
sNfL might be useful as a biomarker at the preataxic stage of SCA1 also due to the fact that it seems to rise rather early during the preataxic stage: it appeared to be increased in preataxic individuals with still normal pontine and cerebellar volumes, at least on volumetric MRI. If confirmed in an independent cohort, this finding suggests that the NfL increase is an early event in a multimodal cascade of biomarker alterations in SCA1, preceding even atrophy in those brain regions shown to be most change-sensitive in volumetric MRI of SCA1.18,19 This notion is in line with recent findings from (murine) SCA3 that showed that the onset of sNfL increase already starts with, or even precedes, the onset of Purkinje cell loss.22 Similar studies with combined assessment of neuropathology and blood NfL are warranted in SCA1 to further corroborate this hypothesis for SCA1.
Our study provides preliminary sample size estimates for future trials of disease-modifying drugs in SCA1, with the reduction of increased sNfL levels used as treatment response biomarker. Our longitudinal assessment of sNfL levels shows that intraindividual biological variation of sNfL levels is likely only a minimal source of noise in observation and intervention trials of SCA1. The use of sNfL levels as an outcome variable might thus help to reduce trial sample sizes compared to clinical and other surrogate outcome measures.14,18,19,30 For instance, our estimates suggest that a total sample size of 14 individuals would suffice to detect therapeutic effect sizes of 50% at the ataxic stage of SCA1.
Our study has several limitations. First, additional longitudinal measurements of NfL levels during the preataxic stage are needed to model the early intraindividual dynamics of NfL levels in SCA1 in more detail, including nonlinear models and sample size estimations at the preataxic stage. Second, validation of our findings (in particular of e.g., the MRI findings) is needed in independent larger multicentric cohorts of individuals with SCA1 like the READISCA cohort.31
Our multicentric study suggests blood NfL levels as biomarkers for stratification of the preataxic stage (particularly for capturing proximity to onset), early neuronal decay at the preataxic stage, and potentially treatment response at the preataxic and ataxic stages in SCA1 and takes the first steps toward a multimodal biomarker-based stratification of the preataxic disease stage of SCA1.
Acknowledgment
The authors thank all participants and their families for their contribution. C.W., L.S., T.K., A.D., R.S., and M.S. are members of the European Reference Network for Rare Neurological Diseases project 739510. This work was supported by the Horizon 2020 Research and Innovation Programme (grant 779257 Solve-RD to M.S. and R.S.), the Clinician Scientist Program of the Medical Faculty Tübingen (480-0-0 to C.W., and 459-0-0 to D.M.), the Bundesministerium für Forschung und Bildung through funding for the TreatHSP network (01GM1905 to R.S.), the European Joint Programme on Rare Diseases (EJP RD) under the EJP RD COFUND-EJP No. 825575 (PROSPAX consortium, to M.S., R.S., and A.D., with M.S. and R.S. hereby supported by the Deutsche Forschungsgemeinschaft). B.M. was supported by the National Scientific Research Programme K 138669 and EFOP 3.6.1-16.2016.00004. The authors thank Elke Stransky for technical assistance, Alejandra Leyva for technical execution of the serum NfL measurements, and Christoph Kessler for support in setting up the CSF NfL reference cohort.
Glossary
- ASO
antisense oligonucleotide
- cNfL
CSF levels of NfL
- CV
coefficient of variation
- EUROSCA
European Integrated Project on Spinocerebellar Ataxias
- LLOQ
lower limit of quantification
- NfL
neurofilament light
- RISCA
Prospective Study of Individuals at Risk for Spinocerebellar Ataxia
- SARA
Scale for the Assessment and Rating of Ataxia
- SCA
spinocerebellar ataxia
- Simoa
single-molecule array
- sNfL
serum levels of NfL
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Editorial, page 821
Study Funding
The authors report no targeted funding.
Disclosure
C. Wilke has nothing to disclose. D. Mengel has served as consultant for Biogen. L. Schöls, H. Hengel, and M. Rakowicz have nothing to disclose. T. Klockgether received consulting fees from Biohaven, Roche, UCB, Uniqure, and Vico Therapeutics. A. Durr is currently receiving grants from the NIH (RO1), French National Hospital Clinical Research Program, Agence nationale de la recherche, Triplet Therapeutics, Biogen, and Verum; serves on the advisory boards of Roche, Triplet Therapeutics, and Biogen; and holds partly a patent on anaplerotic therapy of Huntington disease and other polyglutamine diseases (B 06291873.5). A. Filla, B. Melegh, and . Schüle have nothing to disclose. K. Reetz has received grants from the German Federal Ministry of Education and Research (01GQ1402, 01DN18022), the German Research Foundation (IRTG 2150), Alzheimer Forschung Initiative eV (NL-18002CB), and Friedreich's Ataxia Research Alliance and honoraria for presentations or advisory boards from Biogen and Roche. H. Jacobi has nothing to disclose. M. Synofzik received consultancy honoraria from Orphazyme Pharmaceuticals and Janssen Pharmaceuticals, both unrelated to the current project and manuscript. He received consultancy honoraria from Ionis Pharmaceuticals on trial planning in SCA1, also unrelated to the current project and manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Robitaille Y, Schut L, Kish SJ. Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype. Acta Neuropathol. 1995;90(6):572-581. [DOI] [PubMed] [Google Scholar]
- 2.Rüb U, Schöls L, Paulson H, et al. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38-66. [DOI] [PubMed] [Google Scholar]
- 3.Paulson HL, Shakkottai VG, Clark HB, Orr HT. Polyglutamine spinocerebellar ataxias: from genes to potential treatments. Nat Rev Neurosci. 2017;18(10):613-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedrich J, Kordasiewicz HB, O'Callaghan B, et al. Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI Insight. 2018;3(21):e123193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kourkouta E, Weij R, González-Barriga A, et al. Suppression of mutant protein expression in SCA3 and SCA1 mice using a CAG repeat-targeting antisense oligonucleotide. Mol Ther Nucleic Acids. 2019;17:601-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter B, Guenther R, Ludolph AC, Hermann A, Otto M, Wurster CD. Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J Neurol Neurosurg Psychiatry. 2019;90(9):1068-1069. [DOI] [PubMed] [Google Scholar]
- 7.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732. [DOI] [PubMed] [Google Scholar]
- 8.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. [DOI] [PubMed] [Google Scholar]
- 9.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54(10):1655-1661. [DOI] [PubMed] [Google Scholar]
- 10.Barro C, Chitnis T, Weiner HL. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. 2020;7(12):2508-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coarelli G, Darios F, Petit E, et al. Plasma neurofilament light chain predicts cerebellar atrophy and clinical progression in spinocerebellar ataxia. Neurobiol Dis. 2021;153:105311. [DOI] [PubMed] [Google Scholar]
- 12.Wilke C, Bender F, Hayer SN, et al. Serum neurofilament light is increased in multiple system atrophy of cerebellar type and in repeat-expansion spinocerebellar ataxias: a pilot study. J Neurol. 2018;265(7):1618-1624. [DOI] [PubMed] [Google Scholar]
- 13.Jacobi H, Reetz K, du Montcel ST, et al. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. Lancet Neurol. 2013;12(7):650-658. [DOI] [PubMed] [Google Scholar]
- 14.Jacobi H, du Montcel ST, Bauer P, et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14(11):1101-1108. [DOI] [PubMed] [Google Scholar]
- 15.Jacobi H, du Montcel ST, Romanzetti S, et al. Conversion of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 to manifest ataxia (RISCA): a longitudinal cohort study. Lancet Neurol. 2020;19:738-747. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz-Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717-1720. [DOI] [PubMed] [Google Scholar]
- 17.Norgren N, Karlsson JE, Rosengren L, Stigbrand T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics. 2002;21(1):53-59. [DOI] [PubMed] [Google Scholar]
- 18.Reetz K, Costa AS, Mirzazade S, et al. Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain. 2013;136(pt 3):905-917. [DOI] [PubMed] [Google Scholar]
- 19.Adanyeguh IM, Perlbarg V, Henry PG, et al. Autosomal dominant cerebellar ataxias: imaging biomarkers with high effect sizes. Neuroimage Clin. 2018;19:858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilke C, Preische O, Deuschle C, et al. Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. J Neurol Neurosurg Psychiatry. 2016;87(11):1270-1272. [DOI] [PubMed] [Google Scholar]
- 21.Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilke C, Haas E, Reetz K, et al. Neurofilaments in spinocerebellar ataxia type 3: blood biomarkers at the preataxic and ataxic stage in humans and mice. EMBO Mol Med. 2020;12:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington's disease: a retrospective cohort analysis. Lancet Neurol. 2017;16(8):601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tezenas du Montcel S, Durr A, Rakowicz M, et al. Prediction of the age at onset in spinocerebellar ataxia type 1, 2, 3 and 6. J Med Genet. 2014;51:479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne LM, Rodrigues FB, Johnson EB, et al. Evaluation of mutant huntingtin and neurofilament proteins as potential markers in Huntington's disease. Sci Transl Med. 2018;10(458):eaat7108. [DOI] [PubMed] [Google Scholar]
- 26.Alagaratnam J, von Widekind S, De Francesco D, et al. Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ Neurol Open. 2021;3(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016;79(1):152-158. [DOI] [PubMed] [Google Scholar]
- 29.van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol. 2019;18(12):1103-1111. [DOI] [PubMed] [Google Scholar]
- 30.Kim DH, Kim R, Lee JY, Lee KM. Clinical, imaging, and laboratory markers of premanifest spinocerebellar ataxia 1, 2, 3, and 6: a systematic review. J Clin Neurol. 2021;17(2):187-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CC, Ashizawa T, Kuo SH. Collaborative efforts for spinocerebellar ataxia research in the United States: CRC-SCA and READISCA. Front Neurol. 2020;11:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data of this article can be accessed on reasonable request addressed to the corresponding authors.