Abstract
Background and Objectives
Functional connectivity (FC) measures can be used to differentiate epileptogenic zones (EZs) from non-EZs in patients with medically refractory epilepsy. Little work has been done to evaluate the stability of stereo-EEG (SEEG) FC measures over time and their relationship with antiseizure medication (ASM) use, a critical confounder in epilepsy FC studies. We aimed to answer the following questions: Are SEEG FC measures stable over time? Are they influenced by ASMs? Are they affected by patient data collection state?
Methods
In 32 patients with medically refractory focal epilepsy, we collected a single 2-minute prospective SEEG resting-state (awake, eyes closed) data set and consecutive 2-minute retrospective pseudo-rest (awake, eyes open) data sets for days 1–7 postimplantation. ASM dosages were recorded for days 1–7 postimplantation and drug load score (DLS) per day was calculated to standardize and compare across patients. FC was evaluated using directed and nondirected measures. Standard clinical interpretation of ictal SEEG was used to classify brain regions as EZs and non-EZs.
Results
Over 7 days, presumed EZs consistently had higher FC than non-EZs when using between imaginary coherence (ImCoh) and partial directed coherence (PDC) inward strength, without accounting for DLS. These measures were demonstrated to be stable over a short-term period of 3 consecutive days with the same DLS. Between ImCoh FC differences between EZs and non-EZs were reduced with DLS decreases, whereas other measures were not affected by DLS. FC differences between EZs and non-EZs were seen during both resting-state and pseudo-rest conditions; ImCoh values were strongly correlated between the 2 conditions, whereas PDC values were not.
Discussion
Inward and nondirected SEEG FC is higher in presumed EZs vs non-EZs and measures are stable over time. However, certain measures may be affected by ASM dose, as between ImCoh differences between EZs and non-EZs are less pronounced with lower doses, and other measures such as PDC are poorly correlated across recording conditions. These findings allow novel insight into how SEEG FC measures may aid surgical localization and how they are influenced by ASMs and other factors.
Epilepsy is a debilitating disorder affecting approximately 1.2% of the population and significantly affects quality of life.1,2 One-third of patients fail to achieve seizure control with antiseizure medications (ASMs) and should be evaluated for surgery.3,4 When preoperative evaluation localizes epileptogenic zones (EZs),5 surgery may lead to seizure freedom and improved quality of life.6,7 Stereo-EEG (SEEG) is a minimally invasive intracranial monitoring method used to localize seizure onset for surgery.8,9 During SEEG, ASMs are typically weaned in the epilepsy monitoring unit (EMU) to elicit seizures to localize EZs.
Clinical interpretation of SEEG may be challenging and incomplete, so attention has been given to functional connectivity (FC) studies to supplement clinical findings and elucidate seizure networks. Several previous studies used resting-state SEEG data to quantify functional FC patterns and differences between EZs and non-EZs, with EZs exhibiting stronger FC patterns compared to other regions.10-14 Imaginary coherence (ImCoh), a nondirected FC measure, can be used to quantify FC between and within brain regions, with EZs demonstrating higher FC.15,16 Partial directed coherence (PDC) is a method to calculate intensity and directionality of neural signals between regions.17 These measures may be useful in characterizing FC patterns of epileptogenic networks and monitoring drug effects.18,19 FC metrics have been increasingly studied in epilepsy and other neurologic disorders and their relationships to clinically relevant measures must be better understood.
Most FC studies in epilepsy are confounded by ASMs, but their influence on FC measures remains unknown. Evaluation of SEEG FC stability over time or as a function of ASM dosage is limited,20,21 although ImCoh and PDC metrics have demonstrated stability in magnetoencephalography (MEG) and scalp EEG studies.22-24 Time and spectral domain features of intracranial EEG (iEEG) fluctuate, but previous studies demonstrated no significant difference in prediction of surgical outcomes with 1-hour time segments collected >4 hours apart or a difference between 1-hour segments and shorter segments as brief as 10 seconds.21,25 Whereas one study demonstrated decreased cortical activity after ASM weaning in iEEG,26 effects of ASMs on SEEG FC measures have not been formally studied.27 Scalp EEG and fMRI studies have demonstrated that ASMs may affect quantitative metrics of power and FC.28 These studies are limited as they evaluate changes over weeks while on-boarding ASMs and do not account for polytherapy.28,29 Polytherapy can be evaluated using a quantitative drug load metric to elucidate whether drug load has a relationship with adverse effects of medications,30-32 but the relationship between drug load and quantitative EEG metrics remains unknown. Finally, many EEG segments are collected during resting-state or awake states but are rarely validated across different patient states.
We aimed to address knowledge gaps by evaluating relationships between SEEG FC measures in EZs and non-EZs and time in EMU, changes in ASM doses, differences in seizure burden, and differences between eyes-closed formal resting-state and eyes-open pseudo-rest states utilized in the current investigation. Ultimately, we aim to improve accurate identification of surgical targets and guide future studies.
Methods
Patients
Thirty-two patients with medically refractory epilepsy underwent SEEG at Vanderbilt University Medical Center (VUMC) between 2018 and 2020. Clinical data were obtained from electronic medical records (EMRs) (Table 1). At time of analysis, 28 (87.5%) patients received surgical treatment.
Table 1.
Demographic and Clinical Data
Standard Protocol Approvals, Registrations, and Patient Consents
The VUMC institutional review board (IRB) approved this investigation and informed written consent was obtained from patients.
Data Collection and Preprocessing
SEEG Data
Physicians used Waypoint Navigator (Neurotargeting and FHC)33 or ROSA ONE (Zimmer Biomet)34 software to plan electrode trajectories according to clinical care. Stereotactic technique was used to implant 10- to 16-contact electrodes (PMT Corporation and Integra) (Table 1). Quantum Amplifier EEG (Natus) was used to record EEG data.
Pseudo-resting-state (“pseudo-rest”) data were collected retrospectively by evaluating SEEG video recordings at days 1–7 postimplantation to identify 10-minute segments when patients were awake with eyes open, sitting quietly with minimal talking, without food or beverage consumption. Data were collected >1 hour away from ictal activity and sequential SEEG samples were collected around the same time of day (i.e., within 2 hours). A 20-minute segment of prospective, eyes-closed resting-state interictal data was recorded on day 1.4 ± 0.8 (mean ± SD) postimplantation using the formal resting state as defined by Raichle and colleagues.10,11,35 SEEG data were recorded at a sampling rate of 1,000 Hz.
Raw SEEG data were preprocessed as previously described.10,11 Using EEGLab, bandpass (1–119 Hz) and notch filters (60 Hz) were applied to raw signals.36 Contacts in CSF, outside brain tissue, or completely in white matter were excluded. Channels with visually identified artifacts were removed. The Desikan-Killiany Atlas37 was used to designate anatomical locations of electrodes verified by 2 neurosurgeon coauthors (D.J.E., D.L.P.). For 2 patients with structural abnormalities, regions were manually designated by one neurosurgeon and verified by another. Electrode pairs within a region were included; pairs across 2 regions were excluded. Epileptogenicity of sampled regions was classified using a binary method to designate regions as presumed EZs or non-EZs,5,38 based upon epileptologists' interpretation of ictal and interictal SEEG data, described in our prior work and guided by criteria of Lüders and colleagues.5,10,11 Throughout the article, EZ and non-EZ are used to refer to presumed EZ and non-EZ regions prior to surgery.
Continuous 2-minute segments of pseudo-rest data for days 1–7 postimplantation and a single continuous 2-minute segment of resting-state data were extracted. Two-minute segments were visually inspected and selected, avoiding interictal spikes and brief electrographic seizures. Prior work has demonstrated no significant differences in FC of 2-minute segments compared to longer segments.11,39 In prior EEG and MEG functional studies, data stationarity was maintained within a 2-minute period, but may be lost with longer intervals, and 2-minute segments were sufficient to produce reliable FC maps.15 The entire 2-minute segment, without windowing, was used to perform all FC analyses for both collection states.
FC analysis was performed on pseudo-rest data for days 1–7 postimplantation when available, for a total of 212 SEEG segments (2 minutes long), with 1 segment used per day per patient. A bipolar montage was used to re-reference SEEG electrode contact pairs using FieldTrip MATLAB toolbox (MathWorks Inc.).40
Seizure Outcome Data
For patients who underwent surgery (n = 28), EMRs were reviewed to determine seizure outcome at last follow-up (Table 1). For patients who underwent resection/ablation, outcome was classified by Engel classification,41 and for those who underwent responsive neurostimulation (RNS), outcome was dichotomized to ≥50% seizure reduction and <50% seizure reduction.
ASM Data
We reviewed EMRs and recorded dosages of each ASM administered per patient during days 1–7 postimplantation of EMU stay over a 24-hour period from 12:00 am to 11:59 pm.
Seizure Burden Data
We defined clinical seizure burden as number of seizures per 24-hour period from 12:00 am to 11:59 pm. Events were classified as seizures if electrophysiologic and clinical correlates were reported. Paucisymptomatic or electrographic-only seizures, when annotated, were excluded, and secondarily confirmed on visual inspection of the segments. EMU reports were evaluated to determine seizure burden for days 1–7 postimplantation, when available, per patient. Seizure burden was ranked from highest to lowest by number of seizures per day. If 2 or more days had equal seizures, the day more closely following a higher clinical seizure burden day was ranked higher.
SEEG FC Measures
Pseudo-rest and resting-state SEEG were used to calculate nondirected FC measures. Within ImCoh was defined as ImCoh of electrode contact pairs within a region to other electrode contact pairs within that same region. Between ImCoh was defined as average ImCoh between electrode contact pairs within a region and electrode contact pairs in other regions. We calculated measures in alpha band (8–12 Hz), because it contains the most prominent spectral power peaks at rest and has high test–retest reliability.42 Greater FC differences between EZs and non-EZs are found in alpha band compared to other bands.11
To assess whether univariate power spectral density metrics would account for our bivariate FC findings, we calculated relative alpha band (8–12 Hz) power of clinically defined EZs and non-EZs. Band power was calculated using MATLAB’s pwelch function. First, we calculated the alpha band power from 8 to 12 Hz, then divided it by the whole band power from 1 to 119 Hz. We z-scored each patient's relative band power across all nodes before comparing on the group level.
Directed FC was evaluated with PDC in alpha band.10,11 PDC inward and outward strength (averaged per brain region) were used to characterize strength and direction of signal synchronicity between 2 regions.43 This measure was most effective at distinguishing between EZs and non-EZs in previous work.10,11
Drug Load Score and Rank Calculations
Composite drug load scores (DLS) were calculated per patient to standardize and compare dosages of scheduled ASMs, excluding rescue benzodiazepines, taken over a 24-hour period, using methods previously described.44 Using the minimum and maximum recommended doses per ASM, as cited by the British National Formulary, a daily DLS was calculated per patient for each ASM (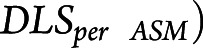 , which was 0 if no ASM was provided, 1 if the minimum dosage was provided, and 10 if the maximum dosage was provided.45 Composite DLS assigned per patient and day, calculated using Equation 1, was the summation of all the ASMs taken by the patient per day.
, which was 0 if no ASM was provided, 1 if the minimum dosage was provided, and 10 if the maximum dosage was provided.45 Composite DLS assigned per patient and day, calculated using Equation 1, was the summation of all the ASMs taken by the patient per day.
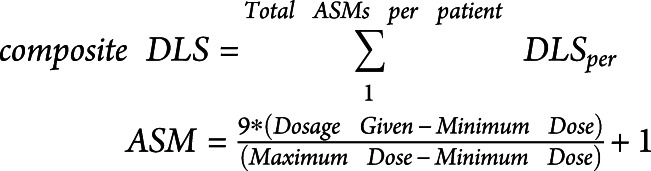 |
A DLS rank order was determined for each patient by reordering days with highest (rank 1) to lowest (rank 7) composite DLS to isolate the effect of drug load.
Statistical Methods
We evaluated data distribution with Anderson-Darling tests. All FC values were found to be normally distributed, so parametric statistical tests were used. DLS and clinical seizure burden data were not normally distributed, therefore we used Wilcoxon signed-rank tests for these comparisons.
Paired-sample, 2-tailed t tests were used when evaluating FC differences between EZs and non-EZs. Pearson correlations were used to evaluate associations between FC measures using resting-state and pseudo-rest data.
Linear mixed effects (LME) models were used to determine trends in FC measures and DLS over time and DLS rank. LME models evaluated for trends accounting for random effect differences in subject-specific factors that may contribute to outcomes.46
MATLAB 2020b was used for statistical analyses, with significance of p < 0.05. When multiple t tests were used to compare FC measures over time and rank, false discovery rate (FDR) correction was used for multiple comparisons. For other tests, Bonferroni-Holm correction was applied for multiple comparisons.
Data Availability
Data are not freely available in a public repository due to restrictions in participant informed consent. However, if approved by our IRB, de-identified data can be made available upon request.
Results
Patient Information
Patients had 17.0 ± 5.3 (mean ± SD) brain regions sampled with 143.0 ± 34 electrode contacts and 11 ± 2.4 electrodes (Table 1). We evaluated 7 days postimplantation of SEEG when available (5 days for 3 patients and 6 days for 6 patients). Mean EMU stay length was 8.8 ± 3.6 days. Each patient was taking 2.8 ± 1.0 unique ASMs over the EMU stay. For days 1–7 postimplantation, patients had 10 ± 9.3 total seizures and a DLS of 5.8 ± 6.8. After clinical evaluation of SEEG, 14 (43.8%) patients were diagnosed with focal neocortical seizure onset, 11 (34.4%) with unilateral mesial temporal onset, and 7 (21.9%) with bilateral mesial temporal onset.
Nondirected and Inward Connectivity Measures Are Higher in EZs Compared to Non-EZs
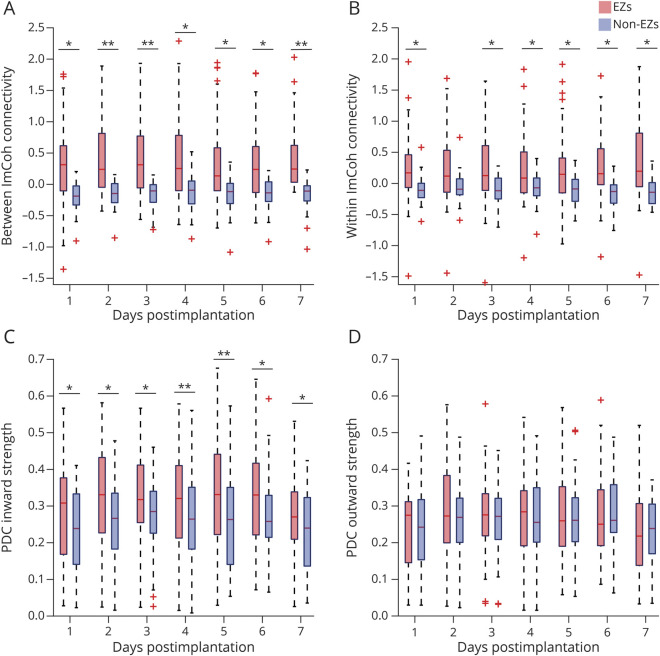
FC differences between presumed EZs and non-EZs were calculated over days 1–7 for each FC measure. Between ImCoh FC was greater in EZs than non-EZs for each day (p = 0.007–0.023, serial paired t tests, FDR-corrected; Figure 1A). Within ImCoh FC was higher for EZs compared to non-EZs each day (p = 0.025–0.036), with a marginal difference on day 2 (p = 0.050; Figure 1B). Likewise, EZs had greater PDC inward strength than non-EZs for days 1–7 (p = 0.009–0.030; Figure 1C). There were no differences in PDC outward strength between EZs and non-EZs on any day (p = 0.20–0.80; paired t tests, FDR-corrected; Figure 1D). Mean z-scored relative alpha band power of EZs (−0.089 ± 0.40) was not different compared to non-EZs (0.008 ± 0.25). This suggests the FC metrics describe a unique increase in correlation between the SEEG timeseries of EZs and other regions, as opposed to an increase in relative band power driving increases in FC. This is in alignment with coherence measurements relying on synchrony of phase shift instead of amplitude.
Figure 1. EZs Consistently Demonstrate Higher Nondirected and Inward Connectivity Than Non-EZs During Consecutive Postimplantation Days.
(A) On postimplantation days 1–7, epileptogenic zones (EZs) demonstrate higher between imaginary coherence (ImCoh) (0.35–0.46 ± 0.58–0.72, mean ± SD) than non-EZs (−0.19 to −0.14 ± 0.20–0.28) (left). (B) Within ImCoh is also higher in EZs (0.24–0.36 ± 0.60–0.73) than non-EZs (−0.13 to −0.055 ± 0.21–0.25) (right). (C) During consecutive postimplantation days, partial directed coherence (PDC) inward strength is higher in EZs (0.27–0.33 ± 0.10–0.16) compared to non-EZs (0.23–0.28 ± 0.10–0.13) (left). (D) PDC outward strength does not differ between EZs (0.23–0.27 ± 0.10–0.13) and non-EZs (0.22–0.27 ± 0.095–0.12) (right). In (A–D), the central line represents the median and the top and bottom box edges indicate the 75th and 25th percentiles, respectively; whiskers demonstrate data extremes and crosses indicate outliers. *p < 0.05, **p < 0.01; serial paired t tests with false discovery rate correction.
An LME model was used to evaluate for trends in FC between EZs and non-EZs over time, which was not significant for any FC measure (p = 0.19–0.91), suggesting measures do not change significantly during EMU stays, without taking medication effects into account. Overall, these results suggest EZs consistently demonstrate higher nondirected and inward-directed FC than non-EZs during EMU stays.
Between ImCoh, but Not PDC, May Be Influenced by ASMs
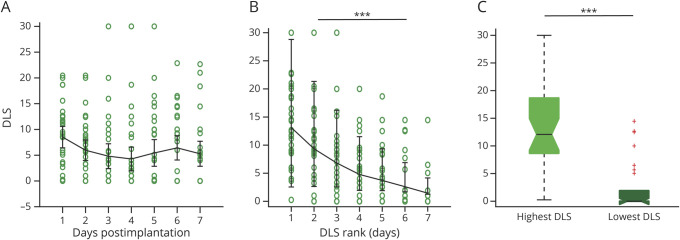
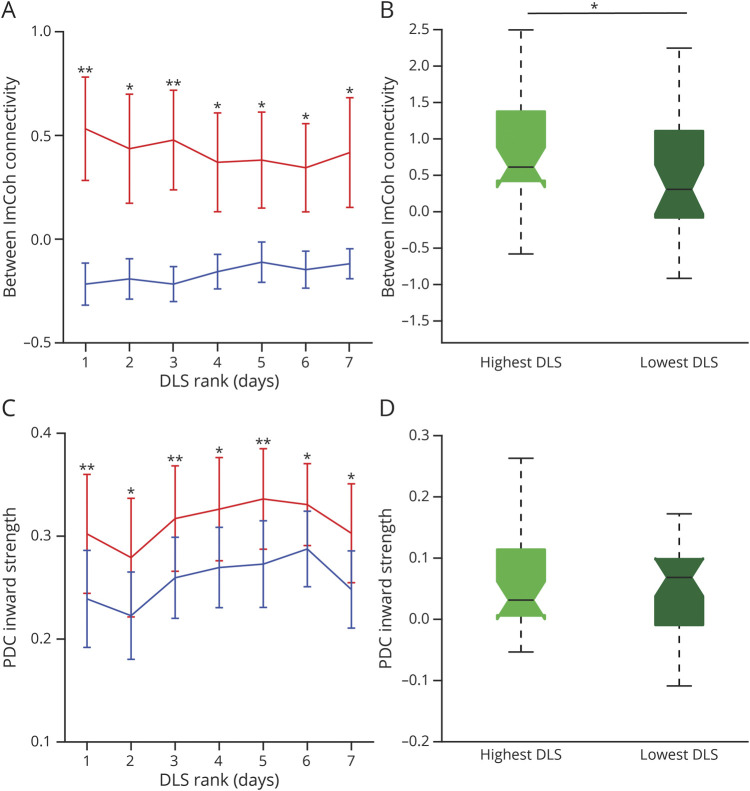
Whereas our results suggest no overall relationship between time and FC, ASM weaning and loading patterns differ greatly between individual SEEG patients. DLS over EMU stay did not differ across days (p = 0.17, LME model; Figure 2A). Therefore, to isolate the relationship between FC and ASMs, we ranked days from highest to lowest DLS. The relationship between FC and DLS was evaluated by calculating differences between EZs and non-EZs over ranked days 1–7 for each FC measure. Two patients not on ASMs during these days were excluded (n = 30). As expected, there was a significant trend in DLS over ranked days (p < 0.001, LME model; Figure 2B), and there was a significant difference in DLS between highest and lowest load days (p < 0.001, Wilcoxon signed-rank; Figure 2C), which we performed as a proof-of-principle analysis to allow us to compare FC measures across ranked DLS days. FC of EZs was higher than non-EZs for each ranked day for between ImCoh (p = 0.0036–0.0230, serial paired t tests, FDR-corrected; Figure 3A) and within ImCoh (p = 0.025–0.034; eFigure 1A, links.lww.com/WNL/B880). PDC inward strength was higher in EZs than non-EZs for each ranked day (p = 0.0018–0.0160; Figure 3C), but PDC outward strength did not differ between EZs and non-EZs (p = 0.33–0.89, t tests, FDR-corrected; eFigure 1C). A trend towards lower FC in EZs and higher FC in non-EZs with decreasing DLS appears when visualizing between ImCoh (Figure 3A), but trends were not appreciated for other measures.
Figure 2. Drug Load Score Trend Is Not Significant Over Time but Is Significant Over Reordered Drug Load Score Rank.
(A) Fluctuations of drug load scores (DLS) over time are shown for postimplantation days 1 (8.5 ± 6.0), 2 (6.0 ± 5.7), 3 (4.8 ± 6.9), 4 (4.3 ± 6.7), 5 (5.4 ± 7.5), 6 (6.4 ± 6.8), and 7 (5.3 ± 7.0). (B) Daily DLS rank ordered from highest to lowest (left), corresponding to rank ordered days 1 (13.1 ± 6.7, mean ± SD), 2 (9.3 ± 6.9), 3 (6.8 ± 6.8), 4 (4.8 ± 5.1), 5 (3.7 ± 5.0), 6 (2.6 ± 4.4), and 7 (1.5 ± 3.3). (C) As a proof-of-concept confirmation, DLS on the highest DLS day (13.1 ± 6.8) are expectedly greater than DLS on the lowest DLS day (2.4 ± 4.4). In (A) and (B), error bars represent 95% CI. In (C), the central line represents the median and the top and bottom box edges indicate the 75th and 25th percentiles, respectively; whiskers demonstrate data extremes and crosses indicate outliers. Wilcoxon signed-rank test. ***p < 0.001.
Figure 3. Connectivity Differences Between EZs and Non-EZs Are More Pronounced on Higher Compared to Lower ASM Doses for Between ImCoh and Stable for PDC Inward.
(A) For ranked days 1–7, values are shown for between imaginary coherence (ImCoh) for epileptogenic zones (EZs) (0.34–0.53 ± 0.59–0.74) and non-EZs (−0.22 to −0.11 ± 0.20–0.28), demonstrating consistently higher EZ connectivity than non-EZ connectivity with a greater difference between EZs and non-EZs on higher compared to lower drug load scores (DLS). (B) For between ImCoh, greater differences in connectivity between EZs and non-EZs were observed on the highest (0.76 ± 0.79) versus lowest (0.49 ± 0.84) DLS day. (C) For ranked days 1–7, partial directed coherence (PDC) inward strength for EZs (0.28–0.34 ± 0.11–0.16) was consistently higher than non-EZs (0.22–0.29 ± 0.10–0.13) and did not appear to be influenced by DLS. (D) For PDC inward, there was no significant difference in connectivity on the highest (0.055 ± 0.074) compared to the lowest (0.053 ± 0.069) DLS day. *p < 0.05, **p < 0.01. For (A) and (C), serial paired t tests with false discovery rate correction. For (B) and (D), single paired t test, Bonferroni-Holm corrected.
For the 3 FC measures differing between EZs and non-EZs, an LME model evaluated for trends in FC differences between EZs and non-EZs across DLS rank. For between ImCoh, we observed a negative relationship between FC and DLS rank (p = 0.033), with smaller differences between EZs and non-EZs for lower DLS. There were no trends between FC and DLS for within ImCoh (p = 0.99) or PDC inward strength (p = 0.41). Overall, FC differences between EZs and non-EZs were larger for the highest DLS rank compared to the lowest DLS rank when examining between ImCoh (p = 0.036; paired t test, Bonferroni-Holm–corrected; Figure 3B), but no differences in other FC measures were observed for the highest vs lowest DLS ranks (p = 0.44–0.54; Figure 3D and eFigure 1.B and 1.D, links.lww.com/WNL/B880). These results suggest between ImCoh may be influenced by ASMs, while other measures do not appear to be influenced by ASMs.
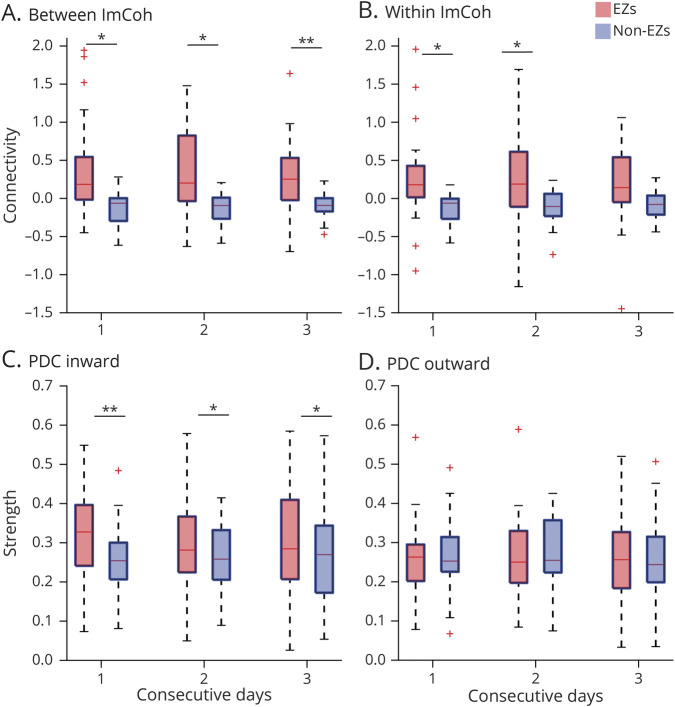
FC Measures Are Stable Over Time Independent of ASMs
We determined stability of FC measures while controlling for DLS. Patients with 3 consecutive days of equal DLS were included (n = 23). Over these 3 days, between ImCoh was higher in EZs than non-EZs (p = 0.0083–0.0250; paired t tests, FDR-corrected; Figure 4A), and a trend toward higher within ImCoh values was noted in EZs vs non-EZs (p = 0.029–0.072; Figure 4B). Also, PDC inward strength was higher in EZs compared to non-EZs over the 3 days (p = 0.0042–0.0170; Figure 4C), but no difference in PDC outward strength was seen between EZs and non-EZs (p = 0.72–0.88; Figure 4D). When visualizing patient-level data, FC appeared relatively stable in EZs and non-EZs across all measures, with least variability seen in ImCoh in non-EZs, and FC did not differ from day to day for any measure (p = 0.55–0.85; LME model; eFigure 2, links.lww.com/WNL/B880). This suggests FC measures are stable over several days when ASMs are constant.
Figure 4. Over Consecutive Days With Consistent ASM Dose, Between ImCoh and PDC Inward Strength Remain Higher in EZs, and Measures Are Stable Over Time.
(A) During 3 consecutive days of consistent drug load scores (DLS), between imaginary coherence (ImCoh) is consistently higher in epileptogenic zones (EZs) (0.31–0.36 ± 0.46–0.65, mean ± SD) than non-EZs (−0.12 to −0.095 ± 0.16–0.20) across all patients. (B) Within ImCoh trends higher in EZs (0.19–0.28 ± 0.54–0.61) compared to non-EZs (−0.12 to −0.087 ± 0.17–0.23). (C) Partial directed coherence (PDC) inward strength is consistently higher in EZs (0.30–0.32 ± 0.12–0.15) than non-EZs (0.25–0.27 ± 0.09–0.12). (D) PDC outward strength does not differ between EZs (0.25–0.27 ± 0.10–0.12) and non-EZs (0.25–0.28 ± 0.09–0.12). In (A–D), the central line represents the median and the top and bottom box edges indicate the 75th and 25th percentiles, respectively; whiskers demonstrate data extremes and crosses indicate outliers. *p < 0.05, **p < 0.01, paired t test, Bonferroni-Holm correction. N = 23 of 32 patients who had a stable and equivalent DLS score over 3 consecutive days.
Connectivity Measures Are Not Affected by Clinical Seizure Burden
We evaluated whether clinical seizure burden influences FC. For each patient, the day with highest (5.8 ± 5.8 seizures) and lowest seizure burden (0 seizures for all patients) were determined and FC was calculated. Highest seizure burden day had a significantly greater number of seizures than lowest seizure burden day (p < 0.001; Wilcoxon signed-rank). For all FC metrics, there were no differences in FC values between highest and lowest seizure days for EZs (p = 0.16–0.32; paired t tests) or non-EZs (p = 0.16–0.93; paired t tests), suggesting FC is not affected by clinical seizure burden.
PDC, but Not ImCoh, May Differ Between Resting-State and Pseudo-Rest Conditions
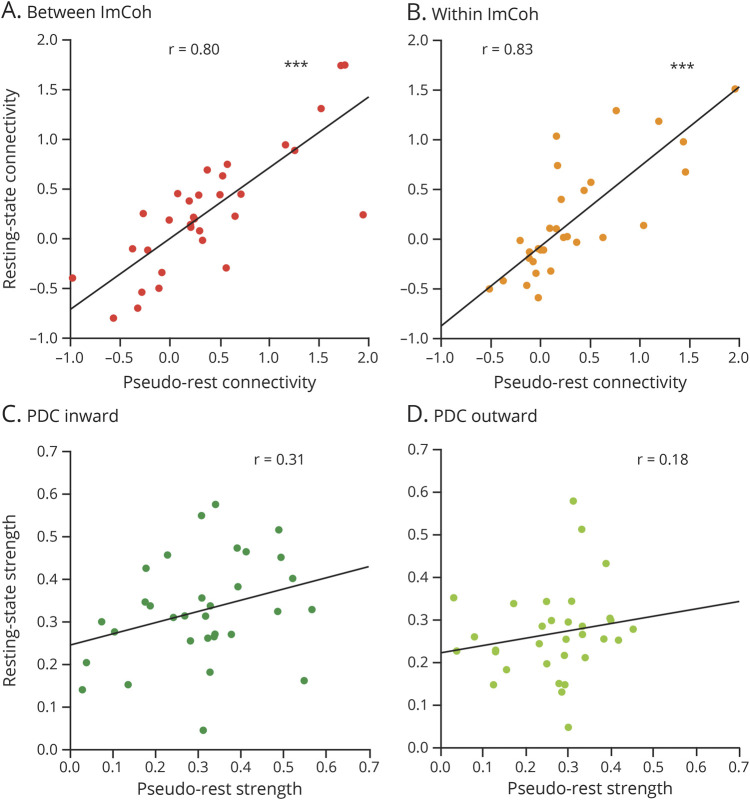
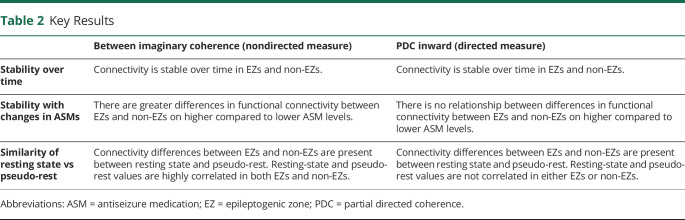
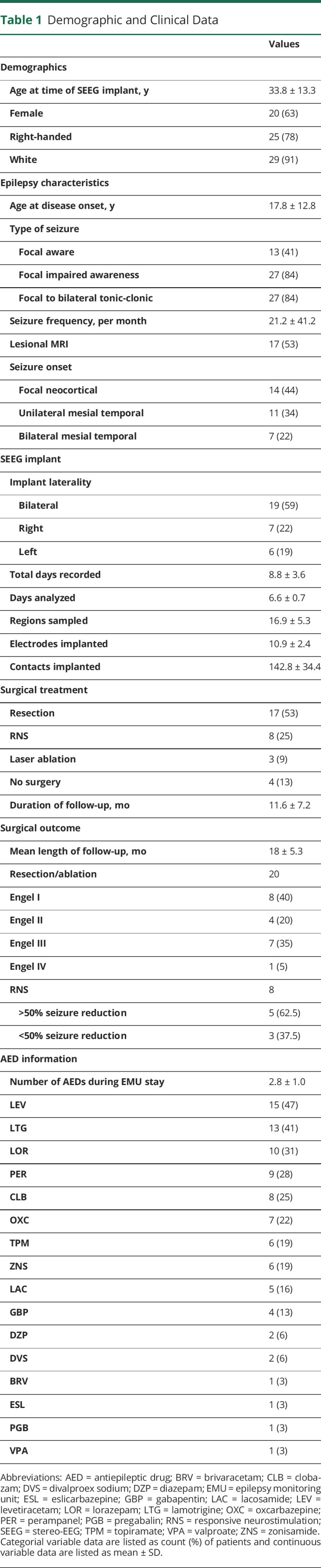
Data used for analysis were collected during retrospectively identified eyes-open pseudo-rest states, whereas prior studies have utilized formal prospective eyes-closed resting states. Thus, we evaluated whether resting-state vs pseudo-rest conditions influence FC measures by comparing resting-state with pseudo-rest measurements collected on the same day (and same DLS; Figure 5 and eFigure 3, links.lww.com/WNL/B880). For between ImCoh, FC measurements were highly correlated between resting-state and pseudo-rest conditions in EZs (r = 0.80, p < 0.001; Bonferroni-Holm–corrected) and non-EZs (r = 0.85, p < 0.001). Similarly, within ImCoh values were highly correlated between resting state and pseudo-rest in EZs (r = 0.83, p < 0.001) and non-EZs (r = 0.75, p < 0.001). However, PDC inward strength measurements were not correlated between the 2 conditions in EZs (r = 0.31, p = 0.17) and non-EZs (r = 0.28, p = 0.12), nor were PDC outward strength measurements correlated in EZs (r = 0.18, p = 0.66) and non-EZs (r = −0.12, p = 0.51). Given that PDC inward strength appears to better stratify EZs and non-EZs, we assessed whether differences between PDC inward strength in EZs vs non-EZs were larger during resting-state or pseudo-rest conditions. Using pseudo-rest recordings, PDC inward strength was higher in EZs (0.31 ± 0.14, mean ± SD) than non-EZs (0.25 ± 0.11; p < 0.001). Utilizing resting-state recordings, however, the difference in PDC inward strength was more pronounced between EZs (0.33 ± 0.12) and non-EZs (0.25 ± 0.11; p < 0.001), suggesting that PDC inward measurements during resting state may be optimal for distinguishing EZs from non-EZs.
Figure 5. ImCoh but Not PDC Connectivity Measures Are Highly Correlated During Pseudo-Rest Vs Resting-State Conditions.
(A) Between imaginary coherence (ImCoh) values during eyes-open pseudo-rest vs eyes-closed resting-state conditions are highly correlated in epileptogenic zones (EZs). (B) Within ImCoh values between these 2 conditions are also strongly correlated in EZs. (C) Partial directed coherence (PDC) inward strength values during pseudo-rest vs resting-state conditions are not correlated in EZs. (D) PDC outward strength values are similarly not correlated between these conditions in EZs. ***p < 0.001, Pearson correlation.
Relationship Between Surgical Outcome and FC
Surgical outcomes were available in 28 patients who underwent resection, ablation, or RNS implantation (Table 1). In a final analysis, we sought to examine EZ connectivity in “confirmed” EZs in patients who underwent resection and achieved an Engel I (seizure-free) outcome, vs “presumed” EZs in individuals who underwent resection and had an Engel II–IV (not seizure-free) outcome. EZ connectivity (between ImCoh and PDC inward strength) in patients who had Engel I outcome after resection (0.35 ± 0.71 and 0.37 ± 0.13, respectively, mean ± SD) did not differ significantly from EZ connectivity in individuals who had Engel II–IV outcome after resection (0.12 ± 0.45 and 0.36 ± 0.12, respectively; p = 0.43 and p = 0.81, respectively, paired t test). However, using either a conservative (0.25) or generous (0.75) effect size, a post hoc power analysis suggests our study is powered at only 7.7% or 30.4%, respectively, to detect a significant difference (α = 0.05) between these smaller patient subgroups (G*Power 3.1.9.6; Heinrich-Heine-Universität Düsseldorf). Therefore, further study with a larger population will be needed to evaluate the relationship between connectivity and confirmed vs presumed EZs in patient subgroups.
Discussion
Our study demonstrates differential effects of ASMs and recording condition on FC measures. PDC inward strength is consistently higher in presumed EZs vs non-EZs independent of ASM dosage, whereas between ImCoh is affected by ASM dosage, with smaller differences between EZs and non-EZs when patients are on lower ASMs doses. All FC measures were stable over days with stable DLS, and clinical seizure burden did not influence FC measures. For most measures, FC was higher in EZs than non-EZs during resting-state and pseudo-rest conditions, although PDC was not correlated between conditions and better distinguished EZs during the eyes-closed resting state, whereas ImCoh recordings were highly correlated between the 2 states (Table 2). These results build upon prior work in a smaller cohort demonstrating a FC model incorporating directed and nondirected FC measures may help identify EZs at the patient level with an accuracy of 84.3% and area under the receiver operating curve of 0.88.10 Understanding stability of these measures and potential influence of ASMs and recording condition will be critical for interpreting them in real-world applications. On a broader scale, confounders influencing FC should be better understood and accounted for in analysis, especially as these methods have become increasingly utilized for research and clinical purposes.
Table 2.
Key Results
Although ASMs are confounders in most FC studies, influence of these agents on FC has rarely been studied. PDC inward strength values appeared stable regardless of ASM dose, whereas between ImCoh better differentiated EZs and non-EZs with higher DLS. Some scalp EEG studies suggest directed FC measures are more reliable and less susceptible to artifacts than nondirected FC measures.47 Increased instability of EEG signal with medication wean, leading to more signal variability and propensity for spike activity, may lead to greater changes and less reliability in ImCoh measures compared to PDC. Also, a simultaneous fMRI-EEG study found increased FC with a general linear model in the ASM-naive state.48 Because there are more non-EZs than EZs in our patients, these results are in alignment with the increased between ImCoh FC of non-EZs exhibited on lowest DLS days.
Whereas the relationship between ASMs and SEEG FC has not been previously reported, one study examining raw iEEG activity found ASM weaning was associated with decreased cortical activity.26 Scalp EEG studies have indicated ASM dose changes result in power changes in different frequency bands during monotherapy with several ASMs,28,29 whereas some studies show no changes in power with onboarding levetiracetam.28 Overall, our findings demonstrating influence of ASMs on certain SEEG FC measures, but not others, warrant further exploration.
Our results demonstrate that between ImCoh and PDC inward consistently exhibit significant differences in EZs vs non-EZs when DLS is stable over 3 consecutive days. Similarly, other studies using MEG and resting-state scalp EEG have demonstrated that ImCoh has varied test–retest reliability depending on the brain network tested in patients with schizophrenia22 and controls.23 Also, in a study measuring FC in controls using MEG, PDC was the most robust directed measure.24 Prior work has suggested tonic inward inhibition of EZs during interictal periods,49 and robustness of directed measures may reflect the importance of signal directionality. Höller et al.47 noted directed measures were more robust across longer time spans, and proposed this is because they take previous signals into account, whereas nondirected metrics do not. Furthermore, Wang et al.21 demonstrated stability of FC in iEEG over 10-second to 1-hour segments collected at different times of day, suggesting the effect of time is minimal. Our study further demonstrates stability over multiple days.
Utilizing retrospective recordings (eyes-open pseudo-rest) for FC measurements may make analyses more accessible to other groups to aid EZ localization without necessitating formal prospective resting-state data collection. Interestingly, we found similar patterns of higher inward directed and nondirected FC in EZs vs non-EZs during pseudo-rest, resembling resting-state results. However, we observed no correlation between PDC measurements during resting-state and pseudo-rest conditions, while ImCoh values were highly correlated between states. Although the etiology for this distinction is unclear, posterior dominant alpha rhythm is more prominent in the eyes-closed state,50 which may potentially influence FC measurements. Although we included analyses in other frequency bands in prior work,11 future studies evaluating potential relationships between recording condition and FC across different frequencies may be valuable. Overall, whereas pseudo-rest conditions may be sufficient to help localize EZs from retrospective recordings, PDC measures may perform better using eyes-closed resting state.
Pseudo-rest SEEG data were collected retrospectively, limiting control and standardization of patient condition and introducing more artifact and variance. Our patients had various lesional types, anatomic locations of EZs, and treatments, adding heterogeneity, but this heterogeneity in our cohort strengthens our results, making them more widely applicable. Our sample size of 32 limits power of subgroup analyses, but to our knowledge is the largest cohort of patients undergoing SEEG whose FC has been analyzed in relation to ASMs.
Another limitation of our study is that we excluded pure electrographic or paucisymptomatic seizures, due to variability in documentation of these seizures (e.g., interpretation of a long interictal discharge vs brief electrographic asymptomatic seizure), and given that our primary interest was in clinical symptomatic seizures. Further studies may consider including these seizure types, when possible, to evaluate any potential influence on FC measures. Induction methods to elicit seizures may affect FC, but seizure induction other than ASM taper was not routinely performed in our patient cohort during days 1–7. Furthermore, EZ designations were determined clinically from ictal recordings interpreted by epileptologists, and as the definition of EZ varies in the literature, there likely exists interrater variability in what is considered an EZ.
Our analysis of 2-minute segments was chosen to avoid interictal spikes, therefore spike burden was not accounted for in our analysis. However, in longer collection segments where spikes cannot be avoided, baseline spike activity and neurophysiology of anatomic regions should be considered and accounted for. Current and prior studies have found differences in EZ FC despite varied anatomical locations of EZs, suggesting these findings are not driven by anatomic location.10,11 Windowing data into shorter time segments may improve the resolution of results and should be performed in future studies. As demonstrated by Wang et al., spatial correlation of contact pairs to minimize signal redundancy due to proximity would likely improve resolution of results, but was not performed in our study and should be considered in future studies.21
We analyzed FC methods in alpha band due to large peaks found in this range and high test–retest reliability,42 yet there may be other discernible FC changes in SEEG data related to ASMs in other bands. FC changes could be related to baseline changes in power as ASMs are weaned and future studies could discern these trends within different frequency bands. Other groups have found unique neurophysiologic patterns of EZ out-links in different types of epilepsy, such as cortical developmental malformations and focal dysplasia.14 Although our patient cohort did not include these pathologies, their analysis may be an interesting future direction. Finally, we examined ImCoh and PDC based on previous work, but plan to explore additional FC paradigms in future studies.
Overall, our results suggest that higher inward directed and nondirected FC may help localize EZs using brief interictal recordings. These FC patterns persist over time and are resilient to ASM changes and patient condition, with some exceptions. ASMs may have greater influence on ImCoh results than PDC, and between ImCoh may best distinguish EZs during the “on”-medication vs “off”-medication state. Conversely, data collection condition may have a greater effect on PDC, such that PDC inward may best distinguish EZs from non-EZs during eyes-closed resting state. These findings are among the first to examine the influence of ASMs and data collection condition on SEEG FC in focal epilepsy and should be considered when choosing testing conditions and analysis paradigms for FC studies. More generally, it is important to consider confounders in FC analysis for broader use in clinical and research purposes.
Glossary
- ASM
antiseizure medication
- DLS
drug load score
- EMR
electronic medical record
- EMU
epilepsy monitoring unit
- EZ
epileptogenic zone
- FC
functional connectivity
- FDR
false discovery rate
- iEEG
intracranial EEG
- ImCoh
imaginary coherence
- IRB
institutional review board
- LME
linear mixed effects
- MEG
magnetoencephalography
- PDC
partial directed coherence
- RNS
responsive neurostimulation
- SEEG
stereo-EEG
- VUMC
Vanderbilt University Medical Center
Appendix. Authors
Study Funding
This work was supported in part by NIH grants R00 NS097618 (D.J.E.), R01 NS112252 (D.J.E.), K23 NS114178 (J.D.R.), and F31 NS106735 (H.F.J.G.), and the Vanderbilt Institute for Surgery and Engineering.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Engel J. Surgical Treatment of the Epilepsies. Raven; 1987. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Epilepsy Data and Statistics. September 30, 2020. https://www.cdc.gov/epilepsy/data/index.html. [Google Scholar]
- 3.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318. [DOI] [PubMed] [Google Scholar]
- 4.Engel J Jr., Wiebe S, French J, et al. . Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003;44:741-751. [DOI] [PubMed] [Google Scholar]
- 5.Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord. 2006;8(Suppl 2):S1–S9. [PubMed] [Google Scholar]
- 6.Englot DJ, Han SJ, Rolston JD, et al. . Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. 2014;14:386-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englot DJ, Raygor KP, Molinaro AM, et al. . Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75:648-645; discussion 655; quiz 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale F, Cossu M, Castana L, et al. . Stereoelectroencephalography. Neurosurgery. 2013;72:353-366. [DOI] [PubMed] [Google Scholar]
- 9.Khoo HM, Hall JA, Dubeau F, et al. . Technical aspects of SEEG and its interpretation in the delineation of the epileptogenic zone. Neurol Med Chir. 2020;60:565-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narasimhan S, Kundassery KB, Gupta K, et al. . Seizure-onset regions demonstrate high inward directed connectivity during resting-state: an SEEG study in focal epilepsy. Epilepsia. 2020;61:2534-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodale SE, González HFJ, Johnson GW, et al. . Resting-state SEEG may help localize epileptogenic brain regions. Neurosurgery. 2020;86:792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartolomei F, Bettus G, Stam CJ, Guye M. Interictal network properties in mesial temporal lobe epilepsy: a graph theoretical study from intracerebral recordings. Clin Neurophysiol. 2013;124:2345-2353. [DOI] [PubMed] [Google Scholar]
- 13.Bettus G, Wendling F, Guye M, et al. . Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58-68. [DOI] [PubMed] [Google Scholar]
- 14.Lagarde S, Roehri N, Lambert I, et al. . Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain. 2018;141:2966-2980. [DOI] [PubMed] [Google Scholar]
- 15.Englot DJ, Hinkley LB, Kort NS, et al. . Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. 2015;138:2249-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292-2307. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Sun Z, Tao R, Li K, Bao G, Yan X. Epileptic seizure detection based on partial directed coherence analysis. IEEE J Biomed Health Inform. 2016;20:873-879. [DOI] [PubMed] [Google Scholar]
- 18.Haneef Z, Levin HS, Chiang S. Brain graph topology changes associated with anti-epileptic drug use. Brain Connect. 2015;5:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian Y, Yang L, Xu W, et al. . Predictors for drug effects with brain disease: shed new light from EEG parameters to brain connectomics. Eur J Pharm Sci. 2017;110:26-36. [DOI] [PubMed] [Google Scholar]
- 20.Herff C, Krusienski DJ, Kubben P. The potential of stereotactic-EEG for brain-computer interfaces: current progress and future directions. Front Neurosci. 2020;14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Sinha N, Schroeder GM, et al. . Interictal intracranial electroencephalography for predicting surgical success: the importance of space and time. Epilepsia. 2020;61:1417-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candelaria-Cook FT, Stephen JM. Test-retest reliability of magnetoencephalography resting-state functional connectivity in schizophrenia. Front Psychiatry. 2020;11:551952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moezzi B, Hordacre B, Berryman C, Ridding MC, Goldsworthy MR. Test-retest reliability of functional brain network characteristics using resting-state EEG and graph theory. bioRxiv. Preprint posted online August 5, 2018. doi: 10.1101/385302 [DOI] [Google Scholar]
- 24.Colclough GL, Woolrich MW, Tewarie PK, Brookes MJ, Quinn AJ, Smith SM. How reliable are MEG resting-state connectivity metrics? Neuroimage. 2016;138:284-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ung H, Baldassano SN, Bink H, et al. . Intracranial EEG fluctuates over months after implanting electrodes in human brain. J Neural Eng. 2017;14:056011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaveri HP, Pincus SM, Goncharova II, et al. . Background intracranial EEG spectral changes with anti-epileptic drug taper. Clin Neurophysiol. 2010;121:311-317. [DOI] [PubMed] [Google Scholar]
- 27.Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335-343. [DOI] [PubMed] [Google Scholar]
- 28.Mecarelli O, Vicenzini E, Pulitano P, et al. . Clinical, cognitive, and neurophysiologic correlates of short-term treatment with carbamazepine, oxcarbazepine, and levetiracetam in healthy volunteers. Ann Pharmacother. 2004;38:1816-1822. [DOI] [PubMed] [Google Scholar]
- 29.Clemens B, Ménes A, Piros P, et al. . Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res. 2006;70:190-199. [DOI] [PubMed] [Google Scholar]
- 30.Joshi R, Tripathi M, Gupta P, Gulati S, Gupta YK. Adverse effects & drug load of antiepileptic drugs in patients with epilepsy: monotherapy versus polytherapy. Indian J Med Res. 2017;145:317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canevini MP, De Sarro G, Galimberti CA, et al. . Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51:797-804. [DOI] [PubMed] [Google Scholar]
- 32.Witt J-A, Nass RD, Baumgartner T, et al. . Does the accumulated antiepileptic drug load in chronic epilepsy reflect disease severity? Epilepsia. 2020;61:2685-2695. [DOI] [PubMed] [Google Scholar]
- 33.D'Haese P-F, Pallavaram S, Li R, et al. . CranialVault and its CRAVE tools: a clinical computer assistance system for deep brain stimulation (DBS) therapy. Med Image Anal. 2012;16:744-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmer Biomet ROSA brain 3.0 robotic surgery system. Biomed Saf Stand. 2020;50:12. [Google Scholar]
- 35.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9-21. [DOI] [PubMed] [Google Scholar]
- 37.Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968-980. [DOI] [PubMed] [Google Scholar]
- 38.Lüders HO. Critical analysis of seizure classifications. Electroencephalogr Clin Neurophysiol. 1993;87:S8. [Google Scholar]
- 39.Chu CJ, Kramer MA, Pathmanathan J, et al. . Emergence of stable functional networks in long-term human electroencephalography. J Neurosci. 2012;32:2703-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engel J Jr. Update on surgical treatment of the epilepsies: summary of the second international palm desert conference on the surgical treatment of the epilepsies (1992). Neurology. 1993;43:1612-1617. [DOI] [PubMed] [Google Scholar]
- 42.Hinkley LBN, Marco EJ, Findlay AM, et al. . The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One. 2012;7:e39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baccalá LA, Sameshima K. Partial directed coherence: a new concept in neural structure determination. Biol Cybern. 2001;84:463-474. [DOI] [PubMed] [Google Scholar]
- 44.Xiao F, Caciagli L, Wandschneider B, Vos S. Cognitive Burden of Anti-seizure Medications: A Language Functional MRI Perspective. Presented at: American Epilepsy Society annual meeting; 2020. [Google Scholar]
- 45.Joint Formulary Committee. British National Formulary. Accessed December 2, 2021. http://www.medicinescomplete.com. [Google Scholar]
- 46.Koerner TK, Zhang Y. Application of linear mixed-effects models in human neuroscience research: a comparison with Pearson correlation in two auditory electrophysiology studies. Brain Sci. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Höller Y, Uhl A, Bathke A, et al. . Reliability of EEG measures of interaction: a paradigm shift is needed to fight the reproducibility crisis. Front Hum Neurosci. 2017;11:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermans K, Ossenblok P, van Houdt P, et al. . Network analysis of EEG related functional MRI changes due to medication withdrawal in focal epilepsy. Neuroimage Clin. 2015;8:560-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tracy JI, Osipowicz K, Spechler P, et al. . Functional connectivity evidence of cortico-cortico inhibition in temporal lobe epilepsy. Hum Brain Mapp. 2014;35:353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Britton JW, Frey LC, Hopp JL, et al. . Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants. American Epilepsy Society; 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not freely available in a public repository due to restrictions in participant informed consent. However, if approved by our IRB, de-identified data can be made available upon request.