Abstract
Background and Objectives
Subarachnoid hemorrhage from cerebral aneurysm remains a devastating disease with high mortality and morbidity. Cerebral aneurysm and its rupture are more prevalent in postmenopausal women and have been postulated to be hormonally influenced. The goal of this study was to investigate the associations of female-specific factors, including reproductive life span, age at menarche, and age at menopause, with the incidence of aneurysmal subarachnoid hemorrhage (aSAH) in women.
Methods
Participants in the Nurses' Health Study were followed up from 1980 or the time of reaching menopause until 2018. Only women with natural menopause or surgical menopause due to bilateral oophorectomy were included. Reproductive life span was defined by subtracting the age at menarche from the age at menopause. Multivariable-stratified proportional hazards models were used to study reproductive life span, age at menarche, and age at menopause with the incidence of aSAH. Multivariable models were adjusted for age, race, smoking, hysterectomy, hypertension, hyperlipidemia, body mass index, hormone therapy use, oral contraceptive use, and parity.
Results
A total of 97,398 postmenopausal women with reproductive life span data were included; 138 participants developed aSAH, which was confirmed on medical record review by a physician. A shorter reproductive life span (≤35 years) was associated with a 2-fold higher incidence of aSAH after multivariable adjustment (hazard ratio [HR] 2.0 [95% CI 1.4–2.8]). Early age at menopause (age <45 years) was similarly associated with a higher risk of aSAH (HR 2.1 [95% CI 1.4–3.1]), but age at menarche was not. Use of oral contraceptives and postmenopausal hormone therapy was not associated with the incidence of aSAH.
Discussion
An earlier age at menopause and a shorter reproductive life span duration (≤35 years) were associated with a higher risk of incident aSAH in women. No associations were noted for age at menarche, parity, oral contraceptive use, or postmenopausal therapy use.
Subarachnoid hemorrhage (SAH) from cerebral aneurysm rupture remains a devastating disease with high mortality and morbidity. Postmenopausal women have a higher incidence of aneurysmal SAH (aSAH) and subsequent complications, including cerebral vasospasm,1-3 than premenopausal women.
Although it remains unclear why the rate of SAH is higher in postmenopausal women, hormonal influences have been postulated in the pathogenesis of cerebral aneurysms and their rupture. Specifically, the loss of estrogens with menopause has been implicated to play a role in the development of cerebral aneurysms.4,5 Some retrospective studies have suggested that earlier age at menopause is associated with increased risk of aneurysmal rupture.6-8 However, existing studies cannot account for up to one-third of individuals who die at the time of aneurysmal rupture. The rarity of aSAH has made prospective studies with sufficient reproductive data difficult to achieve. Using a large prospective cohort study, the Nurses' Health Study (NHS), we investigated the association between reproductive life span, age at menarche, age at menopause, and the use of oral contraceptives or postmenopausal hormone therapy and aSAH in women.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the Partners Institutional Review Board of the Brigham and Women's Hospital (Boston, MA) for human experimentation, and participants gave informed consent to be included in the study.
Study Population
The NHS is a large prospective cohort study with long-term follow-up and detailed characterization of reproductive and medical history. The NHS was established in 1976 and included 121,701 female registered nurses 30 to 55 years of age living across the United States. Baseline data were collected in 1976, and questionnaires were mailed to participants biennially to collect information on medical history, including SAH, and lifestyle factors, including height, weight, menopausal status, age at menopause, and hormone therapy use. Baseline reproductive life span variables were first gathered in 1980. The follow-up rate is >90%.
Women were eligible for the current analysis beginning in 1980 if they were postmenopausal or at the time of reporting menopause during follow-up. Our study population excluded women with aSAH occurrence before 1980 (n = 33), death before 1980 (n = 751), and missing year of birth (n = 170). Due to the primary goals of the study, we excluded those with missing age at menarche (n = 958) and those with age at menarche >18 years (n = 71) because delays may have had pathologic causes. Due to an inability to assign a reliable date of menopause, participants with no postmenopausal data (n = 5,756) were excluded, as were women who had a hysterectomy before menopause but did not undergo bilateral oophorectomy (n = 16,564). After exclusions, 97,398 participants with reproductive life span data were followed up for 38 years to 2018, of whom 138 had experienced a documented aSAH.
Reproductive Life Span
Age at menarche, defined as the first menstrual period, was self-reported on the baseline 1976 questionnaire. Age at menopause was defined as the age when the participant's menstrual period ceased permanently and was recorded biennially on questionnaires. In addition, menopause due to surgical intervention (hysterectomy with bilateral oophorectomy) was reported. Women with hysterectomy without bilateral oopherectomy before menopause were excluded from analyses of age at menopause and reproductive life span because age at menopause could not be accurately established in these individuals. Self-reported menopause status and age at menopause were previously validated in a subsample of the NHS participants, with 98.8% reproducibility of menopause status.9 Duration of reproductive life span was calculated by subtracting age at menarche from age at menopause. On the basis of prior association of reproductive variables with cardiovascular disease,10 dichotomous cut points were created: reproductive life span was dichotomized as ≤35 or >35 years; age at menopause was categorized as <45 and ≥45 years; and age at menarche was categorized as ≤11 and >11 years. Postmenopausal hormone therapy use was queried on biennial questionnaires and defined as current, past, or never use. Parity was defined as the number of pregnancies lasting at least 6 months or number of children on questionnaires from 1976 through 1984. Oral contraceptive use was queried on biennial questionnaires and categorized as never or former use.
Additional aSAH Risk Factors
Medical, reproductive, and lifestyle risk factors were obtained from biennial questionnaires. Age and race were recorded from the initial baseline data collection in 1976. Smoking, hypertension, hypercholesterolemia, diabetes, and history of hysterectomy were assessed from physician reports and updated on biennial questionnaires. Body mass index (BMI) was calculated from self-reported weight and height. Self-reported weights have previously been demonstrated to be highly correlated with measured weights (r = 0.97).11 BMI was categorized into 4 groups: <18.5, 18.5 to <25, 25 to <30, and ≥30 kg/m2.
Aneurysmal SAH
On each biennial questionnaire, participants were asked about recent medical history, including stroke. Deaths were reported by the postal service or next of kin or by search of the National Death Index. Follow-ups for deaths have been shown to be >98% with all sources combined.12 Medical records were requested from all participants who reported stroke and reviewed by physicians to classify the type of stroke, including SAH. To confirm aneurysmal origin of SAH, documentation of aneurysm by angiogram, CT, or MRI was required. SAHs without documented aneurysms were not included in the current analyses.
Statistical Analysis
For each participant, person-time was allocated according to the categories of reproductive variables from the earliest questionnaire after menopause occurrence to the date of diagnosis of aSAH, death, or the end of follow-up, whichever came first. Stratified proportional hazards models were performed to examine the association of reproductive life span, age at menopause, and age at menarche with aSAH. Multivariable proportional hazards models were additionally performed for clinically relevant factors, including race, smoking status, hysterectomy, hypertension, BMI, postmenopausal hormone therapy use, oral contraceptive use, and parity. All covariates were time-varying variables, except for race and ethnicity and menopause type, which were time invariant. Postmenopausal hormone therapy and parity had missing values (<3% missing values), and an indicator variable for missing was generated and treated as a separate category in analyses. All other variables had no missing values. A sensitivity analysis was performed in the subgroup of participants who underwent natural menopause and those with information available on alcohol intake. A 2-sided value of p < 0.05 was considered statistically significant. All data analyses were performed with SAS software (version 9.4 for UNIX; SAS Institute Inc, Cary, NC).
Data Availability
Due to the sensitive nature of the data collected for this study, data not published within this article may be shared (anonymized) at the request of any qualified investigator trained in human participant confidentiality protocols by sending to the NHS (nhsaccess@channing.harvard.edu).
Results
Characteristics of the Study Population and aSAH
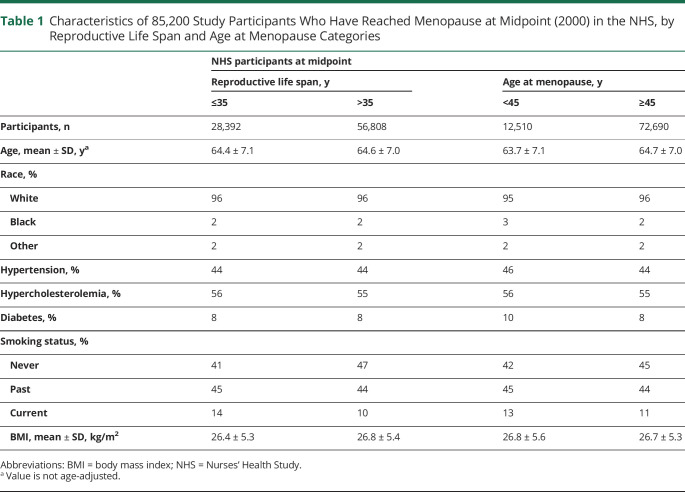
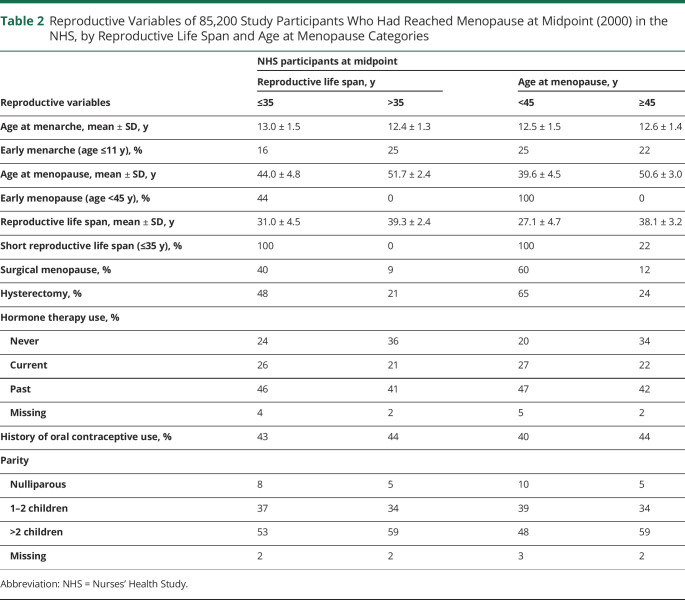
A total of 97,398 participants were followed up for 38 years from 1980 to 2018. During 2,607,921 person-years of follow-up, we documented 138 incident cases of aSAH. Tables 1 and 2 give population characteristics of the study participants who had reached menopause at the midpoint of the study (2000). There was a higher percent of surgical menopause (40% vs 9%) and hysterectomy (48% vs 21%) in the cohort with a short reproductive life span (age ≤35 years). Similarly, surgical menopause (60% vs 12%) and hysterectomy (65% vs 24%) were higher in the cohort with shorter age at menopause (age <45 years). During follow-up, 138 participants developed aSAH. At the time of aSAH, the mean age was 61.6 ± 8.7 years (Figure 1).
Table 1.
Characteristics of 85,200 Study Participants Who Have Reached Menopause at Midpoint (2000) in the NHS, by Reproductive Life Span and Age at Menopause Categories
Table 2.
Reproductive Variables of 85,200 Study Participants Who Had Reached Menopause at Midpoint (2000) in the NHS, by Reproductive Life Span and Age at Menopause Categories
Figure 1. Age of Participants Who Developed aSAH (n = 138).
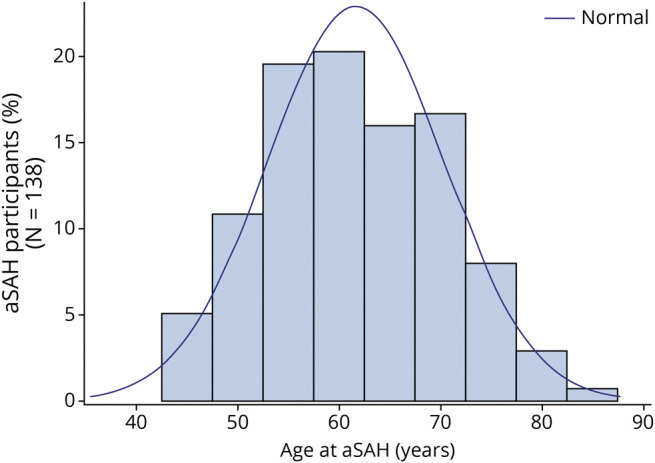
aSAH = aneurysmal subarachnoid hemorrhage.
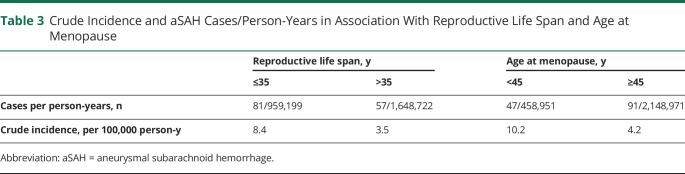
The incidence of aSAH in our study was 5 per 100,000 person-years (Table 3). When stratified by reproductive life span, the aSAH incidence was 8 per 100,000 person-years for the short reproductive life span and 3 per 100,000 person-years for long reproductive life span. Younger and older women at menopause had an aSAH incidence of 10 and 4 per 100,000 person-years, respectively.
Table 3.
Crude Incidence and aSAH Cases/Person-Years in Association With Reproductive Life Span and Age at Menopause
Association Between Reproductive Life Span and aSAH
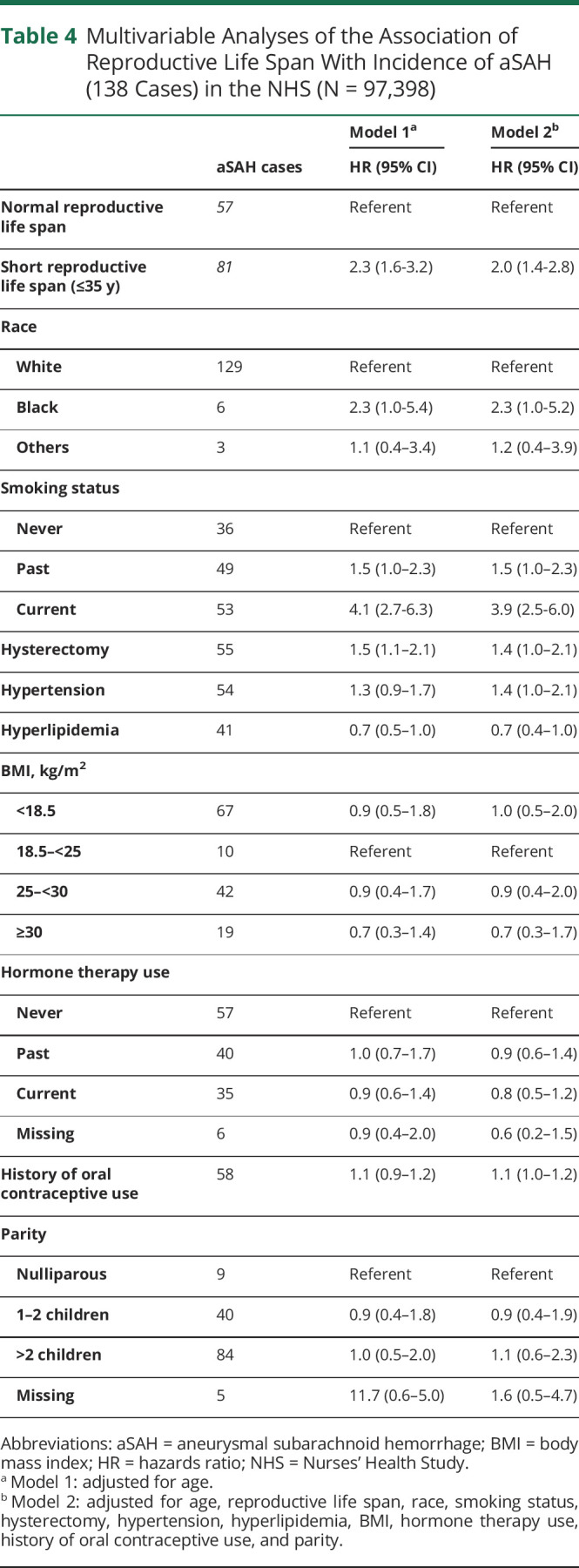
Table 4 presents the associations between reproductive life span and incidence of aSAH. In the age-adjusted model (model 1), women with a shorter reproductive life span (≤35 years) had a higher risk of aSAH compared to those with longer reproductive life span (>35 years) (hazard ratio [HR] 2.3 [95% CI 1.6–3.2]). A 2-fold risk remained (HR 2.0 [95% CI 1.4–2.8]) after multivariable adjustment for race, smoking status, hysterectomy, hypertension, hyperlipidemia, BMI, hormone therapy use, oral contraceptive use, and parity (model 2). Hormone therapy use, oral contraceptive use, and parity were not associated with aSAH in the multivariable analyses. We confirmed previously noted associations aSAH and smoking, finding a 4-fold higher risk of aSAH among current smokers after multivariable adjustment (HR 3.9 [95% CI 2.5–6.0]) compared to never smokers. Women who identified as Black exhibited a 2-fold increased risk of aSAH compared to those who identified as White in multivariable models (HR 2.3 [95% CI 2.5–6.0]).
Table 4.
Multivariable Analyses of the Association of Reproductive Life Span With Incidence of aSAH (138 Cases) in the NHS (N = 97,398)
To investigate whether the observed association was influenced by the shorter reproductive life span among those with surgical menopause, a sensitivity analysis was performed, restricting to those with natural menopause. Shorter reproductive life span remained associated with an increased risk of aSAH among those who had natural menopause (multivariable HR 2.5 [95% CI 1.6–3.8]).
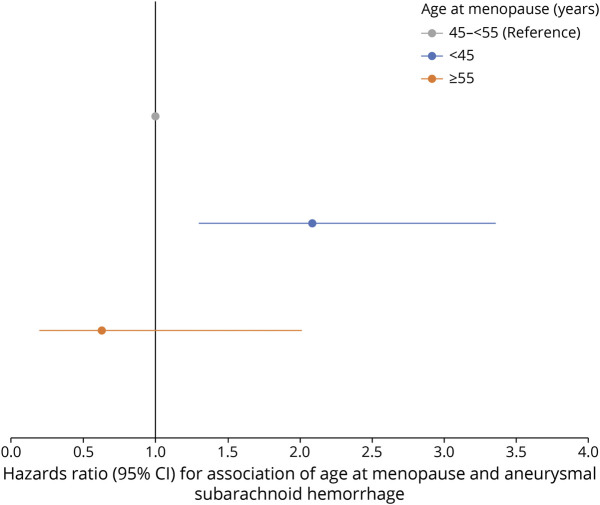
Association Between Age at Menopause and Menarche and aSAH
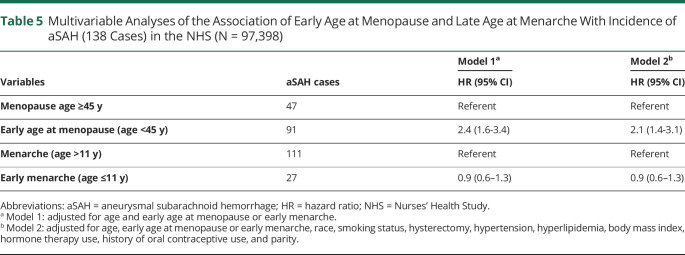
In age-adjusted analyses, early age at menopause (<45 years) was also associated with aSAH (HR 2.4 [95% CI 1.6–3.4]) (Table 5, model 1). After multivariable adjustment, early menopause remained significantly associated with aSAH (HR 2.1 [95% CI 1.4–3.1]) (Figure 2). In a sensitivity analysis restricted to women who had natural menopause, age at menopause <45 years was associated with a 3-fold increased risk of aSAH compared to older age at menopause (multivariable HR 3.0 [95% CI 1.9–4.8]).
Table 5.
Multivariable Analyses of the Association of Early Age at Menopause and Late Age at Menarche With Incidence of aSAH (138 Cases) in the NHS (N = 97,398)
Figure 2. Association of Age at Menopause (<45 and >55 years vs 45–55 years) and aSAH in the NHS (N = 97,398).
aSAH = aneurysmal subarachnoid hemorrhage; NHS = Nurses’ Health Study.
Younger age at menarche (age ≤ 11) was not associated with the incidence of aSAH (multivariable HR 0.9 [95% CI 0.6–1.3], Table 5, model 2).
Discussion
In this prospective cohort study, we examined the association between reproductive exposures and the incidence of aSAH in women. We found that a shorter duration of a woman's reproductive life span and a younger age at menopause were associated with a higher risk of aSAH. These associations remained significant after adjustment for medical comorbid conditions and lifestyle factors. In addition, the observed associations were consistent with those observed among a subgroup of women with natural menopause. No associations were noted for age at menarche, parity, oral contraceptive use, or postmenopausal therapy use.
The overall global incidence of aSAH has been reported to be an average of 7.9 per 100,000 person-years, with the global SAH incidence declining from 10.2 to 6.1 per 100,000 person-years from 1980 to 2010, with the declining trend attributed to decreases in systolic blood pressure and smoking prevalence.13,14 In our study population, we report an incidence of 5.3 per 100,000 person-years, with a higher incidence of aSAH among women with a shorter reproductive life span (8 per 100,000 person-years) and younger age at menopause (10 per 100,000 person-years).
Postmenopausal women are at a disproportionately higher risk for the development of cerebral aneurysms.2,15-18 Estrogens are thought to play a protective role against the development of cerebral aneurysms through regulation of the vasculature and the inflammatory process.4 The endogenous estrogens estradiol and estrone bind to estrogen receptors (ESR1 and ESR2) in the cell nucleus to mediate long-term alteration of downstream gene expression. These receptors have been identified in human cerebral vascular endothelial cells and demonstrated in in situ studies to interact with estrogens.19,20
The production of estrogen dramatically declines with menopause, which has been postulated to explain the increased development of cerebral aneurysms in women after their fourth and fifth decades of life.17,21 Murine models support this theory and have demonstrated increased intracranial aneurysm development with the loss of estrogen by ovariectomy and the restoration of its protective affect after the administration of estrogen agonists.5 However, the study of estrogens and aSAH is more complex and insufficient in humans. Several case-control studies have suggested an association between early menopause and the development of aSAH,6,8,21 but these studies were retrospective in nature. Case-control studies are limited by not being able to ascertain reproductive data from fatal aSAH cases (which may account for up to one-third of aSAHs) and may suffer from recall bias. Although an association between reproductive life span and increased risk of ischemic stroke has been reported,22 it has not been previously explored for aSAH. Using a prospective cohort, we found an association between early age at menopause and shorter reproductive life span with aSAH. We did not observe an association between the age at menarche and aSAH.
The association of postmenopausal hormone therapy use and the development of cerebral aneurysms and aSAH remains controversial. While some studies have found a protective association for aSAH with hormone therapy,17,23-25 others have found the opposite association with increased aSAH.26 The differences observed may be due to several factors, including missing information on the type of postmenopausal hormone therapy used, duration of use, and the evolution of hormone therapy agents over time. There are currently no guidelines recommending for or against the use of postmenopausal hormone therapy with respect to cerebral aneurysms. The largest evaluation of postmenopausal hormone therapy and SAH was in the Women's Health Initiative Study, which analyzed 93,676 women in the Observational Study and 114 SAH outcomes. The rate of SAH was higher in women who were current users of hormone therapy, but a higher rate was not observed for women who used estrogen-only therapy. However, the study did not examine aSAH.26 Our study found no association between postmenopausal hormone therapy use and aSAH. Because of our limited sample size, we were unable to perform subgroup analyses among users of estrogen-only or estrogen-progesterone hormone therapy or on the association with duration of hormone therapy use. We hope that future studies can address this issue.
Multiple studies have suggested an association between SAH and the use of oral contraceptives.25,27,28 A meta-analysis of 21 studies found an increased risk of SAH, albeit a weak association.27 This effect was not observed, however, when limited to aSAH.23,29 We did not find an association with oral contraceptive use and aSAH. aSAHs have a different etiology than other forms of SAH, which may explain the lack of an association in this subgroup. Furthermore, most aSAHs occur later in life, and the association with oral contraceptive use may have been masked by other factors such as hormone therapy use and reproductive life span. The association with parity, pregnancy, and SAH is similarly controversial. While several studies have found a protective effect of parity against SAH,30,31 others have found increased number of children to be associated with SAH.32 A systematic review of 16 studies did not find an association of SAH with pregnancy.25 A case-control study with aSAH also did not find an association with the number of births, age at pregnancy, or age at first birth.29 Similar to this study, we did not find an association with parity and aSAH.
Several limitations should be considered. First, we observed a relatively small number of aSAHs due to the rarity of aSAH; however, this represents the largest prospective analysis of reproductive factors and aSAH. Furthermore, our study included only individuals with aSAH and not individuals with undiagnosed or unruptured cerebral aneurysms or SAH for which an aneurysmal source could not be confirmed. We chose aSAH so that the mechanisms would be more specific, but this may have contributed to the limited sample size in this study. We performed sensitivity analyses excluding women with surgical menopause and found similar associations for reproductive life span and younger age at menopause in this group with natural menopause; however, the number of cases was too small to examine surgical menopause alone. Moreover, the high percent of female nurses of European ancestry may also limit the generalizability of these findings to other racial and ethnic groups. The study also does not account for factors known to be associated to aSAH that were not collected on the questionnaires such as family history of SAH or intracranial aneurysms. While alcohol use has been associated with an increase in aSAH,33,34 none of the cases with aSAH in our study had self-reported alcohol dependence. The NHS included a questionnaire for number of drinks per week in a year, but responses for 42% of the aSAH cases were missing and therefore not included in the final analysis. In a separate sensitivity analysis including only those with reported drinks per week, short reproductive life span remained significantly associated with aSAH cases (HR 1.9 [95% CI 1.3–2.7]). Moreover, the results of this study do not evaluate the risk of aSAH in women who were assigned male sex at birth or intersex individuals or their risks associated with hormone therapy treatments. Despite the limitations, the strengths of the current study are its prospective nature and detailed reproductive, medical, and lifestyle information, which prior retrospective studies were unable to provide.
Despite aSAH being a relatively rare event, we found that shorter reproductive life span and younger age at menopause were associated with the development of aSAH in women, supporting a role of estrogens in the pathogenesis of aSAH.
Glossary
- aSAH
aneurysmal SAH
- BMI
body mass index
- HR
hazard ratio
- NHS
Nurses' Health Study
- SAH
subarachnoid hemorrhage
Appendix. Authors
Study Funding
Supported by Brigham and Women's Hospital, BPN044-2491, as well as NHS UM1 CA186107 and R01 HL088521.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Lin B, Chen W, Ruan L, et al. Sex differences in aneurysm morphologies and clinical outcomes in ruptured anterior communicating artery aneurysms: a retrospective study. BMJ Open. 2016;6(4):e009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000;31(2):392-397. [DOI] [PubMed] [Google Scholar]
- 3.Rosalind Lai PM, Gormley WB, Patel N, Frerichs KU, Aziz-Sultan MA, Du R. Age-dependent radiographic vasospasm and delayed cerebral ischemia in women after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;130:e230-e235. [DOI] [PubMed] [Google Scholar]
- 4.Harrod CG, Batjer HH, Bendok BR. Deficiencies in estrogen-mediated regulation of cerebrovascular homeostasis may contribute to an increased risk of cerebral aneurysm pathogenesis and rupture in menopausal and postmenopausal women. Med Hypotheses. 2006;66(4):736-756. [DOI] [PubMed] [Google Scholar]
- 5.Tada Y, Wada K, Shimada K, et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63(6):1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown S. Early menopause linked to increased risk of cerebral aneurysm. Menopause Int. 2012;18(3):97-98. [DOI] [PubMed] [Google Scholar]
- 7.Ding C, Toll V, Ouyang B, Chen M. Younger age of menopause in women with cerebral aneurysms. J Neurointerv Surg. 2013;5(4):327-331. [DOI] [PubMed] [Google Scholar]
- 8.Wang YX, He J, Zhang L, et al. A higher aneurysmal subarachnoid hemorrhage incidence in women prior to menopause: a retrospective analysis of 4,895 cases from eight hospitals in China. Quant Imaging Med Surg. 2016;6(2):151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319-325. [DOI] [PubMed] [Google Scholar]
- 10.Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11):e006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466-473. [DOI] [PubMed] [Google Scholar]
- 12.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837-839. [DOI] [PubMed] [Google Scholar]
- 13.Etminan N, Chang HS, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76(5):588-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87(11):1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminogo M, Yonekura M, Shibata S. Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke. 2003;34(1):16-21. [DOI] [PubMed] [Google Scholar]
- 16.Nabaweesi-Batuka J, Kitunguu PK, Kiboi JG. Pattern of cerebral aneurysms in a Kenyan population as seen at an urban hospital. World Neurosurg. 2016;87:255-265. [DOI] [PubMed] [Google Scholar]
- 17.Stober T, Sen S, Anstatt T, Freier G, Schimrigk K. Direct evidence of hypertension and the possible role of post-menopause oestrogen deficiency in the pathogenesis of berry aneurysms. J Neurol. 1985;232(2):67-72. [DOI] [PubMed] [Google Scholar]
- 18.Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women: a population-based case-control study. Ann Intern Med. 1994;121(3):168-173. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Liu Z, Gou Y, et al. Estradiol mediates vasculoprotection via ERRalpha-dependent regulation of lipid and ROS metabolism in the endothelium. J Mol Cell Cardiol. 2015;87:92-101. [DOI] [PubMed] [Google Scholar]
- 20.Tu J, Jufri NF. Estrogen signaling through estrogen receptor beta and G-protein-coupled estrogen receptor 1 in human cerebral vascular endothelial cells: implications for cerebral aneurysms. Biomed Res Int. 2013;2013:524324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabuchi S. Relationship between postmenopausal estrogen deficiency and aneurysmal subarachnoid hemorrhage. Behav Neurol. 2015;2015:720141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demel SL, Kittner S, Ley SH, McDermott M, Rexrode KM. Stroke risk factors unique to women. Stroke. 2018;49(3):518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D; Australasian Cooperative Research on Subarachnoid Hemorrhage Study Group. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: an international population-based, case-control study. Stroke. 2001;32(3):606-612. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Ouyang B, Goldstein-Smith L, Feldman L. Oral contraceptive and hormone replacement therapy in women with cerebral aneurysms. J Neurointerv Surg. 2011;3(2):163-166. [DOI] [PubMed] [Google Scholar]
- 25.Algra AM, Klijn CJ, Helmerhorst FM, Algra A, Rinkel GJ. Female risk factors for subarachnoid hemorrhage: a systematic review. Neurology. 2012;79(12):1230-1236. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Malik AA, Saeed O, Defillo A, Sherr GT, Suri MF. Hormone replacement therapy and the risk of subarachnoid hemorrhage in postmenopausal women. J Neurosurg. 2016;124(1):45-50. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SC, Colford JM Jr, Gress DR. Oral contraceptives and the risk of subarachnoid hemorrhage: a meta-analysis. Neurology. 1998;51(2):411-418. [DOI] [PubMed] [Google Scholar]
- 28.Petitti DB, Wingerd J. Use of oral contraceptives, cigarette smoking, and risk of subarachnoid haemorrhage. Lancet. 1978;2(8083):234-235. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto K, Horisawa R, Kawamura T, et al. Menstrual and reproductive factors for subarachnoid hemorrhage risk in women: a case-control study in Nagoya, Japan. Stroke. 2001;32(12):2841-2844. [DOI] [PubMed] [Google Scholar]
- 30.Levin S. Matiasmenos. S Afr Med J. 2003;93(9):666. [PubMed] [Google Scholar]
- 31.Yang CY, Chang CC, Kuo HW, Chiu HF. Parity and risk of death from subarachnoid hemorrhage in women: evidence from a cohort in Taiwan. Neurology. 2006;67(3):514-515. [DOI] [PubMed] [Google Scholar]
- 32.Jung SY, Bae HJ, Park BJ, Yoon BW; Acute Brain Bleeding Analysis Study Group. Parity and risk of hemorrhagic strokes. Neurology. 2010;74(18):1424-1429. [DOI] [PubMed] [Google Scholar]
- 33.Can A, Castro VM, Ozdemir YH, et al. Alcohol consumption and aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2018;9(1):13-19. [DOI] [PubMed] [Google Scholar]
- 34.Juvela S, Hillbom M, Numminen H, Koskinen P. Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke. 1993;24(5):639-646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the sensitive nature of the data collected for this study, data not published within this article may be shared (anonymized) at the request of any qualified investigator trained in human participant confidentiality protocols by sending to the NHS (nhsaccess@channing.harvard.edu).